Keywords: acute renal failure, pediatrics, pediatric nephrology
Abstract
Significance Statement
Serum creatinine is not a sensitive biomarker for neonatal AKI because it is confounded by maternal creatinine level, gestational age, and neonatal muscle mass. In this multicenter cohort study of 52,333 hospitalized Chinese neonates, the authors proposed serum cystatin C–related criteria (CyNA) for neonatal AKI. They found that cystatin C (Cys-C) is a robust and sensitive biomarker for identifying AKI in neonates who are at an elevated risk of in-hospital mortality and that CyNA detects 6.5 times as many cases as the modified Kidney Disease Improving Global Outcomes creatinine criteria. They also show that AKI can be detected using a single test of Cys-C. These findings suggest that CyNA shows promise as a powerful and easily applicable tool for detecting AKI in neonates.
Background
Serum creatinine is not a sensitive biomarker for AKI in neonates. A better biomarker-based criterion for neonatal AKI is needed.
Methods
In this large multicenter cohort study, we estimated the upper normal limit (UNL) and reference change value (RCV) of serum cystatin C (Cys-C) in neonates and proposed cystatin C–based criteria (CyNA) for detecting neonatal AKI using these values as the cutoffs. We assessed the association of CyNA-detected AKI with the risk of in-hospital death and compared CyNA performance versus performance of modified Kidney Disease Improving Global Outcomes (KDIGO) creatinine criteria.
Results
In this study of 52,333 hospitalized neonates in China, Cys-C level did not vary with gestational age and birth weight and remained relatively stable during the neonatal period. CyNA criteria define AKI by a serum Cys-C of ≥2.2 mg/L (UNL) or an increase in Cys-C of ≥25% (RCV) during the neonatal period. Among 45,839 neonates with measurements of both Cys-C and creatinine, 4513 (9.8%) had AKI detected by CyNA only, 373 (0.8%) by KDIGO only, and 381 (0.8%) by both criteria. Compared with neonates without AKI by both criteria, neonates with AKI detected by CyNA alone had an increased risk of in-hospital mortality (hazard ratio [HR], 2.86; 95% confidence interval [95% CI], 2.02 to 4.04). Neonates with AKI detected by both criteria had an even higher risk of in-hospital mortality (HR, 4.86; 95% CI, 2.84 to 8.29).
Conclusions
Serum Cys-C is a robust and sensitive biomarker for detecting neonatal AKI. Compared with modified KDIGO creatinine criteria, CyNA is 6.5 times more sensitive in identifying neonates at elevated risk of in-hospital mortality.
Introduction
AKI is common in hospitalized neonates, particularly those in neonatal intensive care unit, and associated with an increased risk of mortality and morbidity in this population.1–4 Although study interest in neonatal AKI has increased greatly over the past decade, advances in clinical diagnosis and management for AKI in neonates have lagged behind that seen in adult and older pediatric patients.5,6 One major factor limiting progress in this field has been the lack of a standardized definition for AKI in neonates, leading to an inability to recognize AKI early and to compare results across studies.5,7
Currently, the diagnosis of AKI is dependent on a rise in serum creatinine (SCr) or a decrease in urine output. Before 2008, most neonatal AKI studies used an arbitrary definition of AKI defined by an absolute SCr of ≥1.5 mg/dl. In 2012, Jetton and Askenazi proposed a neonatal AKI definition on the basis of the Kidney Disease Improving Global Outcomes (KDIGO) definition for adults8 with modifications.9 Other neonatal studies have used modifications of the RIFLE or the AKI Network criteria for adults.10–12 All these definitions define AKI by a given magnitude of rise in SCr in a short period of time that have been shown to be associated with increased risk of adverse outcomes, mostly in-hospital mortality, in epidemiological studies. However, the unique kidney physiology of neonates creates challenges for the use of SCr as the marker of AKI in these patients. Neonatal SCr initially reflects maternal values and decreases over subsequent weeks after birth at different rates depending on gestational age.5,6 In addition, SCr concentration also reflects creatinine production, which is proportional to muscle mass and has a considerable intraindividual and interindividual variation.13 Therefore, SCr values have to be adjusted for body height and body composition to reflect renal function in the pediatric age groups. Furthermore, neonatal AKI commonly present non-oliguria, making urine output an insensitive marker.9
Cystatin C (Cys-C) is a cationic protein with a molecular weight of 13.3 kD produced at a constant rate by all nucleated body cells14 and eliminated from the circulation almost exclusively by glomerular filtration.15 A study in children13 has shown that Cys-C correlates better with GFR than SCr and reflects GFR independent of body height. Unlike SCr, Cys-C does not cross the blood placental barrier.16 Neonatal serum Cys-C originates almost exclusively in the neonate, making it a promising alternative to SCr for evaluating renal function in neonates. Currently, the reference interval of serum Cys-C in neonates have only been reported in a few small studies,17–19 and the reference change value (RCV) of serum Cys-C in neonates have not been investigated.
In this large multicenter cohort study including 52,333 hospitalized neonates with measurement of Cys-C, we provided evidence for serum Cys-C as a biomarker for neonatal AKI. First, we assess the impacts of gestational age, birthweight, and days after birth on the level of Cys-C and estimated upper normal limit (UNL) and RCV of serum Cys-C in neonates. Then, we proposed Cys-C–based criteria (CyNA) for detecting neonatal AKI on the basis of both Cys-C level and the change in Cys-C using UNL and RCV as the cutoffs, respectively. Finally, we assessed the performance of CyNA in detecting neonatal AKI and the association of CyNA-detected AKI with the risk of in-hospital death and compared the performance with that using the KDIGO creatinine criteria. Our study suggests that serum Cys-C is a robust and sensitive biomarker for detecting neonatal AKI.
Methods
Study Population and Data Source
The study sample was drawn from the China Renal Data System (CRDS). CRDS is a cooperative network formed by the regional medical centers across China with the aim of facilitating clinical research on kidney disease. Each participating center exported from its proprietary hospital information systems the demographic and the clinical data of the patients who had been hospitalized between 2000 and 2021. The exported data were cleaned up, standardized, anonymized, and pooled at the CRDS datacenter located at the National Clinical Research Center of Kidney Disease in Guangzhou. The demographic data included sex and date of birth. The clinical data included admission and discharge date and division of each hospitalization, diagnosis codes, operation and procedure codes, results of laboratory assays, imaging and histological reports, prescriptions, and medical records of both in-patient and out-patient visits. All laboratories of the participating centers had passed the annual External Quality Assessment by the Chinese National Center for Clinical Laboratories. As of November 1, 2021, the CRDS databases included data of more than seven million patients from 19 medical centers. In this study, we selected from the CRDS database neonates who had at least one measurement of serum Cys-C within 28 days after birth and excluded those with congenital renal failure (ICD10 code: P96.0), congenital renal artery stenosis or malformation (Q27.1, Q27.2), and congenital malformation of the urinary system (Q60.x–Q64.x). The flowchart of sample selection is presented in Supplemental Figure 1. The Medical Ethics Committee of Nanfang Hospital approved the study and waived patients' informed consent.
Distribution and UNL of Serum Cys-C in Neonates
We excluded neonates who died within 28 days after birth or required dialysis and selected the first available serum Cys-C measurement from each qualified neonate for the distribution analyses of serum Cys-C. We computed and drew the Gaussian kernel density estimates of serum Cys-C on the day of birth stratified by gestational age (≥37 versus <37 weeks) and birth weight (≥2500 versus <2500 g). We calculated and drew the box plots of serum Cys-C and SCr stratified by day after birth and visually compared the day-specific medians of serum Cys-C stratified by sex, gestational age, and birth weight. We estimated the mean (μ1) and the variance () of serum Cys-C in neonates using the Huber robust method20 and calculated the UNL of Cys-C as μ1+1.64×s1 which corresponds to the 95th percentile of Cys-C under normal assumption. As a sensitivity analysis, we built a linear regression model of Cys-C adjusting for comorbidities in neonates, obtained the adjusted value of Cys-C in each neonate by removing the estimated effects of comorbidities, and used the adjusted Cys-C values to calculate the UNL of Cys-C.
Distribution of and the Reference Value for Serum Cys-C Change in Neonates
We selected the first pair of Cys-C values from each neonate who had at least two Cys-C measurements with no more than 7 days apart and excluded those who died within 28 days after birth or required dialysis. We drew the Bland-Altman plot of the paired Cys-C values. We binned the Cys-C pairs by paired average with a bin size of 0.1. In each bin, we used the Huber robust method to estimate the mean () and the variance () of the relative change of Cys-C (Δ), which is defined as the paired difference divided by the paired average, i.e., , where c1 and c2 were a pair of Cys-C values and r was the ratio of the paired values (c2/c1). The RCV for Cys-C ratio (c2/c1), defined as the 95th percentile of the distribution, was calculated as , where . The 95% confidence interval (95% CI) of RCV for Cys-C ratio was estimated using 5000 bootstrap samples. As a sensitivity analysis, we also built a linear regression model of adjusting for comorbidities in neonates, obtained the adjusted value of in each neonate by removing the estimated effects of comorbidities, and used adjusted to calculate the RCV for Cys-C ratio.
Definition and Detection of Neonatal AKI
We proposed a Cys-C–based algorithm for detecting neonatal AKI (CyNA), which considers a Cys-C level of ≥2.2 mg/L (the UNL) or an increase of ≥25% in Cys-C (the RCV) as neonatal AKI. We also used the modified KDIGO creatinine criteria to detect neonatal AKI, which requires an increase of ≥50% or ≥26.5 umol/L in SCr.8 We screened neonates for AKI according to either CyNA or KDIGO criteria. In the screening, a marker value (Cys-C or creatinine) was compared with a baseline value which was dynamically defined as the running minimal value preceding the measurement of interest.9
Time to AKI was treated as interval censored, and the cumulative incidence of AKI over time was estimated using the Turnbull method.21
Association between AKI Status and In-Hospital Mortality
We used the Kaplan-Meier method to estimate the cumulative incidence of in-hospital mortality stratified by AKI status according to CyNA criteria and its two components (Cys-C level and Cys-C ratio), respectively. We estimated the nonlinear association between Cys-C level and the risk of in-hospital mortality using a penalized spline in a Cox proportional hazard regression model with adjustment for sex, intensive care unit (ICU), and common comorbidities (see Supplemental Methods). In the model, Cys-C and admission to ICU were coded as time-dependent covariates. We also estimated the nonlinear association between Cys-C ratio and the risk of in-hospital death using a similar Cox model with additional adjustment for the first value of Cys-C.
We compared the performance of CyNA and KDIGO criteria in a subset of neonates who had measurements of both Cys-C and SCr at the same time. We classified AKI status into one of the categories denoted by C−K−, C−K+, C+K−, and C+K+ according to the presence (+) and the absence (−) of AKI detected by CyNA (C) and KDIGO (K), respectively. We estimated the hazard ratios (HRs) and the corresponding confidence intervals of the AKI status for the risk of in-hospital death using a Cox regression model with adjustment for sex, admission to ICU, and common comorbidities, in which AKI status and ICU were coded as time-dependent covariates.
In all of the above Cox models, we first tested the proportional hazards assumption. For the covariates that the proportional hazards assumption did not hold, we performed stratified Cox regression analyses accordingly.
All statistical analyses were performed using R version 4.0.3.
Results
Study Population
The study population included 52,333 hospitalized neonates who had testing on serum Cys-C and did not have known congenital disease in the urinary system (Supplemental Figure 1), of whom 14,484 had multiple testing of Cys-C. The characteristics of the study population stratified by the testing frequency are summarized in Table 1.
Table 1.
Characteristics of the study population
| Variables | Single Cys-C Testing, n=37,849 |
≥2 Cys-C Testing n=14,484 |
P Value |
|---|---|---|---|
| Female | 16,231 (42.9) | 5964 (41.2) | <0.001 |
| Cys-C, mg/L | 1.60 (1.39–1.84) | 1.70 (1.47–1.96) | <0.001 |
| Creatinine, umol/L | 47 (33–64) | 58 (42–74) | <0.001 |
| Length of hospital stay, d | 8 (6–12) | 19 (12–33) | <0.001 |
| Gestational age | <0.001 | ||
| ≥37 wk | 27,775 (73.4) | 6042 (41.7) | |
| 28–36 wk | 9875 (26.1) | 8227 (56.8) | |
| <28 wk | 199 (0.5) | 215 (1.5) | |
| Low birthweight | 7142 (18.9) | 6358 (43.9) | <0.001 |
| Birth asphyxia | 2755 (7.3) | 2520 (17.4) | <0.001 |
| Neonatal respiratory distress syndrome | 5124 (13.5) | 4926 (34.0) | <0.001 |
| Congenital cardiovascular disease | 7753 (20.5) | 4659 (32.2) | <0.001 |
| Congenital pneumonia | 6938 (18.3) | 4558 (31.5) | <0.001 |
| Neonatal jaundice | 19,451 (51.4) | 4925 (34.0) | <0.001 |
| Perinatal infection | 8995 (23.8) | 4028 (27.8) | <0.001 |
| Bacterial sepsis | 3198 (8.4) | 2019 (13.9) | <0.001 |
| Respiratory failure | 1043 (2.8) | 1470 (10.1) | <0.001 |
| Heart failure | 24,767 (6.5) | 1921 (13.3) | <0.001 |
| Required intensive care | 23,874 (63.1) | 12,433 (85.8) | <0.001 |
| Required dialysis | 24 (0.1) | 54 (0.4) | <0.001 |
| Neonatal death | 165 (0.4) | 133 (0.9) | <0.001 |
Cys-C, creatinine, and length of stay are expressed as median (interquartile range), others as No. (%). Cys-C, Cystatin C.
In general, compared with neonates who had single Cys-C testing, those with multiple testing were more likely being preterm and of low birthweight, having severe comorbidities and death, and needing intensive care and long hospital stay. Neonatal jaundice was the most frequent complication in the single-testing group and the only complication listed in Table 1 that had a higher prevalence in the single-testing group than that in the multiple-testing group. It is noteworthy that two thirds of the neonates in our study population needed intensive care. The levels of serum Cys-C and creatinine in the multiple-testing group were also higher than that in the single-testing group.
Estimating the UNL of Serum Cys-C in Neonates
We estimated the distribution of serum Cys-C using the first testing value from 51,978 neonates who survived the neonatal period and did not require dialysis. Serum level of Cys-C on the first day of birth was approximately normally distributed, and the distribution in neonates was essentially the same regardless of gestational age and birthweight (Supplemental Figure 2).
Unlike SCr, Cys-C level did not change significantly over the first 4 weeks after birth. In our study population, the daily median of Cys-C within the first 2 days was 1.73 mg/L, dropped to 1.55 mg/L on day 3, and remained steady at this level until the fourth week (Figure 1). The between-day variation of Cys-C, indexed by the SD of the daily means of Cys-C, was only 0.06 mg/L, which was very small compared with the within-day SD of 0.42 mg/L. By contrast, the daily median level of SCr dropped from 63 umol/L (0.72 mg/dl) on the first day to 24 umol/L (0.27 mg/dl) at the end of week 4. In the analysis of variance, gestational age, birthweight, and days after birth combined accounted for 2.3% and 24.3% of the total variance of Cys-C and SCr, respectively, in neonates.
Figure 1.
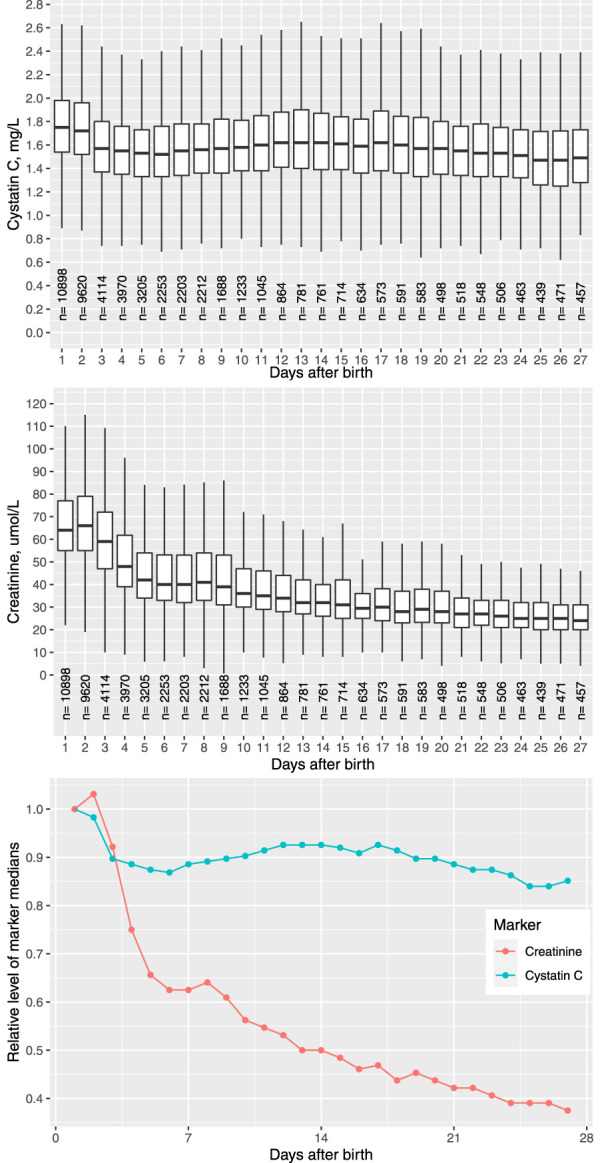
Distribution of serum Cystatin C (Cys-C) and creatinine stratified by day after birth in the neonates. Figure 1 can be viewed in color online at www.jasn.org.
Because the distribution of Cys-C did not vary substantially across time, we proposed a time-invariant UNL of Cys-C for the neonates, using 95th percentile under normal distribution as the cutoff. The estimated UNL of Cys-C in our study population was 2.2 mg/L. To assess the impact of comorbidities on the estimated UNL, we re-estimated the UNL of Cys-C using the adjusted Cys-C values that removed the effect of comorbidities. The UNL estimated from the adjusted Cys-C was 2.1 mg/L, which was very close to the estimate from the unadjusted Cys-C values, indicating the robustness of our estimation method. The strata-specific UNLs of Cys-C estimated among neonates of different gestational age were also comparable (Supplemental Table 1).
Estimating the RCV of Cys-C in Neonates
The Bland-Altman plot of 9571 independent pairs of Cys-C testing taken within ≤7 days apart is presented in Supplemental Figure 3. The distribution of relative pair difference was similar across the whole spectrum of pair average. The concordance correlation coefficient between the paired Cys-C values was 0.54 (95% CI, 0.53 to 0.55), and the bias correction factor was 0.94.
We estimated the RCVs for the Cys-C ratio (c2/c1) and the bootstrap confidence intervals stratified by the paired averages and found that the pairs of different Cys-C levels shared similar estimated RCVs (Figure 2). Within a wide range of Cys-C level from 1.0 to 2.3 mg/L, the estimated RCVs fall into a narrow range between 1.2 and 1.3. RCV value of 1.25 was within the bootstrap confidence intervals of RCV across the whole spectrum of Cys-C levels examined. The strata-specific RCVs of Cys-C estimated among neonates of different gestational age were also comparable (Supplemental Table 2). As a result, we proposed 1.25 as the universal RCV for the Cys-C ratio regardless of the Cys-C level and gestational age.
Figure 2.
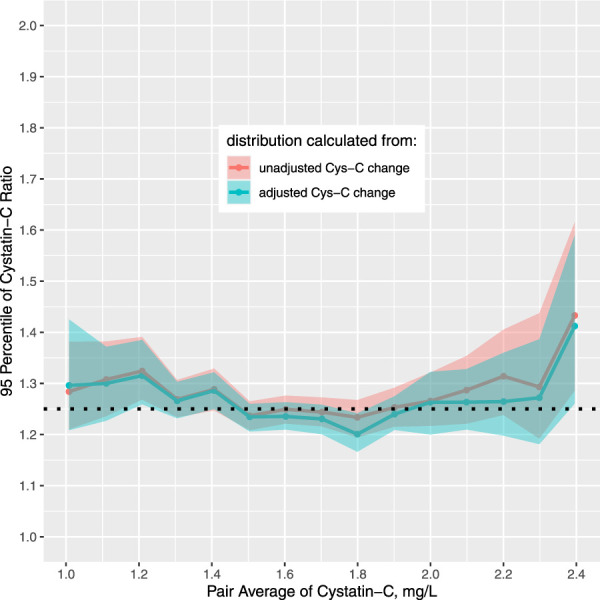
RCVs of Cys-C in neonates were similar across a wide spectrum of Cys-C levels. The shaded area denotes the 95% intervals of the estimated RCVs. The dotted black horizontal line indicates the overall RCV of Cys-C ratio, which was 1.25. Figure 2 can be viewed in color online at www.jasn.org.
To examine the impact of comorbidities on the estimation of RCV, we also estimated the RCVs using the adjusted values of paired change that removed the effect of the comorbidities (Figure 2). The RCVs estimated from the adjusted paired change essentially overlapped with that from the unadjusted ones, suggesting the robustness of our estimation method.
Detecting Neonatal AKI Using Cys-C–Based Criteria
In the absence of congenital disorders in the urinary system, a high level of serum Cys-C beyond UNL in neonates would suggest with high probability of AKI rather than chronic kidney lesions occurred during fetal development. Therefore, we proposed a Cys-C–based algorithm for detecting neonatal AKI (CyNA criteria) on the basis of both Cys-C level and the change in Cys-C, which defines neonatal AKI by a serum Cys-C of ≥2.2 mg/L (the estimated UNL) or an increase in Cys-C of ≥25% (the estimated RCV) during the neonatal period.
Among 52,333 neonates, 6336 (12.1%) AKI cases were detected by CyNA criteria. The curve of cumulative AKI incidence is presented in Supplemental Figure 4. The cumulative incidence of AKI and the 95% CI at day 1, 8, 15, and 28 was 7.0% (6.8%–7.3%), 11.2% (10.7%–13.3%), 17.1% (16.2%–18.0%), and 23.8% (23.0%–25.8%), respectively. It is noteworthy that a significant portion of AKI was estimated to occur within the first day after birth. The highest incidence of early onset of AKI was observed in the neonates with birth asphyxia and respiratory distress syndrome (Supplemental Figure 5).
Among 45,839 neonates who had measurement of both Cys-C and SCr, 4513 (9.8%) had AKI detected by CyNA only, 373 (0.8%) by KDIGO only, and 381 (0.8%) by both criteria. Overall, CyNA identified 6.5 times as many cases as the modified KDIGO creatinine criteria. A significant portion of AKI cases detected by KDIGO but not CyNA (C−/K+) was observed in the neonates with low initial SCr values as the incidence rate of KDIGO-detected AKI was substantially inflated in those neonates (Supplemental Figure 6).
Association of AKI with In-Hospital Mortality
Of 52,333 neonates, 298 (0.57%) died during hospitalization. In a Cox model adjusted for all comorbidities, both Cys-C level and the Cys-C change were associated with the risk of in-hospital mortality in a nonlinear and dose-dependent fashion (Figure 3A). The risk of in-hospital death was already significantly increased when Cys-C level reached UNL and Cys-C change reached RCV.
Figure 3.
Higher Cys-C level and Cys-C ratio were associated with an increased risk of in-hospital death. (A) The effect of Cys-C and the Cys-C ratio on the risk of in-hospital mortality estimated using a Cox regression model with adjustment for admission to ICU and comorbidities. Cys-C ratio and admission to ICU were coded as time-varying variables. A restricted cubic spline was used to allow for nonlinear effect. The gray areas cover the 95% CIs of the estimated effects. The dotted vertical lines indicate the UNL (left) and the RCV (right) of Cys-C. (B) Kaplan-Meier curves of in-hospital death stratified by initial level of Cys-C and increase in Cys-C. Left: The neonates with initial Cys-C value above UNL had a significantly higher cumulative incidence of in-hospital death. Right: The K-M curve was estimated in neonates with multiple Cys-C measurement and whose initial Cys-C value was below UNL. The neonates with Cys-C ratio above RCV had a significantly higher cumulative incidence of in-hospital death. The shaded areas cover the 95% confidence intervals (CIs) of the estimates. Figure 3 can be viewed in color online at www.jasn.org.
Among 6336 neonates who had CyNA-detected AKI, 96 (1.5%) died. In comparison, 202 (0.44%) death events occurred in 45,997 neonates who did not have AKI. The Kaplan-Meier curves of cumulative in-hospital mortality in neonates stratified by the initial Cys-C level (≥2.2 versus <2.2 mg/L) and by level of Cys-C increase (≥25% versus <25%) are shown in Figure 3B. After adjusting all comorbidities, CyNA-detected AKI was associated with a 1.9-fold increase (HR, 2.91; 95% CI, 2.24 to 3.78) in the risk of in-hospital mortality (Supplemental Table 3).
The in-hospital mortalities stratified by a combination of CyNA-detected and KDIGO-detected AKI status in 45,839 neonates with measurements of both Cys-C and SCr are summarized in Table 2. After adjusting admission to ICU and comorbidities, AKI detected by CyNA only (C+/K−) was associated with a significantly increased risk of in-hospital mortality compared with those without AKI by both criteria (C−/K−), with an adjusted HR of 2.86 (95% CI, 2.02 to 4.04). In comparison, the HR for those detected by KDIGO only (C−/K+) was lower at 2.15 (95% CI, 0.96 to 4.81). Neonates with AKI detected by both criteria (C+/K+) had the highest risk of in-hospital mortality (HR, 4.86; 95% CI, 2.84 to 8.29).
Table 2.
In-hospital mortality by AKI status
| AKI Statusa | n | Death | 1000 Person Days | Death Per 100,000 Person Days | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|
| C−/K− | 40,572 | 137 | 626.7 | 21.9 | — | — |
| C−/K+ | 373 | 7 | 8.0 | 87.8 | 5.64 (2.61 to 12.2) | 2.15 (0.96 to 4.81) |
| C+/K− | 4513 | 51 | 83.5 | 61.1 | 4.25 (3.05 to 5.92) | 2.86 (2.02 to 4.04) |
| C+/K+ | 381 | 22 | 8.9 | 247.0 | 11.8 (7.30 to 19.2) | 4.86 (2.84 to 8.29) |
HR, hazard ratio; 95% CI, 95% confidence interval.
AKI status is presented as a combination of absence (−) or presence (+) of AKI detected by CyNA (C) and KDIGO (K) criteria, respectively. Hazard ratios were estimated in the neonates with measurements of both Cystatin C and serum creatinine using a Cox proportional hazard model with adjustment for sex, admission to intensive care unit (ICU), and common comorbidities. AKI status and admission to ICU were coded as time-dependent covariates in the model.
Disscussion
Efficiently detecting AKI in neonates has been a great challenge. In the current study, we provide high-quality evidence for using serum Cys-C as a biomarker for neonatal AKI in a large cohort of hospitalized neonates. We have estimated the UNL and RCV of serum Cys-C and proposed Cys-C–based criteria (CyNA) for detecting neonatal AKI. CyNA could detect 5.5 times more AKI cases than the modified KDIGO criteria. More importantly, these additional AKIs detected by CyNA beyond KDIGO were associated with a significantly increased risk of in-hospital mortality even after adjusting for admission to ICU and comorbidities.
Currently, there has been no consensus on defining AKI in neonates. The modified KDIGO criteria on the basis of SCr change has often been used in the epidemiological studies on neonatal AKI.1,22,23 However, SCr is not a good biomarker for neonatal AKI. Neonatal SCr initially reflects maternal values and decreases dramatically during the first 4 weeks after birth. In our study population, the median of SCr was 63 umol/L on the first day after birth and dropped to 24 umol/L at the end of fourth week. By contrast, serum Cys-C was quite steady during the same period such that the between-day variation was only a small fraction of the within-day variation. Furthermore, we have shown that gestational age and birthweight had minimal impact on Cys-C level in neonates. In this regard, Cys-C is a more robust marker for perturbance of kidney function in neonates than SCr.
There have been no gold standard definitions for AKI in both adults and children. Traditionally, the creatinine criteria for AKI were empirically established on the basis of the association between the level of increase in SCr and the risk of adverse outcomes, mostly in-hospital mortality, in epidemiological studies. We have previously proposed an RCV-based SCr criterion for pediatric AKI (pROCK) on the basis of a simple concept that an acute increase in SCr above the upper limit of normal variability should be a consistent index of a decline in renal function.24 In validation, pROCK outperformed both KDIGO and pRIFLE criteria in identifying true AKI cases that were at high risk of in-hospital death in a larger sample of pediatric inpatients. As expected, in the current study the RCV-based Cys-C criterion also performed well in detecting neonatal AKI. In addition, AKI may frequently occur at birth because of medical procedures, stress, and complications during delivery. In many of these cases, the level of Cys-C before AKI cannot be easily determined, which could cause delay and even failure in detecting AKI using the criteria on the basis of Cys-C change. Most of the hospitalized neonates in our cohort only received one blood testing, and the criteria on the basis of change in biomarkers will have no power to detect AKI in these cases. In the absence of congenital malformation of the urinary system or immaturity of the kidney, elevated Cys-C level in neonates would be suggestive of AKI. For this reason, we used elevated Cys-C level as an independent criterion for neonatal AKI.
The cutoff value for AKI definition is usually established on the basis of the association between the level of increase in SCr and the risk of adverse outcomes and the need to balance the tradeoff between sensitivity and specificity. Using the KDIGO creatinine criteria, most studies reported an AKI incidence rate of 10%–20% among the general hospitalized adults and a much higher rate in those who need intensive care. In our own study, using the KDIGO creatinine criteria, the incidence rate of AKI was 12% and 20%, respectively, among the hospitalized adults and children (excluding neonates).25,26 Using CyNA criteria, the incidence rate of AKI was 12.1% among neonates, which is in line with that observed in adults and children using the KDIGO criteria and five times more than that detected by the modified KDIGO creatinine criteria in neonates. The association between CyNA-detected AKI and in-hospital mortality (adjusted HR, 2.91; 95% CI, 2.24 to 3.78) in neonates was slightly lower than that observed in adults and children using the KDIGO criteria. Our study suggests that CyNA criteria in neonates performed similarly to the KDIGO creatinine criteria in children and adult with respect to the detected rate of AKI and the level of association between the detected AKI and in-hospital mortality.
In our study, we derived the UNL and the RCV of Cys-C from a large cohort of hospitalized neonates instead of healthy neonates. To reduce the influence of selection bias on the estimates, we use a robust method for estimating the distribution parameters. In addition, we have performed sensitivity analyses on UNL and RCV estimation by using the adjusted Cys-C values or the adjusted Cys-C change values that removed the effects of comorbidities. The UNL estimated from the sensitivity analysis was only slightly lower than that from the original estimate (2.1 versus 2.2 mg/L), and the RCVs estimated from the sensitivity analysis were essentially the same as that from the original estimates. There were only a few reports on the reference interval of Cys-C in neonates, usually neonatal patients without known renal diseases or severe comorbidities. In a study of 50 term and 58 preterm neonates, Harmoinen et al.17 reported a reference interval of 1.34–2.57 mg/L and 1.36–2.23 mg/L, respectively, for the preterm and the term neonates. In a study of 90 term neonates, Mikulic et al.18 reported a reference interval of 0.93–3.15 mg/L and 1.5–3.36 mg/L, respectively, for girls and boys. Tong et al.19 investigated gestational and age-specific Cys-C reference intervals in 1044 neonates, and the reference intervals were generally within the range of 1.3–2.3 mg/L for the neonates whose gestational age was older than 28 weeks. Our estimate of UNL (2.2 mg/L) was consistent with the above reported reference intervals, except that from the study by Mikulic which reported a much wider reference interval. We believe the UNL and the RCV estimated in our study would be in close proximity to those from the healthy neonates and applicable to the normal infants.
The study has several limitations. First, we did not use urine output in defining AKI because the urine output data were not available in most neonates. It is challenging to measure urine output accurately, and the thresholds on the basis of older children and adults may not perform well in neonates.27 Second, as we have discussed above, we estimated the UNL and the RCV from the hospitalized neonates rather than healthy ones. Our sensitivity analyses suggest that the impact of this selection bias on the accuracy of the estimates may be limited. Furthermore, the reference values estimated from the hospitalized neonates could capture the potential variabilities in Cys-C change associated with the environment and interventions during hospitalization and hence might be a more appropriate index for defining hospital-acquired AKI in neonates. Third, the study was performed in Chinese neonates. Whether the estimated UNL and RCV are applicable to other ethnic groups remains to be validated.
In conclusion, we consider serum Cys-C a robust and sensitive biomarker for detecting neonatal AKI. Together with the fact that AKI can be detected using a single test and that Cys-C assay has been routinely performed in many clinical laboratories, CyNA could be a powerful and easily applicable tool for detecting AKI in neonates.
Supplementary Material
Footnotes
X.X. and S.N. contributed equally to this work.
Disclosures
F.F. Hou reports Consultancy: AstraZeneca; and Honoraria: AstraZeneca. H. Liu reports Research Funding: National Natural Science Foundation of China. X. Xu reports Employer: Fujun Genetics, Shanghai, China; and Ownership Interest: Apple Inc. All remaining authors have nothing to disclose.
Funding
This study is supported by grants from the National Key R&D Program of China (2021YFC2500200 to X.X.), the National Natural Science Foundation of China (81970586 to X.X.), the National Natural Science Foundation of China (Key Program) (82030022 to F.F.H.), the Research Fund Program of Guangdong Provincial Clinical Research Center for Kidney Disease (2020B1111170013 to F.F.H.), the Research Fund Program of Guangdong Provincial Key Laboratory of Renal Failure Research (2017B030314036 to F.F.H.), Guangdong Key Program of Precision Medicine (202204 to F.F.H.), and the National Natural Science Foundation of China (81900626 to S.N.).
Author Contributions
Conceptualization: Fan Fan Hou, Xin Xu.
Data curation: Yue Cao, Ruixuan Chen, Peiyan Gao, Qi Gao, Yanqin Li, Fan Luo, Sheng Nie, Licong Su, Ruqi Xu, Xiaodong Zhang, Shiyu Zhou.
Formal analysis: Sheng Nie, Xin Xu.
Funding acquisition: Fan Fan Hou, Sheng Nie, Xin Xu.
Methodology: Xin Xu.
Project administration: Sheng Nie, Xin Xu.
Resources: Chunbo Chen, Fan Fan Hou, Yaozhong Kong, Guisen Li, Hua Li, Bicheng Liu, Huafeng Liu, Yongjun Shi, Guobin Su, Ying Tang, Qijun Wan, Jianping Weng, Gang Xu, Hong Xu, Qiongqiong Yang, Yan Zha, Yilun Zhou.
Supervision: Fan Fan Hou.
Writing – original draft: Fan Fan Hou.
Writing – review & editing: Fan Fan Hou, Sheng Nie, Xin Xu.
Data Sharing Statement
Please contact Dr. Hou at ffhouguangzhou@163.com for access to the deidentified individual-level data.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E389.
Supplemental Methods. List of comorbidities (ICD10 codes) adjusted in the Cox proportional hazard models for in-hospital death.
Supplemental Figure 1. Flowchart of the study population.
Supplemental Figure 2. Density curves of serum Cys-C on the day of birth stratified by gestational age and birthweight.
Supplemental Figure 3. Bland-Altman plot of 9,571 pairs of Cys-C testing in neonates.
Supplemental Figure 4. Cumulative incidence of neonatal AKI detected by CyNA and the modified KDIGO criteria.
Supplemental Figure 5. Incidence of CyNA-detected AKI in neonates stratified by comorbidities and onset time of AKI.
Supplemental Figure 6. Incidence of by CyNA and KDIGO criteria stratified by the first creatinine level in neonates.
Supplemental Table 1. The estimated UNLs of Cys-C in the neonates stratified by gestational age.
Supplemental Table 2. The estimated RCVs of Cys-C ratio in the neonates stratified by gestational age.
Supplemental Table 3. Neonatal mortality by status of CyNA-detected AKI.
References
- 1.Jetton JG Boohaker LJ Sethi SK, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184–194. doi: 10.1016/s2352-4642(17)30069-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlton JR Boohaker L Askenazi D, et al. Incidence and risk factors of early onset neonatal AKI. Clin J Am Soc Nephrol. 2019;14(2):184–195. doi: 10.2215/CJN.03670318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zwiers AJ de Wildt SN Hop WC, et al. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Crit Care. 2013;17(4):R151. doi: 10.1186/cc12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Patil N, Ambalavanan N. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol. 2013;28(4):661–666. doi: 10.1007/s00467-012-2369-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jetton JG Guillet R Askenazi DJ, et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr. 2016;4:68. doi: 10.3389/fped.2016.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selewski DT Charlton JR Jetton JG, et al. Neonatal acute kidney injury. Pediatrics. 2015;136(2):e463–e473. doi: 10.1542/peds.2014-3819 [DOI] [PubMed] [Google Scholar]
- 7.Zappitelli M Ambalavanan N Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. 2017;82(4):569–573. doi: 10.1038/pr.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ISN. Kidney Disease: Improving global outcomes (KDIGO) acute kidney injury work group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 9.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. 2012;24(2):191–196. doi: 10.1097/mop.0b013e32834f62d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46(4):630–635. doi: 10.1016/j.jpedsurg.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 11.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69(4):354–358. doi: 10.1203/pdr.0b013e31820b95ca [DOI] [PubMed] [Google Scholar]
- 12.Blinder JJ Goldstein SL Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143(2):368–374. doi: 10.1016/j.jtcvs.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 13.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–1953. doi: 10.1093/clinchem/38.10.1933 [DOI] [PubMed] [Google Scholar]
- 14.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C–a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101(5):875–881. doi: 10.1542/peds.101.5.875 [DOI] [PubMed] [Google Scholar]
- 15.Bökenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol. 1998;12(2):125–129. doi: 10.1007/s004670050419 [DOI] [PubMed] [Google Scholar]
- 16.Cataldi L, Mussap M, Bertelli L, Ruzzante N, Fanos V, Plebani M. Cystatin C in healthy women at term pregnancy and in their infant newborns: relationship between maternal and neonatal serum levels and reference values. Am J Perinatol. 1999;16(6):287–295. doi: 10.1055/s-2007-993874 [DOI] [PubMed] [Google Scholar]
- 17.Harmoinen A, Ylinen E, Ala-Houhala M, Janas M, Kaila M, Kouri T. Reference intervals for cystatin C in pre- and full-term infants and children. Pediatr Nephrol. 2000;15(1-2):105–108. doi: 10.1007/s004670000421 [DOI] [PubMed] [Google Scholar]
- 18.Mikulic V Rogic D Mikulic I, et al. Cystatin C concentration during the first three postnatal days in healthy term newborns. Z Geburtshilfe Neonatol 2022;226(3):193–196. doi: 10.1055/a-1727-6309 [DOI] [PubMed] [Google Scholar]
- 19.Tong C Liu Y Wu Y, et al. Gestational and age-specific cystatin C reference intervals for newborns. Am J Perinatol. 2022;39(15):1654–1658. doi: 10.1055/s-0041-1724000 [DOI] [PubMed] [Google Scholar]
- 20.Huber PJ. Robust Statistics. John Wiley and Sons; 1981. [Google Scholar]
- 21.Turnbull BW. The empirical distribution function with arbitrarily grouped censored and truncated data. J R Stat Soc Ser B (Methodological). 1976;38(3):290–295. doi: 10.1111/j.2517-6161.1976.tb01597.x [DOI] [Google Scholar]
- 22.Nada A, Bonachea EM, Askenazi DJ. Acute kidney injury in the fetus and neonate. Semin Fetal Neonatal Med. 2017;22(2):90–97. doi: 10.1016/j.siny.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorga SM, Murphy HJ, Selewski DT. An update on neonatal and pediatric acute kidney injury. Curr Pediatr Rep. 2018;6(4):278–290. doi: 10.1007/s40124-018-0184-5 [DOI] [Google Scholar]
- 24.Xu X Nie S Zhang A, et al. A new criterion for pediatric AKI based on the reference change value of serum creatinine. J Am Soc Nephrol. 2018;29(9):2432–2442. doi: 10.1681/ASN.2018010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X Nie S Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10(9):1510–1518. doi: 10.2215/CJN.02140215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X Nie S Zhang A, et al. Acute kidney injury among hospitalized children in China. Clin J Am Soc Nephrol. 2018;13(12):1791–1800. doi: 10.2215/CJN.00800118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Mul A Parvex P Héneau A, et al. Urine output monitoring for the diagnosis of early-onset acute kidney injury in very preterm infants. Clin J Am Soc Nephrol. 2022;17(7):949–956. doi: 10.2215/CJN.15231121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact Dr. Hou at ffhouguangzhou@163.com for access to the deidentified individual-level data.




