Abstract
Background
Guidelines recommend using multiple drugs in patients with heart failure (HF) with reduced ejection fraction, but there is a paucity of real‐world data on the simultaneous initiation of the 4 pharmacological pillars at discharge after a decompensation event.
Methods and Results
A retrospective data mart, including patients diagnosed with HF, was implemented. Consecutively admitted patients with HF with reduced ejection fraction were selected through an automated approach and categorized according to the number/type of treatments prescribed at discharge. The prevalence of contraindications and cautions for HF with reduced ejection fraction treatments was systematically assessed. Logistic regression models were fitted to assess predictors of the number of treatments (≥2 versus <2 drugs) prescribed and the risk of rehospitalization.
A population of 305 patients with a first episode of HF hospitalization and a diagnosis of HF with reduced ejection fraction (ejection fraction, <40%) was selected. At discharge, 49.2% received 2 current recommended drugs, β‐blockers were prescribed in 93.4%, while a renin‐angiotensin system inhibitor or an angiotensin receptor–neprilysin inhibitor was prescribed in 68.2%. A mineralocorticoid receptor antagonist was prescribed in 32.5%, although none of the patients showed contraindications to mineralocorticoid receptor antagonist prescription. A sodium‐glucose cotransporter 2 inhibitor could be prescribed in 71.1% of patients. On the basis of current recommendations, 46.2% could receive the 4 foundational drugs at discharge. Renal dysfunction was associated with <2 foundational drugs prescribed. After adjusting for age and renal function, use of ≥2 drugs was associated with lower risk of rehospitalization during the 30 days after discharge.
Conclusions
A quadruple therapy could be directly implementable at discharge, potentially providing prognostic advantages. Renal dysfunction was the main prevalent condition limiting this approach.
Keywords: 4 pillars, cautions, comprehensive therapy, contraindications, heart failure with reduced ejection fraction
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- ARNi
angiotensin receptor–neprilysin inhibitor
- ESC
European Society of Cardiology
- HFrEF
heart failure with reduced ejection fraction
- MRA
mineralocorticoid receptor antagonist
- SGLT2i
sodium‐glucose cotransporter 2 inhibitor
Clinical Perspective.
What Is New?
Discharge from an acute decompensation event offers a safe and valuable opportunity for the simultaneous initiation of the 4 foundational treatments in heart failure with reduced ejection fraction.
What Are the Clinical Implications?
Simultaneous initiation of 4 treatments may have an impact on reducing rehospitalization within the vulnerable phase after discharge, prompting ad‐hoc pragmatic trials testing this strategy.
Data‐driven methods can support the implementation of evidence‐based recommendations in heart failure.
On the basis of several landmark randomized clinical trials, current guidelines recommend that patients with heart failure with reduced ejection fraction (HFrEF) should receive a quadruple therapy with an angiotensin‐converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB) or an angiotensin receptor–neprilysin inhibitor (ARNi), a β‐blocker, a mineralocorticoid receptor antagonist (MRA), and a sodium‐glucose cotransporter 2 inhibitor (SGLT2i). 1 A conventional approach has been sequencing the initiation of drugs following the historical order of their development, adding one medication to the other once up titration to target dose for each therapy is completed. This approach is time requiring and may delay or even hamper the achievement of a comprehensive combination with all the disease‐modifying therapies available in HFrEF.
There is emerging consensus that rapid sequencing or simultaneous initiation of the 4 pharmacological pillars confers higher protection to patients. 2 , 3 , 4 , 5 Indeed, these drugs show independent and additive benefits and may mutually improve tolerance of each other. 2 Nonetheless, implementation of guidelines' recommendations is limited by common underuse and underdosing of effective therapies, a phenomenon known as “clinical inertia,” because of concern that starting 4 treatments simultaneously may expose patients to higher risk of adverse effects. 6
More important, randomized clinical trials and subsequent real‐world observational studies mainly focused on the stable outpatient setting, whereas there is paucity of data on initiating the 4 pharmacological pillars of HFrEF during hospitalization or at discharge. 7
Studies show that the 30 days after an episode of worsening heart failure (HF) represent a highly vulnerable phase. 8 Because the “4 pillars” showed meaningful benefits within almost 4 weeks after initiation, providing patients with an early and comprehensive protection is key to improve clinical outcomes.
In our retrospective observational study, we perform a cross‐sectional assessment of patients with HFrEF at the moment of discharge after an episode of HF hospitalization, to evaluate, as proportions, the prevalence of contraindications and cautions to the use of current recommended therapies, based on the definitions provided by the 2021 European Society of Cardiology (ESC) Guidelines on HF. 1 In addition, we assess the association between contraindications/cautions and the number of drugs prescribed, as well as the association between number of treatments received and the risk of rehospitalization within 30 days.
Methods
Data will be available on request.
Real‐World Evidence Automated Data Extraction Framework: Gemelli Heart Failure Data Mart
Given the intrinsic complexity of HF as a chronic disease (functional impact, risk factors, lifestyle implications, concurrent comorbidities, and concomitant therapies), Policlinico A. Gemelli has designed and implemented a high‐performance technology infrastructure, the “Gemelli Heart Failure Data Mart,” to collect clinical, laboratory, imaging, and on‐site contact data of patients with HF treated at Policlinico A. Gemelli, starting from 2019.
The method of the Gemelli Heart Failure Data Mart has been previously described. 9 It is an evidence‐focused project to develop and maintain this big data repository with a retrospective design, as a result of a collaborative effort among the clinical staff and Gemelli Data Science and Artificial Intelligence Laboratory Generator Real‐World Data. 10 Similarly, our institutional facility has realized other disease‐specific data marts, as previously published elsewhere. 11
The Gemelli Heart Failure Data Mart accommodates heterogeneous data carefully transformed in a standardized format following systematic validation processes. Clinical, laboratory, and imaging notes, in the format of unstructured text, are extracted into structured variables with the application of natural language processing and text mining algorithms, to integrate heterogeneous sources of medical data extracted from a real‐world setup. Clinicians and data scientists collaborated closely for the manual annotation/validation of these data, such as clinical conditions (contraindications or adverse conditions) and comorbidities, to fulfill high data quality standards, carefully defined per study and type of concept (Data S1).
Setting and Cohort Selection
We performed a retrospective observational study on a cohort of consecutive patients with HFrEF discharged from a tertiary referral center university hospital. To focus on a contemporary cohort, based on electronic health records, we identified patients hospitalized with a primary diagnosis of HF (International Classification of Diseases, Ninth Revision [ICD‐9] code 428.*) between January 2019 and December 2021.
When multiple records were present, we selected first registration with HF as a primary diagnosis, assessing the therapeutic approach adopted during the first event.
We excluded patients who died during hospitalization. Cohort selection and subsequent analysis were approved by a local ethics committee.
Variables
We defined contraindications and cautions to ACEi/ARB/ARNi, β‐blockers, MRA, and SGLT2i based on the descriptions provided by the 2021 European Society of Cardiology Guidelines on HF 1 (Table S1).
Overall, 28 variables, including administrative and clinical structured data, were selected from Gemelli Heart Failure Data Mart. Integrated data sources included echocardiogram reports, clinical history, and clinical diaries (day hospital and inpatient visits), clinical notes during outpatient visits, observations, laboratory examinations, emergency visit reports, and discharge letters. We extracted clinical characteristics from notes and discharge letters applying text‐mining algorithms and further combining the results to secondary ICD‐9 diagnosis codes registered during hospitalization. Diseases and comorbidities were assigned according to the latest guidelines and further confirmed and validated following the Joint Commission International standards, because our center is an accredited institution. Similarly, medications, as well as contraindications and cautions to their use, were analyzed applying text‐mining algorithms to discharge letters. The Gemelli Heart Failure Data Mart is compliant to the CODE‐electronic healthcare record framework, 12 and an overview of data extraction, as well as of data quality assurance, is provided with Figure S1 and Data S1, respectively. Briefly, to ensure data accuracy, 2 clinicians (D.R. and A.R.) manually validated data extracted from text for the entire patient cohort. All conditions were actively searched throughout clinical diaries and reports. When multiple values for clinical measures were present, we selected the last available value up to discharge, to reflect patient status when treatments were prescribed. Table S2 provides a detailed description of the variables selected and used in the study.
More important, in the outcome analysis, we consider hypotension and significant renal dysfunction as defined for ACEi/ARB/ARNi prescription.
Statistical Analysis
Patients who met the study selection criteria were divided into 4 groups based on drug type (ACEi/ARB/ARNi, β‐blocker, MRA, and SGLT2i), and into 5 categories based on the number of drugs received at discharge (no pillar, 1 pillar, 2 pillars, 3 pillars, and 4 pillars). Only treatments recommended by the 2016 and 2021 European Society of Cardiology HF Guidelines were considered. 1 , 13
Continuous variables were reported as median and interquartile range (IQR), and categorical variables were reported as counts and proportions (percentages). Baseline characteristics were assessed in the entire cohort and compared across categories of number of drugs prescribed using Kruskal‐Wallis test for continuous variables and Pearson χ2 test for categorical variables.
Unadjusted and adjusted odds ratios (ORs) with 95% CIs were calculated by fitting univariate and multivariate logistic regression models, respectively, to assess the predictors of number of drugs prescribed at discharge (≥2 versus <2 drugs). In addition, we performed a sensitivity analysis focusing on the prescription of ACEi/ARB/ARNi (ie, treatments recommended for all patients with HFrEF by the 2016 ESC Guidelines on HF). 13
We evaluated the association between ≥2 drugs prescribed at discharge with all‐cause 30‐day risk of rehospitalization in our center by unadjusted and adjusted (age and renal function) logistic regression models. For this analysis, elective outpatient visits were not considered.
In multivariate models, missing data were handled by chained equation multiple imputation. Variables included in multiple imputation model were laboratory values given their lower missing rates. Multivariate analysis included demographics (age and sex), clinical variables (heart rate), laboratory values (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide], estimated glomerular filtration rate [eGFR], potassium, and hemoglobin), comorbidities (diabetes), and most prevalent cautions and contraindications (hyperkalemia, significant renal dysfunction, hypotension, and heart block). Moreover, we applied feature selection based on backward feature elimination method, maintaining only significant variables in the final models. Missing rates for baseline characteristics are reported per variable in Table S3, whereas missing values for contraindications/cautions are reported in Table S4.
Statistical analysis was performed using Python version 3.8.12 with main packages: pandas for data processing, scipy for statistical tests, statsmodels for modeling, matplotlib, scikit‐learn for data imputation, and zepid for reporting. In addition, we used SAS for extract, transform, load data extraction and text mining pipelines and doccano application for data validation. P<0.05 was considered statistically significant.
Results
Considering 2019 to 2021 as a reference period for the scope of this retrospective observational study, Gemelli Heart Failure Data Mart included 1951 patients (Figure 1) and was accompanied by a variable set of ≈100 variables captured along inpatient and outpatient visits. The final population consisted of 305 patients with a confirmed HFrEF diagnosis on admission based on an echocardiographic assessment of ejection fraction.
Figure 1. Flowchart of cohort selection.
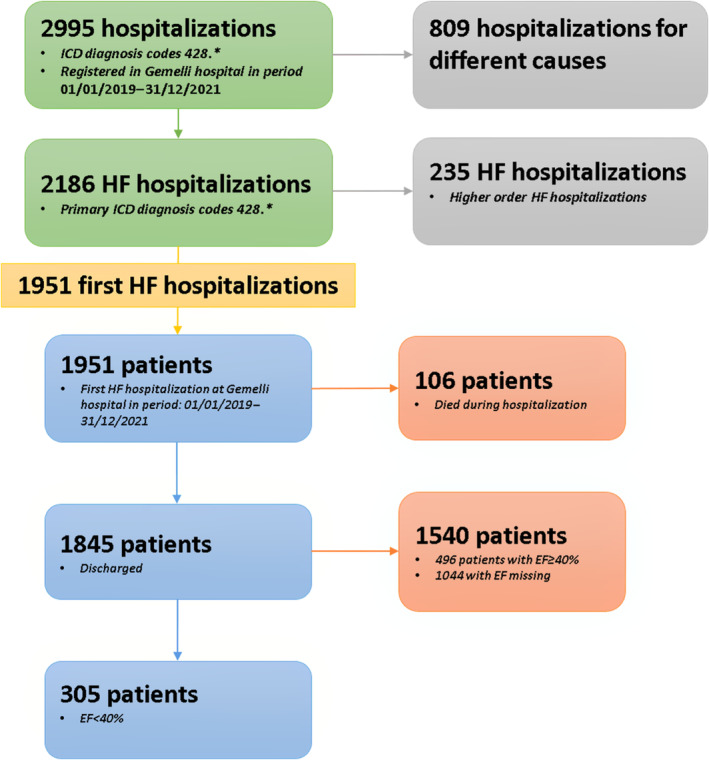
EF indicates ejection fraction; HF, heart failure; and ICD, International Classification of Diseases.
Baseline Characteristics
Baseline characteristics of the overall population are reported in Table S3. The median age of the overall population was 73 years (IQR, 63–81 years), and 74% of patients were men. Overall, 1.6% of patients did not receive any pillar, 25% of patients received only 1 drug, 49.2% of patients were on 2 recommended treatments, 21.6% of patients were prescribed with 3 drugs, and 2.6% of patients were discharged on all 4 pharmacological pillars (Figure 2). At discharge, >90% of patients received a β‐blocker, whereas an ACEi/ARB/ARNi was prescribed in 68.2% of patients and an MRA was prescribed in 32.5% of patients (Figure 3). In 34.4% and 60.0% of patients, an ACEi/ARB/ARNi and a β‐blocker, respectively, were already used at admission (Figure S2). Patients receiving a higher number of drugs were younger and had a higher eGFR, whereas patients using a lower number of drugs presented with higher NT‐proBNP values and worse renal function (Table 1). There were no statistically significant differences in potassium levels among the groups.
Figure 2. Proportion of patients per number of foundational treatments prescribed and prescriptible based on absence of contraindications.
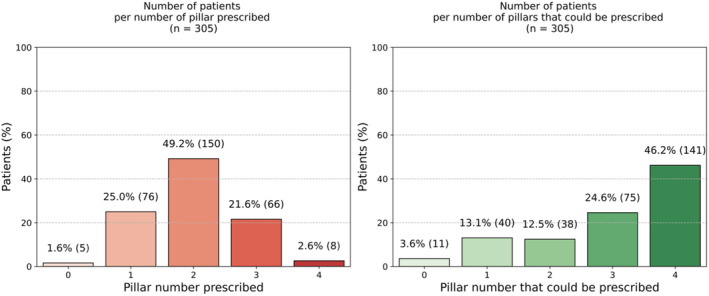
Figure 3. Proportion of patients per drug type.
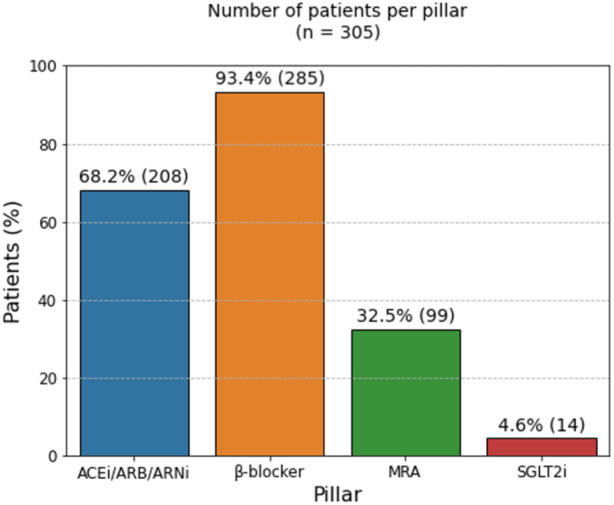
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; and SGLT2I, sodium‐glucose cotransporter 2 inhibitor.
Table 1.
Baseline Characteristics According to the Number of Foundational Drugs Prescribed at Discharge
| Variables | No pillar (n=5 [1.6%]) | 1 Pillar (n=76 [25.0%]) | 2 Pillars (n=150 [49.2%]) | 3 Pillars (n=66 [21.6%]) | 4 Pillars (n=8 [2.6%]) | P value |
|---|---|---|---|---|---|---|
| Demographics/organizational/socioeconomic | ||||||
| Age, median (IQR), y | 80.0 (73.0–81.0) | 75.0 (68.0–81.0) | 72.5 (62.0–79.8) | 70.0 (61.0–80.0) | 74.0 (70.0–79.5) | 0.146 |
| Male sex | 5 (100.0) | 58 (76.3) | 111 (74.0) | 46 (69.7) | 6 (75.0) | 0.625 |
| Education | 0.844 | |||||
| No education | 0 (0.0) | 1 (1.3) | 0 (0.0) | 2 (3.0) | 0 (0.0) | |
| Primary | 0 (0.0) | 10 (13.2) | 19 (12.7) | 10 (15.2) | 1 (12.5) | |
| Secondary | 3 (60.0) | 32 (42.1) | 61 (40.7) | 27 (40.9) | 4 (50.0) | |
| Higher | 1 (20.0) | 12 (15.8) | 15 (10.0) | 5 (7.6) | 1 (12.5) | |
| Married | 3 (60.0) | 46 (60.5) | 79 (52.7) | 35 (53.0) | 3 (37.5) | 0.669 |
| Clinical | ||||||
| NYHA class | 0.732 | |||||
| I | 0 (0.0) | 1 (1.3) | 2 (1.3) | 0 (0.0) | 0 (0.0) | |
| II | 0 (0.0) | 4 (5.3) | 5 (3.3) | 3 (4.5) | 0 (0.0) | |
| III | 0 (0.0) | 9 (11.8) | 20 (13.3) | 8 (12.1) | 2 (25.0) | |
| IV | 1 (20.0) | 5 (6.6) | 4 (2.7) | 4 (6.1) | 0 (0.0) | |
| Heart rate, median (IQR), bpm | 62.0 (49.0–88.0) | 72.0 (66.0–79.8) | 73.0 (65.0–80.0) | 74.0 (69.0–79.6) | 72.0 (70.0–77.0) | 0.869 |
| Atrioventricular block | 0.681 | |||||
| I | 0 (0.0) | 3 (3.9) | 4 (2.7) | 2 (3.0) | 0 (0.0) | |
| II | 0 (0.0) | 1 (1.3) | 1 (0.7) | 2 (3.0) | 1 (12.5) | |
| III | 0 (0.0) | 2 (2.6) | 6 (4.0) | 1 (1.5) | 0 (0.0) | |
| Systolic blood pressure, median (IQR), mm Hg | 110.0 (110.0–120.0) | 116.9 (105.0–130.0) | 115.0 (108.0–120.0) | 115.8 (110.0–130.0) | 132.5 (129.0–140.0) | 0.019* |
| BMI, median (IQR), kg/m2 | 25.9 (22.0–30.9) | 26.2 (24.0–29.1) | 25.3 (23.0–28.5) | 25.7 (24.0–28.7) | 24.7 (22.0–27.0) | 0.637 |
| Laboratory values, median (IQR) | ||||||
| Hemoglobin, g/dL | 11.7 (11.0–12.9) | 12.4 (11.0–13.8) | 12.8 (11.0–15.0) | 13.7 (12.0–15.0) | 13.8 (12.0–14.9) | 0.037* |
| NT‐proBNP, pg/mL | 9146.5 (1542.0–16 798.5) | 8570.0 (3149.0–16 297.5) | 3281.0 (1248.0–6847.0) | 2427.0 (939.0–6347.0) | 7359.0 (5202.0–14 105.5) | 0.000* |
| eGFR, mL/min per 1.73 m2 | 27.2 (22.0–54.5) | 49.8 (28.0–68.4) | 64.9 (49.0–83.6) | 67.4 (48.0–86.4) | 57.2 (36.0–77.4) | 0.000* |
| Potassium, mEq/L | 4.3 (4.0–4.4) | 4.0 (4.0–4.5) | 4.0 (4.0–4.4) | 4.1 (4.0–4.4) | 4.4 (4.0–4.5) | 0.891 |
| History and comorbidities | ||||||
| Diabetes | 3 (60.0) | 26 (34.2) | 35 (23.3) | 23 (34.8) | 6 (75.0) | 0.007* |
| Pulmonary disease | 1 (20.0) | 28 (36.8) | 35 (23.3) | 16 (24.2) | 2 (25.0) | 0.271 |
| Malignant disease | 0 (0.0) | 21 (27.6) | 25 (16.7) | 11 (16.7) | 1 (12.5) | 0.212 |
| Hypertension | 3 (60.0) | 59 (77.6) | 85 (56.7) | 45 (68.2) | 6 (75.0) | 0.031* |
| Hepatic disease | 0 (0.0) | 0 (0.0) | 3 (2.0) | 2 (3.0) | 0 (0.0) | 0.663 |
| Treatments | ||||||
| β‐Blockers | 0 (0.0) | 64 (84.2) | 147 (98.0) | 66 (100.0) | 8 (100.0) | 0.000* |
| ACEi | 0 (0.0) | 5 (6.6) | 22 (14.7) | 13 (19.7) | 2 (25.0) | 0.135 |
| ARB | 0 (0.0) | 0 (0.0) | 24 (16.0) | 12 (18.2) | 1 (12.5) | 0.004* |
| ARNi | 0 (0.0) | 4 (5.3) | 81 (54.0) | 39 (59.1) | 5 (62.5) | 0.000* |
| MRA | 0 (0.0) | 3 (3.9) | 26 (17.3) | 62 (93.9) | 8 (100.0) | 0.000* |
| SGLT2i | 0 (0.0) | 0 (0.0) | 1 (0.7) | 5 (7.6) | 8 (100.0) | 0.000* |
| Diuretics | 4 (80.0) | 67 (88.2) | 129 (86.0) | 65 (98.5) | 8 (100.0) | 0.056 |
| Digoxin | 0 (0.0) | 3 (3.9) | 14 (9.3) | 2 (3.0) | 0 (0.0) | 0.270 |
| Statin | 4 (80.0) | 39 (51.3) | 65 (43.3) | 33 (50.0) | 7 (87.5) | 0.067 |
| Acetylsalicylic acid | 1 (20.0) | 20 (26.3) | 45 (30.0) | 13 (19.7) | 2 (25.0) | 0.622 |
Data are given as number (percentage) unless otherwise indicated. All included variables reported at the date of discharge. ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; bpm, beats per minute; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
P‐value <0.05.
On the basis of the presence/absence of contraindications to recommended drugs, a comprehensive approach with all 4 recommended treatments could be prescribed in 46.2% of patients (Figure 2).
Most patients on a drug combination with 2 drugs used ACEi/ARB/ARNi and a β‐blocker (40.3% of the overall population), whereas patients on a triple therapy with ACEi/ARB/ARNi, β‐blocker, and MRA were 20% (Table 2).
Table 2.
Patterns of Prescription According to Combinations Used and Drug Type and Presence/Absence of Contraindications
| Pillars | Patients (n=305) |
|---|---|
| ACEi/ARB/ARNi+β‐blocker | 123 (40.3) |
| β‐Blocker | 64 (21.0) |
| ACEi/ARB/ARNi+MRA+β‐blocker | 61 (20.0) |
| β‐Blocker+MRA | 23 (7.5) |
| ACEi/ARB/ARNi | 9 (3.0) |
| ACEi/ARB/ARNi+MRA+β‐blocker+SGLT2i | 8 (2.6) |
| ACEi/ARB/ARNi+β‐blocker+SGLT2i | 4 (1.3) |
| MRA | 3 (1.0) |
| ACEi/ARB/ARNi+MRA | 3 (1.0) |
| β‐Blocker+MRA+SGLT2i | 1 (0.3) |
| β‐Blocker+SGLT2i | 1 (0.3) |
| SGLT2i | 0 (0.0) |
| No pillar | 5 (1.7) |
Data are given as number (percentage). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; and SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
Prevalence of Contraindications and Cautions to ACEi/ARB/ARNi Use
In the overall population, 13.8% of patients were on ACEi, 12.1% were on ARB, and 42.3% were on ARNi (Table S3). Among patients using an ACEi/ARB/ARNi, median eGFR was 71.0 mL/min per 1.73 m2 (IQR, 62.0–91.5 mL/min per 1.73 m2), median potassium was 4.1 mEq/L (IQR, 3.7–4.4 mEq/L), and median systolic blood pressure was 116 mm Hg (IQR, 110–125 mm Hg).
On the basis of the presence/absence of contraindications, an ACEi/ARB/ARNi could be prescribed in 98.4% of patients. In the absence of contraindications, it was not prescribed in 31.5% of patients (Table 3).
Table 3.
Patterns of Prescription According to Drug Type and Presence/Absence of Contraindications
| Pillar | Prescribed | Possible to prescribe* | Possible to prescribe but not prescribed* |
|---|---|---|---|
| ACEi/ARB/ARNi | 208 (68.2) | 300 (98.4) | 96 (31.5) |
| β‐Blocker | 285 (93.4) | 284 (93.1) | 18 (5.9) |
| MRA | 99 (32.5) | 305 (100.0) | 206 (67.5) |
| SGLT2i | 14 (4.6) | 230 (75.4) | 217 (71.1) |
Data are given as number (percentage). Total patients (n=305). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; MRA, mineralocorticoid receptor antagonist; and SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
Prescriptibility based on the absence of contraindications.
In the overall population, 13.1% and 11.8% of patients showed significant renal dysfunction and hypotension, respectively (ie, cautions to ACEi/ARB/ARNi treatment) (Figure 4). Significant renal dysfunction was the main prevalent condition in patients not receiving an ACEi/ARB/ARNi (Table S4). Among patients showing significant renal dysfunction, 82.5% of patients did not receive the drug, whereas an ACEi/ARB/ARNi was not prescribed in 55% of patients with hyperkalemia or hypotension.
Figure 4. Prevalence of most frequent contraindications and cautions to life‐saving therapies in heart failure with reduced ejection fraction.
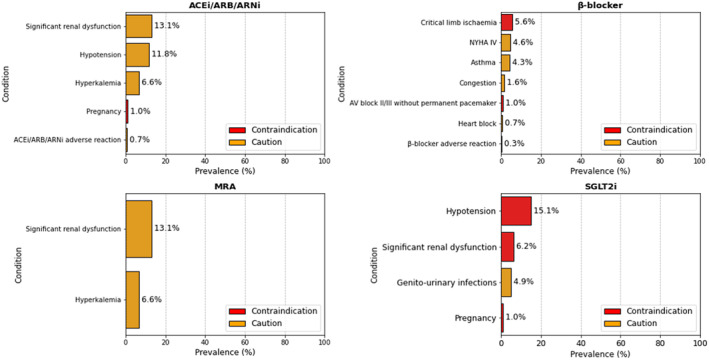
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; AV, atrioventricular; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; and SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
Prevalence of Contraindications and Cautions to β‐Blocker Use
Median eGFR of patients using a β‐blocker was 64.5 mL/min per 1.73 m2 (IQR, 43.8–88.3 mL/min per 1.73 m2), median potassium was 4.1 mEq/L (IQR, 3.7–4.4 mEq/L), and median systolic blood pressure was 155 mm Hg (IQR, 110–125 mm Hg).
On the basis of the presence/absence of contraindications, a β‐blocker could be prescribed in 93.1% of patients. There were 5.9% of patients who did not receive a β‐blocker at discharge, in the absence of contraindications (Table 3).
In the overall population, 5.6% of patients presented with critical limb ischemia (ie, a contraindication to β‐blocker use), whereas 4.6% of patients had New York Heart Association class IV, which is considered a caution for this treatment. Congestion was present in 1.6% of patients (Figure 4).
A β‐blocker was prescribed in 88% of patients with critical limb ischemia, whereas only one patient with New York Heart Association class IV did not receive a β‐blocker at discharge. All patients with congestion were prescribed a β‐blocker, whereas all patients with heart rate <50 beats per minute were not discharged on the drug (Table S4).
Prevalence of Contraindications and Cautions to MRA Use
Median eGFR of patients using an MRA was 72.5 mL/min per 1.73 m2 (IQR, 57.5–89.5 mL/min per 1.73 m2), median potassium was 4.1 mEq/L (IQR, 3.7–4.4 mEq/L), and median systolic blood pressure was 120 mm Hg (IQR, 110–130 mm Hg).
Although no patients showed contraindications to the use of an MRA, it was not prescribed in 67.5% of patients (Table 2).
As cautions to MRA use, significant renal dysfunction and hyperkalemia were present in 13.1% and 6.6% of patients, respectively (Figure 4). Among patients with significant renal dysfunction, an MRA was prescribed in 27.5%. Among patients with hyperkalemia, 25% received the drug at discharge (Table S4).
Prevalence of Contraindications and Cautions to SGLT2i Use
Median eGFR of patients using an SGLT2i was 76.0 mL/min per1.73 m2 (IQR, 71.0–95.0 mL/min per1.73 m2), median potassium was 4.4 mEq/L (IQR, 4.1–4.8 mEq/L), and median systolic blood pressure was 127.5 mm Hg (IQR, 110–134 mm Hg).
Considering the absence of contraindications, an SGLT2i could be prescribed in 75.4% of patients (Table 3).
Hypotension (systolic blood pressure <95 mm Hg or symptomatic hypotension) and significant renal dysfunction (eGFR <20 mL/min per 1.73 m2) were present in 15.1% and 6.2% of patients, respectively. Genitourinary infections were reported in 4.9% of patients (Figure 4).
Predictors of Drug Prescription at Discharge
Significant variables per outcome are reported in Table S5. In the unadjusted analysis, renal insufficiency and higher age were associated with <2 foundational drugs prescribed (OR, [95% CI], 0.14 [0.07–0.29] and 0.97 [0.95–0.99], respectively) (Table 4). After applying backward feature elimination and maintaining only significant variables, renal insufficiency was an independent predictor of <2 prescribed (adjusted OR [95% CI], 0.16 [0.08–0.32]) (Table 4).
Table 4.
Predictors of Number of Foundational Drugs Prescribed
| Predictor | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P value | Baseline, median (IQR) |
|---|---|---|---|---|
| eGFR <30 mL/min per 1.73 m2, yes vs no | 0.14 (0.07–0.29) | 0.16 (0.08–0.32) | 0.000/0.000 | 2 (1–2) |
| Age, per 1‐y increase | 0.97 (0.95–0.99) | 0.98 (0.95–1.00) | 0.006/0.047 | |
| NT‐proBNP, per 100‐pg/mL increase | 0.99 (0.99–1.00) | 0.001 | ||
| Hemoglobin, per 1‐g/dL increase | 1.18 (1.06–1.33) | 0.004 | ||
| eGFR, per 10–mL/min per 1.73 m2 increase | 1.36 (1.21–1.53) | 0.000 |
Drug number ≥2. eGFR indicates estimated glomerular filtration rate; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and OR, odds ratio.
In a sensitivity analysis, renal insufficiency (eGFR <30 mL/min per 1.73m2), hypotension, and higher age were predictors of not receiving an ACEi/ARB/ARNi in both unadjusted and adjusted logistic regression models (Table S6).
Risk of Rehospitalization Within 30 Days According to Pillar Number
Rehospitalization within 30 days occurred in 4.9% of 305 cohort patients. A total of 0.7% of patients received no drug at discharge, 2.6% of patients used 1 drug, and 1.6% of patients were discharged on 2 drugs. No rehospitalization events occurred in patients receiving 3 or 4 treatments at discharge.
On the basis of univariate logistic regression models, use of ≥2 pillars was associated with lower risk of rehospitalization within 30 days in the overall population (unadjusted OR [95% CI], 0.16 [0.05–0.49]), as well as in age and renal function subgroups (Figure 5 and Figure S3).
Figure 5. Risk of rehospitalization within 30 days.
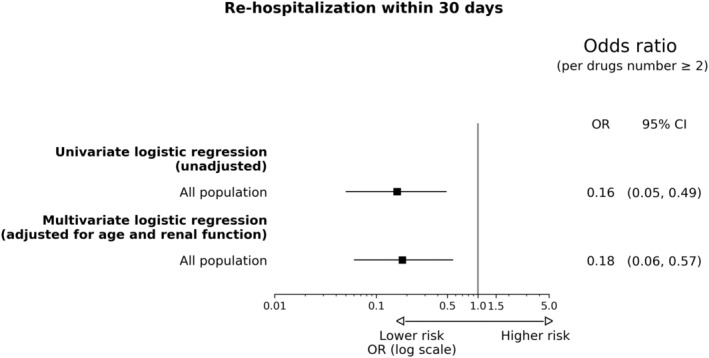
OR indicates odds ratio.
In multivariable analysis, adjusting for age and renal function, patients receiving ≥2 pillars at discharge showed lower risk of 30‐day rehospitalization (adjusted OR [95% CI], 0.18 [0.06–0.57]) (Figure 5).
Discussion
Main Findings
We used a high‐performance technology infrastructure enabling the analysis of heterogeneous data sources transformed in a standardized format following systematic validation processes, in a contemporary cohort of patients with HFrEF at the moment of discharge after an episode of HF hospitalization, thereby showing that:
On the basis of the presence/absence of contraindications, a quadruple therapy could be directly implementable at discharge after a decompensation event;
The use of a higher number of drugs at discharge may be associated with a lower risk of rehospitalization at 30 days.
Our retrospective observational study proves that data‐driven methods can accelerate evidence‐based research and decision support in chronic diseases. Clinicians used the data mart, currently integrated in the research pipeline and clinical practice at our institution, to select a cohort of inpatients with HFrEF during 2019 to 2021, together with a selected set of variables covering different domains (demographics, clinical data, laboratory values, clinical history, comorbidities, and a refined list of treatments).
Implementation of guideline‐directed medical therapy is a major challenge in HFrEF. 4 Underusing and underdosing of recommended treatments are common, and clinicians might be usually concerned that starting 4 treatments simultaneously exposes patients to higher risk of adverse effects. A recent survey found that 84% of the cardiologists participating in the study considered feasible starting all 4 recommended treatments simultaneously. 14 In our study, we explore the feasibility of a comprehensive pharmacological approach in patients discharged after an HF hospitalization event, considering the possibility of a simultaneous initiation of all 4 pillars of HFrEF therapy. On the basis of the absence of contraindications to drugs, this approach could have been implemented in ≈46% of patients.
The 2016 ESC Guidelines on HF recommended using a combination of ACEi/ARB/ARNi and β‐blocker in all patients with HFrEF, adding MRA only in patients still symptomatic after up titration to maximum tolerated doses of the aforementioned drugs. 13 In our cohort, there were 40.3% of patients using a combination with ACEi/ARB/ARNi and β‐blocker. Although those using a β‐blocker were >90% of the overall population, there were ≈30% of patients not receiving an ACEi/ARB/ARNi despite the absence of clear contraindications. SGLT2i could have been prescribed at discharge in ≈70% of patients with HFrEF.
Renal insufficiency was the main predictor of a lower number of drugs prescribed, whereas use of ≥2 medications at discharge suggested lower risk of rehospitalization within 30 days in both unadjusted and adjusted (age and kidney function) analysis. Although being limited to rehospitalization in our center, our outcome analysis is consistent with several meta‐analyses showing that combining treatments is associated with incremental benefits in HFrEF 15 , 16 and with a recent randomized clinical trial testing an intensive treatment strategy after acute HF. 17 Nonetheless, our results should be interpreted cautiously given the potential presence of residual confounders.
Generalizability
The baseline characteristics of this study population share similarities with representative larger cohorts of inpatients with HF at discharge. Median age was 73 years in our study, whereas mean age was 72 years in a study from the CAN‐HF (Canadian Heart Failure Registry). 18 The prevalence of female sex (≈30%) is similar in other contemporary registries, whereas the baseline eGFR is slightly higher in our patients (61.5 versus 54.4 mL/min per 1.73 m2 in CAN‐HF). 18 , 19
When compared with STRONG‐HF, a major clinical trial testing rapid up titration after an acute HF admission, age, male prevalence, and comorbidity burden in our population were higher, with also lower systolic blood pressure at baseline. 17
In aggregate, the characteristics of our population are consistent with real‐world cohorts, but also reflect that stricter inclusion criteria are used in pragmatic trials.
Barriers in ACEi/ARB/ARNi and β‐Blocker Prescription
Hospitalization offers a critical framework to optimize medical therapy in patients with HFrEF, with the possibility of starting all recommended medications in hemodynamically stable and clinically euvolemic patients. Namely, expert consensus documents have pointed out that a β‐blocker should not be started yet in patients showing signs or symptoms of congestion, whereas a renin‐angiotensin system inhibitor is usually better tolerated when patients are not hypovolemic. 20 In our study, the proportion of eligible patient receiving a β‐blocker at discharge is high, with >90% of patients discharged. Previous studies from the OPTIMIZE‐HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure), the GWTG‐HF (Get With The Guidelines–Heart Failure), and the VICTORIA (Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction) registries reported that patients discharged on a β‐blocker were 72.9%, 94.6%, and 75.1%, respectively. 19 , 21 , 22 In our study group, prevalence of congestion was low (<2%), which may justify a high use of β‐blocker at discharge.
In sharp contrast, use of an ACEi/ARB/ARNi was <70% (ie, lower than in the GWTG‐HF registry), with 90.5% of patients receiving an ACEi/ARB, 23 but similar to the proportion observed in the more recent VICTORIA registry, with 65% of patients discharged on ACEi/ARB/ARNi and ≈30% patients without contraindications and not treated, which is consistent to our findings. 19 Other observational studies from Europe reported a prescription rate of ACEi/ARB of around 77%. 24 , 25
In our study, age, hypotension and renal insufficiency were independent predictors of nonuse of an ACEi/ARB/ARNi at discharge. In the GWTG‐HF registry, there were 19.5% of hospitalized patients who discontinued ACEi/ARB and 23.4% who did not start an ACEi/ARB because of renal insufficiency, whereas hypotension was documented in 8.7% of patients. 23 It must be mentioned that patients with advanced age and lower eGFR are less represented in randomized clinical trials and that several studies observed a survival benefit irrespectively of a significant (>30%) elevation in creatinine levels. 26 A careful management of drug's initiation with low doses and later titration to maximum tolerated dosages may increase patients' protection and improve clinical outcomes.
Notably, the PIONEER‐HF (Comparison of Sacubitril‐Valsartan Versus Enalapril on Effect on NT‐proBNP in Patients Stabilized From an Acute Heart Failure Episode) trial and the TRANSITION study support in‐hospital and early‐after discharge initiation of ARNi in patients stabilized after acute HF. 27 , 28 In our single‐center study, 42.3% of patients were on ARNi, whereas there were 2.3% patients discharged on ARNi in the GWTG‐HF registry and 11% in the VICTORIA registry. 19 Our cross‐sectional assessment at discharge prevented us from identifying the specific patterns of ARNi in‐hospital management. However, despite the risk of selection bias present in our study, our findings confirm the feasibility of ARNi use at discharge among a high‐risk real‐world cohort of inpatients with HF.
In‐Hospital Initiation of MRA and SGLT2i
Contemporary registries identified underuse of MRA in patients with HFrEF. 29 , 30 In addition, in‐hospital initiation of MRA has not been extensively explored in previous investigations. The proportion of patients receiving MRA in our study (32.5%) was lower than the proportion reported by the VICTORIA registry (40.5%) and the ESC‐HF Long‐Term Registry (55.3%). 19 , 24 Although MRA underuse may be associated with a perceived higher risk of hyperkalemia and renal function deterioration, previous ESC HF Guidelines recommended starting MRA in patients still symptomatic after up titration of an ACEi/ARB/ARNi and a β‐blocker. Because the latest guidelines recommend using MRA in all patients with HFrEF, it is valuable to observe the absence of absolute contraindications in a group of high‐risk patients, pointing to a greater feasibility of use at discharge. This finding is consistent with an ad‐hoc randomized clinical investigation focusing on MRA use during hospitalization. 31 Also, previous studies showed that MRA use was associated with better outcomes after discharge. 32 , 33
Reports on in‐hospital initiation of SGLT2i are limited. We provide novel data on SGLT2i eligibility in a cohort of high‐risk inpatients with HFrEF, with 71.1% of patients who could receive the drug. This is based on the inclusion criteria of previous randomized clinical trials testing SGLT2i, leading us to define a systolic blood pressure <95 mm Hg and an eGFR <20 mL/min per 1.73 m2 as contraindications to SGLT2i use. These conditions should be considered within the framework of new ongoing investigations, including randomized clinical trials focusing on inpatients, but also in light of the high tolerability profile that SGLT2i showed to date, with negligible effects on blood pressure and no higher risk of symptomatic hypotension or kidney injury. 34 This safety profile, together with no need of up titrating SGLT2i dose, offers an opportunity of implementing SGLT2i treatment at discharge, which is a key time to provide an early and effective protection to patients.
Study Limitations
Although providing important data on a debated topic, our study also has important limitations that should be mentioned.
First, we report a single‐center experience, but there is high heterogeneity in therapeutic approaches across different countries and care providers. Thus, the external validity of our findings must be interpreted accordingly. Also, our institution is a tertiary referral center. A risk of selection bias toward inpatients with worse clinical status could be present. However, our study population may also offer relevant insights on more vulnerable patients (ie, a more challenging situation for prescribing a comprehensive pharmacological therapy at discharge). A note of caution is that patients with worse status may have been more likely to receive more intense therapies, or to have higher risk of rehospitalization in the 30 days after discharge (indication bias), but also patients with better status could have had longer time to get an event (survival bias) or could have been prone to receive more treatments because of their stable conditions and a higher tolerability profile. Although we evaluate a short‐term outcome and the former bias might be more relevant, we cannot rule out that both biases occurred in our study, affecting outcomes in opposite directions.
Censoring for competing events in our outcome analysis was limited by data availability. We were not able to assess vital status of patients after discharge, and hospitalization records were available only from our institution. We cannot exclude that patients have been lost at follow‐up because of hospitalization elsewhere or death during the follow‐up time. Nonetheless, reducing 30‐day rehospitalization by early‐treatment strategies is relevant to avoiding patients' deterioration. 8 Our data prompt further ad‐hoc prospective studies to formally assess that starting a comprehensive therapy with the 4 pharmacological pillars of HFrEF at discharge is a safe and effective strategy to avoid unnecessary delays in therapeutic initiation and to improve clinical outcomes.
When multiple records were present, we selected the first HF hospitalization as baseline event. However, we were not able to discriminate whether these patients were totally naïve for worsening HF or had previously undergone HF visit/hospitalization in other centers. More important, 60% of patients were already treated with a β‐blocker at admission, but the lack of dosing data does not allow us to assess whether these treatments were used for other conditions at risk of HF (eg, hypertension).
Being also a retrospective observational study, despite adjusting for known confounders, we cannot exclude the presence of unknown confounders affecting the association between clinical conditions, drugs' prescription, and risk of rehospitalization.
In addition, data on treatment initiation during hospitalization could not be included, and our analysis is a cross‐sectional assessment at admission and discharge. However, in‐hospital therapy trajectories (ie, switching or de‐escalation of disease‐modifying therapies) are another important factor affecting clinical outcomes, and it is relevant to consider whether treatments are initiated during hospitalization versus after discharge. This is particularly important when considering ARNi use, which has been recommended in patients still symptomatic after using an ACEi or ARB. Of note, 42% of patients were on ARNi, suggesting that these patients may not have been totally naïve for an HF diagnosis. Also, reasons for not prescribing recommended treatments are not routinely reported in discharge letters in our center. Two clinicians carefully validated all clinical diaries to retrieve the presence of adverse reactions or absolute contraindications, and it is unlikely that outstanding major conditions (like angioedema) were not reported, if present. However, given the retrospective design of the study, a standardized form to assess the presence/absence of contraindications and cautions to drugs' use was not used. Thus, missing rates could be estimated only for those conditions based on structured data. For missing laboratory values, we used multiple imputation to reduce bias and increase external validity, but subgroup analyses to explore whether data were missing at random or missing not at random could not be efficiently performed because of limited power for these subgroups, given the low occurrence of missing values. Another point is our focus on simultaneous initiation of drugs, whereas a rapid sequencing strategy might have been implemented in the weeks after discharge.
We were not able to report doses of medications because these data are highly unstructured and heterogeneous in our data sources. However, there is consensus to start with low dose of renin‐angiotensin system inhibitor, ARNi, β‐blockers, and MRA in naïve patients, considering that even low doses are effective. In our center, standard operating procedures entail starting with low dose of multiple medications in patients with HF at discharge. Indeed, using a higher number of drugs might be associated with better outcomes compared with sequential initiation and up titration of single drugs to target dose. Nonetheless, treatments' tolerability and titration to target dose remain key elements of the therapeutic management of patients with HFrEF, and an association between dose and outcomes is present also when therapies are combined. 6 , 35
Last, we have retrospectively evaluated patients by assessing whether they could be eligible to contemporary recommendations, but SGLT2i agents were included in ESC Guidelines on HF only in 2021, and previous ESC Guidelines on HF recommended using MRA only in patients with worse clinical status. 1 , 13 Contraindications/cautions are based on trial inclusion criteria, mainly focusing on stable outpatients, and a comprehensive combination of 4 treatments might not have been the standard background therapy in these studies. Although there is evidence that HF treatments may have synergistic and protective effects, ad hoc studies will help underscore the safety profile of using multiple medications simultaneously.
Conclusions
In a contemporary cohort of inpatients with HFrEF, based on the presence/absence of contraindications to recommended treatments, a quadruple therapy could be started in ≈46% of patients. Renal dysfunction was the main prevalent condition limiting the achievement of a more comprehensive therapeutic approach. Use of a higher number of drugs was associated with lower risk of rehospitalization within 30 days after discharge. Reports from larger cohorts and prospective studies focusing on treatments' initiation strategies in hospitalized patients are required to confirm the generalizability of these findings and to assess tailored approaches with specific patients' profiles.
Sources of Funding
This study received partial funding from the Italian Ministry for University and Research under the Program Piano Operativo Nazionale “Research and Innovation” supporting the development of the artificial intelligence platform “Gemelli Generator” at Policlinico Universitario A. Gemelli IRCCS in Rome (Italy).
Disclosures
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
Supporting information
Data S1
Tables S1–S6
Figures S1–S3
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029071
For Sources of Funding and Disclosures, see page 12 and 13.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure‐optimizing therapy with the need for speed. JAMA Cardiol. 2021;6:743–744. doi: 10.1001/jamacardio.2021.0496 [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJV, Packer M. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence‐based medicine. Circulation. 2021;143:875–877. doi: 10.1161/CIRCULATIONAHA.120.052926 [DOI] [PubMed] [Google Scholar]
- 4. Sharma A, Verma S, Bhatt DL, Connelly KA, Swiggum E, Vaduganathan M, Zieroth S, Butler J. Optimizing foundational therapies in patients with HFrEF: how do we translate these findings into clinical care? JACC Basic Transl Sci. 2022;7:504–517. doi: 10.1016/j.jacbts.2021.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen L, Jhund PS, Docherty KF, Vaduganathan M, Petrie MC, Desai AS, Køber L, Schou M, Packer M, Solomon SD, et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur Heart J. 2022;43:2573–2587. doi: 10.1093/eurheartj/ehac210 [DOI] [PubMed] [Google Scholar]
- 6. D'Amario D, Rodolico D, Rosano GMC, Dahlström U, Crea F, Lund LH, Savarese G. Association between dosing and combination use of medications and outcomes in heart failure with reduced ejection fraction: data from the Swedish Heart Failure Registry. Eur J Heart Fail. 2022;24:871–884. doi: 10.1002/ejhf.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7:1–12. doi: 10.1016/j.jchf.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–229. doi: 10.1038/nrcardio.2015.14 [DOI] [PubMed] [Google Scholar]
- 9. D'Amario D, Laborante R, Delvinioti A, Lenkowicz J, Iacomini C, Masciocchi C, Luraschi A, Damiani A, Rodolico D, Restivo A, et al. GENERATOR HEART FAILURE DataMart: an integrated framework for heart failure research. Front Cardiovasc Med. 2023;10:1104699. doi: 10.3389/fcvm.2023.1104699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damiani A, Masciocchi C, Lenkowicz J, Capocchiano ND, Boldrini L, Tagliaferri L, Cesario A, Sergi P, Marchetti A, Luraschi A, et al. Building an artificial intelligence laboratory based on real world data: the experience of Gemelli Generator. Front Comput Sci. 2021;3:3. doi: 10.3389/fcomp.2021.768266 [DOI] [Google Scholar]
- 11. Murri R, Masciocchi C, Lenkowicz J, Fantoni M, Damiani A, Marchetti A, Sergi PDA, Arcuri G, Cesario A, Patarnello S, et al. A real‐time integrated framework to support clinical decision making for covid‐19 patients. Comput Methods Programs Biomed. 2022;217:106655. doi: 10.1016/j.cmpb.2022.106655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotecha D, Asselbergs FW, Achenbach S, Anker SD, Atar D, Baigent C, Banerjee A, Beger B, Brobert G, Casadei B, et al. CODE‐EHR best practice framework for the use of structured electronic healthcare records in clinical research. Eur Heart J. 2022;43:3578–3588. doi: 10.1093/eurheartj/ehac426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 14. Fauvel C, Bonnet G, Mullens W, Giraldo CIS, Mežnar AZ, Barasa A, Tokmakova M, Shchendrygina A, Costa FM, Mapelli M, et al. Sequencing and titrating approach of therapy in heart failure with reduced ejection fraction following the 2021 European Society of Cardiology guidelines: an international cardiology survey. Eur J Heart Fail. 2023;25:213–222. doi: 10.1002/ejhf.2743 [DOI] [PubMed] [Google Scholar]
- 15. Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD. Estimating lifetime benefits of comprehensive disease‐modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. doi: 10.1016/S0140-6736(20)30748-0 [DOI] [PubMed] [Google Scholar]
- 16. Tromp J, Ouwerkerk W, van Veldhuisen DJ, Hillege HL, Richards AM, van der Meer P, Anand IS, Lam CSP, Voors AA. A systematic review and network meta‐analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10:73–84. doi: 10.1016/j.jchf.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 17. Mebazaa A, Davison B, Chioncel O, Cohen‐Solal A, Diaz R, Filippatos G, Metra M, Ponikowski P, Sliwa K, Voors AA, et al. Safety, tolerability and efficacy of up‐titration of guideline‐directed medical therapies for acute heart failure (STRONG‐HF): a multinational, open‐label, randomised, trial. The Lancet. 2022;400:1938–1952. doi: 10.1016/S0140-6736(22)02076-1 [DOI] [PubMed] [Google Scholar]
- 18. Moghaddam N, Hawkins Nathaniel M, McKelvie R, Poon S, Joncas Sebastien X, MacFadyen J, Honos G, Wang J, Rojas‐Fernandez C, Kok M, et al. Patient eligibility for established and novel guideline‐directed medical therapies after acute heart failure hospitalization. JACC Heart Fail. 2023;11:596–606. doi: 10.1016/j.jchf.2022.10.013 [DOI] [PubMed] [Google Scholar]
- 19. Greene SJ, Ezekowitz JA, Anstrom KJ, Demyanenko V, Givertz MM, Pina IL, O'Connor CM, Koglin J, Roessig L, Hernandez AF, et al. Medical therapy during hospitalization for heart failure with reduced ejection fraction: the VICTORIA registry. J Card Fail. 2022;28:1063–1077. doi: 10.1016/j.cardfail.2022.02.011 [DOI] [PubMed] [Google Scholar]
- 20. Hollenberg SM, Warner Stevenson L, Ahmad T, Amin VJ, Bozkurt B, Butler J, Davis LL, Drazner MH, Kirkpatrick JN, Peterson PN, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74:1966–2011. doi: 10.1016/j.jacc.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 21. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Dosing of beta‐blocker therapy before, during, and after hospitalization for heart failure (from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure). Am J Cardiol. 2008;102:1524–1529. doi: 10.1016/j.amjcard.2008.07.045 [DOI] [PubMed] [Google Scholar]
- 22. Krantz MJ, Ambardekar AV, Kaltenbach L, Hernandez AF, Heidenreich PA, Fonarow GC; Get With The Guidelines Steering Committee and Hospitals. Patterns and predictors of evidence‐based medication continuation among hospitalized heart failure patients (from Get With The Guidelines‐Heart Failure). Am J Cardiol. 2011;107:1818–1823. doi: 10.1016/j.amjcard.2011.02.322 [DOI] [PubMed] [Google Scholar]
- 23. Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, Smith EE, Heidenreich P, Hernandez AF, Yancy CW, et al. Initiation, continuation, or withdrawal of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.004675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maggioni AP, Anker SD, Dahlstrom U, Filippatos G, Ponikowski P, Zannad F, Amir O, Chioncel O, Leiro MC, Drozdz J, et al. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134 [DOI] [PubMed] [Google Scholar]
- 25. Carubelli V, Lombardi C, Specchia C, Peveri G, Oriecuia C, Tomasoni D, Di Pasquale M, Inciardi R, Garrafa E, Metra M. Adherence and optimization of angiotensin converting enzyme inhibitor/angiotensin II receptors blockers and beta‐blockers in patients hospitalized for acute heart failure. ESC Heart Fail. 2021;8:1944–1953. doi: 10.1002/ehf2.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003;138:917–924. doi: 10.7326/0003-4819-138-11-200306030-00013 [DOI] [PubMed] [Google Scholar]
- 27. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E, Investigators P‐H. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 28. Wachter R, Senni M, Belohlavek J, Straburzynska‐Migaj E, Witte KK, Kobalava Z, Fonseca C, Goncalvesova E, Cavusoglu Y, Fernandez A, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. doi: 10.1002/ejhf.1498 [DOI] [PubMed] [Google Scholar]
- 29. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 30. Savarese G, Carrero JJ, Pitt B, Anker SD, Rosano GMC, Dahlstrom U, Lund LH. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: an analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail. 2018;20:1326–1334. doi: 10.1002/ejhf.1182 [DOI] [PubMed] [Google Scholar]
- 31. Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA‐HF randomized clinical trial. JAMA Cardiol. 2017;2:950–958. doi: 10.1001/jamacardio.2017.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maisel A, Xue Y, van Veldhuisen DJ, Voors AA, Jaarsma T, Pang PS, Butler J, Pitt B, Clopton P, de Boer RA. Effect of spironolactone on 30‐day death and heart failure rehospitalization (from the COACH study). Am J Cardiol. 2014;114:737–742. doi: 10.1016/j.amjcard.2014.05.062 [DOI] [PubMed] [Google Scholar]
- 33. Hernandez AF, Mi X, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Qualls LG, Peterson ED, Fonarow GC, Curtis LH. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. doi: 10.1001/jama.2012.14795 [DOI] [PubMed] [Google Scholar]
- 34. Rao VN, Murray E, Butler J, Cooper LB, Cox ZL, Fiuzat M, Green JB, Lindenfeld J, McGuire DK, Nassif ME, et al. In‐hospital initiation of sodium‐glucose cotransporter‐2 inhibitors for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;78:2004–2012. doi: 10.1016/j.jacc.2021.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ouwerkerk W, Teng TK, Tromp J, Tay WT, Cleland JG, van Veldhuisen DJ, Dickstein K, Ng LL, Lang CC, Anker SD, et al. Effects of combined renin‐angiotensin‐aldosterone system inhibitor and beta‐blocker treatment on outcomes in heart failure with reduced ejection fraction: insights from BIOSTAT‐CHF and ASIAN‐HF registries. Eur J Heart Fail. 2020;22:1472–1482. doi: 10.1002/ejhf.1869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S6
Figures S1–S3


