Keywords: kidney biopsy, epidemiology and outcomes, nephron, CKD, renal morphology
Abstract
Significance Statement
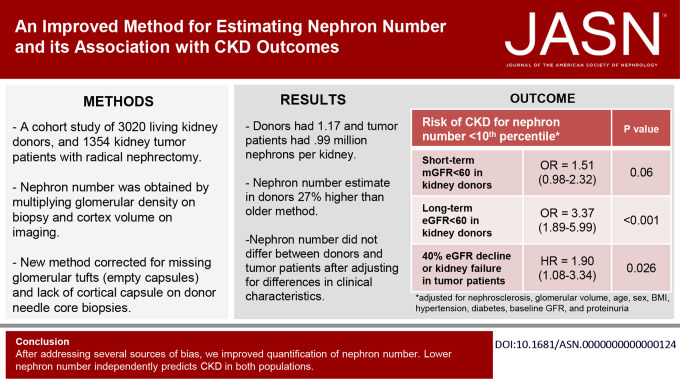
Nephron number currently can be estimated only from glomerular density on a kidney biopsy combined with cortical volume from kidney imaging. Because of measurement biases, refinement of this approach and validation across different patient populations have been needed. The prognostic importance of nephron number also has been unclear. The authors present an improved method of estimating nephron number that corrects for several biases, resulting in a 27% higher nephron number estimate for donor kidneys compared with a prior method. After accounting for comorbidities, the new nephron number estimate does not differ between kidney donors and kidney patients with tumor and shows consistent associations with clinical characteristics across these two populations. The findings also indicate that low nephron number predicts CKD independent of biopsy and clinical characteristics in both populations.
Background
Nephron number can be estimated from glomerular density and cortical volume. However, because of measurement biases, this approach needs refinement, comparison between disparate populations, and evaluation as a predictor of CKD outcomes.
Methods
We studied 3020 living kidney donors and 1354 patients who underwent radical nephrectomy for tumor. We determined cortex volume of the retained kidney from presurgical imaging and glomerular density by morphometric analysis of needle core biopsy of the donated kidney and wedge sections of the removed kidney. Glomerular density was corrected for missing glomerular tufts, absence of the kidney capsule, and then tissue shrinkage on the basis of analysis of 30 autopsy kidneys. We used logistic regression (in donors) and Cox proportional hazard models (in patients with tumor) to assess the risk of CKD outcomes associated with nephron number.
Results
Donors had 1.17 million nephrons per kidney; patients with tumor had 0.99 million nephrons per kidney. A lower nephron number was associated with older age, female sex, shorter height, hypertension, family history of ESKD, lower GFR, and proteinuria. After adjusting for these characteristics, nephron number did not differ between donors and patients with tumor. Low nephron number (defined by <5th or <10th percentile by age and sex in a healthy subset) in both populations predicted future risk of CKD outcomes independent of biopsy and clinical characteristics.
Conclusions
Compared with an older method for estimating nephron number, a new method that addresses several sources of bias results in nephron number estimates that are 27% higher in donors and 1% higher in patients with tumor and shows consistency between two populations. Low nephron number independently predicts CKD in both populations.
Introduction
Nephron number is a fundamental property of kidney health,1 but there are limited options for determining nephron number in living humans.2,3 We and others previously developed a method to estimate nephron number from the product of cortical volume (determined on contrast-enhanced computerized tomography [CT]) and glomerular density on kidney biopsy (glomerular density) in a cohort of living kidney donors.4,5 We subsequently showed that low nephron number (<5th percentile for age) was predictive of detectable albuminuria at 4 months after donation6 and of eGFR <45 ml/min per 1.73 m2 at 10 years after donation.7
However, there are several problems we have identified with this approach. First, we used a published correction factor to account for biopsy tissue shrinkage from formalin fixation and paraffin embedding (FFPE)5 and from loss of arterial perfusion pressure.8 The original study from which tissue shrinkage from FFPE was determined was on the basis of needle core kidney biopsies from eight rats.9 Needle core kidney tissue is prone to stretching artifact that may bias the determination of shrinkage, and human kidney tissue shrinkage may differ from that in rats. Second, we identified a calculation error in how we corrected for tissue shrinkage. Specifically, instead of dividing glomerular density by 1.43 (for 43% shrinkage from FFPE) and 1.268 (for 26.8% shrinkage from loss of perfusion), we should have divided glomerular density by 1.75=1/(1−0.43) for shrinkage from FFPE and 1.366=1/(1−0.268) for shrinkage from loss of perfusion. Third, we calculated glomerular density on kidney biopsy using glomerular tuft profiles. However, some glomerular profiles are an empty glomerular capsule with a missing tuft that was displaced during tissue processing. Fourth, glomerular density is higher near the renal capsule because the descending tubules of more superficial glomeruli disperse deeper glomeruli apart but needle core biopsies vary in their cortical depth.4 Thus, needle biopsies need to be standardized to account for higher superficial glomerular density near the capsule even when the capsule is not present. Fifth, we assumed that partial glomerular tufts bisected by the needle should be counted as half a glomerulus, and this assumption should be tested. Sixth, we had not accounted for variation in kidney image slice thickness on determining kidney cortical volume.
Perhaps the most concerning problem with this approach for estimating nephron number is lack of validation against an independent method. There are currently limited options for independent validation in living humans, particularly at the sample sizes needed for meaningful analysis.2 Nonetheless, showing consistency of nephron number estimates in a patient population with large wedge sections rather than needle core biopsies to define glomerular density would provide supportive evidence to this approach. While available in some living kidney donor programs,10,11 patients who undergo a radical (complete) nephrectomy for tumor also provide an opportunity to study large wedge sections of kidney parenchyma.12 Patients with tumor also provide an opportunity to study nephron number in older age ranges and with comorbidities not present in living kidney donors. The risk of adverse kidney outcomes with low nephron number in patients with tumor can also be assessed.
Thus, we performed a study to improve our method to estimate nephron number accounting for several measurement biases with the prior method. We applied this new method to both living kidney donors and tumor nephrectomy patients with two primary objectives—first, to determine whether nephron number estimates were consistent between living kidney donors and patients with tumor after accounting for differences in clinical characteristics and second, to determine how nephron number associates with clinical characteristics and how it predicts adverse kidney outcomes in both populations.
Methods
Study Populations
This Aging Kidney Anatomy study relates structural measures on kidney biopsy and kidney CT/magnetic resonance imaging (MRI) imaging to clinical characteristics and outcomes among living kidney donors and patients who undergo radical nephrectomy for tumor.6,7,13,14 The study was approved by the Mayo Clinic Institutional Review Board.
The living kidney donors are from three sites (Mayo Clinic, Minnesota; Mayo Clinic, Arizona; and Cleveland Clinic, Ohio) where they underwent an intraoperative biopsy of the donated kidney during transplant surgery from 2000 to 2019. Living kidney donors were selected on the basis of their health as previously described.4,15 Briefly, acceptable criteria for donation varied by era and site and, in general, included 24-hour urine albumin <30 mg/24 hours and a measured GFR (mGFR) normal for age.16 Mild hypertension in older donors and moderate obesity (body mass index [BMI] 30–35 kg/m2; occasionally up to 40 kg/m2 in older donors) were allowed. Hypertension was defined as a diagnosis of hypertension before donation, an office systolic blood pressure of 140–159 mm Hg, a diastolic blood pressure of 90–99 mm Hg, or the use of one antihypertensive medication (with or without a thiazide diuretic). Potential donors with more severe hypertension, diabetes, or cardiovascular disease were not accepted as donors.
Patients with kidney tumor are from Mayo Clinic, Minnesota, where they underwent a radical unilateral nephrectomy for a kidney tumor from 2000 to 2019. None had metastatic lesions or positive lymph nodes at the time of surgery. We excluded tumor patients with extensive renal vein thrombosis, a diffuse specific kidney disease on histology, diffuse tubulointerstitial inflammation (involving most of the cortex and medulla), severe ischemia with percentage of interstitial fibrosis and tubular atrophy (IFTA) >90% of the cortex, a large focal scar, end stage kidney histology (thin and completely scarred cortex), or kidney failure within 4 months after nephrectomy.14 We did not exclude patients who had evidence of mild-moderate diabetic nephropathy or patients who had minimal segmental glomerulosclerosis.
Clinical Characteristics
Demographics and preoperative clinical characteristics of both donors and patients with tumor were abstracted from the medical record. This included age, sex, race, height, BMI, serum creatinine (corrected to standardized values if assayed before standardization), and hypertension. Hypertension in both donors and patients with tumor was defined by an office blood pressure of >140/90 mm Hg or use of antihypertensive medications to lower blood pressure. Known family history of ESKD was defined by being related to the kidney recipient and was only available in donors (donors unrelated to the recipient and patients with tumor were categorized as no known family history of ESKD). Diabetes mellitus as reported in clinical notes was abstracted from the medical record in patients with tumor (diabetes mellitus was not permitted in donors). Both donors and patients with tumor had serum creatinine–based eGFR using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation without adjustment for race.17 Donors also had mGFR by urinary iothalamate clearance.
Clinical proteinuria data are inherently complicated. We primarily used 24-hour urine protein estimated from a spot urine protein-to-osmolality ratio18 because this was available in most Mayo Clinic donors and patients with tumor. For Mayo Clinic donors without urine protein-to-osmolality ratios, we estimated 24-hour urine protein from protein-to-creatinine ratios19 and then measured 24-hour urine protein values were used as available. All Cleveland Clinic donors had a measured 24-hour urine protein that was reported as <160 mg and were assigned the median 24-hour urine protein value of 62 mg on the basis of Mayo Clinic donors with 24-hour urine protein <160 mg. Higher levels of urine protein within the normal range are not necessarily pathological in donors because of the requirement of a urine albumin of <30 mg.20 Thus, 24-hour urine protein in donors was used to define an abnormal threshold (95th percentile), and this value was used to dichotomize 24-hour urine protein for both cohorts. A measured 24-hour urine albumin level was also available in most Mayo Clinic donors. Missing values for 24-hour urine protein (1.4% of donors and 13.3% of patients with tumor) and 24-hour urine albumin (19.9% of donors) were imputed using multivariate imputation by chained equations.21
Kidney Microstructure
For kidney donors, an 18-gauge needle core biopsy of the kidney cortex was obtained at the time of transplantation, fixed in formalin, and embedded in paraffin. For patients with tumor, the stored formalin-fixed whole-kidney specimen was retrieved to obtain a large wedge section (far from the tumor) that was then embedded in paraffin. A 2–3-µm–thick section was cut from the paraffin embedded tissue block, stained for periodic acid-Schiff, and scanned into high-resolution digital images (Aperio AT2 system scanner, Leica Microsystems, Inc., Buffalo Grove, IL; http://www.aperio.com). Only donors with biopsy sections that had no obvious compression artifacts, at least 2 mm2 of the cortex area, and at least four glomeruli were studied.22
Biopsy images were annotated by investigators at Mayo Clinic, MN, while masked to clinical characteristics. Training data and reproducibility studies were used to ensure consistency between different investigators as previously reported.4 The scanned digital images were magnified with ImageScope software (version 12.2.2.5015 Aperio) onto a large touch-screen tablet, and the cortex and each glomerular tuft cross-sectional profile (separately for non-sclerosed and globally sclerosed) were manually traced with a pen. In addition, all missing tufts as evidenced by empty Bowman's capsule were counted (sometimes, an isolated glomerular tuft would be seen in the empty space adjacent to the tissue for these biopsy sections or be even displaced elsewhere in the tissue) (Supplemental Figure 1). Smaller tissue area associated with a higher percentage of missing glomeruli at all three sites for donor needle biopsies (Minnesota: rs=−0.13, P = 0.0001; Arizona: rs=−0.13, P = 0.002; Ohio: rs=−0.17, P = 0.001), but not for the much larger nephrectomy biopsies (rs=0.01, P = 0.82). In donors, partial glomeruli (bisected by the needle) were counted as 0.675, the mean ratio of the partial tuft area to the complete tuft area (Supplemental Methods). We then used Weibel and Gomez stereology models23 to calculate the volumetric non-sclerosed glomerular tuft density (referred to as glomerular density) and the mean non-sclerosed glomerular tuft volume (referred to as glomerular volume). To account for glomerular capsules with missing tufts, glomerular density was corrected using the mean tuft area of glomerular profiles with tufts for those without tufts (Supplemental Methods and Supplemental Figure 2). Cortex per glomerulus, a measure of nephron size, was defined as the reciprocal of this corrected glomerular density.
The number of globally sclerotic glomerular (GSG) profiles per slide was divided by the total number of glomerular profiles (including those with missing tufts) per slide to calculate the %GSG. The IFTA was manually traced for the entire cortex to calculate the proportion of the cortex area (%IFTA) and the number of IFTA foci, as previously described.13 The IFTA foci density was calculated by dividing the number of distinct IFTA foci by the traced cortical area.14
Kidney Cortex and Tumor Volume
For donors, predonation CT and for patients with tumor, presurgery CT or MRI images were analyzed. The cortical volume of the retained kidney and, in patients with tumor, the tumor volume were determined after segmentation using a semiautomated image processing tool (ITK-SNAP software, version 2.2; University of Pennsylvania, Philadelphia, PA) as previously reported.15 Among CT or MRI scans with poor cortical-medullary differentiation (200 [6.6%] donors and 661 [48.8%] patients with tumor), the total kidney parenchymal volume (cortex and medulla) was segmented. Cortical volume was estimated from kidney volume using a linear regression model that also adjusted for slice thicknesses >2.5 mm (Supplemental Methods).15
Glomerular Density Correction for Bias
The glomerular density of donor needle core biopsies without the capsule was increased by 1.77 glomeruli/mm3 to reflect the estimated glomerular density if the capsule was present (Supplemental Methods). To measure a shrinkage factor for FFPE, a needle core biopsy was not used because of tissue stretchiness making it difficult to measure a consistent length before FFPE. Instead, square cortical wedge sections of approximately 100 mm2 were obtained on 30 autopsy patients without severe kidney disease or macroscopic deformities. A digital image was obtained before FFPE to measure the area. Then, after formalin fixation for 30 days followed by embedding in paraffin, a digital image after FFPE was obtained to measure the area (Supplemental Figure 3). The two-dimensional tissue shrinkage was used to calculate the true three-dimensional tissue shrinkage, and the mean value (1.365) was used as the shrinkage factor (Supplemental Table 1).
Nephron Number Calculation
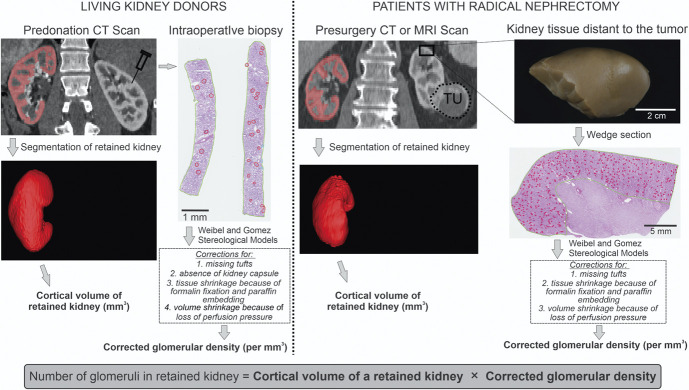
The corrected glomerular density of the biopsied kidney in both cohorts was calculated by obtaining glomerular density (per mm3), recalculating its value corrected for any missing tufts, adding 1.77 per mm3 if the kidney capsule was missing, dividing by 1.365 for the FFPE shrinkage factor, and dividing by 1.366 to account for shrinkage from loss of perfusion pressure8 (Figure 1). Nephron number was then calculated from corrected glomerular density times cortical volume of the retained kidney. Nephron number was also calculated using the previously published old method4 (Supplemental Methods).
Figure 1.
Method used to estimate nephron number. The retained kidney cortex (red) on the pre-nephrectomy imaging study was segmented to determine cortical volume. The biopsy of the removed kidney was used to calculate volumetric non-sclerotic glomerular density. This included corrections for missing tufts (empty glomerular capsule), and in donors, absence of kidney capsule on the biopsy section. The glomerular density was divided by correction factors to account for our new estimate of tissue shrinkage with FFPE and for volume shrinkage due to loss of perfusion pressure.8 Then this “true” glomerular density was multiplied by cortical volume to estimate nephron number. Figure 1 can be viewed in color online at www.jasn.org.
Outcomes
As part of clinical care, donors returned to their donation center for a standardized follow-up visit a median of 4.4 months after donation and CKD outcomes were identified using a mGFR of either <60 or <45 ml/min per 1.73 m2.6 The donors were also surveyed a median of 10.5 years after donation to obtain their most recent serum creatinine that was used to identify CKD outcomes by an eGFR of either <60 or <45 ml/min per 1.73 m2.7 We previously found good correlation (r=0.85) and no significant bias between serum creatinine levels that were self-reported versus those in the medical record for the subset with follow-up care at Mayo Clinic, Minnesota (n=82).7 For patients with tumor, a time-to-event analysis was possible because these patients return for visits with serum creatinine every 3–6 months during the first year after nephrectomy and subsequently once or twice per year. A post-nephrectomy baseline eGFR was defined by the eGFR closest to but preceding 4 months after the nephrectomy. Progressive CKD was defined as the first occurrence of either a 40% decline in eGFR from the post-nephrectomy baseline eGFR, dialysis, kidney transplantation, or eGFR<10 ml/min per 1.73 m2 through June 1, 2022. Kidney failure was defined as the first date of dialysis, kidney transplantation, or onset of eGFR <10 ml/min per 1.73 m2.
Statistical Analyses
Age was categorized into 18–39, 40–49, 50–59, 60–69, 70–79, and 80–89 years (patients with tumor only). Linear regression was used to evaluate the relationship of nephron number with age (continuous spline with a knot at 70 years because few donors were older than 70 years), sex, height, BMI, hypertension, diabetes (patients with tumor only), family history of ESKD (donors only), mGFR (donors only), eGFR, abnormal 24-hour urine protein, 24-hour urine albumin (donors only), and tumor volume (patients with tumor only). A multivariable model assessed for an independent association of each clinical characteristic with nephron number. A test for interaction was used to determine whether these multivariable adjusted associations differed between kidney donors and patients with tumor. A multivariable model subsequently identified clinical characteristics that independently associated with nephron number in the combined population of donors and patients with tumor. This model also assessed whether there was any difference in nephron number between kidney donors and patients with tumor after adjustment for differences in clinical characteristics. Nephron number and the associations of clinical characteristics with nephron number were compared between this new method and the old method.4 The nephron number and %GSG in each age group was compared with the youngest age group stratified by sex and by donor versus patient with tumor. Age- and sex-based thresholds for low nephron number were defined by the 5th and 10th percentiles in a healthy subset (no family history of ESKD, hypertension, 24-hour urine protein >500 mg, diabetes, eGFR <60 ml/min per 1.73 m2 if 60 years or older, or eGFR <45 ml/min per 1.73 m2 if younger than 60 years).
Logistic regression was used to determine the risk of CKD outcomes in donors as predicted by nephron number, low nephron number, %GSG, %IFTA, and IFTA foci density. Cox proportional hazard regression was used to assess risk of progressive CKD or kidney failure outcomes in patients with kidney tumor censoring at last eGFR, death, or cancer recurrence. Models adjusted for nephrosclerosis measures reflective of nephron loss (%GSG, %IFTA, and IFTA foci density) were further adjusted for glomerular volume and then even further adjusted for clinical characteristics.
We identified the subset of 35 donors with two separate needle core biopsies and 68 patients with tumor with two separate wedge biopsies (Supplemental Figure 4). To assess reproducibility, we calculated the within-person coefficient of variation (CV) for glomerular density. All statistical analyses were performed using BlueSky Statistics software version 7.40 (BlueSky Statistics LLC, Chicago, IL) and R (RStudio) version 3.4.2. A P value of <0.05 was considered significant.
Results
Clinical and Biopsy Characteristics of Cohorts
There were 3020 living kidney donors and 1354 tumor nephrectomy patients studied in the cross-sectional analysis with subsets that had longitudinal data for outcome analyses (Figure 2). The demographic, clinical, and biopsy characteristics of kidney donors and patients with tumor are presented and compared in Table 1. Compared with kidney donors, patients with tumor were older by a mean of 20 years and were proportionally more male, obese, diabetic, and hypertensive and had lower eGFR and higher 24-hour urine protein. With wedge biopsy sections, patients with tumor had 21-fold more cortex area, more glomeruli, and a much lower percentage of missing glomerular tufts. Patients with tumor also had larger glomerular tuft volume, more cortex per glomerulus, higher %GSG, higher %IFTA, and higher IFTA foci density than donors. Among patients with tumor, the median tumor volume was 144 cm3 (interquartile range [IQR], 59–330 cm3). Cortical volume of the retained kidney was larger in patients with tumor than donors. Among 20 patients with tumor who had both CT and MRI (median 15 days apart), there was good agreement in the retained kidney parenchymal volume (between-test CV of 1.8%).
Figure 2.
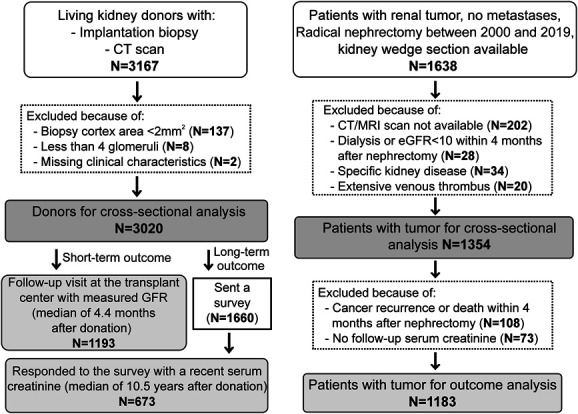
Selection of study sample for both living kidney donors and patients with kidney tumor.
Table 1.
Pre-nephrectomy clinical characteristics and biopsy findings of living kidney donors and patients with tumor
| Clinical Characteristic or Biopsy Finding | Kidney Donors (N=3020) |
Patients with Tumor (N=1354) |
P Value |
|---|---|---|---|
| Mean (SD), Median [IQR], or n (%) | |||
| Demographic | |||
| Age, yr | 44.1 (12.1) | 64.2 (11.9) | <0.001 |
| Men, % | 1235 (40.9%) | 877 (64.8%) | <0.001 |
| Height, cm | 170.5 (9.6) | 172.5 (10.0) | <0.001 |
| Race | <0.001 | ||
| White or unknown | 2772 (91.8%) | 1294 (95.5%) | |
| Black | 92 (3.0%) | 23 (1.7%) | |
| American Indian/Alaskan Native | 48 (1.6%) | 15 (1.1%) | |
| Asian | 43 (1.4%) | 5 (0.4%) | |
| Other | 65 (2.2%) | 17 (1.3%) | |
| CKD risk factors | |||
| Diabetes mellitus, % | 0 (0%) | 193 (14.3%) | <0.001 |
| Hypertension, % | 437 (14.5%) | 923 (68.2%) | <0.001 |
| Known family history of ESKD, % | 1522 (50.4%) | 0 (0%) | <0.001 |
| BMI, kg/m2 | 27.5 (4.8) | 30.6 (6.7) | <0.001 |
| Kidney function | |||
| mGFR, ml/min/1.73 m2a | 103.4 (18.5) | N/A | |
| eGFR, ml/min/1.73 m2 | 100.0 (16.2) | 72.6 (19.5) | <0.001 |
| 24-h urine albumin, mgb | 4.0 (7.3) | N/A | |
| 24-h urine protein, mgc | 63 [52–94] | 172 [98–355] | <0.001 |
| >165 mg (95th percentile in donors) | 147 (4.9%) | 607 (51.7%) | <0.001 |
| >500 mg | 0 (0.0%) | 213 (18.1%) | <0.001 |
| Biopsy measures of the removed kidney | |||
| Biopsy cortex area, mm2 | 6.5 (3.0) | 137.2 (57.8) | <0.001 |
| Glomerular tuft volume, mm3 | 0.0026 (0.0011) | 0.0028 (0.0010) | <0.001 |
| Missing glomerular tuft profiles, % | 16.2 (11.2) | 3.5 (3.5) | <0.001 |
| Corrected number of glomerular tufts | 20.4 (11.1) | 346.0 (156.7) | <0.001 |
| Glomerular density, per mm3 | 21.4 (7.9) | 15.4 (5.8) | <0.001 |
| Cortex per glomerulus, mm3 | 0.054 (0.022) | 0.075 (0.031) | <0.001 |
| No. of observed glomerular tuftsd | 17.3 (10.1) | 334.1 (152.8) | <0.001 |
| No. of missing glomerular tuftse | 3.1 (2.3) | 11.9 (13.2) | <0.001 |
| No. of GSGs on biopsy | 0.6 (1.0) | 32.1 (37.2) | <0.001 |
| GSGs of all glomeruli, % | 2.9 (5.4) | 8.9 (8.9) | <0.001 |
| 0%–10% | 2755 (91.2%) | 952 (70.3%) | |
| 11%–25% | 238 (7.9%) | 314 (23.2%) | |
| >25% | 27 (0.9%) | 88 (6.5%) | |
| IFTA, % | 0.3 (1.2) | 3.9 (7.5) | <0.001 |
| IFTA density, foci per cm2 cortex | 4.4 (11.8) | 26.0 (21.1) | <0.001 |
| CT/MRI measures | |||
| MRI instead of CT imaging | 0 (0%) | 167 (12.3%) | <0.001 |
| Measured cortical volume of retained kidney, mm3 | 103,100 (21,526) | 123,616 (34,629) | <0.001 |
| Measured or estimated cortical volume of retained kidney, mm3 | 104,100 (22,205) | 124,037 (37,916) | <0.001 |
| Tumor volume in patients with tumor, mm3 | N/A | 144,480 [58,907–330,440] | |
mGFR, measured GFR; GSG, globally sclerotic glomerular; IFTA, interstitial fibrosis and tubular atrophy, IQR, interquartile range; CT, computerized tomography; MRI, magnetic resonance imaging; BMI, body mass index; N/A, not available.
Measured GFR used only at Mayo Clinic, Rochester, and Cleveland Clinic sites.
Data available in 2420 donors only.
Data available in 2978 donors and 1174 patients with tumor. Of Mayo Clinic donors, urine protein was estimated from the protein-osmolality ratio in 2099 donors and from the protein-to-creatinine ratio in 338 donors and 150 donors had measured 24-hour urine protein. All 391 Cleveland Clinic donors were assigned a median value from the Mayo Clinic donors.
For donors, this represents mean 15.7 complete observed tufts+0.675 (mean 2.3 observed partial tufts bisected by a needle).
For donors, this represents mean 1.9 complete empty capsules with missing tufts+0.675 (mean 1.8 empty partial capsules missing tuft bisected by a needle).
Calculation of Nephron Number
An example of the calculation for nephron number in both cohorts using the mean glomerular density of women aged 50–69 years with no known family history of ESKD is presented in Table 2. In this subgroup, the cortex per glomerulus was 35.1% larger in patients with tumor than donors (P < 0.001) and the total cortical volume was 22.6% larger in patients with tumor than donors (P < 0.001). Because the larger nephrons (cortex per glomerulus) were proportionally larger than the larger cortex, this resulted in a lower nephron number in patients with tumor compared with donors (−8.6%, P = 0.003). In the full cohorts, there were a mean of 1.17 (95% confidence interval [CI], 1.16 to 1.19) million nephrons in donors and 0.99 (95% CI, 0.97 to 1.01) million nephrons in patients with tumor per kidney. This compared with 0.92 (95% CI, 0.90 to 0.93) million nephrons in donors and 0.98 (95% CI, 0.96 to 1.01) million nephrons in patients with tumor using the old method that did not correct for measurement biases. The within-person CV for glomerular density was 23.3% for donors and 9.7% for patients with tumor. In combination with the previously established within-person CV of 6% for cortical volume,24 the CV for nephron number was 29.3% for donors and 15.7% for patients with tumor. Nephron number did not differ between 2820 donors with measured cortical volume versus 200 donors with estimated cortical volume (1.17 versus 1.18 million, P = 0.78) or between 693 patients with tumor with measured cortical volume versus 661 patients with tumor with estimated cortical volume (0.98 versus 1.01 million, P = 0.21). Finally, nephron number did not differ between 1187 patients with tumor who had CT versus 167 patients with tumor who had MRI to determine cortical volume (1.00 versus 0.96 million, P = 0.24).
Table 2.
Example of calculating nephron number in the retained kidney using the mean values in both cohorts for women between ages 50–69 years with no family history of ESKD
| Calculating Nephron Number Steps | Living Kidney Donors (n=390) |
Patients with Kidney Tumor (n=256) |
Percentage Difference | P Value | |
|---|---|---|---|---|---|
| Glomerular density corrections | Correction factor | Glomerular density per mm3 | Glomerular density per mm3 | ||
| Uncorrected glomerular density with only observed tufts | 17.3 | 16.3 | −5.8% | 0.091 | |
| Correction for missing tufts | Patient-specific | 20.8 | 16.9 | −18.8% | <0.0001 |
| Correction for absence of the kidney capsule | +1.77 in those without the capsule | 22.4 | 16.9 | −24.6% | <0.0001 |
| Correction for tissue shrinkage due to paraffin embedding | Divide by 1.366 | 16.4 | 12.4 | −24.4% | <0.0001 |
| Correction for the loss of tissue perfusion pressure | Divide by 1.365 | 12.0 | 9.1 | −24.2% | <0.0001 |
| Corrected cortex per glomerulus, mm3 | Reciprocal of glomerular density | 0.094 | 0.127 | +35.1% | |
| Calculating nephron number | |||||
| Cortical volume, mm3 | 88,824 | 108,894 | +22.6% | <0.0001 | |
| Nephron number per kidney | 1,053,980 | 963,636 | −8.6% | 0.003 | |
| Nephron number per kidney using the old method4 | 830,472 | 957,229 | +15.3% | <0.0001 | |
Clinical Characteristics Associated with Nephron Number
In both donor and tumor patient populations, older age, female, shorter height, hypertension, and lower eGFR associated with lower nephron number (Table 3). In patients with tumor, higher proteinuria associated with lower nephron number. In donors, lower mGFR had a stronger association with lower nephron number than did lower eGFR. Diabetes trended toward lower nephron number in patients with tumor, but did not achieve statistical significance. Tumor size did not affect nephron number. Multivariable analyses identified independent clinical characteristics associated with nephron number and assessed whether these associations differed between donors and patients with tumor. In multivariable adjusted analyses, there was no significant difference between the two cohorts in the association of nephron number with age, sex, height, BMI, hypertension, or proteinuria (Table 4). However, lower eGFR did associate more strongly with lower nephron number in patients with tumor than it did in donors, and family history of ESKD was now associated with lower nephron number in donors. Associations with nephron number were similar using the old method4 compared with this new method in donors (Supplemental Table 2). In the combined sample, the independent predictors of lower nephron number were older age, female sex, shorter height, hypertension, family history of ESKD, lower eGFR, and higher proteinuria (Table 5). There was no significant difference in nephron number between donors and patients with tumor after adjustment for clinical characteristics. In analysis limited to participants with measured cortical volume, findings were similar, except for proteinuria >165 mg, which was only borderline significant (P = 0.07).
Table 3.
Unadjusted association of clinical characteristics with nephron number in the retained kidney in 3020 living kidney donors and 1354 patients with kidney tumor
| Clinical Characteristic | Living Kidney Donors | Patients with Kidney Tumor | ||
|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | |
| Age, per 10 yr (up to age 70 yr) | −65,680 | <0.0001 | −88,180 | <0.0001 |
| Age, per 10 yr (>70 yr) | −64,971 | 0.81 | −167,039 | <0.0001 |
| Male | 136,515 | <0.0001 | 129,636 | <0.0001 |
| Height, per SD | 72,943 | <0.0001 | 94,808 | <0.0001 |
| BMI, per SD | 12,738 | 0.13 | 16,602 | 0.13 |
| Hypertension | −100,428 | <0.0001 | −122,611 | <0.0001 |
| Diabetes | — | — | −55,181 | 0.08 |
| Family history of ESKD | −28,713 | 0.08 | — | — |
| mGFR, per SDb | 78,022 | <0.0001 | — | — |
| eGFR, per SD | 64,169 | <0.0001 | 139,198 | <0.0001 |
| 24-h urine albumin, per SDc | 7638 | 0.36 | — | — |
| 24-h protein >165 mgd | −34,486 | 0.37 | −93,398 | <0.0001 |
| Tumor volume per doubling | — | — | 5301 | 0.37 |
BMI, body mass index, mGFR, measured GFR.
Measured GFR imputed in 520 donors.
Twenty-four–hour urine albumin imputed in 600 donors.
Twenty-four–hour urine protein imputed in 42 donors and 180 patients with tumor.
Table 4.
Multivariable-adjusted association of clinical characteristics with nephron number in retained kidney in 3020 donors and 1354 patients with tumor
| Clinical Characteristic | Living Kidney Donors | Patients with Kidney Tumor | Test for Interaction | ||
|---|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | ||
| Age per 10 yr (up to age 70 yr) | −44,704 | <0.0001 | −40,479 | 0.002 | 0.68 |
| Age, per 10 yr (>70 yr) | −39,778 | 0.44 | −97,446 | 0.0002 | 0.80 |
| Male | 61,677 | 0.007 | 41,778 | 0.16 | 0.43 |
| Height, per SD | 50,522 | <0.0001 | 56,637 | <0.0001 | 0.62 |
| BMI, per SD | 15,370 | 0.06 | 7658 | 0.75 | 0.40 |
| Hypertension | −60,992 | 0.011 | −34,292 | 0.14 | 0.26 |
| Diabetes | — | — | −48,421 | 0.10 | — |
| Family history of ESKD | −78,687 | <0.0001 | — | — | — |
| eGFR, per SD | 38,624 | 0.001 | 96,154 | <0.0001 | <0.0001 |
| 24 h protein >165 mg | −27,354 | 0.46 | −53,211 | 0.009 | 0.43 |
BMI, body mass index.
Each characteristic adjusted for each other characteristic, but with separate models for each cohort ×characteristic interaction.
Table 5.
Independent clinical characteristics associated with nephron number in retained kidney in the combined sample of both kidney donors and patients with tumor
| Characteristic | All Donors and Tumor Patients (N=4374) | Only Donors and Tumor Patients with Measured Cortical Volume (N=3513) | ||
|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | |
| Age per 10 yr (up to age 70 yr) | −38,473a | <0.0001a | −43,007a | <0.0001a |
| Age, per 10 yrs (>70 yr) | −111,786a | <0.0001a | −94,378a | 0.01a |
| Male | 56,546a | 0.002a | 52,230a | 0.01a |
| Height per SD | 53,663a | <0.0001a | 57,402a | <0.0001a |
| BMI per SD | 13,292 | 0.052 | 13,006 | 0.11 |
| Hypertension | −54,958a | 0.001a | −45,839a | 0.02a |
| Diabetes | −48,776 | 0.14 | −18,848 | 0.70 |
| Family history of ESKD | −80,394a | <0.0001a | −85,142a | <0.0001a |
| eGFR per SD | 74,558a | <0.0001a | 72,255a | <0.0001a |
| 24 h urine protein >165 mg | −52,027a | 0.007a | −43,588 | 0.07 |
| Patients with tumor versus donors | −3354 | 0.88 | −32,258 | 0.25 |
BMI, body mass index.
The mean SD across both populations was used (9.76 cm for height, 5.7 kg/m2 for body mass index, 21.6 ml/min/1.73m2 for eGFR).
Nephron Number Trends with Age
Nephron number and %GSG by each age and sex group were compared between donors and patients with tumor using 18–39 years as the reference group. Donors with a family history of ESKD were excluded in this analysis such that the donors clearly represented the lower risk cohort for kidney disease. In each age group, donors had more nephrons than patients with tumor and men had more nephrons than women (Supplemental Table 3). The proportional loss of nephrons with increasing age relative to the youngest age group was similar in donors and patients with tumor. There was a similar pattern of more %GSG in patients with tumor than donors and an increase in %GSG with older age in both donors and patients with tumor (Supplemental Table 4). However, the proportional increase in %GSG with age was less than the proportional loss of nephrons with age in both donors and patients with tumor. Supplemental Figure 5 shows smooth spline fits of nephron loss or increase in glomerulosclerosis with age in men and women. We defined age and sex-specific thresholds for low nephron number on the basis of a combined relatively healthy subset of 1240 donors and 327 patients with tumor (Table 6).
Table 6.
Age-based thresholds used to define low nephron number per kidney based on a combined sample (N=1240 donors and N=327 patients with tumor) after excluding those with diabetes, hypertension, family history of ESKD, 24 hours urine protein >500 mg/d, eGFR <60 ml/min per 1.73 m2 for age <60 years, and eGFR <45 ml/min per 1.73 m2 for age ≥60 years
| Age (yr) | Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | 5th Percentiles | 10th Percentiles | n | 5th Percentiles | 10th Percentiles | |||||
| Threshold | n (%) Abnormal | Threshold | n (%) Abnormal | Threshold | n (%) Abnormal | Threshold | n (%) Abnormal | |||
| 18–39 | 198 | <832,551 | 10 (5.1) | <868,983 | 20 (10.1) | 257 | <597,963 | 13 (5.1) | <718,571 | 26 (10.1) |
| 40–49 | 162 | <685,956 | 9 (5.6) | <765,753 | 17 (10.5) | 276 | <574,069 | 14 (5.1) | <688,871 | 28 (10.1) |
| 50–59 | 162 | <681,376 | 9 (5.6) | <739,822 | 17 (10.5) | 229 | <542,527 | 12 (5.2) | <658,520 | 23 (10.0) |
| 60–69 | 91 | <621,889 | 5 (5.5) | <672,435 | 9 (9.9) | 112 | <483,515 | 6 (5.4) | <539,805 | 12 (10.7) |
| 70+ | 49 | <553,210 | 3 (6.1) | <575,894 | 5 (10.2) | 31 | <430,338 | 2 (6.5) | <483,885 | 4 (12.9) |
Low Nephron Number as a Predictor of CKD
There were 1753 donors who returned to their transplant center for a post-donation follow-up visit a median of 4.4 months after donation, of which 1193 had a mGFR. Low nephron number predicted a mGFR of <60 ml/min per 1.73 m2 (n=387) with and without adjustment for nephrosclerosis, glomerular volume, and clinical characteristics (Table 7). Findings were similar but less statistically significant with mGFR <45 ml/min per 1.73 m2 (n=38) as the outcome. Kidney donors were sent a survey, of whom 673 returned the survey with a follow-up serum creatinine a median 10.5 years after donation. Low nephron number predicted an eGFR of <60 ml/min per 1.73 m2 (n=228) with and without adjustment for nephrosclerosis, glomerular volume, and clinical characteristics (Table 8). Findings were similar with eGFR <45 ml/min per 1.73 m2 (n=32) as the outcome. Among patients with tumor, 1183 were followed for 112 progressive CKD events that occurred after a median (IQR) 3.9 (2.0–7.0) years and for 30 kidney failure events that occurred after a median (IQR) 4.2 (2.2–7.3) years. The median (IQR) number of follow-up serum creatinine values was 14 (9–26) per patient. Low nephron number (threshold) predicted progressive CKD after adjustment for nephrosclerosis, glomerular volume, and clinical characteristics (Table 9 and Figure 3). Findings were similar but less statistically significant with the kidney failure outcome.
Table 7.
Nephron number of retained kidney as a predictor of short-term follow-up measured GFR <60 or <45 ml/minutes per 1.73 m2
| Median Follow-Up of 4.4 mo by Clinical Care (n=1193) | measured GFR <60 (n=387) | measured GFR <45 (n=38) | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Unadjusted | ||||
| Nephron number, per 100,000 | 0.91 (0.89 to 0.94) | <0.0001 | 0.84 (0.76 to 0.93) | 0.0005 |
| Nephron number <5th percentile | 1.54 (1.02 to 2.34) | 0.04 | 0.92 (0.28 to 3.06) | 0.90 |
| Nephron number <10th percentile | 1.51 (1.08 to 2.12) | 0.02 | 1.99 (0.92 to 4.28) | 0.08 |
| % GSG, per SD | 1.31 (1.17 to 1.47) | <0.0001 | 1.59 (1.32 to 1.91) | <0.0001 |
| IFTA, per 5% | 4.11 (1.94 to 8.72) | 0.0002 | 1.18 (0.54 to 2.57) | 0.67 |
| IFTA foci density, per SD | 1.31 (1.17 to 1.46) | <0.0001 | 1.21 (0.99 to 1.49) | 0.07 |
| Adjusted for nephrosclerosis measuresb | ||||
| Nephron number, per 100,000 | 0.92 (0.89 to 0.95) | <0.0001 | 0.87 (0.78 to 0.96) | 0.006 |
| Nephron number <5th percentile | 1.46 (0.95 to 2.24) | 0.08 | 0.70 (0.20 to 2.50) | 0.58 |
| Nephron number <10th percentile | 1.45 (1.03 to 2.05) | 0.03 | 1.62 (0.72 to 3.65) | 0.24 |
| Further adjusted for glomerular volume | ||||
| Nephron number, per 100,000 | 0.89 (0.86 to 0.93) | <0.0001 | 0.83 (0.75 to 0.93) | 0.001 |
| Nephron number <5th percentile | 1.56 (0.99 to 2.44) | 0.05 | 0.72 (0.19 to 2.70) | 0.63 |
| Nephron number <10th percentile | 1.55 (1.07 to 2.23) | 0.02 | 1.75 (0.75 to 4.11) | 0.19 |
| Further adjusted for clinical characteristicsc | ||||
| Nephron number, per 100,000 | 0.95 (0.91 to 0.99) | 0.02 | 0.89 (0.79 to 1.01) | 0.06 |
| Nephron number <5th percentile | 1.65 (0.97 to 2.81) | 0.07 | 0.85 (0.23 to 3.12) | 0.80 |
| Nephron number <10th percentile | 1.51 (0.98 to 2.32) | 0.06 | 2.06 (0.85 to 4.98) | 0.11 |
OR, odds ratio; CI, confidence interval; GSG, globally sclerotic glomerular; IFTA, interstitial fibrosis and tubular atrophy.
%GSG, %IFTA and interstitial fibrosis and tubular atrophy foci density.
Age, follow-up time, sex, and baseline GFR.
Table 8.
Nephron number of retained kidney as a predictor of short-term follow-up measured GFR <60 or <45 ml/minutes per 1.73 m2
| Median Follow-Up of 10.5 yr by Survey (n=673) | estimated GFR <60 (n=228) | estimated GFR <45 (n=32) | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Unadjusted | ||||
| Nephron number, per 100,000 | 0.92 (0.88 to 0.96) | <0.0001 | 0.85 (0.76 to 0.95) | 0.003 |
| Nephron number <5th percentile | 2.49 (1.36 to 4.55) | 0.003 | 5.29 (2.23 to 12.6) | 0.0002 |
| Nephron number <10th percentile | 2.72 (1.69 to 4.36) | <0.0001 | 5.06 (2.37 to 10.8) | <0.0001 |
| %GSG, per SD | 1.35 (1.16 to 1.58) | 0.0001 | 1.31 (1.01 to 1.70) | 0.04 |
| IFTA, per 5% | 3.94 (1.47 to 10.6) | 0.007 | 1.79 (1.03 to 3.09) | 0.04 |
| IFTA foci density, per SD | 1.19 (1.03 to 1.36) | 0.01 | 1.15 (0.90 to 1.47) | 0.27 |
| Adjusted for nephrosclerosis measuresb | ||||
| Nephron number, per 100,000 | 0.92 (0.89 to 0.97) | 0.0004 | 0.86 (0.77 to 0.96) | 0.008 |
| Nephron number <5th percentile | 2.38 (1.28 to 4.42) | 0.006 | 4.90 (1.99 to 12.1) | 0.0006 |
| Nephron number <10th percentile | 2.68 (1.65 to 4.36) | <0.0001 | 4.91 (2.22 to 10.8) | <0.0001 |
| Further adjusted for glomerular volume | ||||
| Nephron number, per 100,000 | 0.91 (0.86 to 0.95) | <0.0001 | 0.88 (0.78 to 0.99) | 0.03 |
| Nephron number <5th percentile | 2.55 (1.35 to 4.85) | 0.004 | 4.07 (1.56 to 10.6) | 0.004 |
| Nephron number <10th percentile | 2.93 (1.77 to 4.87) | <0.0001 | 4.29 (1.86 to 9.86) | 0.0006 |
| Further adjusted for clinical characteristicsc | ||||
| Nephron number, per 100,000 | 0.94 (0.89 to 0.99) | 0.04 | 0.92 (0.81 to 1.04) | 0.19 |
| Nephron number <5th percentile | 2.61 (1.26 to 5.38) | 0.009 | 4.00 (1.44 to 11.1) | 0.008 |
| Nephron number <10th percentile | 3.37 (1.89 to 5.99) | <0.0001 | 4.68 (1.92 to 11.4) | 0.0007 |
OR, odds ratio; CI, confidence interval; GSG, globally sclerotic glomerular; IFTA, interstitial fibrosis and tubular atrophy.
%GSG, %IFTA and interstitial fibrosis and tubular atrophy foci density.
Age, follow-up time, sex, and baseline GFR.
Table 9.
Nephron number of retained kidney as a predictor of progressive CKD among 1183 patients with kidney tumor with no cancer recurrence, death, or dialysis during the first 4 months post-nephrectomy surgery
| Predictor | Progressive CKD | Kidney Failure | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Unadjusted | ||||
| Nephron number, per 100,000 | 0.90 (0.85 to 0.95) | 0.0001 | 0.80 (0.71 to 0.90) | 0.0002 |
| Nephron number <5th percentile | 3.86 (2.52 to 5.90) | <0.0001 | 6.79 (3.27 to 14.12) | <0.0001 |
| Nephron number <10th percentile | 3.15 (2.10 to 4.74) | <0.0001 | 7.12 (3.47 to 14.60) | <0.0001 |
| % GSG, per SD | 1.59 (1.41 to 1.80) | <0.0001 | 1.80 (1.49 to 2.19) | <0.0001 |
| IFTA, per 5% | 1.20 (1.10 to 1.30) | <0.0001 | 1.25 (1.09 to 1.44) | 0.002 |
| IFTA foci density, per SD | 1.56 (1.34 to 1.80) | <0.0001 | 1.68 (1.28–2.21) | 0.0002 |
| Adjusted for nephrosclerosis measuresb | ||||
| Nephron number, per 100,000 | 0.95 (0.90–1.00) | 0.058 | 0.88 (0.77 to 1.00) | 0.048 |
| Nephron number <5th percentile | 2.51 (1.54–4.08) | 0.0002 | 3.83 (1.62 to 9.06) | 0.002 |
| Nephron number <10th percentile | 2.13 (1.34–3.38) | 0.001 | 4.54 (2.01 to 10.22) | 0.0003 |
| Further adjusted for glomerular volume | ||||
| Nephron number, per 100,000 | 0.99 (0.94–1.05) | 0.76 | 0.98 (0.87 to 1.11) | 0.77 |
| Nephron number <5th percentile | 1.73 (1.00–3.02) | 0.05 | 1.71 (0.62 to 4.73) | 0.30 |
| Nephron number <10th percentile | 1.45 (0.86–2.47) | 0.17 | 2.32 (0.90 to 5.96) | 0.08 |
| Further adjusted for clinical characteristicsc | ||||
| Nephron number, per 100,000 | 0.99 (0.92–1.06) | 0.74 | 1.04 (0.92 to 1.19) | 0.52 |
| Nephron number <5th percentile | 2.32 (1.27–4.26) | 0.006 | 1.44 (0.46 to 4.50) | 0.53 |
| Nephron number <10th percentile | 1.90 (1.08–3.34) | 0.026 | 2.12 (0.77 to 5.87) | 0.15 |
Progressive CKD was defined as eGFR reduction by 40% from post-nephrectomy baseline, dialysis, or kidney transplantation. Sample size was 1183 patients with 112 progressive CKD events, and 30 kidney failure events. HR, hazard ratio; 95% CI, 95% confidence interval; GSG, globally sclerosed glomeruli; IFTA, interstitial fibrosis/tubular atrophy.
%GSG, %IFTA and interstitial fibrosis and tubular atrophy foci density.
Age, sex, body mass index, hypertension, diabetes, post-surgery baseline eGFR, and proteinuria (continuous).
Figure 3.
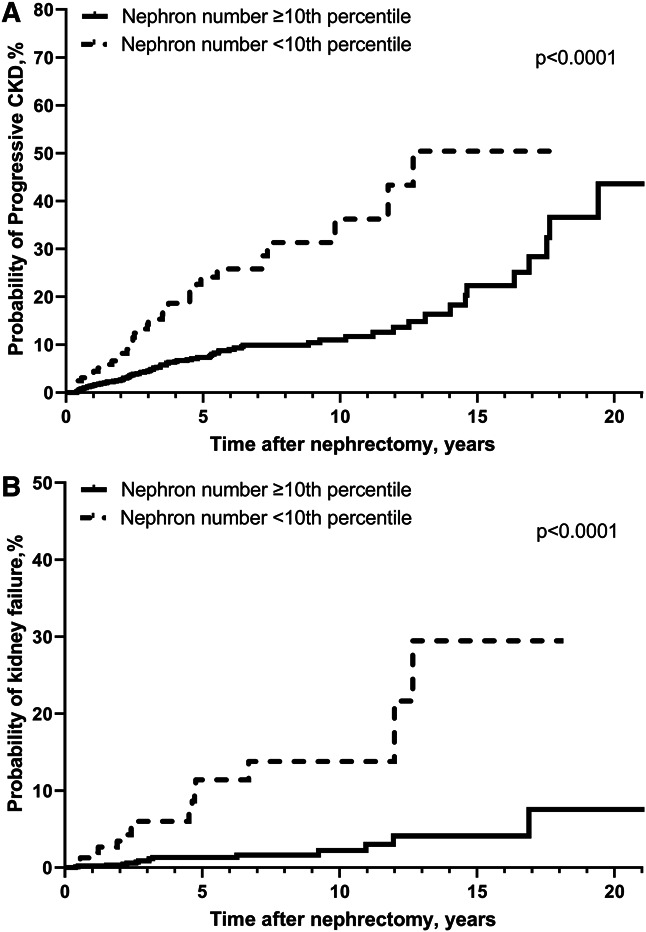
Risk of outcomes with low nephron number among kidney tumor patients. Low nephron number of retained kidney (less than age-sex-specific tenth percentile in healthy subset) predicted progressive CKD (A) and kidney failure (B) (Log-rank P < 0.0001 for both).
Discussion
This study found that a new method of estimating nephron number from cortical volume on imaging and glomerular density on biopsy led to higher nephron number estimates than the old method by 27% in donors and 1% in patients with tumor. This new method corrected for bias in glomerular density estimates from missing glomerular tufts, absence of the kidney capsule on needle core biopsies, counting of partial glomeruli, and thicker kidney axial image sections and used a more accurate FFPE shrinkage factor on the basis of human autopsy kidney sections. Supportive of this new method, there was no detectable difference in nephron number between donors and patients with tumor after accounting for the differences in clinical characteristics that associate with nephron number. This is consistent with prior work showing that there was also not detectable difference in glomerular volume between these two populations after accounting for differences in clinical characteristics.25 Except for eGFR, clinical characteristics did not differ in their strength of association with nephron number between donors and patients with tumor. Nephron loss of 38%–45% is evident in patients aged 80–89 years compared with 18–39 years and is not explained by 16%–20% higher glomerulosclerosis in patients aged 80–89 years. Low nephron number was an independent predictor of both short- and long-term CKD in donors and of both progressive CKD and kidney failure in patients with tumor.
Using 30 large wedge sections from human autopsy kidney specimens, we more precisely estimated kidney tissue shrinkage from FFPE. However, after also correcting for the prior math error, the net correction for tissue shrinkage (FFPE and loss of perfusion) by the new method (1.365×1.366=1.86) compared with the old method (1.43×1.268=1.81) was minimally different. Corrections from counting partial glomeruli bisected by the biopsy needle as 0.675 rather than 0.5 glomeruli and correcting for imaging slice thickness also had only a modest effect on nephron number estimates. The largest effect on nephron number estimates with the new method was inclusion of missing glomerular tufts in the calculation of glomerular density and correction for the higher density of glomeruli with the capsule in needle biopsies that lacked the capsule. These correction factors increased nephron number in donors, but had little effect on the nephron number calculation in nephrectomy patients. The new estimate of a 1.17 million nephrons per kidney in living kidney donors is higher than the 901,000 nephrons per kidney in 420 autopsy patients using the dissector fractionator method.26,27 However, the selection on health that occurs with living kidney donation (such as requiring normal GFR, no proteinuria, no cardiovascular disease, no diabetes, and no moderate-severe hypertension) was not made (or even possible) in these autopsy patients, and CKD risk factors were present.28–30 Our estimate of a mean 990,000 nephrons per kidney in patients with tumor was more consistent with estimates from past autopsy studies.
A somewhat unexpected finding was the frequency of missing glomerular tufts, particularly in donor kidney biopsies with a small parenchymal area. We carefully reviewed the protocols for processing donor and tumor patient tissue biopsies, and there are several potential steps where glomerular tufts may be lost. After a needle core biopsy is obtained, glomerular tufts on the edge may have had their hilum transected such that they are no longer physically attached to the parenchyma. Even an empty glomerular capsule surrounded by tissue on cross-section may represent a lost tuft from shallow sectioning near the surface of the tissue cylinder. Because the smaller donor sections have proportionally more cut edges per tissue area, there is a higher probability of a glomerular tuft on the edge being lost. The FFPE process itself involves washing tissue samples in a series of graded alcohol, xylene, and paraffin, and unattached or weakly attached glomerular tufts may be dislodged. Even after FFPE, sheer stress during the sectioning may dislocate tufts connected to the capsule only by paraffin. Finally, during the automated staining process, the plane of slides is vertical and the slide is slightly vibrated. Reagents dissolve paraffin, which may lead to tufts attached only by paraffin being dislocated. The same amount of reagent and stain washes across the tissue section, regardless of the parenchymal area, and this may lead to more dislocation by the wash effect with smaller tissue samples.
Several clinical characteristics had an independent association with nephron number and were consistent between donors and patients with tumor. We have previously discussed some of these: Shorter height and family history of ESKD likely reflect lower nephron endowment; older age likely reflects subsequent nephron loss due to nephrosclerosis; and GFR likely reflects both nephron endowment and loss.4 The larger sample size of this study with inclusion of patients with tumor allowed for the discovery of additional independent clinical characteristics that associate with nephron number. First, nephron number declines more rapidly with age after 70 years, and this is consistent with higher rates of GFR decline after 70 years.31 Female is also an independent risk factor for lower nephron number possibly because of lower nephron endowment at birth with lower needs for metabolic waste clearance than men.32 Hypertension and proteinuria >165 mg/24 hours were independent risk factors for lower nephron number that likely reflect nephron loss due to nephrosclerosis with compensatory glomerulomegaly and glomerular hyperfiltration.25,33 Diabetes was similar to hypertension in strength of association with low nephron number, but did not achieve statistical significance possibly because of the much lower prevalence than hypertension.
Interestingly, lower eGFR had a stronger strength of association with lower nephron number in patients with tumor than in donors. This is likely because of imprecision and inaccuracy with eGFR in healthy donors. Serum creatinine is proportionally more reflective of muscle mass in kidney donors than in populations inclusive of persons with kidney disease.34–37 Supportive of this hypothesis, lower nephron number had a stronger strength of association with lower mGFR than with lower eGFR in kidney donors. Thus, it is possible that the true association between nephron number and GFR is not different between donors and patients with tumor. When these two populations were combined into one cohort, we did not find a difference in nephron number between donors and patients with tumor after adjusting for these clinical characteristics. This is despite donors presenting with good health and tumor patients with cancer and comorbidity and despite donors having small-needle core tissue biopsies and patients with tumor having 20-fold larger wedge tissue biopsies.
Lower nephron number is clinically relevant because it is a predictor of adverse kidney outcomes. Lower nephron number of the retained kidney associated with mGFR <60ml/min per 1.73 m2 at 4 months after kidney donation and with eGFR <60 or <45 ml/min per 1.73 m2 at 10.5 years after kidney donation. We had previously showed similar findings in donors using our old method for nephron number.6,7 We further extend these findings by showing that lower nephron number also predicts progressive CKD and kidney failure in patients with tumor. Patients with lower nephron number have less reserves to increase GFR in the retained kidney after nephrectomy. Maladaptive, enlarged, and hyperfiltering nephrons attempt to maintain GFR, but nephron overload followed by nephron loss with GFR decline ensues.38 Some of this risk with lower nephron number may be captured by detection of nephrosclerosis and larger glomeruli on biopsy and by clinical characteristics, including CKD risk factors, eGFR, and proteinuria. However, increased risk persisted despite adjusting for these biopsy and clinical factors such that lower nephron number is an important pathway for CKD not captured by current biopsy and clinical evaluations. Nonetheless, the cost and risks associated with both kidney biopsy and contrast-enhanced kidney imaging, the tedious segmentation of glomeruli and the kidney cortex, and the imprecision of the nephron number estimate limit the clinical utility of this method in determining nephron number. However, it may still be useful in clinical research to understand how nephron number and single nephron GFR (GFR divided by nephron number)39 varies across states of health and disease or with different medications.
There are also limitations with this method. The Weibel and Gomez stereology models used to calculate glomerular density approximates the shape of glomeruli to be spheres rather than their true shape.23 While we are able to improve estimation of tissue shrinkage from FFPE with a reasonably large sample of human autopsy kidneys, we still rely on a pig model to estimate tissue shrinkage with loss of perfusion.8 The cross-sectional area of missing glomerular tufts is imputed, and it is possible that some of the empty capsules represent urinary space rather than a dislocated tuft. However, visual inspection of the images and the association of percentage of missing tufts with smaller tissue area is consistent with dislocated tuft loss. Ideally, a new technology would process kidney biopsy tissue samples in a manner that prevents tuft loss. While our approach corrected for the higher glomerular density near the kidney capsule in biopsy tissue samples that lacked the capsule, this is unlikely to correct for all variability in glomerular density related to the depth of needle core biopsies. The precision of nephron number was substantially improved with a large wedge kidney biopsy section rather than the 20-fold smaller needle core kidney biopsy section with uncertain depth and more missing glomerular tufts (repeat-test CV of 15.7% versus 29.6%), but there are limited clinical settings where a large wedge tissue section of the kidney can be obtained in living humans. While these CVs are high for clinical use, identifying individuals with low nephron number may still have some clinical utility; identifying abnormal albuminuria is useful, despite a repeat-test CV of 19% for urine albumin measures.40 There may be unaccounted variation in nephron number estimates because of variability in the tissue sectioning by microtome or more subtle tissue compression artifact. Finally, we corrected cortex volume for images with thicker slices (>2.5 mm), but there may be other sources of variation related to imaging protocols. We estimated rather than measured the cortex volume in persons with poor corticomedullary differentiation on their kidney imaging. Nevertheless, analysis limited to those with directly measured cortex volume did not substantively differ from analysis performed in all participants.
In summary, using two independent cohorts with differing kidney biopsy types, we demonstrated that healthy donors have more nephrons than tumor patients with comorbidities. After accounting for differences in demographics and comorbidities, nephron number was similar between donors and patients with tumor. Low nephron number is prognostically important because it captures CKD risk not captured by thorough assessment of kidney biopsy pathology, kidney function, and CKD risk factors. Technological advances are needed for a less invasive and more precise estimate of nephron number. Such technology has the potential to revolutionize the assessment of kidney health in patient care.
Supplementary Material
Acknowledgments
We thank Miloš Denić for assistance with computer algorithms for processing of biopsy annotations data.
Disclosures
A.D. Rule reports Patents or Royalties: UpToDate; and Advisory or Leadership Role: JASN—Associate Editor, Mayo Clinic Proceedings—Section Editor, and NIDDK—Urological Diseases of America Contract Management Board. Because A.D. Rule is an associate editor of the JASN, he was not involved in the peer-review process for this manuscript. A guest editor oversaw the peer-review and decision-making process for this manuscript. V. Sharma reports Consultancy: Immunity Bio—scientific advisory board; and Ownership Interest: Immunity Bio. M.D. Stegall reports Consultancy: eGenesis, Hansa, and Novartis; and Research Funding: Janssen, Talaris, and Veloxis. All remaining authors have nothing to disclose.
Funding
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Author Contributions
Conceptualization: Andrew D. Rule
Funding Acquisition: Andrew D. Rule
Data curation: Aidan F. Mullan, Anthony C. Luehrs
Investigation Joshua Augustine, Aleksandar Denic, Timothy L. Kline, Andrew D. Rule, Vidit Sharma, R. Houston Thompson, Luke D. Wilson.
Formal analysis: Aleksandar Denic, Aidan F. Mullan, Andrew D. Rule.
Writing – original draft: Aleksandar Denic, Andrew D. Rule.
Writing – review & editing: Mariam P. Alexander, Joshua Augustine, Aleksandar Denic, Anthony C. Luehrs, Aidan F. Mullan, Andrew D. Rule, Vidit Sharma, Mark D. Stegall, R. Houston Thompson, Luke D. Wilson.
Data Sharing Statement
There are some restrictions for these data as follows: Data in these studies are from patient medical records and require IRB approval for use in research. We are open to collaborations from investigators who submit to us a research proposal. Potential overlap, expense, and time are factors we will consider with such proposals.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E388.
Supplemental Table 1. A summary of 30 autopsy patients with a calculated ratio of volumetric shrinkage for wedge sections. Three-dimensional shrinkage factor was obtained by dividing the area of fresh wedge with the area after fixation, and the results is exponentiated to the power of 1.5 (Eq. 4). A mean of all 30 shrinkage factors is obtained at the end.
Supplemental Table 2. Sensitivity analysis: clinical characteristics of nephron number per kidney in living donors using the old versus new method in calculating nephron number.
Supplemental Table 3. Number of nephrons in the retained kidney in kidney donors (excluding those with a family history of ESKD) and patients with tumor with age by sex.
Supplemental Table 4. Percentage change in number of globally sclerotic glomeruli (GSGs) in kidney donors (excluding those with a family history of ESRD) and patients with tumor with age by sex.
Supplemental Figure 1. (A) Example from a needle biopsy with five empty Bowman's capsules. (B) Example of a biopsy with two empty Bowman's capsules and a nearby floating tuft. (C) Example of an open Bowman's capsule and slightly displaced tuft. (D) A rare example of a folded glomerular tuft that cannot be reliably traced. (E) Another rare example of a displaced tuft within an artery lumen.
Supplemental Figure 2. An example of a needle biopsy with three missing glomerular tufts (black arrowheads show three empty Bowman's capsules). Below the figure is the calculation of the uncorrected non-sclerotic glomerular (NSG) density and then corrected for the three missing glomeruli.
Supplemental Figure 3. Representative photographs of a fresh (A) and paraffin-embedded wedge section (B) after 30 days in formalin. In both A and B, the 1-cm scale is identical, and % volumetric shrinkage was estimated from the yellow-traced area of a fresh tissue block, and green-traced area of the same block in paraffin 30 days later.
Supplemental Figure 4. An example of a case where the biopsy slide contains 2 separate wedge sections. Cortex areas (green traces), non-sclerotic glomeruli (red traces on the left, blue traces on the right), and globally sclerotic glomeruli (pink traces on both) were manually traced, and data used to assess reproducibility between two sections.
Supplemental Figure 5. In living kidney donors without a family history of ESKD and patients who underwent a radical nephrectomy, nephron number per kidney declines with older age in (A) men and (B) women. Percentage of glomerulosclerosis increases with older age in both populations in (C) men and (D) women.
References
- 1.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–347. doi: 10.1093/ajh/1.4.335 [DOI] [PubMed] [Google Scholar]
- 2.Denic A, Elsherbiny H, Rule AD. In-vivo techniques for determining nephron number. Curr Opin Nephrol Hypertens. 2019;28(6):545–551. doi: 10.1097/MNH.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertram JF Cullen-McEwen LA Egan GF, et al. Why and how we determine nephron number. Pediatr Nephrol. 2014;29(4):575–580. doi: 10.1007/s00467-013-2600-y [DOI] [PubMed] [Google Scholar]
- 4.Denic A Lieske JC Chakkera HA, et al. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. 2017;28(1):313–320. doi: 10.1681/ASN.2016020154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulladosa X, Moreso F, Narvaez JA, Grinyo JM, Seron D. Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol. 2003;14(10):2662–2668. doi: 10.1097/01.ASN.0000088025.33462.b0 [DOI] [PubMed] [Google Scholar]
- 6.Issa N Vaughan LE Denic A, et al. Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant. 2019;19(7):1989–1998. doi: 10.1111/ajt.15259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merzkani MA Denic A Narasimhan R, et al. Kidney microstructural features at the time of donation predict long-term risk of chronic kidney disease in living kidney donors. Mayo Clinic Proc. 2021;96(1):40–51. doi: 10.1016/j.mayocp.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerman LO, Bentley MD, Bell MR, Rumberger JA, Romero JC. Quantitation of the in vivo kidney volume with cine computed tomography. Invest Radiol. 1990;25(11):1206–1211. doi: 10.1097/00004424-199011000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Miller PL, Meyer TW. Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990;63(6):862–866. https://europepmc.org/article/med/2255192 [PubMed] [Google Scholar]
- 10.Hoang K Tan JC Derby G, et al. Determinants of glomerular hypofiltration in aging humans. Kidney Int. 2003;64(4):1417–1424. doi: 10.1046/j.1523-1755.2003.00207.x [DOI] [PubMed] [Google Scholar]
- 11.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC. The association of predonation hypertension with glomerular function and number in older living kidney donors. J Am Soc Nephrol. 2015;26(6):1261–1267. doi: 10.1681/ASN.2014030304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seibel J, Rebibou JM, Legendre M. Can total nephron number predict progressive CKD after radical nephrectomy? J Am Soc Nephrol. 2020;32(2):517. doi: 10.1681/ASN.2020111585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denic A Elsherbiny H Mullan AF, et al. Larger nephron size and nephrosclerosis predict progressive CKD and mortality after radical nephrectomy for tumor and independent of kidney function. J Am Soc Nephrol. 2020;31(11):2642–2652. doi: 10.1681/ASN.2020040449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricaurte Archila L Denic A Mullan AF, et al. A higher foci density of interstitial fibrosis and tubular atrophy predicts progressive CKD after a radical nephrectomy for tumor. J Am Soc Nephrol. 2021;32(10):2623–2633. doi: 10.1681/ASN.2021020267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denic A Alexander MP Kaushik V, et al. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis. 2016;68(1):58–67. doi: 10.1053/j.ajkd.2015.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poggio ED Rule AD Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75(10):1079–1087. doi: 10.1038/ki.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA Eneanya ND Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson DM, Anderson RL. Protein–osmolality ratio for the quantitative assessment of proteinuria from a random urinalysis sample. Am J Clin Pathol. 1993;100(4):419–424. doi: 10.1093/ajcp/100.4.419 [DOI] [PubMed] [Google Scholar]
- 19.Marco Mayayo MP, Martinez Alonso M, Valdivielso Revilla JM, Fernandez-Giraldez E. A new gender-specific formula to estimate 24-hour urine protein from protein to creatinine ratio. Nephron. 2016;133(4):232–238. doi: 10.1159/000447604 [DOI] [PubMed] [Google Scholar]
- 20.Melchinger H Calderon-Gutierrez F Obeid W, et al. Urine uromodulin as a biomarker of kidney tubulointerstitial fibrosis. Clin J Am Soc Nephrol. 2022;17(9):1284–1292. doi: 10.2215/CJN.04360422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 22.Hoy WE, Samuel T, Hughson MD, Nicol JL, Bertram JF. How many glomerular profiles must be measured to obtain reliable estimates of mean glomerular areas in human renal biopsies? J Am Soc Nephrol. 2006;17(2):556–563. doi: 10.1681/ASN.2005070772 [DOI] [PubMed] [Google Scholar]
- 23.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol. 1962;17(2):343–348. doi: 10.1152/jappl.1962.17.2.343 [DOI] [PubMed] [Google Scholar]
- 24.Wang X Vrtiska TJ Avula RT, et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85(3):677–685. doi: 10.1038/ki.2013.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denic A Mathew J Nagineni VV, et al. Clinical and pathology findings associate consistently with larger glomerular volume. J Am Soc Nephrol. 2018;29(7):1960–1969. doi: 10.1681/ASN.2017121305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertram JF, Hughson MD, Puelles VG, Hoy WE. Chapter 14 - variation in human nephron number and association with disease. In: Little MH, editor. Kidney Development, Disease, Repair and Regeneration. Academic Press; 2016:167–175. [Google Scholar]
- 27.Hoy WE, Ingelfinger JR, Hallan S, Hughson MD, Mott SA, Bertram JF. The early development of the kidney and implications for future health. J Dev Orig Health Dis. 2010;1(4):216–233. doi: 10.1017/S204017441000022X [DOI] [PubMed] [Google Scholar]
- 28.Hughson MD, Gobe GC, Hoy WE, Manning RD, Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52(1):18–28. doi: 10.1053/j.ajkd.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 29.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70(1):104–110. doi: 10.1038/sj.ki.5000397 [DOI] [PubMed] [Google Scholar]
- 30.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69(4):671–678. doi: 10.1038/sj.ki.5000041 [DOI] [PubMed] [Google Scholar]
- 31.Schaeffner ES Ebert N Kuhlmann MK, et al. Age and the course of GFR in persons aged 70 and above. Clin J Am Soc Nephrol. 2022;17(8):1119–1128. doi: 10.2215/CJN.16631221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ, III. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75(10):1071–1078. doi: 10.1038/ki.2008.698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgin JB Bitzer M Wickman L, et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol. 2015;26(12):3162–3178. doi: 10.1681/ASN.2014080752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rule AD Gussak HM Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43(1): 112–119. doi: 10.1053/j.ajkd.2003.09.026 [DOI] [PubMed] [Google Scholar]
- 35.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. doi: 10.7326/0003-4819-141-12-200412210-00009 [DOI] [PubMed] [Google Scholar]
- 36.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol. 2011;6(8):1963–1972. doi: 10.2215/CJN.02300311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rule AD, Glassock RJ. GFR estimating equations: getting closer to the truth? Clin J Am Soc Nephrol. 2013;8(8):1414–1420. doi: 10.2215/CJN.01240213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luyckx VA Rule AD Tuttle KR, et al. Nephron overload as a therapeutic target to maximize kidney lifespan. Nat Rev Nephrol. 2022;18(3):171–183. doi: 10.1038/s41581-021-00510-7 [DOI] [PubMed] [Google Scholar]
- 39.Denic A Mathew J Lerman LO, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376(24):2349–2357. doi: 10.1056/nejmoa1614329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20(2):436–443. doi: 10.1681/ASN.2008030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are some restrictions for these data as follows: Data in these studies are from patient medical records and require IRB approval for use in research. We are open to collaborations from investigators who submit to us a research proposal. Potential overlap, expense, and time are factors we will consider with such proposals.