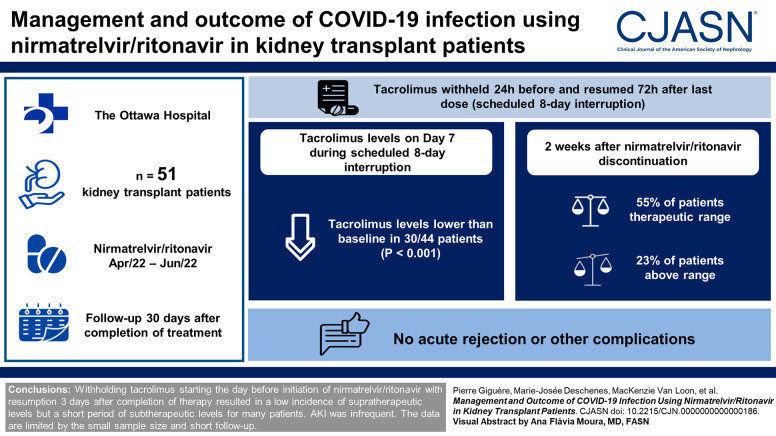
Visual Abstract
Keywords: nirmatrelvir/ritonavir, kidney transplantation, tacrolimus, cyclosporine, COVID-19
Abstract
Background
Nirmatrelvir/ritonavir has been shown to reduce the risk of coronavirus disease 2019 (COVID-19)–related complications in patients at high risk for severe COVID-19. However, clinical experience of nirmatrelvir/ritonavir in the transplant recipient population is scattered due to the complex management of drug–drug interactions with calcineurin inhibitors. We describe the clinical experience with nirmatrelvir/ritonavir at The Ottawa Hospital kidney transplant program.
Methods
Patients who received nirmatrelvir/ritonavir between April and June 2022 were included and followed up to 30 days after completion of treatment. Tacrolimus was withheld for 24 hours and resumed 72 hours after the last dose of nirmatrelvir/ritonavir (on day 8) on the basis of the drug level the day before. The first 30 patients had their dose adjusted according to drug levels performed twice in the first week and as needed thereafter. Subsequently, a simplified algorithm with less frequent calcineurin inhibitor–level monitoring was implemented. Outcomes, including tacrolimus-level changes, serum creatinine and AKI (defined as serum creatinine increase by 30%), and clinical outcomes were described globally and compared between algorithms.
Results
Fifty-one patients received nirmatrelvir/ritonavir. Tacrolimus levels drawn at the first time point, 7 days after withholding of calcineurin inhibitor, and 2 days after discontinuing nirmatrelvir/ritonavir were within the therapeutic target in 17/44 (39%), subtherapeutic in 21/44 (48%), and supratherapeutic in 6/44 (14%). Two weeks after, 55% were within the therapeutic range, 23% were below, and 23% were above it. The standard and simplified algorithms provided similar tacrolimus level (median 5.2 [4.0–6.2] µg/L versus 4.8 [4.3–5.7] µg/L, P = 0.70). There were no acute rejections or other complications.
Conclusions
Withholding tacrolimus starting the day before initiation of nirmatrelvir/ritonavir with resumption 3 days after completion of therapy resulted in a low incidence of supratherapeutic levels but a short period of subtherapeutic levels for many patients. AKI was infrequent. The data are limited by the small sample size and short follow-up.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_07_10_CJN_POD_EP15_071023.mp3
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has had a great burden of morbidity and mortality among immunocompromised individuals. Solid organ transplant recipients, including kidney transplant recipients, have been particularly affected. In 2020, approximately one in six deaths among kidney transplant patients in the United States was due to COVID-19, with an overall higher mortality rate compared with previous years within the transplant population.1 It is estimated that in kidney transplant patients, early antibody formation after two doses of a COVID-19 vaccine occurs in only 35% of recipients and can vary significantly among kidney transplant recipients.2 Recent reports involving the newer Omicron variant suggest that despite a higher incidence of severe acute respiratory syndrome coronavirus 2 infection, hospitalization and mortality were lower compared with previous waves.3,4 Nonetheless, solid organ transplant recipients maintained a greater mortality risk compared with the general population.
Nirmatrelvir/ritonavir, a medication approved for the early treatment of COVID-19, has proven to be efficacious in preventing the development of severe COVID-19.5 Transplant recipients have been significantly affected by COVID-19 but have had inconsistent access to early outpatient treatment with nirmatrelvir/ritonavir because of interactions with post-transplant immunosuppressive therapy, particularly calcineurin inhibitors. There is a lack of specific guidance on dose adjustment to allow for safe concurrent therapy. Ritonavir is a potent cytochrome P450 (CYP3A4) inhibitor, whereas calcineurin inhibitors, such as tacrolimus and cyclosporine, are substrates of CYP3A4. A few case reports have reported inadvertent coadministration of nirmatrelvir/ritonavir with a calcineurin inhibitor, resulting in calcineurin inhibitor levels ten-fold or higher and AKI along with other serious adverse effects.6,7 The product monograph simply recommends that while nirmatrelvir/ritonavir therapy is being taken, increased monitoring of the immunosuppressive agent's concentration should occur.5 The American Society of Transplantation (AST) did publish a statement with advice to hold tacrolimus and reduce cyclosporine to 20% for the duration of nirmatrelvir/ritonavir therapy, but there is no guidance for when and how to reinstate calcineurin inhibitors.8 In addition, the AST also suggests using either monoclonal antibodies or remdesivir as the initial options before considering nirmatrelvir/ritonavir. Owing to this concern, health care professionals have remained hesitant to recommend and use nirmatrelvir/ritonavir in transplant patients.
Guidance for calcineurin inhibitors with nirmatrelvir/ritonavir is focused on the 5-day duration of therapy.9 However, ritonavir CYP3A4 inhibition is irreversible and, therefore, persists for some days after discontinuation.10 Hence, early initiation of calcineurin inhibitor at baseline dose may lead to supratherapeutic levels and toxicity. Late initiation or lower doses may lead to subtherapeutic levels and acute rejection. With the Omicron wave, the only effective monoclonal antibody therapeutic is bebtelovimab, which was not approved nor available in Canada. The other option was the intravenous formulation of remdesivir, which requires three daily doses of intravenous administration, which was logistically challenging.11 Hence, we preferentially and deliberately used nirmatrelvir/ritonavir in our program for the past few months. The purpose of this study was to report our experience of nirmatrelvir/ritonavir use to help other transplant professionals and patients in this situation.
Methods
Setting
A retrospective chart review of kidney transplant patients at The Ottawa Hospital who received nirmatrelvir/ritonavir was conducted. We included eligible kidney transplant recipients who developed COVID-19 and were treated with nirmatrelvir/ritonavir from March 31, 2022, to June 9, 2022, with 30-day follow-up data available.
Population
Patients were considered eligible for COVID-19 therapeutics at our center on the basis of the Science Table Ontario guidance.12 Patients are considered for COVID-19 therapeutics, regardless of vaccination status, if they have a higher risk of adverse outcomes due to COVID-19 infection. Risk factors include immunosuppression, comorbid conditions, and age. As kidney transplant recipients are on lifetime immunosuppression, they should be considered for COVID-19 therapeutics. During this period, the monoclonal antibodies available were not considered adequate for the circulating variants (i.e., sotrovimab for the Omicron variant, and bebtelovimab and molnupiravir were not available in Canada) and the guidance suggested the preferred use of nirmatrelvir/ritonavir.13 Nirmatrelvir/ritonavir was started within 5 days of symptom onset or positive test result, whichever came first.
Procedures
The kidney transplant program developed and followed a protocol for early identification of patients eligible to receive nirmatrelvir/ritonavir, review and modification of administered medications, and immunosuppressive medication management. All 1145 transplant recipients were sent communications advising them to contact the transplant clinic in case of COVID-19 symptoms or positive tests. Approximately 80% of the patients received the communications using a secure patient portal. Patients not registered on the portal received letters through email. Patients may also have accessed this information through updates that were posted on the program's website.
On confirmation of a positive COVID-19 test, a pharmacist reviewed the patient's medication list to assess any relevant drug interactions. Certain drugs were considered contraindication for nirmatrelvir/ritonavir use (e.g., amiodarone and phenytoin), whereas others required dose adjustment. Any decisions for adjustment of nonimmunosuppressive agents were performed in consultation with the transplant nephrologist and the transplant pharmacist.
For the standard dose adjustment strategy for patients on tacrolimus, nirmatrelvir/ritonavir was started 24 hours after the last dose of tacrolimus. The tacrolimus was held during the 5-day course of nirmatrelvir/ritonavir, and for 2 days following, after which the tacrolimus level was drawn (day 7). Tacrolimus was then restarted according to this level the next morning (day 8). For patients on cyclosporine, the dose was reduced to 20% of the baseline dose when nirmatrelvir/ritonavir was started. The cyclosporine level was then drawn approximately 2 days after the end of nirmatrelvir/ritonavir treatment (day 7), and the dose was adjusted accordingly. Subsequent levels were drawn, typically every 3–4 days, until target was met. Mycophenolate was held for all patients, and the steroid dose was increased for 1 week (or more in the case of persistent severe COVID-19–related symptoms) as per standard local practice. The outpatient laboratory was notified when a patient was expected, and they were segregated in a designated area from other patients. This procedure required numerous laboratory visits during the isolation period, which was challenging for some patients and resource intensive for the clinic.
On May 2022, a new simplified algorithm was implemented to limit the frequency of laboratory visits. The first post-therapy level was drawn at day 2 poststopping nirmatrelvir/ritonavir (same as standard protocol), and subsequent levels were drawn a week later, and at weekly intervals thereafter, if needed. The dosing after resumption of calcineurin inhibitor was also different from the standard algorithm, per Table 1.
Table 1.
Simplified protocol for resuming tacrolimus and cyclosporine dosing after nirmatrelvir/ritonavir treatment
| Medication | Medication Adjustment According to Protocol |
|---|---|
| Tacrolimus trough concentrations targeta | |
| Subtherapeutic (<4 μg/L) | Resumed at 75% of baseline dose for 3 d, then 100% baseline dose resumed thereafter |
| Therapeutic (4–6 μg/L) | Resumed at 50% of baseline dose for 3 d, then 100% baseline dose resumed thereafter |
| Supratherapeutic (>6 μg/L) | Resumed at 33% of baseline dose for 3 d, then 100% baseline dose resumed thereafter |
| Cyclosporine | |
| Subtherapeutic/therapeuticb | Resumed at 100% of baseline dose |
| Supratherapeuticc | Continued at 20% of baseline dose for 2 d, then 100% of baseline dose thereafter |
Post-transplant >6 months.
Subtherapeutic cyclosporine levels are defined as trough levels (C0) below 50 µmol/L and peak levels (C2) below 400 µmol/L. Therapeutic levels are trough levels between 50 and 150 µmol/L and peak levels between 400 and 600 µmol/L.
Supratherapeutic levels are defined as trough levels above 150 µmol/L and peak levels above 600 µmol/L.
Data Collection and Analysis
A list of kidney transplant patients who developed COVID-19 and were prescribed nirmatrelvir/ritonavir had been maintained. These patients were tracked throughout the treatment period and followed up to 30 days post-treatment. Descriptive statistics are used to describe patient characteristics and outcomes. Outcomes included drug levels for sirolimus and calcineurin inhibitors, kidney function, and hospitalization or mortality. Kidney function was measured at every follow-up visit. Kidney dysfunction was defined as any rise of serum creatinine by 30% above baseline during the follow-up period. A manual chart review was performed to ascertain cause and attribute reason for kidney dysfunction (e.g., acute rejection, calcineurin inhibitor toxicity, natural progression, and COVID-19). Data were abstracted from the hospital electronic medical record (EPIC systems, Verona, WI). Research ethics approval was obtained from the Ottawa Health Sciences Research Ethics Board (20220402-01H). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Results
Fifty-one patients were included in the analysis (Table 2) and followed up for 30 days. All patients except one (combined liver/kidney transplant) were using extended-release tacrolimus (Advagraf, Astellas Pharma Inc.) as part of the immunosuppressive treatment. Twenty-one patients were followed in the period after implementation of the simplified protocol for calcineurin inhibitor reinitiation. The main interacting comedications were calcium channel blockers and statins (Table 2).
Table 2.
Baseline characteristics
| Parameter | Standard Algorithm n=30 | Simplified Algorithm n=21 | Total N=51 |
|---|---|---|---|
| Age, yr, mean (SD) | 53 (16) | 53 (16) | 53 (16) |
| Male sex, N (%) | 20 (67) | 12 (57) | 32 (63) |
| First kidney transplant, N (%) | 24 (80) | 16 (76) | 40 (78) |
| Living donor, N (%) | 15 (50) | 12 (57) | 27 (52.9) |
| Time since transplant, mo, median (IQR) | 95 (45–172) | 41 (19–99) | 77 (28–155) |
| Induction agent, N (%) | |||
| Antithymocyte globulin | 10 (33) | 3 (14) | 13 (26) |
| Antithymocyte globulin+basiliximab | 4 (13) | 5 (24) | 9 (18) |
| Basiliximab | 8 (27) | 12 (57) | 20 (39) |
| Unknown | 8 (27) | 1 (5) | 9 (18) |
| Baseline serum creatinine, mg/dl, mean (SD) | 1.53 (0.69) | 1.55 (0.77) | 1.54 (0.75) |
| Immunosuppressants, N (%) a | |||
| Tacrolimus | 24 (80) | 20 (95) | 44 (86) |
| Cyclosporine | 5 (17) | 1 (5) | 6 (12) |
| Sirolimus | 1 (3) | 1 (5) | 2 (4) |
| Mycophenolate | 22 (73) | 18 (85) | 40 (78) |
| Azathioprine | 2 (7) | 2 (10) | 4 (8) |
| COVID-19 vaccination status, N (%) | |||
| One dose | 0 | 1 (5) | 1 (2) |
| Two doses | 4 (13) | 0 | 4 (8) |
| Three doses | 12 (40) | 6 (3) | 18 (35) |
| Four doses | 14 (47) | 14 (67) | 28 (55) |
| Days between symptom onset and nirmatrelvir/ritonavir start, median (IQR) | 4 (2–4) | 2 (1–4) | 3 (2–4) |
| Interacting medications, N (%) | |||
| Calcium channel blockers | |||
| Amlodipine | 16 (53) | 11 (52) | 27 (53) |
| Diltiazem | 0 | 1 (5) | 1 (2) |
| Nifedipine | 0 | 3 (15) | 3 (6) |
| Statins | |||
| Rosuvastatin | 3 (10) | 1 (5) | 4 (8) |
| Atorvastatin | 18 (58) | 13 (62) | 31 (61) |
| Other | |||
| Tamsulosin | 2 (7) | 1 (5) | 3 (6) |
| Zopiclone | 1 (3) | 1 (5) | 2 (4) |
| Apixaban | 1 (3) | 1 (5) | 2 (4) |
| Colchicine | 1 (3) | 2 (10) | 3 (6) |
| No. of interacting comedications, median (range) | 2 (1–5) | 2 (1–4) | 2 (1–5) |
IQR, interquartile range; COVID-19, coronavirus disease 2019.
One patient was on both tacrolimus and sirolimus.
Tacrolimus
The results of tacrolimus management are summarized (Tables 3 and 4, Supplemental Figure 1). At the first measurement postdiscontinuation of nirmatrelvir/ritonavir (median 2 days after therapy cessation), calcineurin inhibitor levels were within therapeutic range in 17/44 (39%), supratherapeutic in 16/44 (14%), and subtherapeutic in 21/44 (48%). When compared with the last calcineurin inhibitor level used as a baseline value, the first level was lower in 30/44 patients (median difference [interquartile range (IQR)], −1.1 µg/L [−2.0 to 0.2], P < 0.001 Wilcoxon signed rank test). For the first 20 patients, before the simplified protocol was implemented, the second follow-up measurement (median 6.5 days after stopping nirmatrelvir/ritonavir) calcineurin inhibitor levels were therapeutic in 8/24 (33%), subtherapeutic in 33%, and supratherapeutic in 33%. On the third monitoring calcineurin inhibitor level, the median plasma concentration was 5.2 µg/L, and 50% (10/20) achieved a level within therapeutic range.
Table 3.
Tacrolimus serum concentration before and after implementation of a standardized protocol
| Standard Algorithm | Simplified Algorithm | P Valuea | ||||
|---|---|---|---|---|---|---|
| Days after Stopping Nirmatrelvir/Ritonavirb | Tacrolimus Concentrationb,c | Days after Stopping Nirmatrelvir/Ritonavir | Tacrolimus Concentrationb,c | |||
| Baseline | 5.1 (4.8–5.7) | Baseline | 5.0 (4.5–5.6) | 0.33 | ||
| Level 1 | 2 (2–2) | 4.0 (3.2–5.1) | Level 1 | 2 (2–3) | 3.7 (3.2–4.9) | 0.70 |
| Level 2 | 7 (6–8) | 4.8 (2.8–7.3) | ||||
| Level 3 | 13 (11–16) | 5.2 (4.0–6.2) | Level 2 | 11 (10–13) | 4.8 (4.3–5.7) | 0.70 |
IQR, interquartile range.
Mann–Whitney U test.
Days after nirmatrelvir and plasma concentrations are median (IQR).
Tacrolimus median (IQR) plasma concentration in ng/L.
Table 4.
Therapeutic level of tacrolimus before and after implementation of a standardized protocol
| Algorithm | Levels | Days after Stopping Nirmatrelvir/Ritonavir | Therapeutic n (%) |
Subtherapeutic n (%) |
Supratherapeutic n (%) |
|---|---|---|---|---|---|
| Standard algorithm | Baseline | 18/24 (75) | 3/24 (13) | 3/24 (13) | |
| 1 | 2 (2–2) | 10/24 (42) | 10/24 (42) | 4/24 (17) | |
| 2 | 7 (6–8) | 8/24 (33) | 8/24 (33) | 8/24 (33) | |
| 3 | 13 (11–16) | 10/20 (50) | 5/20 (25) | 5/20 (25) | |
| Simplified algorithm | Baseline | 13/20 (65) | 3/20 (15) | 4/20 (20) | |
| 1 | 2 (2–3) | 7/20 (35) | 11/20 (55) | 2/20 (10) | |
| 2 | 11 (10–13) | 12/20 (60) | 4/20 (20) | 4/20 (20) |
Therapeutic range for tacrolimus is between 4 and 6 ng/L.
In the second period of the analysis, calcineurin inhibitor monitoring was changed to minimize therapeutic drug monitoring and standardize dose resumption on the first follow-up level. The second level was drawn on average 11 days after nirmatrelvir/ritonavir discontinuation and 9 days after calcineurin inhibitor restarted. This simplified strategy resulted in a similar proportion of patients within therapeutic range.
The median time (range) to return to stable dosing with the standard algorithm versus the simplified strategy was 13.5 (10–30) and 12.5 (6–30) days. The median number (range) of tacrolimus levels was 4 (2–8) and 3 (2–6), respectively (P < 0.002, Mann–Whitney test).
Eight patients had their first follow-up tacrolimus level later than 2 days after completion of nirmatrelvir/ritonavir. All had levels lower than their baseline value (median −2.0 µg/L), and six of these were subtherapeutic. All patients with subtherapeutic levels did eventually reach therapeutic levels during follow-up with no clinical consequences at up to 30 days of follow-up.
Two patients failed to follow instructions and experienced toxic (>10 µg/L) tacrolimus level on their first follow-up levels. One patient took tacrolimus simultaneously with nirmatrelvir/ritonavir on the first day of treatment. The tacrolimus level was 28.9 µg/L 1 day after the end of nirmatrelvir/ritonavir. The other patient restarted tacrolimus while on day 4 of nirmatrelvir/ritonavir. The tacrolimus level was 75.9 µg/L on day 3 postcompletion of nirmatrelvir/ritonavir. In both cases, the tacrolimus level eventually normalized, and no kidney dysfunction or clinical toxicity from tacrolimus was reported.
Cyclosporine
Six patients studied were taking cyclosporine. Unlike sirolimus and tacrolimus, the cyclosporine doses were not held but were reduced to approximately 20% of the original dose. Four of the six patients were observed before the initiation of the simplified protocol. Of the four patients observed before the initiation of the protocol, three of the patients were able to restart their original baseline dose within three blood levels. One of the four patients before protocol had supratherapeutic levels on finishing nirmatrelvir/ritonavir. This patient's dose was further reduced; however, their level returned to the therapeutic level within the next week. Their normal dose was resumed after the fifth blood level, which occurred 24 days postnirmatrelvir/ritonavir. Two of the six patients were observed postprotocol. Both patients were returned to their original baseline doses after the first initial drug level and maintained on it as per protocol.
Sirolimus
Two patients studied were on sirolimus. One of the two patients was also on tacrolimus. Similar to the tacrolimus group, sirolimus was held for the duration of the nirmatrelvir/ritonavir therapy. In one patient, sirolimus was resumed at 50% of the dose for 7 days (administered every other day) and increased at full dose thereafter. The other patient was unfortunately lost to follow-up after the level was taken. For both patients, the sirolimus level ranged between 2.6 and 5.9 µg/L at all times.
Kidney Function
The median (IQR) change in serum creatinine for patients on tacrolimus was −0.02 (−0.13 to 0.06), −0.02 (−0.09 to 0.07), and −0.04 (−0.10 to 0.06) mg/dl for the first three levels, respectively. Increasing creatinine after nirmatrelvir/ritonavir treatment occurred in 29/44 (66%) patients. For this group, the median (IQR) peak serum creatinine was 0.14 (0.06–0.20) occurring 9 days after discontinuation of nirmatrelvir/ritonavir (Supplemental Figure 2 and Supplemental Table 1). Four patients developed kidney dysfunction (defined as 30% higher creatinine at any time point above baseline, details in Supplemental Appendix). One patient had an obstructing kidney stone, and two had no identifiable explanation for AKI, of which one returned by 30 days and the other did not. The final patient had poor oral intake, requiring temporary discontinuation of diuretics. Kidney function returned to baseline in another 10 days. This may have been related to loss of appetite and dysgeusia from nirmatrelvir. None of the patients had acute rejection or clinical calcineurin inhibitor toxicity.
Hospitalization and Mortality
During the treatment period and the 30-day follow-up, no patients were hospitalized, and none of them died. There were also no reported clinical complications resulting from the other medications (chiefly calcium channel blockers and statins) that were held for the duration of the treatment.
Discussion
In this study, we described the results of a modified protocol that included an 8-day hold of calcineurin inhibitor the day before nirmatrelvir/ritonavir start. We observed a low incidence of tacrolimus toxicity and a good safety profile as demonstrated by the low incidence of AKI. There were no episodes of acute rejection during follow-up.
Management of COVID-19 infections in transplant patients is complex, in part due to polypharmacy and the risk for severe drug–drug interactions. There have already been several case reports and case series of calcineurin inhibitor toxicity after the use of nirmatrelvir/ritonavir in the transplant population, when the calcineurin inhibitor was continued inappropriately, or when there was a 1–2-day overlap between calcineurin inhibitor and nirmatrelvir/ritonavir.6,14–16 These concerns have led to recommendations from the National Institute of Health as well as the AST statement that suggest the use of remdesivir or monoclonal antibodies as first-line agents in the transplant populations.8,17 However, remdesivir must be given intravenously for 3 days, which presents logistical problems and may not be available at all centers. As mentioned before, the efficacy of most monoclonal antibodies with the currently circulating variants was poor. Nonetheless, there are reports of successful use of nirmatrelvir/ritonavir in this setting if performed carefully.18,19 However, these reports lack in providing further instructions for monitoring and providing dosing recommendations for calcineurin inhibitors after COVID-19 treatment is completed.
An algorithm was recently proposed based on a simulation model, which recommends administering a one-time tacrolimus dose equivalent to 1/8th of the total initial daily dose on day 1 of nirmatrelvir/ritonavir therapy and then holding tacrolimus during the remaining 4 days of treatment. The tacrolimus dose is then resumed at 50% of the initial dose starting at the end of day 1 post–COVID-19 treatment, 75% of the initial dose the following day and full dose the next day.20 Here, we report our empiric experience, which includes a simple standardized dosing titration of tacrolimus and cyclosporine adapted to the first follow-up serum levels. First, we were interested in finding the optimal timing for the tacrolimus level after completion of nirmatrelvir/ritonavir. Recovery of CYP3A4 activity after discontinuation of ritonavir is gradual, and it may take up to 4 days before 90% of the inhibition disappears.21 As tacrolimus is a sensitive substrate of CYP3A4 and p-glycoprotein, resuming usual dose of tacrolimus too early may result in toxic concentration and potentially calcineurin inhibitor toxicity. Our results confirm that levels drawn 2 days after ritonavir is stopped are approximately comparable with levels before initiating nirmatrelvir/ritonavir treatment. Levels drawn later than 2 days postnirmatrelvir/ritonavir treatment showed a decline in tacrolimus concentrations to being slightly lower than baseline values. Our proposed algorithm confirms that a gradual increase of the tacrolimus dose back to pretreatment dose is feasible and safe, without the need for repeated tacrolimus level monitoring during the first week post-treatment. We also noted that nearly half of the patients had suboptimal levels during the first week. These levels were close to the lower limit of the target range, and quickly corrected. This decline was temporary and did not translate into graft rejection. The simulation model suggestion of a one-time 1/8th dose of tacrolimus might obviate this but might come at a cost of supratherapeutic concentration in a proportion of patients.
It is important to highlight the importance of adequate follow-up with patients to ensure proper understanding of the procedures. Multiple instructions were communicated to patients who are nursing intensive and may not be available for all centers. Despite this, two patients inadvertently took tacrolimus during nirmatrelvir/ritonavir treatment. Fortunately, despite the elevated tacrolimus levels in these cases, no graft dysfunction occurred. Fewer patients were on cyclosporine, which prevents extrapolation or validation of our current protocol.
Nirmatrelvir/ritonavir was associated with low rates of hospital admissions and mortality in this study. No patients were admitted to the hospital, and none died. All patients were able to complete COVID-19 treatment as prescribed. Although our patient outcomes were good, the safe prescription of this medication and monitoring of patients came at a cost of a significant increase in the workload of pharmacists, nurses, and physicians in our program.
Standard limitations to observational studies apply to this cohort analysis. The absence of a control group limits the interpretation about the efficacy. Patients had to have access to COVID-19 testing and to be knowledgeable enough to contact the transplant program to be able to be prescribed nirmatrelvir/ritonavir. Patients who might have been hospitalized before being able to start nirmatrelvir/ritonavir would be underrepresented in this study. We do not have detailed levels of calcineurin inhibitor levels during the course of COVID-19 and nirmatrelvir/ritonavir therapy, preventing granular knowledge of calcineurin inhibitor levels during the 8-day discontinuation period. Our patient population did not include patients with recent transplantation and were almost exclusively receiving extended-release tacrolimus formulation. Extrapolation of these results outside this context should be performed carefully. These results are also short term in nature, and longer-term consequences of potential subtherapeutic levels will need to be studied. Nevertheless, the use of nirmatrelvir/ritonavir with the described protocol was quite safe, and there were no episodes of either clinical calcineurin inhibitor toxicity or any acute rejection during the 30-day follow-up.
In this study, withholding tacrolimus for an 8-day period starting the day before and continuing 3 days after was associated with a low frequency of supratherapeutic levels and AKI but with a short period of subtherapeutic levels of unclear significance. Thus, nirmatrelvir/ritonavir may be used in transplant recipients receiving calcineurin inhibitor after taking appropriate steps to hold calcineurin inhibitors and monitor levels; however, we still need higher-quality comparative effectiveness data, as well as long-term safety data of the approach described here.
Supplementary Material
Disclosures
T. Fairhead reports honoraria from Alexion and Astellas; advisory or leadership roles for Alexion, Astellas, and AstraZeneca; and speakers bureau for Alexion. P. Giguère reports consultancy for Gilead Sciences and Merck. S. Hiremath reports research salary support from the Department of Medicine, University of Ottawa; serving on the Editorial Boards of American Journal of Hypertension, American Journal of Kidney Disease, and Canadian Journal of Cardiology; and serving on the Board of Directors of NephJC (not-for-profit educational entity; unpaid volunteer position). G. Knoll reports serving on the Editorial Board of Canadian Journal of Kidney Health and Disease. M. McGuinty reports research funding from VBI. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Marie-Josée Deschenes, Todd Fairhead, Pierre Giguère.
Data curation: Swapnil Hiremath, MacKenzie Van Loon.
Formal analysis: Marie-Josée Deschenes, Pierre Giguère, MacKenzie Van Loon.
Methodology: Marie-Josée Deschenes, Todd Fairhead, Pierre Giguère, Swapnil Hiremath, Greg Knoll, Michaeline McGuinty, Rinu Pazhekattu, MacKenzie Van Loon.
Project administration: Marie-Josée Deschenes, Todd Fairhead, Pierre Giguère, Swapnil Hiremath, Jolanta Karpinski, Namrata Parikh.
Supervision: Swapnil Hiremath, Stephanie Hoar, Jolanta Karpinski, Greg Knoll, Michaeline McGuinty.
Validation: Pierre Giguère.
Writing – original draft: Marie-Josée Deschenes, Todd Fairhead, Pierre Giguère, Swapnil Hiremath, Stephanie Hoar, Jolanta Karpinski, Greg Knoll, Jessica McDougall, Michaeline McGuinty, Namrata Parikh, Rinu Pazhekattu, MacKenzie Van Loon.
Writing – review & editing: Pierre Giguère, Swapnil Hiremath.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to containing information that could compromise the privacy of the participants.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B765.
Supplemental Figure 1. Individualized tacrolimus plasma concentrations.
Supplemental Figure 2. Individualized serum creatinine data per blood draw in patients on tacrolimus.
Supplemental Table 1. Peak serum creatinine changes in patients on tacrolimus.
References
- 1.Mohan S, King KL, Husain SA, Schold JD. COVID-19-Associated mortality among kidney transplant recipients and candidates in the United States. Clin J Am Soc Nephrol. 2021;16(11):1695–1703. doi: 10.2215/CJN.02690221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr EJ Kronbichler A Graham-Brown M, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6(9):2292–2304. doi: 10.1016/j.ekir.2021.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochran W Shah P Barker L, et al. COVID-19 clinical outcomes in solid organ transplant recipients during the Omicron surge. Transplantation. 2022;106(7):e346–e347. doi: 10.1097/tp.0000000000004162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanego F Vigara LA Alonso M, et al. Trends in COVID-19 outcomes in kidney transplant recipients during the period of Omicron variant predominance. Transplantation. 2022;106(6):e304–e305. doi: 10.1097/tp.0000000000004126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfizer Canada ULC. Paxlovid (Nirmatrelvir/Ritonavir) Product Monograph. 2002
- 6.Rose DT Gandhi SM Bedard RA, et al. Supratherapeutic tacrolimus concentrations with nirmatrelvir/ritonavir in solid organ transplant recipients requiring hospitalization: a case series using rifampin for reversal. Open Forum Infect Dis. 2022;9(7):ofac238. doi: 10.1093/ofid/ofac238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young C, Papiro T, Greenberg JH. Elevated tacrolimus levels after treatment with nirmatrelvir/ritonavir (Paxlovid) for COVID-19 infection in a child with a kidney transplant. Pediatr Nephrol. 2022;38(4):1387–1388. doi: 10.1007/s00467-022-05712-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Society of Transplantation. AST Statement on Oral Antiviral Therapy for COVID-19 for Organ Transplant Recipients. Accessed August 29, 2022. https://www.myast.org/sites/default/files/AST%20Statement%20on%20Oral%20Antiviral%20Therapy%20for%20COVID%20Jan%204%20%282%29.pdf. [Google Scholar]
- 9.Fishbane S, Hirsch JS, Nair V. Special considerations for paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am J Kidney Dis. 2022;79(4):480–482. doi: 10.1053/j.ajkd.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal S, Agarwal SK. Lopinavir-ritonavir in SARS-CoV-2 infection and drug-drug interactions with cardioactive medications. Cardiovasc Drugs Ther. 2021;35(3):427–440. doi: 10.1007/s10557-020-07070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottlieb RL Vaca CE Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/nejmoa2116846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ontario COVID-19 Science Advisory Table. Nirmatrelvir/Ritonavir (Paxlovid): What Prescribers and Pharmacists Need to Know (2002). Accessed August 29, 2022. https://covid19-sciencetable.ca/sciencebrief/nirmatrelvir-ritonavir-paxlovid-what-prescribers-and-pharmacists-need-to-know-3-0/. [Google Scholar]
- 13.Takashita E Kinoshita N Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against covid-19 Omicron variant. New Engl J Med. 2022;386(10):995–998. doi: 10.1056/nejmc2119407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prikis M, Cameron A. Paxlovid (Nirmatelvir/Ritonavir) and tacrolimus drug-drug interaction in a kidney transplant patient with SARS-2-CoV infection: a case report. Transplant Proc. 2022;54(6):1557–1560. doi: 10.1016/j.transproceed.2022.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berar Yanay N, Bogner I, Saker K, Tannous E. Paxlovid-tacrolimus drug-drug interaction in a 23-year-old female kidney transplant patient with COVID-19. Clin Drug Investig. 2022;42(8):693–695. doi: 10.1007/s40261-022-01180-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindauer KE, Hamel AG. Case report: nirmatrelvir/ritonavir and tacrolimus in a kidney transplant recipient with COVID-19. Am Fam Physician. 2022;105(6):569–570. [PubMed] [Google Scholar]
- 17.National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines (2002). National Institutes of Health. Accessed August 11 2022. https://www.covid19treatmentguidelines.nih.gov/. [PubMed] [Google Scholar]
- 18.Salerno DM Jennings DL Lange NW, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;22(8):2083–2088. doi: 10.1111/ajt.17027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang AX, Koff A, Hao D, Tuznik NM, Huang Y. Effect of nirmatrelvir/ritonavir on calcineurin inhibitor levels: early experience in four SARS-CoV-2 infected kidney transplant recipients. Am J Transplant. 2022;22(8):2117–2119. doi: 10.1111/ajt.16997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaitre F. Yes we can (use nirmatrelvir/ritonavir even in high immunological risk patients treated with immunosuppressive drugs). Clin Pharmacokinet. 2022;61(8):1071–1073. doi: 10.1007/s40262-022-01158-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stader F Khoo S Stoeckle M, et al. Stopping lopinavir/ritonavir in COVID-19 patients: duration of the drug interacting effect. J Antimicrob Chemother. 2020;75(10):3084–3086. doi: 10.1093/jac/dkaa253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to containing information that could compromise the privacy of the participants.