Abstract
Background
Prevention strategies targeting standard modifiable cardiovascular risk factors (SMuRFs; diabetes, hypertension, smoking, hypercholesterolemia) are critical to improving cardiovascular disease outcomes. However, acute myocardial infarction (AMI) among individuals who lack 1 or more SMuRFs is not uncommon. Moreover, the clinical characteristics and prognosis of SMuRFless individuals are not well characterized.
Methods and Results
We analyzed AMI hospitalizations from 2000 to 2014 captured by the ARIC (Atherosclerosis Risk in Community) study community surveillance. AMI was classified by physician review using a validated algorithm. Clinical data, medications, and procedures were abstracted from the medical record. Main study outcomes included short‐ and long‐term mortality within 28 days and 1 year of AMI hospitalization. Between 2000 and 2014, a total of 742 (3.6%) of 20 569 patients with AMI were identified with no documented SMuRFs. Patients without SMuRFs were less likely to receive aspirin, nonaspirin antiplatelet therapy, or beta blockers and less often underwent angiography and revascularization. Compared with those with one or more SMuRFs, patients without SMuRFs had significantly higher 28‐day (odds ratio, 3.23 [95% CI, 1.78–5.88]) and 1‐year (hazard ratio, 2.09 [95% CI, 1.29–3.37]) adjusted mortality. When examined across 5‐year intervals from 2000 to 2014, the incidence of 28‐day mortality significantly increased for patients without SMuRFs (7% to 15% to 27%), whereas it declined for those with 1 or more SMuRFs (7% to 5% to 5%).
Conclusions
Individuals without SMuRFs presenting with AMI have an increased risk of all‐cause mortality with an overall lower prescription rate for guideline‐directed medical therapy. These findings highlight the need for evidence‐based pharmacotherapy during hospitalization and the need to discover new markers and mechanisms for early risk identification in this population.
Keywords: acute coronary syndrome, acute myocardial infarction, cardiovascular disease, chest pain, standard modifiable risk factors
Subject Categories: Cardiovascular Disease, Risk Factors, Epidemiology, Primary Prevention
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities Study
- GDMT
guideline‐directed medical therapy
- SMuRF
standard modifiable cardiovascular risk factors
Clinical Perspective.
What Is New?
Whether the clinical characteristics, management and prognosis differ for hospitalized patients with acute myocardial infarction presenting without versus with standard modifiable risk factors is uncertain.
In this population‐based surveillance, individuals without standard modifiable risk factors presenting with acute myocardial infarction had an increased risk of all‐cause mortality with an overall lower prescription of guideline‐directed medical therapy.
Across the 15‐year interval under surveillance, the incidence of 28‐day mortality significantly increased for patients without standard modifiable risk factors, whereas it declined for those with 1 or more standard modifiable risk factors.
What Are the Clinical Implications?
Our findings highlight the need for optimal management and evidence‐based pharmacotherapy during hospitalization and the need to discover new markers and mechanisms for early identification of risk in this population.
The standard modifiable cardiovascular risk factors (SMuRFs) for cardiovascular disease (eg, hypertension, diabetes, hyperlipidemia, and cigarette smoking) are central inputs for several validated cardiovascular risk scores. 1 , 2 , 3 , 4 Primary and secondary prevention strategies targeting these modifiable risk factors for coronary heart disease are crucial and have resulted in significant treatment improvement along with a substantial reduction in morbidity and mortality. 4 , 5 However, coronary heart disease remains one of the leading causes of death in the world. 6
Importantly, cardiovascular diseases, especially myocardial infarction, are not uncommon in the absence of SMuRFs. 7 , 8 , 9 , 10 A significant proportion of patients presenting with acute myocardial infarction (AMI) have no history of SMuRFs at or greater than diagnostic thresholds. 7 Previous studies have shown that the proportion of patients without SMuRFs among patients presenting with acute coronary syndrome ranged widely from 11% to 27%. 8 , 9 , 10 Furthermore, this group was observed to have a higher rate of in‐hospital mortality compared with patients with 1 or more SMuRFs. 10 , 11 , 12 Although contemporary data suggest an increase in the proportion of patients without SMuRFs in recent years, 8 , 9 , 10 the clinical characteristics and prognosis of individuals without SMuRFs who are hospitalized with AMI have not been studied extensively. A better characterization of this patient population will have important implications for identifying optimal approaches to manage AMI risk in this group.
Although several studies have been undertaken in Europe and Australia, there are limited data evaluating the characteristics and prognosis of patients without SMuRFs in patients hospitalized with AMI, especially in the United States. In this study, we analyze SmuRFless AMI hospitalizations from 4 US regions that were captured by the ARIC (Atherosclerosis Risk in Communities) study.
METHODS
The ARIC Study Data
The ARIC study's data are owned by the National Heart Lung and Blood Institute and are publicly available to qualified investigators with an approved manuscript proposal and data use agreement.
The ARIC Study Community Surveillance
The ARIC study includes a cohort population and several community surveillance populations. 13 The present analysis is based on the AMI community surveillance population of patients hospitalized with AMI. From 1987 to 2014, the ARIC study conducted community surveillance of AMI hospitalization in 4 geographically different regions of the United States: Forsyth County, North Carolina; Washington County, Maryland; Jackson, Mississippi; and Minneapolis, Minnesota. Hospitalizations were randomly sampled within prespecified strata based on the ARIC community, discharge code, age, sex, and race (Black or White) and reviewed by a panel of physicians. All surveillance activities involved in this study were approved by local institutional review boards. The surveillance did not warrant patient consent as personal identifiers were redacted from the analytic data set. For the purpose of this analysis, hospitalizations were limited to discharge dates between January 1, 2000 and December 31, 2014. Because we aimed to examine temporal trends in the presentation, management, and outcomes of SMuRFless admissions, we analyzed a consistent age category (35–74 years) for each of the 15 years under consideration.
Acute Myocardial Infarction Classification
As we have previously described, 14 events were classified by the ARIC study as definite, probable, suspected, or no AMI, based on ECG evidence, presence of chest pain, and levels of cardiac biomarkers. Details of the standardized algorithm are published. 15 Classification of an event as definite or probable AMI required the presence of at least 1 of the following: (1) evolving diagnostic ECG pattern, (2) diagnostic ECG pattern and abnormal biomarkers (≥2× upper limit of normal), (3) cardiac pain and abnormal biomarkers (≥2× upper limit of normal), (4) cardiac pain and equivocal biomarkers (exceeding the upper limit of normalbut <2× the upper limit of normal) with evolving ST‐segment/T‐wave pattern or diagnostic ECG pattern, or (5) abnormal biomarkers with evolving ST‐segment/T‐wave pattern. The first, third, and last 12‐lead ECG during hospitalization were obtained from the medical record and coded electronically at the Minneapolis ECG Reading Center and were used for classification of ST‐segment–elevation myocardial infarction (STEMI) or non–STEMI.
Modifiable Risk Factors and Clinical Data Abstraction
Clinical data were collected from the hospital record by trained abstractors following a standardized, well‐defined protocol, using the physician notes, laboratory reports, medical history, and discharge summaries. Similar to previous investigations, 7 , 8 , 9 , 10 we considered smoking, hypertension, diabetes, and hypercholesterolemia to be conventional, modifiable risk factors for AMI. For this analysis, smoking was defined as “current” smoking. Hypertension was classified by documented diagnosis in the medical history. Diabetes was classified by a physician diagnosis or diabetic medication use. Hypercholesterolemia was inferred by administration of lipid‐lowering therapies. Patients lacking all 4 risk factors were considered SMuRFless; those with 1 or more factors were classified as SMuRF.
Presenting conditions (chest pain, acute pulmonary edema/congestive heart failure, cardiogenic shock, and ventricular fibrillation/cardiac arrest) were abstracted from the hospital chart. Origin of chest pain was determined by review of physician notes, with abstraction limited to chest pain of cardiac origin. Laboratory values were abstracted from the medical record by recording the first and worst value during hospitalization. We estimated the glomerular filtration rate using the Chronic Kidney Disease‐Epidemiology Collaboration formula and the first abstracted serum creatinine. Chronic kidney disease was defined by an estimated glomerular filtration rate <60 mL/min per 1.73 m2 or receipt of dialysis. Cerebrovascular history was abstracted as prior stroke or transient ischemic attack. Prior coronary revascularization was defined by history of percutaneous coronary intervention or coronary artery bypass graft.
Therapies
Medications were abstracted if administered during hospitalization or prescribed at hospital discharge. In‐hospital interventions, such as invasive angiography or coronary revascularization were abstracted if performed during the AMI hospitalization.
Mortality
All‐cause mortality was ascertained within 28 days and 1 year of hospital admission by the ARIC study, by linking hospitalizations with the National Death Index.
Statistical Analysis
All statistical analyses were carried out using SAS 9.4 (SAS Institute; Cary, NC). Statistical tests and models were weighted by the inverse of the sampling probability. Continuous variables were assessed for normality and compared using the difference in least square means from weighted linear regression and categorical variables were compared using Rao‐Scott χ2 tests. Temporal trends in the administration of AMI therapies (aspirin, nonaspirin antiplatelets, beta blockers, invasive angiography, and coronary revascularization), 28‐day and 1‐year all‐cause mortality were visually plotted for groups with and without SMuRFs across 5‐year intervals (2000–2004, 2005–2009, 2010–2014) with significance of trends analyzed using logistic regression and the Cochran–Armitage test for trend, regressing on year categories as an ordinal variable. Potential modification by SMuRFless versus SMuRF classifications was tested by multiplicative interaction. Adjusted associations of therapy administration for SMuRFless relative to SMuRF classification were analyzed by multivariable logistic regression, with odds ratios (ORs) converted into relative risks (RR) and 95% CIs. Models were adjusted for race, geographic region, and year of admission. The adjusted associations of SMuRFless versus SMuRF classification with mortality were analyzed by multivariable logistic or Cox regression, for short‐term (in‐hospital or 28‐day) and long‐term (1‐year) mortality outcomes, respectively. Mortality models were fully adjusted for demographics (age, race, sex, geographic region, and year of admission), comorbid conditions (history of chronic kidney disease and transient ischemic attack/stroke) and therapies (aspirin, antiplatelets, beta blockers, and coronary revascularization) during the hospital admission.
RESULTS
Between 2000 and 2014, 20 569 patients were hospitalized for AMI in the ARIC Study Community Surveillance. The study population selection flow chart is shown in Figure S1. Of the entire sample, 742 (3.6%) lacked SMuRFs documented before or during the AMI hospitalization. Relative to those with SMuRFs, patients without SMuRFs were more often White (75% versus 63%) and female (48% versus 37%), but the mean age was 60 years for both groups (Table). Compared with individuals with 1 or more SMuRFs, patients without SMuRFs were less likely to present with chest pain (62% versus 78%) or ST‐segment–elevation (9% versus 15%) but more often presented with ventricular fibrillation (16% versus 7%). Furthermore, a slightly higher proportion of patients without SMuRFs had onset of acute symptoms after hospitalization (6% versus 4%), compared with those with SMuRFs. Additionally, patients without SMuRFs were less likely to have a prior history of myocardial infarction (16% versus 31%), stroke (3% versus 11%), or kidney disease (33% versus 41%) as compared with those with 1 or more SMuRFs. Pregnancy‐related AMI, which may be a cause of spontaneous coronary artery dissection in the absence of standard modifiable risk factors, was uncommon both for SMuRFless (0.2%) and SMuRF (3%) presentations.
Table .
Demographics and Clinical Characteristics of Patients Hospitalized With Acute Myocardial Infarction, Presenting With and Without Standard Modifiable Risk Factors. The Community Surveillance Component of the Atherosclerosis Risk in Communities Study, 2000 to 2014
| Characteristic | SMuRFless | SMuRF | P value |
|---|---|---|---|
| N=742 | N=19 827 | ||
| Demographics | |||
| Age, y (mean ± SD) | 60 ± 18 | 60 ± 14 | 0.8 |
| Female sex | 354 (48%) | 7268 (37%) | 0.008 |
| White race | 554 (75%) | 12 463 (63%) | 0.004 |
| Presentation | |||
| ST‐elevation | 66 (9%) | 2889 (15%) | 0.005 |
| Chest pain | 462 (62%) | 15 405 (78%) | <0.0001 |
| Acute pulmonary edema/heart failure | 251 (34%) | 5737 (29%) | 0.2 |
| Cardiogenic shock | 29 (4%) | 648 (3%) | 0.2 |
| Ventricular fibrillation/arrest | 116 (16%) | 1388 (7%) | 0.001 |
| Chest pain onset after arrival | 45 (6%) | 847 (4%) | 0.006 |
| Pregnancy related hospitalization | 1 (0.2%) | 7 (3%) | 0.1 |
| Medical history | |||
| Current smoking | 0 | 7768 (39%) | … |
| Hypertension | 0 | 15 559 (78%) | … |
| Diabetes | 0 | 8255 (42%) | … |
| Hypercholesterolemia (statin therapy) | 0 | 14 874 (75%) | … |
| Chronic kidney disease* | 178 (33%) | 6188 (41%) | 0.1 |
| Stroke/transient ischemic attack | 24 (3%) | 2246 (11%) | <0.0001 |
| Prior myocardial infarction | 121 (16%) | 6031 (31%) | 0.0002 |
| Prior revascularization | 96 (13%) | 6398 (32%) | <0.0001 |
Chronic kidney disease missing for 5066 because serum creatinine not routinely abstracted before 2004 and not assayed for all patients hospitalized with acute myocardial infarction.
SMuRF indicates standard modifiable cardiovascular risk factors; and SMuRFless, patients lacking all 4 standard modifiable risk factors.
Administration of Acute Myocardial Infarction Therapies
When aggregated across 2000 to 2014, SMuRFless patients were less likely to be administered aspirin (70% versus 89%; P<0.0001), nonaspirin antiplatelet therapy (24% versus 58%; P<0.0001), or beta blockers (69% versus 87%; P<0.0001) during the hospitalization, and less often underwent invasive angiography (40% versus 58%; P<0.0001) or coronary revascularization (16% versus 44%; P<0.0001) compared with those with 1 or more risk factors. The differential prescription of AMI therapies persisted after adjustment for race, age, sex, geographic region, and year of admission. With adjustments, patients without SMuRFs had a 63% lower probability of receiving nonaspirin antiplatelet therapy (RR, 0.37 [95% CI, 0.29–0.47]), 24% lower probability of receiving aspirin (RR, 0.76 [95% CI, 0.64–0.85]), 22% lower probability of receiving beta blockers (RR, 0.78 [95% CI, 0.67–0.87]), 38% lower probability of undergoing invasive angiography (RR, 0.62 [95% CI, 0.49–0.76]), and 67% lower probability of coronary revascularization interventions (RR, 0.33 [95% CI, 0.24–0.44]).
Temporal Trends in Acute Myocardial Infarction Therapies
When examined across 5‐year intervals (2000–2004, 2005–2009, 2010–2014), patients without SMuRFs had a dramatic decline in the receipt of nonaspirin antiplatelet therapy (44% to 19% to 10%; P for trend<0.0001), along with a temporal decline in invasive angiography (55% to 29% to 36%; P for trend=0.05) and revascularization procedures (36% to 9% to 5%; P for trend<0.0001), Figure 1. A similar declining trend was also observed among patients with 1 or more SMuRFs. However, on comparison, patients presenting with AMI in the absence of SMuRFs had a steeper temporal decline in the administration of antiplatelet therapies (P‐interaction <0.0001) or undergoing coronary revascularization (P‐interaction <0.0001) relative to those with 1 or more SMuRFs.
Figure 1. Temporal trends in administration of therapies to patients hospitalized with acute myocardial infarction, presenting with and without standard modifiable risk factors.
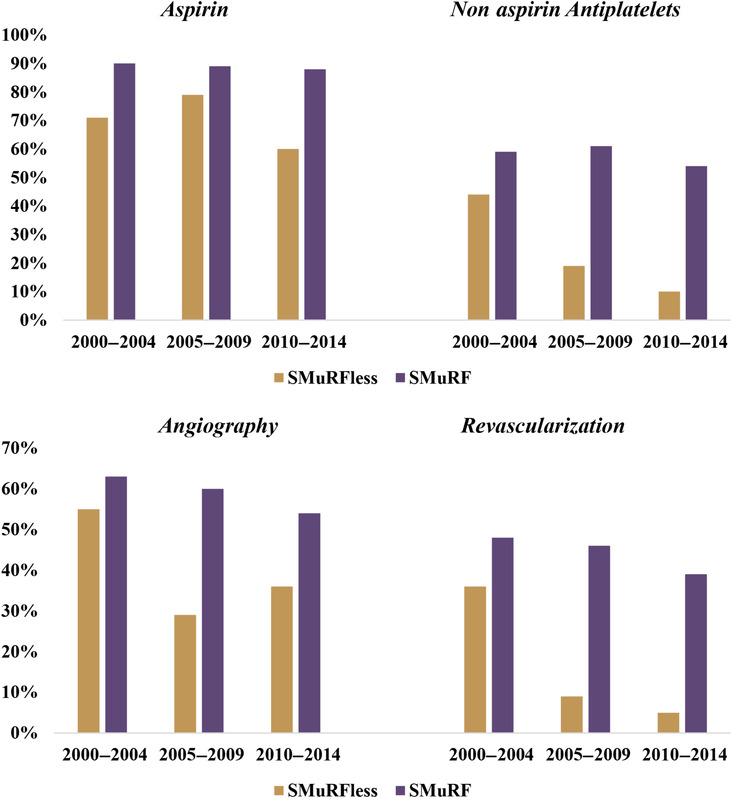
Nonaspirin antiplatelets include P2Y12 inhibitors (cangrelor, clopidogrel, prasugrel, ticagrelor, ticlopidine), glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, tirofiban), phosphodiesterase 3 inhibitors (cilostazol), phosphodiesterase 5 inhibitors (dipyridamole), and protease‐activated receptor‐1 antagonists (vorapaxar). Revascularization includes percutaneous coronary intervention and coronary artery bypass graft. SMuRF indicates standard modifiable cardiovascular risk factors; and SMuRFless, patients lacking all 4 standard modifiable risk factors.
Temporal Trends in Standard Modifiable Risk Factors
Among the subset (N=19 827) of patients hospitalized across 2000 to 2014 with 1 or more SMuRFs, the most common comorbidity was hypertension (78%), followed by hypercholesterolemia (75%), diabetes (42%), and current smoking (39%). When examined over time, there was a significant increase in the annual prevalence of diabetes (37% in 2000 to 45% in 2014; P for trend<0.0001), hypertension (74% to 83%; P for trend<0.0001), and hypercholesterolemia (60% to 79%; P for trend<0.0001), Figure 2. However, the annual proportion of patients who were current smokers did not appreciably change over time.
Figure 2. Annual trends in comorbidities among patients hospitalized with acute myocardial infarction and presenting with 1 or more standard modifiable risk factor.

Demographic Trends in Standard Modifiable Risk Factors
When stratified by race and sex groups, the annual prevalence of hypertension steadily increased from 2000 to 2014 for Black women (87% in 2000 to 93% in 2014; P for trend=0.01) but not for White women. However, there was an increasing trend in the annual prevalence of hypercholesterolemia for both Black women (43% to 73%; P for trend=0.009) and White women (63% to 84%; P for trend<0.0001) from 2000 to 2014. Among men, there was a significant increase in the annual prevalence of diabetes for both Black men (33% to 43%; P for trend=0.004) and White men (34% to 37%; P for trend=0.005). Similarly, the annual prevalence of hypercholesterolemia increased for Black men (48% to 74%; P for trend=0.0001) and White men (67% to 85%; P for trend<0.0001) across 2000 to 2014. The annual prevalence of hypertension increased only among White men. The annual prevalence of current smoking significantly decreased among Black men (54% to 40%; P for trend=0.05) and Black women (56% to 22%; P for trend=0.04) whereas no change was observed among the White men or women.
Mortality Outcomes
Overall, there were 1229 all‐cause deaths within 28 days of AMI hospitalization and 2671 deaths within 1 year. Compared with those with 1 or more SMuRFs, patients without SMuRFs had a significantly higher 28‐day (16% versus 6%; P<0.0001) and 1‐year (24% versus 13%; P=0.0006) mortality, Figure 3. After adjustment for age, race, sex, year of admission, and geographic region, SMuRFless presentation was associated with an approximately 3.5 times higher likelihood of 28‐day mortality (OR, 3.57 [95% CI, 2.06–6.18]) relative to SMuRF. The higher likelihood of 28‐day mortality for patients without SMuRFs persisted after additional adjustments for chronic kidney disease, history of transient ischemic attack/stroke, and therapies (OR, 3.23 [95% CI, 1.78–5.88]). When considering long‐term outcomes, SMuRFless presentation was associated with twice the demographic‐adjusted 1‐year mortality (hazard ratio [HR], 2.32 [95% CI, 1.54–3.51]) relative to a SMuRF presentation; an association that persisted after adjusting for chronic kidney disease, transient ischemic attack/stroke, and therapies (HR, 2.09 [95% CI, 1.29–3.37]).
Figure 3. Twenty‐eight‐day and 1‐year survival for patients hospitalized with acute myocardial infarction, presenting with and without standard modifiable risk factors.

SMuRF indicates standard modifiable cardiovascular risk factors; and SMuRFless, patients lacking all 4 standard modifiable risk factors.
When examined across 5‐year intervals (2000–2004, 2005–2009, 2010–2014), the incidence of 28‐day mortality significantly increased over time for patients without SMuRFs (7% to 15% to 27%; P for trend=0.007) whereas it declined for patients with 1 or more SMuRFs (7% to 5% to 5%; P for trend=0.003). The 1‐year mortality was also observed to increase over time for patients with a SMuRFless presentation (17% to 25% to 29%), but the rate of increase was less gradual and did not attain significance (P for trend=0.2), Figure 4.
Figure 4. Temporal trends in 28‐day and 1‐year mortality for patients hospitalized with acute myocardial infarction, presenting with and without standard modifiable risk factors.

SMuRF indicates standard modifiable cardiovascular risk factors; and SMuRFless, patients lacking all 4 standard modifiable risk factors.
DISCUSSION
This analysis from the ARIC study community surveillance highlights the importance of a subgroup of patients who, despite having no traditional risk factors, present with AMI. We observed that (1) of the total AMI hospitalizations, 3.6% of patients with AMI had no documented SMuRFs before or during hospitalization; (2) during hospitalization, patients without SMuRFs had a lower rate of administration of guideline‐directed medical therapy (GDMT) such as aspirin, antiplatelets, and beta blockers and less often underwent angiography and revascularization than those with 1 or more SMuRFs; (3) additionally, when analyzed across 2000 to 2014, patients without 1 or more SMuRFs had a significantly greater decline in receiving antiplatelet therapy as well as undergoing angiography or revascularization; (4) importantly, patients in the SMuRFless group had greater rates of 28‐day and 1‐year mortality as compared with those with 1 or more SMuRFs; and (5) when examined across 2000 to 2014, there was a significant increasing temporal trend in the rates of 28‐day mortality in the population without SMuRFs, whereas we observed a declining short‐term mortality rate in patients with 1 or more SMuRFs.
In our study, the proportion of patients without SMuRFs hospitalized with AMI was ~4%, which is lower than previous reports. 10 , 16 , 17 Some of these discrepancies may be due to differences in study populations. For example, the YOUNG‐MI registry, which was limited to patients <50 years old who presented with a type 1 AMI reported 17% lacking SMuRFs. 18 Another single‐center retrospective study reported an increase in the proportion of SMuRFless patients without SMuRFs with STEMI from 11% in 2006 to 26% in 2014. 16 Similarly, a large Australian registry reported an increasing trend in patients without SMuRFs with STEMI from 14% in 1999 to 23% in 2017. 10 Aside from differences in study populations, the lower proportion in our study could also be due to detailed clinical phenotyping during hospitalization as well as the in‐hospital abstraction of medical records by trained physicians specific to the ARIC study community surveillance.
One of the key findings of our study is the greater rate of short‐ and long‐term mortality observed in the patients without SMuRFs hospitalized with AMI compared with those with 1 or more SMuRFs. Consistent with our findings, studies from the Australian and Canadian registries have also reported a higher in‐hospital mortality rate in the patients without SMuRFs presenting with STEMI. 10 , 12 Similarly, a retrospective analysis in the SWEDEHEART (the Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) registry also observed a significantly higher 30‐day‐all‐cause mortality in patients without SMuRFs presenting with STEMI. 9 On the contrary, a retrospective analysis in patients with non–STEMI observed a lower in‐hospital all‐cause as well as cardiac mortality in patients without SMuRFs, relative to patients with 1 or more SMuRFs. 19 , 20 However, previous studies have specifically analyzed either STEMI or non–STEMI, which makes it difficult to generalize these findings in the overall acute coronary syndrome. Our study is the first to include a large surveillance of diverse patients presenting with AMI, irrespective of ST‐segment presentation.
Several potential factors could contribute to the increased mortality in our population of patients without SMuRFs hospitalized with AMI. We observed a lower prescription of GDMT (eg, aspirin, nonaspirin antiplatelets, and beta blockers) for patients without SMuRFs, which is consistent with reports from the Swedish myocardial infarction registry. 9 This suboptimal secondary prevention method could be one of the contributing factors for a greater rate of mortality in this population. In contrast, prescription of evidence‐based medical therapy was more closely adhered to for individuals with 1 or more SMuRFs, consistent with studies in recent years. 10 , 21 On the other hand, arrhythmia could also be a likely cause of increased mortality in this population. It is plausible that the suboptimal prescription rates of GDMT such as beta blockers could be one of the potential causes of arrhythmia in these patients, considering the beneficial effects of beta blockers in reducing mortality in patients with AMI. 22 , 23 Additionally, our survival analysis shows an early separation of cumulative mortality events. It is thus conceivable that this early separation could be largely contributed by arrhythmia, which is known to be frequent in early phases after STEMI. 24 These findings reiterate the significance of initiating GDMT in the early post‐AMI phase to prevent mortality, irrespective of the risk factor status. Alternatively, the worse prognosis of patients without SMuRFs could also be attributed to the differential prevalence of the AMI types, although we were unable to differentiate type 1 from type 2 MI in this study.
To date, there has been a lack of attention toward the application of noninvasive imaging to detect subclinical vascular disease in low‐risk patients. In our study, only 40% of the patients without SMuRFs underwent angiography on hospitalization. A recent study reported evidence of atherosclerosis in 50% of the adults without SMuRFs. 25 Another study performed in asymptomatic individuals with no SMuRFs demonstrated evidence of coronary artery calcification in 32% of individuals, out of whom 12% had moderate or severe coronary artery calcification. 26 Although standard guidelines do not recommend screening with coronary artery calcium or computed tomography angiography in patients with low‐risk scores, these findings warrant a greater emphasis on modifying existing imaging guidelines to include patients with low overall risk.
Furthermore, our study findings contradict the general presumption that AMI could be prevented to a large extent by adequately managing modifiable risk factors. Although it is imperative to identify and address the high burden of atherosclerotic risk factors, our study emphasizes the need to unravel new biological mechanisms responsible for the underlying disease in the population without SMuRFs, as well as discover novel biomarkers that could effectively identify individuals at high risk of atherosclerotic cardiovascular events in their early phase of life. 27 , 28 Furthermore, several large‐scale genome‐wide association studies have demonstrated that the majority of genetic loci associated with coronary artery disease are not associated with traditional risk factors. Out of the 55 loci associated with coronary artery disease, more that 66% are not associated with traditional risk factors 29 ; this points toward the significant contribution of several undiscovered disease pathways leading to coronary artery disease. Notably, previously validated polygenic risk scores could be effectively used to stratify a patient's risk largely independent of the individual's SMuRFs and improve risk prediction over traditional risk scores. 30 Such approaches could promote early identification of the individuals at risk without SMuRFs and provide opportunities for strategic intervention through lifestyle modifications and pharmacotherapies.
Our study has some limitations that warrant discussion. The ARIC Community Surveillance Study is localized to 4 US communities, so it may not be generalizable to the total population in the United States. Trends in risk factors may be influenced by temporal changes in diagnostic testing and documentation over time. Substance use, such as amphetamines, cocaine, and marijuana, 17 , 31 and the use of prescription stimulants was not abstracted from the medical record. Potential risk factors such as air pollution and vaping as well as polygenic risk scores were not considered due to unavailability. Classifying hypertension or hypercholesterolemia based on the documented diagnoses by the treating physician might have been limited by underdiagnosis. Additionally, insufficient data were abstracted to differentiate type 1 from type 2 MI, which warrants further analysis. Although a higher prevalence of arrhythmia was observed in patients without SMuRFs, the ARIC study did not abstract ejection fraction at discharge. Despite these limitations, our study also has several noteworthy strengths. The ARIC study provides a large, multiyear surveillance of 4 geographically diverse US communities, promoting generalizability of the results and allowing an analysis of contemporary trends that spans several decades. Certified abstractors meticulously collected clinical and laboratory values following standardized well‐defined protocols. AMI was classified and adjudicated by physician review of the medical records and mortality outcomes verified by the National Death Index.
Conclusions
In conclusion, the patients without SMuRFs hospitalized with AMI have a lower GDMT prescription rate and worse prognosis than patients with at least 1 SMuRF. Targeted interventions and prevention strategies could help improve the prognosis of patients hospitalized with AMI with a SMuRFless presentation.
Sources of Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I).
Disclosures
Dr Bhatt discloses the following relationships—advisory board: Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; board of directors: Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; chair: Inaugural Chair, American Heart Association Quality Oversight Committee; consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique‐Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY‐DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the National Institutes of Health‐funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol‐Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co‐Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol‐Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald's Heart Disease); Site Co‐Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda. Ron Blankstein has received research support for Amgen Inc, Novartis Inc, Beren Inc. Dr. Qamar has received research support from the NorthShore Auxiliary Research Scholar fund, NorthShore CardioDiabetes Pilot Grant, Novo Nordisk, Idorsia Pharmaceuticals, and Novartis. The remaining authors have no disclosures to report.
Supporting information
Figure S1
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
This article was sent to Daniel Edmundowicz, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027851
For Sources of Funding and Disclosures, see page 9 and 10.
References
- 1. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J III. Factors of risk in the development of coronary heart disease—six year follow‐up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33 [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds risk score. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 3. Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 4. Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47:4–24. doi: 10.2105/ajph.47.4_pt_2.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piironen M, Ukkola O, Huikuri H, Havulinna AS, Koukkunen H, Mustonen J, Ketonen M, Lehto S, Airaksinen J, Antero Kesäniemi Y, et al. Trends in long‐term prognosis after acute coronary syndrome. Eur J Prev Cardiol. 2017;24:274–280. doi: 10.1177/2047487316679522 [DOI] [PubMed] [Google Scholar]
- 6. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/s0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and Other Societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Figtree GA, Vernon ST. Coronary artery disease patients without standard modifiable risk factors (SMuRFs)‐a forgotten group calling out for new discoveries. Cardiovasc Res. 2021;117:e76–e78. doi: 10.1093/cvr/cvab145 [DOI] [PubMed] [Google Scholar]
- 9. Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, Delatour V, Leósdóttir M, Hagström E. Mortality in STEMI patients without standard modifiable risk factors: a sex‐disaggregated analysis of SWEDEHEART registry data. Lancet. 2021;397:1085–1094. doi: 10.1016/s0140-6736(21)00272-5 [DOI] [PubMed] [Google Scholar]
- 10. Vernon ST, Coffey S, D'Souza M, Chow CK, Kilian J, Hyun K, Shaw JA, Adams M, Roberts‐Thomson P, Brieger D, et al. ST‐segment‐elevation myocardial infarction (STEMI) patients without standard modifiable cardiovascular risk factors‐how common are they, and what are their outcomes? J Am Heart Assoc. 2019;8:e013296. doi: 10.1161/jaha.119.013296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV Jr, Ornato JP, Zalenski RJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–2127. doi: 10.1001/jama.2011.1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang JY, Goodman SG, Saltzman I, Wong GC, Huynh T, Dery JP, Leiter LA, Bhatt DL, Welsh RC, Spencer FA, et al. Cardiovascular risk factors and In‐hospital mortality in acute coronary syndromes: insights from the Canadian Global Registry of Acute Coronary Events. Can J Cardiol. 2015;31:1455–1461. doi: 10.1016/j.cjca.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 13. Wright JD, Folsom AR, Coresh J, Sharrett AR, Couper D, Wagenknecht LE, Mosley TH, Ballantyne CM, Boerwinkle EA, Rosamond WD, et al. The ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2021;77:2939–2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arora S, Stouffer GA, Kucharska‐Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL, et al. Twenty year trends and sex differences in Young adults hospitalized with acute myocardial infarction. Circulation. 2019;139:1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/circheartfailure.111.963199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vernon ST, Coffey S, Bhindi R, Soo Hoo SY, Nelson GI, Ward MR, Hansen PS, Asrress KN, Chow CK, Celermajer DS, et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur J Prev Cardiol. 2017;24:1824–1830. doi: 10.1177/2047487317720287 [DOI] [PubMed] [Google Scholar]
- 17. DeFilippis EM, Bajaj NS, Singh A, Malloy R, Givertz MM, Blankstein R, Bhatt DL, Vaduganathan M. Marijuana use in patients with cardiovascular disease. J Am Coll Cardiol. 2020;75:320–332. doi: 10.1016/j.jacc.2019.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh A, Collins Bradley L, Gupta A, Fatima A, Qamar A, Biery D, Baez J, Cawley M, Klein J, Hainer J, et al. Cardiovascular risk and statin eligibility of Young adults after an MI. J Am Coll Cardiol. 2018;71:292–302. doi: 10.1016/j.jacc.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moledina SM, Rashid M, Nolan J, Nakao K, Sun LY, Velagapudi P, Wilton SB, Volgman AS, Gale CP, Mamas MA. Addressing disparities of care in non‐ST‐segment elevation myocardial infarction patients without standard modifiable risk factors: insights from a nationwide cohort study. Eur J Prev Cardiol. 2021;29:zwab200. doi: 10.1093/eurjpc/zwab200 [DOI] [PubMed] [Google Scholar]
- 20. Parwani P, Bhatt DL. In NSTEMI, are patients without SMuRFs real? Eur J Prev Cardiol. 2022;29:1081–1083. doi: 10.1093/eurjpc/zwab210 [DOI] [PubMed] [Google Scholar]
- 21. Fraticelli L, Kleitz O, Claustre C, Eydoux N, Peiretti A, Tazarourte K, Bonnefoy‐Cudraz E, Dussart C, El Khoury C. Comparison of the pathways of care and life courses between first‐time ST‐elevation myocardial infarction (STEMI) and STEMI with prior MI: findings from the OSCAR registry. BMJ Open. 2020;10:e038773. doi: 10.1136/bmjopen-2020-038773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersson C, Shilane D, Go AS, Chang TI, Kazi D, Solomon MD, Boothroyd DB, Hlatky MA. β‐Blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol. 2014;64:247–252. doi: 10.1016/j.jacc.2014.04.042 [DOI] [PubMed] [Google Scholar]
- 23. Bugiardini R, Cenko E, Ricci B, Vasiljevic Z, Dorobantu M, Kedev S, Vavlukis M, Kalpak O, Puddu PE, Gustiene O, et al. Comparison of early versus delayed Oral β blockers in acute coronary syndromes and effect on outcomes. Am J Cardiol. 2016;117:760–767. doi: 10.1016/j.amjcard.2015.11.059 [DOI] [PubMed] [Google Scholar]
- 24. Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, Harsanyi A, Rouleau JL, Maggioni A, Kober L, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938 [DOI] [PubMed] [Google Scholar]
- 25. Fernández‐Friera L, Fuster V, López‐Melgar B, Oliva B, García‐Ruiz JM, Mendiguren J, Bueno H, Pocock S, Ibáñez B, Fernández‐Ortiz A, et al. Normal LDL‐cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol. 2017;70:2979–2991. doi: 10.1016/j.jacc.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 26. Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT, Agatston A, Blumenthal RS, Nasir K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the multi‐ethnic study of atherosclerosis. Eur Heart J. 2014;35:2232–2241. doi: 10.1093/eurheartj/eht508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kott KA, Vernon ST, Hansen T, Yu C, Bubb KJ, Coffey S, Sullivan D, Yang J, O'Sullivan J, Chow C, et al. Biobanking for discovery of novel cardiovascular biomarkers using imaging‐quantified disease burden: protocol for the longitudinal, prospective, BioHEART‐CT cohort study. BMJ Open. 2019;9:e028649. doi: 10.1136/bmjopen-2018-028649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vernon ST, Hansen T, Kott KA, Yang JY, O'Sullivan JF, Figtree GA. Utilizing state‐of‐the‐art "omics" technology and bioinformatics to identify new biological mechanisms and biomarkers for coronary artery disease. Microcirculation. 2019;26:e12488. doi: 10.1111/micc.12488 [DOI] [PubMed] [Google Scholar]
- 29. Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al. Large‐scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 2018;72:1883–1893. doi: 10.1016/j.jacc.2018.07.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeFilippis EM, Singh A, Divakaran S, Gupta A, Collins BL, Biery D, Qamar A, Fatima A, Ramsis M, Pipilas D, et al. Cocaine and marijuana use among Young adults with myocardial infarction. J Am Coll Cardiol. 2018;71:2540–2551. doi: 10.1016/j.jacc.2018.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


