ABSTRACT
Objective
To describe and compare the clinical characteristics and outcomes of patients admitted to intensive care units during the first and second waves of the COVID-19 pandemic.
Methods
In this retrospective single-center cohort study, data were retrieved from the Epimed Monitor System; all adult patients admitted to the intensive care unit between March 4, 2020, and October 1, 2021, were included in the study. We compared the clinical characteristics and outcomes of patients admitted to the intensive care unit of a quaternary private hospital in São Paulo, Brazil, during the first (May 1, 2020, to August 31, 2020) and second (March 1, 2021, to June 30, 2021) waves of the COVID-19 pandemic.
Results
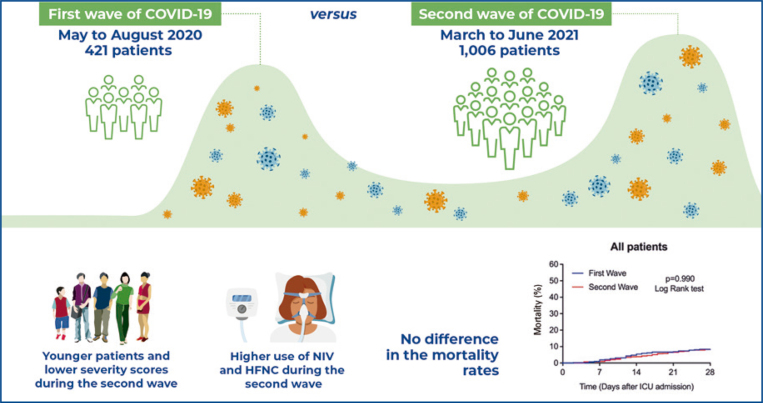
In total, 1,427 patients with COVID-19 were admitted to the intensive care unit during the first (421 patients) and second (1,006 patients) waves. Compared with the first wave group [median (IQR)], the second wave group was younger [57 (46-70) versus 67 (52-80) years; p<0.001], had a lower SAPS 3 Score [45 (42-52) versus 49 (43-57); p<0.001], lower SOFA Score on intensive care unit admission [3 (1-6) versus 4 (2-6); p=0.018], lower Charlson Comorbidity Index [0 (0-1) versus 1 (0-2); p<0.001], and were less frequently frail (10.4% versus 18.1%; p<0.001). The second wave group used more noninvasive ventilation (81.3% versus 53.4%; p<0.001) and high-flow nasal cannula (63.2% versus 23.0%; p<0.001) during their intensive care unit stay. The intensive care unit (11.3% versus 10.5%; p=0.696) and in-hospital mortality (12.3% versus 12.1%; p=0.998) rates did not differ between both waves.
Conclusion
In the first and second waves, patients with severe COVID-19 exhibited similar mortality rates and need for invasive organ support, despite the second wave group being younger and less severely ill at the time of intensive care unit admission.
Keywords: Coronavirus infections; COVID-19; SARS-CoV-2; Respiration, artificial; Noninvasive ventilation; Extracorporeal membrane oxygenation; Critical care outcomes; Mortality; Intensive care units
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has become a major public health concern, with almost half a billion cases diagnosed and over six million deaths worldwide.(1) During this pandemic, waves of increased numbers of patients newly diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been reported worldwide, with varying degrees of disease severity and pressure on healthcare systems.(2)
Since the first SARS-CoV-2 infection was confirmed in São Paulo, Brazil,(3) over 31 million cases and 660 thousand deaths due to COVID-19 have been registered.(1) Brazil’s first wave of COVID-19 occurred between March and November 2020, and the most prevalent SARS-CoV-2 variants were B.1.1.28 and B.1.1.33.(4)The second wave occurred between February and October 2021, when the most prevalent SARS-CoV-2 variants were P.2 and P.1 (Gamma).(4)During both waves, an abrupt increase in new COVID-19 cases across different Brazilian regions imposed enormous pressure on the healthcare system, which had been under strain since the beginning of the pandemic.(5)
Studies comparing the epidemiological characteristics and outcomes of both waves in different countries have reported conflicting results.(4-10) While some authors reported lower mortality rates during the second wave,(6,8) others found no significant differences in mortality(9,10) or reported poorer clinical outcomes, such as increased mortality among younger age groups and an increased proportion of patients requiring mechanical ventilation.(5,7) A cross-sectional study of a large nationwide database of hospitalized patients in Brazil showed that, compared with the first wave, the second wave was characterized by increased demand for intensive care unit (ICU) admissions, increased use of noninvasive ventilation (NIV), invasive mechanical ventilation (MV), and increased hospital mortality.(4)
The clinical characteristics and outcomes of patients with COVID-19 in Brazil vary significantly across the country, mainly because of social and economic disparities and different levels of access to the health system.(4,11) Studies on the epidemiological characteristics and outcomes of patients with severe COVID-19 admitted to the ICU in private hospitals in Brazil during the first two waves of the pandemic are limited.
OBJECTIVE
To describe and compare the epidemiological and clinical characteristics, resource use, and outcomes of patients with COVID-19 admitted to the intensive care unit during the first and second waves of the COVID-19 pandemic in São Paulo, Brazil.
METHODS
Study design
This was a single-center retrospective cohort study. The study was approved by the local ethics committee of Hospital Israelita Albert Einstein; the need for informed consent was waived (CAAE: 30797520.6.0000.0071; # 4.562.815). This study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement.(12)
Setting
This study was conducted in a private quaternary care hospital in São Paulo, Brazil. The Hospital Israelita Albert Einstein has 706 beds. Of these, 37 were open medical-surgical adult ICU beds, and 81 were adult step-down unit beds. The total ICU operational capacity designated to support patients with severe COVID-19 requiring intensive care increased during the first and second waves, reaching 81 and 159 ICU beds, respectively.
Study participants
Consecutive adult (≥18 years) patients admitted to the ICU from March 4, 2020, to October 1, 2021, and diagnosed with COVID-19 were eligible for inclusion in this study. Laboratory confirmation of SARS-CoV-2 infection was based on a positive reverse transcription polymerase chain reaction (RT-PCR) assay.(13)
Patient management
The criteria for ICU admission and institutional protocol for severe SARS-CoV-2 infection management have been published elsewhere.(14,15)
Data collection and study variables
All study data were retrieved from the Epimed Monitor System® (Epimed Solutions, Rio de Janeiro, Brazil), an electronic structured case report form in which trained ICU case managers entered patient data prospectively.(16) Collected variables included demographics, comorbidities, Simplified Acute Physiology Score (SAPS 3 Score)(17) at ICU admission, Sequential Organ Failure Assessment (SOFA) Score(18) at ICU admission, Charlson Comorbidity Index,(19) Modified Frailty Index (MFI),(20,21) resource use and organ support [vasopressors, NIV, high flow nasal cannula (HFNC), MV, renal replacement therapy (RRT) and extracorporeal membrane oxygenation (ECMO)] during ICU stay, destination at hospital discharge, ICU and hospital length of stay (LOS), and ICU and in-hospital mortality.
Definitions
We defined the first wave period as the time from May 1, 2020, to August 31, 2020 (first wave group) and the second wave from March 1, 2021, to June 30, 2021 (second wave group). These two periods correspond to four consecutive months in 2020 and 2021, respectively, with the highest number of patients with COVID-19 admitted to the ICU.
Statistical analysis
Categorical variables were presented as absolute and relative frequencies. Continuous variables were presented as medians with interquartile ranges (IQR). Normality was assessed using the Kolmogorov-Smirnov test.
The first- and second-wave groups were compared. Categorical variables were compared with the χ2 test or Fisher’s exact test as appropriate. Continuous variables were compared using an independent Student’s t test or the Mann-Whitney U test in cases of non-normal distribution. Mortality on day 28 of the pooled patients and mortality stratified according to the use of mechanical ventilation, RRT, and ECMO were analyzed using the Kaplan-Meier method. Patients discharged from the hospital before day 28 were considered alive on day 28.
Two-tailed tests were used, and statistical significance was set at p<0.05. All analyses were performed using the IBM (SPSS) Statistics for Macintosh, version 27 (IBM Corp., Armonk, NY, USA), and GraphPad Prism version 9.3.0 (GraphPad Software Inc., San Diego, CA, USA) was used for graph plotting.
RESULTS
From March 4, 2020, to October 1, 2021, 2,566 patients with COVID-19 were admitted to the ICU. Of them, 1,427 were admitted during the first (421 patients) and second (1,006 patients) COVID-19 waves.
Patient characteristics
The baseline characteristics of the patients admitted during the first and second waves are shown in table 1. Compared with the first wave group, the second wave group was younger [57 (46-70) versus 67 (52-80) years; p<0.001]. More often, they were men (69.9% versus 63.9%; p=0.032), had a lower SAPS 3 Score [45 (42-52) versus 49 (43-57); p<0.001], a lower SOFA Score [3 (1-6) versus 4 (2-6); p=0.018], lower Charlson Comorbidity Index [0 (0-1) versus 1 (0-2); p<0.001], were less frequently frail (10.4% versus 18.1%; p<0.001), and had less frequently congestive heart failure (3.5% versus 7.2%; p=0.012).
Table 1. Baseline characteristics of studied patients.
| Characteristics | All n=1,427 | First wave n=421 | Second wave n=1,006 | p value* |
|---|---|---|---|---|
| Age, years (median, IQR) | 59 (47-73) | 67 (52-80) | 57 (46-70) | <0.001# |
| Men, n/total n (%) | 972/1,427 (68.1) | 269/421 (63.9) | 703/1,006 (69.9) | 0.032& |
| SAPS 3 Score (median, IQR) | 46 (42-54) | 49 (43-57) | 45 (42-52) | <0.001# |
| SOFA Score (median, IQR)$ | 3 (1-6) | 4 (2-6) | 3 (1-6) | 0.018# |
| CCI (median, IQR) | 0 (0-1) | 1 (0-2) | 0 (0-1) | <0.001# |
| MFI, points (median, IQR)‡ | 1 (0-2) | 1 (0-2) | 1 (0-2) | <0.001# |
| Non-frail | 612/1,416 (43.2) | 153/421 (36.3) | 459/995 (46.1) | <0.001& |
| Pre-frail | 625/1,416 (44.1) | 192/421 (45.6) | 433/995 (43.5) | |
| Frail | 179/1,416 (12.6) | 76/421 (18.1) | 103/995 (10.4) | |
| Underlying disease, n/total n (%) | ||||
| Systemic hypertension | 623/1,083 (57.5) | 195/335 (58.2) | 428/748 (57.2) | 0.812& |
| Diabetes mellitus | 328/1,083 (30.3) | 113/335 (33.7) | 215/748 (28.7) | 0.114& |
| Asthma | 68/1,083 (6.3) | 23/335 (6.9) | 45/748 (6.0) | 0.691& |
| Cancer | 72/1,083 (6.6) | 23/335 (6.9) | 49/748 (6.6) | 0.952& |
| Congestive heart failure | 50/1,083 (4.6) | 24/335 (7.2) | 26/748 (3.5) | 0.012& |
| COPD | 66/1,083 (6.1) | 17/335 (5.1) | 49/748 (6.6) | 0.423& |
| Chronic kidney disease | 45/1,083 (4.2) | 20/335 (6.0) | 25/748 (3.3) | 0.066& |
| Chronic kidney disease requiring RRT | 18/1,083 (1.7) | 6/335 (1.8) | 12/748 (1.6) | 1.000& |
| Hematologic cancer | 21/1,083 (1.9) | 12/335 (3.6) | 9/748 (1.2) | 0.017& |
| Metastatic cancer | 9/1,083 (0.8) | 4/335 (1.2) | 5/748 (0.7) | 0.604& |
| Days from hospital to ICU admission | 1 (0-2) | 0 (0-2) | 1 (0-3) | <0.001# |
| Support at ICU admission, n/total n (%) | ||||
| Non-invasive ventilation | 581/1,425 (40.8) | 69/421 (16.4) | 512/1,004 (51.0) | <0.001& |
| Mechanical ventilation | 122/1,425 (8.6) | 49/421 (11.6) | 73/1,004 (7.3) | 0.010& |
| Vasopressors | 100/1,425 (7.0) | 35/421 (8.3) | 65/1,004 (6.5) | 0.260& |
| Renal replacement therapy | 4/1,425 (0.3) | 2/421 (0.5) | 2/1,004 (0.2) | 0.727& |
Values represent median (IQR) or n/n total (%).
* P values were calculated using; # Mann-Whitney U test; & χ2 test; $ data available for 827 patients (First Wave: 295 patients, Second Wave: 532 patients); ‡ patients were categorized according to their MFI values into Non-frail (MFI=0), Pre-frail (MFI=1-2) or Frail (MFI≥3).
SAPS 3: Simplified Acute Physiology Score 3, scores range from 0 to 217, with higher scores indicating more severe illness and higher risk of death; SOFA Score: Sequential Organ Failure Assessment Score, ranges from 0 to 24, with higher scores indicating more severe organ dysfunction; CCI: Charlson Comorbidity Index, based on a point scoring system (from 0 to 37) for the presence of specific associated diseases and is used for prognosis of lethality; MFI: Modified Frailty Index, values from 1 to 11, scored by assigning 1 point for each frailty components (11 possible comorbidities or deficits). COPD: chronic obstructive pulmonary disease; RRT: renal replacement therapy; ICU: intensive care unit.
The median (IQR) number of days from hospital admission to ICU admission was higher in the second wave [1 (0-3) versus 0 (0-2); p<0.001] than in the first. On ICU admission, patients admitted during the second wave received NIV more frequently (51.0% versus 16.4%; p<0.001) and mechanical ventilation less frequently (7.3% versus 11.6%; p=0.010) than those admitted during the first wave (Table 1).
Resource use
During the ICU stay, the second-wave group used more NIV (81.3% versus 53.4%; p<0.001) and HFNC (63.2% versus 23.0%; p<0.001). The median number of days on MV and the proportion of patients requiring MV, vasopressors, RRT, ECMO, or tracheostomy did not differ between the two waves (Table 2).
Table 2. Resource use.
| Resource | All n=1,427 | First wave n=421 | Second wave n=1,006 | p value* |
|---|---|---|---|---|
| Support during ICU stay, n/total n (%) | ||||
| Non-invasive ventilation | 1,043/1,427 (73.1) | 225/421 (53.4) | 818/1,006 (81.3) | <0.001& |
| Mechanical ventilation | 494/1,427 (34.6) | 137/421 (32.5) | 357/1,006 (35.5) | 0.315& |
| Vasopressors | 434/1,427 (30.4) | 143/421 (34.0) | 291/1,006 (28.9) | 0.068& |
| High flow nasal cannula | 733/1,427 (51.4) | 97/421 (23.0) | 636/1,006 (63.2) | <0.001& |
| Renal replacement therapy | 149/1,427 (10.4) | 35/421 (8.3) | 114/1,006 (11.3) | 0.108& |
| ECMO | 20/1,427 (1.4) | 6/421 (1.4) | 14/1,006 (1.4) | 1.000& |
| Tracheostomy, n/total n (%) | 92/1,427 (6.4) | 26/421 (6.2) | 66/1,006 (6.6) | 0.879& |
| MV duration (days), median (IQR) | 9 (6-23) | 9 (5-15) | 10 (6-24) | 0.170# |
Values represent median (IQR) or n/total n (%).
* P values were calculated using; & χ2 test; # Mann-Whitney U test.
ICU: intensive care unit; ECMO: extracorporeal membrane oxygenation; MV: mechanical ventilation.
Clinical outcomes
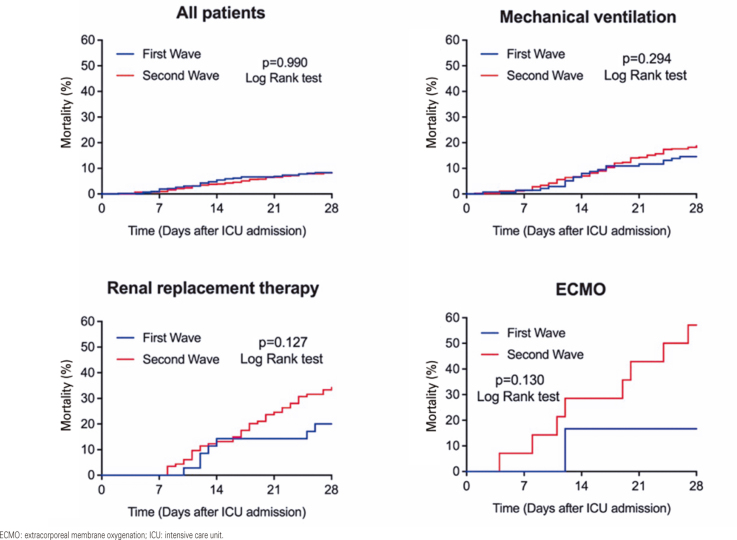
The ICU (11.3% versus 10.5%; second and first waves, respectively; p=0.696) and in-hospital mortality (12.3% versus 12.1%, second and first waves, respectively; p=0.998) rates did not differ between patients admitted during the second and first waves (Table 3 and Figure 1). Compared with the first wave, the second wave had a longer length of ICU [9 (5-16) days versus 8 (4-15) days; p=0.009] and hospital [13 (9-22) days versus 12 (8-22) days; p=0.031] stay (Table 3).
Table 3. Clinical outcomes stratified according to the use of invasive support.
| Outcomes | All n=1,427 | First wave n=421 | Second wave n=1,006 | p value* |
|---|---|---|---|---|
| Destination at hospital discharge, n/total n (%) | 0.452& | |||
| Home | 1,220/1,422 (85.8) | 358/421 (85.0) | 862/1,001 (86.1) | |
| Home-care | 15/1,422 (1.1) | 7/421 (1.7) | 8/1,001 (0.8) | |
| Transfer to another hospital | 13/1,422 (0.9) | 5/421 (1.2) | 8/1,001 (0.8) | |
| Death | 174/1,422 (12.2) | 51/421 (12.1) | 123/1,001 (12.3) | |
| Palliative care | 24/1,422 (1.7) | 16/421 (3.8) | 8/1,001 (0.8) | <0.001& |
| ICU mortality | 158/1,427 (11.1) | 44/421 (10.5) | 114/1,006 (11.3) | 0.696& |
| Hospital mortality | 174/1,422 (12.2) | 51/421 (12.1) | 123/1,001 (12.3) | 0.998& |
| ICU LOS (days), median (IQR) | 8 (5-16) | 8 (4-15) | 9 (5-16) | 0.009# |
| Hospital LOS (days), median (IQR) | 13 (9-22) | 12 (8-22) | 13 (9-22) | 0.031# |
| According to the use of MV | ||||
| Patients who received MV, n/total n (%) | 494/1,427 (34.6) | 137/421 (32.5) | 357/1,006 (35.5) | |
| ICU mortality | 128/494 (25.9) | 30/137 (21.9) | 98/357 (27.5) | 0.252& |
| Hospital mortality | 138/491 (28.1) | 36/137 (26.3) | 102/354 (28.8) | 0.654& |
| ICU LOS (days), median (IQR) | 18 (13-29)§ | 18 (12-30)§ | 18 (13-29)§ | 0.735# |
| Hospital LOS (days), median (IQR) | 24 (16-38)§ | 24 (15-41)§ | 24 (16-37)§ | 0.836# |
| Patients who did not receive MV, n/total n (%) | 933/1,427 (65.4) | 284/421 (67.5) | 649/1,006 (64.5) | |
| ICU mortality | 30/933 (3.2) | 14/284 (4.9) | 16/649 (2.5) | 0.078& |
| Hospital mortality | 36/931 (3.9) | 15/284 (5.3) | 21/647 (3.2) | 0.194& |
| ICU LOS (days), median (IQR) | 6 (3-9) | 5 (2-9) | 6 (4-9) | 0.001# |
| Hospital LOS (days), median (IQR) | 11 (8-14) | 10 (7-14) | 11 (8-14) | 0.012# |
| According to the use of RRT | ||||
| Patients who received RRT, n/total n (%) | 149/1,427 (10.4) | 35/421 (8.3) | 114/1,006 (11.3) | |
| ICU mortality | 76/149 (51.0) | 13/35 (37.1) | 63/114 (55.3) | 0.092& |
| Hospital mortality | 80/149 (53.7) | 17/35 (48.6) | 63/114 (55.3) | 0.617& |
| ICU LOS (days), median (IQR) | 26 (15-42)¶ | 25 (12-48)¶ | 27 (16-40)¶ | 0.975# |
| Hospital LOS (days), median (IQR) | 34 (18-56)¶ | 36 (17-56)¶ | 33 (18-57)¶ | 0.525# |
| Patients who did not receive RRT, n/total n (%) | 1,278/1,427 (89.6) | 386/421 (91.7) | 892/1,006 (88.7) | |
| ICU mortality | 82/1,278 (6.4) | 31/386 (8.0) | 51/892 (5.7) | 0.154& |
| Hospital mortality | 94/1,273 (7.4) | 34/386 (8.8) | 60/887 (6.8) | 0.244& |
| ICU LOS (days), median (IQR) | 8 (4-14) | 7 (3-13) | 8 (5-14) | 0.033# |
| Hospital LOS (days), median (IQR) | 13 (9-19) | 12 (8-19) | 13 (9-19) | 0.053# |
| According to the use of ECMO | ||||
| Patients who received ECMO, n/total n (%) | 20/1,427 (1.4) | 6/421 (1.4) | 14/1,006 (1.4) | |
| ICU mortality | 10/20 (50.0) | 1/6 (16.7) | 9/14 (64.3) | 0.141$ |
| Hospital mortality | 11/20 (55.0) | 2/6 (33.3) | 9/14 (64.3) | 0.336$ |
| ICU LOS (days), median (IQR) | 25 (16-45)‡ | 37 (22-55)† | 25 (12-40)‡ | 0.353# |
| Hospital LOS (days), median (IQR) | 31 (16-49)‡ | 49 (27-56)† | 26 (12-46)Ψ | 0.153# |
| Patients who did not receive ECMO, n/total n (%) | 1,407/1,427 (98.6) | 415/421 (98.6) | 992/1,006 (98.6) | |
| ICU mortality | 148/1,407 (10.5) | 43/415 (10.4) | 105/992 (10.6) | 0.977& |
| Hospital mortality | 163/1,402 (11.6) | 49/415 (11.8) | 114/987 (11.6) | 0.963& |
| ICU LOS (days), median (IQR) | 8 (5-16) | 8 (4-15) | 9 (5-16) | 0.007# |
| Hospital LOS (days), median (IQR) | 13 (9-21) | 12 (8-21) | 13 (9-21) | 0.020# |
Values represent median (IQR) or n/total n (%).
* P values were calculated using; # Mann-Whitney U test; & χ2 test; $ Fisher’s Exact test; § p<0.001 (Mann-Whitney U test) versus patients who did not receive MV; ¶ p<0.001 (Mann-Whitney U test) versus patients who did not receive RRT; ‡ p<0.001 (Mann-Whitney U test) versus patients who did not receive ECMO; † p<0.005 (Mann-Whitney U test) versus patients who did not receive ECMO; Ψ p=0.016 (Mann-Whitney U test) versus patients who did not receive ECMO.
ICU: intensive care unit; LOS: length of stay; MV: mechanical ventilation; RRT: renal replacement therapy; ECMO: extracorporeal membrane oxygenation.
Figure 1. Mortality at day 28 of pooled patients and according to the need of invasive organ support.
ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit.
Intensive care unit mortality, in-hospital mortality, and ICU and hospital LOS did not differ between patients who received MV, RRT, or ECMO during the first and second waves (Table 3 and Figure 1). Nevertheless, compared to the first wave, the second wave had longer ICU and hospital stays for patients who did not receive MV, RRT, or ECMO (Table 3).
DISCUSSION
The main findings of this study were that, both the first and second COVID-19 waves had similar mortality rates and the use of invasive resources, despite the second wave patients being younger and less severely ill at ICU admission, according to the SAPS 3 and SOFA Scores. Moreover, during the second wave, patients had longer hospital stays before ICU admission and longer ICU and hospital stays.
Studies from Europe have compared the waves of the COVID-19 pandemic.(9,10) A study from France reported no difference in ICU mortality between the first and second waves. However, they observed a lower proportion of patients requiring invasive mechanical ventilation and a lower rate of thrombotic events in the second wave.(9) In another study from Switzerland, mortality and the need for ICU admission were similar in both waves despite the higher proportion of younger patients admitted to the ICU during the second wave.(10)
Unlike developed countries,(6,8-10) Africa(7) and Brazil,(4,5) had a more aggressive second wave than the first wave as the demand for hospital admissions increased, and the proportion of patients requiring advanced respiratory support was higher.
In contrast to other studies conducted in Brazil,(4,5) we did not observe increased ICU and in-hospital mortality rates when the second and first COVID-19 waves were compared. Additionally, the observed mortality in both waves of our cohort was lower than that reported previously.(4,5) The observed lower mortality rate in our study compared to other studies(4,22,23) may be related to ICU characteristics, i.e., organizational factors(24) and ICU staffing patterns,(25) and discrepancies in COVID-19 outcomes across the country, which may be explained by social, political, and economic disparities across the regions affecting the availability of ICU beds and the period between disease onset/need for organ support and hospital/ICU admission.
Similar to our study, Contou et al. reported a longer time between hospital and ICU admissions during the second wave than during the first wave.(9) This finding may be explained by the delayed need for endotracheal intubation and MV, potentially related to the early administration of glucocorticoids and increased use of NIV and HFNC during the second wave. Knowledge of evidence-based treatment with glucocorticoids was not available during the first COVID-19 wave. Meanwhile, during the second wave, the benefits of this therapy had already been disseminated and incorporated into clinical practice.(26)
We observed increased use of NIV before ICU admission and increased use of NIV and HFNC during ICU stay in the second wave. A recent adaptive randomized controlled trial showed a significantly lower rate of the composite endpoint (tracheal intubation or mortality within 30 days) in patients with COVID-19 with acute hypoxemic respiratory failure randomized to receive continuous positive airway pressure than in patients randomized to receive conventional oxygen therapy.(27) Nevertheless, compared with patients who received conventional oxygen therapy, those randomized to receive HFNC did not exhibit improved outcomes.(27) One may postulate that the increased use of HFNC during the second wave may have affected the timing of endotracheal intubation and the initiation of MV, which has been associated with poor clinical outcomes.(28) Nevertheless, this hypothesis warrants further investigation.
We observed that patients admitted to the ICU during the second wave were younger than patients admitted to the ICU during the first wave [mean difference: 7.3 years; 95%CI: 5.3-9.3 years; p<0.001). The increased hospitalization among younger patients during the second COVID-19 wave may be related to the vaccination campaign in Brazil, where older adults were prioritized to receive the vaccine first.(4) Another possible explanation is related to the behavior of P.1 (gamma) and P.2 variants, which were the prevalent COVID-19 variants during the second wave in Brazil, among younger age groups.(4,29,30) According to a study conducted in European countries, patients infected with the P.1 variant who were younger than 60 years had a higher risk of hospitalization and ICU admission than older patients.(31)
A recent meta-analysis of 42 studies and 423,117 patients hospitalized with SARS-CoV-2 infection showed that increased age, male sex, smoking, and the presence of comorbidities such as chronic obstructive pulmonary disease, cardiovascular disease, diabetes, systemic hypertension, obesity, cancer, and acute kidney injury were significantly associated with COVID-19 mortality.(32) Similarly, in our study, we observed that older age and a higher Charlson Comorbidity Index were independently associated with increased COVID-19 mortality.
Our study had several limitations. First, because the study was performed in a single ICU in a private quaternary care hospital in Brazil, the results may not be generalizable to other ICUs in Brazil or outside the country. Second, because there is no standard definition of COVID-19 wave boundaries, we arbitrarily defined the beginning and end of the first and second waves in our study. This may have affected our results and precluded a comparison with other authors. Third, we did not collect detailed data on SARS-CoV-2 variants, which may affect clinical outcomes in patients with severe COVID-19.
Further, we used the SAPS 3 Score to quantify the severity of illness at the time of ICU admission, which may not fully reflect the severity of COVID-19 in our analysis.(33) Also, vaccination against SARS-CoV-2 may have affected clinical outcomes during the second wave. Nevertheless, data on the patients’ vaccination status were not recorded, precluding us from further exploring this hypothesis. Additionally, the small sample size of patients with COVID-19 who received ECMO and the nature of the subgroup analysis precluded us from proposing mechanistic explanations for our findings. Finally, this was an observational, retrospective, single-center study. Therefore, hypothesis generation must be considered.
CONCLUSION
In the first and second waves, patients with severe COVID-19 exhibited similar mortality rates and need for invasive organ support, despite the patients in the second wave being younger and less severely ill at the time of intensive care unit admission.
ACKNOWLEDGMENTS
We thank the intensive care unit physicians, nursing staff, physical therapists, and all the multidisciplinary team members at Hospital Israelita Albert Einstein, who managed the patients during the COVID-19 pandemic. The authors thank Helena Spalic for proofreading this manuscript.
Footnotes
In Brief
We compared the characteristics and outcomes of 1,427 patients with COVID-19 admitted to the intensive care unit during the first and second waves of the COVID-19 pandemic. Both waves had similar mortality rates and need for invasive organ support, despite the patients in the second wave being younger and less severely ill at the time of intensive care unit admission.
Highlights
We compared characteristics between patients admitted during the first and second waves.
There were 1,427 intensive care unit patients with COVID-19: 421 (first wave) and 1,006 (second wave).
The patients in the second wave were younger and less severely ill at the time of intensive care unit admission.
Patients exhibited similar mortality rates and need for invasive organ support.
REFERENCES
- 1.World Health Organization (WHO) Weekly epidemiological update on COVID-19 - 27 April 2022 (Edition 89) Geneva: WHO; 2022. [cited 2023 Apr 3]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-april-20222022 . [Google Scholar]
- 2.Van Damme W, Dahake R, Delamou A, Ingelbeen B, Wouters E, Vanham G, et al. The COVID-19 pandemic: diverse contexts; different epidemics-how and why? BMJ Glob Health. 2020;5(7):e003098. doi: 10.1136/bmjgh-2020-003098. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teich VD, Klajner S, Almeida FA, Dantas AC, Laselva CR, Torritesi MG, et al. Epidemiologic and clinical features of patients with COVID-19 in Brazil. einstein (São Paulo) 2020;18:eAO6022. doi: 10.31744/einstein_journal/2020AO6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeiser FA, Donida B, Costa CA, Ramos GO, Scherer JN, Barcellos NT, et al. First and second COVID-19 waves in Brazil: a cross-sectional study of patients’ characteristics related to hospitalization and in-hospital mortality. 100107Lancet Reg Health Am. 2022;6 doi: 10.1016/j.lana.2021.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastos LS, Ranzani OT, Souza TM, Hamacher S, Bozza FA. COVID-19 hospital admissions: brazil’s first and second waves compared. Lancet Respir Med. 2021;9(8):e82–e83. doi: 10.1016/S2213-2600(21)00287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito S, Asai Y, Matsunaga N, Hayakawa K, Terada M, Ohtsu H, et al. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salyer SJ, Maeda J, Sembuche S, Kebede Y, Tshangela A, Moussif M, et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021;397(10281):1265–1275. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coccia M. The impact of first and second wave of the COVID-19 pandemic in society: comparative analysis to support control measures to cope with negative effects of future infectious diseases. 111099Environ Res. 2021;197 doi: 10.1016/j.envres.2021.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contou D, Fraissé M, Pajot O, Tirolien JA, Mentec H, Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? 3Crit Care. 2021;25(1) doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfisberg S, Gregoriano C, Struja T, Kutz A, Koch D, Bernasconi L, et al. Comparison of characteristics, predictors and outcomes between the first and second COVID-19 waves in a tertiary care centre in Switzerland: an observational analysis. w20569Swiss Med Wkly. 2021;151(3132) doi: 10.4414/smw.2021.20569. [DOI] [PubMed] [Google Scholar]
- 11.Corrêa TD, Midega TD, Timenetsky KT, Cordioli RL, Barbas CS, Silva M, Júnior, et al. Clinical characteristics and outcomes of COVID-19 patients admitted to the intensive care unit during the first year of the pandemic in Brazil: a single center retrospective cohort study. einstein (São Paulo) 2021;19:eAO6739. doi: 10.31744/einstein_journal/2021AO6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 13.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. 2000045Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Erratum in: Euro Surveill. 2020;25(14): Erratum in: Euro Surveill. 2020;25(30): Erratum in: Euro Surveill. 2021;26(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrêa TD, Matos GF, Bravim BA, Cordioli RL, Garrido AD, Assuncao MS, et al. Intensive support recommendations for critically-ill patients with suspected or confirmed COVID-19 infection. einstein (São Paulo) 2020;18:eAE5793. doi: 10.31744/einstein_journal/2020AE5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrêa TD, Matos GF, Bravim BA, Cordioli RL, Garrido AD, Assuncao MS, et al. Comment to: Intensive support recommendations for critically-ill patients with suspected or confirmed COVID-19 infection. einstein (São Paulo) 2020;18:eCE5931. doi: 10.31744/einstein_journal/2020CE5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zampieri FG, Soares M, Borges LP, Salluh JI, Ranzani OT. The Epimed Monitor ICU Database®: a cloud-based national registry for adult intensive care unit patients in Brazil. Rev Bras Ter Intensiva. 2017;29(4):418–426. doi: 10.5935/0103-507X.20170062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR, SAPS 3 Investigators SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. Erratum in: Intensive Care Med. 2006;32(5):796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183(1):104–110. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Zampieri FG, Iwashyna TJ, Viglianti EM, Taniguchi LU, Viana WN, Costa R, Corrêa TD, Moreira CE, Maia MO, Moralez GM, Lisboa T, Ferez MA, Freitas CE, Carvalho CB, Mazza BF, Lima MF, Ramos GV, Silva AR, Bozza FA, Salluh JF, Soares M, ORCHESTRA Study Investigators Association of frailty with short-term outcomes, organ support and resource use in critically ill patients. Intensive Care Med. 2018;44(9):1512–1520. doi: 10.1007/s00134-018-5342-2. [DOI] [PubMed] [Google Scholar]
- 22.Marcolino MS, Ziegelmann PK, Souza-Silva MV, Nascimento IJ, Oliveira LM, Monteiro LS, Sales TL, Ruschel KB, Martins KP, Etges AP, Molina I, Polanczyk CA, Brazilian COVID-19 Registry Investigators Clinical characteristics and outcomes of patients hospitalized with COVID-19 in Brazil: Results from the Brazilian COVID-19 registry. Int J Infect Dis. 2021;107:300–310. doi: 10.1016/j.ijid.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza-Silva MV, Ziegelmann PK, Nobre V, Gomes VM, Etges AP, Schwarzbold AV, et al. Hospital characteristics associated with COVID-19 mortality: data from the multicenter cohort Brazilian Registry. Intern Emerg Med. 2022;17(8):2299–2313. doi: 10.1007/s11739-022-03092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares M, Bozza FA, Angus DC, Japiassú AM, Viana WN, Costa R, et al. Organizational characteristics, outcomes, and resource use in 78 Brazilian intensive care units: the ORCHESTRA study. Intensive Care Med. 2015;41(12):2149–2160. doi: 10.1007/s00134-015-4076-7. [DOI] [PubMed] [Google Scholar]
- 25.Zampieri FG, Salluh JI, Azevedo LC, Kahn JM, Damiani LP, Borges LP, Viana WN, Costa R, Corrêa TD, Araya DE, Maia MO, Ferez MA, Carvalho AG, Knibel MF, Melo UO, Santino MS, Lisboa T, Caser EB, Besen BA, Bozza FA, Angus DC, Soares M, ORCHESTRA Study Investigators ICU staffing feature phenotypes and their relationship with patients’ outcomes: an unsupervised machine learning analysis. Intensive Care Med. 2019;45(11):1599–1607. doi: 10.1007/s00134-019-05790-z. [DOI] [PubMed] [Google Scholar]
- 26.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, Bradley JM, Dark P, Dave C, De Soyza A, Dennis AV, Devrell A, Fairbairn S, Ghani H, Gorman EA, Green CA, Hart N, Hee SW, Kimbley Z, Madathil S, McGowan N, Messer B, Naisbitt J, Norman C, Parekh D, Parkin EM, Patel J, Regan SE, Ross C, Rostron AJ, Saim M, Simonds AK, Skilton E, Stallard N, Steiner M, Vancheeswaran R, Yeung J, McAuley DF, RECOVERY-RS Collaborators Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients With Acute Hypoxemic Respiratory Failure and COVID-19: the RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327(6):546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. 121Crit Care. 2021;25(1) doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujino T, Nomoto H, Kutsuna S, Ujiie M, Suzuki T, Sato R, et al. Novel SARS-CoV-2 Variant in Travelers from Brazil to Japan. Emerg Infect Dis. 2021;27(4):1243–1245. doi: 10.3201/eid2704.210138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naveca FG, Nascimento V, Souza VC, Corado AL, Nascimento F, Silva G, et al. COVID-19 in Amazonas, Brazil, was driven by the persistence of endemic lineages and P.1 emergence. Nat Med. 2021;27(7):1230–1238. doi: 10.1038/s41591-021-01378-7. [DOI] [PubMed] [Google Scholar]
- 31.Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, Gonzalez G, Garcia-Leon A, Crispie F, O’Connor L, Murphy N, Mossong J, Vergison A, Wienecke-Baldacchino AK, Abdelrahman T, Riccardo F, Stefanelli P, Di Martino A, Bella A, Lo Presti A, Casaca P, Moreno J, Borges V, Isidro J, Ferreira R, Gomes JP, Dotsenko L, Suija H, Epstein J, Sadikova O, Sepp H, Ikonen N, Savolainen-Kopra C, Blomqvist S, Möttönen T, Helve O, Gomes-Dias J, Adlhoch C, COVID study groups Characteristics of SARS-CoV-2 variants of concern B.1.1.7, B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. 2100348Euro Surveill. 2021;26(16) doi: 10.2807/1560-7917.ES.2021.26.16.2100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. 855BMC Infect Dis. 2021;21(1) doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtz P, Bastos LS, Salluh JI, Bozza FA, Soares M. SAPS-3 performance for hospital mortality prediction in 30,571 patients with COVID-19 admitted to ICUs in Brazil. Intensive Care Med. 2021;47(9):1047–1049. doi: 10.1007/s00134-021-06474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]