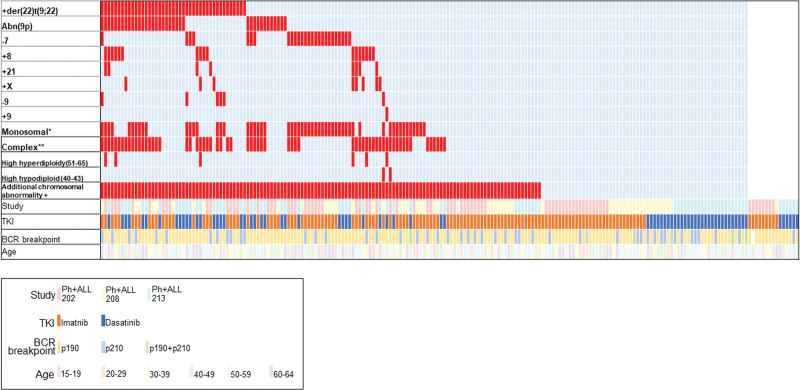
Additional chromosomal abnormalities (ACAs) are found in 60%–70% of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) cases. Although some high-risk chromosomal and genetic abnormalities have recently been reported,1,2 they have not been established as risk factors.3 The purpose of this study was to explore the prognostic importance of ACAs for Ph+ALL.
This study combined 3 Japan Adult Leukemia Study Group (JALSG) prospective studies in the era of tyrosine kinase inhibitors (TKIs): 77 registered in the Ph+ALL202 study, 55 registered in the Ph+ALL208 study, and 74 registered in the Ph+ALL213 study. The detailed treatment schedules for each study are described elsewhere.4-6 The Ph+ALL202 study was the first JALSG study using imatinib for Ph+ALL. In the Ph+ALL208 study, imatinib was also used as a TKI, but the emphasis was on the combination of chemotherapy and imatinib, and the duration of imatinib monotherapy was shorter compared with the Ph+ALL202 study. In the Ph+ALL213 study, dasatinib was used instead of imatinib, and the induction phase was separated into 2 steps (Supplemental Methods). In this study, we used chromosomal data reviewed in the final analysis of each study. A complex karyotype was defined by the presence of at least 3 abnormalities (ie, t[9;22] and 2 or more additional aberrations) as previously described.7-9
We analyzed data from 206 de novo adult Ph+ALL patients (Suppl. Table S1). The median age at diagnosis was 45 years, white blood cell count at diagnosis was 23,510/μL, and the TKI of initial treatment was imatinib in 132 patients (64.1%) and dasatinib in 74 patients (35.9%). An ACA was identified in 63.6% of all patients; the most common structural chromosomal abnormality was +der(22)t(9;22) (32.8% of patients with an ACA). A complex karyotype was observed in 48.1% of patients with an ACA. Of 152 evaluable patients, 121 (79.6%) achieved molecular complete remission (CR) at 3 months. Allogeneic stem cell transplantation (allo-SCT) in the first CR (CR1) was performed in 138 (67.0%) of 206 patients.
Regarding the overlap of ACAs, the pairwise analysis showed significant cooccurrence of +der(22)t(9;22) with both abnormal 9p and a complex karyotype (Figure 1; Suppl. Figure S1). Among 43 patients with +der(22)t(9;22), 67.4% also had a complex karyotype. On the contrary, +der(22)t(9;22) was observed in 46.0% of 63 patients with a complex karyotype.
Figure 1.
Landscape of additional chromosomal abnormalities. *Monosomal karyotype means 1 or more autosomal monosomy in addition to t(9;22) (−7 and −9 described above were also counted as monosomal). **Complex karyotype means 2 or more additional aberrations in addition to t(9;22) (ACAs described earlier were also counted as complex when there were at least 2 aberrations in addition to t[9;22]). ACA = additional chromosomal abnormalities.
At 5 years, the overall survival (OS) was 58.8% (95% confidence interval [CI], 51.4%-65.4%) in all 206 patients. OS was significantly higher in patients treated with dasatinib compared with those treated with imatinib (dasatinib: 74.2% [95% CI, 61.7%-83.1%] versus imatinib: 50.6% [95% CI, 41.6%-59.0%] at 5 y; P = 0.002). OS with a landmark at 3 months was significantly different between patients who achieved molecular CR at 3 months and those who did not (molecular CR(+): 65.7% [95% CI, 56.6%-73.4%] versus molecular CR(−): 43.5% [95% CI, 24.8%-60.9%] at 5 y; P = 0.02).
At 5 years, leukemia-free survival (LFS) was 51.0% (95% CI, 43.6%-57.9%) in 198 patients who achieved CR1. LFS tended to be higher in patients treated with dasatinib compared with those treated with imatinib (dasatinib: 55.7% [95% CI, 42.6%-66.9%] versus imatinib: 47.6% [95% CI, 38.5%-56.2%] at 5 y; P = 0.07). LFS with a landmark at 3 months was significantly different between patients who achieved molecular CR at 3 months and those who did not (molecular CR(+): 55.5% [95% CI, 46.3%-63.8%] versus molecular CR(−): 32.6% [95% CI, 15.7%-50.8%] at 5 y; P = 0.02).
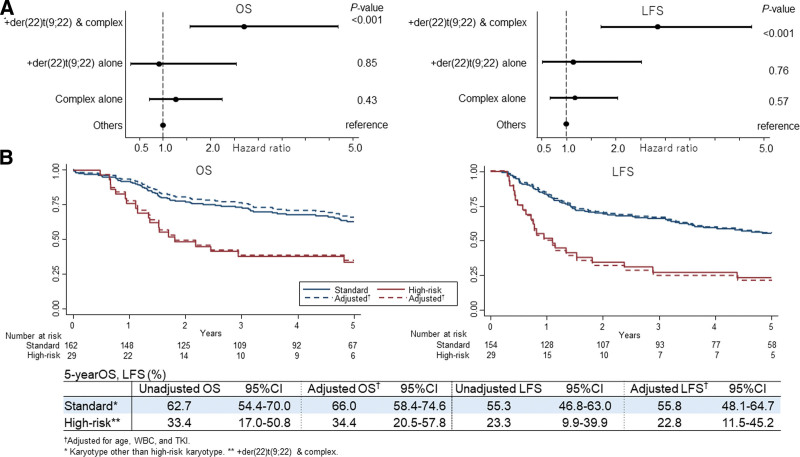
When divided into 4 groups of chromosomal abnormalities, (1) both +der(22)t(9;22) and a complex karyotype; (2) +der(22)t(9;22) alone; (3) a complex karyotype alone; and (4) others, OS and LFS were worse in patients with both +der(22)t(9;22) and complex karyotype (Suppl. Figure S2). In multivariate analysis, while coexistence of +der(22)t(9;22) and a complex karyotype was a significant risk factor for both OS and LFS, +der(22)t(9;22) alone or a complex karyotype alone were not significant risk factors (Figure 2A).
Figure 2.
Risk of additional chromosomal abnormalities. (A) Forest plots for the adjusted hazard ratios and 95% confidence intervals in the multivariate analysis. (B). Survival according to the risk of ACAs. All covariates other than karyotype included in multivariate models were age, WBC, and TKI. ACA = additional chromosomal abnormalities; TKI = tyrosine kinase inhibitor; WBC = white blood cell.
When the coexistence of +der(22)t(9;22) and a complex karyotype were considered high risk and the others are considered standard risk, both OS and LFS were significantly worse in high-risk patients compared with standard-risk patients (Figure 2B).
Patient characteristics by chromosomal risk are shown in Suppl. Table S2. There were no significant differences in baseline characteristics between the standard- and high-risk patients. The CR1 achievement rate was not significantly different between the standard- and high-risk patients (95.1% versus 100%; P = 0.22). In addition, the molecular CR rate at 3 months was not significantly different between the standard- and high-risk patients (79.8% versus 78.2%; P = 0.86). However, among patients who relapsed after achieving CR1, CR duration was significantly shorter in high-risk patients compared with standard-risk patients (0.68 versus 1.1 y; P = 0.046). The OS of high-risk patients was worse than that of low-risk patients, even when censored at the time of allo-SCT (1-y adjusted OS: 73.3% [95% CI, 54.6%-98.3%] versus 95.7% [95% CI, 92.0%-99.5%]).
Regarding the type of TKIs, although OS was significantly higher in standard-risk patients treated with dasatinib compared with standard-risk patients treated with imatinib (dasatinib: 85.2% [95% CI, 72.5%-92.3%] versus imatinib: 51.9% [95% CI, 41.7%-61.2%] at 5 y; P < 0.001), no significant differences in OS were observed between high-risk patients treated with dasatinib and high-risk patients treated with imatinib when post hoc analyses were performed (dasatinib: 33.7% [95% CI, 9.5%-60.4%] versus imatinib: 31.3% [95% CI, 11.4%-53.7%] at 5 y; P = 0.55). Similarly, although LFS tended to be higher in standard-risk patients treated with dasatinib compared with standard-risk patients treated with imatinib (dasatinib: 65.4% [95% CI, 49.8%-77.2%] versus imatinib: 49.3% [95% CI, 38.9%-58.9%] at 5 y; P = 0.06), no significant differences in LFS were observed between high-risk patients treated with dasatinib and high-risk patients treated with imatinib when post hoc analyses were performed (dasatinib: 19.2% [95% CI, 3.3%-45.0%] versus imatinib: 25.0% [95% CI, 7.8%-45.0%] at 5 y; P = 0.54).
Although it has been reported that +der(22)t(9;22) is an ACA with a poor prognosis1, we clarified that prognoses differ depending on the coexistence of a complex karyotype. It is known that an ACA is often observed in Ph+ALL patients, but reports of whether ACAs affect prognosis are inconsistent: some studies have reported that ACAs were a poor prognostic factor7,9 whereas others have reported that ACAs were not a poor prognostic factor.10-12 Considering the types and combinations of ACAs, we were able to clarify a subgroup of ACAs with poor prognosis in Ph+ALL.
The cooccurrence of +der(22)t(9;22) and a complex karyotype was associated with a poor prognosis. In this high-risk combination of chromosomal abnormalities, not only hematological CR but also molecular CR could be achieved with the same probability as patients with standard risk, but the risk was characterized by early recurrence. In addition, improved survival with dasatinib instead of imatinib, which was observed in standard-risk patients, was not observed in high-risk patients. In this study, a complex karyotype was defined as the presence of at least 3 abnormalities, which meant that high-risk patients had 1 or more other abnormalities in addition to Ph of the main cell lineage and +der(22)t(9;22). It has been reported that ponatinib, a third-generation TKI, and/or blinatumomab, a bispecific T-cell engager, improves treatment results for Ph+ALL patients.13,14 Because ponatinib is a multikinase inhibitor and blinatumomab is an immunotherapy drug, both of which act in addition to BCR-ABL1 kinase, they may be effective for Ph+ALL patients with high-risk chromosomal abnormalities. As ponatinib was approved in 2016 for relapsed/refractory Ph+ALL and blinatumomab was approved in 2018 for relapsed/refractory ALL in Japan, the effects of these new drugs will be clarified in future clinical trials.
Recently, the effect of genetic aberrations on prognosis has been reported by clinical studies of Ph+ALL. The presence of additional copy number alterations (CNAs) with the cooccurrence of IKZF1 plus CDKN2A/B and/or PAX5 has been associated with poor survival.2,14 In another study, we are conducting genetic analysis using preserved specimens from clinical studies of Ph+ALL conducted at JALSG. Considering that several chromosomal abnormalities are reported to be associated with CNAs in ALL,15 there may be genetic aberrations associated with the high-risk combination of ACAs identified in this study. The elucidation of associated genetic abnormalities may lead to new targeted therapies.
Data from 3 prospective studies conducted at JALSG in the TKI era were analyzed in this study. Although some treatment improvements have been made between the studies, the principals of the chemotherapy regimens remained the same. Therefore, pooling data from the 3 studies was considered suitable for identifying leukemias with high-risk biological characteristics using a large number of cases that are difficult to accumulate in a single clinical study. As a result, we were able to identify a high-risk ACA combination that was less frequent (14%) by analyzing >200 de novo Ph+ALL patients.
In conclusion, the coexistence of +der(22)t(9;22) and a complex karyotype was identified as a high-risk combination of ACAs in Ph+ALL. Multiple ACAs are often observed in Ph+ALL, leading to identifying this subgroup with a poor prognosis. It was characterized by early relapse, although remission could be achieved at the same rate as standard-risk Ph+ALL. Further molecular genetic elucidation and the establishment of effective therapeutic strategies are warranted.
PARTICIPATING INSTITUTION
| ID | Name of Institution |
|---|---|
| 1001 | Hematology and Rheumatology, Nihon University Itabashi Hospital |
| 1005 | Hematology Division, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital |
| 1006 | Department of Hematology, Nagoya University Hospital |
| 1012 | Department of Hematology, Komaki City Hospital |
| 1013 | Department of Hematology/Oncology, Nagoya ekisaikai hospital |
| 1014 | Department of Hamatology and Oncology, JA Aichi Konan Kosei Hospital |
| 1015 | Department of Hematology, Okazaki City Hospital |
| 1017 | Hematology, Yokkaichi Municipal Hospital |
| 1018 | Division of Hematology and Oncology, Toyohashi Municipal Hospital |
| 1023 | Ichinomiya Municipal Hospital |
| 1026 | Department of Hematology and Cell Therapy, Aichi Cancer Center |
| 1027 | Department of Hematology, Japanese Red Cross Nagoya First Hospital |
| 1028 | Department of Hematology, Fujita Health University Hospital |
| 1029 | Department of Hematology, Mie University Hospital |
| 1030 | Division of Hematology/Oncology, Suzuka General Hospital |
| 1031 | Department of Hematology, Suzuka Kaisei Hospital |
| 1034 | Department of Hematology, Japanese Red Cross Ise Hospital |
| 1036 | Department of Hematology and Rheumatology, Kindai University Hospital |
| 1037 | Department of Hematology, Osaka International Cancer Institute |
| 1038 | Hematology, Hiroshima Red Cross and Atomic-bomb Suvivors Hospital |
| 1040 | Department of hematology, Nagasaki University Hospital |
| 1041 | Department of Hematology, Sasebo City General Hospital |
| 1042 | Department of Hematology, Kumamoto University Hospital |
| 1044 | Dep of Hematology and oncology, Kumamoto City hospital |
| 1046 | Clinical Hematology branch, Jichi Medical University Hospital |
| 1050 | Department of Hematology and Oncology, Okayama University Hospital |
| 1052 | Department of Haematology, National Hospital Organization Okayama Medical Centre |
| 1053 | Department of Medicine, Okayama Rosai Hospital |
| 1054 | Dept. of Hematology/Oncology, Okayama City Hospital |
| 1056 | Department of Hematology, Chugoku Central Hospital |
| 1057 | Division of Hematology, Gunma University Hospital |
| 1061 | Department of Hematology, National Hospital Organization Shibukawa Medical Center |
| 1062 | Department of Hematology, Fujioka Genaral Hospital |
| 1063 | Department of Hematology and Oncology, University of Fukui Hospital |
| 1064 | Department of Hematology/Oncology, Kurashiki Central Hospital (Ohara HealthCare Foundation) |
| 1065 | Internal Medicine, Japanese Red Cross Fukui Hospital |
| 1069 | Department of Hematology, National Cancer Center Hospital |
| 1072 | Department of Hemato-Oncology, Saitama Medical University International Medical Center |
| 1073 | Division of Hematology, Department of Internal Medicine, Hyogo College of Medicine. |
| 1075 | Department of Hematology, Kawasaki Medical School Hospital |
| 1077 | Department of Hematology and Blood Transfusion, Kochi Health Sciences Center |
| 1078 | Division of Hematology, Ehime Prefectural Central Hospital |
| 1079 | Department of Hematology, Chiba University Hospital |
| 1080 | Internal Medicine, Japan Community Health care Organization Funabashi Central Hospital |
| 1081 | Department of Hematology, Chiba Aoba Municipal Hospital |
| 1082 | Department of Hematology, Chibaken Siseikai Narashino Hospital |
| 1083 | Department of Respiratory Medicine, Allergology and Hematology, Nara Medicai University Hospital |
| 1085 | Division of Clinical Oncology/ Hematology, Department of Internal Medicine, The Jikei University Daisan Hospital |
| 1086 | Hematology and Oncology, Dokkyo Medical University Hospital |
| 1087 | Hematology, National Hospital Organization Nagoya Medical Center |
| 1089 | Department of Hematology, Ohta Nishinouchi Hospital, Ohta General Hospital Foundation |
| 1090 | Department of hematology, Kochi Medical School Hospital |
| 1092 | Hematology, Shiga University of Medical Science |
| 1099 | Department of Hematology, National Cancer Center Hospital East |
| 1100 | Department of Hematology and Oncology, Anjo Kosei Hospital |
| 1101 | Division of Hematology and Oncology, St. Marianna University School of Medicine |
| 1102 | Department of Internal Medicine, Division of Hematology, Yokohama City Seibu Hospital, St. Marianna University School of Medicine |
| 1104 | Department of Hematology, JCHO Kyoto Kuramaguchi Medical Center |
| 1105 | Internal Medichine, Japan Community Health care Organization Kobe Central Hospital |
| 1109 | Department of Hematology, Shinshu University Hospital |
| 1110 | Department of Hematology, Nagano Red Cross Hospital |
| 1111 | Department of Hematology, Tokyo Women’s Medical University |
| 1113 | Division of Hematology, Internal Medicine 3, Hamamatsu Univesity School of Medicine |
| 1116 | Department of Hematology and Rheumatology, Kagoshima University Hospital |
| 1119 | Division of Hematology, Kanazawa University Hospital |
| 1121 | Internal Medicine, Keiju Medical Center |
| 1122 | Keiju Kanazawa Hospital |
| 1124 | Division of Hematology, Toyama City Hospital |
| 1126 | Department of Hematology, Ishikawa Prefectural Central Hospital |
| 1128 | Department of Hematology, Tokyo Medical University Hospital |
| 1129 | Department of Hematology, Kyorin University Hospital |
| 1130 | Department of Hematology, Hokkaido University Hospital |
| 1132 | Blood Disorders Center, Aiiku Hospital |
| 1133 | Department of Hematology, Sapporo Hokuyu Hospital Institute for Artificial Organs, Transplantation & Cell Therapy |
| 1137 | Dept. of Hematology, Asahikawa City Hospital |
| 1144 | Divison of Hematology, Saiseikai Maebashi Hospital |
| 1145 | Hematology and Oncology, Nagoya City University Hospital |
| 1148 | Hematology and Oncology, Tokai University School of Medicine |
| 1149 | Department of Hematology, EBINA GENERAL HOSPITAL |
| 1150 | Third Department of Internal Medicine, Yamaguchi University School of Medicine |
| 1152 | Department of Hematology, Yamaguchi Grand Medical Center |
| 1153 | Hematology/Oncology, Research Hospital, The Institute of Medical Science, The University of Tokyo |
| 1156 | Department of Hematology, Hematopoietic Cell Transplantation, Osaka City University Hospital |
| 1157 | Department of Hematology, Fuchu Hospital, Osaka |
| 1160 | Hematology and Oncology, Osaka University Hospital |
| 1161 | Department of Hematology and Oncology, The University of Tokyo Hospital |
| 1163 | Department of Hematology, Niigata University Medical and Dental General Hospital |
| 1164 | Department of Hematology, Oita University Hospital |
| 1165 | Hematology, Oita Prefectural Hospital |
| 1166 | National Hospital Organization Kyushu Cancer Center |
| 1168 | Division of Hematology, Department of Internal Medicine, Aichi Medical University Hospital |
| 1169 | Department of Hematology, Kitasato University Hospital |
| 1170 | Division of Hematology, Yamagata University Hospital |
| 1171 | Hematology, Almeida Memorial Hospital |
| 1172 | Hematology, Oitaken Kouseiren Tsurumi Hospital |
| 1173 | Department of Hematology, Kumamoto Shinto General Hospital |
| 1178 | Department of Hematology, Kyushu Medical Center |
| 1181 | Department of Hematology, Iizuka Hospital |
| 1183 | Division of Hematology, Department of Medicine, Keio University School of Medicine |
| 1184 | Department of Hematology, Aomori Prefectural Central Hospital |
| 1186 | Department of Hematology, Hyogo Cancer Center |
| 1187 | Department of Hematology, Kyoto Prefectural University of Medicine |
| 1191 | Department of Hematology, Osaka City General Hospital |
| 1192 | Division of Hematology, National Defense Medical College Hospital |
| 1193 | Hematology, Akita University Hospital |
| 1194 | department of hematology, Kanazawa medical center |
| 1195 | Department of Hematology, Ogaki Municipal Hospital |
| 1196 | Hematology, Hakodate Municipal Hospital |
| 1197 | Department of Hematology, NTT Medical Center Tokyo |
| 1198 | Department of Hematology and Clinical Immunology, Yokohama City University Hospital |
| 1199 | Department of Hematology and Rheumatology, Tohoku University Hospital |
| 1200 | Department of Hematology, Hiroshima University Hospital |
| 1203 | Department Of Hematology, Yokohama City University Medical Center |
| 1204 | Department of Hematology/Oncology, Kanagawa Cancer Center |
| 1206 | Department of Hematology, Fujisawa City Hospital |
| 1210 | Dept. of Hematology, Shizuoka Red Cross Hospital |
| 1212 | Division of Hematology, Kagawa University |
| 1213 | Department of Hematology, Juntendo University School of Medicine |
| 1214 | Department of Hematology & Immunology, Kanazawa Medical University Hospital |
| 1215 | Department of Hematology, National Hospital Organization Nagasaki Medical Center |
| 1216 | Department of Hematology, National Hospital Organization Osaka Minami Medical Center |
| 1223 | Division of Medical Oncology/Hematology, Department of Medicine, Kobe University Hospital |
| 1225 | Department of Hematology, Imamura General Hospital |
| 1227 | Department of Hematology, Miyagi Cancer Center |
| 1228 | Hematology, Ehime University Hospital |
| 1229 | Internal Medicine, Tokyo Metropolitan Bokutoh Hospital |
| 1230 | Department of Hematology, Takarazuka Municipal Hospital |
| 1231 | Division of Hematology, National Hospital Organization, Matsumoto Medical Center |
| 1232 | Hematology Division, Internal Medicine, Kagawa Prefectural Central Hospital |
| 1236 | Department of Hematology, sakaide city hospital |
| 1237 | Department of Hematology and Immunology, Ohtsu Red Cross Hospital |
| 1240 | Department of Hematology, Osaki Citizen Hospital |
| 1241 | Department of Hematology, Tokyo Medical and Dental University Hospital |
| 1242 | Division of Hematology, Saitama Medical Center, Jichi Medical University |
| 1243 | Division of Hematology and Stem Cell Transplantation, Shizuoka Cancer Center |
| 1244 | Division of Hematology, National Center for Global Health and Medicine |
| 1245 | Department of Hematology, National Hospital Organization Hokkaido Cancer Center |
| 1246 | Department of Internal Medicine, Uegahara Hospital |
| 1248 | Department of Hematology, Tokyo Metropolitan Health and Medical Treatment Corporation, Tama-Hokubu Medical Center |
| 1251 | Department of Hematology, Yokohama City Minato Red Cross Hospital |
| 1253 | department of Hematology, National Hospital Organization Kure Medical Center |
| 1254 | Department of Hematology and Oncology, Japanese Red Cross Nagoya Daini Hospital |
| 1255 | Department of Hematology/Oncology, University of Yamanashi |
| 1256 | department of hematology, Heartlife hospital |
| 1257 | Hematologic oncology, NHO Shikoku Cancer Center |
| 1258 | Department of Hematology, Japanese Red Cross Musashino Hospital |
| 1259 | Department of Hematology, Kagawa Rosai Hospital |
| 1260 | Department of Hematology, Saitama Medical Center, Saitama Medical University |
| 1261 | Department of Hematology, PL General Hospital |
| 1262 | Internal Medicine (Hematology), Toyama Prefectural Central Hospital |
| 1265 | Osaka Saiseikai Nakatsu Hospital |
| 1266 | Matsusaka Chuo General Hospital |
| 1267 | Department of Hematology, National Hospital Organization Disaster Medical Center |
| 1268 | Hematologu/Oncology, Yamato Municipal Hospital |
| 1269 | Department of Hematology NHO Hiroshimanishi Medical Center |
| 1270 | Department of oncology/hematology, Shimane university hospital |
| 1271 | Department of Hematology, Otemae Hospital |
| 1272 | hematology, Tokyo Medical University Hachioji Medical Center |
| 1273 | Hematology/Oncology, Nakagami Hospital |
| 1274 | Department of Hematology, Matsushita memorial hospital |
| 1275 | Department of Hematology, University of Tsukuba Hospital |
| 1277 | Department of Hematology, Tottori Prefectural Central Hospital |
| 1278 | Department of Hematology, Ibaraki Prefectural Central Hospital |
| 1279 | Division of Hematology and Blood transfusion, Tokyo Metropolitan Ohtsuka Hospital |
| 1280 | Department of Hematology, Toyota Kosei Hospital |
| 1281 | Department of Hematology and Oncology, Tosei General Hospital |
| 1282 | Department of Hematology, National Hospital Organization Mito Medical Center |
| 1283 | Department of Hematology, Tsuchiura Kyodo General Hospital |
| 1284 | Division of Hematology and Oncology, Department of Internal Medicine, Saga University Hospital |
| 1285 | Department of Hematology and Oncology, Hitachi general hospital |
| 1286 | Department of Hematology, Yamanashi prefectural central hospital |
| 1287 | Department of Hematology and Oncology, Fukui Prefectural Hospital |
| 1288 | Dept. Int. Med., Showa Inan General Hospital |
| 1289 | Department of Hematology, National Center for Geriatrics and Gerontology |
| 1291 | Deparment of Hematology, Sendai Medical Center, National Hospital Organization |
| 1294 | Internal Medicine, Japan Community Health care Organization Kyushu Hospital |
| 1295 | Hematology, Faculty of Medicine, University of Miyazaki Hospital |
| 1297 | Medicine and Biosystemic Science, Kyushu University Hospital |
| 1301 | Department of Hematology, Fukushima Medical University Hospital |
| 1302 | Department of Medical Oncology, Hematology and Infectious Diseases, Fukuoka University Hospital |
| 1303 | Department of Hematology and Oncology, Nagoya City West Medical Center |
| 1305 | Department of Hematology, Yokohama Municipal Citizen’s hospital |
| 1306 | Hematology, JA Toride Medical Center |
| 1307 | 2nd Department of Internal Medicine(Hematology), University of Ryukyus Hospital |
| 1308 | Department of Hematology, Saiseikai Yokohama Nanbu Hospital |
| 1309 | Department of Hematology, Oami Municipal hospital |
| 1310 | Department of Hematology, Japanese Red Cross Osaka Hospital |
| 1311 | Department of Hematology, Sapporo Medical University Hospital |
| 1313 | Department of Hematology, Japanese Red Cross Kyoto Daiichi Hospital |
| 1314 | Hematology/Oncology, Kansai Medical University Hospital |
| 1315 | Division of Hematology, Shonan Kamakura General Hospital |
| 1316 | Department of Hematology, Kyoto University Hospital |
| 1317 | Department of Internal Medicine (Hematology), Toyonaka municipal hospital |
| 1319 | Department of Hematology, Kansai Electric Power Hospital |
| 1320 | Division of Hematology, Department of Internal Medicine, Kyoto-Katsura Hospital |
| 1321 | Department of Hematology and Rheumatology, Saiseikai Noe Hospital |
| 1323 | Department of Hematology and Oncology, Takatsuki Red Cross Hospital |
| 1324 | Medical Oncology/Hematology, Kakogawa Central City Hospital |
| 1325 | Department of Hematology, Kobe City Medical Center General Hospital |
| 1326 | Department of Hematology, Toyama Red Cross Hospital |
| 1327 | Department of Hematology, Nippon Medical School Hospital |
| 1328 | Department of Hematology, Japanese Red Cross, Kyoto Daini Hospital |
| 1329 | Department of Hematology, Nagaoka Red Cross Hospital |
| 1331 | Department of Internal Medicine (Hematology), Niigata Prefectural Central Hospital |
| 1332 | Department of Hematology/Oncology, Tokai University Hachioji Hospital |
| 1333 | Department of Hematology, Kyoto City Hospital |
| 1334 | Department of Hematology, Mitsui Memorial Hospital |
| 1335 | Department of Hematology, Rinku General Medical Center |
| 1336 | Department of Hematology, Japanese Red Cross Okayama Hospital |
| 1337 | Department of Hematology, Asahi General Hospital |
| 1338 | Department of Hematology, Osaka General Hospital of West Japan Railway Company |
| 1339 | Division of Hematology Oncology, Japanese Red Cross Narita Hospital |
| 1340 | Department of Hematology and Oncology, Nagoya City East Medical Center |
| 1341 | Division of Hematology, Department of Medicine, Showa University School of Medicine |
| 1342 | Hematology, Kindai University Nara Hospital |
| 1343 | Department of Hematology, Tottori University Hospital |
| 1344 | Division of Hematology, Tokyo-Kita Medical Center |
| 1345 | Division of Clinical Oncology/ Hematology, Department of Internal Medicine, The Jikei University Hospital |
| 1346 | Division of Clinical Oncology/ Hematology, Department of Internal Medicine, The Jikei University Kashiwa Hospital |
| 1347 | University Of Occupational And Environmental Health, Japan |
| 1348 | Department of Hematology and Oncology, Nagoya Memorial Hosptial, Japan |
| 1349 | Department of Hematology, Tokyo Metropolitan Police Hospital, Japan |
ACKNOWLEDGMENTS
The authors thank all of the physicians and staff of the hospitals participating in the study, as well as all staff of JALSG and the Japanese Data Center for Hematopoietic Cell Transplantation.
AUTHOR CONTRIBUTIONS
SN, IS, SF, YH, and YA designed the research, performed the statistical analysis, interpreted the data, and wrote the article. N Doki, S Kurahashi, YU, N Dobashi, TM, YT, and MT provided the data of patients. YA, S Kako, TI, and TF collected the data of patients regarding TRUMP database. SO, YI, HK, IM, YM collected the data of patients regarding JALSG studies. All authors reviewed and approved the final draft.
DISCLOSURES
YH reports honoraria from Kyowa Kirin Co., Ltd., Bristol-Myers Squibb, and Novartis Pharma KK., and speakers bureau from Kyowa Kirin Co., Ltd. SF reports honoraria from Bristol-Myers-Squibb, Astellas Pharma Inc., Nippon Shinyaku, Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Novartis Pharma KK, MSD K.K., Sanofi K.K., Janssen, SymBio Pharma, Kyowa Kirin Co., Ltd., AstraZeneca, CSL Behring K.K, Meiji Seika Pharma, AbbVie Inc, Takeda Pharmaceutical Co. Ltd., and Chugai Pharmaceutical Co., Ltd., and research funding from Shionogi & CO., Ltd., Kyowa Hakko Kirin, Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Asahi-Kasei Pharma, and Daiichi Sankyo Co., Ltd. YU reports honoraria from Otsuka Pharmaceutical Co., Ltd., and Sanofi K.K. N. Dobashi reports research funding from Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Kyowa Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Abbvie GK, Takeda Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., and Pfizer Inc., and paid expert testimony from Otsuka Pharmaceutical Co., Ltd. TM reports research funding from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Sumitomo Pharma Co., Ltd.; Honoraria: Amgen K.K., Nippon Becton Dickinson Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma KK., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., and TOPPAN INC. YA reports honoraria from Novartis Pharma KK., Kyowa Kirin Co., Ltd., Abbvie GK; Astellas Pharma Inc., Mochida Pharmaceutical Co., Ltd., and Meiji Seika Pharma Co., Ltd. S. Kako reports honoraria from Novartis Pharma KK. And Bristol-Myers-Squibb. HK reports research funding from Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Zenyaku Kogyo Co., Ltd., Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Perseus Proteomics Inc., CURED Co., Ltd., Astellas Pharma Inc., Asahi Kasei Corporation, Abbvie Inc., Nippon Shinyaku, Co., Ltd., JCR Pharmaceuticals Co., Ltd., and Takeda Pharmaceutical Co. Ltd., and honoraria from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Daiichi Sankyo Co., Ltd., Astellas Pharma Inc., Abbvie Inc., Nippon Shinyaku Co., Ltd., AstraZeneca plc., Novartis Pharma KK., SymBio Pharmaceuticals Ltd.., Bristol-Myers Squibb K.K., Amgen Inc., Meiji Seika Pharma Co., Ltd., Pfizer Inc., Nippon Kayaku Co., Ltd., and Towa Pharmaceutical Co., Ltd. IM reports consultancy from Otsuka Pharmaceutical Co., Ltd., research funding from Otsuka Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co. Ltd., Shionogi & Co., Ltd., Asahi Kasei Pharma Corp., Eisai Co., Ltd., Daiichi Sankyo Co., Ltd., Astellas Pharma Inc., Nippon Shinyaku Co., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co. Ltd., Sanofi K.K., Mitsubishi Tanabe Pharma Corp., Novartis Pharma KK., Janssen Pharmaceutical K.K., Abbvie GK, SymBio Pharmaceuticals Ltd., Pfizer Japan Inc., and Alexion Pharmaceuticals, Inc., and speakers bureau from Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co. Ltd., Novartis Pharma KK., Janssen Pharmaceutical K.K., Abbvie GK, Pfizer Japan Inc., Bristol-Myers Squibb K.K., SymBio Pharmaceuticals Ltd., and AstraZeneca plc. YM reports research funding from Nippon Shinyaku Co., Ltd., Novartis Pharma KK., Abbvie GK, Astellas Pharma Inc., Sumitomo Dainippon Pharma Co., Ltd., SymBio Pharmaceuticals Ltd., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Kyowa Kirin Co., Ltd., Pfizer Inc., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co. Ltd., Daiichi Sankyo Co., Ltd., Janssen Pharmaceutical K.K., and Celgene Corp., and honoraria from Sumitomo Dainippon Pharma Co., Ltd. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grant Number JP 20K08730 and the Japan Agency for Medical Research and Development Grant Number JP 21ck0106624 and JP23lk1503005.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Short NJ, Kantarjian HM, Sasaki K, et al. Poor outcomes associated with +der(22)t(9;22) and -9/9p in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia receiving chemotherapy plus a tyrosine kinase inhibitor. Am J Hematol. 2017;92:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiaretti S, Ansuinelli M, Vitale A, et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol. Haematologica. 2021;106:1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi T, Huang X, Zhu L, et al. Adult Ph-positive acute lymphoblastic leukemia-current concepts in cytogenetic abnormalities and outcomes. Am J Cancer Res. 2020;10:2309–2318. [PMC free article] [PubMed] [Google Scholar]
- 4.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24:460–466. [DOI] [PubMed] [Google Scholar]
- 5.Fujisawa S, Mizuta S, Akiyama H, et al. Phase II study of imatinib-based chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Am J Hematol. 2017;92:367–374. [DOI] [PubMed] [Google Scholar]
- 6.Sugiura I, Doki N, Hata T, et al. Dasatinib-based 2-step induction for adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6:624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wetzler M, Dodge RK, Mrózek K, et al. Additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a study of the Cancer and Leukaemia Group B. Br J Haematol. 2004;124:275–288. [DOI] [PubMed] [Google Scholar]
- 8.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113:4153–4162. [DOI] [PubMed] [Google Scholar]
- 9.Aldoss I, Stiller T, Cao TM, et al. Impact of additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousselot P, Coude MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akahoshi Y, Mizuta S, Shimizu H, et al. Additional cytogenetic abnormalities with Philadelphia chromosome-positive acute lymphoblastic leukemia on allogeneic stem cell transplantation in the tyrosine kinase inhibitor Era. Biol Blood Marrow Transplant. 2018;24:2009–2016. [DOI] [PubMed] [Google Scholar]
- 12.Heerema NA, Harbott J, Galimberti S, et al. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia. 2004;18:693–702. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16:1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foà R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383:1613–1623. [DOI] [PubMed] [Google Scholar]
- 15.Moorman AV, Barretta E, Butler ER, et al. Prognostic impact of chromosomal abnormalities and copy number alterations in adult B-cell precursor acute lymphoblastic leukaemia: a UKALL14 study. Leukemia. 2022;36:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]