ABSTRACT
Cerebral small vessel disease is highly prevalent, particularly in marginalized communities, and its incidence is expected to increase given the aging global population. Cerebral small vessel disease contributes to risk for stroke, vascular cognitive impairment and dementia, late‐life depression, and gait disorders. A growing body of evidence suggests that adverse outcomes, including cerebral small vessel disease, caused by traditional cardiovascular risk factors are at least partly mediated by epigenetic changes, some of them already beginning during fetal development. Societal and health care access inequities, summarized under the umbrella term social determinants of health, put a higher burden of cardiovascular risk factors on marginalized populations and expose them to an increased risk for adverse outcomes. Social epigenetics has begun to deliver solid evidence that social determinants of health lead to distinct epigenetic signatures that potentially mediate the biological effect of environmental exposures on cardiovascular risk factors. Here, we provide a review of the most recent advances in the epigenetics of cerebral small vessel disease risk factors and social determinants of health and call for research efforts combining insights from both fields to reach a deeper understanding of the causal pathways, ultimately facilitating discovery of new treatment targets for a disease whose burden is magnified by existing health disparities.
Keywords: cardiovascular risk factors, cerebral small vessel disease, epigenetics, social determinants of health, social epigenomics
Subject Categories: Cerebrovascular Disease/Stroke, Epigenetics
Nonstandard Abbreviations and Acronyms
- CpG
cytosine‐guanine
- CSVD
cerebral small vessel disease
- GD
gestational diabetes
- ICH
intracerebral hemorrhage
- MR
Mendelian randomization
- T2D
type 2 diabetes
Cerebral small vessel disease (CSVD) is a chronic progressive disorder of the microvasculature perfusing the brain. The 2 most common forms of sporadic CSVD, arteriolosclerosis and cerebral amyloid angiopathy, account for the vast majority of CSVD manifestations. 1 Sporadic CSVDs are responsible for a quarter of all ischemic strokes 2 and most intracerebral hemorrhages (ICHs). 3 However, CSVD also commonly manifests in a subacute fashion with cognitive impairment, neuropsychiatric symptoms, gait impairment, depression, and parkinsonism as common presenting complaints. 3 CSVD that presents in a subacute fashion is often diagnosed by distinct radiographic markers, such as covert brain infarcts, white matter hyperintensities, cerebral microbleeds, or enlarged perivascular spaces. 4 Although hypertension is the most important risk factor for CSVD, other cardiovascular risk factors such as diabetes, smoking, and hypercholesterolemia have also been associated with CSVD. 5 CSVD is highly prevalent and is expected to increase further in an aging population. 6 When considering both asymptomatic and symptomatic individuals, total CSVD prevalence has a similar or even higher burden of disease in the general population than heart disease. 7
Social determinants of health (SDOH) is an umbrella term encompassing the “conditions in which people are born, grow, live, work, and age,” as recently stated by the World Health Organization. 8 SDOH are thought to have direct and indirect effects on the onset of diseases. 9 Despite global improvements in health care, striking sex, ethnic, socioeconomic, and geographic disparities persist in mortality rates and causes of death, including CSVD. 8 , 9 Globally, individuals with lower socioeconomic status (SES) are more likely to have cardiovascular risk factors, such as smoking, hypertension, obesity, stress, and other factors that are associated with poor health outcomes such as unstable working/living conditions, low education level, and limited access to health care. 10 In the United States, SDOH play an important role in the development of cardiovascular risk factors 10 that increase the risk for CSVD. However, SDOH may also contribute to increased prevalence of CSVD through other biological or nonbiological pathways.
Although the identified risk factors for CSVD show overlap with those for cardiovascular disease (CVD), prevention efforts remain behind. Primary prevention of cerebrovascular disease has not yet been established, partly because robust and practical screening parameters are lacking for CSVD and continue to rely on magnetic resonance imaging markers. Distinct epigenetic signatures of CSVD or CSVD risk factors could serve as biomarkers to enable the identification of asymptomatic, high‐risk individuals with incipient CSVD or as potential treatment targets for primary prevention of CSVD manifestations.
In addition to direct increase in prevalence of cardiovascular risk factors, SDOH has been thought to contribute to disease onset through biological ramifications. 11 To better understand this relationship, researchers have been attempting to explain the social and environmental effects on the body, specifically, through regulation of gene expression, a concept called epigenetics. This relatively new domain, social epigenomics, aims to further understand the biological modifications resulting from social and environmental factors that ultimately contribute to health outcomes. 12
One of the major challenges of the association of epigenetic signatures with disease phenotypes is to assign causality. If epigenetic modifications mediate the risk on disease caused by environmental exposures, they could be interesting as therapeutic targets; however, if they are caused by risk factors or diseases themselves, they could serve as biomarkers. This question of causality must be addressed to deepen our understanding of pathogenesis or potential therapeutic targets. Mendelian randomization is a powerful approach for investigating the direction of causality that leverages genetic variants, which are static since conception and thus not prone to confounding in the way that epigenetic signatures can be. 13
In this review, we summarize recent advances on epigenetics related to CSVD, focusing particularly on the following: epigenetics of cardiovascular risk factors, epigenetics of SDOH‐related risk factors, the analytical approaches useful to dissect their complex associations, and on the current gaps that need to be filled to effectively understand and ultimately address these biological manifestations of health disparities in CSVD.
EPIGENETICS
Epigenetics is generally accepted as “the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence.” 14 DNA methylation and microRNA (miRNA) expression, 2 common types of epigenetic changes, impact gene expression through modification of gene promoters and regulation of mRNA abundance. 15 In this review we will focus on these 2 epigenetic mechanisms.
DNA methylation mostly occurs at cytosine‐guanine (CpG) sites. Generally, DNA methylation in promoter regions is inversely correlated with transcription levels (Figure 1A). Several affordable methods for measuring either whole‐genome (locus‐specific methylation) or global methylation (percentage of methylated cytosine relative to cytosine content) with high‐throughput microarrays are now available in addition to traditional bisulfite sequencing, 16 making DNA methylation the most frequently studied epigenetic modification.
Figure 1. Effects of 2 of the most common epigenetic modifications, on gene expression: DNA methylation (A) and noncoding (nc)RNAs (B).
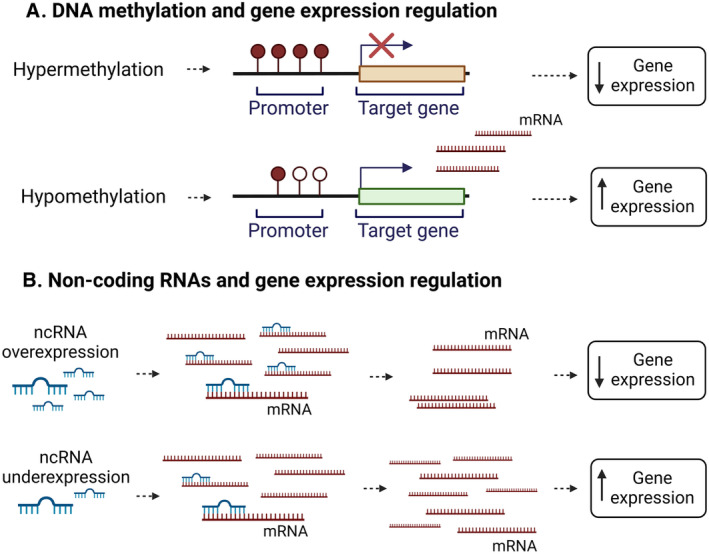
Noncoding RNAs, including miRNAs, have been discovered relatively recently as important epigenetic controllers of gene expression affecting multiple genes or even large gene networks simultaneously. Specific miRNAs bind to target mRNAs, resulting in mRNA cleavage and degradation, ultimately leading to translation repression. 15 (Figure 1B).
EPIGENETIC MECHANISMS OF CSVD AND ITS RISK FACTORS
Cerebral Small Vessel Disease and Its Proxies
Few studies have specifically investigated debilitating CSVD phenotypes such as small‐vessel stroke and ICH. An epigenome‐wide association study of ischemic stroke and its subtypes found many signals for atherothrombotic, cardioembolic, and undetermined stroke, but few for small vessel stroke. 17 The largest DNA methylation study of almost 10 000 individuals investigating magnetic resonance imaging–derived white matter hyperintensity, a radiographic proxy for hypertensive CSVD, has revealed dysregulation of genes involved in blood–brain barrier, immune response, extracellular matrix organization, and lipid and lipoprotein metabolism, suggesting antihyperlipidemic agents as possible target drugs for CSVD. 18 Biological age as measured by DNA methylation in whole blood mediates 43% of the association of chronological age with white matter hyperintensity burden, indicating a significant role of DNA methylation in its pathogenesis. 19 Another study found specific circulating blood miRNAs significantly associated with CSVD phenotypes that could potentially serve as biomarkers for CSVD cognitive impairment; however, sample size and prediction capability were limited. 20 Future studies evaluating epigenetic mechanisms as biomarkers for CSVD screening and prioritization of drug targets in diverse samples are urgently needed. Given that the data linking clinical and radiographic CSVD phenotypes to epigenetic changes are relatively limited at present, we will next describe epigenetic studies investigating demonstrated risk factors for CSVD, because amelioration of these morbidities could limit CSVD progression in at‐risk individuals. Selected publications are listed in the Table.
Table 1.
Selected Publications on Epigenomics of Cerebral Small Vessel Diseases and Related Traits and Their Main Risk Factors
| Trait | References |
|---|---|
| Cerebral small vessel disease and its proxies |
“An Epigenome‐Wide Association Study of DNA Methylation and Ischemic Stroke Risk” 17 “Epigenetic and Integrative Cross‐Omics Analyses of Cerebral White Matter Hyperintensities on MRI” 18 “Epigenetic Clock Explains White Matter Hyperintensity Burden Irrespective of Chronological Age” 19 “Circulating MicroRNAs as Potential Biomarkers for the Identification of Vascular Dementia Due to Cerebral Small Vessel Disease” 20 |
| Blood pressure |
“Genome‐Wide DNA Methylation Meta‐Analysis of Blood Pressure” 26 “Epigenetic Control of 11 Beta‐Hydroxysteroid Dehydrogenase 2 Gene Promoter Is Related to Human Hypertension” 24 “A Genome‐Wide Methylation Study on Essential Hypertension in Young African American Males” 27 “Noncoding RNAs in Hypertension” 31 “Circulating Levels of MicroRNAs Associated With Hypertension: A Cross‐Sectional Study in Male and Female South African Participants” 34 “Increased Expression of miR‐33a in Monocytes from Mexican Hypertensive Patients in Elevated Carotid Intima‐Media Thickness” 35 “Blood Pressure and Expression of MicroRNAs in Whole Blood” 36 “MicroRNA‐21 and Hypertension” 32 “Up‐Regulation of Circular RNA hsa_circ_0037909 Promotes Essential Hypertension” 33 |
| Type 2 diabetes |
“Fine‐Mapping Type 2 Diabetes Loci to Single‐Variant Resolution Using High‐Density Imputation and Islet‐Specific Epigenome Maps” 38 “Novel Epigenetic Determinants of Type 2 Diabetes in Mexican‐American Families” 40 “Regulatory Roles of MicroRNAs in Diabetes” 41 “Epigenetics of Type 2 Diabetes Mellitus and Weight Change—A Tool for Precision Medicine?” 39 |
| Cigarette smoking |
“The Role of Cigarette Smoke‐Induced Epigenetic Alterations in Inflammation” 42 “Epigenetic Signatures of Cigarette Smoking” 43 “Changes in DNA Methylation at the Aryl Hydrocarbon Receptor Repressor May Be a New Biomarker for Smoking” 44 “Associations Between Maternal Tobacco Smoke Exposure and the Cord Blood DNA Methylome” 45 “Maternal Smoking During Pregnancy Induces Persistent Epigenetic Changes Into Adolescence, Independent of Postnatal Smoke Exposure and Is Associated With Cardiometabolic Risk” 46 |
| Lipids |
“Epigenetics of Lipid Phenotypes” 47 “Epipolymorphisms Within Lipoprotein Genes Contribute Independently to Plasma Lipid Levels in Familial Hypercholesterolemia” 48 “DNA Methylation Signatures Link Prenatal Famine Exposure to Growth and Metabolism” 49 |
Blood Pressure
Genome‐wide association studies, an unbiased approach examining millions of single‐nucleotide polymorphisms in large cohorts or case–control studies, have improved our understanding of blood pressure genomics and identified >1000 genomic loci robustly associated with blood pressure traits. Although discovered variants explain only a small proportion of blood pressure heritability (variance in the trait explained by genetics) and thus far have not led to clinical use of novel therapeutics, these loci are enriched for association with DNA methylation, suggesting a pivotal role for epigenetics, and specifically, DNA methylation as a mediator between genetics and complex traits such as blood pressure. 21 Although still expensive, investing in epigenetics studies might accelerate biological insights compared with further increasing genome‐wide association studies sample size.
DNA methylation profiles have been associated with elevated blood pressure and ischemic stroke. 22 In vivo, in vitro, and human studies have shown a role of DNA methylation in the pathogenesis of hypertension. 23 , 24 , 25 Recent studies found associations between systolic and diastolic blood pressure and DNA methylation among participants of European, Hispanic, and Black descent. 26 , 27 Differences between methylated sites and regions associated with blood pressure across racial and ethnic groups point to different molecular pathways. Although no definitive causal mechanisms have been identified, these differences provide hints that epigenetic mechanisms are likely to be involved in the observed elevated cardiovascular disease risk experienced by individuals of South Asian ancestry compared with European individuals. 28 Furthermore, some of these hypertension‐associated methylation changes are influenced by environmental exposures. For instance, prenatal exposure to air pollutants is associated with change in postnatal DNA methylation patterns, 29 and even experimental exposure of humans to air pollution for only 130 minutes changes short‐term methylation profiles and is associated with higher systolic and diastolic blood pressure after exposure. 30
Beyond DNA methylation, there is growing evidence that small noncoding RNA levels contribute to pathogenesis of hypertension. 31 Studies of miRNAs provide evidence for numerous genes and biological processes involved in the regulatory network of hypertension, with distinct profiles for essential hypertension, nocturnal hypertension, pulmonary hypertension, and pulmonary arterial hypertension. 31 Distinct profiles of miRNAs have achieved good diagnostic performance for essential hypertension, 32 , 33 possibly impacting the risk of CSVD and hinting at a future role of miRNAs as both diagnostic markers and therapeutic targets. 31 Although it is too early to predict whether these investigations will transform clinical care, they may yield more robust biological insights because they have been performed across multiple ancestries, 34 , 35 , 36 unlike early endeavors of genome‐wide association studies that were restricted to individuals of European ancestry.
Type 2 Diabetes
The prevalence of type 2 diabetes (T2D) has been rapidly increasing and is expected to double by 2060. 37 T2D is a highly heritable trait, but similar to hypertension, well‐powered genome‐wide association studies have yielded a lower heritability estimate than expected from twin studies. 38 One hypothesis to explain this missing heritability is that gene–gene interactions, gene–environment interactions, and epigenetics likely contribute to its pathogenesis of those traits. 39 For instance, epigenetic modifications explained an additional 7.8% of the heritability of T2D in a Mexican cohort. 40 There have been numerous epigenetic studies in T2D, and DNA methylation patterns in pancreatic islets, human skeletal muscle, adipose tissue, and liver have been linked to insulin resistance and T2D. DNA methylation markers in blood have been established as biomarkers that associate robustly with incident T2D. 39 Beyond DNA methylation, miRNAs also contribute to the pathogenesis of T2D through modification of expression in gene networks that influence pancreatic β‐cell function and insulin resistance. 41 These findings may prove useful for future primary prevention efforts that could leverage epigenetic tests for diabetes risk stratification, or lead to drugs that inhibit the development of insulin resistance.
Cigarette Smoking
Cigarette smoking is a major risk factor for cardiovascular disease, chronic obstructive pulmonary disease, and cancer. Smoking is one of the most comprehensively investigated environmental exposures in the epigenetics literature. 42 Researchers have investigated the association of smoking during adulthood with epigenetic signatures, and the association of smoking during pregnancy with epigenetic modifications of placenta, cord blood, and tissue of offspring with resultant impacts on longitudinal outcomes. Some epigenetic mechanisms mediating the adverse effects of smoking on health outcomes could lead to future drug targets to mitigate long‐term sequelae of smoking, even as concurrent efforts continue to identify new ways to help patients quit altogether.
Effects of Smoking in Adulthood
Cigarette smoking in adulthood has a large effect on DNA methylation, both global 43 and locus specific, 44 and this discovery has been validated in well‐powered cohorts of individuals of many different ancestries. Within 5 years after smoking cessation, the majority of the methylation changes return to the patterns of individuals who never smoked, but some remain altered for up to 30 years, suggesting that there are both short‐ and long‐term epigenetic effects after exposure to cigarette smoke. 43
miRNAs and long noncoding RNAs also have distinctly different profiles in smokers versus nonsmokers, both in humans and in mice, and these alterations have been found in both peripheral blood mononuclear cells and lung tissue. 42 Although those changes are predominantly transient and vanish after smoking cessation, more research is needed to better understand the potential role of noncoding RNAs as biomarkers or treatment targets to mitigate the deleterious effects of smoking either in chronic smokers or those who have achieved cessation.
Effects of Smoking During Pregnancy
Maternal smoking during pregnancy has been associated with methylation changes in cord blood of newborns. 45 Smoking exposure during pregnancy is associated with persistent changes in offspring DNA methylation of genes involved in cardiometabolic risk factors, as well as pathways involved in development, cancer, and nicotine dependence. These DNA methylation changes continued to associate with diastolic blood pressure, triglycerides, and high‐density lipoprotein levels in adolescence, illustrating potential biological mediators of heritable, but not genotype‐conferred, risk factors. 46
Lipids
Epigenome‐wide association studies of DNA methylation have identified specific CpG sites strongly associated with lipid levels. 47 Among individuals with familial hypercholesterolemia, DNA methylation patterns influence low‐density lipoprotein cholesterol levels and appear to account for a large portion of the variance in the trait that is not explained by genetics. 48 Different methylation patterns of CPT1A have been associated with birth weight and serum low‐density lipoprotein cholesterol levels later in life in a cohort of offspring who were subjected to famine conditions in utero, suggesting that epigenetic modifications are potential mediators of prenatal malnutrition and long‐term adverse health outcomes. 49 Epigenetic modifications of gene transcription could reveal novel drug targets undetectable by conventional genetic analyses.
SOCIAL EPIGENETICS
Social epigenetics is a relatively new field of study that focuses on understanding the impact of physical and social environments on gene expression. 12 This shifts the focus from the genetic associations, which are static, to the more dynamic epigenetic associations, which are able to account for both internal and external environmental exposures and changes. 50 Studies have found associations between epigenetic and, for instance, healthy age‐related physiological processes. 51 In contrast, epigenetic changes induced by environmental risk factors, such as air pollution, smoking, and stress, have been associated with higher risk of a variety of diseases, including cancer, neurodegenerative diseases, and CVD. 52 , 53 Advances in identifying epigenetic risk factors have also served to highlight underlying causal environmental and social factors, including SDOH.
SDOH Are Associated With Distinct Epigenetic Signatures
The recent progress in social epigenetics research has led to a better understanding of the associations between epigenetic signatures of psychosocial stressors and the heritability of these epigenetic associations. As hypothesized by social epidemiologists, 54 stress and traumas can be inherited from one generation to the following. DNA methylation patterns have been shown to be altered based on exposure to environmental factors such as psychosocial stressors, racism and discrimination, socioeconomic status, and neighborhood social environment. 12 Despite advances in understanding epigenetic signatures resulting from social determinants, less is known about how they are linked to health outcomes. More recently, studies have found differences in DNA methylation patterns between Black and individuals of other race categories at risk for preterm birth 55 and prostate, 56 breast, and colon cancers. 57 A link connecting social epigenetics and CSVD has yet to be established.
It has been hypothesized that individuals' social context and lifetime stressors exposure, reflected by epigenetic changes, can contribute to increase the overall risk of cardiovascular disease and, in absence of adequate interventions, to reinforce health inequities (Figure 2). 58 The following sections therefore summarize the most recent studies about specific epigenetic signatures that have been found to mediate the relationship between social risk factors and CVSD risk factors.
Figure 2. Schematic overview of the role played by epigenetic modifications in mediating the association between social determinants of health (SDOH) and cerebral small vessel diseases (CSVDs).
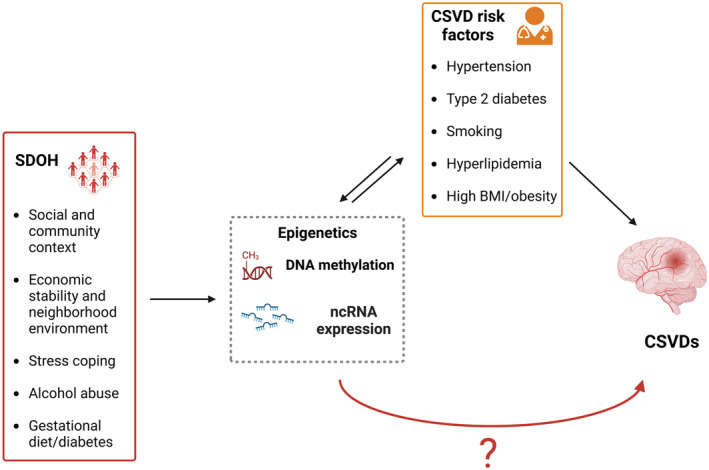
Although an indirect link has been identified, specific SDOH epigenetic signatures directly accounting for higher CSVD risk still need to be unraveled. BMI indicates body mass index; and nc, noncoding.
Social Epigenomics and Risk Factors for CSVD
To date, few studies have investigated the association between SDOH and CSVD, potentially due to the complex and multifaceted dimensions of SDOH. For example, the Healthy People Framework 2030 characterizes SDOH into 5 domains of economic stability, education access and quality, health care access and quality, neighborhood environment, and social and community context. 59 Prior studies use older terminology, such as SES, to imply measurement across multiple domains of SDOH such as economic stability and neighborhood environment. However, growing literature suggests that moving beyond these former descriptions, including race‐based classifications, may be more helpful in understanding why health inequities exist. Cross‐disciplinary collaborations across SDOH and CSVD research teams are needed to ensure research at this intersection is performed in accordance with best practices.
SDOH: Social and Community Context
The largest efforts to describe the impact of social and community context have been made in survivors of ICH, the most severe and overt manifestation of CSVD. In the United States, race and ethnicity have been used as an imperfect proxy for social and community context. 60 Survivors of ICH of self‐reported Black or Hispanic race and ethnicity have higher average blood pressure after ICH. 61 In addition, Black and Hispanic survivors of ICH consistently had more severe CSVD on magnetic resonance imaging at the time of ICH, an independent risk factor for recurrent stroke and cognitive decline. 62 As a result, US Black and Hispanic survivors of ICH were at the highest risk for recurrent stroke after ICH. 61 Other researchers leveraging large administrative data sets have confirmed this higher risk for stroke recurrence among US Black and Asian individuals to move beyond racial and ethnic classifications alone. 63
SDOH: Economic Stability and Neighborhood Environment
Prior studies have grouped the SDOH of economic stability and neighborhood environment as SES, to include neighborhood‐level social characteristics, which has significant associations with cardiovascular risk factors such as obesity, hypertension, diabetes, and smoking, among others. 64 Elevated and constant exposure to 4 established markers of SES (everyday life stressors related to income level, employment status, educational attainment, and environmental factors) have been associated with health outcomes via epigenetic changes. 64 DNA methylation levels in genes linked to stress reactivity and inflammation have been found to be associated with neighborhood‐related effects, such as neighborhood socioeconomic disadvantage and neighborhood social environment. 65 To explore the link between SES and cardiovascular risk, Wang et al 66 conducted an epigenome‐wide mediation analysis that highlighted 43 DNA methylation sites showing significant evidence of mediating the association between neighborhood socioeconomic disadvantage and high‐density cholesterol levels. The identified mediators showed enrichment for genes involved in monocyte‐to‐macrophage differentiation and macrophage polarity among others, corroborating the established role of inflammation in CVD. 66
Low SES has been associated with dysregulations of pathways involved in stress response, as shown by the detection of higher levels of stress hormones, 67 and a higher predisposition to chronic inflammation. 68 Epigenetic changes have been postulated to mediate this association, but never fully validated, likely because of a lack of statistical power due to different definitions or categorization of SES factors across studies, leading to sometimes contradictory results. 69 , 70 , 71 , 72 A recent study by Needham et al 73 in the Multi‐Ethnic Study of Atherosclerosis cohort leveraged a larger sample size of well‐characterized participants and was able to detect significant associations between low SES and DNA methylation in several candidate genes known to be involved in stress reactivity and inflammation.
The identification of epigenetics changes in inflammation and stress response pathways mediating associations between SES and cardiovascular factors unites multiple lines of evidence supporting a role for inflammation in cardiovascular and CSVD pathophysiology. 74 In addition to the identification of candidate SES‐modified pathways, recent studies of DNA methylation changes over time suggest that the biological impact of experiencing low SES during childhood could be mitigated, if an individual's socioeconomic status improves. 73 If further confirmed, these observations could provide evidence to support interventions to improve SES with the explicit goal of reducing health inequities within marginalized populations. Further studies are warranted to further untangle the complex relationship between differences in SES‐related health status, epigenetic changes, and health outcomes.
Alcohol Use and Cholesterol
Alcohol use disorder is an umbrella term that includes conditions such as alcohol dependence, addiction, and abuse. 75 Alcohol use disorder has been associated with race and ethnicity, lack of access to health care, a disadvantaged social environment, low SES, and unemployment. 12 Factors such as improvement in financial situation, social support, and access to health care have been associated with alcohol use disorder recovery rates. 76
Chronic alcohol consumption is associated with a variety of epigenetic modifications including DNA methylation and miRNA expression, in a variety of organs. 77 Epigenetic modifications have also recently been proposed to underlie the higher cardiovascular risk associated with alcohol consumption. After performing cross‐tissue and cross‐phenotypic genome‐wide methylomic variation analysis in patients with alcohol use disorder, Lohoff et al 78 identified a differentially methylated region in the promoter of the PCSK9 gene associated with alcohol‐induced epigenetic regulation of PCSK9 expression levels, and proposed this as a possible novel mechanism linking alcohol abuse and cardiovascular risk. In this study, alcohol use appeared to modulate PCSK9 expression levels in a dose‐dependent fashion, with low/moderate use leading to downregulation and chronic high use resulting in upregulation. These epigenetic variations mirror the J‐shaped trend often seen in cohort studies assessing alcohol consumption and cardiovascular risk wherein small amounts of consumption are protective and larger amounts are detrimental. 78 , 79 The epigenetic changes observed by Lohoff et al could at least partially explain this phenomenon, with PCSK9 downregulation being protective, and upregulation contributing to higher cardiovascular risk. 78 PCSK9 is a well‐known regulator of cholesterol metabolism, so much so that PCSK9 inhibitors are used to treat resistant hypercholesterolemia. 78 , 80
Gestational Diet and Diabetes/Obesity
Over the years many epidemiologic studies have shown that an adverse nutritional intrauterine environment, related for instance to wartime or economic crises, is associated with increased risk of metabolic and cardiovascular disorders in the offspring's lifetime. 81 In a cohort of individuals exposed to the historic Dutch famine in utero, Heijmans et al identified hypomethylation of the maternally imprinted IGF2 (insulin‐like growth‐factor II) gene, which plays a key role in human growth and development, potentially making IGF2 epigenetic regulation a biological link between birth weight and fetal metabolic programming with obesity onset later in life. 82 A similar study by Waterland et al in Gambian populations that experience seasonally fluctuating nutritional supply showed that exposure to a nutritionally unfavorable periconceptional environment could result in permanent epigenetic changes, potentially responsible for T2D, cardiovascular disease, and obesity onset later in life. 83
Epigenetic modifications were also hypothesized to be the part of the pathway through which maternal obesity and diabetes associate with increased risk of cardiometabolic outcomes, obesity, and T2D in offspring. 84 Multiple studies have validated this hypothesis, identifying specific epigenetic signatures attributable to in utero fetal modifications possibly explaining the higher predisposition observed. 85 , 86 Studies focusing on gestational diabetes (GD), one of the most frequent complications of pregnancy, 87 have found altered DNA methylation signatures in genes with established association with obesity in the offspring of individuals with GD, possibly explaining at least a portion of the increased predisposition to obesity in offspring of mothers with GD. 88 In vivo experiments support these results, as well as the observation of altered methylation of genes involved in metabolism processes in obese adults. 88 These findings have been further corroborated by a recent study that compared DNA methylation profiles of GD offspring controls, detecting 13 CpGs associated with GD. 89 Interestingly, the genes involved regulate lipid metabolism and endocrine pathways, further reinforcing the hypothesis that GD‐related epigenetic alterations is a mechanism in the maternal transmission of metabolic diseases. 89 An additional study in the Pima Indian population, which has a high prevalence of T2D, showed that in utero exposure to T2D led to altered methylation at multiple loci that ultimately led to impaired insulin secretion, and to increased risk of obesity and T2D in offspring. 90
Additional studies have examined the impact of maternal hyperglycemia and prepregnancy body mass index (BMI) on offspring obesity rates. Allard et al found that maternal hyperglycemia was associated with the hypomethylation of LEP (leptin) gene, an adipokine crucial for energy balancing processes, which may lead to a higher predisposition to excessive adiposity later in life. 91 In another study, Sharp et al found that prepregnancy maternal BMI was associated with changes in DNA methylation in newborns' blood, as well as in adolescents. 92 Despite a large cohort size in that study, the detected effects were small, highlighting the need of larger, better‐powered cohorts to better understand the epigenetic influence of maternal BMI on offspring health status, independent of confounding genetic or environmental factors.
MENDELIAN RANDOMIZATION IN EPIGENETICS
As technological advancements have moved epigenetic research from a candidate gene approach to epigenome‐wide association studies, there has been a shift toward an omics approach to analyses, with epigenomic data being interpreted in the context of genomics, proteomics, and other omics lines of evidence, working toward an increasingly holistic approach to understanding biological processes and their genomic influences. Epigenomics can benefit from application of Mendelian randomization (MR), an approach that allows inference of causal relationships between an exposure and an outcome of interest. In genetics, MR methods consider genetic variants, which are static, as an unbiased tool to assess causality between modifiable exposures and health outcomes to better understand causal mechanisms in the development of disease. 93 In CSVD, we and others have leveraged MR approaches to identify causal associations of risk factors for multiple related phenotypes. 94 , 95 Epigenetic analyses building on these causal associations could provide a more complete understanding of processes such as kidney disease, lipid levels, and obesity in the pathogenesis and progression of CSVD.
Studies have leveraged MR to infer whether DNA methylation could act as a causal mediator of the relationship between environmental exposures and health outcomes. Most analyses have adopted the 2‐step MR method, proposed by Relton and Davey Smith, who first performed an MR analysis to assess the causal association between the exposure and DNA methylation, followed by a second step to infer the causal relationship between the epigenetic mediator and the selected outcome. 13 An example of this approach relevant to CSVD and SDOH is a study by Juhn et al 96 that investigated the mediating role of epigenetics in the established association between cigarette smoking and inflammation. In a cohort of 822 Black individuals, they performed a 2‐step epigenetic MR to test the hypothesis that cigarette smoking changes DNA methylation patterns, ultimately leading to increased inflammatory response. The first MR analysis leveraged previously detected genetic variants strongly associated with cigarette smoking as instruments to establish the causal association between current smoking and examined DNA methylation of these variants, allowing identification of significant associations between DNA methylation levels of 2 CpG sites neighboring F2RL3 and GPR15 genes. In the second step, MR was used to test the existence of causal associations between the 2 CpGs detected in step 1, and inflammatory markers. This second step confirmed a significant association between F2RL3 CpGs and interleukin‐18 concentration, suggesting that F2RL3 hypomethylation plays a causal role in mediating the increase in interleukin‐18 levels caused by cigarette smoking. This result thereby demonstrates a plausible biological mechanism between cigarette smoking and inflammation and potentially even prioritizes methylation of F2RL3 as a therapeutic target to reduce smoking‐related vascular inflammation. 96
Methylation quantitative trait loci are genetic loci that influence DNA methylation at specific sites, and are thus considered as proxies of DNA methylation. Because methylation quantitative trait loci are encoded within germline DNA and are therefore not modifiable, they can also be used to infer genetically correlated causality in epigenetic associations. 17 Studies have investigated methylation quantitative trait loci at a genome‐wide level to help determine whether and which alterations of DNA methylation are a consequence or a cause of BMI levels, showing that many epigenetic alterations are likely a consequence of high BMI, rather than the cause. 97 An epigenetic MR study in T2D systematically testing methylation quantitative trait loci found new epigenetic signatures that influence T2D, although further analyses will be required to exclude the presence of horizontal pleiotropic effects as confounders. 98
Although future studies with larger sample size are needed, integration of multiomics data with MR approaches has been shown to be a potent tool to identify the directional role of epigenetics mediating environmental exposures and health outcomes. This is particularly significant in light of the dynamic nature of DNA methylation which, although complicating analytical approaches due to modifiability, makes it by that same virtue a promising target for novel therapeutic strategies.
DISCUSSION
In this review we sought to provide an update of epigenetic research, emphasizing the role that epigenetic modifications play in mediating the impact of SDOH on risk factors relevant to CSVD, which exhibits outcome disparities in communities that are at risk for adverse SDOH. Great progress has been made in the past few decades, boosting our knowledge of epigenetic mechanisms, as well as their association with diseases, including CSVD. 26 , 27 Parallel discoveries have also been made in the field of social epigenomics, unraveling the biological mechanisms by which environmental exposures may lead to disease onset. 99
Particularly in the context of CSVD, studies have identified specific epigenetic signatures linking exposure to a particular SDOH to increased risk of CSVD risk factors. 84 , 91 Future work is warranted to unravel the presence of a clear, direct, and causal connection between SDOH‐related epigenetic modifications and CSVDs. Finding this missing link could be crucial not only to unravel new pathogenic mechanisms and novel drug targets, but also to decrease global CSVD burden and eliminate disparities seen in CSVD among disadvantaged populations.
To achieve the goals of identifying epigenetic changes linking SDOH and CSVD, some challenges remain. Despite striking sex, racial and ethnic, socioeconomic, and geographic disparities in health outcomes, genomic research still lacks significant representation of marginalized populations. It is crucial that future studies to enroll historically underrepresented groups to make meaningful contributions to the eradication of health inequalities, as is being successfully implemented in the National Institutes of Health–sponsored All of Us Study (researchallofus.org). A second major challenge consists in reaching the statistical power to identify significant and replicable findings, which requires the aggregation of larger sample sizes for relevant SDOH exposures as well as health outcomes. This can be further limited by the lack of phenotypic harmonization across studies, especially measures of SDOH, which limits the ability to meta‐analyze data generated by different groups. Another aspect to be considered in this regard is also the synchronization between the observation of exposures and blood sample collection, which must be carefully planned to overcome potential limitations due to the highly dynamic nature of DNA methylation patterns. 100
CONCLUSIONS
Despite being a disease with considerable disparity in prevalence among marginalized communities, epigenetic studies focusing on the associations between SDOH and CSVD are lacking. Current epigenetic research is limited to SDOH and cardiovascular risk factors, which has yielded important insights about the role of epigenetics in mediating the relationship between social exposures, cardiovascular risk factors, and outcomes. Future studies should investigate epigenetic signatures of CSVD in larger, more diverse cohorts, with standardized SDOH and cardiovascular risk factor ascertainment, to gain better biological insights into a disease whose burden is magnified by existing health disparities.
Sources of Funding
C.D.A. is supported by National Institutes of Health R01NS103924, U01NS069673; American Heart Association 18SFRN34250007, 21SFRN812095; and the Massachusetts General Hospital McCance Center for Brain Health for this work. J.R. receives research grants from the National Institutes of Health and the American Heart Association‐Bugher Foundation. C.D.L. and M.E.C. are supported by the National Institutes of Health U01NS069763.
Disclosures
C.D.A. has received sponsored research support from Bayer AG and has consulted for ApoPharma unrelated to this work. J.R. reports compensation from National Football League for expert witness services and from Takeda Development Center Americas and Boehringer Ingelheim for consultant services, all unrelated to this work. The remaining authors have no disclosures to report.
Acknowledgments
The figures were created with Biorender.com.
This article was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1. Chojdak‐Łukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: a review. Adv Clin Exp Med. 2021;30:349–356. doi: 10.17219/acem/131216 [DOI] [PubMed] [Google Scholar]
- 2. Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491 [DOI] [PubMed] [Google Scholar]
- 3. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92:1146–1156. doi: 10.1212/WNL.0000000000007654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bos D, Wolters FJ, Darweesh SKL, Vernooij MW, de Wolf F, Ikram MA, Hofman A. Cerebral small vessel disease and the risk of dementia: a systematic review and meta‐analysis of population‐based evidence. Alzheimers Dement. 2018;14:1482–1492. doi: 10.1016/j.jalz.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Chen Q, Chen J, Yang N, Zheng K. Risk factors of cerebral small vessel disease: a systematic review and meta‐analysis. Medicine (Baltimore). 2021;100:e28229. doi: 10.1097/MD.0000000000028229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging. 2006;16:243–251. doi: 10.1111/j.1552-6569.2006.00047.x [DOI] [PubMed] [Google Scholar]
- 7. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 8. Commission on Social Determinants of Health . Closing the gap in a generation: health equity through action on the social determinants of health. Final report of the Commission on Social Determinants of Health. World Health Organization; 2008.
- 9. Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129(Suppl 2):19–31. doi: 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreatsoulas C, Anand SS. The impact of social determinants on cardiovascular disease. Can J Cardiol. 2010;26:8C–13C. doi: 10.1016/s0828-282x(10)71075-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tung J, Gilad Y. Social environmental effects on gene regulation. Cell Mol Life Sci. 2013;70:4323–4339. doi: 10.1007/s00018-013-1357-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mancilla VJ, Peeri NC, Silzer T, Basha R, Felini M, Jones HP, Phillips N, Tao M‐H, Thyagarajan S, Vishwanatha JK. Understanding the interplay between health disparities and epigenomics. Front Genet. 2020;11:903. doi: 10.3389/fgene.2020.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Relton CL, Davey Smith G. Two‐step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41:161–176. doi: 10.1093/ije/dyr233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu C‐T, Morris JR. Genes, genetics, and epigenetics: a correspondence. Science. 2001;293:1103–1105. doi: 10.1126/science.293.5532.1103 [DOI] [PubMed] [Google Scholar]
- 15. Wei J‐W, Huang K, Yang C, Kang C‐S. Non‐coding RNAs as regulators in epigenetics (review). Oncol Rep. 2017;37:3–9. doi: 10.3892/or.2016.5236 [DOI] [PubMed] [Google Scholar]
- 16. Li S, Tollefsbol TO. DNA methylation methods: global DNA methylation and methylomic analyses. Methods. 2021;187:28–43. doi: 10.1016/j.ymeth.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cullell N, Soriano‐Tarraga C, Gallego‐Fabrega C, Carcel‐Marquez J, Torres‐Aguila NP, Muino E, Lledos M, Llucia‐Carol L, Esteller M, Castro de Moura M, et al. DNA methylation and ischemic stroke risk: an epigenome‐wide association study. Thromb Haemost. 2022;122:1767–1778. doi: 10.1055/s-0042-1749328 [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Knol MJ, Wang R, Mishra A, Liu D, Luciano M, Teumer A, Armstrong N, Bis JC, Jhun MA, et al. Epigenetic and integrative cross‐omics analyses of cerebral white matter hyperintensities on MRI. Brain. 2022;146:492–506. doi: 10.1093/brain/awac290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jimenez‐Balado J, Giralt‐Steinhauer E, Fernandez‐Perez I, Rey L, Cuadrado‐Godia E, Ois A, Rodriguez‐Campello A, Soriano‐Tarraga C, Lazcano U, Macias‐Gomez A, et al. Epigenetic clock explains white matter hyperintensity burden irrespective of chronological age. Biology (Basel). 2022;12:33. doi: 10.3390/biology12010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prabhakar P, Chandra SR, Christopher R. Circulating microRNAs as potential biomarkers for the identification of vascular dementia due to cerebral small vessel disease. Age Ageing. 2017;46:861–864. doi: 10.1093/ageing/afx090 [DOI] [PubMed] [Google Scholar]
- 21. Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, Kelly TN, Saleheen D, Lehne B, Leach IM, et al. Trans‐ancestry genome‐wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet. 2015;47:1282–1293. doi: 10.1038/ng.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei LK, Sutherland H, Au A, Camilleri E, Haupt LM, Gan SH, Griffiths LR. A potential epigenetic marker mediating serum folate and vitamin B12 levels contributes to the risk of ischemic stroke. Biomed Res Int. 2015;2015:167976. doi: 10.1155/2015/167976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alikhani‐Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11 beta‐hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114:1146–1157. doi: 10.1172/JCI21647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, Carletto A, Pattini P, Corrocher R, Olivieri O. Epigenetic control of 11 beta‐hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199:323–327. doi: 10.1016/j.atherosclerosis.2007.11.029 [DOI] [PubMed] [Google Scholar]
- 25. Riviere G, Lienhard D, Andrieu T, Vieau D, Frey BM, Frey FJ. Epigenetic regulation of somatic angiotensin‐converting enzyme by DNA methylation and histone acetylation. Epigenetics. 2011;6:478–489. doi: 10.4161/epi.6.4.14961 [DOI] [PubMed] [Google Scholar]
- 26. Richard MA, Huan T, Ligthart S, Gondalia R, Jhun MA, Brody JA, Irvin MR, Marioni R, Shen J, Tsai PC, et al. DNA methylation analysis identifies loci for blood pressure regulation. Am J Hum Genet. 2017;101:888–902. doi: 10.1016/j.ajhg.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Falkner B, Zhu H, Shi H, Su S, Xu X, Sharma AK, Dong Y, Treiber F, Gutin B, et al. A genome‐wide methylation study on essential hypertension in young African American males. PLoS One. 2013;8:e53938. doi: 10.1371/journal.pone.0053938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kazmi N, Elliott HR, Burrows K, Tillin T, Hughes AD, Chaturvedi N, Gaunt TR, Relton CL. Associations between high blood pressure and DNA methylation. PLoS One. 2020;15:e0227728. doi: 10.1371/journal.pone.0227728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breton CV, Yao J, Millstein J, Gao L, Siegmund KD, Mack W, Whitfield‐Maxwell L, Lurmann F, Hodis H, Avol E, et al. Prenatal air pollution exposures, DNA methyl transferase genotypes, and associations with newborn LINE1 and Alu methylation and childhood blood pressure and carotid intima‐media thickness in the Children's Health Study. Environ Health Perspect. 2016;124:1905–1912. doi: 10.1289/EHP181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bellavia A, Urch B, Speck M, Brook RD, Scott JA, Albetti B, Behbod B, North M, Valeri L, Bertazzi PA, et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: findings from controlled human exposure experiments. J Am Heart Assoc. 2013;2:e000212. doi: 10.1161/JAHA.113.000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jusic A, Devaux Y; Action EUCC . Noncoding RNAs in hypertension. Hypertension. 2019;74:477–492. doi: 10.1161/HYPERTENSIONAHA.119.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Wei Y, Wang Z. microRNA‐21 and hypertension. Hypertens Res. 2018;41:649–661. doi: 10.1038/s41440-018-0071-z [DOI] [PubMed] [Google Scholar]
- 33. Bao X, He X, Zheng S, Sun J, Luo Y, Tan R, Zhao J, Zhong F, Zhang L. Up‐regulation of circular RNA hsa_circ_0037909 promotes essential hypertension. J Clin Lab Anal. 2019;33:e22853. doi: 10.1002/jcla.22853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matshazi DM, Weale CJ, Erasmus RT, Kengne AP, Davids SFG, Raghubeer S, Davison GM, Matsha TE. Circulating levels of microRNAs associated with hypertension: a cross‐sectional study in male and female South African participants. Front Genet. 2021;12:710438. doi: 10.3389/fgene.2021.710438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torres‐Paz YE, Huesca‐Gomez C, Sanchez‐Munoz F, Martinez‐Alvarado R, Soto ME, Torres‐Tamayo M, Fuentevilla‐Alvarez G, Gamboa R. Increased expression of miR‐33a in monocytes from Mexican hypertensive patients in elevated carotid intima‐media thickness. J Hum Hypertens. 2018;32:681–690. doi: 10.1038/s41371-018-0102-x [DOI] [PubMed] [Google Scholar]
- 36. Zhang Z, Joyce BT, Kresovich JK, Zheng Y, Zhong J, Patel R, Zhang W, Liu L, Dou C, McCracken JP, et al. Blood pressure and expression of microRNAs in whole blood. PLoS One. 2017;12:e0173550. doi: 10.1371/journal.pone.0173550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin J, Thompson TJ, Cheng YJ, Zhuo X, Zhang P, Gregg E, Rolka DB. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr. 2018;16:9. doi: 10.1186/s12963-018-0166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, et al. Fine‐mapping type 2 diabetes loci to single‐variant resolution using high‐density imputation and islet‐specific epigenome maps. Nat Genet. 2018;50:1505–1513. doi: 10.1038/s41588-018-0241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ling C, Bacos K, Rönn T. Epigenetics of type 2 diabetes mellitus and weight change—a tool for precision medicine? Nat Rev Endocrinol. 2022;18:433–448. doi: 10.1038/s41574-022-00671-w [DOI] [PubMed] [Google Scholar]
- 40. Kulkarni H, Kos MZ, Neary J, Dyer TD, Kent JW, Göring HHH, Cole SA, Comuzzie AG, Almasy L, Mahaney MC, et al. Novel epigenetic determinants of type 2 diabetes in Mexican‐American families. Hum Mol Genet. 2015;24:5330–5344. doi: 10.1093/hmg/ddv232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feng J, Xing W, Xie L. Regulatory roles of microRNAs in diabetes. Int J Mol Sci. 2016;17:1729. doi: 10.3390/ijms17101729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zong D, Liu X, Li J, Ouyang R, Chen P. The role of cigarette smoke‐induced epigenetic alterations in inflammation. Epigenetics Chromatin. 2019;12:65. doi: 10.1186/s13072-019-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Joehanes R, Just AC, Marioni RE, Pilling LC, Reynolds LM, Mandaviya PR, Guan W, Xu T, Elks CE, Aslibekyan S, et al. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9:436–447. doi: 10.1161/CIRCGENETICS.116.001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Philibert RA, Beach SRH, Lei M‐K, Brody GH. Changes in DNA methylation at the aryl hydrocarbon receptor repressor may be a new biomarker for smoking. Clin Epigenetics. 2013;5:19. doi: 10.1186/1868-7083-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Howe CG, Zhou M, Wang X, Pittman GS, Thompson IJ, Campbell MR, Bastain TM, Grubbs BH, Salam MT, Hoyo C, et al. Associations between maternal tobacco smoke exposure and the cord blood CD4+ DNA methylome. Environ Health Perspect. 2019;127:47009. doi: 10.1289/EHP3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rauschert S, Melton PE, Burdge G, Craig JM, Godfrey KM, Holbrook JD, Lillycrop K, Mori TA, Beilin LJ, Oddy WH, et al. Maternal smoking during pregnancy induces persistent epigenetic changes into adolescence, independent of postnatal smoke exposure and is associated with cardiometabolic risk. Front Genet. 2019;10:770. doi: 10.3389/fgene.2019.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sayols‐Baixeras S, Irvin MR, Arnett DK, Elosua R, Aslibekyan SW. Epigenetics of lipid phenotypes. Curr Cardiovasc Risk Rep. 2016;10:31. doi: 10.1007/s12170-016-0513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guay S‐P, Brisson D, Lamarche B, Gaudet D, Bouchard L. Epipolymorphisms within lipoprotein genes contribute independently to plasma lipid levels in familial hypercholesterolemia. Epigenetics. 2014;9:718–729. doi: 10.4161/epi.27981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, Slieker RC, Stok AP, Thijssen PE, Müller F, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014;5:5592. doi: 10.1038/ncomms6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xiao F‐H, Wang H‐T, Kong Q‐P. Dynamic DNA methylation during aging: a "prophet" of age‐related outcomes. Front Genet. 2019;10:107. doi: 10.3389/fgene.2019.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fuke C, Shimabukuro M, Petronis A, Sugimoto J, Oda T, Miura K, Miyazaki T, Ogura C, Okazaki Y, Jinno Y. Age related changes in 5‐methylcytosine content in human peripheral leukocytes and placentas: an HPLC‐based study. Ann Hum Genet. 2004;68:196–204. doi: 10.1046/j.1529-8817.2004.00081.x [DOI] [PubMed] [Google Scholar]
- 52. Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Tarantini L, Sparrow D, Vokonas P, Schwartz J. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ho S‐M, Johnson A, Tarapore P, Janakiram V, Zhang X, Leung Y‐K. Environmental epigenetics and its implication on disease risk and health outcomes. ILAR J. 2012;53:289–305. doi: 10.1093/ilar.53.3-4.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Krieger N. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol. 2001;30:668–677. doi: 10.1093/ije/30.4.668 [DOI] [PubMed] [Google Scholar]
- 55. Salihu HM, Das R, Morton L, Huang H, Paothong A, Wilson RE, Aliyu MH, Salemi JL, Marty PJ. Racial differences in DNA‐methylation of CpG sites within preterm‐promoting genes and gene variants. Matern Child Health J. 2016;20:1680–1687. doi: 10.1007/s10995-016-1967-3 [DOI] [PubMed] [Google Scholar]
- 56. Devaney JM, Wang S, Furbert‐Harris P, Apprey V, Ittmann M, Wang BD, Olender J, Lee NH, Kwabi‐Addo B. Genome‐wide differentially methylated genes in prostate cancer tissues from African‐American and Caucasian men. Epigenetics. 2015;10:319–328. doi: 10.1080/15592294.2015.1022019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chitrala KN, Hernandez DG, Nalls MA, Mode NA, Zonderman AB, Ezike N, Evans MK. Race‐specific alterations in DNA methylation among middle‐aged African Americans and Whites with metabolic syndrome. Epigenetics. 2020;15:462–482. doi: 10.1080/15592294.2019.1695340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saban KL, Mathews HL, DeVon HA, Janusek LW. Epigenetics and social context: implications for disparity in cardiovascular disease. Aging Dis. 2014;5:346–355. doi: 10.14336/AD.2014.0500346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion . Healthy People 2030. Available at: https://health.gov/healthypeople/priority‐areas/social‐determinants‐health. Accessed March 28, 2023. [PubMed]
- 60. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407–411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rodriguez‐Torres A, Murphy M, Kourkoulis C, Schwab K, Ayres AM, Moomaw CJ, Young Kwon S, Berthaud JV, Gurol ME, Greenberg SM, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018;91:e37–e44. doi: 10.1212/WNL.0000000000005729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Castello JP, Pasi M, Abramson JR, Rodriguez‐Torres A, Marini S, Demel S, Gilkerson L, Kubiszewski P, Charidimou A, Kourkoulis C, et al. Contribution of racial and ethnic differences in cerebral small vessel disease subtype and burden to risk of cerebral hemorrhage recurrence. Neurology. 2021;96:e2469–e2480. doi: 10.1212/WNL.0000000000011932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leasure AC, King ZA, Torres‐Lopez V, Murthy SB, Kamel H, Shoamanesh A, Al‐Shahi Salman R, Rosand J, Ziai WC, Hanley DF, et al. Racial/ethnic disparities in the risk of intracerebral hemorrhage recurrence. Neurology. 2020;94:e314–e322. doi: 10.1212/WNL.0000000000008737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith JA, Zhao W, Wang X, Ratliff SM, Mukherjee B, Kardia SLR, Liu Y, Roux AVD, Needham BL. Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the Multi‐Ethnic Study of Atherosclerosis. Epigenetics. 2017;12:662–673. doi: 10.1080/15592294.2017.1341026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang YZ, Zhao W, Ammous F, Song Y, Du J, Shang L, Ratliff SM, Moore K, Kelly KM, Needham BL, et al. DNA methylation mediates the association between individual and neighborhood social disadvantage and cardiovascular risk factors. Front Cardiovasc Med. 2022;9:848768. doi: 10.3389/fcvm.2022.848768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Janicki‐Deverts D, Cohen S, Adler NE, Schwartz JE, Matthews KA, Seeman TE. Socioeconomic status is related to urinary catecholamines in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosom Med. 2007;69:514–520. doi: 10.1097/PSY.0b013e3180f60645 [DOI] [PubMed] [Google Scholar]
- 68. Stringhini S, Batty GD, Bovet P, Shipley MJ, Marmot MG, Kumari M, Tabak AG, Kivimäki M. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: the Whitehall II prospective cohort study. PLoS Med. 2013;10:e1001479. doi: 10.1371/journal.pmed.1001479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lam LL, Emberly E, Fraser HB, Neumann SM, Chen E, Miller GE, Kobor MS. Factors underlying variable DNA methylation in a human community cohort. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17253–17260. doi: 10.1073/pnas.1121249109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McGuinness D, McGlynn LM, Johnson PCD, MacIntyre A, Batty GD, Burns H, Cavanagh J, Deans KA, Ford I, McConnachie A, et al. Socio‐economic status is associated with epigenetic differences in the pSoBid cohort. Int J Epidemiol. 2012;41:151–160. doi: 10.1093/ije/dyr215 [DOI] [PubMed] [Google Scholar]
- 71. Tehranifar P, Wu H‐C, Fan X, Flom JD, Ferris JS, Cho YH, Gonzalez K, Santella RM, Terry MB. Early life socioeconomic factors and genomic DNA methylation in mid‐life. Epigenetics. 2013;8:23–27. doi: 10.4161/epi.22989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Subramanyam MA, Diez‐Roux AV, Pilsner JR, Villamor E, Donohue KM, Liu Y, Jenny NS. Social factors and leukocyte DNA methylation of repetitive sequences: the multi‐ethnic study of atherosclerosis. PLoS One. 2013;8:e54018. doi: 10.1371/journal.pone.0054018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Needham BL, Smith JA, Zhao W, Wang X, Mukherjee B, Kardia SLR, Shively CA, Seeman TE, Liu Y, Diez Roux AV. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: the multi‐ethnic study of atherosclerosis. Epigenetics. 2015;10:958–969. doi: 10.1080/15592294.2015.1085139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Low A, Mak E, Rowe JB, Markus HS, O'Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. doi: 10.1016/j.arr.2019.100916 [DOI] [PubMed] [Google Scholar]
- 75. Schuckit MA. Alcohol‐use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X [DOI] [PubMed] [Google Scholar]
- 76. Swan JE, Aldridge A, Joseph V, Tucker JA, Witkiewitz K. Individual and community social determinants of health and recovery from alcohol use disorder three years following treatment. J Psychoactive Drugs. 2021;53:394–403. doi: 10.1080/02791072.2021.1986243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ciafrè S, Carito V, Ferraguti G, Greco A, Chaldakov GN, Fiore M, Ceccanti M. How alcohol drinking affects our genes: an epigenetic point of view. Biochem Cell Biol. 2019;97:345–356. doi: 10.1139/bcb-2018-0248 [DOI] [PubMed] [Google Scholar]
- 78. Lohoff FW, Sorcher JL, Rosen AD, Mauro KL, Fanelli RR, Momenan R, Hodgkinson CA, Vendruscolo LF, Koob GF, Schwandt M, et al. Methylomic profiling and replication implicates deregulation of PCSK9 in alcohol use disorder. Mol Psychiatry. 2018;23:1900–1910. doi: 10.1038/mp.2017.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta‐analysis. J Am Coll Cardiol. 2010;55:1339–1347. doi: 10.1016/j.jacc.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 80. Pokhrel B, Yuet WC, Levine SN. PCSK9 Inhibitors. StatPearls. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 81. Schulz LC. The Dutch hunger winter and the developmental origins of health and disease. Proc Natl Acad Sci USA. 2010;107:16757–16758. doi: 10.1073/pnas.1012911107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Waterland RA, Kellermayer R, Laritsky E, Rayco‐Solon P, Harris RA, Travisano M, Zhang W, Torskaya MS, Zhang J, Shen L, et al. Season of conception in rural Gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–398. doi: 10.1530/REP-10-0077 [DOI] [PubMed] [Google Scholar]
- 85. Quilter CR, Cooper WN, Cliffe KM, Skinner BM, Prentice PM, Nelson L, Bauer J, Ong KK, Constância M, Lowe WL, et al. Impact on offspring methylation patterns of maternal gestational diabetes mellitus and intrauterine growth restraint suggest common genes and pathways linked to subsequent type 2 diabetes risk. FASEB J. 2014;28:4868–4879. doi: 10.1096/fj.14-255240 [DOI] [PubMed] [Google Scholar]
- 86. Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome‐wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet. 2015;24:3021–3029. doi: 10.1093/hmg/ddv013 [DOI] [PubMed] [Google Scholar]
- 87. Koning SH, Hoogenberg K, Lutgers HL, van den Berg PP, Wolffenbuttel BHR. Gestational diabetes mellitus:current knowledge and unmet needs. J Diabetes. 2016;8:770–781. doi: 10.1111/1753-0407.12422 [DOI] [PubMed] [Google Scholar]
- 88. El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, Korenkov M, Aretz M, Zechner U, Lehnen H, Haaf T. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62:1320–1328. doi: 10.2337/db12-0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hjort L, Martino D, Grunnet LG, Naeem H, Maksimovic J, Olsson AH, Zhang C, Ling C, Olsen SF, Saffery R, et al. Gestational diabetes and maternal obesity are associated with epigenome‐wide methylation changes in children. JCI Insight. 2018;3:e122572. doi: 10.1172/jci.insight.122572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen P, Piaggi P, Traurig M, Bogardus C, Knowler WC, Baier LJ, Hanson RL. Differential methylation of genes in individuals exposed to maternal diabetes in utero. Diabetologia. 2017;60:645–655. doi: 10.1007/s00125-016-4203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Allard C, Desgagné V, Patenaude J, Lacroix M, Guillemette L, Battista MC, Doyon M, Ménard J, Ardilouze JL, Perron P, et al. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 2015;10:342–351. doi: 10.1080/15592294.2015.1029700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sharp GC, Salas LA, Monnereau C, Allard C, Yousefi P, Everson TM, Bohlin J, Xu Z, Huang R‐C, Reese SE, et al. Maternal BMI at the start of pregnancy and offspring epigenome‐wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum Mol Genet. 2017;26:4067–4085. doi: 10.1093/hmg/ddx290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Burgess S, Foley CN, Zuber V. Inferring causal relationships between risk factors and outcomes from genome‐wide association study data. Annu Rev Genomics Hum Genet. 2018;19:303–327. doi: 10.1146/annurev-genom-083117-021731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayerhofer E, Malik R, Parodi L, Burgess S, Harloff A, Dichgans M, Rosand J, Anderson CD, Georgakis MK. Genetically predicted on‐statin LDL response is associated with higher intracerebral haemorrhage risk. Brain. 2022;145:2677–2686. doi: 10.1093/brain/awac186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Georgakis MK, Malik R, Anderson CD, Parhofer KG, Hopewell JC, Dichgans M. Genetic determinants of blood lipids and cerebral small vessel disease: role of high‐density lipoprotein cholesterol. Brain. 2020;143:597–610. doi: 10.1093/brain/awz413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jhun MA, Smith JA, Ware EB, Kardia SLR, Mosley TH, Turner ST, Peyser PA, Park SK. Modeling the causal role of DNA methylation in the association between cigarette smoking and inflammation in African Americans: a 2‐step epigenetic Mendelian randomization study. Am J Epidemiol. 2017;186:1149–1158. doi: 10.1093/aje/kwx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, Tsai P‐C, Ried JS, Zhang W, Yang Y, et al. Epigenome‐wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Richardson TG, Haycock PC, Zheng J, Timpson NJ, Gaunt TR, Davey Smith G, Relton CL, Hemani G. Systematic Mendelian randomization framework elucidates hundreds of CpG sites which may mediate the influence of genetic variants on disease. Hum Mol Genet. 2018;27:3293–3304. doi: 10.1093/hmg/ddy210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Howard G, Cushman M, Howard VJ, Kissela BM, Kleindorfer DO, Moy CS, Switzer J, Woo D. Risk factors for intracerebral hemorrhage: the REasons for geographic and racial differences in stroke (REGARDS) study. Stroke. 2013;44:1282–1287. doi: 10.1161/STROKEAHA.111.000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brown KM, Hui Q, Huang Y, Taylor JY, Prescott L, de Mendoza VB, Crusto C, Sun YV. Association between stress and coping with DNA methylation of blood pressure‐related genes among African American women. Chronic Stress (Thousand Oaks). 2019;3:2470547019879088. doi: 10.1177/2470547019879088 [DOI] [PMC free article] [PubMed] [Google Scholar]


