Abstract
Background
Studies in mice and small patient subsets implicate metabolic dysfunction in cardiac remodeling in aortic stenosis, but no large comprehensive studies of human metabolism in aortic stenosis with long‐term follow‐up and characterization currently exist.
Methods and Results
Within a multicenter prospective cohort study, we used principal components analysis to summarize 12 echocardiographic measures of left ventricular structure and function pre–transcatheter aortic valve implantation in 519 subjects (derivation). We used least absolute shrinkage and selection operator regression across 221 metabolites to define metabolic signatures for each structural pattern and measured their relation to death and multimorbidity in the original cohort and up to 2 validation cohorts (N=543 for overall validation). In the derivation cohort (519 individuals; median age, 84 years, 45% women, 95% White individuals), we identified 3 axes of left ventricular remodeling, broadly specifying systolic function, diastolic function, and chamber volumes. Metabolite signatures of each axis specified both known and novel pathways in hypertrophy and cardiac dysfunction. Over a median of 3.1 years (205 deaths), a metabolite score for diastolic function was independently associated with post–transcatheter aortic valve implantation death (adjusted hazard ratio per 1 SD increase in score, 1.54 [95% CI, 1.25–1.90]; P<0.001), with similar effects in each validation cohort. This metabolite score of diastolic function was simultaneously associated with measures of multimorbidity, suggesting a metabolic link between cardiac and noncardiac state in aortic stenosis.
Conclusions
Metabolite profiles of cardiac structure identify individuals at high risk for death following transcatheter aortic valve implantation and concurrent multimorbidity. These results call for efforts to address potentially reversible metabolic biology associated with risk to optimize post–transcatheter aortic valve implantation recovery, rehabilitation, and survival.
Keywords: aortic stenosis, metabolomics, outcomes, remodeling
Subject Categories: Metabolism, Translational Studies
Nonstandard Abbreviations and Acronyms
- AS
aortic stenosis
- LASSO
least absolute shrinkage and selection operator
- PC
principal component
- PCA
principal components analysis
- TAVI
transcatheter aortic valve implantation
Clinical Perspective.
What Is New?
Metabolite signatures identified known and novel pathways of cardiac remodeling across a large sample of individuals with severe aortic stenosis referred for transcatheter aortic valve implantation.
What Are the Clinical Implications?
A metabolic signature related to diastolic function was independently associated with post–transcatheter aortic valve implantation death and systemic multimorbidity (“frailty”) measures, suggesting links between cardiac and noncardiac states in advanced heart disease.
While transcatheter aortic valve implantation (TAVI) improves outcome in individuals with symptomatic severe aortic stenosis (AS), 1 there remains significant residual risk following TAVI, marked by persistent poor quality of life and rehospitalization for heart failure (HF). 2 , 3 As the application of TAVI in sicker populations with greater systemic comorbidity advances, identifying biological mechanisms of residual post‐TAVI risk has become increasingly important, with risk assessment metrics based on clinical factors, cardiac‐specific biomarkers, myocardial structure, and systemic illness. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 In this space, the growth of high‐throughput methods for broad biochemical phenotyping (“omics”) has enabled a search for circulating biomarkers in a “hypothesis‐free” discovery approach that may identify those individuals with reduced myocardial resilience, delineate underlying biological mechanisms, and identify opportunities for intervention to mitigate risk following TAVI. Our group and others have identified a series of metabolic pathways potentially linked to adverse remodeling in severe AS, 12 , 13 several of which have been linked to systemic phenotypes previously relevant to post‐TAVI risk (eg, “frailty”). 14 Many of these “omic” studies are small, limited in phenotypic breadth, and lacking in significant follow‐up to assess the relation between metabolism, adverse phenotypes, and long‐term outcome. Here, we address these central limitations by quantifying a broad circulating metabolome alongside detailed echocardiographic quantification of cardiac structure/function in individuals with symptomatic severe AS to delineate metabolic signatures and pathways related to cardiac remodeling and their relation to death and systemic multimorbidity. Our goal was to identify metabolic signatures of composite echocardiographic measures of cardiac remodeling characteristic in AS, using these metabolites to understand potential pathways and prognosis in AS.
Methods
The data underlying this article will be shared upon reasonable request to the corresponding author.
Clinical Characterization
Our derivation cohort is a multicenter, prospective observational cohort study of adults with symptomatic severe AS undergoing TAVI between May 2014 and February 2017 (Data S1). Severe AS was defined according to American Society of Echocardiography guidelines: peak velocity ≥4 m/s, indexed aortic valve area <0.6 cm2/m2, or mean gradient ≥40 mm Hg. 15 We included participants in our derivation cohort whose pre‐TAVI echocardiogram was transferred to our core laboratory for analysis with a pre‐TAVI blood sample available. In addition to standard clinical and demographic indices, measures of systemic multimorbidity were obtained (eg, grip strength, gait speed, lung function, albumin, hemoglobin). Subjects in the derivation cohort underwent transthoracic echocardiography before TAVI (median, 39 days; interquartile range, 21–72). Echocardiograms were electronically transferred to our core laboratory to quantify left ventricular (LV) structure and function according to American Society of Echocardiography guidelines: LV ejection fraction (LVEF), LV stroke volume index, LV internal dimensions and volumes at end‐diastole and end‐systole, LV mass, relative wall thickness, mean transmitral E/e′, tissue Doppler S velocity of lateral mitral annulus, left atrial volume, and mean aortic valve gradient. 15
To test the relation of metabolic signatures of cardiac structure/function with death, we leveraged 2 additional cohorts: (1) a set of 286 individuals with blood samples from our parent multicenter, prospective observational cohort study who did not have full core lab‐adjudicated echocardiographic phenotyping for analysis (therefore not included in derivation cohort); and (2) a single‐center, prospective observational cohort study of 257 individuals with blood samples enrolled at Barnes‐Jewish Hospital (St Louis, MO) with symptomatic severe AS who underwent TAVI between 2010 and 2015 (Figure S1). AS severity was defined according to American Society of Echocardiography criteria.
A complete assessment of vital status was performed between March and June 2020 for the multicenter cohort 16 (both derivation and validation) and between November and December 2016 for the single‐center cohort (validation).
Metabolite Profiling
Blood samples were obtained before TAVI in all participants. Metabolite profiling was performed via standard liquid chromatography–mass spectrometry techniques, details of which are summarized in Data S2. 17
Analytic Methods
Generation of Composite Patterns of Cardiac Remodeling
Our analytic flow is shown in Figure 1. Our goal was to identify those metabolites related to cardiac structure/function in AS. Both “supervised” (phenotypic measures to “supervise” selection of metabolites related to that phenotype) and “unsupervised” methods (metabolome first reduced into sets of interrelated metabolites, agnostic to phenotype) have been used recently in this regard to study phenotype‐metabolome relation in HF. 18 To build optimized models for composite measures of remodeling, we chose least absolute shrinkage and selection operator (LASSO) regression, in which >200 metabolites would be independent variables in models for a composite measure of cardiac structure/function (a supervised analytic approach). In contrast to some prior approaches focused on limited measures of cardiac remodeling (eg, LVEF, LV mass 12 ), our experimental design incorporates measurement of multiple, related measures of remodeling by echocardiography to provide a more holistic assessment of cardiac structure/function. We used principal components analysis (PCA) to generate 3 composite axes of cardiac remodeling/function, resulting in participants having a principal component (PC) “score” for each axis (see Data S1 for further details).
Figure 1. Overall study design.
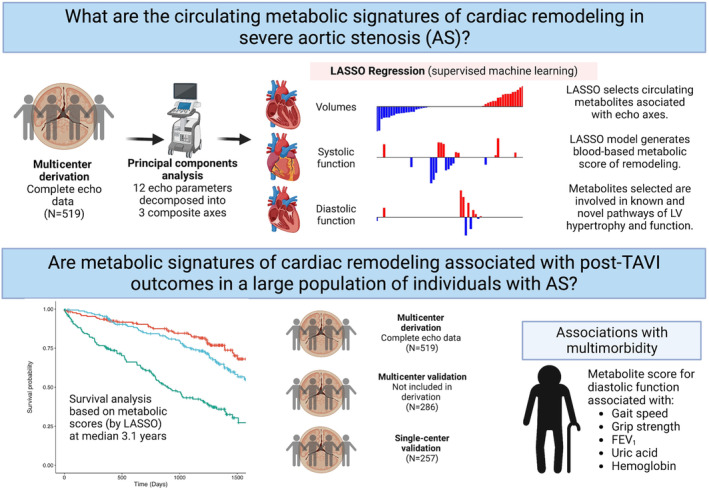
FEV1 indicates forced expiratory volume in the first second; LASSO, least absolute shrinkage and selection operator; LV, left ventricular; and TAVI, transcatheter aortic valve implantation. Created with BioRender.com.
LASSO Regression
To generate parsimonious models for each composite axis defined by PCA, we used LASSO regression (with 10‐fold cross validation to optimize hyper parameters; R package “glmnet” 19 [R project; www.rproject.org]), with 3 PCA‐based scores as the dependent variables and all metabolites as independent variables for selection. Metabolites were log‐transformed to reduce skewness and standardized. LASSO models were used to generate “metabolite scores” (blood‐based surrogates of the composite axes from PCA) in downstream analysis. To assess the pathway importance of each of the selected metabolites, we used pathway analysis (MetaboAnalyst 5.0; https://www.metaboanalyst.ca; accessed August 18, 2022) using the KEGG metabolome as reference 20 and literature search. The metabolites quantified in the single‐center validation cohort did not completely match the metabolites measured in our multicenter cohort. Accordingly, we refit the LASSO models for use in the single‐center cohort (see Data S1).
Survival Analysis
We standardized PC and metabolite scores for use in Cox models for all‐cause death. Proportionality assumption for the metabolite scores were tested using the cox.zph function (R package “survival”) and examined Schoenfeld residuals for any suspected violation (by P value). For those with potential violations, models using robust standard errors were generated, yielding similar results to the original models. Hazard ratios (HRs) are reported per 1 SD difference in score, with standard 95% confidence intervals. Cox models were adjusted for age, sex, body mass index, diabetes (defined as hemoglobin A1c ≥6.5% or reported history), coronary artery disease (defined as prior myocardial infarction, prior revascularization or atherosclerotic disease in ≥1 coronary arteries), history of atrial fibrillation/flutter, estimated glomerular filtration rate, cardiac troponin‐T, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide). In the single‐center validation cohort, we adjusted for the same variables except cardiac troponin‐T or NT‐proBNP due to unavailability) and B‐type natriuretic peptide. To assess whether the metabolite‐based scores would be independent of key echocardiographic phenotypes of risk following TAVI, we further adjusted for mean aortic valve gradient and LVEF in a sensitivity analysis. Biomarkers were log‐transformed for use in Cox models.
Analyses were performed in R. All subjects provided informed consent as part of this research cohort. Institutional review boards at Vanderbilt University Medical Center and Massachusetts General Hospital approved this study.
Results
Sample Characteristics
Characteristics of the 519 participants in our multicenter derivation cohort are reported in Table. The median age was 84 years, with 45% women and 95% White individuals. The median Society of Thoracic Surgeons score was 4.2%. There was a high prevalence of both cardiovascular and noncardiovascular comorbidity, as well as abnormalities in cardiac structure (eg, LV hypertrophy), systolic (eg, LVEF), and diastolic function (elevated NT‐proBNP, elevated E/e′). Characteristics of the validation cohorts were similar to our derivation cohort (Table).
Table 1.
Baseline Characteristics of the Multicenter Derivation, Multicenter Validation, and Single‐Center Validation Cohorts
| Characteristic | Derivation (N=519) | Multicenter validation (n=286) | Single‐center validation (n=257) |
|---|---|---|---|
| Age, y | 84 (78–88) | 82 (75–87) | 83 (76–89) |
| Sex, female | 233 (45) | 121 (42) | 128 (50) |
| Race | |||
| Black | 17 (3.3) | 1 (0.3) | |
| Asian | 6 (1.2) | 2 (0.7) | |
| White | 495 (95) | 282 (99) | 251 (98) |
| Body mass index, kg/m2 | 27.3 (23.8–31.4) | 27.7 (24.6–32.9) | 27.6 (23.9–31.3) |
| Diabetes | 184 (36); 0.2 | 128 (45) | 100 (39) |
| History of atrial fibrillation or flutter | 200 (39); 0.4 | 122 (43); 0.3 | 104 (40) |
| Coronary artery disease | 368 (71) | 191 (67) | 218 (85) |
| New York Heart Association class | |||
| I | 16 (3.3); 6.2 | 10 (3.7); 4.9 | |
| II | 140 (29); 6.2 | 47 (17); 4.9 | |
| III | 272 (56); 6.2 | 184 (68); 4.9 | |
| IV | 59 (12); 6.2 | 31 (11); 4.9 | |
| Society of Thoracic Surgeons score | 4.2 (2.9–6.3) | 3.9 (2.6–6.3) | 8.3 (5.0–12.6) |
| Echocardiographic measures before TAVI | |||
| LV mass index, g/m2 | 108 (91–126) | 106 (91–127); 49 | |
| LV hypertrophy by ASE criteria | |||
| None | 266 (51) | 68 (47); 49 | |
| Mild | 87 (17) | 33 (23); 49 | |
| Moderate | 69 (13) | 20 (14); 49 | |
| Severe | 97 (19) | 24 (17); 49 | |
| LV ejection fraction (%) | 61 (53–66) | 60 (52–65); 9.1 | 60 (45–66); 0.4 |
| LV ejection fraction <50% | 106 (20) | 55 (21); 9.1 | 79 (31); 0.4 |
| Stroke volume index, mL/m2 | 37 (30–45) | 36 (28–43); 44 | |
| Stroke volume index <35 mL/m2 | 222 (43) | 76 (48); 44 | |
| LV end‐diastolic internal diameter, mm | 44 (40–49) | 43 (39–49); 49 | |
| LV end‐systolic internal diameter, mm | 29 (25–35) | 29 (25–36); 50 | |
| LV end‐diastolic volume index, mL/m2 | 44 (35–54) | 45 (34–56); 58 | |
| LV end‐systolic volume index, mL/m2 | 17 (12–25) | 17 (12–29); 58 | |
| Left atrial volume index, mL/m2 | 35 (27–47) | 39 (31–49); 48 | |
| LV tissue Doppler S lateral annulus, cm/s | 6.5 (5.3–7.8) | 6.4 (5.4–7.3); 62 | |
| Average transmitral E/e′ | 18 (14–24) | 18 (14–24); 72 | |
| Relative wall thickness | 0.54 (0.45–0.64) | 0.56 (0.49–0.65); 49 | |
| Interventricular septum thickness, mm | 12.6 (11.4–14.3) | 13.2 (11.7–14.7); 49 | |
| Posterior wall thickness, mm | 11.8 (10.6–13.3) | 12.5 (10.7–13.9); 49 | |
| Aortic valve area, cm2 | 0.72 (0.60–0.85); 0.2 | 0.73 (0.59–0.89); 12 | 0.70 (0.50–0.80); 1.2 |
| Aortic valve area index, cm2/m2 | 0.39 (0.31–0.45); 0.2 | 0.37 (0.30–0.46); 12 | |
| Aortic valve mean gradient, mm Hg | 39 (32–50) | 38 (30–48); 8.0 | 41 (35–47); 0.4 |
| Peak aortic velocity, m/s | 4.09 (3.65–4.58); 0.2 | 4.01 (3.59–4.49); 13 | |
| Moderate–severe aortic regurgitation | 49 (9.4) | 26 (9.1) | |
| Moderate–severe mitral regurgitation | 72 (14) | 36 (13) | |
| Laboratory measures before TAVI | |||
| eGFR, mL/min per 1.73 m2 | 54 (41–69); 0.4 | 54 (42–70); 1.0 | 55 (41–71) |
| NT‐proBNP, ng/mL | 1303 (622–3374); 3.5 | 1249 (560–2758); 2.1 | |
| High‐sensitivity cardiac troponin, ng/mL | 25 (16–41); 3.5 | 24 (15–42); 2.1 | |
| B‐type natriuretic peptide, pg/mL | 293 (154–658); 2.3 | ||
Continuous variables are reported as median (25th–75th percentile); % missing, if any. Categorical variables are reported as n (%); % missing. ASE indicates American Society of Echocardiography; eGFR, estimated glomerular filtration rate; LV, left ventricular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and TAVI, transcatheter aortic valve implantation.
Defining Composite Axes of Remodeling Using PCA of Echocardiographic Data
We observed significant interrelation across echocardiographic measures (Figure S2), motivating our approach to use PCA as an unsupervised method to summarize these related measures into composite “axes” of remodeling (see Analytic Methods). We identified 3 PCs that explained 65% of the variance in the data (Figure S3). Loadings for each of the 12 individual echocardiographic measures for each PC are shown in Figure 2A. The first PC was mostly weighted on LV volumes and LVEF (accordingly labeled “volumes”). The second PC was more highly loaded on stroke volume index and LVEF (accordingly labeled “systolic function”). The third PC was mostly loaded on E/e′ and left atrial volume (accordingly labeled “diastolic function”). When we visualized the 12 echocardiographic measures across our derivation cohort (Figure 2B), we observed (1) significant heterogeneity across individuals in cardiac structure/function (consistent with clinical practice) and (2) differences in PCA‐based scores across individuals that appeared to track with this heterogeneity (color patterns on horizontal color bars, Figure 2B). These 3 PCA‐based scores of composite axes of cardiac structure/function were carried forward to guide metabolic discovery.
Figure 2. Interindividual heterogeneity in cardiac structure before TAVI.
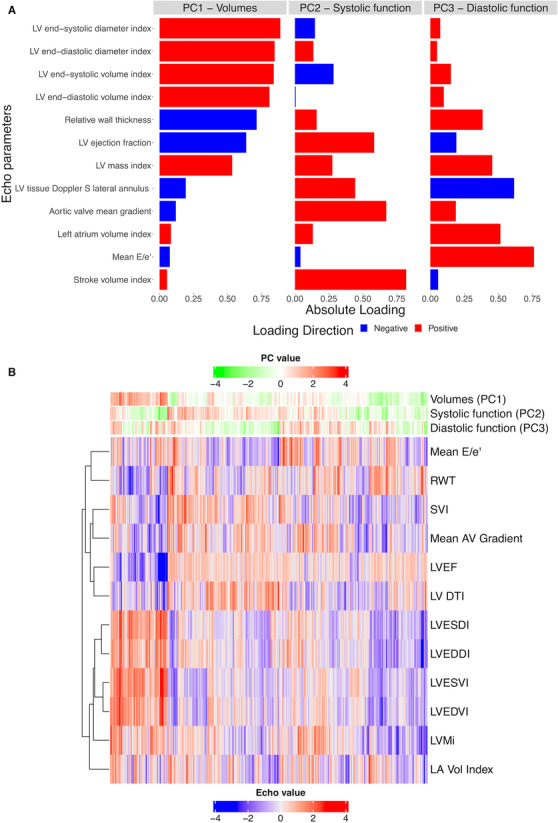
A, Results of PCA of 12 echocardiographic measures. The bar plot indicates the PCA‐based loadings for each echocardiographic measure for each PC. B, Clustered heatmap demonstrating individuals across echocardiographic measures, with PC scores for each individual represented as heatbars across the top of the heatmap. Each column is a participant, and rows represent phenotypes. AV indicates aortic valve; LA Vol, left atrium volume; LV DTI, tissue Doppler S velocity of lateral mitral annulus; LV, left ventricular; LVEDDI, left ventricular end‐diastolic diameter index; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESDI, left ventricular end‐systolic diameter index; LVESVI, left ventricular end‐systolic volume index; LVMi, left ventricular mass index; PC, principal component; RWT, relative wall thickness; and SVI, stroke volume index.
Metabolic Signature of Cardiac Structure/Function in AS
We next aimed to define metabolomic correlates for cardiac structure/function, using the PCA‐based composite axes in our derivation cohort. Sixty unique metabolites were selected in LASSO models (Table S1). Coefficients for metabolites from LASSO models (Figure S4) were used to generate metabolite‐based scores for each PCA‐based axis. We observed a moderate correlation of the metabolite scores with their parent PC scores (Figure S5; Spearman rho range, 0.41–0.47). We did not observe a clinically significant difference in metabolite scores by sex (Figure S6) and only a weak correlation with age (Figure 3; Tables S2 and S3). Individual metabolites across the 3 metabolite scores included metabolites previously implicated in cardiac structure and metabolism (long‐chain acylcarnitines, 12 branched‐chain amino acids 21 ), as well as many not previously implicated in HF or AS (Table S1). Examining 52 metabolites with identifiable Human Metabolome Database identifiers across all 3 metabolite scores, we found several key enriched pathways (Figure S7): arginine metabolism (implicated in nitric oxide metabolism 22 ) and pantothenate and coenzyme A metabolism (central to aerobic metabolic flux 23 ), in addition to several others not widely previously implicated in cardiac remodeling.
Figure 3. Metabolite scores are associated with measures of multimorbidity.
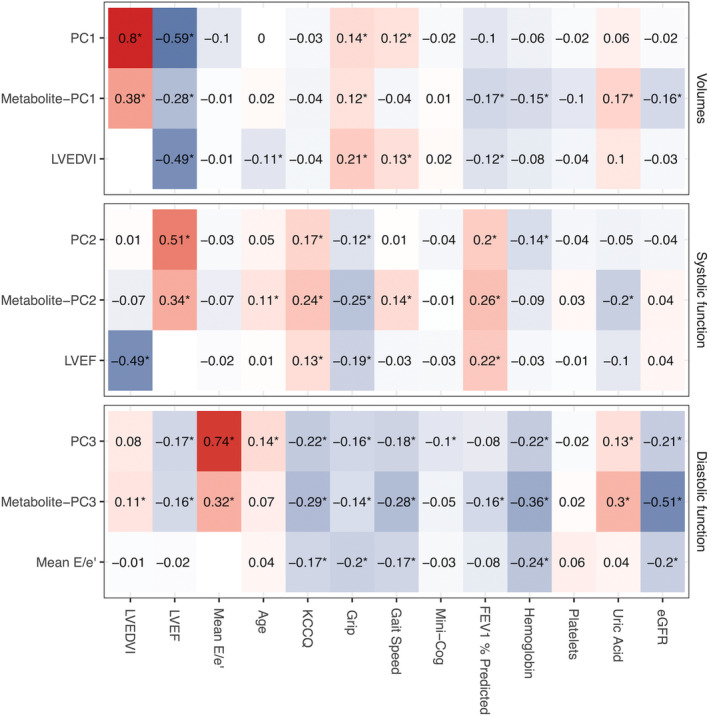
Spearman correlation between each metabolite PC scores and available measures of multimorbidity in the multicenter derivation cohort. Exemplary echocardiographic variables are also included (LVEDVI for volumes, PC1; LVEF for systolic function, PC2; Mean E/e′ for diastolic function, PC3) to demonstrate the relation between each metabolite score with its exemplary LV phenotype. Raw data are reported in Table S2. eGFR indicates estimated glomerular filtration rate; FEV1, forced expiratory volume in the first second; KCCQ, Kansas City Cardiomyopathy Questionnaire summary score; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; Mini‐Cog, Mini‐Cog score for dementia; and PC, principal component. *indicates a false discovery rate <0.05 (Benjamini–Hochberg method).
Metabolite Scores of Cardiac Structure/Function in AS Are Associated With Death
We next measured the association of PC and metabolite scores with all‐cause death in our derivation cohort (median follow‐up, 3.1 years; 25th–75th percentile; 1.6–3.8 years; 205 deaths; Table S4). We did not observe meaningful violation of proportionality in Cox models (see Methods). While neither metabolite‐based nor PC scores of LV volumes and systolic function were strongly and consistently related to outcome (Figure 4A), both the PC and metabolite‐based scores of diastolic function were related to death. After adjustment for clinical risk factors and cardiac biomarkers, the metabolite score of diastolic function was associated with death (HR per 1 SD increase in score, 1.54 [95% CI, 1.25–1.90]; P<0.001), which remained significant with further adjustments for mean aortic valve gradient and LVEF (HR per 1 SD increase, 1.55 [95% CI, 1.26–1.92]; P<0.001). The PC score for diastolic function was directionally consistent (but not statistically significant). We observed similar results in the multicenter validation cohort (median follow‐up, 2.6 years; 25th–75th percentile; 1.0–3.7 years; 121 deaths; Figure 4B). Using recalibrated metabolite scores in our single‐center validation (recalibration fit [R2] between 46% and 87%; Table S5; Figure S8), we observed an adjusted association between the metabolite score for PC3 (diastolic function)—but not PC1 or PC2—with death (median follow‐up after TAVI, 1.6 years; 25th–75th percentile; 0.9–2.8 years; 93 events; Figure 4B and Figure S9).
Figure 4. Association of metabolite scores and parent phenotype scores with death.
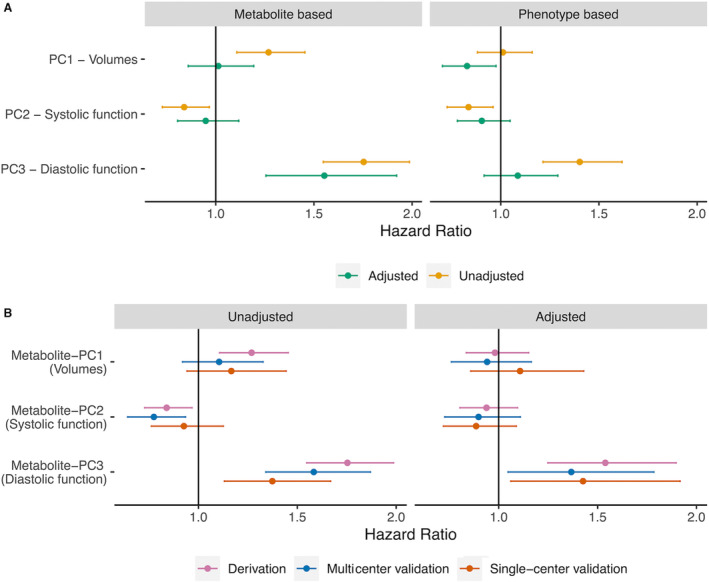
Results of Cox models are displayed with 95% CIs (model results in Table S5). A, Phenotype‐based PC scores demonstrated limited associations with death after adjustment. The metabolite score for diastolic function (PC3) was associated with all‐cause death. B, The association of each metabolite score with death in our replication cohorts, demonstrating replication of the prognostic association of our diastolic function metabolite score (PC3) with death in the multicenter and single‐center validation cohorts. The effect sizes appear in a similar range as with the derivation cohort. PC indicates principal component.
Metabolites Associated With Cardiac Structure/Function Are Also Related to Functional Impairment Across Multiple Noncardiac Organ Systems
Despite the focus on cardiac structure/function to post‐TAVI outcome, 7 , 24 the importance of a holistic approach to assessing post‐TAVI outcome (the concept of multimorbidity and frailty) is increasingly recognized as a major contributor to post‐TAVI outcome not reversed completely by reduction in HF and restoration of cardiac performance. 9 The finding that a metabolite score reflecting diastolic function (PC3)—a cardiac phenotype related in HF to other systems (eg, sarcopenia, obesity, inflammation 18 )—was associated with death across cohorts with adjustment for known post‐TAVI risk was intriguing (Figure 4). We tested the hypothesis that metabolite‐based scores may reflect noncardiac measures relevant to risk (distributions of multimorbid measures are reported in Table S6). We observed a relation between the metabolite scores and multiorgan debility, particularly for the metabolite score corresponding to diastolic function (PC3; Figure 3, Tables S2 and S3). A higher metabolite score for diastolic function was related to higher uric acid (a marker of systemic inflammation 25 ), lower hemoglobin, and lower physical and pulmonary performance (Benjamini–Hochberg false discovery rate <5% for all). Given that this metabolite score was not strongly related to age or sex (Figure 3, Figure S6), we excluded potential for confounding by age or sex, though other unmeasured confounders remain possible. Nevertheless, these analyses suggest that circulating metabolites of cardiac risk may also reflect additional systemic multimorbidity, potentially accounting for the prognostic performance of these metabolite patterns following TAVI.
Discussion
While TAVI has revolutionized the care of individuals with severe AS, residual risk following TAVI remains a significant clinical problem, 2 , 3 , 6 , 26 reflecting multiple cardiac and noncardiac factors relevant to recovery and prognosis. While efforts in other advanced cardiac conditions (eg, HF) have been the subject of broad efforts to delineate mechanisms and biomarkers of risk, studies in AS are limited by study size and biomarker and outcome characterization (beyond the short‐term and standard clinical biomarkers). Here, we performed (to our knowledge) the largest study of individuals with symptomatic severe AS referred for TAVI before their procedure (1062 individuals), integrating comprehensive, core laboratory–adjudicated echocardiographic measures with a broad metabolome to identify metabolic correlates of cardiac structure/function and their relation to death and morbidity. Our principal findings were 3‐fold. First, using integrative statistical approaches, we defined metabolite signatures of 3 echocardiographic axes of remodeling (volumes, systolic and diastolic function), with the metabolite‐based score corresponding to diastolic function related to long‐term outcomes independent of known clinical risk factors, including markers of ischemia and hemodynamic stress. Second, metabolites reflecting each axis of cardiac remodeling identified both known and novel pathways related to remodeling. Third, a metabolite score that was constructed to reflect diastolic function was also related to systemic measures of organ‐level debility and dysfunction, including skeletal muscle, pulmonary, hematologic, and inflammatory systems. Collectively, in the largest cohort of individuals with metabolite profiling in AS, these findings underscore the relevance of systemic metabolism on both cardiac and noncardiac prognosis following TAVI.
Limited studies among patients with severe AS have demonstrated relationships between circulating metabolites (or their changes 13 , 27 ) and markers of adverse LV remodeling. 12 , 13 Based on the importance of metabolism on cardiac function in previous work from our group 12 and others, 28 , 29 we conducted one of the largest studies of metabolite profiling in severe AS, uniting detailed, core laboratory–adjudicated cardiac phenotyping with a broad metabolome to define metabolic features associated with the pre‐TAVI cardiac state. In our analysis, molecular signatures reflecting LV volumes and diastolic function (PC1 and PC3, respectively) included metabolites implicated in LV remodeling 12 (choline), hypertrophy 30 (uracil), survival among patients with HF 31 (kynurenine), and coronary artery disease 32 , 33 (S‐adenosyl‐L‐homocysteine, C18:2 acylcarnitine). The metabolite signatures reflecting LV systolic function (PC2) included metabolites that appear protective against ischemia reperfusion injury 34 (N‐oleoyl dopamine) and pressure overload–associated injury 35 (α‐ketoglutaric acid). These results are consistent with prior work demonstrating a relationship between long‐chain acylcarnitines and LV remodeling in 44 patients with severe AS 12 and a correlation between acylcarnitines and improvement of LVEF after TAVI in 30 patients. 27 Our results are consistent with other reports suggesting a role for nitric oxide metabolism in AS 13 and incident HF. 36 A particularly interesting finding is the association of leucine and valine with diastolic function (PC3). Impaired metabolism of branched‐chain amino acids has been implicated in the pathogenesis of HF, and pharmacological enhancement of this metabolic pathway may be of benefit. 21 Importantly, several metabolites have not been widely reported in AS‐related LV remodeling, suggesting potential for new discovery (Table S1).
In addition to metabolite relations, we found a striking relation between the metabolite score for PC3—the metabolic signature related to post‐TAVI all‐cause death—and measures of multiorgan function. These differences did not seem to be driven by differences in metabolite scores by sex (Figure S6) or age (Figure 3). One explanation may be due to a shared inflammatory pathophysiology of cardiac fibrosis/diastolic impairment and systemic organ function, 37 especially given the relation of persistent diastolic dysfunction 24 and underlying fibrosis 38 , 39 , 40 on death following TAVI. An important corollary is the potential for reversibility in these cardiac phenotypes: Given the potential for rapid improvement in hemodynamics following TAVI, a shared metabolic signature between cardiac and noncardiac phenotypes may also identify individuals with lower metabolic “resiliency” to stress in noncardiac organs—“multimorbidity frailty.” 41 Indeed, studies in humans and mice have identified shared factors relevant to cardiac hypertrophy responses to pressure overload and skeletal muscle metabolism, 14 suggesting the potential for shared molecular pathways common to both cardiac and systemic “remodeling” following TAVI.
In effect, our findings provide molecular context for the emerging importance of cardiac‐dependent and cardiac‐independent frailty in advanced heart disease. 42 While TAVI can immediately improve hemodynamics, it does not necessarily translate to improved frailty, 43 which is prognostically central. 9 Certainly, we do not advocate the use of these broad approaches to limit application of TAVI, given its dramatic impact on outcome. 44 , 45 , 46 , 47 , 48 Nevertheless, approaches that quantify functional biomarkers of a systemic metabolic state (like this one) move the goalpost from identifying risk markers to limit TAVI toward efforts to understand potentially reversible biology associated with risk that represent opportunities for intervention. Whether additional interventions on this multimorbidity can reverse, or halt, some of these key biological processes remains an area of intense interest.
The results of this study should be viewed in the context of its design. While we explored concurrent phenotypes and metabolites (potential for reverse causation), the association of metabolite‐based scores with long‐term outcome increases confidence as to their validity. We did not evaluate for the confounding effects of medications. In addition, we did not examine changes in cardiac structure following TAVI. While evidence suggests that persistent fibrosis following TAVI may be central to future HF, 49 further studies in this cohort and others are required to understand how baseline pre‐TAVI metabolism and changes following TAVI correspond to the capacity for reverse remodeling in the heart (and other organs). The cohorts studied underwent TAVI ≥5 years ago, which may limit generalizability to contemporary cohorts. Importantly, this study was performed in a predominantly White population, limiting generalizability to other racial and ethnic groups. Future studies with greater racial diversity that are based on the effects of intervention on a broad cardiac and noncardiac phenome will be helpful to delve further into the mechanistic and pathway relevance of selected metabolites.
In conclusion, we identified metabolite signatures of 3 distinct axes of cardiac structure/function, one of which was linked to long‐term death after TAVI. Strikingly, a metabolic pattern of diastolic function was associated with multiorgan morbidity and frailty, highlighting an overlapping role of metabolism in cardiac and noncardiac states in advanced heart disease more generally. Studies to evaluate the effect of TAVI on metabolism—including adjunctive interventions to modify specific metabolic pathways—are needed to expand potential routes to optimize post‐TAVI outcome.
Sources of Funding
Metabolite quantification was funded by National Heart, Lung and Blood Institute, National Institutes of Health (R01‐HL151838) (to Dr Elmariah). Echocardiographic phenotyping was funded by the Doris Duke Clinical Research Foundation (to Dr Lindman).
Disclosures
Dr Murthy has received grant support from Siemens Healthineers, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Aging, National Heart, Lung, and Blood Institute, and the American Heart Association. He has received other research support from NIVA Medical Imaging Solutions. He owns stock in Eli Lilly, Johnson & Johnson, Merck, Bristol‐Myers Squibb, and Pfizer and stock options in Ionetix. He has received research grants and speaking honoraria from Quart Medical. Dr Fearon receives institutional research support from Edwards, Abbott, Boston Scientific, and Medtronic. Dr Gillam is an advisor to Bracco Diagnostics, Philips, and Edwards Lifesciences and directs an imaging core laboratory with contracts with Edwards Lifesciences, Medtronic, and Abbott (no direct compensation). Dr Whisenant is a consultant at Edwards Lifescience and Medtronic. Dr Zajarias is a consultant at Edwards Lifescience. Dr Welt formerly sat on the Medtronic advisory board. Dr Coylewright received research funding from Edwards LifeSciences. Dr Piana serves on the data safety monitoring boards for Abbott Medical and Baim Cardiovascular Research Institute. Dr Gupta receives research support from Imara Inc. Dr Shah has received grant support from the National Institutes of Health and the American Heart Association and has served as a consultant in the past 12 months for Amgen and Cytokinetics. He is a coinventor on a patent for ex‐RNA signatures of cardiac remodeling. Dr Lindman has served on the scientific advisory board for Roche Diagnostics and has received research grants from the National Institutes of Health (R01AG073633), Edwards Lifesciences, and Roche Diagnostics. Dr. Elmariah has received research grants from Edwards Lifesciences, Medtronic, and Abbott and consulting fees from Edwards Lifesciences. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S6
Figures S1–S9
References 50–81
Acknowledgments
The investigators thank the study participants for their contributions of time and biospecimens.
This manuscript was sent to Sakima A. Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029542
For Sources of Funding and Disclosures, see page 11.
References
- 1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 2. Arnold SV, Cohen DJ, Dai D, Jones PG, Li F, Thomas L, Baron SJ, Frankel NZ, Strong S, Matsouaka RA, et al. Predicting quality of life at 1 year after transcatheter aortic valve replacement in a real‐world population. Circ Cardiovasc Qual Outcomes. 2018;11:e004693. doi: 10.1161/CIRCOUTCOMES.118.004693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vemulapalli S, Dai D, Hammill BG, Baron SJ, Cohen DJ, Mack MJ, Holmes DR Jr. Hospital resource utilization before and after transcatheter aortic valve replacement: the STS/ACC TVT registry. J Am Coll Cardiol. 2019;73:1135–1146. doi: 10.1016/j.jacc.2018.12.049 [DOI] [PubMed] [Google Scholar]
- 4. Hermiller JB Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo J, Huang J, Popma JJ; CoreValve United States Clinical Investigators . Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:343–352. doi: 10.1016/j.jacc.2016.04.057 [DOI] [PubMed] [Google Scholar]
- 5. Schoechlin S, Schulz U, Ruile P, Hein M, Eichenlaub M, Jander N, Neumann FJ, Valina C. Impact of high‐sensitivity cardiac troponin T on survival and rehospitalization after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2021;98:E881–E888. doi: 10.1002/ccd.29781 [DOI] [PubMed] [Google Scholar]
- 6. Yoshijima N, Saito T, Inohara T, Anzai A, Tsuruta H, Shimizu H, Fukuda K, Naganuma T, Mizutani K, Yamawaki M, et al. Predictors and clinical outcomes of poor symptomatic improvement after transcatheter aortic valve replacement. Open Heart. 2021;8:e001742. doi: 10.1136/openhrt-2021-001742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzales H, Douglas PS, Pibarot P, Hahn RT, Khalique OK, Jaber WA, Cremer P, Weissman NJ, Asch FM, Zhang Y, et al. Left ventricular hypertrophy and clinical outcomes over 5 years after TAVR: an analysis of the PARTNER trials and registries. JACC Cardiovasc Interv. 2020;13:1329–1339. doi: 10.1016/j.jcin.2020.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Stein EJ, Fearon WF, Elmariah S, Kim JB, Kapadia S, Kumbhani DJ, Gillam L, Whisenant B, Quader N, Zajarias A, et al. Left ventricular hypertrophy and biomarkers of cardiac damage and stress in aortic stenosis. J Am Heart Assoc. 2022;11:e023466. doi: 10.1161/JAHA.121.023466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR study. J Am Coll Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024 [DOI] [PubMed] [Google Scholar]
- 10. Kano S, Yamamoto M, Shimura T, Kagase A, Tsuzuki M, Kodama A, Koyama Y, Kobayashi T, Shibata K, Tada N, et al. Gait speed can predict advanced clinical outcomes in patients who undergo transcatheter aortic valve replacement: insights from a Japanese multicenter registry. Circ Cardiovasc Interv. 2017;10:e005088. doi: 10.1161/CIRCINTERVENTIONS.117.005088 [DOI] [PubMed] [Google Scholar]
- 11. Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, et al. Development and validation of a risk prediction model for In‐hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. doi: 10.1001/jamacardio.2015.0326 [DOI] [PubMed] [Google Scholar]
- 12. Elmariah S, Farrell LA, Furman D, Lindman BR, Shi X, Morningstar JE, Rhee EP, Gerszten RE. Association of acylcarnitines with left ventricular remodeling in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. JAMA Cardiol. 2018;3:242–246. doi: 10.1001/jamacardio.2017.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Driel BO, Schuldt M, Algul S, Levin E, Guclu A, Germans T, Rossum ACV, Pei J, Harakalova M, Baas A, et al. Metabolomics in severe aortic stenosis reveals intermediates of nitric oxide synthesis as most distinctive markers. Int J Mol Sci. 2021;22:3569. doi: 10.3390/ijms22073569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11:eaau8680. doi: 10.1126/scitranslmed.aau8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 16. Perry AS, Stein EJ, Biersmith M, Fearon WF, Elmariah S, Kim JB, Clark DE, Patel JN, Gonzales H, Baker M, et al. Global longitudinal strain and biomarkers of cardiac damage and stress as predictors of outcomes after transcatheter aortic valve implantation. J Am Heart Assoc. 2022;11:e026529. doi: 10.1161/JAHA.122.026529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou B, Xiao JF, Tuli L, Ressom HW. LC‐MS‐based metabolomics. Mol BioSyst. 2012;8:470–481. doi: 10.1039/c1mb05350g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanders‐van Wijk S, Tromp J, Beussink‐Nelson L, Hage C, Svedlund S, Saraste A, Swat SA, Sanchez C, Njoroge J, Tan RS, et al. Proteomic evaluation of the comorbidity‐inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS‐HFpEF study. Circulation. 2020;142:2029–2044. doi: 10.1161/CIRCULATIONAHA.120.045810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedman JH, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009;37:W652–W660. doi: 10.1093/nar/gkp356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, et al. Catabolic defect of branched‐chain amino acids promotes heart failure. Circulation. 2016;133:2038–2049. doi: 10.1161/CIRCULATIONAHA.115.020226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Czumaj A, Szrok‐Jurga S, Hebanowska A, Turyn J, Swierczynski J, Sledzinski T, Stelmanska E. The pathophysiological role of CoA. Int J Mol Sci. 2020;21:9057. doi: 10.3390/ijms21239057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ong G, Pibarot P, Redfors B, Weissman NJ, Jaber WA, Makkar RR, Lerakis S, Gopal D, Khalique O, Kodali SK, et al. Diastolic function and clinical outcomes after transcatheter aortic valve replacement: PARTNER 2 SAPIEN 3 registry. J Am Coll Cardiol. 2020;76:2940–2951. doi: 10.1016/j.jacc.2020.10.032 [DOI] [PubMed] [Google Scholar]
- 25. Spiga R, Marini MA, Mancuso E, Di Fatta C, Fuoco A, Perticone F, Andreozzi F, Mannino GC, Sesti G. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF‐kappaB signaling pathway in HepG2 cells. Arterioscler Thromb Vasc Biol. 2017;37:1241–1249. doi: 10.1161/ATVBAHA.117.309128 [DOI] [PubMed] [Google Scholar]
- 26. Tamburino C, Barbanti M, D'Errigo P, Ranucci M, Onorati F, Covello RD, Santini F, Rosato S, Santoro G, Fusco D, et al. 1‐year outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT study. J Am Coll Cardiol. 2015;66:804–812. doi: 10.1016/j.jacc.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 27. Haase D, Baz L, Bekfani T, Neugebauer S, Kiehntopf M, Mobius‐Winkler S, Franz M, Schulze PC. Metabolomic profiling of patients with high gradient aortic stenosis undergoing transcatheter aortic valve replacement. Clin Res Cardiol. 2021;110:399–410. doi: 10.1007/s00392-020-01754-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng ML, Wang CH, Shiao MS, Liu MH, Huang YY, Huang CY, Mao CT, Lin JF, Ho HY, Yang NI. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65:1509–1520. doi: 10.1016/j.jacc.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 29. Hunter WG, Kelly JP, McGarrah RW III, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. 2016;5:5. doi: 10.1161/JAHA.115.003190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossi A, Aussedat J, Olivares J, Ray A, Verdys M. Pyrimidine nucleotide metabolism in cardiac hypertrophy. Eur Heart J. 1984;5:155–162. doi: 10.1093/eurheartj/5.suppl_f.155 [DOI] [PubMed] [Google Scholar]
- 31. Lund A, Nordrehaug JE, Slettom G, Solvang SH, Pedersen EK, Midttun O, Ulvik A, Ueland PM, Nygard O, Giil LM. Plasma kynurenines and prognosis in patients with heart failure. PLoS One. 2020;15:e0227365. doi: 10.1371/journal.pone.0227365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Z, Wang L, Zhan Y, Xie C, Xiang Y, Chen D, Wu Y. Clinical value and expression of Homer 1, homocysteine, S‐adenosyl‐l‐homocysteine, fibroblast growth factors 23 in coronary heart disease. BMC Cardiovasc Disord. 2022;22:215. doi: 10.1186/s12872-022-02554-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deda O, Panteris E, Meikopoulos T, Begou O, Mouskeftara T, Karagiannidis E, Papazoglou AS, Sianos G, Theodoridis G, Gika H. Correlation of serum acylcarnitines with clinical presentation and severity of coronary artery disease. Biomol Ther. 2022;12:354. doi: 10.3390/biom12030354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhong B, Wang DH. N‐oleoyldopamine, a novel endogenous capsaicin‐like lipid, protects the heart against ischemia‐reperfusion injury via activation of TRPV1. Am J Physiol Heart Circ Physiol. 2008;295:H728–H735. doi: 10.1152/ajpheart.00022.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. An D, Zeng Q, Zhang P, Ma Z, Zhang H, Liu Z, Li J, Ren H, Xu D. Alpha‐ketoglutarate ameliorates pressure overload‐induced chronic cardiac dysfunction in mice. Redox Biol. 2021;46:102088. doi: 10.1016/j.redox.2021.102088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tahir UA, Katz DH, Zhao T, Ngo D, Cruz DE, Robbins JM, Chen ZZ, Peterson B, Benson MD, Shi X, et al. Metabolomic profiles and heart failure risk in black adults: insights from the Jackson heart study. Circ Heart Fail. 2021;14:e007275. doi: 10.1161/CIRCHEARTFAILURE.120.007275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021;128:1451–1467. doi: 10.1161/CIRCRESAHA.121.318159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thornton GD, Musa TA, Rigolli M, Loudon M, Chin C, Pica S, Malley T, Foley JRJ, Vassiliou VS, Davies RH, et al. Association of myocardial fibrosis and stroke volume by cardiovascular magnetic resonance in patients with severe aortic stenosis with outcome after valve replacement: the British Society of Cardiovascular Magnetic Resonance AS700 study. JAMA Cardiol. 2022;7:513–520. doi: 10.1001/jamacardio.2022.0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Everett RJ, Treibel TA, Fukui M, Lee H, Rigolli M, Singh A, Bijsterveld P, Tastet L, Musa TA, Dobson L, et al. Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol. 2020;75:304–316. doi: 10.1016/j.jacc.2019.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papanastasiou CA, Kokkinidis DG, Kampaktsis PN, Bikakis I, Cunha DK, Oikonomou EK, Greenwood JP, Garcia MJ, Karamitsos TD. The prognostic role of late gadolinium enhancement in aortic stenosis: a systematic review and meta‐analysis. JACC Cardiovasc Imaging. 2020;13:385–392. doi: 10.1016/j.jcmg.2019.03.029 [DOI] [PubMed] [Google Scholar]
- 41. Ijaz N, Buta B, Xue QL, Mohess DT, Bushan A, Tran H, Batchelor W, deFilippi CR, Walston JD, Bandeen‐Roche K, et al. Interventions for frailty among older adults with cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Flint KM, Matlock DD, Lindenfeld J, Allen LA. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail. 2012;5:286–293. doi: 10.1161/CIRCHEARTFAILURE.111.963215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gouda P, Paterson C, Meyer S, Shanks M, Butler C, Taylor D, Tyrrell B, Welsh R. Effects of transcatheter aortic valve implantation on frailty and quality of life. CJC Open. 2020;2:79–84. doi: 10.1016/j.cjco.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bing R, Everett RJ, Tuck C, Semple S, Lewis S, Harkess R, Mills NL, Treibel TA, Prasad S, Greenwood JP, et al. Rationale and design of the randomized, controlled early valve replacement guided by biomarkers of left ventricular decompensation in asymptomatic patients with severe aortic stenosis (EVOLVED) trial. Am Heart J. 2019;212:91–100. doi: 10.1016/j.ahj.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 45. Banovic M, Putnik S, Penicka M, Doros G, Deja MA, Kockova R, Kotrc M, Glaveckaite S, Gasparovic H, Pavlovic N, et al. Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis: the AVATAR trial. Circulation. 2022;145:648–658. doi: 10.1161/CIRCULATIONAHA.121.057639 [DOI] [PubMed] [Google Scholar]
- 46. Tastet L, Tribouilloy C, Marechaux S, Vollema EM, Delgado V, Salaun E, Shen M, Capoulade R, Clavel MA, Arsenault M, et al. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J Am Coll Cardiol. 2019;74:550–563. doi: 10.1016/j.jacc.2019.04.065 [DOI] [PubMed] [Google Scholar]
- 47. Lindman BR, Dweck MR, Lancellotti P, Genereux P, Pierard LA, O'Gara PT, Bonow RO. Management of asymptomatic severe aortic stenosis: evolving concepts in timing of valve replacement. JACC Cardiovasc Imaging. 2020;13:481–493. doi: 10.1016/j.jcmg.2019.01.036 [DOI] [PubMed] [Google Scholar]
- 48. Lindman BR, Lindenfeld J. Prevention and mitigation of heart failure in the treatment of calcific aortic stenosis: a unifying therapeutic principle. JAMA Cardiol. 2021;6:993–994. doi: 10.1001/jamacardio.2021.2082 [DOI] [PubMed] [Google Scholar]
- 49. Zhang C, Liu J, Qin S. Prognostic value of cardiac magnetic resonance in patients with aortic stenosis: a systematic review and meta‐analysis. PLoS One. 2022;17:e0263378. doi: 10.1371/journal.pone.0263378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lewis GD, Wei R, Liu E, Yang E, Shi X, Martinovic M, Farrell L, Asnani A, Cyrille M, Ramanathan A, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118:3503–3512. doi: 10.1172/JCI35111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Landoni G, Zangrillo A, Lomivorotov VV, Likhvantsev V, Ma J, De Simone F, Fominskiy E. Cardiac protection with phosphocreatine: a meta‐analysis. Interact Cardiovasc Thorac Surg. 2016;23:637–646. doi: 10.1093/icvts/ivw171 [DOI] [PubMed] [Google Scholar]
- 52. Balestrino M. Role of creatine in the heart: health and disease. Nutrients. 2021;13:1215. doi: 10.3390/nu13041215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khlebnikov AI, Schepetkin IA, Quinn MT. Quantitative structure‐activity relationships for small non‐peptide antagonists of CXCR2: indirect 3D approach using the frontal polygon method. Bioorg Med Chem. 2006;14:352–365. doi: 10.1016/j.bmc.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 54. Al Hageh C, Rahy R, Khazen G, Brial F, Khnayzer RS, Gauguier D, Zalloua PA. Plasma and urine metabolomic analyses in aortic valve stenosis reveal shared and biofluid‐specific changes in metabolite levels. PLoS One. 2020;15:e0242019. doi: 10.1371/journal.pone.0242019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jou S, Patel H, Oglat H, Zhang R, Zhang L, Ells P, Nappi A, El‐Hajjar M, DeLago A, Torosoff M. The prevalence and prognostic implications of pre‐procedural hyperbilirubinemia in patients undergoing transcatheter aortic valve replacement. Heart Vessel. 2020;35:1102–1108. doi: 10.1007/s00380-020-01588-y [DOI] [PubMed] [Google Scholar]
- 56. Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, et al. Alpha‐hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5:e10883. doi: 10.1371/journal.pone.0010883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marszalek‐Grabska M, Walczak K, Gawel K, Wicha‐Komsta K, Wnorowska S, Wnorowski A, Turski WA. Kynurenine emerges from the shadows–current knowledge on its fate and function. Pharmacol Ther. 2021;225:107845. doi: 10.1016/j.pharmthera.2021.107845 [DOI] [PubMed] [Google Scholar]
- 58. Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, et al. The pyruvate‐lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab. 2021;33:629–648. doi: 10.1016/j.cmet.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guasch‐Ferre M, Hu FB, Ruiz‐Canela M, Bullo M, Toledo E, Wang DD, Corella D, Gomez‐Gracia E, Fiol M, Estruch R, et al. Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (prevention with Mediterranean diet) Study. J Am Heart Assoc. 2017;6:e006524. doi: 10.1161/JAHA.117.006524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, et al. 12,13‐diHOME: an exercise‐induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 2018;27:1111–1120. doi: 10.1016/j.cmet.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raad M, AlBadri A, Wei J, Mehta PK, Maughan J, Gadh A, Thomson L, Jones DP, Quyyumi AA, Pepine CJ, et al. Oxidative stress is associated with diastolic dysfunction in women with ischemia with no obstructive coronary artery disease. J Am Heart Assoc. 2020;9:e015602. doi: 10.1161/JAHA.119.015602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leary PJ, Tedford RJ, Bluemke DA, Bristow MR, Heckbert SR, Kawut SM, Krieger EV, Lima JA, Masri CS, Ralph DD, et al. Histamine H2 receptor antagonists, left ventricular morphology, and heart failure risk: the MESA study. J Am Coll Cardiol. 2016;67:1544–1552. doi: 10.1016/j.jacc.2016.01.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen J, Hong T, Ding S, Deng L, Abudupataer M, Zhang W, Tong M, Jia J, Gong H, Zou Y, et al. Aggravated myocardial infarction‐induced cardiac remodeling and heart failure in histamine‐deficient mice. Sci Rep. 2017;7:44007. doi: 10.1038/srep44007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mate‐Munoz JL, Lougedo JH, Garnacho‐Castano MV, Veiga‐Herreros P, Lozano‐Estevan MDC, Garcia‐Fernandez P, de Jesus F, Guodemar‐Perez J, San Juan AF, Dominguez R. Effects of beta‐alanine supplementation during a 5‐week strength training program: a randomized, controlled study. J Int Soc Sports Nutr. 2018;15:19. doi: 10.1186/s12970-018-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stefani GP, Capalonga L, da Silva LR, Dal Lago P. Beta‐alanine and l‐histidine supplementation associated with combined training increased functional capacity and maximum strength in heart failure rats. Exp Physiol. 2020;105:831–841. doi: 10.1113/EP088327 [DOI] [PubMed] [Google Scholar]
- 66. Holm J, Ferrari G, Holmgren A, Vanky F, Friberg O, Vidlund M, Svedjeholm R. Effect of glutamate infusion on NT‐proBNP after coronary artery bypass grafting in high‐risk patients (GLUTAMICS II): a randomized controlled trial. PLoS Med. 2022;19:e1003997. doi: 10.1371/journal.pmed.1003997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sivakumar R, Babu PV, Shyamaladevi CS. Aspartate and glutamate prevents isoproterenol‐induced cardiac toxicity by alleviating oxidative stress in rats. Exp Toxicol Pathol. 2011;63:137–142. doi: 10.1016/j.etp.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 68. Zheng Y, Hu FB, Ruiz‐Canela M, Clish CB, Dennis C, Salas‐Salvado J, Hruby A, Liang L, Toledo E, Corella D, et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvencion con DIeta MEDiterranea (PREDIMED) trial. J Am Heart Assoc. 2016;5:e003755. doi: 10.1161/JAHA.116.003755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Krylova IB, Selina EN, Bulion VV, Rodionova OM, Evdokimova NR, Belosludtseva NV, Shigaeva MI, Mironova GD. Uridine treatment prevents myocardial injury in rat models of acute ischemia and ischemia/reperfusion by activating the mitochondrial ATP‐dependent potassium channel. Sci Rep. 2021;11:16999. doi: 10.1038/s41598-021-96562-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Salmani M, Alipoor E, Navid H, Farahbakhsh P, Yaseri M, Imani H. Effect of l‐arginine on cardiac reverse remodeling and quality of life in patients with heart failure. Clin Nutr. 2021;40:3037–3044. doi: 10.1016/j.clnu.2021.01.044 [DOI] [PubMed] [Google Scholar]
- 71. Deidda M, Piras C, Dessalvi CC, Locci E, Barberini L, Torri F, Ascedu F, Atzori L, Mercuro G. Metabolomic approach to profile functional and metabolic changes in heart failure. J Transl Med. 2015;13:297. doi: 10.1186/s12967-015-0661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shimada YJ, Batra J, Kochav SM, Patel P, Jung J, Maurer MS, Hasegawa K, Reilly MP, Fifer MA. Difference in metabolomic response to exercise between patients with and without hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2021;14:246–255. doi: 10.1007/s12265-020-10051-2 [DOI] [PubMed] [Google Scholar]
- 73. Abbas ZSB, Latif ML, Dovlatova N, Fox SC, Heptinstall S, Dunn WR, Ralevic V. UDP‐sugars activate P2Y14 receptors to mediate vasoconstriction of the porcine coronary artery. Vasc Pharmacol. 2018;103‐105:36–46. doi: 10.1016/j.vph.2017.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR, et al. Metabolomic analysis of pressure‐overloaded and infarcted mouse hearts. Circ Heart Fail. 2014;7:634–642. doi: 10.1161/CIRCHEARTFAILURE.114.001151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng S, Rhee EP, et al. 2‐Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest. 2013;123:4309–4317. doi: 10.1172/JCI64801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saremi A, Howell S, Schwenke DC, Bahn G, Beisswenger PJ, Reaven PD, Investigators V. Advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA diabetes trial and follow‐up study. Diabetes Care. 2017;40:591–598. doi: 10.2337/dc16-1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hannoush H, Introne WJ, Chen MY, Lee SJ, O'Brien K, Suwannarat P, Kayser MA, Gahl WA, Sachdev V. Aortic stenosis and vascular calcifications in alkaptonuria. Mol Genet Metab. 2012;105:198–202. doi: 10.1016/j.ymgme.2011.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Russ M, Jauk S, Wintersteiger R, Andra M, Brcic I, Ortner A. Investigation of antioxidative effects of a cardioprotective solution in heart tissue. Mol Cell Biochem. 2019;461:73–80. doi: 10.1007/s11010-019-03591-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. 2019;18:86. doi: 10.1186/s12933-019-0892-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bullo M, Papandreou C, Garcia‐Gavilan J, Ruiz‐Canela M, Li J, Guasch‐Ferre M, Toledo E, Clish C, Corella D, Estruch R, et al. Tricarboxylic acid cycle related‐metabolites and risk of atrial fibrillation and heart failure. Metabolism. 2021;125:154915. doi: 10.1016/j.metabol.2021.154915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tang X, Liu J, Dong W, Li P, Li L, Lin C, Zheng Y, Hou J, Li D. The cardioprotective effects of citric acid and L‐malic acid on myocardial ischemia/reperfusion injury. Evid Based Complement Alternat Med. 2013;2013:820695. doi: 10.1155/2013/820695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6
Figures S1–S9
References 50–81


