Abstract
Background
In‐stent restenosis (ISR) is commonly encountered even in the era of contemporary percutaneous coronary intervention (PCI). There is a paucity of data on the comparative outcomes of PCI for ISR lesions versus de novo lesions.
Methods and Results
An electronic search was conducted for MEDLINE, Cochrane, and Embase through August 2022 for studies comparing the clinical outcomes after PCI for ISR versus de novo lesions. The primary outcome was major adverse cardiac events. Data were pooled using a random‐effects model. The final analysis included 12 studies, with a total of 708 391 patients, of whom 71 353 (10.3%) underwent PCI for ISR. The weighted follow‐up duration was 29.1 months. Compared with de novo lesions, PCI for ISR was associated with a higher incidence of major adverse cardiac events (odds ratio [OR], 1.31 [95% CI, 1.18–1.46]). There was no difference on a subgroup analysis of chronic total occlusion lesions versus none (P interaction=0.69). PCI for ISR was associated with a higher incidence of all‐cause mortality (OR, 1.03 [95% CI, 1.02–1.04]), myocardial infarction (OR, 1.20 [95% CI, 1.11–1.29]), target vessel revascularization (OR, 1.42 [95% CI, 1.29–1.55]), and stent thrombosis (OR, 1.44 [95% CI, 1.11–1.87]), but no difference in cardiovascular mortality (OR, 1.04 [95% CI, 0.90–1.20]).
Conclusions
PCI for ISR is associated with higher incidence of adverse cardiac events compared with PCI for de novo lesions. Future efforts should be directed toward prevention of ISR and exploring novel treatment strategies for ISR lesions.
Keywords: de novo lesions, in‐stent restenosis, percutaneous coronary intervention
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- CTO
chronic total occlusion
- DES
drug‐eluting stent
- ISR
in‐stent restenosis
- MACE
major adverse cardiac events
- TVR
target vessel revascularization
Clinical Perspective.
What Is New?
Percutaneous coronary intervention (PCI) for in‐stent restenosis was associated with a higher incidence of risk‐adjusted major adverse cardiac events compared with PCI for de novo lesions at a median of ≈30 months.
PCI for in‐stent restenosis was associated with higher incidences of all‐cause mortality, myocardial infarction, target vessel revascularization, and stent thrombosis.
There was no evidence of interaction for the outcomes for chronic total occlusion versus non‐chronic total occlusion.
What Are the Clinical Implications?
In‐stent restenosis is not a benign entity, and major efforts should be directed toward optimizing index PCI procedures.
Further research is warranted to evaluate the outcomes of PCI of in‐stent restenosis compared with de novo lesions using state‐of‐the‐art PCI techniques, including routine intravascular imaging, transradial access, and advanced modalities such as laser atherectomy, brachytherapy, and drug‐coated balloons.
Percutaneous coronary intervention (PCI) remains the cornerstone in the management of patients with acute coronary syndrome and those with chronic coronary syndrome refractory to medical management. 1 The use of metallic coronary stents (ie, drug eluting and bare metal) has revolutionized this space during the past 2 decades. 2 However, metallic coronary stents have introduced another disease (ie, in‐stent restenosis [ISR]). 3 ISR could present clinically with either chronic coronary syndrome or acute coronary syndrome. 2 Although the rates of ISR have decreased considerably with the more widespread use of drug‐eluting stents (DES), especially second–generation, ISR remains a challenge even with DES, and the incidence is up to 5% to 15% after 5 years. 4 , 5 Despite the large number of PCIs performed annually in the United States and worldwide, data evaluating the long‐term outcomes with PCI for ISR are limited. Moreover, comparative data on the outcomes associated with PCI with DES for ISR versus de novo lesions are limited. In that context, we aimed to perform a comprehensive meta‐analysis investigating the comparative long‐term outcomes with PCI for ISR versus de novo lesions.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Data Sources and Search Strategy
We conducted a digital search of the following databases: MEDLINE, Cochrane, and Embase, through July 2022, using the terms “in‐stent restenosis,” “de novo stenosis,” “revascularization,” and “percutaneous coronary intervention” separately and in combination to identify studies that evaluated the outcomes with PCI for ISR versus de novo coronary artery lesions. We also conducted a simultaneous search of the abstracts presented at major societal meetings (American College of Cardiology, European Society of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions meetings) using similar keywords through August 2022. We searched the bibliographies of the retrieved studies, as well as ClinicalTrials.gov, to capture eligible studies that were not obtained through the initial search. This systematic review and meta‐analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (Table S1). 6 A protocol for this meta‐analysis was prospectively registered at PROSPERO (CRD42022364832). This study was deemed exempt from institutional review board evaluation, because it is a study‐level meta‐analysis.
Selection Criteria
We included studies that compared the clinical outcomes with PCI for ISR versus de novo coronary lesions. We included randomized or observational studies. In studies with multiple reports, we used data from the longest follow‐up. We excluded studies that only reported outcomes after PCI for ISR without a comparator group of de novo PCI. Two investigators (I.M. and M.E.) conducted an independent screening, and discrepancies among investigators were resolved by consensus.
Data Extraction
Two independent investigators (I.M. and M.E.) extracted the following data: the study design, baseline characteristics, intervention strategies, and clinical outcomes. In case of discrepancies among investigators, this was resolved by consensus.
Outcomes
The primary outcome of the study was the composite of major adverse cardiovascular events (MACE). The secondary outcomes included all‐cause mortality, cardiovascular mortality, myocardial infarction (MI), repeat ischemia‐driven target vessel revascularization (TVR), and stent thrombosis. Definitions of outcomes were adopted as per each study.
Assessment of the Quality of the Included Studies
Because all of the included studies were observational, we assessed the quality of the included studies using the Newcastle‐Ottawa quality assessment scale. Accordingly, the quality of the studies was classified as very good, good, satisfactory, or unsatisfactory corresponding to a score of 9 to 10, 7 to 8, 5 to 6, or 0 to 4 points, respectively. 7
Statistical Analysis
Interrater agreement between the 2 researchers conducting study selection and data extraction was evaluated using κ coefficient. A random‐effects model was used, and data were pooled using the DerSimonian‐Laird inverse variance method. A random‐effects model provides more conservative results than a fixed‐effects model and assumes that each sample comes from a different population, and that the effects in these populations may also differ. Meta‐analysis was conducted using pooled effect sizes from the included studies using risk‐adjusted data when available. Statistical heterogeneity among the included studies was evaluated using I2 statistics and Cochran Q test. I2 statistic values <25%, 25% to 50%, and >50% corresponded to low, moderate, and high degree of heterogeneity, respectively. 8 , 9 Publication bias was assessed using the Egger test. 10 A prespecified subgroup analysis was conducted for the primary outcome according to chronic total occlusion (CTO) versus non‐CTO coronary lesions. Sensitivity analysis for the primary outcome was conducted after excluding studies without risk‐adjusted outcomes and studies with less than good quality based on the Newcastle‐Ottawa scale. In the prespecified study protocol, we planned to report summary estimates using risk ratios. After conclusion of the study search, the studies meeting the selection criteria were mainly observational studies, and there were no available randomized clinical trials. As such, we decided to report summary estimates using odds ratios (ORs). 11 P values were considered statistically significant if ≤0.05. Statistical analyses were conducted using RevMan 5.0 software (Cochrane Collaboration, Oxford, UK).
Results
Included Studies
The study selection process is outlined in Figure 1. The final analysis included 12 studies published between 2011 to 2021, with a total of 708 391 patients: 71 353 patients underwent PCI for ISR, and 637 038 patients underwent PCI for de novo lesions. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 There was good interrater agreement between the 2 researchers performing study selection (κ=0.79; standard error=0.09) and data extraction (κ=0.81; standard error=0.06). The weighted follow‐up duration was 29.8 months. The study characteristics are outlined in Table 1. Six studies were retrospective studies, and the remaining 6 studies were prospective studies. Four of the included studies exclusively evaluated patients with PCI for CTO. 12 , 13 , 16 , 21 PCI with stenting was used in the ISR group in 46% to 49% of patients in 3 studies, 12 , 17 , 20 75% to 89% in 3 studies, 13 , 14 , 16 and 97% to 100% in the remaining studies. Stents used during PCI procedures were exclusively DES in 7 studies, 12 , 16 , 18 , 19 , 20 , 22 , 23 predominantly DES in 3 other studies, 14 , 17 , 21 and in 2 studies the types of stents were not reported. 13 , 15 Baseline characteristics of included patients and procedural characteristics appear in Table 2 and Table 3. The weighted mean age was 64.3 years and included predominantly men.
Figure 1. Study flowchart.
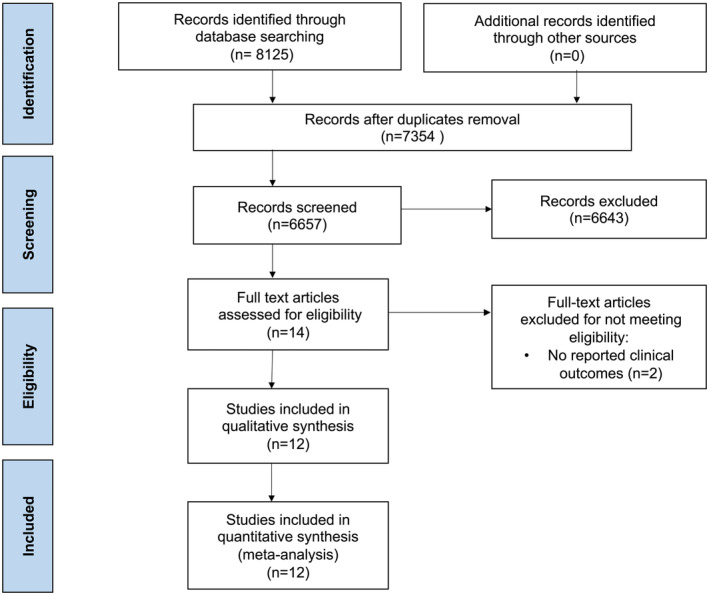
Table 1.
Characteristics of Included Studies
| Study | Year | Study design | No. | PCI with stents % | DES % | Use of IVUS | Use of OCT | Focal ISR % | Diffuse ISR % | Occlusive ISR % | Follow‐up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdelwahab et al 18 | 2011 | Prospective multicenter | 872/4272 | 97.7/97.9 | 100/100 | NA | NA | NA | NA | 4.9 | 12.4 mo |
| Richardt et al 23 | 2013 | Prospective multicenter | 281/3194 | 100/100 | 100/100 | NA | NA | NA | NA | NA | 2 y |
| Gao et al 22 | 2013 | Retrospective single center | 1300/27 211 | 100/100 | 100/100 | 4.1/3.5 | NA | NA | NA | NA | 17 mo |
| Shimonaga et al 15 | 2015 | Prospective single center | 34/87 | NA | NA | NA | NA | 53 | 47 | 0 | In‐hospital |
| Redfors et al 19 | 2017 | Prospective multicenter | 840/7742 | 100/100 | 100/100 | 42.3/38.8 | NA | NA | NA | 6 | 2 y |
| Buchanan et al 17 | 2018 | Retrospective single center | 472/1888 | 46.6/95.7 | 81.7/93.2 | 54/61 | NA | NA | NA | NA | 12 mo |
| Lee et al 16 | 2020 | Retrospective multicenter | 164/1208 | 89.1/93.3 | 100/100 | NA | NA | NA | NA | 100 | 5 y |
| Takeuchi et al 20 | 2021 | Retrospective single center | 280/1258 | 49.3/87.9 | 100/100 | 79.6/91.6 | 27.9/11.1 | NA | NA | NA | 2 y |
| Tamez et al 14 | 2021 | Retrospective multicenter | 66 718/586 586 | 80.6/93 | 91.1/77.2 | NA | NA | NA | NA | NA | 4 y |
| Tang et al 12 | 2021 | Prospective single center | 69/357 | 48.4/79.4 | 100/100 | 17.9/15 | NA | NA | NA | 100 | 2 y |
| Wang et al 13 | 2021 | Retrospective single center | 212/2447 | 75.5/73.9 | NA | 6.8/6.8 | NA | NA | NA | 100 | 5 y |
| Azzalini et al 21 | 2017 | Prospective multicenter | 111/788 | 100/100 | 97.9/87.9 | 9.9/13.3 | NA | NA | NA | 100 | 15.7 mo |
DES indicates drug‐eluting stent; ISR, in‐stent restenosis; IVUS, intravascular ultrasound; NA, not applicable; OCT, optical coherence tomography and PCI, percutaneous coronary intervention.
Table 2.
Baseline Characteristics of Included Patients
| Study | Age, y, mean±SD | Men % | Tobacco use % | HTN % | Diabetes % | Prior MI % | GFR | FH of CAD % | HLD % | ACS % |
|---|---|---|---|---|---|---|---|---|---|---|
| Abdelwahab et al 18 | 66.2±10.4/65±10.5 | 75.9/74.4 | 16.2/23.5 | 86.9/83.3 | 28.8/32.2 | 49.8/26.2 | NA | 39.5/35.5 | 84.5/79.6 | 22/27.3 |
| Richardt et al 23 | 65.3±10.5/63.6±11.1 | 77.6/77.4 | 14.6/25.8 | 79.0/68.0 | 31.0/27.9 | 53.9/25.3 | NA | 36.2/32.0 | 81.1/62.4 | 47.3/56.0 |
| Gao et al 22 | 57.31±10.94/57.75±10.73 | 83.2/78.6 | 42.1/31.7 | 47.2/40.3 | 21.3/15.0 | 33.2/24.4 | NA | 4.2/3.4 | 34.5/26.3 | 63.4/60.2 |
| Shimonaga et al 15 | 71.1±9.8/70.8±8.4 | 64.7/80.5 | 55.9/69 | 82.4/90.8 | 67.6/58.6 | 47.1/18.4 | NA | NA | 73.5/73.6 | 0/0 |
| Redfors et al 19 | 63.6±10.9/63.6±10.9 | 76.5/73.8 | 19.9/22.9 | 90.2/78.5 | 37.0/31.9 | 48.2/22.7 | 92.8±38.4/94.2±37.7 | NA | 89.4/72.7 | 47.9/52.1 |
| Buchanan et al 17 | 65±11/66±11 | 68/64 | 16/16 | 96/97 | 47/45 | 53/52 | NA | NA | 95/96 | 67/59 |
| Lee et al 16 | 59.1±10.3/62.2±10.9 | 69.5/77.6 | 22/31.5 | 53.7/62.8 | 30.5/41.4 | 42.7/16.2 | 68.3±33.2/68.4±37.2 | NA | 49.4/49.3 | 40.9/31.7 |
| Takeuchi et al 20 | 69.4±10.0/67.9±11.2 | 83.9/80.0 | 16.4/21.7 | 83.9/70.0 | 54.7/39.2 | NA | 64.6±27.5/70.9±25.8 | 28.6/27.3 | 86.1/72.4 | 11.8/28.2 |
| Tamez et al 14 | 74.2±6.8/74.6±7.0 | 65.7/62.7 | 12.6/13.7 | 92.5/85.8 | 43.1 /36.1 | 50.4 /24.7 | NA | 21.2 /19.1 | 92.7/79.8 | 63.9/64.7 |
| Tang et al 12 | 64.8±9.8/63.1±11.6 | 82.6/83.8 | 39.1/44.8 | 69.6/70.3 | 50.7/46.5 | 58.0/17.9 | 93.3±27.2 /94.9±27.4 | NA | 71.0/61.3 | 56.5/56.3 |
| Wang et al 13 | 56.80±10.26/57.23±10.52 | 81.1/83.6 | 36.8/41.7 | 59.4/65.3 | 33.0/31.4 | 59.0/40.2 | NA | NA | 87.3/84.0 | NA |
| Azzalini et al 21 | 65.1±10.3/65.1±10.8 | 82.9/87.1 | 21.6/23.3 | 79.4/74.3 | 41.3/37.3 | 70.4/45.1 | 82.7±26.5/83.6±28.5 | NA | 85.0/79.8 | 18.2/18.3 |
ACS indicates acute coronary syndrome; CAD, coronary artery disease; FH, family history; GFR, glomerular filtration rate; HLD, hyperlipidemia HTN, hypertension; MI, myocardial infarction and NA, not applicable.
Table 3.
Procedure Characteristics
| Study | Lesion length, mm, mean±SD or median (IQR) | LM disease >50% | Px LAD disease % | Any LAD disease % | RCA disease % | LCX disease % | SVG disease % | Radial access % | Rotational atherectomy % | Laser atherectomy % | Scoring/cutting balloon % | Contrast volume , mL | Procedural duration , min | No. of stents , mean±SD or % | Average stent length mm | Average stent diameter |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdelwahab et al 18 | 18 (12–23)/15 (10–20) | 1.3/1.3 | NA | 45.1/50.4 | 31.2/25.7 | 22.5/20.7 | 5.6/4.8 | NA | NA | NA | NA | NA | NA | NA | 20 (16–28)/18 (13–24) | 3 (2.8–3)/3 (2.8–3) |
| Richardt et al 23 | 18.0±12.9/16.6±10.1 | 1.6/1.9 | NA | 36.7/43.9 | 34.6/28.7 | 22.9/24.0 | 3.6/1.4 | NA | NA | NA | NA | NA | NA | NA | 22.1±6.4/20.9±6.6 | 3.2±0.5/3.1±0.5 |
| Gao et al 22 | 23.79±15.57/25.32±16.28 | 3.7/3.6 | NA | 40.5/43.1 | 34.0/30.8 | 20.5/22.3 | 1.3/0.3 | 70.1/80.6 | NA | NA | 3.8/1.2 | NA | NA | 2.04±1.09/1.90±1.04 (stents per patient) | 29.95±17.71/30.73±17.77 | 3.02±0.43/3.06±0.47 |
| Shimonaga et al 15 | 14.6±7.0/22.0±11.7 | NA | NA | 44.1/55.2 | 32.4/24.1 | 23.5/20.7 | NA | NA | NA | NA | NA | NA | NA | 0/1.3±0.5 | 0/27.5±14.6 | 0/3.09±0.49 |
| Redfors et al 19 | 27.3±20.7/27.1±20.0 | 3.3/3.8 | NA | 41.8/46.5 | 44.3/36.3 | 29.8/31.1 | 9.4/4.5 | 5.2/4.3 | NA | NA | NA | NA | NA | 1.71±1.03/1.70±1.00 | 33.1±23.5/32.4±22.2 | NA |
| Buchanan et al 17 | NA | 1.8/3 | NA | 21/36 | 33/32 | 20/28 | 23/0 | NA | 1.0/4.9 | 1.6/0.3 | 19.0/5.4 | NA | NA | 1.17±0.87/1.29±0.95 | 19±6.4/19±6.7 | NA |
| Lee et al 16 | 16.4±11.1/19.6±14.1 | NA | NA | 43.3/38.3 | 38.4/40.8 | 18.3/20.9 | NA | NA | NA | NA | NA | 190.3±89.2/216.2±123.6 | Fluoroscopic time 40.1±64.4/25.5±26.1 | 1.8±0.5/1.84±0.6 | 30.2±14.9/30.2±17.1 | 2.94±0.4/3.08±0.9 |
| Takeuchi et al 20 | 21.8±12.2/21.5±11.9 | NA | NA | 40.4/52.0 | 36.8/27.8 | 18.2/16.4 | 0.4/0.5 | NA | 3.6/6.4 | NA | 69.6/40.9 | NA | NA | 49.3/87.9 | NA | NA |
| Tamez et al 14 | 18.46±11.22/18.40±10.57 | NA | NA | NA | NA | NA | NA | NA | 0.9/1.6 | 0.5/0.1 | 17.3/3.8 | NA | NA | DES G1/G2 76 939 (90.7%), 640 432 (77.7%); and BMS G1/G2 7876 (9.3%), 184 142 (22.3%) | 19.20±7.5518.53±7.06 | 2.99±0.50 .96±0.52 |
| Tang et al 12 | NA | 1.4/5.9 | NA | 39.1/44.8 | 50.7/46.8 | 26.1/26.6 | NA | 77.5/81.5 | NA | NA | NA | 239 (180, 280)/250 (200, 300) | NA | NA | NA | NA |
| Wang et al 13 | 24.2±17.3/16.9±11.5 | 83.5/84.9 | NA | NA | NA | NA | 51.09±30.72/56.90±37.91 | 75.4/73.9 | 26.19±5.28/26.10±5.25 | 3.00±0.41/2.91±0.40 | ||||||
| Azzalini et al 21 | Lesion length >20 mm: 68.5%/42.5% | NA | NA | 27.0/28.6 | 55.0/51.8 | 18.0/19.6 | NA | 34.2/42.7 | NA | NA | NA | 302±134/332±137 | 115±75/128±69 (min) | 2.34±1.31/2.20±1.27 (stents per patient) | 77.3±49.7/69.1±40.7 | NA |
BMS indicates bare‐metal stent; DES, drug‐eluting stent; IQR, interquartile range; LAD, left anterior descending artery; LM, left main coronary artery; NA, not applicable; Px, proximal; RCA, right coronary artery and SVG, saphenous vein graft.
The quality of included studies is outlined in Table S2. Based on the Newcastle‐Ottawa scale, all studies were considered to be of very good quality, except for 1 study, which was considered as good quality. 13
Outcomes
MACE was reported in 11 studies (Table S3). Adjusted data were pooled from 9 studies as outlined in Table S4. Compared with de novo PCI, PCI with ISR was associated with a higher incidence of MACE (OR, 1.31 [95% CI, 1.18–1.46]; I2=92%; Q=127.61, P<0.001) (Figure 2). Funnel plot inspection showed no publication bias (P=0.16) (Figure S1). Subgroup analysis showed no significant interaction according to CTO versus non‐CTO lesions (P interaction=0.69). Sensitivity analyses excluding studies not reporting risk‐adjusted estimates (ie, Richardt et al 23 and Wang et al 13 ) (OR, 1.34 [95% CI, 1.20–1.50]) showed similar results (Figure S2).
Figure 2. Forrest plot for long‐term major adverse cardiovascular events after PCI of ISR vs de novo lesions.
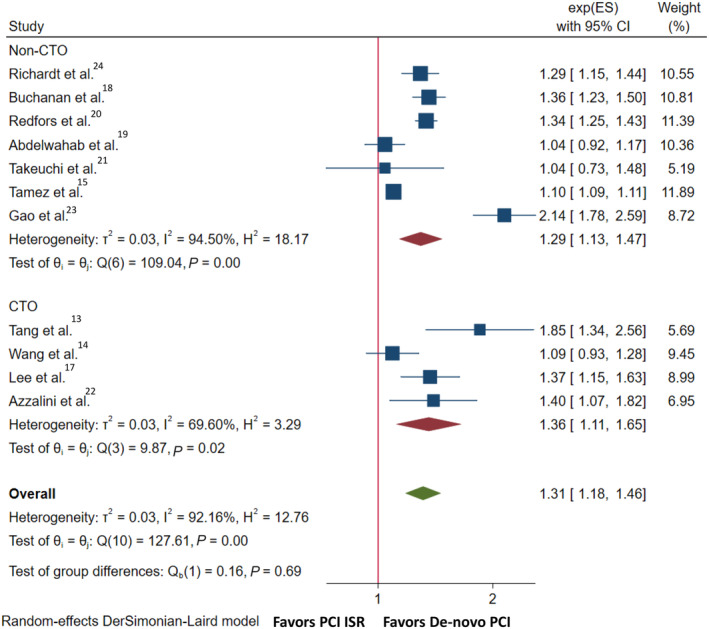
CTO indicates chronic total occlusion; exp(ES), Exponentiated log effect‐size; ISR, in‐stent restenosis; and PCI, percutaneous coronary intervention.
All‐cause mortality was reported in 7 studies. There was a higher incidence of all‐cause mortality among the ISR versus the de novo group (OR, 1.03 [95% CI, 1.02–1.04]; I2=0%; Q=4.86, P=0.56). Subgroup analysis showed no significant interaction according to CTO versus non‐CTO lesions (P interaction=0.91) (Figure 3).
Figure 3. Forrest plot for secondary outcomes after PCI of ISR vs de novo lesions.
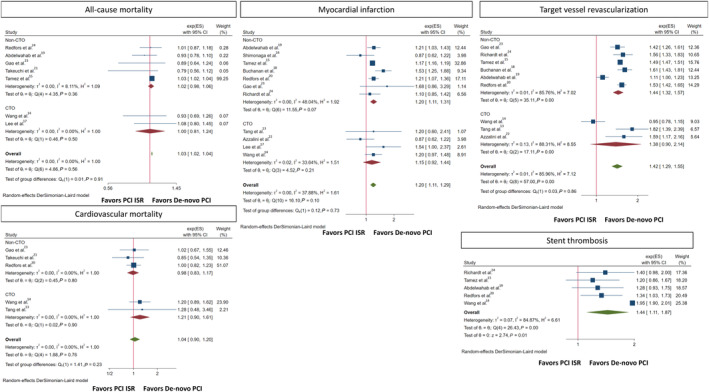
CTO indicates chronic total occlusion; exp(ES), Exponentiated log effect‐size; ISR, in‐stent restenosis; and PCI, percutaneous coronary intervention.
Cardiovascular mortality was reported in 5 studies.2,3,5,8 There was no difference in cardiovascular mortality between the ISR and de novo groups (OR, 1.04 [95% CI, 0.90–1.20]; I2=0%; Q=1.88, P=0.76). MI was reported in 11 studies (definition of MI according to each study is reported in Table S5). There was a higher incidence of MI among the ISR versus de novo groups (OR, 1.20 [95% CI, 1.11–1.29]; I2=38%; Q=16.10, P=0.10). Subgroup analysis showed no significant interaction according to CTO versus non‐CTO lesions (P interaction=0.73). TVR was reported in 9 studies.1–5,9 Compared with de novo lesions, the ISR group had a higher incidence of TVR (OR, 1.42 [95% CI, 1.29–1.55]; I2=86%; Q=57.00, P<0.001). Subgroup analysis showed no significant interaction according to CTO versus non‐CTO lesions (P interaction=0.86). Stent thrombosis was reported in 5 studies.3–5,9 Analysis showed a higher incidence of stent thrombosis with PCI for ISR versus de novo lesions (OR, 1.44 [95% CI, 1.11–1.87]; I2=84%; Q=26.43, P<0.001) (Figure 3).
Discussion
In this meta‐analysis of 12 studies including 708 391 patients, we evaluated the outcomes of PCI of ISR versus de novo lesions. The salient findings were: (1) PCI for ISR was associated with a higher incidence of risk‐adjusted MACE compared with PCI for de novo lesions at a median of ≈30 months. (2) This was driven by a higher incidence of all‐cause mortality, MI, TVR, and stent thrombosis in the ISR group. (3) There was no evidence of interaction for the outcomes in the CTO versus non‐CTO subgroups (Figure 4).
Figure 4. Summary of the outcomes with PCI for ISR vs de novo lesions.
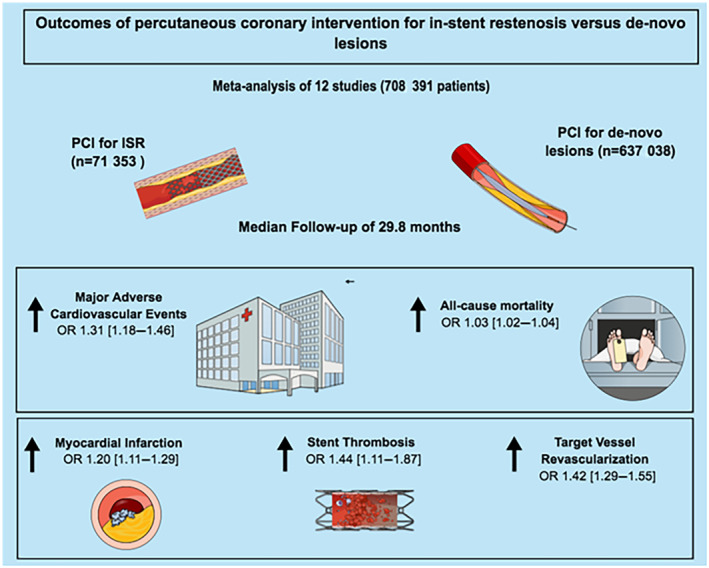
ISR indicates in‐stent restenosis; OR, odds ratio; and PCI, percutaneous coronary intervention.
ISR PCI represents about 10% of all PCI in the contemporary DES era. 24 Patients with ISR present in the form of acute coronary syndrome in more than two‐thirds of patients and tend to present more often with unstable angina and less often with MI than de novo coronary lesions. 17 , 25 Clinicians have attempted to stratify different classes of ISR, either angiographically or by mechanism. 26 , 27 Focal patterns, which were predominantly treated with angioplasty and stenting, were associated with better clinical outcomes compared with more diffuse lesion patterns or CTOs. 26
In this study, we pooled the totality of available data for the outcomes of PCI for ISR, compared with PCI for de novo lesions. This analysis is the largest to date, and showed a higher incidence of adverse clinical events, including all‐cause mortality, after PCI for ISR versus de novo lesions. These findings are line with results of individual studies. The higher risk of adverse events with PCI for ISR is probably related to the unique pathophysiology of ISR lesions as well as the challenging nature of interventions for ISR lesions. ISR lesions have a distinctive pathophysiology, which is characterized by neointimal hyperplasia and neoatherosclerosis, 28 as opposed to the atherosclerotic plaque formation with fibro‐fatty calcified core in de novo coronary artery disease. Optical coherence tomography studies have demonstrated the neointimal changes that occur with time inside DES. These include increased neointimal thickness and transformation from a homogenous to heterogenous or lipid‐laden intima, with formation of cholesterol‐rich plaques and thin‐cap atheromas. 29 , 30 These neoatherosclerotic changes occur more frequently and earlier in DES than bare‐metal stents and play a role in DES ISR. 31 , 32 Lipid‐laden neointima also plays a role in the increased risk of late stent thrombosis with DES, in addition to delayed neointimal coverage of stent struts. 33 , 34 Although new‐generation everolimus‐eluting stents demonstrate better vessel healing and neointimal stent coverage than first‐generation sirolimus‐ and paclitaxel‐eluting stents, they have a similar prevalence of neoatherosclerosis. 35 In addition to vessel remodeling following DES, mechanical factors, such as stent under expansion due to underlying calcification and malposition, also play a role in ISR. 36 Patient‐related factors such as diabetes and renal failure have also been reported to be independent risk factors for ISR. 37 , 38
Given its unique pathophysiology and complex clinical presentation, intervening on ISR portends a challenging subset of PCI procedures. Different intervention techniques are used in clinical practice, which are mainly based on clinical presentation, type of ISR lesions (ie, focal versus diffuse), and mechanism of ISR lesions. A mechanism‐driven approach should be always considered when selecting a strategy for treatment of ISR, including restenting, balloon angioplasty, cutting and scoring balloons, rotational atherectomy, brachytherapy, or using drug‐coated balloons. 5 Even with the wide variety of interventions, 2 modalities are preferred, repeat PCI with DES or angioplasty with drug‐coated balloons. 39 , 40 In the current meta‐analysis, the proportion of PCI with stenting among the ISR group varied across different studies from 48.4% to 100%, mainly with DES. Comparatively, a national cohort study of >5 million patients observed that 23.5% of patients with ISR did not receive DES and were more likely to receive other treatments. 24 Restenting ISR with DES, specifically everolimus stents, was found to be superior in both angiographic and clinical outcomes compared with other PCI methods. 41 , 42 However, operators should be mindful of multilayered stenting, and recurrent DES implantations could exacerbate a cycle of restenosis. Drug‐coated balloons, although providing less lumen gain than restenting, are also a viable option, because they do not add a stent layer and still provide favorable outcomes. 43 , 44 Coronary artery bypass graft should ultimately be considered in patients with diffuse, recurrent ISR with associated multivessel disease, but this approach may be limited due to poor target vessels. 45 The use of intracoronary imaging to guide PCI can optimize lesion preparation, stent deployment, and may assist with identifying the mechanism of stent failure, reducing the risk of MACE compared with angiography‐guided PCI. 46 Limited data were reported in the current analysis on the use of intracoronary imaging during PCI for ISR. Further research is warranted to evaluate the outcomes of PCI of ISR compared with de novo lesions using the state‐of‐the‐art PCI techniques, including routine intravascular imaging, transradial access, and using advanced modalities such as laser atherectomy, brachytherapy, and drug‐coated balloons.
In this analysis, the higher risk of adverse events with PCI for ISR, was observed irrespective of ISR morphology (ie, CTO or non‐CTO). CTO ISR lesions represent the extreme end of the spectrum of ISR lesions, and share similar morphological patterns, including neointimal hyperplasia, neoatherosclerosis, and stent under expansion in CTO ISR. PCI for CTO ISR lesions entails different technical considerations. At 1 side, the previously deployed stent acts as a roadmap of the target vessel and also doubles as a guard against coronary dissection during PCI of the CTO. 16 However, the previous stent may obstruct the path of the wire and lead to suboptimal reentry for PCI. Lee et al additionally observed that stenting of ISR in CTO was a risk factor for MI and TVR, possibly explained by an abnormal vessel reaction and thrombus formation attributed to multilayered stenting. 16
The collective data in this study demonstrated that PCI for ISR is associated with worse outcomes compared with de novo lesions. This highlights the need for optimization of index PCI procedures to reduce the chances of future ISR by adopting best practices such as adequate lesion preparation and intracoronary image‐guided interventions. Future directions should be directed toward increasing awareness for adoption of these best practices, and toward exploring novel/alternative approaches for PCI of ISR.
Limitations
The current analysis has certain limitations. First, due to the observational nature of included studies, there is an inherent risk for selection bias. In our analysis, we have pooled risk‐adjusted effect sizes to minimize this risk. Nevertheless, residual confounding cannot be ruled out. Second, there was a considerable degree of statistical heterogeneity for some of the study outcomes. Also, there were some variabilities among the included studies in the definition of the primary outcome. Nevertheless, we have adopted a random‐effects model to mitigate the effects of such heterogeneity. Third, some of the included studies only reported data for patients who underwent stent implantation, and data for unsuccessful PCIs were not reported. This might have introduced selection bias by not reporting data for patients with unsuccessful PCIs or ISR with anatomies not amenable to stenting. Fourth, patients with recurrent ISR may be treated with different approaches and management strategies, contributing to heterogeneity of outcomes. Insufficient patient‐level data precluded further subgroup analyses. Finally, the use of intracoronary imaging and laser atherectomy were either not reported or extremely low in the included studies in our analysis. This could impact the external generalizability of the findings to contemporary practice, given the viable role of these modalities in contemporary management of ISR lesions.
Conclusions
Compared with de novo lesions, PCI of ISR was associated with a higher incidence of risk‐adjusted MACE at a weighted follow‐up of 29.8 months. This was mainly driven by a higher incidence of all‐cause mortality, MI, repeat revascularization, and stent thrombosis. This association was demonstrated regardless of the nature of ISR (ie, CTO versus non‐CTO). These findings crystallize the notion that both ISR itself and PCI for ISR are not benign entities, and major efforts should be directed toward optimizing index PCI procedures.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S2
This article was sent to Katherine C. Wu, MD, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029300
For Sources of Funding and Disclosures, see page 11.
References
- 1. Lawton JS, Tamis‐Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e4–e17. doi: 10.1161/CIR.0000000000001060 [DOI] [PubMed] [Google Scholar]
- 2. Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151:1260–1264. doi: 10.1016/j.ahj.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 3. Virmani R, Farb A. Pathology of in‐stent restenosis. Curr Opin Lipidol. 1999;10:499–506. doi: 10.1097/00041433-199912000-00004 [DOI] [PubMed] [Google Scholar]
- 4. Lowe HC, Oesterle SN, Khachigian LM. Coronary in‐stent restenosis: current status and future strategies. J Am Coll Cardiol. 2002;39:183–193. doi: 10.1016/S0735-1097(01)01742-9 [DOI] [PubMed] [Google Scholar]
- 5. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in‐stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545 [DOI] [PubMed] [Google Scholar]
- 6. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 7. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2000. Accessed October 22, 2022. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 8. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elbadawi A, Elgendy IY, Saad M, Megaly M, Mentias A, Abuzaid AS, Shahin HI, Goswamy V, Abowali H, London B. Meta‐analysis of trials on prophylactic use of levosimendan in patients undergoing cardiac surgery. Ann Thorac Surg. 2018;105:1403–1410. doi: 10.1016/j.athoracsur.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 10. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knol M, Algra A, Groenwold R. How to deal with measures of association: a short guide for the clinician. Cerebrovasc Dis. 2012;33:98–103. doi: 10.1159/000334180 [DOI] [PubMed] [Google Scholar]
- 12. Tang G, Zheng N, Yang G, Li H, Ai H, Zhao Y, Sun F, Zhang H. Procedural results and long‐term outcomes of percutaneous coronary intervention for in‐stent restenosis chronic total occlusion compared with de novo chronic total occlusion. Int J Gen Med. 2021;14:5749–5758. doi: 10.2147/IJGM.S328332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang T, Guan H, Tian T, Guan C, Bai Y, Hu Y, Yuan J, Qiao S, Xu B, Yang W. Thirty‐day and 5‐year results of percutaneous coronary intervention for in‐stent restenotic chronic total occlusion lesions: data from 2,659 consecutive patients. Catheter Cardiovasc Interv. 2021;97:1016–1024. doi: 10.1002/ccd.29585 [DOI] [PubMed] [Google Scholar]
- 14. Tamez H, Secemsky EA, Valsdottir LR, Moussa ID, Song Y, Simonton CA, Gibson CM, Popma JJ, Yeh RW. Long‐term outcomes of percutaneous coronary intervention for in‐stent restenosis among Medicare beneficiaries. EuroIntervention. 2021;17:e380–e387. doi: 10.4244/EIJ-D-19-01031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimonaga T, Kurisu S, Watanabe N, Ikenaga H, Higaki T, Iwasaki T, Ishibashi K, Dohi Y, Fukuda Y, Kihara Y. Myocardial injury after percutaneous coronary intervention for in‐stent restenosis versus de novo stenosis. Intern Med. 2015;54:2299–2305. doi: 10.2169/internalmedicine.54.5003 [DOI] [PubMed] [Google Scholar]
- 16. Lee SH, Cho JY, Kim JS, Lee HJ, Yang JH, Park JH, Hong SJ, Choi RK, Choi S‐H, Gwon H‐C. A comparison of procedural success rate and long‐term clinical outcomes between in‐stent restenosis chronic total occlusion and de novo chronic total occlusion using multicenter registry data. Clin Res Cardiol. 2020;109:628–637. doi: 10.1007/s00392-019-01550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchanan KD, Torguson R, Rogers T, Xu L, Gai J, Ben‐Dor I, Suddath WO, Satler LF, Waksman R. In‐stent restenosis of drug‐eluting stents compared with a matched group of patients with de novo coronary artery stenosis. Am J Cardiol. 2018;121:1512–1518. doi: 10.1016/j.amjcard.2018.02.033 [DOI] [PubMed] [Google Scholar]
- 18. Abdel‐Wahab M, Nienaber CA, Mostafa AE, Sabin G, Tebbe U, Hochadel M, Senges J, Akin I, Kuck K‐H, Hamm C, et al; German Drug–Eluting Stent (DES.DE) registry. Clinical outcome of percutaneous treatment of in‐stent restenosis with drug‐eluting stents: results from the first phase of the prospective multicentre German DES.DE registry. EuroIntervention. 2011;7:201–208. doi: 10.4244/EIJV7I2A34 [DOI] [PubMed] [Google Scholar]
- 19. Redfors B, Généreux P, Witzenbichler B, Maehara A, Weisz G, McAndrew T, Mehran R, Kirtane AJ, Stone GW. Percutaneous coronary intervention of lesions with in‐stent restenosis: a report from the ADAPT‐DES study. Am Heart J. 2018;197:142–149. doi: 10.1016/j.ahj.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi M, Dohi T, Fukase T, Nishio R, Takahashi N, Endo H, Kato Y, Okai I, Iwata H, Okazaki S, et al. Comparison of clinical outcomes between percutaneous coronary intervention for de novo lesions versus in‐stent restenosis lesions. Cardiovasc Interv Ther. 2022;37:324–332. doi: 10.1007/s12928-021-00792-5 [DOI] [PubMed] [Google Scholar]
- 21. Azzalini L, Dautov R, Ojeda S, Benincasa S, Bellini B, Giannini F, Chavarría J, Pan M, Carlino M, Colombo A, et al. Procedural and long‐term outcomes of percutaneous coronary intervention for in‐stent chronic total occlusion. J Am Coll Cardiol Intv. 2017;10:892–902. doi: 10.1016/j.jcin.2017.01.047 [DOI] [PubMed] [Google Scholar]
- 22. Gao Z, Xu B, Yang YJ, Yuan JQ, Chen J, Chen JL, Qiao SB, Wu YJ, Yan HB, Agao RL. Long‐term outcomes of drug‐eluting stent therapy for in‐stent restenosis versus de novo lesions. J Interv Cardiol. 2013;26:550–555. doi: 10.1111/joic.12069 [DOI] [PubMed] [Google Scholar]
- 23. Richardt G, Leschke M, Abdel‐Wahab M, Toelg R, El‐Mawardy M, Serruys PW, Silber S, Windecker S, Belardi JA, Neumann F‐J, et al. Clinical outcomes of the resolute zotarolimus‐eluting stent in patients with in‐stent restenosis: 2‐year results from a pooled analysis. J Am Coll Cardiol Intv. 2013;6:905–913. doi: 10.1016/j.jcin.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 24. Moussa ID, Mohananey D, Saucedo J, Stone GW, Yeh RW, Kennedy KF, Waksman R, Teirstein P, Moses JW, Simonton C. Trends and outcomes of restenosis after coronary stent implantation in the United States. J Am Coll Cardiol. 2020;76:1521–1531. doi: 10.1016/j.jacc.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 25. Magalhaes MA, Sa M, Chen F, Torguson R, Omar AF, Loh JP, Escarcega RO, Lipinski MJ, Baker NC, Kitabata H, et al. Clinical presentation and outcomes of coronary in‐stent restenosis across 3‐stent generations. Circ Cardiovasc Interv. 2014;7:768–776. doi: 10.1161/CIRCINTERVENTIONS.114.001341 [DOI] [PubMed] [Google Scholar]
- 26. Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in‐stent restenosis: classification and implications for long‐term outcome. Circulation. 1999;100:1872–1878. doi: 10.1161/01.CIR.100.18.1872 [DOI] [PubMed] [Google Scholar]
- 27. Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug‐eluting stents: a new classification system based on disease mechanism to guide treatment and state‐of‐the‐art review. Circ Cardiovasc Interv. 2019;12:e007023. doi: 10.1161/CIRCINTERVENTIONS.118.007023 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura D, Yasumura K, Nakamura H, Matsuhiro Y, Yasumoto K, Tanaka A, Matsunaga‐Lee Y, Yano M, Yamato M, Egami Y, et al. Different neoatherosclerosis patterns in drug‐eluting‐and bare‐metal stent restenosis—optical coherence tomography study. Circ J. 2019;83:313–319. doi: 10.1253/circj.CJ-18-0701 [DOI] [PubMed] [Google Scholar]
- 29. Kim J‐S, Hong M‐K, Shin D‐H, Kim B‐K, Ko Y‐G, Choi D, Jang Y. Quantitative and qualitative changes in DES‐related neointimal tissue based on serial OCT. JACC Cardiovasc Imaging. 2012;5:1147–1155. doi: 10.1016/j.jcmg.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 30. Hou J, Jia H, Liu H, Han Z, Yang S, Xu C, Schmitt J, Zhang S, Yu B, Jang I‐K. Neointimal tissue characteristics following sirolimus‐eluting stent implantation: OCT quantitative tissue property analysis. Int J Cardiovasc Imaging. 2012;28:1879–1886. doi: 10.1007/s10554-012-0031-7 [DOI] [PubMed] [Google Scholar]
- 31. Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, Kolodgie FD, Finn AV, Virmani R. The pathology of neoatherosclerosis in human coronary implants: bare‐metal and drug‐eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. doi: 10.1016/j.jacc.2011.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goto K, Takebayashi H, Kihara Y, Hagikura A, Fujiwara Y, Kikuta Y, Sato K, Kodama S, Taniguchi M, Hiramatsu S. Appearance of neointima according to stent type and restenotic phase: analysis by optical coherence tomography. EuroIntervention. 2013;9:601–607. doi: 10.4244/EIJV9I5A96 [DOI] [PubMed] [Google Scholar]
- 33. Higo T, Ueda Y, Oyabu J, Okada K, Nishio M, Hirata A, Kashiwase K, Ogasawara N, Hirotani S, Kodama K. Atherosclerotic and thrombogenic neointima formed over sirolimus drug‐eluting stent: an angioscopic study. JACC Cardiovasc Imaging. 2009;2:616–624. doi: 10.1016/j.jcmg.2008.12.026 [DOI] [PubMed] [Google Scholar]
- 34. Miyazaki S, Hiasa Y, Takahashi T, Yano Y, Minami T, Murakami N, Mizobe M, Tobetto Y, Nakagawa T, Chen PM, et al. In vivo optical coherence tomography of very late drug‐eluting stent thrombosis compared with late in‐stent restenosis. Circ J. 2011;76:E1745. doi: 10.1016/S0735-1097(11)61745-2 [DOI] [PubMed] [Google Scholar]
- 35. Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, et al. Pathology of second‐generation everolimus‐eluting stents versus first‐generation sirolimus‐and paclitaxel‐eluting stents in humans. Circulation. 2014;129:211–223. doi: 10.1161/CIRCULATIONAHA.113.001790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ikenaga H, Ishihara M, Dai K, Nakama Y, Ohtani T. Mechanisms of very late stent thrombosis after drug‐eluting stent implantation: findings from coronary angioscopy and optical coherence tomography. JACC Cardiovasc Imaging. 2011;4:1217–1219. doi: 10.1016/j.jcmg.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 37. Nakamura D, Dohi T, Ishihara T, Kikuchi A, Mori N, Yokoi K, Shiraki T, Mizote I, Mano T, Higuchi Y. Predictors and outcomes of neoatherosclerosis in patients with in‐stent restenosis. EuroIntervention. 2021;17:489–496. doi: 10.4244/EIJ-D-20-00539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rathore S, Terashima M, Katoh O, Matsuo H, Tanaka N, Kinoshita Y, Kimura M, Tuschikane E, Nasu K, Ehara M, et al. Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention. 2009;5:349–354. doi: 10.4244/V5I3A55 [DOI] [PubMed] [Google Scholar]
- 39. Sousa‐Uva M, Neumann F‐J, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2019;55:4–90. doi: 10.1093/ejcts/ezy289 [DOI] [PubMed] [Google Scholar]
- 40. Elgendy IY, Mahmoud AN, Elgendy AY, Mojadidi MK, Elbadawi A, Eshtehardi P, Pérez‐Vizcayno MJ, Wayangankar SA, Jneid H, Anderson RD, et al. Drug‐eluting balloons versus everolimus‐eluting stents for in‐stent restenosis: a meta‐analysis of randomized trials. Cardiovasc Revasc Med. 2019;20:612–618. doi: 10.1016/j.carrev.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 41. Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez‐Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, et al. Percutaneous coronary interventional strategies for treatment of in‐stent restenosis: a network meta‐analysis. Lancet. 2015;386:655–664. doi: 10.1016/S0140-6736(15)60657-2 [DOI] [PubMed] [Google Scholar]
- 42. Alfonso F, Pérez‐Vizcayno MJ, Cuesta J, García del Blanco B, García‐Touchard A, López‐Mínguez JR, Masotti M, Zueco J, Cequier A, Velázquez M, et al. 3‐year clinical follow‐up of the RIBS IV clinical trial: a prospective randomized study of drug‐eluting balloons versus everolimus‐eluting stents in patients with in‐stent restenosis in coronary arteries previously treated with drug‐eluting stents. J Am Coll Cardiol Intv. 2018;11:981–991. doi: 10.1016/j.jcin.2018.02.037 [DOI] [PubMed] [Google Scholar]
- 43. Scheller B, Clever YP, Kelsch B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Speck U, Böhm M, et al. Long‐term follow‐up after treatment of coronary in‐stent restenosis with a paclitaxel‐coated balloon catheter. J Am Coll Cardiol Intv. 2012;5:323–330. doi: 10.1016/j.jcin.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 44. Indermuehle A, Bahl R, Lansky AJ, Froehlich GM, Knapp G, Timmis A, Meier P. Drug‐eluting balloon angioplasty for in‐stent restenosis: a systematic review and meta‐analysis of randomised controlled trials. Heart. 2013;99:327–333. doi: 10.1136/heartjnl-2012-302945 [DOI] [PubMed] [Google Scholar]
- 45. Moustapha A, Assali A, Sdringola S, Vaughn W, Fish R, Rosales O, Schroth G, Krajcer Z, Smalling R, Anderson H, et al. Event‐free survival after revascularization in patients with in‐stent restenosis: coronary artery bypass surgery may be better than percutaneous intervention. Am J Cardiol. 2001;88:54G. [Google Scholar]
- 46. Buccheri S, Franchina G, Romano S, Puglisi S, Venuti G, D'Arrigo P, Francaviglia B, Scalia M, Condorelli A, Barbanti M, et al. Clinical outcomes following intravascular imaging‐guided versus coronary angiography–guided percutaneous coronary intervention with stent implantation: a systematic review and Bayesian network meta‐analysis of 31 studies and 17,882 patients. J Am Coll Cardiol Intv. 2017;10:2488–2498. doi: 10.1016/j.jcin.2017.08.051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S2


