Abstract
Background
It remains unclear if white matter hyperintensity (WMH) on magnetic resonance imaging adds relevant cerebrovascular prognostic information beyond vascular risk factors and demographics alone.
Methods and Results
We performed a post hoc analysis of hypertensive individuals in SPRINT‐MIND (Systolic Blood Pressure Intervention Trial–Memory and Cognition in Decreased Hypertension). The primary outcome was incident stroke or cognitive impairment (mild cognitive impairment or dementia). We fit logistic regression models with the predictors of Atherosclerotic Cardiovascular Disease Risk Score, age, sex, race, education, current cigarette smoking, and the SPRINT‐MIND randomization arm. WMH was subsequently included in the model to determine if it improved area under the receiver operating curve using the DeLong test. We used a structural equation model to determine the indirect effect on the primary outcome mediated through WMH. We included 727 individuals (mean age at baseline 67.7±8.4 years, 61.1% were men, 62.6% were non‐Hispanic White, and mean years of follow‐up was 3.6±0.9). Of the 727 individuals, 67 (9.2%) developed incident stroke or cognitive decline. The area under the receiver operating curve of the baseline model (without WMH) was 0.75 (95% CI, 0.70–0.81), and after the addition of WMH it increased to 0.81 (95% CI, 0.76–0.86) (P=0.004 for difference). The mediation analysis showed that 26.3% of the vascular risk's effect on the primary outcome is indirectly mediated through WMH.
Conclusions
In adult hypertensive individuals, we found that the addition of WMH to models predicting incident stroke or cognitive impairment improved the prognostic ability above vascular risk and demographics alone to a level consistent with excellent prediction.
Registration Information
REGISTRATION: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01206062.
Keywords: brain magnetic resonance imaging, cognitive impairment, stroke, vascular risk, white matter hyperintensity
Subject Categories: Cerebrovascular Disease/Stroke, Cognitive Impairment, Intracranial Hemorrhage, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- SPRINT‐MIND
Systolic Blood Pressure Intervention Trial–Memory and Cognition in Decreased Hypertension
- WMH
white matter hyperintensity
Clinical Perspective.
What Is New?
In adult hypertensive individuals, we found that the addition of white matter hyperintensity volume on magnetic resonance imaging of the brain to models predicting incident stroke or cognitive impairment improved the prognostic ability above vascular risk and demographics alone to a level consistent with excellent prediction.
What Are the Clinical Implications?
The clinical implications are that magnetic resonance imaging–detected white matter hyperintensity, a marker of cerebral small vessel disease often available in routine clinical care, adds meaningful and independent risk assessment when considering vascular outcomes specific to the brain.
Brain health is central to healthy aging. 1 , 2 The prevalence of magnetic resonance imaging (MRI)‐detected white matter hyperintensity (WMH) is a neuroimaging manifestation of cerebral microvascular disease that increases with age, and it is prevalent in at least half of community‐dwelling individuals >60 years of age. 3 , 4 , 5 , 6 In a recent meta‐analysis, moderate or advanced WMH was a stronger biomarker of the risk of cognitive impairment and stroke. 7 Vascular risk factors, such as hypertension, are strongly linked to the extent of WMH in an individual. 8 These data suggest that WMH is on the causal pathway linking vascular risk factors to the clinical end points of cognitive impairment and stroke. However, it is unclear whether WMH adds relevant prognostic information beyond vascular risk factors alone.
In clinical practice, vascular risk (including stroke risk) is typically quantified using the Pooled Cohort Equations Cardiovascular Risk (Atherosclerotic Cardiovascular Disease [ASCVD] Risk Score), consistent with American College of Cardiology/American Heart Association guidelines. 9 , 10 Vascular risk and risk scores are also highly correlated with the emergence of cognitive impairment. 11 , 12 However, prior research has not determined if the addition of WMH to models predicting stroke and cognitive impairment, the primary neurologic manifestations of vascular risk, improves their accuracy.
Methods
We performed a post hoc cohort study of the SPRINT‐MIND (Systolic Blood Pressure Intervention Trial–Memory and Cognition in Decreased Hypertension) study, using a deidentified data set that is publicly available at https://biolincc.nhlbi.nih.gov/studies/sprint/. 13
The hypothesis of our study is that WMH will significantly improve the discriminative ability compared with vascular risk alone in models fit to the primary outcome of incident stroke or cognitive impairment, defined as incident mild cognitive impairment or dementia, in adults with the vascular risk factor of hypertension. We included adult hypertensive individuals ≥50 years of age (SPRINT [Systolic Blood Pressure Intervention Trial] enrollment criterion) with a baseline study MRI in SPRINT‐MIND, which had 3‐dimensional volumetric quantification of WMH using methodology that has previously been described. 14 In short, WMH was quantified in the SPRINT‐MIND study on a 1‐mm isotropic fluid‐attenuated inversion recovery sequence acquired on a 3T MRI scanner using an automated pipeline for skull stripping and correction of inhomogeneities. WMH burden was subsequently quantified using a deep learning–based segmentation method and was quality checked by a neuroradiologist. 14 We did not exclude individuals based on advanced age or prior cardiovascular events.
The primary outcome of incident stroke, ischemic and hemorrhagic, was formally adjudicated in SPRINT, 15 as were mild cognitive impairment and dementia in SPRINT‐MIND, both using methodology that has been previously described, and relied on screening followed by committee adjudication. 13 The stroke adjudication was based on the World Health Organization definition and includes ischemic stroke (American Heart Association brain infarction criteria 16 ), intracerebral/intraparenchymal hemorrhage, intraventricular hemorrhage, or subarachnoid hemorrhage. 17
Statistical Analysis
We report baseline demographics for the full cohort and compare them between those with versus without the primary outcome with the χ2 or Fisher exact test for categorical variables, Mann‐Whitney U test for ordinal variables, and Student t test for interval variables. We fit logistic regression models to the outcome of incident stroke/cognitive impairment. We verified that the models did not have multicollinearity, defined as a variance inflation factor <5, met the Hosmer‐Lemeshow goodness‐of‐fit assumption (P≥0.05, 10 groups specified), and had a Brier score <0.1 (10 groups specified). 18 , 19 We transformed age from an interval variable to ordinal (<60, 60–69, 70–79, ≥80 years), because the variance inflation factor was 16.03 as an interval variable, and in ordinal categories it met the criteria of <5.
The baseline model included vascular risk, defined as the 2014 version of the ASCVD Risk Score, 10 with and without WMH. Both WMH and vascular risk were transformed into deciles due to right skew. It was not possible to use established ASCVD risk cut points (0%–4.9% low‐risk, 5%–7.49% borderline risk, 7.5%–19.9% intermediate risk, ≥20% high risk) because of low counts below intermediate risk. In a second model, we further adjusted for age (<60, 60–69, 70–79, ≥80 years), sex, race, education (less than college, college, graduate school), current cigarette smoking, and SPRINT randomization arm (intensive versus standard blood pressure control). These covariates were selected a priori and confirmed to be valid confounders using least absolute shrinkage and selection operator methodology. 20 Although the calculation of the ASCVD Risk Score uses age, sex, race, and smoking, the values of these covariates provide additional information and did not introduce multicollinearity.
To determine the improvement after the addition of WMH, we compare the models' area under the receiver operating curve (AUC) using the DeLong test and report the relative integrated discrimination index as well as a decision curve analysis. 21 , 22 , 23 In models containing WMH, a relative integrated discrimination index that is greater than the inverse of the number of variables in the model without WMH indicates that WMH is a better predictor than the average of the other variables. 24 To confirm the predictive performance of our models, we also used 5‐fold cross‐validation to generate a cross‐validated AUC that applied the bootstrap procedure to obtain a bias‐corrected 95% CI. 25 We report the consistency between the average AUC of the 5‐fold cross‐validated model and the actual AUC of the model.
As a sensitivity analysis, we fit a time‐to‐event model to the primary outcome and compare the Somers D with a 95% CI for the same models with and without WMH to determine if the addition of WMH improves prediction of failure time. To calculate Somers D after Cox proportional hazards models we used the somersd package in Stata 17.0 (StataCorp, College Station, TX), with estimates assumed to have a t distribution with n–1 degrees of freedom and Fisher Z transformation, consistent with recommendations for comparing time‐to‐event models. 26 We also report the AUC of the time‐to‐event models at 1800 days using the stroccurve package. 27 Finally, as an exploratory analysis, we used structural equation model to determine the direct effect of vascular risk and WMH on the risk of the primary outcome and the indirect effect mediated through WMH, which was bootstrapped 100 times. All statistical analyses were performed in Stata 17.0, and significance was defined as a 2‐sided P<0.05.
Standard Protocol Approvals, Registrations, and Participant Consents
All participating sites in SPRINT‐MIND obtained approval from a local or central institutional review board. The study was conducted according to Good Clinical Practice and the Declaration of Helsinki. Participants provided written informed consent before enrollment. The study is registered with ClinicalTrials.gov (NCT01206062).
Results
Of the 767 individuals enrolled in the SPRINT‐MIND MRI substudy, we included 727 and excluded 40 who did not have enough data to calculate vascular risk. The mean age at baseline was 67.7±8.4 years, 61.1% were men, 62.6% were non‐Hispanic White (31.0% Black, 4.8% Hispanic, 1.6% other including Asian, American Indian or Alaska Native, and Native Hawaiian or Other Pacific Islander), 57.2% had less than a college diploma, 13.5% were current smokers, the median vascular risk was 16.8% (11.1–28.0), 5.1% died during follow‐up, and the median (interquartile range) years of follow‐up was 3.8 (3.3–4.1) (Table). Violin plots of the WMH volume by deciles and vascular risk by deciles is seen in Figure 1. WMH volume was positively correlated with vascular risk (correlation coefficient, 0.28; P<0.001). Of the 727 total individuals, 67 (9.2%) developed incident stroke or cognitive decline, of which 10 events were stroke, 14 were dementia, and 42 were mild cognitive impairment.
Table .
Baseline Demographics in the Full Cohort and in Those With Versus Without the Primary Outcome
| Variable | Full cohort, n=727 | Incident stroke or cognitive impairment, n=67 | No incident stroke or cognitive impairment, n=660 | P value* |
|---|---|---|---|---|
| Age, y, mean±SD | 67.7±8.4 | 70.7±8.3 | 67.4±8.3 | 0.002 |
| Male sex, n (%) | 444 (61.1%) | 42 (62.7%) | 402 (60.9%) | 0.776 |
| Race and ethnicity, n (%) | 0.514 | |||
| White | 455 (62.6%) | 37 (55.2%) | 418 (68.3%) | |
| Black | 225 (31.0%) | 24 (35.8%) | 201 (30.5%) | |
| Hispanic | 35 (4.8%) | 5 (7.5%) | 30 (4.5%) | |
| Other† | 12 (1.6%) | 1 (1.5%) | 11 (1.7%) | |
| Education, n (%) | 0.003 | |||
| Less than college degree | 416 (57.2%) | 49 (73.1%) | 367 (55.6%) | |
| College degree | 121 (16.7%) | 12 (17.9%) | 109 (16.5%) | |
| Graduate school | 190 (26.1%) | 6 (9.0%) | 184 (27.9%) | |
| Current smoker, n (%) | 98 (13.5%) | 14 (20.9%) | 84 (12.7%) | 0.064 |
| Intensive blood pressure control, n (%) | 385 (53.0%) | 42 (62.7%) | 343 (52.0%) | 0.094 |
| White matter hyperintensity volume, median, IQR | 3.2, 1.5–6.1 | 5.6, 2.4–15.7 | 3.1, 1.5–5.8 | <0.001 |
| Atherosclerotic Cardiovascular Disease Risk Score, median, IQR | 16.8, 11.1–28.0 | 23.8, 15.6–37.9 | 16.3, 10.8–26.9 | <0.001 |
IQR indicates interquartile range.
P values are derived with the χ2 or Fisher exact test for categorical variables, Mann–Whitney U test for ordinal variables, and Student t test for interval variables.
Other includes Asian, American Indian or Alaska Native, and Native Hawaiian or Other Pacific Islander.
Figure 1. Violin plot of the deciles of white matter hyperintensity (top) and vascular risk (Atherosclerotic Cardiovascular Disease Risk Score) (bottom).
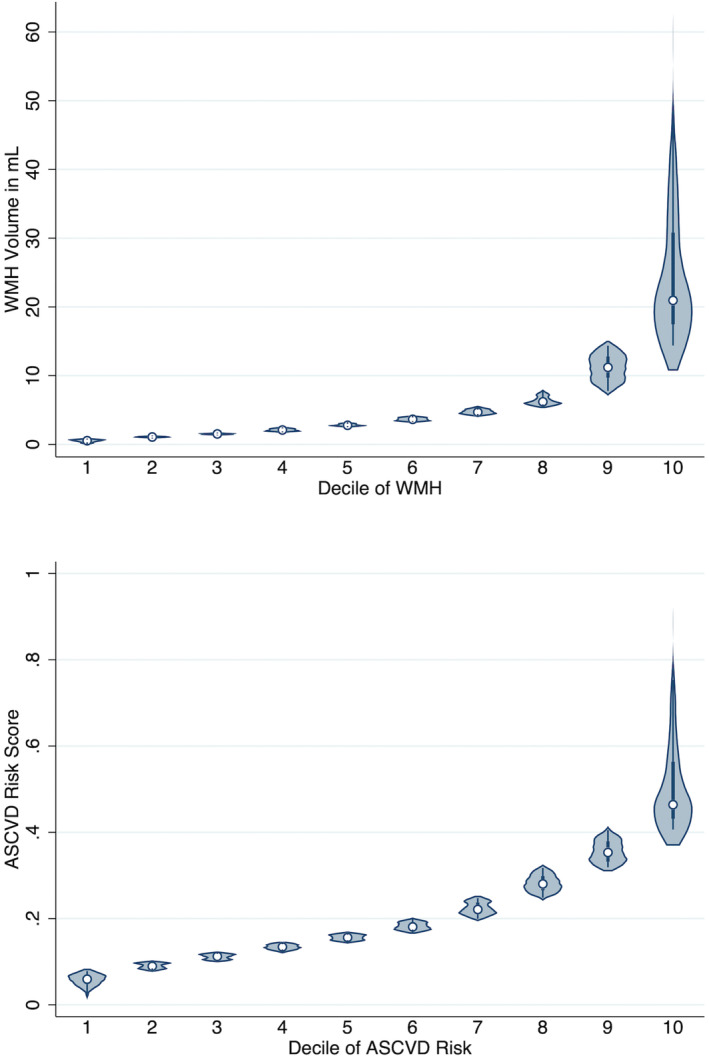
ASCVD indicates Atherosclerotic Cardiovascular Disease; and WMH, white matter hyperintensity.
In the baseline model, vascular risk without WMH, the Brier score was 0.081 and Hosmer‐Lemeshow P value was 1.0, whereas with WMH it was 0.078 and P=0.078, respectively. In the adjusted model, without WMH, the Brier score was 0.078 and Hosmer‐Lemeshow P value was 0.497, whereas with WMH it was 0.074 and P=0.794, respectively. Without WMH the AUC of the baseline model was 0.67 (95% CI, 0.60–0.73) and with WMH was 0.75 (95% CI, 0.69–0.80) (P=0.001 for difference). The 5‐fold cross‐validated AUCs for the models without and with WMH were concordant at 0.67 (95% CI, 0.55–0.70) and 0.75 (95% CI, 0.65–0.79) (Figure 2). In the model adjusted for vascular risk, age, sex, race, education, smoking, and randomization arm, the AUC without WMH was 0.75 (95% CI, 0.70–0.81), and after the addition of WMH it rose to 0.81 (95% CI, 0.76–0.86) (P=0.004 for difference). The 5‐fold cross‐validated AUCs for the models without and with WMH were concordant at 0.75 (95% CI, 0.66–0.79) and 0.81 (95% CI, 0.72–0.84) (Figure 3).
Figure 2. Five‐fold cross‐validation of the baseline model's area under the receiver operating curve seen for the model with Atherosclerotic Cardiovascular Disease Risk Score (top) and after the addition of deciles of white matter hyperintensity (bottom).
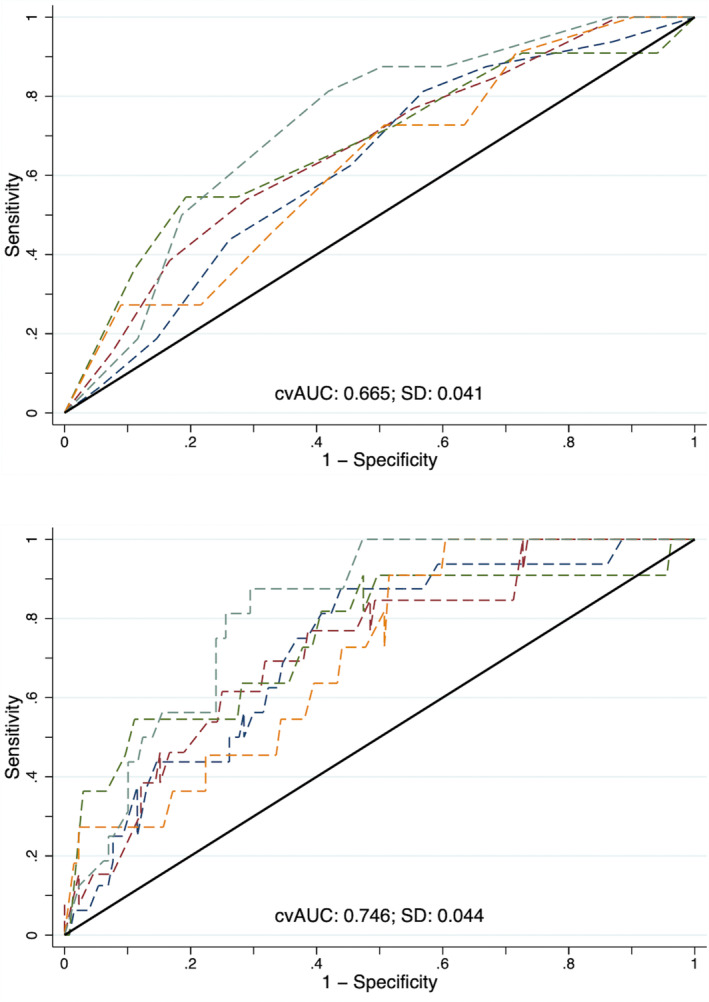
cvAUC indicates cross‐validated area under the receiver operating curve.
Figure 3. Five‐fold cross‐validation of the adjusted model's area under the receiver operating curve seen for the model without white matter hyperintensity (top) and after the addition of deciles of white matter hyperintensity (bottom).
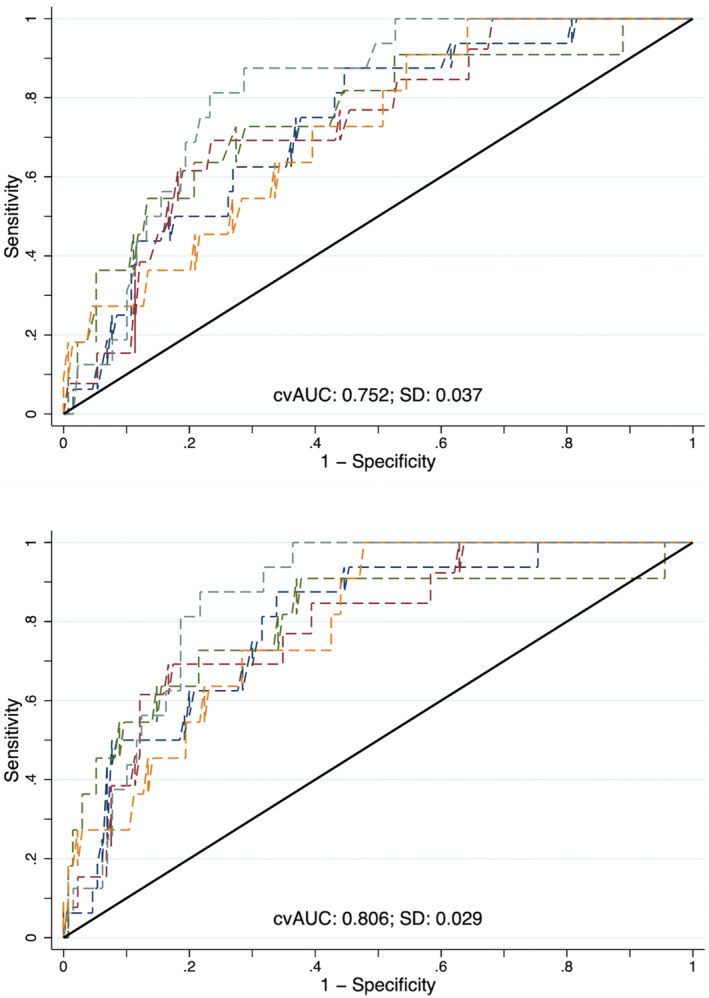
cvAUC indicates cross‐validated area under the receiver operating curve.
The relative integrated discrimination index of the baseline model comparison was 1.13 (95% CI, 0.72–1.54), which is greater than the inverse of the number of variables without WMH (1 of 1=1), indicating that WMH improves the model's prediction (P<0.001). Likewise, in the adjusted model, the relative integrated discrimination index is 0.51 (95% CI, 0.27–0.75), which exceeds 1 of 7=0.143, also indicating that WMH improves the model's prediction (P<0.001). The decision curve analyses are seen in Figure 4, confirming that the addition of WMH improved the ability to predict incident stroke or cognitive decline across all threshold probabilities.
Figure 4. Decision curve analysis for the baseline model of vascular risk (top) and the adjusted model (bottom), shown both with and without white matter hyperintensity.
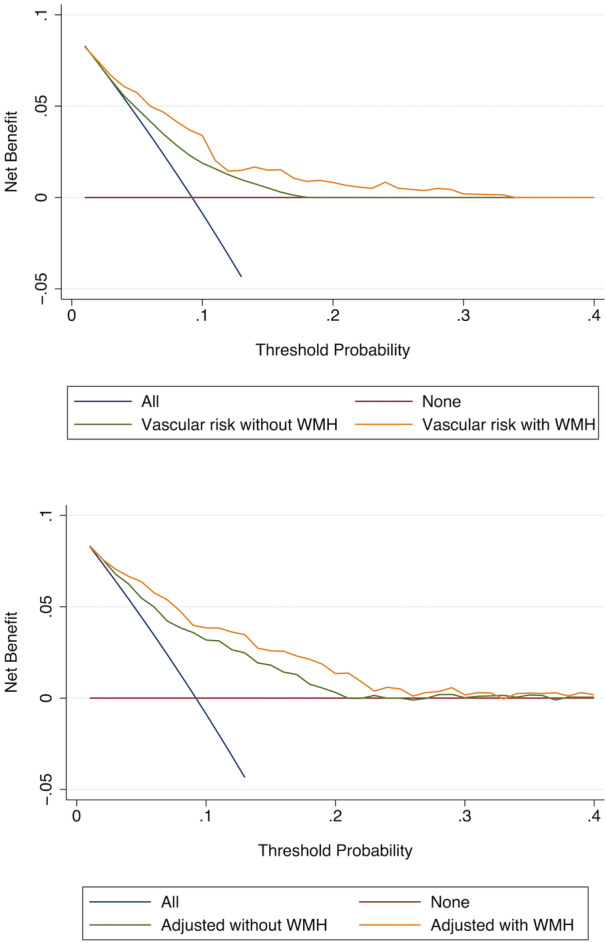
WMH indicates white matter hyperintensity.
In the baseline time‐to‐event models, we found that Somers D improved from 0.34 (95% CI, 0.10–0.49) with vascular risk to 0.53 (95% CI, 0.38–0.67) when WMH was added (P=0.002 for improvement). In the adjusted model without WMH, the Somers D was 0.55 (95% CI, 0.40–0.70), whereas after the addition of WMH it was 0.70 (95% CI, 0.54–0.86) (P=0.003 for improvement). The AUC for the baseline time‐to‐event model was 0.68, but with WMH it increased to 0.74.
The structural equation model mediation analysis showed that there was complementary partial mediation through WMH (Figure 5). In that model, both vascular risk and WMH have significant direct effects on the primary outcome, and vascular risk has a direct effect on WMH, but 26.3% of its effect on the primary outcome is indirectly mediated through WMH.
Figure 5. Structural equation model showing the direct effects of deciles of vascular risk and white matter hyperintensity on the primary outcome and of vascular risk on white matter hyperintensity.
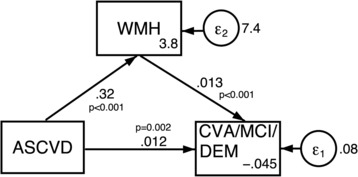
ASCVD indicates Atherosclerotic Cardiovascular Disease; CVA, cross‐validated area; DEM, dementia; MCI, mild cognitive impairment; and WMH, white matter hyperintensity.
Discussion
Our results suggest that WMH on MRI is a valuable prognostic measure for cerebrovascular risk assessment. WMH has been associated with an increased risk of stroke, cognitive decline, dementia, and death. 28 Thus, the discovery of WMH is a finding of clinical importance, and based on its demonstrated prognostic value, it should also be considered an indication to screen for potential cerebrovascular risk and events. Additionally, because of its heritability, 29 WMH could help identify genetic risk factors that lead to vascular pathologies and thus personalize preventative approaches.
Estimating vascular risk is a critical component of preventative medicine and a validated method of measuring the extent of vascular risk for a given individual. We have shown a link between vascular risk and WMH volume. However, vascular risk can be assessed solely through demographic and clinical variables, whereas the identification of WMH requires an MRI scan. Given the robust positive correlation between the 2, by screening for vascular risk one can identify the groups of patients in need of an MRI scan to assess the presence and extent of WMH. The combination of these 2 metrics could be used to increase prognostic ability and develop an effective treatment plan.
In the Action to Control Cardiovascular Risk in Diabetes–Memory in Diabetes cohort, WMH progression was lower in the intensive versus standard blood pressure reduction randomization arm (∆WMH between groups, 0.49 mL), 12 consistent with what was reported in SPRINT‐MIND (∆WMH between groups, 0.54 mL). 14 , 30 The Regression of Cerebral Artery Stenosis trial showed that statins could slow WMH progression, Prevention of Dementia by Intensive Vascular care indicated that a nurse‐led multimodal intervention reduced WMH progression in patients with severe WMH at baseline, Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability reported cognitive benefit to a multimodal intervention including lifestyle optimization, and Prevention of Decline in Cognition after Stroke Trial indicated that combined intensive blood pressure and lipid lowering could improve cognitive outcomes. 31 , 32 , 33 , 34 Despite these promising data, the optimal medical treatment for prevention of WMH remains uncertain. Nonetheless, assessing the extent of WMH in individuals to obtain a picture of their vascular risk is a valuable tool for establishing preventative approaches to improve an individual's overall brain health.
Our study has limitations, including that this is a post hoc analysis of a data set that was not created to answer this hypothesis, which introduces bias. We also used the ASCVD Risk Score for a purpose that it was not explicitly intended for. The creation of a composite outcome including all‐cause stroke and cognitive impairment may have reduced the association between exposure and outcome in our study, but that bias would favor the null hypothesis. Finally, although SPRINT‐MIND enrolled a racially diverse cohort, it excluded individuals with prior history of stroke or diabetes, so the results of our analysis are not generalizable.
In adult hypertensive individuals, we found that the addition of WMH to models predicting incident stroke or cognitive impairment improved the prognostic ability above vascular risk and demographics alone to a level consistent with excellent prediction. The implication of this finding is that MRI‐detected WMH, a marker of cerebral small vessel disease often available in routine clinical care, adds meaningful and independent risk assessment when considering vascular outcomes specific to the brain. Because many individuals will have a standard‐of‐care MRI of their brain for other clinical purposes, such as stroke or cognitive workup, WMH could be included in future risk scores focusing on brain health.
Sources of Funding
Dr de Havenon is funded by National Institutes of Health–National Institute of Neurological Disorders and Stroke K23NS105924, R01NS130189; Dr Sheth by National Institutes of Health–National Institute of Neurological Disorders and Stroke U01NS106513, R01NS11072, R01NR018335, R03NS112859, U24NS107215, U24NS107136, and the American Heart Association 17CSA33550004.
Disclosures
Dr de Havenon has received investigator‐initiated clinical research funding from the American Academy of Neurology, has received consultant fees from Integra and Novo Nordisk, has equity in TitinKM and Certus, and receives author fees from UpToDate. Dr Sheth reports funding from Biogen, Novartis, Bard, Hyperfine, Astrocyte, Alva Health, NControl, and is Data and Safety Monitoring Board Chair for Zoll. The remaining authors have no disclosures to report.
Acknowledgments
The authors acknowledge the National Heart, Lung, and Blood Institute and the SPRINT investigators for making the trial's data set publicly available. This article was prepared using SPRINT‐MIND research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of SPRINT‐MIND or the National Heart, Lung, and Blood Institute.
This article was sent to Neel S. Singhal, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 8.
See Editorial by Karvelas and Elahi
References
- 1. Cole JH, Marioni RE, Harris SE, Deary IJ. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry. 2019;24:266–281. doi: 10.1038/s41380-018-0098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorelick PB, Furie KL, Costantino I, Smith EE, Waddy SP, Lloyd‐Jones DM, Hee‐Joon B, Ann BM, Martin D, Duncan PW, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mosley TH, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, Folsom AR, Cooper LS, Burke GL, Liao D, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88 [DOI] [PubMed] [Google Scholar]
- 4. Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral small‐vessel disease and decline in information processing speed, executive function and memory. Brain J Neurol. 2005;128:2034–2041. doi: 10.1093/brain/awh553 [DOI] [PubMed] [Google Scholar]
- 5. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MMB; Rotterdam Scan Study . Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2 [DOI] [PubMed] [Google Scholar]
- 6. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066 [DOI] [PubMed] [Google Scholar]
- 7. Debette S, Schilling S, Duperron M‐G, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta‐analysis. JAMA Neurol. 2019;76:81–94. doi: 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Mosley TH. Blood pressure and white‐matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 11. Von CBF, Josefsson M, Wåhlin A, Nyberg L, Karalija N. Association of cardiovascular risk trajectory with cognitive decline and incident dementia. Neurology. 2022;98:e2013–e2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song R, Pan K‐Y, Xu H, Qi X, Buchman AS, Bennett DA, Xu W. Association of cardiovascular risk burden with risk of dementia and brain pathologies: a population‐based cohort study. Alzheimers Dement. 2021;17:1914–1922. doi: 10.1002/alz.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SPRINT MIND Investigators for the SPRINT Research Group ; Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. SPRINT MIND Investigators for the SPRINT Research Group ;Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. Association of Intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322:524–534. doi: 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Group TSR . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJB, Culebras A, Elkind MSV, George MG, Hamdan AD, Higashida RT, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218 [DOI] [PubMed] [Google Scholar]
- 18. Assel M, Sjoberg DD, Vickers AJ. The Brier score does not evaluate the clinical utility of diagnostic tests or prediction models. Diagn Progn Res. 2017;1:19. doi: 10.1186/s41512-017-0020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer–Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67–80. doi: 10.1002/sim.5525 [DOI] [PubMed] [Google Scholar]
- 20. Ribbing J, Nyberg J, Caster O, Jonsson EN. The lasso—a novel method for predictive covariate model building in nonlinear mixed effects models. J Pharmacokinet Pharmacodyn. 2007;34:485–517. doi: 10.1007/s10928-007-9057-1 [DOI] [PubMed] [Google Scholar]
- 21. Barkhordari M, Padyab M, Hadaegh F, Azizi F, Bozorgmanesh M. Stata modules for calculating novel predictive performance indices for logistic models. Int J Endocrinol Metab. 2016;14:e26707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 23. Vickers AJ, van Calster B, Steyerberg EW. A simple, step‐by‐step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18. doi: 10.1186/s41512-019-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pencina MJ, D'Agostino RB, Pencina KM, Janssens ACJW, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol. 2012;176:473–481. doi: 10.1093/aje/kws207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luque‐Fernandez MA, Maringe C, Nelson P. CVAUROC: Stata module to compute cross‐validated area under the curve for ROC analysis after predictive modelling for binary outcomes. Statistical Software Components. 2022. https://ideas.repec.org/c/boc/bocode/s458324.html.
- 26. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata J. 2010;10:339–358. doi: 10.1177/1536867X1001000303 [DOI] [Google Scholar]
- 27. Cattaneo M, Malighetti P, Spinelli D. Estimating receiver operative characteristic curves for time‐dependent outcomes: the stroccurve package. Stata J. 2017;17:1015–1023. doi: 10.1177/1536867X1801700415 [DOI] [Google Scholar]
- 28. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sachdev PS, Thalamuthu A, Mather KA, Ames D, Wright MJ, Wen W, Bowden J, Lee T, Brodaty H, Crawford J, et al. White matter hyperintensities are under strong genetic influence. Stroke. 2016;47:1422–1428. doi: 10.1161/STROKEAHA.116.012532 [DOI] [PubMed] [Google Scholar]
- 30. de Havenon A, Majersik JJ, Tirschwell DL, McNally JS, Stoddard G, Rost NS. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology. 2019;92:e1168–e1175. doi: 10.1212/WNL.0000000000007093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bath PM, Scutt P, Blackburn DJ, Ankolekar S, Krishnan K, Ballard C, Burns A, Mant J, Passmore P, Pocock S, et al. Intensive versus guideline blood pressure and lipid lowering in patients with previous stroke: main results from the pilot “Prevention of Decline in Cognition after Stroke Trial” (PODCAST) randomised controlled trial. PLoS One. 2017;12:e0164608. doi: 10.1371/journal.pone.0164608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mok VCT, Lam WWM, Fan YH, Wong A, Ng PW, Tsoi TH, Yeung V, Wong KS. Effects of statins on the progression of cerebral white matter lesion: post hoc analysis of the ROCAS (Regression of Cerebral Artery Stenosis) study. J Neurol. 2009;256:750–757. doi: 10.1007/s00415-009-5008-7 [DOI] [PubMed] [Google Scholar]
- 33. Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 34. van Dalen JW, Moll van Charante EP, Caan MWA, Scheltens P, Majoie CBLM, Nederveen AJ, van Gool WA, Richard E. Effect of long‐term vascular care on progression of cerebrovascular lesions: magnetic resonance imaging substudy of the PreDIVA trial (Prevention of Dementia by Intensive Vascular Care). Stroke. 2017;48:1842–1848. doi: 10.1161/STROKEAHA.117.017207 [DOI] [PubMed] [Google Scholar]


