Abstract
We have used the patch clamp technique to characterize whole-cell currents in spheroplasts isolated from a trk1Δ trk2Δ strain of Saccharomyces cerevisiae which lacks high- and moderate-affinity K+ uptake capacity. In solutions in which extracellular divalent cation concentrations were 0.1 mM, cells exhibited a large inward current. This current was not the result of increasing leak between the glass pipette and membrane, as there was no effect on the outward current. The inward current comprised both instantaneous and time-dependent components. The magnitude of the inward current increased with increasing extracellular K+ and negative membrane potential but was insensitive to extracellular anions. Replacing extracellular K+ with Rb+, Cs+, or Na+ only slightly modulated the magnitude of the inward current, whereas replacement with Li+ reduced the inward current by approximately 50%, and tetraethylammonium (TEA+) and choline were relatively impermeant. The inward current was blocked by extracellular Ca2+ and Mg2+ with apparent Kis (at −140 mV) of 363 ± 78 and 96 ± 14 μM, respectively. Furthermore, decreasing cytosolic K+ increased the magnitude of the inward current independently of the electrochemical driving force for K+ influx, consistent with regulation of the inward current by cytosolic K+. Uptake of 86Rb+ by intact trk1Δ trk2Δ cells was inhibited by extracellular Ca2+ with a Ki within the range observed for the inward current. Furthermore, increasing extracellular Ca2+ from 0.1 to 20 mM significantly inhibited the growth of these cells. These results are consistent with those of the patch clamp experiments in suggesting that low-affinity uptake of alkali cations in yeast is mediated by a transport system sensitive to divalent cations.
Yeast cells maintain a stable K+ content and growth rate in a wide range of extracellular K+ concentrations (18, 23). Consequently, the mechanisms responsible for K+ uptake must be capable of adapting to a wide range of external K+ concentrations. This is reflected in the broad range of apparent Km values measured for K+ uptake in Saccharomyces cerevisiae growing in different nutritional conditions. The Km values for K+ uptake vary from 15 μM during K+ starvation to 5 mM in cells growing at low millimolar K+ concentrations (18, 23) to values as high as 50 mM in cells growing in K+ concentrations exceeding 100 mM (20).
High- and moderate-affinity K+ uptake in S. cerevisiae is mediated by the products of two genes, TRK1 and TRK2 (11, 20). Consequently, trk1Δ trk2Δ cells possess only low-affinity K+ uptake and exhibit a growth rate much lower than that observed for wild-type yeast in media containing less than 10 mM extracellular K+ (11, 20).
The identity of the low-affinity K+ uptake pathway in yeasts is unknown. In the few electrophysiological studies performed on yeasts, only two currents have been reported at the plasma membrane; (i) a small instantaneous inward current in Schizosaccharomyces pombe (13) and S. cerevisiae (4) which is probably mediated by the TRK gene products and (ii) a time-dependent outward current mediated by a K+-selective outward rectifier (3, 6) which is the product of TOK1 (10, 12, 29). In plants, low-affinity K+ uptake is thought to be mediated by K+-selective influx channels (14, 21). However, the characteristics of the pathway which mediates low-affinity K+ uptake in yeast have not been reported.
In previous electrophysiological experiments on yeast, plasma membrane currents were measured in high (≥5 mM) extracellular Ca2+ and Mg2+ (e.g., references 3, 5, and 13). In the present study, we applied the patch clamp technique to spheroplasts isolated from a trk1Δ trk2Δ strain of S. cerevisiae and observed whole-cell cation-selective inward currents which are revealed by reducing extracellular divalent cation concentrations to 0.1 mM. K+ uptake and growth experiments suggest that the inward current mediates low-affinity K+ uptake in intact yeast cells. Inward currents with characteristics very similar to those reported here were also described for S. cerevisiae at the 42nd American Biophysical Society Annual Meeting (7).
MATERIALS AND METHODS
All chemicals were supplied by Sigma (Poole, United Kingdom) unless otherwise stated.
Yeast culture and spheroplast isolation.
A trk1Δ trk2Δ double-deletion strain of S. cerevisiae (WΔ3; MATa ura3 his3 trp1 ade2 leu2 trk1Δ::LEU2 trk2Δ::HIS3) was used unless otherwise stated. Cells were grown overnight at 30°C, with shaking at 100 rpm in 30 ml of liquid medium (LM; 2% [wt/vol] glucose, yeast nitrogen broth [Difco Laboratories], 100 mM KCl) supplemented with the appropriate amino acids. A method based on that described by Bertl and Slayman (2) was used for spheroplast isolation. Cells were harvested from 10 ml of suspension culture by centrifugation (188 × g for 5 min). The cell pellet was resuspended in 10 ml of buffer A (50 mM KH2PO4, 40 mM 2-mercaptoethanol; adjusted to pH 7.0 with KOH), pelleted again, resuspended in 4 ml of buffer B (1.2 M sorbitol, 50 mM KH2PO4, 40 mM 2-mercaptoethanol, 400 U of lyticase, 1 U of chitinase, and 2,000 U of β-glucuronidase per ml; adjusted to pH 7.0 with KOH), and incubated at 30°C, with shaking at 100 rpm. After 90 min, the digest was centrifuged at 188 × g for 5 min, and the pellet was resuspended in 2 ml of ice-cold buffer C (2 M sorbitol, 1 mM CaCl2, 50 mM KH2PO4, 40 mM 2-mercaptoethanol; adjusted to pH 7.0 with KOH). Below this was layered 2 ml of ice-cold buffer D (buffer C, with 2 M sorbitol replaced with 1.5 M sucrose and 0.5 M sorbitol), followed by 2 ml of ice cold buffer E (buffer C, with 2 M sorbitol replaced with 2 M sucrose). This sucrose step gradient was centrifuged at 488 × g for 3 min, and clean spheroplasts were collected from the top layer. Spheroplasts were washed in 5 ml of buffer F (1 M sorbitol, 10 mM HEPES, 1 mM CaCl2; adjusted to pH 7.0 with KOH) and centrifuged at 188 × g for 5 min. The pellet was resuspended in 1 ml of buffer F and stored on ice. Spheroplasts with diameters of 4 to 5 μm were used.
Electrophysiology.
All recordings were made in a continuously perfused chamber in which a glass coverslip formed the base to which the spheroplasts adhered loosely. Patch pipettes were fabricated on a two-stage puller (Kopf Instruments, Tujunga, Calif.) from borosilicate glass (Kimax-51; Kimax Products, Vineland, N.J.). To reduce pipette capacitance, electrodes were coated with a 50% (wt/wt) mixture of mineral oil and Parafilm (American National Can Co., Chicago, Ill.). Pipette resistances varied between 5 and 50 MΩ, depending on the solution used in the pipette. An Ag-AgCl reference electrode was connected to the bath via a 2 M KCl agar bridge. Whole-cell currents were recorded at room temperature (approximately 20°C) with an RK-400 amplifier (Biologique, Claix, France) connected to an A/D converter (Digidata 1200; Axon Instruments, Foster City, Calif.). Recording and storage of data was controlled by the software package pClamp 6.03 (Axon Instruments) and a 486 personal computer. Data were filtered at 1 kHz. Results are presented as means ± standard errors of the means (SEM).
Solutions used in electrophysiology.
All solutions were filtered (0.2-μm pore diameter; Millipore) before use and were adjusted to 600 mosmol kg−1 with sorbitol. Seals in excess of 12 GΩ were formed in sealing solution that contained 10 mM KCl, 10 mM CaCl2, 5 mM MgCl2, and 5 mM HEPES-Tris base (pH 7.4). After the whole-cell configuration (indicated by an increase in capacitance of between 0.5 to 0.7 pF) was obtained, the solution was replaced by a standard bath solution (SBS; 0.1 mM CaCl2, 5 mM morpholineethanesulfonic acid [MES]-choline base, pH 5.5) containing various concentrations of K2SO4 or KCl. Pipettes were filled with either K+-depleted medium (LKM; 10 mM KCl, 2 mM MgCl2, 3 mM MgATP, 10 mM HEPES, 4 mM EGTA, 22 mM choline base [pH 7.4]) or K+-replete medium (HKM; 100 mM KCl, 5 mM MgCl2, 3 mM K2ATP, 10 mM HEPES, 4 mM EGTA, 23 mM KOH [pH 7.4]). Ionic equilibrium potentials were calculated after correction for ionic activity with GEOCHEM-PC (17a). K+ activities in LKM and HKM were 8.6 and 97.6 mM, respectively.
86Rb+ uptake experiments.
WΔ3 cells were grown overnight at 30°C, with shaking at 100 rpm, in 60 ml of LM, harvested by centrifugation (188 × g for 5 min), and washed with deionized water. Cells were repelleted and resuspended in 10 ml of reaction buffer (2% [wt/vol] glucose, 50 mM KCl, 5 mM HEPES-choline base [pH 7.0]) supplemented with 0.1 mM EGTA or CaCl2 ranging from 0.1 to 20 mM. The assay was started by the addition of 86Rb+ (0.1 μCi/ml; Amersham International, Amersham, United Kingdom) and shaking at 100 rpm at room temperature (ca. 20°C). 86Rb+ was used as a tracer for K+ and assumed to simulate K+ uptake. The reaction was stopped after 10 min by filtering 1 ml of cell suspension onto nitrocellulose filters (0.45-μm pore diameter; Millipore) and washing with 30 ml of wash solution (50 mM RbCl, 5 mM HEPES-choline base [pH 7.0]). Radioactivity on the filters was then counted.
Growth experiments.
WΔ3 cells were grown overnight at 30°C in 30 ml of liquid SD, prepared as described by Sherman et al. (24) but containing 200 μM Mg2+ and 100 μM Ca2+, and supplemented with the appropriate amino acids and 100 mM KCl. One milliliter of liquid culture of cells was added to 7 ml of SD or SD supplemented with 20 mM Ca2+ and incubated at 30°C, with shaking at 100 rpm. Optical density of cell cultures was measured at 600 nm to determine cell density.
RESULTS
Inward current revealed in low extracellular Ca2+.
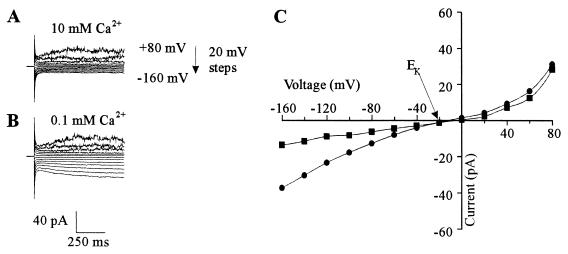
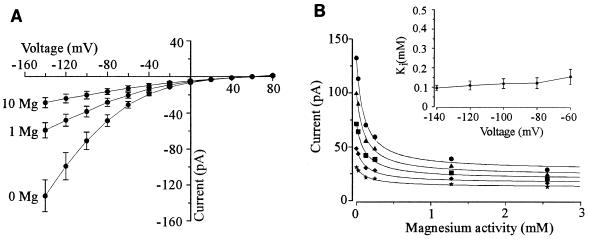
Using 129 mM cytosolic K+ (HKM in the pipette) and 10 mM extracellular Ca2+, we always observed time-dependent (TD) spiky outward currents and very little inward current in WΔ3 cells (n = 81) (Fig. 1A and C). The outward current has been characterized previously (3, 6) and shown to be mediated by an outwardly rectifying K+ channel encoded by the TOK1 gene (10, 12, 29). In contrast, the inward current represents a resistance of approximately 14 GΩ, and hence a significant portion of this current might result from the seal conductance between the glass pipette and plasma membrane (see below).
FIG. 1.
Whole-cell currents in yeast spheroplasts are revealed at low extracellular Ca2+ concentrations. Pipette medium was HKM. (A) Current traces in response to voltage pulses ranging from +80 to −160 mV in 20-mV steps. Holding potential was −30 mV. Bath solution was SBS supplemented with 10 mM CaCl2 and 25 mM K2SO4. (B) Like panel A except that bath solution was SBS supplemented with 25 mM K2SO4. (C) Steady-state current-voltage relationship of currents in the presence of 0.1 (•) and 10 mM (■) extracellular Ca2+ derived from panels A and B.
All cells exhibited an increase in the inward current on reducing extracellular Ca2+ to 0.1 mM (n = 59) (Fig. 1A and C). The magnitude of the outward current was unaffected by reducing extracellular Ca2+, indicating that the enhanced inward current was not the result of increasing leak current associated with the seal between the glass pipette and the plasma membrane. Reducing cytosolic K+ from 129 to 10 mM (LKM in the pipette) significantly increased the magnitude of the inward current (compare Fig. 1 with Fig. 2 and see below). Thus, unless otherwise stated, the inward current was investigated with 10 mM cytosolic K+. It is noteworthy that the outward current is no longer observed in spheroplasts when cytosolic K+ is 10 mM, consistent with the outward current being carried by K+ (3).
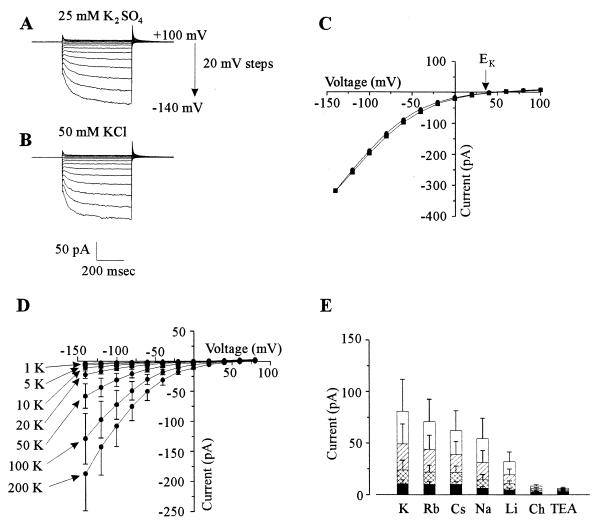
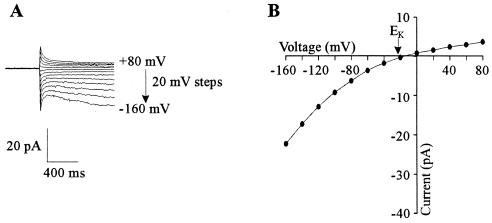
FIG. 2.
Whole-cell cation-selective inward currents in yeast spheroplasts. Pipette solution was LKM. (A) Current traces in response to voltage pulses ranging from +100 to −140 mV in 20-mV steps. Holding potential was +40 mV. Bath solution was SBS supplemented with 25 mM K2SO4. (B) As panel A except that bath solution was SBS supplemented with 50 mM KCl. (C) Steady-state current-voltage (I-V) relationship of currents in the presence of either 25 mM K2SO4 (•) or 50 mM KCl (■) in the bath. Data are taken from traces shown in panels A and B. (D) I-V relationship of currents in the presence of a range of extracellular K+ concentrations from 1 to 200 mM. Data are the mean values (±SEM) of five separate cells. Bath solution was SBS supplemented with appropriate concentrations of K2SO4. (E) Effects of various cations on inward current at different voltages. Data are the mean values (±SEM) from four separate cells. Current values are shown for −140 (open bars), −100 (hatched bars), −60 (double hatched bars), and −20 (solid bars) mV. Ch, choline.
The inward current had two distinct components, one instantaneous and one TD. The TD current was observed only at potentials negative of approximately −20 mV. The TD current activated very rapidly, and thus its activation was partially obscured by the capacitance currents associated with charging the plasma membrane. Consequently, it was not possible to describe the activation kinetics of the TD current or accurately separate it from the instantaneous current. Hence, unless otherwise stated, total inward current was investigated.
Selectivity.
The reversal potential (Erev) of the inward current closely followed changes in the equilibrium potential for K+ (EK). With 129 mM cytosolic K+ and 50 mM extracellular K+, the Erev was approximately −12 mV (Fig. 1), much closer to EK (−22 mV) than the reversal potential for any other ion in the bath or pipette medium. Furthermore, with 10 mM cytosolic K+ and 50 mM extracellular K+, Erev was approximately equal to EK (+40 mV; Fig. 2C). This is consistent with the instantaneous current being carried predominantly by K+.
The selectivity of the TD component of the inward current is ideally determined by measuring the reversal potential of its deactivation (tail) currents (see reference 22 for method). However, tail currents could not reliably be measured in the present study because, as with inward current activation, deactivation of the TD current was very rapid and obscured by the whole-cell capacitance current (Fig. 2A and B). Thus, the following experiments were performed to determine which ions were responsible for carrying the inward current. Replacing 25 mM extracellular K2SO4 with 50 mM KCl (Fig. 2A to C) did not affect either the activation kinetics, the Erev, or the magnitude of the inward current. The inward currents were similarly independent of extracellular H+ in the pH range 5.5 to 7.0 (data not shown). Increasing extracellular K+ from 1 to 200 mM increased the magnitude of both the instantaneous and TD inward currents (Fig. 2D). Thus, it was concluded that the instantaneous and TD inward currents were carried predominately by K+ ions.
Replacing extracellular K+ with Rb+, Cs+, or Na+ only slightly suppressed the inward current, whereas Li+ reduced the inward current to approximately half of that for K+. By contrast, TEA+ and choline were relatively impermeant (Fig. 2E). A conductivity sequence of K+ > Rb+ > Cs+ > Na+ > Li+ (Eisenman sequence IV) was apparent. The conductance for the various monovalent cations was independent of voltage (Fig. 2E), indicating that the instantaneous and TD components of the inward current exhibited similar selectivities among the monovalent cations. This suggests that the same transporters mediate both the TD and instantaneous components of the inward current.
Block by divalent cations.
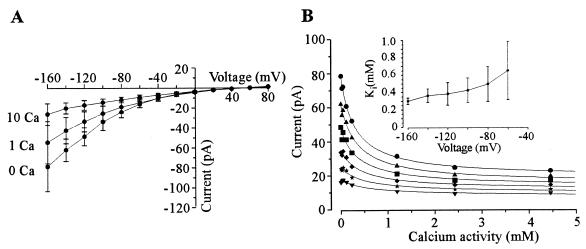
The inward current carried by K+ was insensitive to extracellular TEA+ up to 50 mM (data not shown) but was sensitive to extracellular Ca2+ and Mg2+. Typically, up to 70% of the inward current was inhibited by increasing extracellular Ca2+ concentrations from 0 to 20 mM, indicating that although the majority of the current is Ca2+ sensitive, a Ca2+-insensitive component is also evident (Fig. 3). The apparent Ki for the Ca2+-sensitive component was derived from equation 1: I = ICa · (1/(1 + ([Ca]/Ki)))+Iins where I is the total current, ICa is the Ca2+-sensitive current, Iins is the Ca2+-insensitive current, Ki is the constant for inhibition of the Ca2+-sensitive current, and [Ca] is extracellular Ca2+ activity. We obtained Ki values at various voltages (Fig. 3B) and found that they did not vary significantly with voltage (i.e., Ki varied between 653 ± 335 μM at −60 mV to 363 ± 78 μM at −140 mV [Fig. 3B, inset]), indicating that the Ca2+ inhibition was voltage independent. With respect to the Ca2+-insensitive component, it was not possible to distinguish between currents resulting from the seal between the plasma membrane and glass pipette and specific cation transporters in the plasma membrane. However, radiotracer experiments (see below and Fig. 7) indicated that at least part of the Ca2+-insensitive current is physiologically relevant.
FIG. 3.
Inhibition of the inward current by extracellular Ca2+. Pipette medium was LKM, and bath solution was SBS supplemented with 25 mM K2SO4, 0.1 mM MgCl2, and the appropriate concentration of CaCl2. For 0 mM Ca2+, bath solution was as above except that CaCl2 was omitted from SBS. Data are the mean values (±SEM) of four separate cells. (A) Current-voltage relationship for currents in the presence of 0, 1, and 10 mM extracellular Ca2+. (B) Inhibition of inward current at −160 (•), −140 (▴), −120 (■), −100 (⧫), −80 (★), and −60 (▾) mV plotted as a function of extracellular Ca2+ activity. Data are fitted with equation 1. Error bars have been omitted for clarity but are of the order of 15 to 25% of the mean. Inset, Ki values plotted as a function of voltage. Data are taken from fits with equation 1.
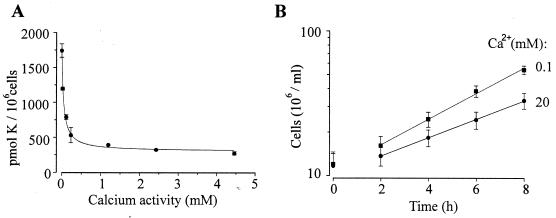
FIG. 7.
Effects of extracellular Ca2+ on K+ uptake and growth in intact cells. (A) K+ uptake plotted as a function of extracellular Ca2+ activity. Data are fitted as Z = ZCa · (1/(1 + ([Ca]/Ki))) + Zins, were Z is the total K+ uptake, ZCa is the Ca2+-sensitive K+ uptake, Zins is the Ca2+-insensitive K+ uptake, Ki is the constant for inhibition of the Ca2+-sensitive K+ uptake and [Ca] is extracellular Ca2+ activity. Ki was 48 ± 1 μM. Results are the means (±SEM) for three separate experiments. (B) Growth of cells in SD medium containing 100 μM (■) and 20 mM (•) extracellular Ca2+. Solid lines represent linear regressions from which doubling times of 203 (■) and 283 (•) min were calculated. Results are the means (±SEM) of eight separate experiments.
A similar but more effective block of the inward current was achieved with extracellular Mg2+ (Fig. 4). Data obtained from inhibition of the inward current by extracellular Mg2+ were fitted with equation 1 and revealed that the Kis for the inhibition of the Mg2+-sensitive current were lower than those observed with Ca2+ but were again relatively voltage insensitive (i.e., the Ki was 155 ± 39 μM at −60 mV, compared to 96 ± 14 μM at −140 mV [Fig. 4B, inset]).
FIG. 4.
Inhibition of the inward current by extracellular Mg2+. Pipette medium was LKM, and bath solution was SBS supplemented with 25 mM K2SO4 and the appropriate concentration of MgCl2. Data are the mean values of four separate cells ± SEM. (A) Current-voltage relationship for currents in the presence of 0, 1, and 10 mM extracellular Mg2+. (B) Inhibition of inward current at −140 (•), −120 (▴), −100 (■), −80 (⧫), and −60 (★) mV plotted as a function of extracellular Mg2+ activity. Data are fitted with equation 1. Error bars have been omitted for clarity but are of the order of 10 to 15% of the mean. Inset; Ki values plotted as a function of voltage. Data are taken from fits with equation 1.
Regulation of the inward current by cytosolic K+.
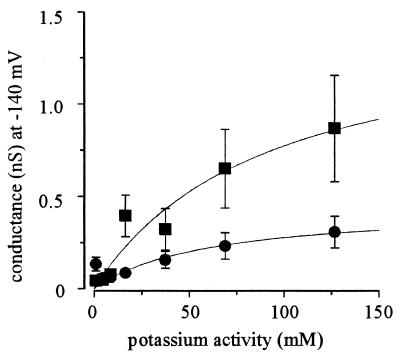
Figures 1 and 2 indicate that decreasing cytosolic K+ increases the magnitude of the inward current. Figure 5 shows the results of an analysis in which the plasma membrane conductance was calculated for extracellular K+ concentrations ranging from 1 to 200 mM (with a permissive extracellular Ca2+ concentration of 0.1 mM) with pipette solutions either of LKM (10 mM cytosolic K+) or HKM (129 mM cytosolic K+). The chord conductance was calculated after steady-state currents were normalized with respect to the electrochemical potential for K+ (see the legend to Fig. 5). The conductance saturates as a function of extracellular K+ activity. Fitting the data with Michaelis-Menten functions yielded maximal conductances of 0.44 ± 0.10 and 1.25 ± 0.31 nS for HKM and LKM, respectively. This finding indicates that the increased inward current observed with low cytosolic K+ (compare Fig. 1 and 2) was not solely the result of increasing the electrochemical potential gradient for K+ influx but rather the result of the inward current regulation by cytosolic K+.
FIG. 5.
Control of the inward current by cytosolic K+. Plasma membrane conductance at −140 mV plotted as a function of extracellular K+ activity. Pipette solutions were LKM and HKM for 10 (■) and 129 (•) mM cytosolic K+, respectively. Conductance was calculated as Iss/(−140 − EK), where Iss is the steady-state current and EK is the equilibrium potential for K+. Data are fitted with Michaelis-Menten functions. For 10 mM cytosolic K+, Km was 90 ± 50 mM and Vmax was 1.25 ± 0.31 nS; for 129 mM cytosolic K+, Km was 62 ± 55 mM and Vmax was 0.44 ± 0.10 nS. Data for 10 mM cytosolic K+ were taken from Fig. 2C.
Does TOK1 mediate the inward current?
Since WΔ3 is a trk1Δ trk2Δ strain, the inward current cannot be mediated by TRK1 or TRK2. However, the possibility remains that the inward current is mediated by the TOK1 gene product. This possibility was tested with a strain of WΔ3 in which the TOK1 gene was additionally deleted (WΔ3-tok1Δ; as WΔ3 except tokΔ::TRP; manufactured by J. Rosamond, Zeneca Pharmaceuticals). Using HKM as the pipette solution, significant outward current was never observed in WΔ3-tok1Δ (n = 15), confirming earlier reports showing that TOK1 exhibited K+-selective outward rectifier activity (10, 12, 29). However, Fig. 6 shows that the inward current was apparent in WΔ3-tok1Δ cells; indeed, all WΔ3-tok1Δ cells tested exhibited this current (n = 15). There was no significant difference in the membrane conductance measured at −140 mV between WΔ3-tok1Δ (0.19 ± 0.11 nS; n = 5) and WΔ3 (0.15 ± 0.06 nS; n = 5) cells when tested with HKM in the pipette and SBS supplemented with 25 mM K2SO4 in the bath. Thus, it was concluded that the K+-selective outward rectifier does not mediate the Ca2+-sensitive inward current.
FIG. 6.
Whole-cell currents in WΔ3-tok1Δ yeast spheroplasts. Pipette medium was HKM, and bath solution was SBS supplemented with 25 mM K2SO4. (A) Current traces in response to voltage pulses ranging from +80 to −160 mV in 20-mV steps. Holding potential was −10 mV. (B) Current-voltage relationship of plasma membrane currents shown in panel A.
Ca2+-inhibitable K+ uptake and growth in intact yeast.
If the inward currents that we measure are responsible for low-affinity K+ uptake, then it should be possible to demonstrate that K+ influx is Ca2+ inhibitable in intact cells. We have used 86Rb+ as a convenient tracer for K+ since the patch clamp experiments indicate that Rb+ has essentially the same permeability properties as K+ (Fig. 2D). Influx of 86Rb+ into intact cells was measured in a background of 50 mM K+ to simulate low-affinity K+ uptake and, as shown in Fig. 7A, was strongly inhibited by Ca2+. The resultant Ki of 48 ± 1 μM (Fig. 7A), which, although lower than that observed for the inward current, is consistent with the patch clamp data. It is also noteworthy that approximately 87% of the low-affinity K+ uptake is Ca2+ sensitive. This indicates that at least some of the Ca2+-sensitive inward current measured with the patch clamp technique is physiologically relevant and not solely the result of leak between the glass pipette and plasma membrane.
The growth of trk1Δ trk2Δ cells is limited by K+ availability and uptake (11). If the inward currents were a major pathway for K+ uptake in WΔ3, then it would be expected that the growth of these cells would depend on the activity of the inward current, which in turn is dependent on extracellular Ca2+. In accord with this assumption, Fig. 7B shows that increasing extracellular Ca2+ from 0.1 to 20 mM Ca2+ reduced the growth rates such that the doubling times increased from 203 to 283 min.
DISCUSSION
Cation-selective current in the plasma membrane of yeast cells.
Reducing extracellular Ca2+ and Mg2+ revealed a large inward monovalent cation current in the plasma membrane of S. cerevisiae. This current is independent of the previously characterized K+ transporters in yeast, namely, TRK1, TRK2, and TOK1. It is known that extracellular Ca2+ aids formation of a seal between the glass pipette and membrane in the patch clamp technique (15). Thus, it is necessary to address the potential criticism that the inward current in the present study may represent the loss of seal. The following characteristics of the inward current in yeast illustrate that the current is not an artifact of the patch clamp technique but rather is mediated by specific transporters in the plasma membrane: (i) strong rectification favoring inward current, (ii) strong selectivity for monovalent cations over anions or H+, (iii) differential permeability among monovalent cations, and (iv) membrane conductance regulated by cytosolic K+. Furthermore, growth and 86Rb+ uptake experiments suggest that the inward current represents the principal pathway for low-affinity K+ uptake in intact trk1Δ trk2Δ cells, thus supporting the conclusion that the inward current has physiological significance.
Madrid et al. (16) have also recently characterized low-affinity monovalent cation uptake in intact trk1Δ trk2Δ cells. Consistent with the present study, they report only slight differences between K+, Na+, Rb+, and Li+ uptake affinities and that uptake was membrane potential sensitive and partially inhibited by extracellular divalent cations; at similar concentrations of extracellular divalent cations, however, they found less than 30% inhibition of Rb+ uptake, compared to 87% inhibition of K+ uptake observed in our study. The reason for this discrepancy is unclear, although it is noteworthy that in the study of Madrid et al. (16), monovalent cation uptake was conducted in a solution buffered to pH 6.0 with MES and Ca(OH)2. In the present study, we show that extracellular Ca2+ inhibits K+ uptake with a Ki of 48 μM, and thus it is likely that in the study of Madrid et al. (16), the Ca2+-sensitive component of low-affinity monovalent cation uptake is mostly blocked.
Physiological roles.
The magnitude of the inward current suggests that it represents a pathway for rapid uptake of K+ in the low-affinity range. The inward current is enhanced when cytosolic K+ is reduced, which may suggest that it functions to rapidly restore cytosolic K+ levels when they become depleted. The regulation by cytosolic K+ might also aid growth at high Ca2+ levels, where uptake tends to be blocked. Consistent with this function, Ramos and Rodriguez-Navarro (19) showed that low-affinity K+ uptake is enhanced in yeast cells which have been treated with azide to reduce their internal K+.
Although the coupling mechanism(s) of the TRK-mediated K+ transport is not known, high-affinity uptake is likely to require high negative membrane potential or possibly additional energization through ion coupling. The inward currents, however, would permit low-capacity K+ uptake without the energetic constraints which are imposed on TRK-mediated K+ uptake. Thus, the inward currents could catalyze high rates of K+ uptake at relatively low membrane potentials, provided that the medium is relatively K+ rich.
The pharmacology and selectivity characteristics of the inward current in yeast are similar to those reported for Na+-permeable channels in higher plant cells. We observed in this study the selectivity sequence K+ > Rb+ > Cs+ > Na+ > Li+, which is similar to that found for the Na+-permeable channel in wheat root cortex (25). Na+-permeable channels have also been found in maize root cortex (22) and barley suspension cells (1). These plant channels are also blocked by extracellular Ca2+ with Kis of between 300 and 400 μM (22, 25), similar to that observed for the block of the inward current in our study. However, contrary to the present report, only instantaneously activating Na+ currents have been recorded in plants.
The Na+-permeable ion channels in plant cells are likely to represent a pathway for Na+ uptake and accumulation, and they are thought to play a central role in determining the Na+ tolerance of higher plants (22, 25). In media containing high concentrations of Na+, yeast cells also accumulate Na+ to high levels (26), indicating that there is a pathway for Na+ uptake in yeast. The TRK1 gene product mediates both high- and moderate-affinity K+ uptake in yeast (20). In its moderate-affinity uptake mode, TRK1 has a ratio of 15 for the Km values for Na+ and K+, but this value rises to 300 when TRK1 switches to high-affinity uptake (18). High extracellular Na+ concentrations induce this transition to the high-affinity uptake state, which reduces the potential for Na+ uptake via TRK1 (18). Hence, although TRK1 mediates Na+ uptake, it is unlikely to represent a significant pathway for Na+ uptake in saline conditions. Indeed, substantial low-affinity Na+ uptake still occurs in trk1Δ mutants (9). The inward current described in the present study might represent the pathway for low-affinity Na+ uptake. In accord with this notion, Na+ tolerance in yeast (including trk1Δ strains) is reduced when extracellular Ca2+ is reduced from 3 to 0.2 mM (9). It is also noteworthy that yeast PMA1 mutants exhibit additional Na+ tolerance because of reduced Na+ uptake (17). PMA1 encodes for the plasma membrane H+ pump. The pump is electrogenic; hence, PMA1 activity is responsible for maintaining a negative membrane potential. Na+ uptake via the low-affinity cation transport pathway described here is passive and thus dependent on the membrane potential. Hence, reduced Na+ uptake by yeast PMA1 mutants is consistent with passive Na+ uptake represented by the inward current.
Molecular identity of the transporter underlying the inward current.
The large magnitude of the inward current suggests that it might be mediated by channel-type molecules which have high turnover rates. Ideally, it should be possible to identify channel-type activity which may underly whole-cell currents in excised patches. However, excised patches from yeast spheroplasts (which are only 4 to 5 μm in diameter) are difficult to obtain and consequently were isolated from only a few cells (n = 5). No single inward channel activity was observed in these patches (data not shown). However, carrier-type mechanisms (for which turnover rates are lower than for channels) could also mediate the inward current if sufficiently large numbers of the carrier-type transporters are present in the plasma membrane. Indeed, TRK-mediated inward currents can be measured in the whole-cell configuration of the patch clamp technique (13), yet it is not known whether these currents are mediated by channel-type or carrier-type molecules. In addition, there are no reports of yeast genes which may encode for a K+ uptake channel. However, recent studies suggest that low-affinity K+ uptake may be mediated by a number of non-K+ transporters which have intrinsic K+ transport capabilities, for example, permeases involved in amino acid, sugar, inositol, or choline uptake (16, 27, 28). K+ uptake has also been reported for the H+/tetracycline antiport in bacteria (8). It is possible that permease-type molecules can mediate the inward current even though the turnover rates of these transporters at first sight seem unlikely to be sufficient to generate the large currents observed in this study.
ACKNOWLEDGMENTS
We thank Alonso Rodríguez-Navarro for the WΔ3 cells, John Rosamond (Zeneca) for the WΔ3-tok1Δ cells, and Stuart J. Dunbar and Frans Maathuis for useful discussions and comments on the manuscript.
This work was funded by a Zeneca grant to D.S.
ADDENDUM IN PROOF
Since submission of this manuscript, currents similar to those described in the present study have been reported in Saccharomyces cerevisiae by Bihler et al. (H. Bihler et al., FEBS Lett. 432:59–64, 1998).
REFERENCES
- 1.Amtmann A, Laurie S, Leigh R, Sanders D. Multiple inward channels provide flexibility in Na+/K+ discrimination at the plasma membrane of barley suspension culture cells. J Exp Bot. 1997;48:481–498. doi: 10.1093/jxb/48.Special_Issue.481. [DOI] [PubMed] [Google Scholar]
- 2.Bertl A, Slayman C L. Cation-selective ion channels in the vacuolar membrane of Saccharomyces cerevisiae: dependence on Ca2+, redox state and voltage. Proc Natl Acad Sci USA. 1990;87:7824–7828. doi: 10.1073/pnas.87.20.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertl A, Slayman C L, Gradmann D. Gating and conductance in an outwardly-rectifying K+ channel from the plasma membrane of Saccharomyces cerevisiae. J Membr Biol. 1993;132:183–199. doi: 10.1007/BF00235737. [DOI] [PubMed] [Google Scholar]
- 4.Bertl A, Anderson J, Slayman C L, Gaber R. Use of Saccharomyces cerevisiae for patch clamp analysis of heterologous membrane proteins: characterisation of Kat1, an inward-rectifying channel from Arabidopsis thaliana and comparison with endogenous yeast channels and carriers. Proc Natl Acad Sci USA. 1995;92:2701–2705. doi: 10.1073/pnas.92.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertl A, Reid J D, Sentenac H, Slayman C L. Functional comparison of plant inward-rectifier channels expressed in yeast. J Exp Bot. 1997;48:405–413. doi: 10.1093/jxb/48.Special_Issue.405. [DOI] [PubMed] [Google Scholar]
- 6.Bertl A, Bihler H, Reid J D, Kettner C, Slayman C L. Physiological characterisation of the yeast plasma membrane outward rectifying K+ channel, DUK1 (TOK1) in situ. J Membr Biol. 1998;162:67–80. doi: 10.1007/s002329900343. [DOI] [PubMed] [Google Scholar]
- 7.Bihler H, Slayman C L, Bertl A. Patch clamp analysis of a novel cation uptake system in Saccharomyces cerevisiae which is independent of DUK1, TRK1 and TRK2. Biophys J. 1998;74:A319. [Google Scholar]
- 8.Dosch D C, Salvacion F F, Epstein W. Tetracycline resistance element of pBR322 mediates potassium transport. J Bacteriol. 1984;160:1188–1190. doi: 10.1128/jb.160.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haro R, Banuelos M A, Quintero F J, Rubio F, Rodriguez-Navarro A. Genetic analysis of sodium exclusion and sodium tolerance in yeast. A model for plants. Physiol Plant. 1993;89:868–874. [Google Scholar]
- 10.Ketchum K A, Joiner W J, Sellers A J, Kaczmarek L K, Goldstein S A N. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–694. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- 11.Ko C H, Gaber R F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romen G, Barhanin J. A pH-sensitive yeast outward rectifier K+ channel with two pore domains and novel gating properties. J Biol Chem. 1996;271:4183–4187. doi: 10.1074/jbc.271.8.4183. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenberg-Frate H, Reid R J, Heyer M, Hofer M. The spTRK gene encodes a potassium specific transport protein TKHP in Schizosaccharomyces pombe. J Membr Biol. 1996;152:169–181. doi: 10.1007/s002329900095. [DOI] [PubMed] [Google Scholar]
- 14.Maathuis F J M, Sanders D. Contrasting roles in ion transport of two K+-channel types in root cells of Arabidopsis thaliana. Planta. 1995;197:456–464. doi: 10.1007/BF00196667. [DOI] [PubMed] [Google Scholar]
- 15.Maathuis F J M, Taylor A R, Assmann S M, Sanders D. Seal promoting solutions and pipette perfusion for patch clamping plant cells. Plant J. 1997;11:891–896. doi: 10.1046/j.1365-313x.1997.11040891.x. [DOI] [PubMed] [Google Scholar]
- 16.Madrid R, Gomez J, Ramos J, Rodriguez-Navarro A. Ectopic potassium uptake in trk1, trk2 mutants of Saccharomyces cerevisiae correlates with a high hyperpolarized membrane potential. J Biochem. 1998;273:14838–14844. doi: 10.1074/jbc.273.24.14838. [DOI] [PubMed] [Google Scholar]
- 17.Nass R, Cunningham W, Rao R. Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+ ATPase. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- 17a.Parker D R, Norvell W A, Chaney R L. GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In: Leoppert R H, Goldberg S, Schwab A P, editors. Chemical equilibrium reaction models. Madison, Wis: American Society of Agronomy; 1994. [Google Scholar]
- 18.Ramos J, Contreras P, Rodriguez-Navarro A. A potassium transport mutant of Saccharomyces cerevisiae. Arch Microbiol. 1985;143:88–93. [Google Scholar]
- 19.Ramos J, Rodriguez-Navarro A. Regulation and interconversion of the potassium transport systems of Saccharomyces cerevisiae as revealed by rubidium transport. Eur J Biochem. 1986;154:307–311. doi: 10.1111/j.1432-1033.1986.tb09398.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramos J, Alijo R, Haro R, Rodríguez-Navarro A. TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae. J Bacteriol. 1994;176:249–252. doi: 10.1128/jb.176.1.249-252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts S K, Tester M. Inward and outward K+-selective currents in the plasma membrane of protoplasts from maize root cortex and stele. Plant J. 1995;8:811–825. [Google Scholar]
- 22.Roberts S K, Tester M. A patch clamp study of Na+ transport in maize roots. J Exp Bot. 1997;48:431–440. doi: 10.1093/jxb/48.Special_Issue.431. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Navarro A, Ramos J. Dual system potassium transport in Saccharomyces cerevisiae. J Bacteriol. 1984;159:940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman F, Fink G, Hinks J. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 25.Tyerman S D, Skerret M, Garrill A, Findlay G P, Leigh R A. Pathways for the permeation of Na+ and Cl− into protoplasts derived from the cortex of wheat roots. J Exp Bot. 1997;48:459–480. doi: 10.1093/jxb/48.Special_Issue.459. [DOI] [PubMed] [Google Scholar]
- 26.Watson T G. Effects of sodium chloride on steady-state growth and metabolism of Saccharomyces cerevisiae. J Gen Microbiol. 1971;64:91–99. doi: 10.1099/00221287-64-1-91. [DOI] [PubMed] [Google Scholar]
- 27.Wright M B, Howell E A, Gaber R F. Amino acid substitutions in membrane-spanning domains of Hol1, a member of the major facilitator superfamily of transporters, confer nonselective cation uptake in Saccharomyces cerevisiae. J Bacteriol. 1997;178:7197–7205. doi: 10.1128/jb.178.24.7197-7205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright M B, Ramos J, Gomez M J, Moulder K, Scherrer M, Munson G, Gaber R F. Potassium transport by amino acid permeases in Saccharomyces cerevisiae. J Biol Chem. 1997;272:13647–13654. doi: 10.1074/jbc.272.21.13647. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X-L, Vaillant B, Loukin S H, Kung C, Saimi Y. YKC1 encodes the depolarisation-activated K+ channel in the plasma membrane of yeast. FEBS Lett. 1995;373:170–176. doi: 10.1016/0014-5793(95)01035-d. [DOI] [PubMed] [Google Scholar]