Abstract
Background
Little is known about the impact of transcatheter mitral valve edge‐to‐edge repair on changes in left ventricular ejection fraction (LVEF) and the effect of an acute reduction in LVEF on prognosis. We aimed to assess changes in LVEF after transcatheter mitral valve edge‐to‐edge repair for both primary and secondary mitral regurgitation (PMR and SMR, respectively), identify rates and predictors of LVEF reduction, and estimate its impact on prognosis.
Methods and Results
In this international multicenter registry, patients with both PMR and SMR undergoing transcatheter mitral valve edge‐to‐edge repair were included. We assessed rates of acute LVEF reduction (LVEFR), defined as an acute relative decrease of >15% in LVEF, its impact on all‐cause mortality, major adverse cardiac event (composite end point of all‐cause death, mitral valve surgery, and residual mitral regurgitation grade ≥2), and LVEF at 12 months, as well as predictors for LVEFR. Of 2534 patients included (727 with PMR, and 1807 with SMR), 469 (18.5%) developed LVEFR. Patients with PMR were older (79.0±9.2 versus 71.8±8.9 years; P<0.001) and had higher mean LVEF (54.8±14.0% versus 32.7±10.4%; P<0.001) at baseline. After 6 to 12 months (median, 9.9 months; interquartile range, 7.8–11.9 months), LVEF was significantly lower in patients with PMR (53.0% versus 56.0%; P<0.001) but not in patients with SMR. The 1‐year mortality was higher in patients with PMR with LVEFR (16.9% versus 9.7%; P<0.001) but not in those with SMR (P=0.236). LVEF at baseline (odds ratio, 1.03 [95% CI, 1.01–1.05]; P=0.002) was predictive of LVEFR for patients with PMR, but not those with SMR (P=0.092).
Conclusions
Reduction in LVEF is not uncommon after transcatheter mitral valve edge‐to‐edge repair and is correlated with worsened prognosis in patients with PMR but not patients with SMR.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT05311163.
Keywords: afterload mismatch, left ventricular ejection fraction, mitral regurgitation
Subject Categories: Valvular Heart Disease, Remodeling
Nonstandard Abbreviations and Acronyms
- COAPT
Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation
- LVEDV
left ventricular end‐diastolic volume
- LVEFR
left ventricular ejection fraction reduction
- MR
mitral regurgitation
- PMR
primary mitral regurgitation
- SMR
secondary mitral regurgitation
- TEER
transcatheter mitral valve edge‐to‐edge repair
Clinical Perspective.
What Is New?
We assessed the rates of left ventricular ejection fraction reduction (LVEFR; defined as a reduction of >15% in left ventricular ejection fraction) following transcatheter mitral valve edge to‐edge repair, predictors of this phenomenon, and its impact on prognosis in primary and secondary mitral regurgitation.
We have included 2534 patients, of whom 469 (18.5%) developed LVEFR.
The 1‐year mortality rates were higher in patients with primary mitral regurgitation with LVEFR (16.9% versus 9.7%; P<0.001) but not in those with secondary mitral regurgitation (P=0.236).
What Are the Clinical Implications?
LVEFR is not uncommon after transcatheter mitral valve edge‐to‐edge repair and is correlated with worsened prognosis in patients with primary mitral regurgitation only.
There is a need to identify mechanisms for LVEFR following mitral transcatheter mitral valve edge‐to‐edge repair, in particular in patients with primary mitral regurgitation.
Transcatheter mitral valve edge‐to‐edge repair (TEER) is considered a safe and important alternative to mitral valve surgery in patients with severe primary and secondary mitral regurgitation (PMR and SMR, respectively) at a high surgical risk. 1 , 2 , 3 , 4 Following surgical mitral valve repair for PMR, improved left ventricular (LV) and left atrial remodeling was demonstrated. 5 However, little is known about the impact of TEER in patients with PMR or those with SMR who are poor candidates for surgery. Studies in these populations are limited and vary in the inclusion criteria and the cause of mitral regurgitation. Some showed no improvement or even reduction in LV ejection fraction (LVEF) following TEER, 6 , 7 , 8 , 9 , 10 whereas in others an improvement in LVEF was shown. 11 , 12 , 13 , 14 , 15 , 16 , 17
Previous studies suggested that an acute reduction in left ventricular function may develop in a significant number of patients undergoing TEER or mitral valve surgery. 17 , 18 , 19 , 20 This so‐called afterload mismatch is thought to occur because of the immediate loss of the low‐resistance leak into the left atrium, leading to an acute change in left ventricular loading conditions. The clinical importance of this phenomenon is not clear yet. It has been studied in a few small, mostly single‐center studies, where it was not defined universally. Currently available data suggested worse prognosis for patients with acute LVEF reduction (LVEFR) in some studies, 18 , 19 , 20 but not in others. 21 Also, in studies including patients undergoing mitral valve surgery, predictors of LVEFR include pulmonary hypertension, atrial fibrillation, low preoperative LVEF, and large left ventricles. 20 , 22 , 23 However, little is known about the causes of LVEFR after TEER. We therefore aimed to learn more about the dynamics in left ventricular function after TEER for both PMR and SMR, identify predictors for an acute reduction in left ventricular function, and understand its impact on prognosis in these 2 different patient populations.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Patients and Data Collection
In this international, multicenter registry (ClinicalTrials.gov identifier: NCT05311163), patients undergoing TEER using the MitraClip percutaneous mitral valve repair (Abbott Vascular, Inc, Santa Clara, CA) were included. Information was collected from a multicenter collaboration in a retrospective manner and incorporated into the MITRA‐EF registry in European and North American centers.
To reflect patients in the real‐world setting, we have decided to include patients with both PMR and SMR undergoing TEER, regardless of cause or baseline LVEF, and study them separately. Exclusion criteria were “mixed cause” of PMR and SMR, single‐leaflet device attachment, immediate conversion to surgery, unavailability of echocardiographic data after discharge, concomitant transcatheter tricuspid repair, active malignant tumor, systemic infection, or cardiogenic shock at presentation. All recruited patients signed an informed consent form following the approval of the institutional review board ethics committee in compliance with the Declaration of Helsinki.
End Points
We measured changes in LVEF before discharge (up to a week after procedure), and 6 to 12 months following the procedure, compared with baseline. LVEFR was defined as an acute relative decrease of ≥15% in LVEF [(LVEF at discharge)/(LVEF at baseline)<0.85]. This value represented the approximate median value of the reduction in LVEF (14.9%) in our study cohort, and was therefore considered as the threshold to define the occurrence of LVEFR. Primary end points were the rates of all‐cause death and major adverse cardiac events (a composite of death, need for mitral valve surgery, or redo percutaneous mitral valve repair and rates of mitral regurgitation grade ≥2) at 12 months. We also measured rates of relative LVEF improvement (by 10% and 15%) after TEER. Secondary outcomes included New York Heart Association classification, heart failure admissions, and need for mitral valve surgery or redo percutaneous mitral valve repair at 12 months. Also included were in‐hospital outcomes, such as procedural success and device success (as defined by the Mitral Valve Academy Research Consortium 24 ), number of devices implanted, mean mitral valve gradient (after TEER), tamponade, right‐to‐left shunt, need for immediate surgery, sepsis, acute coronary syndrome, vascular complication or gastrointestinal bleeding, stroke, acute renal failure, length of hospitalization, and hospital death. Finally, we assessed for independent determinants of the occurrence of LVEFR.
Statistical Analysis
Normality of distribution of continuous variables was explored using the Kolmogorov‐Smirnov and Shapiro‐Wilk tests. Continuous variables following a normal distribution are reported as mean±SD and were compared using Student t‐test (paired or unpaired), whereas those not following a normal distribution are presented as median and interquartile range and were compared using the Mann‐Whitney U test for independent groups and Wilcoxon signed‐rank test for paired comparisons. Categorical variables are reported as counts and percentages and were compared using the χ2 or Fisher exact test, as appropriate.
Survival rate free from clinical end points was estimated using the Kaplan‐Meier method, and the differences between groups were calculated using the log‐rank test. Binary logistic regression analysis was performed to identify the univariate and multivariable predictors of LVEFR. Variables with P<0.25 on univariate analysis were included in the final multivariable model. In logistic regression analysis, the model fit and predictive power were validated using the Hosmer‐Lemeshow goodness‐of‐fit test for logistic regression. Each result is expressed as an odds ratio (OR) and corresponding 95% CI. Finally, Cox proportional hazards regression analysis was performed to determine independent predictors of mortality at 12 months, accounting for known baseline cardiovascular risk differences, which included the following: age, sex, cause of mitral regurgitation (MR) (SMR or PMR), LVEF, diabetes, MR severity, pulmonary artery systolic pressure, left atrial pressure, right ventricular dysfunction, renal failure (defined as glomerular filtration rate <50 mL/min per 1.73 m2, according to the Modification of Diet in Renal Disease formula), and LVEFR. The scaled Schoenfeld residuals test verified the proportional hazard assumption.
In addition, we sought to assess the correlation between patients with “disproportionate MR,” based on the ratio of effective regurgitant orifice area (EROA)/left ventricular end‐diastolic volume (LVEDV), 25 on rates of LVEFR. We also compared rates of LVEFR in patients who fit the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) Trial criteria versus those who did not, according to the following parameters: LVEF between 20% and 50%, left ventricular end–systolic dimeter (LVESD) ≤70 mm, tricuspid regurgitation (TR) less than severe, tricuspid annular plane systolic excursion ≥15 mm, systolic pulmonary artery pressure ≤70 mm Hg, and hemodynamic stability. 4 , 26 Finally, to potentially allocate patients with SMR who fit the criteria of heart failure with preserved ejection fraction, thus also uncovering patients with atrial SMR, we decided to analyze those with LVEF >50% at baseline separately.
For all analyses, a 2‐sided P<0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS version 28.0 (IBM Corp, Armonk, NY).
RESULTS
Of 2932 patients screened in 16 centers, 2534 were enrolled in the final study cohort. At baseline, 727 (28.7%) experienced symptomatic PMR, and 1807 (71.3%) experienced SMR. Of the entire cohort, 469 (18.5%) of the patients fit the definition of LVEFR following TEER: 152 (20.9%) of the patients with PMR, and 317 (17.5%) of the patients with SMR (Figure 1).
Figure 1. Flowchart of the present study.

LVEF indicates left ventricular ejection fraction; LVEFR, left ventricular ejection fraction reduction; PMR, primary mitral regurgitation; SLDA, single‐leaflet device attachment; SMR, secondary mitral regurgitation; and TEER, transcatheter mitral valve edge‐to‐edge repair.
Clinical Baseline Characteristics
Patients with PMR were older than patients with SMR (79.0±9.2 versus 71.8±8.9 years; P<0.001), consisted of fewer women (38.0% versus 49.9%; P<0.001), and were less likely to have diabetes (15.1% versus 35.6%; P<0.001) and other cardiovascular risk factors, but more had previous oncological disease or valvular surgery (Table 1). Both the Society of Thoracic Surgeons Score for mortality and the EuroSCORE II were higher for patients with SMR (6.7±3.6 versus 6.1±3.8 [P<0.001] and 7.9±3.8 versus 7.5±3.7 [P=0.04], respectively). Rates of LVEFR were higher in patients with PMR (20.9% versus 17.5%; P=0.02). For both PMR and SMR, patients with LVEFR were similar to controls in most baseline characteristics, including age (79.0±9.4 versus 78.9±9.1 years for PMR [P=0.359] and 71.7±9.1 versus 71.9±8.8 years for SMR [P=0.282]), sex (37.8% versus 38.1% were women in the PMR group [P=0.429] and 50.1% versus 49.7% were women in the SMR group [P=0.223]), New York Heart Association classification (P=0.376 for PMR and 0.283 for SMR), and surgical risk (Society of Thoracic Surgeons score for mortality, 6.2±3.2 versus 6.1±4.0 [P=0.457] in patients with PMR and 6.6±3.7 versus 6.7±3.6 [P=0.625] in patients with SMR; and EuroSCORE II 7.4±3.4 versus 7.5±3.7 [P=0.822] and 7.8±4.1 versus 7.9±3.7 [P=0.183], respectively; Table 1).
Table 1.
Baseline Characteristics
| Parameter | PMR LVEFR (n=152) | PMR controls (n=575) | P value | SMR LVEFR (n=317) | SMR controls (n=1490) | P value | PMR vs SMR |
|---|---|---|---|---|---|---|---|
| P value | |||||||
| Age, mean±SD, y | 79.0±9.4 | 78.9±9.1 | 0.359 | 71.7±9.1 | 71.9±8.8 | 0.282 | <0.001 |
| Female sex, % | 37.8 | 38.1 | 0.429 | 50.1 | 49.7 | 0.223 | <0.001 |
| Diabetes, % | 14.2 | 15.6 | 0.162 | 35.5 | 35.6 | 0.983 | <0.001 |
| Hypertension, % | 70.9 | 71.0 | 0.852 | 71.7 | 71.6 | 0.536 | 0.420 |
| Smoking, % | 25.4 | 25.7 | 0.293 | 36.8 | 36.7 | 0.691 | 0.004 |
| Dyslipidemia, % | 53.7 | 55.3 | 0.272 | 59.9 | 61.1 | 0.173 | 0.121 |
| Chronic kidney disease, % | 52.6 | 52.2 | 0.473 | 62.1 | 62.9 | 0.568 | <0.001 |
| Hemoglobin, mean±SD, g/dL | 12.1±2.4 | 12.2±2.2 | 0.835 | 12.0±2.1 | 12.1±2.4 | 0.706 | 0.742 |
| Past cerebrovascular accident, % | 10.6 | 10.8 | 0.346 | 12.1 | 11.9 | 0.230 | 0.215 |
| Peripheral arterial disease, % | 10.7 | 10.9 | 0.453 | 16.4 | 16.6 | 0.325 | <0.001 |
| Anemia, % | 46.4 | 48.2 | 0.134 | 49.9 | 49.3 | 0.236 | 0.320 |
| COPD, % | 16.3 | 17.0 | 0.122 | 18.1 | 18.4 | 0.323 | 0.273 |
| Oncological disease, % | 19.3 | 18.2 | 0.146 | 13.5 | 13.1 | 0.542 | <0.001 |
| Coronary artery disease, % | 30.1 | 29.3 | 0.311 | 59.4 | 58.7 | 0.230 | <0.001 |
| Previous CABG, % | 17.8 | 18.0 | 0.572 | 27.9 | 28.1 | 0.446 | <0.001 |
| Past valvular surgery, % | 18.0 | 18.1 | 0.627 | 14.1 | 13.9 | 0.264 | <0.001 |
| Atrial fibrillation, % | 61.5 | 61.1 | 0.276 | 52.4 | 51.9 | 0.204 | 0.002 |
| Pacemaker/ICD implant, % | 15.2 | 14.9 | 0.283 | 54.6 | 54.8 | 0.427 | <0.001 |
| NYHA baseline, % | 0.548 | ||||||
| Class I | 4.7 | 5.0 | 0.376 | 4.9 | 5.1 | 0.283 | |
| Class II | 18.9 | 21.2 | 20.2 | 19.6 | |||
| Class III | 58.3 | 53.8 | 58.5 | 54.7 | |||
| Class IV | 18.1 | 20.0 | 16.4 | 20.6 | |||
| STS score, mean±SD | 6.2±3.2 | 6.1±4.0 | 0.457 | 6.6±3.7 | 6.7±3.6 | 0.625 | <0.001 |
| EuroSCORE II, mean±SD | 7.4±3.4 | 7.5±3.7 | 0.822 | 7.8±4.1 | 7.9±3.7 | 0.183 | 0.002 |
| β‐Blockers, % | 66.1 | 66.2 | 0.862 | 88.8 | 85.1 | 0.120 | <0.001 |
| ACEi/ARB, % | 47.2 | 47.0 | 0.921 | 75.6 | 76.2 | 0.241 | <0.001 |
| MRA, % | 36.1 | 34.5 | 0.108 | 42.1 | 40.3 | 0.145 | <0.001 |
| ARNi, % | 2.6 | 2.5 | 0.324 | 9.8 | 10.2 | 0.322 | <0.001 |
| Diuretics, % | 80.1 | 81.2 | 0.422 | 92.1 | 92.2 | 0.945 | <0.001 |
| SGLT2i, % | 12.1 | 10.4 | 0.332 | 16.4 | 15.5 | 0.572 | <0.001 |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor/neprilysin inhibitor; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter‐defibrillator; LVEFR, left ventricular ejection fraction reduction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PMR, primary mitral regurgitation; SGLT2i, sodium‐glucose transport protein 2 inhibitor; SMR, secondary mitral regurgitation; and STS, Society of Thoracic Surgeons.
Change in LVEF Over Time
Mean LVEF was 56.0±14.0% for PMR and 33.0±10.4% for SMR at baseline. In the first postprocedural echocardiography study (mean, 1.4±0.4 days after TEER), the mean LVEF was 52.3±13.9% for PMR and 32.1±10.3% for SMR, whereas at follow‐up (median, 9.9; interquartile range, 7.8–11.9 months after TEER), it was 53.0±14.5% for PMR and 33.1±10.9% for SMR. The decrease in mean LVEF was not statistically significant for patients with SMR, whereas the reduction in LVEF for patients with PMR was significant at both time points (P<0.001 for both; Figure 2).
Figure 2. Mean left ventricular ejection fraction (LVEF) according to mitral regurgitation cause.

PMR indicates primary mitral regurgitation; and SMR, secondary mitral regurgitation.
There were also differences in the rates of patients who have improved LVEF after the procedure: it improved by 10% following TEER in 21.1% of the patients with SMR, compared with 10.9% of patients with PMR, and by 15% in 17.6% versus 7.6%, respectively. After 12 months, LVEF improved by 10% in 30.4% of the patients with SMR versus 13.3% of patients with PMR, and by 15% in 25.1% versus 9.8%, respectively (P<0.001 for all). There were no differences in the rates of MR improvement (P=0.733 for PMR, and P=0.343 for SMR). The residual mitral regurgitation was numerically higher in the PMR group, but not statistically significant (rates of MR ≥2 were 17.8% versus 11.9% in patients with SMR before discharge [P=0.092] and 18.1% versus 13.8% after 6 to 12 months [P=0.120]; Figure 3). Other echocardiographic and hemodynamic data are presented in Table 2.
Figure 3. Degree of mitral regurgitation (MR) before and after transcatheter mitral valve edge‐to‐edge repair.

PMR indicates primary mitral regurgitation; and SMR, secondary mitral regurgitation.
Table 2.
Echocardiographic and Hemodynamic Information
| Parameter | PMR LVEFR (n=152) | PMR controls (n=575) | P value | SMR LVEFR (n=317) | SMR controls (n=1490) | P value | PMR vs SMR |
|---|---|---|---|---|---|---|---|
| P value | |||||||
| Mitral regurgitation, mean±SD | 3.8±0.4 | 3.8±0.5 | 0.958 | 3.7±0.5 | 3.8±0.4 | 0.930 | <0.001 |
| Mitral valve area, mean±SD, cm | 5.2±1.4 | 5.2±1.5 | 0.890 | 5.4±1.8 | 5.4±2.1 | 0.794 | 0.127 |
| Vena contracta, mean±SD, mm | 6.8±2.3 | 6.6±2.1 | 0.281 | 7.1±2.0 | 6.5±2.1 | 0.009 | 0.208 |
| Pulmonary vein flow reversal, % | 44.1 | 44.5 | 0.746 | 47.1 | 45.6 | 0.126 | 0.322 |
| LV ejection fraction, mean±SD, % | 56.8±12.4 | 55.4±14.9 | 0.008 | 34.8±10.6 | 32.4±10.3 | <0.001 | <0.001 |
| LV end‐diastolic diameter, mean±SD, mm | 53.5±14.6 | 52.0±15.1 | 0.145 | 62.1±15.7 | 59.4±13.1 | 0.074 | <0.001 |
| LV end‐systolic diameter, mean±SD, mm | 37.7±14.7 | 37.4±15.4 | 0.573 | 47.2±16.1 | 47.7±15.5 | 0.382 | <0.001 |
| LV end‐diastolic volume, mean±SD, mL | 137.9±52.1 | 140.1±54.3 | 0.174 | 199.9±91.3 | 193.8±88.6 | 0.249 | <0.001 |
| LV end‐systolic volume, mean±SD, mL | 66.1±30.1 | 64.7±29.2 | 0.283 | 142.8±71.4 | 140.2±69.3 | 0.332 | <0.001 |
| LV mass index, mean±SD, g/m | 152.1±71.4 | 151.3±68.3 | 0.392 | 202.5±91.3 | 199.2±94.1 | 0.271 | <0.001 |
| Left atrial diameter, mean±SD, mm | 36.1±21.4 | 36.2±23.1 | 0.847 | 38.1±23.7 | 37.9±19.7 | 0.476 | 0.495 |
| Left atrial area, mean±SD, cm2 | 34.7±11.1 | 34.4±10.9 | 0.578 | 34.1±10.1 | 34.2±10.2 | 0.455 | 0.386 |
| Left atrial volume index, mean±SD, mL/m2 | 62.3±30.1 | 64.2±29.4 | 0.482 | 65.1±30.3 | 65.5±30.7 | 0.732 | 0.117 |
| Left atrial pressure, mean±SD, mm Hg | 17.2±7.3 | 16.9±8.4 | 0.327 | 18.2±7.1 | 17.8±8.1 | 0.224 | 0.090 |
| Left atrial pressure V‐wave, mean±SD, mm Hg | 33.1±12.1 | 32.6±14.1 | 0.076 | 31.7±12.5 | 31.2±13.7 | 0.312 | 0.472 |
| Systolic pulmonary artery pressure, mean±SD, mm Hg | 47.4±16.7 | 48.1±15.9 | 0.271 | 50.1±16.1 | 49.4±15.8 | 0.101 | 0.331 |
| Right ventricular dysfunction, % | 22.8 | 21.9 | 0.254 | 44.1 | 43.1 | 0.358 | <0.001 |
| TAPSE, mean±SD, mm | 18.2±7.2 | 17.9±6.9 | 0.162 | 15.3±6.1 | 15.1±7.1 | 0.234 | <0.001 |
| Systolic blood pressure, mean±SD, mm Hg | 121.2±19.9 | 119.9±19.4 | 0.159 | 115.1±18.1 | 115.6±19.1 | 0.321 | 0.226 |
| Diastolic blood pressure, mean±SD, mm Hg | 71.3±13.1 | 70.7±11.2 | 0.294 | 68.7±12.9 | 68.3±14.4 | 0.513 | 0.445 |
| Heart rate, mean±SD, 1/min | 72.2±13.1 | 72.0±14.9 | 0.582 | 72.7±14.1 | 72.6±14.3 | 0.314 | 0.932 |
| Reduction in degree of regurgitation, mean±SD | 2.3±0.8 | 2.3±0.9 | 0.733 | 2.2±0.9 | 2.2±0.1 | 0.343 | 0.424 |
| Rates of residual mitral regurgitation of ≥2, % | 11.3 | 11.1 | 0.385 | 10.1 | 9.9 | 0.218 | 0.293 |
LV indicates left ventricular; LVEFR, left ventricular ejection fraction reduction; PMR, primary mitral regurgitation; SMR, secondary mitral regurgitation; and TAPSE, tricuspid annular plane systolic excursion.
Predictors for LVEFR
In patients with PMR, predictors for a reduction in LVEF (the Hosmer‐Lemeshow goodness‐of‐fit test P=0.450) include baseline LVEF (OR, 1.03 [95% CI, 1.01–1.05 for each additional 1% in LVEF]; P=0.002), left atrial pressure V‐waves >30 mm Hg (OR, 1.22 [95% CI, 1.02–1.55]; P=0.042), right ventricular dysfunction (OR, 1.22 [95% CI, 1.02–1.45]; P=0.034), and systolic blood pressure >120 mm Hg on presentation (OR, 1.32 [95% CI, 1.12–1.76]; P<0.001; Table 3). In patients with SMR (the Hosmer‐Lemeshow goodness‐of‐fit test P=0.822), baseline LVEF had no independent impact on LVEFR (P=0.092). However, left atrial pressure >30 mm Hg remained a significant predictor for LVEFR (OR,1.35 [95% CI, 1.11–1.61]; P=0.005), as did right ventricular dysfunction (OR, 1.26 [95% CI, 1.10–1.42]; P=0.044) and systolic blood pressure >120 mm Hg (OR, 1.41 [95% CI, 1.09–2.03]; P<0.001; Table 4).
Table 3.
Predictors of Acute LVEFR in Patients With PMR
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, each additional year | 1.32 (0.94–1.65) | 0.108 | 1.35 (0.90–3.22) | 0.274 |
| Female sex | 1.24 (0.88–1.72) | 0.180 | 1.21 (0.82–1.96) | 0.372 |
| LV ejection fraction at baseline, each additional 1% | 1.04 (1.02–1.08) | <0.001 | 1.03 (1.01–1.05) | 0.002* |
| LV end‐diastolic diameter >55 mm | 1.06 (0.85–1.41) | 0.245 | 0.88 (0.57–1.71) | 0.370 |
| Atrial fibrillation | 1.32 (0.98–1.68) | 0.094 | 1.25 (0.80–2.03) | 0.628 |
| PAPs >50 mm Hg | 1.30 (0.94–1.78) | 0.114 | 1.22 (0.83–2.44) | 0.831 |
| Left atrial pressure V‐wave >30 mm Hg | 1.24 (1.05–1.48) | 0.022 | 1.22 (1.02–1.55) | 0.042* |
| Right ventricular dysfunction | 1.44 (1.11–1.83) | 0.008 | 1.22 (1.02–1.45) | 0.034* |
| Systolic blood pressure >120 mm Hg | 1.33 (1.09–1.60) | <0.001 | 1.32 (1.12–1.76) | <0.001* |
| Residual mitral regurgitation ≥2 | 0.84 (0.49–1.27) | 0.202 | 0.93 (0.44–2.30) | 0.449 |
LV indicates left ventricular; LVEFR, left ventricular ejection fraction reduction; OR, odds ratio; PAPs, pulmonary artery systolic pressure; and PMR, primary mitral regurgitation.
P<0.05.
Table 4.
Predictors of Acute LVEFR in Patients With SMR
| Variable | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, each additional year | 1.32 (0.98–1.68) | 0.120 | 1.24 (0.83–1.49) | 0.483 |
| Female sex | 1.12 (0.78–1.43) | 0.244 | 1.09 (0.62–3.01) | 0.573 |
| LV ejection fraction at baseline, each additional 1% | 1.05 (1.01–1.12) | 0.040 | 1.04 (0.99–1.11) | 0.092 |
| LV end‐diastolic diameter > 55mm | 1.08 (0.76–1.50) | 0.248 | 0.95 (0.67–2.04) | 0.446 |
| Atrial fibrillation | 1.11 (0.99–1.25) | 0.082 | 1.07 (0.91–1.33) | 0.357 |
| PAPs >50 mm Hg | 1.34 (1.01–1.66) | 0.035 | 1.32 (0.99–1.69) | 0.101 |
| Left atrial pressure V‐wave >30 mm Hg | 1.38 (1.20–1.56) | 0.008 | 1.35 (1.11–1.61) | 0.005* |
| Right ventricular dysfunction | 1.27 (1.02–1.51) | 0.010 | 1.26 (1.10–1.42) | 0.044* |
| Systolic blood pressure >120 mm Hg | 1.42 (1.18–1.68) | <0.001 | 1.41 (1.09–2.03) | <0.001* |
| Residual mitral regurgitation ≥2 | 0.86 (0.55–1.17) | 0.220 | 0.81 (0.38–1.84) | 0.386 |
LV indicates left ventricular; LVEFR, left ventricular ejection fraction reduction; OR, odds ratio; PAPs, pulmonary artery systolic pressure; and SMR, secondary mitral regurgitation.
Denotes P<0.05.
In 2 further analyses within the SMR group, we sought to assess the impact of “disproportionate MR” (according to the EROA/LVEDV ratio) and “COAPT Trial–like” characteristics on rates of LVEFR. In both cases, no correlation was found (P=0.183 for EROA/LVEDV ratio and P=0.892 in the comparison of 1102 COAPT Trial–like patients and 705 non–COAPT Trial–like controls). Finally, rates of LVEFR were also not different in the comparison of 197 (10.9%) patients with SMR with LVEF >50% versus those with LVEF <50% at baseline (P=0.291).
Clinical Outcomes
In patients with PMR, all‐cause mortality at 12 months was significantly higher for patients with LVEFR (16.9% versus 9.7%; P<0.001; Figure 4 and Table S1), as were rates of major adverse cardiac events (24.5% versus 14.6%; P=0.006; Table S1). In fact, death rates were already higher during admission for patients with PMR with LVEFR (4.2% versus 2.8%; P=0.043). Hospitalization rates for heart failure were also higher (20.4% versus 11.6%; P=0.015). In patients with SMR, there were no major differences in mortality (P=0.236; Figure 5 and Table S1) or major adverse cardiac events (31.5% versus 30.1%; P=0.582). Other secondary outcomes, including rates of New York Heart Association classification 3 to 4, needed for repeated TEER or mitral valve surgery, were not different between the patients with LVEFR and controls in either PMR or SMR. Notably, patients with LVEFR were treated with more clips in both groups (1.9±0.6 versus 1.7±0.6 in the PMR group [P=0.008] and 1.9±0.7 versus 1.7±0.7 in the SMR group [P=0.020]; Table S1).
Figure 4. The impact of left ventricular ejection fraction reduction (LVEFR) on all‐cause death at 12 months in patients with primary mitral regurgitation.
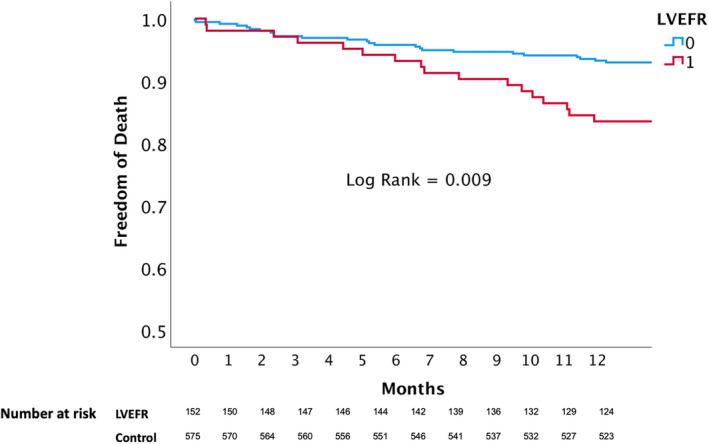
Figure 5. The impact of left ventricular ejection fraction reduction (LVEFR) on all‐cause death at 12 months in patients with secondary mitral regurgitation.

Following Cox proportional hazards regression analysis of the whole cohort, adjusting for confounding factors, age (hazard ratio [HR], 1.08 for each additional year [95% CI, 1.02–1.20]; P=0.013), PMR cause (HR, 0.84 [95% CI, 0.69–0.97]; P=0.003), LVEF at baseline (HR, 0.93 for each additional 1% [95% CI, 0.70–0.97]; P=0.020), left atrial pressure V‐wave >30 mm Hg (HR, 1.29 [95% CI, 1.01–2.03]; P=0.041), and renal failure (HR, 1.96 [95% CI, 1.31–2.44]; P<0.001) were predictors of mortality at 12 months. However, LVEFR (HR, 1.48 [95% CI, 0.88–2.21]; P=0.110; Table 5) was not significantly associated with mortality. However, within the PMR group, LVEFR was independently associated with mortality at 12 months (HR, 1.37 [95% CI, 1.04–1.76]; P=0.040; Tables S2 and S3).
Table 5.
Predictors of 12‐Month All‐Cause Mortality
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age, each additional year | 1.08 (1.02–1.20) | 0.013* |
| Female sex | 1.21 (0.80–2.32) | 0.356 |
| Cause of MR, primary | 0.84 (0.69–0.97) | 0.003* |
| LV ejection fraction at baseline, each 1% | 0.93 (0.70–0.97) | 0.020* |
| Diabetes | 1.28 (0.76–1.91) | 0.364 |
| MR severity | 1.45 (0.98–2.14) | 0.533 |
| PAPs >50 mm Hg | 1.34 (0.79–2.37) | 0.322 |
| Left atrial pressure V‐wave >30 mm Hg | 1.29 (1.01–2.03) | 0.041* |
| Right ventricular dysfunction | 1.30 (0.81–1.84) | 0.235 |
| Renal failure | 1.96 (1.31–2.44) | <0.001* |
| LVEFR | 1.48 (0.88–2.21) | 0.110 |
HR indicates hazard ratio; LV, left ventricular; LVEFR, left ventricular ejection fraction reduction; MR, mitral regurgitation; and PAPs, pulmonary artery systolic pressure.
Denotes P<0.05.
DISCUSSION
In this study, which assessed the impact of transcatheter mitral valve edge‐to‐edge repair on left ventricular function, we found that >20% of patients with PMR and 17.5% of patients with SMR incurred an acute reduction in LVEF soon after TEER. The ventricular function of patients with PMR is impacted differently by TEER than those with SMR: although both groups demonstrate a significant rate of LVEFR after TEER, in patients with SMR, this rate is significantly lower. Nevertheless, a heterogenous response of the left ventricle was apparent over time, especially in patients with SMR, where an almost 2‐fold rate of the patients demonstrated an improvement in LVEF after TEER, compared with patients with PMR. Yet, as expected, the initial LVEF is much higher in patients with PMR, and these dynamics are somewhat anticipated. Finally, the presence of LVEFR is independently associated with an increased risk of adverse events in patients with PMR, whereas in patients with SMR, it confers no increased risk.
Previous studies assessing the dynamics in LV function after TEER were incongruent. In the EVEREST (Endovascular Valve Edge–to–Edge Repair Study) trial, among 107 patients who underwent cardiac catheterization before and immediately following TEER, cardiac output and stroke volume increased, whereas systemic vascular resistance decreased. LVEF was reduced in these patients with PMR, from 59.8±8.3% to 56.3±9.4%. 10 In one small recent study of patients with SMR, a decrease in LV end‐diastolic and LV end‐systolic diameters was observed after TEER, mean pulmonary artery pressures were reduced, and LVEF was unchanged. Again, cardiac output increased. 6 In another study of 130 patients with mixed cause, in whom 54% had reduced LVEF (<40%), varying results according to LVEF range were observed: in patients with middle or preserved ejection fraction, reverse remodeling with reduced LV dilatation and increased contractility was reported. On the other hand, in patients with reduced ejection fraction, there was no reverse remodeling and no improvement in LV function. 7 Another small study (n=43) found no changes in LVEF following TEER, but a reduction in pulmonary artery pressures was noted. 8 Pleger et al assessed 1‐year outcomes in 41 patients with SMR (LVEF=33±3%), and found significantly reduced left atrial volume and LV end‐systolic diameter, as well as significantly increased LVEF. 12 In the COAPT Trial, mean LVEF actually deteriorated following TEER and in the control arm, albeit at a lower rate in the study group (reduction of 5.6±1.2% versus 8.8±1.1% of control; P=0.048). 27
For predictors of change in LV function and dimensions, one study suggested reverse remodeling only in patients with lower values of logistic EuroSCORE and Society of Thoracic Surgeons scores, LVEDV index, right ventricular end‐systolic area, and systolic pulmonary artery pressure at baseline. In multivariable analysis, only systolic pulmonary artery pressure remained an independent predictor of improvement. 28 Another study using cardiovascular magnetic resonance to assess extent and predictors of reverse remodeling (defined by reduction of LVEDV index >15% compared with baseline) demonstrated improvement in 34% of the patients, predicted by improvement in MR volume and MR fraction. 9 Recently, Hagnas et al assessed immediate changes in left ventricular function in 399 patients with SMR, and showed that in most it had not changed, whereas in close to 10% it had slightly improved (by only 1%), and in a similar rate it had deteriorated. Patients with improved LVEF had lower mean LVEF at baseline (26% versus 35%; P<0.001), as well as a higher EuroSCORE II. Notably, decreased postprocedural LVEF was associated with a higher risk for mortality, whereas improved LVEF was protective, compared with unchanged LVEF, 19 as opposed to the findings in our study for this same population.
In our study, by far the largest to date to assess changes in LVEF following TEER, we noted a difference in both the dynamics and the clinical impact of LVEF decline according to cause. It is imperative to mention that PMR and SMR are completely different patient populations, as was also apparent in this study, experiencing MR attributable to valvular disease in the first and predominantly ventricular disease in the latter. In addition, the rates of residual MR ≥2 were somewhat (but not significantly) higher in the PMR group. More important, residual MR was not independently associated with LVEFR in patients with either PMR or SMR. Nevertheless, we have seen that, although in patients with SMR, the decrease in LVEF was temporary, and did not impact prognosis, in patients with PMR, there was a 3.0% absolute reduction in the ejection fraction at discharge, which persisted after 6 to 12 months. In addition, for those patients with PMR who had a ≥15% reduction in LVEF immediately following TEER, a significantly higher risk for death was noted. Predictors for LVEFR in patients with PMR included a higher LVEF, high left atrial pressure V‐waves, right ventricular dysfunction, and systolic blood pressure >120 mm Hg at presentation. In patients with SMR, a higher LVEF was not independently associated with LVEFR.
The reduction in LVEF following TEER may represent the response to the rapid increase in left ventricular afterload, attributable to the rapid loss of the low‐resistance “shunt” into the left atrium. Afterload is thought to be mitigated by mitral regurgitation, which, in turn, increases myocardial contraction and may lead to underestimation of the severity of myocardial impairment. 29 However, this theory is not fully accepted, as some models demonstrate a high impedance to the retrograde flow through the mitral valve in patients with chronic severe MR, exceeding the impedance to forward flow. 30 More important, there are known hemodynamic differences between PMR and SMR, 29 , 31 , 32 which perhaps could explain the differences in LVEFR and its prognostic significance in our study. In patients with PMR, who had preserved left ventricular systolic function, the immediate impact of abruptly augmented afterload after TEER appears to be greater compared with patients with SMR. The fact that elevated systemic blood pressure is independently associated with LVEFR in our study supports that theory. However, the exact pathophysiological processes that explain the reduction in LVEF, as well as the observed differences between PMR and SMR (including the ventricular response after TEER), warrant further investigation. Another putative mechanism is takotsubo syndrome, or stress‐induced cardiomyopathy, which was previously described after both mitral valve replacement and TEER, 33 , 34 , 35 and may be the cause of LVEFR in some patients.
As for the impact of LVEFR on outcomes, previous studies have shown that LVEFR following MVR or TEER is associated with worse outcomes. 18 , 19 , 20 , 22 , 23 Several theories are possible to explain the differential impact of LVEFR in PMR versus SMR. First, the relative decrease of 15% in LVEF is a larger absolute reduction in ejection fraction for patients with PMR, who begin with a mean LVEF of around 55%, whereas in patients with SMR, whose mean LVEF is 32.7% at baseline, the relative decrease translates to a lower absolute reduction. In addition, although the mechanism of MR in patients with PMR is primarily attributable to a diseased valve, myocardial disease has been reported as well, including hypertrophy, myocardial fibrosis, and adverse remodeling. 36 , 37 , 38 The rapid decrease in LVEF could represent more severe underlying pathologic feature of the left ventricle. These changes are likely underdiagnosed in cases of PMR, are possibly undertreated, and may contribute to the worse prognosis of these patients.
The main limitation of this study is in its observational design. We also had no comprehensive information on hemodynamic measurements, in particular about the components of the true afterload, which could have contributed to the understanding of LVEFR in these patients. In addition, the definition used for LVEFR was arbitrary, as there is no consensus on the cutoffs for patients with PMR or SMR. As in other studies, we based our definition on the median of change in LVEF. Finally, measurements are based on echocardiography performed at different time points before and after TEER, and not at uniform schedule, as would have occurred in a prospective study. Finally, information from cardiac magnetic resonance imaging could have added important information on left ventricular remodeling and myocardial fibrosis, and could distinguish stress‐induced cardiomyopathy from other myocardial diseases.
CONCLUSIONS
In conclusion, we found that acute reduction in LV performance occurs in around 20% of patients undergoing TEER. This phenomenon is persistent after 1 year and independently increases the risk for all‐cause mortality and major adverse cardiac events in PMR, whereas in patients with SMR, it is reversible and does not seem to affect prognosis.
APPENDIX
MITRA‐EF group
The MITRA‐EF group includes the investigators Leor Perl, Mark Kheifets, Amos Levi, Yaron Shapira, Shmuel Schwartzenberg, Ran Kornowski (Rabin Medical Center, Petah‐Tikva, Israel), Ascione Guido, Eustachio Agricola, Paolo Denti, Francesco Melillo, Matteo Montorfano, Francesco Maisano (IRCCS San Raffaele Hospital, Milan, Italy), Mirjam Gauri Wild (Bad Krozingen, Germany, and Bern University Hospital, Bern, Switzerland), Fabien Praz (Bern University Hospital), Antonio Popolo Rubbio, Francesco Bedogni (IRCCS Policlinico San Donato, San Donato Milanese, Italy), Federico De Marco (IRCCS Policlinico San Donato and Centro Cardiologico Monzino IRCCS, Milan, Italy), Ronen Beeri (Hadassah‐Hebrew University Medical Center, Jerusalem, Israel), Mony Shuvy (Hadassah‐Hebrew University Medical Center and Shaare Zedek Medical Center, Jerusalem, Israel), Xavier Freixa, Juan Carlos de la Fuente Mancera (IDIBAPS, University of Barcelona, Barcelona, Spain), Arturo Giordano, Filippo Finizio, Nicola Corcione (Pineta Grande Hospital, Castel Volturno, Caserta, Italy), Nicolas M. Van Mieghem, J. F. W. Ooms (Erasmus University Medical Center, Rotterdam, the Netherlands), Neil Fam, Cormac O'Connor (St. Michael's Hospital, Toronto, Ontario, Canada), Stefan Toggweiler (Luzerner Kantonsspital, Lucerne, Switzerland), Stefano Pidello, Fabrizio D'Ascenzo, Filippo Angelini (Città della Salute e della Scienza Hospital, Turin, Italy), Dan Haberman (Kaplan Medical Center, Rehovot, Israel), Gabriele Crimi, Italo Porto (IRCCS, AOU San Martino IST, Genoa, Italy), Ottavia Cozzi, Antonio Mangieri, Damiano Regazzoli (IRCCS Humanitas Research Hospital, Rozzano‐Milan, Italy), Francesco Giannini, Paolo Cimaglia, Filippo Flamigni (GVM Care and Research Maria Cecilia Hospital, Cotignola, Italy), and Giuseppe Tarantini and Giulia Masiero (University of Padua, Padua, Italy).
Sources of Funding
None.
Disclosures
Dr Perl is a consultant to Edwards Lifesciences and an Abbott clinical events committee (CEC) member. Dr Praz received travel expenses from Abbott Vascular, Polares Medical, and Edwards Lifesciences. Dr Shuvy is a proctor and consultant for Abbott and Edwards Lifesciences. Dr Toggweiler is a consultant and proctor for Abbott Vascular, Medtronic, Biosensors, and Boston Scientific; is a consultant for Medira, AtHeart Medical, Polares Medical, Veosource, Shockwave, and Teleflex; has received institutional research grants from Boston Scientific, Fumedica, and Biosensors; and holds equity in Hi‐D Imaging. Dr Porto reports consultant or speaker fees from Biotronik, ABIOMED, Terumo, Philips, Sanofi, Amgen, Daiichi‐Sankyo, AstraZeneca, Bayer, and PILVEFR. Dr Maisano received grant and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, and Terumo; received consulting fees, honoraria personal, and institutional support from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, and Mtex; and received royalty income/intellectual property rights from Edwards Lifesciences Shareholder (including share options) of Cardiogard, Cardiovalve, Magenta, SwissVortex, Transseptal solutions, 4Tech, and Perifect. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Acknowledgments
The authors thank Dr Nicola Corcione, Dr Antonio Mangieri, Dr Damiano Regazzoli, Dr Paolo Cimaglia, Dr Filippo Flamigni, and Dr Giulia Masiero for their immeasurable contribution to this study.
This article was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029735
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Leor Perl, Email: leorperl@gmail.com, Email: leorper@clalit.org.il.
the MITRA‐EF study:
Leor Perl, Mark Kheifets, Amos Levi, Yaron Shapira, Shmuel Schwartzenberg, Ran Kornowski, Ascione Guido, Eustachio Agricola, Paolo Denti, Francesco Melillo, Matteo Montorfano, Francesco Maisano, Mirjam Gauri Wild, Fabien Praz, Antonio Popolo Rubbio, Francesco Bedogni, Federico De Marco, Ronen Beeri, Mony Shuvy, Xavier Freixa, Juan Carlos de la Fuente Mancera, Arturo Giordano, Filippo Finizio, Nicola Corcione, Nicolas M. Van Mieghem, J. F. W. Ooms, Neil Fam, Cormac O’Connor, Stefan Toggweiler, Stefano Pidello, Fabrizio D’Ascenzo, Filippo Angelini, Dan Haberman, Gabriele Crimi, Italo Porto, Ottavia Cozzi, Antonio Mangieri, Damiano Regazzoli, Francesco Giannini, Paolo Cimaglia, Filippo Flamigni, Giuseppe Tarantini, and Giulia Masiero
References
- 1. Feldman T, Foster E, Glower DD, Glower DG, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355 [DOI] [PubMed] [Google Scholar]
- 2. Franzen O, Baldus S, Rudolph V, Meyer S, Knap M, Koschyk D, Treede H, Barmeyer A, Schofer J, Costard‐Jäckle A, et al. Acute outcomes of MitraClip therapy for mitral regurgitation in high‐surgical‐risk patients: emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur Heart J. 2010;31:1373–1381. doi: 10.1093/eurheartj/ehq050 [DOI] [PubMed] [Google Scholar]
- 3. Whitlow PL, Feldman T, Pedersen WR, Lim DS, Kipperman R, Smalling R, Bajwa T, Herrmann HC, Lasala J, Maddux JT, et al. Acute and 12‐month results with catheter‐based mitral valve leaflet repair. J Am Coll Cardiol. 2012;59:130–139. doi: 10.1016/j.jacc.2011.08.067 [DOI] [PubMed] [Google Scholar]
- 4. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 5. Song BG, On YK, Jeon E‐S, Kim D‐K, Lee S‐C, Park SW, Oh JK, Sung KI, Park P. Atrioventricular reverse remodeling after valve repair for chronic severe mitral regurgitation: 1‐year follow‐up. Clin Cardiol. 2010;33:630–637. doi: 10.1002/clc.20782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barth S, Hautmann MB, Kerber S, Gietzen F, Zacher M, Halbfass P, Müller P, Schade A, Deneke T, Diegeler A, et al. Hemodynamic improvement at three months after MitraClip® treatment in end‐stage heart failure patients with functional mitral regurgitation. J Heart Valve Dis. 2016;25:475–482. [PubMed] [Google Scholar]
- 7. Schrage B, Kalbacher D, Schwarzl M, Rübsamen N, Waldeyer C, Becher PM, Tigges E, Burkhoff D, Blankenberg S, Lubos E, et al. Distinct hemodynamic changes after interventional mitral valve edge‐to‐edge repair in different phenotypes of heart failure: an integrated hemodynamic analysis. J Am Heart Assoc. 2018;7:e007963. doi: 10.1161/JAHA.117.007963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kretzler L, Große S, Wiedemann S, Wunderlich C, Nowak C, Riedel C, Sieger T, Schoen S. The effects of interventional mitral valve repair using the MitraClip system on the results of pulmonary function testing, pulmonary pressure and diffusing capacity of the lung. BMC Cardiovasc Disord. 2021;21:235. doi: 10.1186/s12872-021-02042-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spieker M, Marpert J, Afzal S, Scheiber D, Bönner F, Horn P, Kelm M, Westenfeld R. Extent and determinants of left ventricular reverse remodeling in patients with secondary mitral regurgitation undergoing MitraClip implantation. Int J Cardiol Heart Vasc. 2021;34:100804. doi: 10.1016/j.ijcha.2021.100804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siegel RJ, Biner S, Rafique AM, Rinaldi M, Lim S, Fail P, Hermiller J, Smalling R, Whitlow PL, Herrmann HC, et al. The acute hemodynamic effects of MitraClip therapy. J Am Coll Cardiol. 2011;57:1658–1665. doi: 10.1016/j.jacc.2010.11.043 [DOI] [PubMed] [Google Scholar]
- 11. Auricchio A, Schillinger W, Meyer S, Maisano F, Hoffmann R, Ussia GP, Pedrazzini GB, van der Heyden J, Fratini S, Klersy C, et al. Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol. 2011;58:2183–2189. doi: 10.1016/j.jacc.2011.06.061 [DOI] [PubMed] [Google Scholar]
- 12. Pleger ST, Schulz‐Schönhagen M, Geis N, Mereles D, Chorianopoulos E, Antaredja M, Lewening M, Katus HA, Bekeredjian R. One year clinical efficacy and reverse cardiac remodelling in patients with severe mitral regurgitation and reduced ejection fraction after MitraClip implantation. Eur J Heart Fail. 2013;15:919–927. doi: 10.1093/eurjhf/hft046 [DOI] [PubMed] [Google Scholar]
- 13. Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Immé S, Mangiafico S, Barbanti M, Ministeri M, Cageggi A, et al. Extended use of percutaneous edge‐to‐edge mitral valve repair beyond EVEREST (Endovascular Valve Edge‐to‐Edge Repair) criteria: 30‐day and 12‐month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc Interv. 2015;8:74–82. doi: 10.1016/j.jcin.2014.07.024 [DOI] [PubMed] [Google Scholar]
- 14. Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Immé S, Mangiafico S, Barbanti M, Ministeri M, Cageggi A, et al. Gender‐related clinical and echocardiographic outcomes at 30‐day and 12‐month follow up after MitraClip implantation in the GRASP registry. Catheter Cardiovasc Interv. 2015;85:889–897. doi: 10.1002/ccd.25715 [DOI] [PubMed] [Google Scholar]
- 15. Papadopoulos K, Ikonomidis I, Chrissoheris M, Chalapas A, Kourkoveli P, Parissis J, Spargias K. MitraClip and left ventricular reverse remodelling: a strain imaging study. ESC Heart Fail. 2020;7:1409–1418. doi: 10.1002/ehf2.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elbelbesy RA, Elsawah AM, Ammar AS, Khamis HA, Shehata IE. Safety and efficacy outcomes at 1 year after MitraClip therapy for percutaneous mitral valve repair in patients with severe mitral regurgitation: the Egyptian experience. Egypt Heart J. 2021;73:42. doi: 10.1186/s43044-021-00166-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brouwer HJ, Den Heijer MC, Paelinck BP, Debonnaire P, Vanderheyden M, Van De Heyning CM, De Bock D, Coussement P, Saad G, Ferdinande B, et al. Left ventricular remodelling patterns after MitraClip implantation in patients with severe mitral valve regurgitation: mechanistic insights and prognostic implications. Eur Heart J Cardiovasc Imaging. 2019;20:307–313. doi: 10.1093/ehjci/jey088 [DOI] [PubMed] [Google Scholar]
- 18. Jogani S, Van de Heyning CM, Paelinck BP, De Bock D, Mertens P, Heidbuchel H, Claeys MJ. Afterload mismatch after MitraClip implantation: intraoperative assessment and prognostic implications. J Invasive Cardiol. 2020;32:88–93. [DOI] [PubMed] [Google Scholar]
- 19. Hagnäs MJ, Grasso C, Di Salvo ME, Caggegi A, Barbanti M, Scandura S, Milici A, Motta G, Bentivegna A, Sardone A, et al. Impact of post‐procedural change in left ventricle systolic function on survival after percutaneous edge‐to‐edge mitral valve repair. J Clin Med. 2021;10:4748. doi: 10.3390/jcm10204748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quintana E, Suri RM, Thalji NM, Daly RC, Dearani JA, Burkhart HM, Li Z, Enriquez‐Sarano M, Schaff HV. Left ventricular dysfunction after mitral valve repair—the fallacy of “normal” preoperative myocardial function. J Thorac Cardiovasc Surg. 2014;148:2752–2760. doi: 10.1016/j.jtcvs.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 21. Melisurgo G, Ajello S, Pappalardo F, Guidotti A, Agricola E, Kawaguchi M, Latib A, Covello RD, Denti P, Zangrillo A, et al. Afterload mismatch after MitraClip insertion for functional mitral regurgitation. Am J Cardiol. 2014;113:1844–1850. doi: 10.1016/j.amjcard.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 22. Varghese R, Itagaki S, Anyanwu AC, Milla F, Adams DH. Predicting early left ventricular dysfunction after mitral valve reconstruction: the effect of atrial fibrillation and pulmonary hypertension. J Thorac Cardiovasc Surg. 2014;148:422–427. doi: 10.1016/j.jtcvs.2013.08.073 [DOI] [PubMed] [Google Scholar]
- 23. Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ, Sarano ME, Orszulak TA. Determinants of early decline in ejection fraction after surgical correction of mitral regurgitation. J Thorac Cardiovasc Surg. 2008;136:442–447. doi: 10.1016/j.jtcvs.2007.10.067 [DOI] [PubMed] [Google Scholar]
- 24. Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, Vranckx P, Mehran R, Kuck K‐H, Leon MB, Piazza N, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions. J Am Coll Cardiol. 2015;66:308–321. doi: 10.1016/j.jacc.2015.05.049 [DOI] [PubMed] [Google Scholar]
- 25. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation. JACC: Cardiovasc Imaging. 2019;12:353–362. doi: 10.1016/j.jcmg.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 26. Adamo M, Fiorelli F, Melica B, D'Ortona R, Lupi L, Giannini C, Silva G, Fiorina C, Branca L, Chiari E, et al. COAPT‐like profile predicts long‐term outcomes in patients with secondary mitral regurgitation undergoing MitraClip implantation. JACC: Cardiovasc Interv. 2021;14:15–25. doi: 10.1016/j.jcin.2020.09.050 [DOI] [PubMed] [Google Scholar]
- 27. Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, et al. Echocardiographic outcomes after transcatheter leaflet approximation in patients with secondary mitral regurgitation. J Am Coll Cardiol. 2019;74:2969–2979. doi: 10.1016/j.jacc.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 28. Cimino S, Maestrini V, Cantisani D, Petronilli V, Filomena D, Mancone M, Sardella G, Fedele F, Lancellotti P, Agati L. 2D/3D echocardiographic determinants of left ventricular reverse remodelling after MitraClip implantation. Eur Heart J Cardiovasc Imaging. 2019;20:558–564. doi: 10.1093/ehjci/jey157 [DOI] [PubMed] [Google Scholar]
- 29. McDonald IG. Echocardiographic assessment of left ventricular function in mitral valve disease. Circulation. 1976;53:865–871. doi: 10.1161/01.CIR.53.5.865 [DOI] [PubMed] [Google Scholar]
- 30. Gaasch WH, Shah SP, Labib SB, Meyer TE. Impedance to retrograde and forward flow in chronic mitral regurgitation and the physiology of a double outlet ventricle. Heart. 2017;103:581–585. doi: 10.1136/heartjnl-2016-309747 [DOI] [PubMed] [Google Scholar]
- 31. Yesildag O, Koprulu D, Yuksel S, Soylu K, Ozben B. Noninvasive assessment of left ventricular end‐diastolic pressure with tissue Doppler imaging in patients with mitral regurgitation. Echocardiography. 2011;28:633–640. doi: 10.1111/j.1540-8175.2011.01393.x [DOI] [PubMed] [Google Scholar]
- 32. El‐Tallawi KC, Zhang P, Azencott R, He J, Xu J, Herrera EL, Jacob J, Chamsi‐Pasha M, Lawrie GM, Zoghbi WA. Mitral valve remodeling and strain in secondary mitral regurgitation: comparison with primary regurgitation and normal valves. JACC Cardiovasc Imaging. 2021;14:782–793. doi: 10.1016/j.jcmg.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 33. Nomura T, Munehisa Y, Nakashima M, Matsumoto T. Takotsubo syndrome following MitraClip procedure. Eur Heart J Case Rep. 2020;4:1–2. doi: 10.1093/ehjcr/ytaa384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pergolini A, Zampi G, Casali G, Madeo A, Visconti CL, Cipullo PL, Pino PG, Musumeci F. Takotsubo syndrome after mitral valve replacement: case report and brief review of the literature. J Cardiothorac Vasc Anesth. 2015;29:431–435. doi: 10.1053/j.jvca.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 35. Färber G, Mühle A, Doenst T, Borger MA, Mohr F‐W. Late onset Takotsubo cardiomyopathy after mitral valve replacement. Thorac Cardiovasc Surg. 2011;59:500–503. doi: 10.1055/s-0030-1270798 [DOI] [PubMed] [Google Scholar]
- 36. Stulak JM, Suri RM, Dearani JA, Burkhart HM, Sundt TM, Enriquez‐Sarano M, Schaff HV. Does early surgical intervention improve left ventricular mass regression after mitral valve repair for leaflet prolapse? J Thorac Cardiovasc Surg. 2011;141:122–129. doi: 10.1016/j.jtcvs.2010.08.068 [DOI] [PubMed] [Google Scholar]
- 37. Edwards NC, Moody WE, Yuan M, Weale P, Neal D, Townend JN, Steeds RP. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ Cardiovasc Imag. 2014;7:946–953. doi: 10.1161/CIRCIMAGING.114.002397 [DOI] [PubMed] [Google Scholar]
- 38. Witkowski TG, Thomas JD, Debonnaire PJMR, Delgado V, Hoke U, Ewe SH, Versteegh MIM, Holman ER, Schalij MJ, Bax JJ, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. 2013;14:69–76. doi: 10.1093/ehjci/jes155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


