White matter hyperintensities (WMHs) pose an enigma in evaluations of brain health and disease. The prevalence of WMH has been estimated to reach 20% to 50% in the general population in midlife, increasing to >90% with advanced age. 1 , 2 Although WMHs can result from a plethora of molecular and cellular abnormalities, they are commonly considered a consequence of cerebral small‐vessel disease (cSVD) in the aging brain, frequently associated with hypertension. 3 , 4 Despite associations of WMHs with upstream risk factors, what lays beneath the surface of this radiographic marker of abnormality is thought to be multifactorial in the aging brain, and an active area of research.
More important, numerous observations of WMH get classified as “incidental” on clinical reads, a label that is reserved for findings observed and considered as unrelated to the primary reason for which the imaging is ordered. There are several assumptions underpinning this classification. First, incidental insinuates that WMH is “benign” and may not matter to risk stratification for the disease under investigation, often neurodegenerative in nature. Second, incidental further underscores the assumption that brain dysfunction is neurocentric. These assumptions persist in part because cSVD does not have a specific clinical syndrome for which studies get ordered. In addition, although studies have repeatedly demonstrated an association between burden of WMH and cognitive impairment and stroke, 2 , 3 the strength of associations has suggested limited value in adding WMH to predictive models, above and beyond cardiovascular risk and disease.
In this issue of the Journal of the American Heart Association (JAHA), de Havenon et al specifically test the value of WMH in predictive models of stroke and cognitive impairment, beyond vascular risk factors. 5 The authors perform post hoc analyses on data from 727 individuals who participated in the SPRINT‐MIND (Systolic Blood Pressure Intervention Trial–Memory and Cognition in Decreased Hypertension) clinical trial. 6 They divide the trial participants into deciles of WMH burden and atherosclerotic cardiovascular disease risk score. Not surprisingly, they find a significant positive association between atherosclerotic cardiovascular disease score and WMH volumes, with a modest correlation coefficient of 0.28, congruent with prior reports. 7 The team then builds logistic regression models and performs receiver operating characteristic curve analyses to test the value of WMH in predicting incident stroke and cognitive impairment, added to vascular risk factors, controlling for sex, age, smoking status, and clinical trial randomization arm. They show that the predictive value of models increases significantly with addition of WMH deciles into baseline and adjusted models. Furthermore, they also demonstrate an association between vascular risk, WMH burden, and time to event for stroke and cognitive impairment. Nonetheless, WMH and vascular risk factors seem to only partially predict these complex clinical outcomes. This could mean that the relationship between risk, early disease, and multifactorial phenotypes, such as WMH, is complex. Furthermore, in structural equation models, the authors demonstrate that vascular risk has both direct and indirect effects on outcomes, with indirect effects being in part mediated through burden of WMH.
In prior literature, hypertension seems to have the strongest association with WMH and cognitive outcomes. The cohort used by de Havenon et al was therefore justifiably composed of individuals with hypertension, because diabetes and prior clinically significant vascular disease were excluded from the SPRINT‐MIND. 6 There are many ways in which blood pressure may relate to WMH. In the Rotterdam Scan Study, a positive association between arterial stiffness and WMH burden was found in those with uncontrolled hypertension, independent of other vascular factors. 8 A similar finding was reported in the age, gene/environment susceptibility‐Reykjiavik study, in which WMH volumes were positively associated with carotid‐femoral pulse wave velocity after adjusting for the most common vascular health parameters. 9 These findings underscore the mechanical injury theory of hypertension in cSVD, where increased pulse pressure, related to arterial stiffness, is thought to cause gradual damage to small‐vessel beds and dysfunction of brain barriers.
Overall, the study by de Havenon et al demonstrates that WMH volumes are important prognostic measures for the clinically significant outcomes of stroke and cognitive impairment, especially for individuals with elevated atherosclerotic cardiovascular disease risk scores. Their study also suggests that WMH is a complex phenotype. Indeed, age, sex, and genetics are all thought to contribute to burden of WMH. 1 , 4 A study based on the Study of Health in Pomerania cohort 10 found both direct and indirect effects of WMH volumes on an index of brain aging. The indirect effects were mediated through vascular risk factors. The fact that there are both vascular and nonvascular associations of WMH with indexes of brain aging suggests that WMH represents a multifactorial phenotype that is only partially attributable to vascular risk and disease. 10
Although global cerebral burden of WMH, examined by de Havenon et al, is used most readily in studies, location of WMH may also matter. Indeed, periventricular WMH and deep WMH may result from diverse pathologic conditions. In multivariate regression models, periventricular WMH has been shown to be associated with deep medullary vein dilatation and indirectly with enlargement of perivascular spaces, whereas deep WMH has been shown to be directly associated with perivascular spaces and cerebral blood flow. 11 These findings suggest that deep WMH may reflect arterial pathologic conditions, and periventricular WMH may reflect venous‐centric pathologic conditions. It is interesting that perivascular spaces are associated with both, potentially suggesting that the glymphatic system is implicated in both. 11 Histopathologic differences have also been assessed. Periventricular WMH is strongly associated with gliosis, thinning of the extracellular matrix, and loss of oligodendrocytes around tortuous venules, whereas deep WMH most commonly represents areas of ischemia around arteriosclerotic vessels, exhibiting astrogliosis, loss of neuronal cells, and parenchymal destruction. 12 With the advent of novel fluid and imaging biomarkers, various areas of WMH can be dissected into the multitude of upstream molecular and cellular causes.
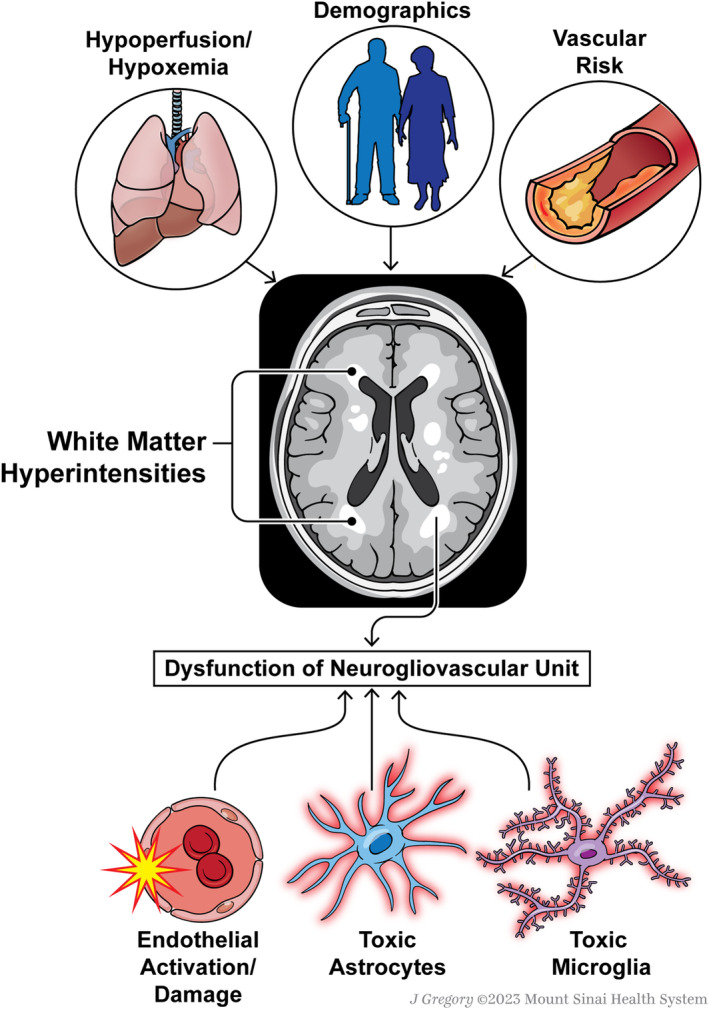
There are pathophysiological factors below the surface of WMH that add additional value to the interpretation of WMH, such as cerebral hypoperfusion and hypoxemia. 11 , 13 , 14 Mismatch between supply and demand can contribute to exacerbations in WMH. A study of elderly patients with cardiac disease demonstrated an inverse correlation between cardiac output and deep WMH volume. 15 Moreover, in the CHS (Cardiovascular Health Study), lower forced expiratory volume in 1 second, a measure of pulmonary health, was associated with increased WMH grade. 4 A study demonstrated that extent of WMH is associated with reduction in cerebral blood flow, decreased capillary density, and decreased oxygenation. 14 In a different study, cerebrovascular reactivity was inversely associated with WMH volumes in patients with suspicion of cSVD, although no associations were found between WMH and cerebral blood flow. 13
Endothelial, mural cell, microglial, astrocytic, and oligodendrocytic dysfunctions can all lead to WMH, as noted in genetic causes of leukodystrophy. Thus, although WMH is a clearly abnormal radiographic finding and common on magnetic resonance imaging of older individuals with vascular risk factors, its pathophysiological origin and prognostic value require the inclusion of additional variables in models, and remain difficult to interpret for patients, clinicians, and researchers. Using a novel molecular biomarker approach, WMH in older individuals with hypertension was shown to be associated with measures of endothelial innate immune inflammation. 16 Other studies have shown that various aspects of blood‐brain barrier dysfunction, an important hallmark of cSVD, are associated with WMH. 17 , 18 In Alzheimer disease, although cerebral amyloid angiopathy is associated with WMH, posterior patches of WMH have been shown to be associated with parietal tau burden rather than vascular pathologic conditions. 19 Thus, the causative diversity of WMH has muddled its interpretation, leaving uncertainty about its predictive value in models, beyond vascular risk factors.
Unsurprisingly, numerous alterations to cellular and physiological components of the brain relate to white matter health and burden of WMH. The neuro‐glio‐vascular unit represents a unique niche in which neurons, astrocytes, microglia, oligodendrocytes, and vascular cells work together to regulate cerebral blood flow and brain function and homeostasis. 20 WMH, therefore, represents a pathologic hallmark that can relate to alterations of glial cells in a hostile vascular microenvironment. 21 Changes in the function of any number of cells composing the neuro‐glio‐vascular unit can lead to WMH and brain dysfunction. For instance, toxic phenotypes undertaken by glia could be an important contributor to brain dysfunction in individuals with WMH, 18 , 22 especially in individuals with hypertension and after stroke. 17
Understanding the multifactorial origins of WMH is critical to the development of treatments for this common radiographic abnormality of the aging brain, with predictive value for cognitive impairment and stroke. A combination of biomarkers, both fluid and imaging, will shed light on the various pathologic features contributing to the development and progression of WMH and the downstream cognitive impairment, stroke, and dementia (Figure). The article by de Havenon et al demonstrates the value of WMH in predictive models, above and beyond vascular risk factors. Their well‐designed and executed study adds to the large body of literature suggesting that WMH is the tip of an iceberg of multiple pathologic features that matter to predictive models of brain health. The great advantage of WMH is that it is slowly progressive and, therefore, early detection could provide the opportunity for early intervention. What is needed next are sets of biomarkers that build more precise diagnostics to advance treatments beyond modification of population‐level vascular risk scores toward n‐of‐one precision therapeutics. With rapid advancements in molecular quantification methods and analytical approaches, there is great hope for a future in which patients can be stratified on the basis of imaging and fluid biomarkers, and prescribed personalized combinatorial treatments to reduce risk, prevent disease progression, and avoid cognitive impairment and strokes.
Figure 1. An overview of factors associated with white matter hyperintensity on brain magnetic resonance imaging, ranging from demographics and medical comorbidities to cellular drivers of disease.
Disclosures
None.
Acknowledgments
We thank Jill K. Gregory for the illustration.
See Article by de Havenon et al.
For Disclosures, see page 4.
This article was sent to Neel S. Singhal, MD, PhD, Associate Editor, for editorial decision and final disposition.
References
- 1. de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roseborough AD, Saad L, Goodman M, Cipriano LE, Hachinski VC, Whitehead SN. White matter hyperintensities and longitudinal cognitive decline in cognitively normal populations and across diagnostic categories: a meta‐analysis, systematic review, and recommendations for future study harmonization. Alzheimers Dement. 2023;19:194–207. doi: 10.1002/alz.12642 [DOI] [PubMed] [Google Scholar]
- 3. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Longstreth WT Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274 [DOI] [PubMed] [Google Scholar]
- 5. de Havenon A, Smith E, Sharma R, Falcone G, Bangad A, Prabhakaran S, Sheth K. Improvement in the prediction of cerebrovascular events with white matter hyperintensity. J Am Heart Assoc. 2023;12:e029374. doi: 10.1161/JAHA.123.029374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. SPRINT MIND Investigators for the SPRINT Research Group ; Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ten Kate M, Sudre CH, den Braber A, Konijnenberg E, Nivard MG, Cardoso MJ, Scheltens P, Ourselin S, Boomsma DI, Barkhof F, et al. White matter hyperintensities and vascular risk factors in monozygotic twins. Neurobiol Aging. 2018;66:40–48. doi: 10.1016/j.neurobiolaging.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 8. Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, Witteman JC, Breteler MM, Mattace‐Raso FU, Ikram MA. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 2012;43:2637–2642. doi: 10.1161/STROKEAHA.111.642264 [DOI] [PubMed] [Google Scholar]
- 9. Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habes M, Erus G, Toledo JB, Bryan N, Janowitz D, Doshi J, Volzke H, Schminke U, Hoffmann W, Grabe HJ, et al. Regional tract‐specific white matter hyperintensities are associated with patterns to aging‐related brain atrophy via vascular risk factors, but also independently. Alzheimers Dement (Amst). 2018;10:278–284. doi: 10.1016/j.dadm.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai J, Sun J, Chen H, Chen Y, Zhou Y, Lou M, Yu R. Different mechanisms in periventricular and deep white matter hyperintensities in old subjects. Front Aging Neurosci. 2022;14:940538. doi: 10.3389/fnagi.2022.940538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685 [DOI] [PubMed] [Google Scholar]
- 13. Blair GW, Thrippleton MJ, Shi Y, Hamilton I, Stringer M, Chappell F, Dickie DA, Andrews P, Marshall I, Doubal FN, et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology. 2020;94:e2258–e2269. doi: 10.1212/WNL.0000000000009483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalby RB, Eskildsen SF, Videbech P, Frandsen J, Mouridsen K, Sorensen L, Jeppesen P, Bek T, Rosenberg R, Ostergaard L. Oxygenation differs among white matter hyperintensities, intersected fiber tracts and unaffected white matter. Brain Commun. 2019;1:fcz033. doi: 10.1093/braincomms/fcz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, Cohen RA. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elahi FM, Harvey D, Altendahl M, Brathaban N, Fernandes N, Casaletto KB, Staffaroni AM, Maillard P, Hinman JD, Miller BL, et al. Elevated complement mediator levels in endothelial‐derived plasma exosomes implicate endothelial innate inflammation in diminished brain function of aging humans. Sci Rep. 2021;11:16198. doi: 10.1038/s41598-021-91759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sole‐Guardia G, Custers E, de Lange A, Clijncke E, Geenen B, Gutierrez J, Kusters B, Claassen J, de Leeuw FE, Wiesmann M, et al. Association between hypertension and neurovascular inflammation in both normal‐appearing white matter and white matter hyperintensities. Acta Neuropathol Commun. 2023;11:2. doi: 10.1186/s40478-022-01497-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, et al. Fibrinogen‐induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McAleese KE, Walker L, Graham S, Moya ELJ, Johnson M, Erskine D, Colloby SJ, Dey M, Martin‐Ruiz C, Taylor JP, et al. Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017;134:459–473. doi: 10.1007/s00401-017-1738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198–1209. doi: 10.1038/s41593-021-00904-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen A, Akinyemi RO, Hase Y, Firbank MJ, Ndung'u MN, Foster V, Craggs LJ, Washida K, Okamoto Y, Thomas AJ, et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post‐stroke dementia. Brain. 2016;139:242–258. doi: 10.1093/brain/awv328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]


