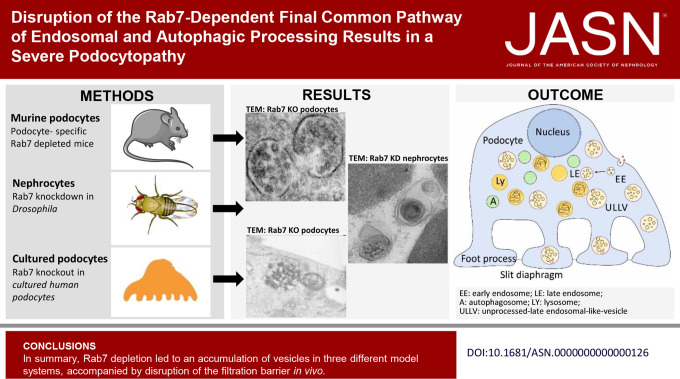
Keywords: vesicle accumulation, foot process effacement, Rab7, podocytes, podocytopathies, proteinuria, lysosome, multivesicular bodies
Abstract
Significance Statement
Endocytosis, recycling, and degradation of proteins are essential functions of mammalian cells, especially for terminally differentiated cells with limited regeneration rates and complex morphology, such as podocytes. To improve our understanding on how disturbances of these trafficking pathways are linked to podocyte depletion and slit diaphragm (SD) injury, the authors explored the role of the small GTPase Rab7, which is linked to endosomal, lysosomal, and autophagic pathways, using as model systems mice and Drosophila with podocyte-specific or nephrocyte-specific loss of Rab7, and a human podocyte cell line depleted for Rab7. Their findings point to maturation and fusion events during endolysosomal and autophagic maturation as key processes for podocyte homeostasis and function and identify altered lysosomal pH values as a putative novel mechanism for podocytopathies.
Background
Endocytosis, recycling, and degradation of proteins are essential functions of mammalian cells, especially for terminally differentiated cells with limited regeneration rates, such as podocytes. How disturbances within these trafficking pathways may act as factors in proteinuric glomerular diseases is poorly understood.
Methods
To explore how disturbances in trafficking pathways may act as factors in proteinuric glomerular diseases, we focused on Rab7, a highly conserved GTPase that controls the homeostasis of late endolysosomal and autophagic processes. We generated mouse and Drosophila in vivo models lacking Rab7 exclusively in podocytes or nephrocytes, and performed histologic and ultrastructural analyses. To further investigate Rab7 function on lysosomal and autophagic structures, we used immortalized human cell lines depleted for Rab7.
Results
Depletion of Rab7 in mice, Drosophila, and immortalized human cell lines resulted in an accumulation of diverse vesicular structures resembling multivesicular bodies, autophagosomes, and autoendolysosomes. Mice lacking Rab7 developed a severe and lethal renal phenotype with early-onset proteinuria and global or focal segmental glomerulosclerosis, accompanied by an altered distribution of slit diaphragm proteins. Remarkably, structures resembling multivesicular bodies began forming within 2 weeks after birth, prior to the glomerular injuries. In Drosophila nephrocytes, Rab7 knockdown resulted in the accumulation of vesicles and reduced slit diaphragms. In vitro, Rab7 knockout led to similar enlarged vesicles and altered lysosomal pH values, accompanied by an accumulation of lysosomal marker proteins.
Conclusions
Disruption within the final common pathway of endocytic and autophagic processes may be a novel and insufficiently understood mechanism regulating podocyte health and disease.
Introduction
Podocytes are an essential component of the glomerular filtration barrier (GFB). To fully cover the surface of glomerular capillaries, they form numerous highly- branched foot processes.1,2 Between these interdigitating foot processes, podocytes build a unique cell–cell contact called the slit diaphragm (SD), which is a crucial part of the GFB.3,4 It has been shown that podocyte depletion or damage of the SD architecture results in proteinuria and glomerular disease that often precede nephrotic syndrome and end-stage kidney disease.5–7
The complex morphology of podocytes requires the efficient transport, targeting, recycling, and turnover of lipids and proteins, indicating that disturbances of these processes might play a key role for podocytopathies. Indeed, the importance of recycling and endocytosis for podocytes has been elucidated in earlier studies: Podocytes lacking crucial endocytic regulators, such as Dynamin (Dnm1, Dnm2), Synaptojanin (Synj1), or Endophilins (Sh3gl1, Sh3gl2, Sh3gl3), develop strong injury phenotypes, including foot process effacement (FPE) and proteinuria.8 Moreover, endocytosis-incompetent mice lacking the phosphatidylinositol 3-kinase Vps34 are unable to form normal GFBs and develop severe podocyte injury.9,10
In support of this, homozygous missense mutations in the human GAPVD1, ANKFY1, and TBC1D8B genes were identified as causes of steroid-resistant nephrotic syndrome. GAPVD1 and ANKFY1 are involved in nephrin trafficking and interact with the GTPase RAB5, a key regulator of early endosomal homeostasis.11 Furthermore, TBC1D8B acts as GTPase-activating protein for RAB11b and controls vesicular recycling in cells.12 Together, these data emphasize the relevance of endocytosis and endosomal recycling for podocyte function and SD maintenance.
Podocytes also engage in a high level of autophagy, a process that ensures the recycling or degradation of nonfunctional cellular components.13,14 Autophagy prevents the noxious accumulation of dysfunctional proteins and organelles, which is particularly necessary for postmitotic cells.15,16 Podocyte-specific knockout of Atg5 (autophagy-related 5) gene leads to proteinuria, podocyte loss, and late-onset glomerulosclerosis in aging mice. Knockout of Atg5 in podocytes also results in increased susceptibility to injury models of glomerular disease.15
Lysosomal maturation is a late step in both endocytic and autophagic processes. However, its relevance for podocyte function and SD maintenance is currently poorly understood. In this study, we address the common end routes of both endocytic and autophagic processes by targeting the Rab7 gene, as this small GTPase is a key regulator of both processes.
Methods
Mice and Genotyping
Experimental methods and protocols involving animals were approved by and conducted in accordance with all guidelines and regulations set forth by the German regional authorities (LANUV; Az.: 84-02.04.2014 A379). Animals were housed under standard specific pathogen-free conditions with free access to tap water and standard animal chow. The conditional Rab7 knockout mice (Rab7fl/fl) were described earlier.17,18 All mice strains used in this study have a C57BL/6 background. To obtain Rab7-depleted podocytes, we bred these mice with NPHS2-Cre lines (PodCre+). DNA isolation and genotyping (Supplemental Table 1) was performed as described in detail by Roy et al. in 2013.17,19 In brief, tissue was incubated in lysis buffer (50 mM KCl, 1.5 mM MgCl2, 10 mM Tris/HCl pH 8.3, 0.45% NP-40, 0.45% Tween 20) containing 0.05 µg/µl proteinase K (Qiagen, Germany) at 56°C overnight (o/n). Proteinase K was then inactivated by incubation at 95°C for 10 minutes, the samples were centrifuged, and the DNA-containing supernatant was used for genotyping. Mice were genotyped as described earlier using primer Rab7 Fwd and Rab7 Rev for Rab7 flox/flox and Cre Fwd and Cre Rev to identify Cre-positive mice. If not otherwise indicated, three animals per group and time point were analyzed. We examined three time points: 2–3-week-old, 4-week-old, and 8-week-old mice. However, 2–3-week-old mice were analyzed between 14 and 18 days after birth.
Tissue Preparation and Histologic Analyses
Kidneys were prepared as described previously.20,21 Periodic acid–Schiff (PAS) sections were digitalized and analyzed using the Panoramic Viewer (3DHISTECH). Cryosections were prepared in 6-µm thick sections and fixed in 4% paraformaldehyde (PFA) for 20 minutes at room temperature (RT). Samples were quenched in PBS containing 100 mM NH4Cl, then washed with PBS, and blocked with PBS containing 5% BSA for 2 hours at RT before applying first antibodies (Supplemental Table 2) for 1 hour (in PBS containing 5% BSA) at RT. Sections were washed in PBS three times. Secondary antibodies were diluted in 5% BSA and applied for 30 minutes. Sections were mounted in Fluoromount (Sigma-Aldrich) and imaged with an Observer Z1 microscope with Apotome 2.0 (ZEISS) with a 63× 1.4 oil immersion objective. Fiji (http://fiji.sc/) and Zen Software (Zeiss GmbH, Jena, Germany) were used to analyze images.
Histopathological assessment of glomerular, tubular, and interstitial phenotypes was performed as previously described.22,23 In brief, semiquantitative scoring schemes were applied on whole slide images (Ventana DP 200 slide scanner, 40× objective) of PAS-stained kidney sections (2-µm sections of formalin-fixed paraffin-embedded [FFPE] tissue). PAS and acid-fuchsin, orange-G, and anilin-blue staining were performed at the Institute of Surgical Pathology, Medical Center University of Freiburg, applying standard diagnostic procedures. A four-tiered score was used for the assessment of glomerular sclerosis (no: no histomorphological alteration of the glomerular tuft, minor: mesangial expansion and/or thickening of the basement membrane without sclerosis, segmental: <50% of tuft area sclerotized, and global: >50% of tuft area sclerotized). For interstitial inflammation (assessing immune cell infiltrates), a four-tiered score was applied (0–3; 0: <5%; 1: >5%–25%; 2: >25%–50%; 3: <50%). Similar four-tiered grading schemes were applied for the assessment of interstitial fibrosis, tubular atrophy, and proteinaceous casts. Full kidney sections of at least five animals per group were analyzed.
Immunofluorescence staining of FFPE tissue was performed as previously described.22,23 In brief, 2-µm FFPE kidney sections were processed by heat-induced antigen retrieval using a pressure cooker and Citrate pH6 (5 minutes) or Tris-EDTA pH9 buffer (10 minutes). Primary antibodies (Rab7, SYNPO, WT1, NPHS1, SQSTM1, LAMP1, see Supplemental Table 2) were diluted in 5% BSA in PBS blocking solution and incubated on kidney section at 4°C for 24 hours. Secondary fluorophore-tagged antibodies (Alexa Fluor Dyes, Thermo Fisher Scientific) and Hoechst 33342 (Thermo Fisher Scientific) were applied for fluorescence labeling. Samples were embedded in ProLong Gold Antifade (Thermo Fisher Scientific) and imaged using a Zeiss Axio Observer microscope (applying an ApoTome.2 device and 100× objective) as previously described in detail.22,23
Transmission Electron Microscopy (TEM) Analyses
TEM microscopy analyses were performed as described previously.24 In brief, kidneys were fixed in 2.5% glutaraldehyde with Sorensen's phosphate buffer and after that postfixed with 1% osmium tetroxide, dehydrated, and embedded in the embedding media epon. In addition, 60-nm ultrathin sections were cut by using Leica Ultracut R ultramicrotome (Vienna, Austria) and counterstained with uranyl acetate in 8% uranyl acetate in bidistilled water and incubated with lead citrate solution. Samples were inspected on a TEM CM10 (Philips, Amsterdam, the Netherlands).
Cells cultivated in 10-cm cell culture dishes were washed with PBS and subsequently fixed in 2% (v/v) formaldehyde and 2.5% (v/v) glutaraldehyde in 100 mM cacodylate buffer, pH 7.4, at 4°C overnight. After washing in PBS, specimens were postfixed in 0.5% (v/v) osmium tetroxide and 1% (w/v) potassium hexacyanoferrate (III) in 0.1 M cacodylate buffer for 2 hours at 4°C followed by washing with distilled water. After dehydration in an ascending ethanol series from 30% to 100% ethanol, specimens were two times incubated in propylenoxide each for 15 minutes. The cells were detached from the surface of the cell culture plate during the propylenoxide treatment. Finally, detached cells were embedded in epon using beem capsules. Ultrathin sections were cut on an ultramicrotome and collected on copper grids. Electron micrographs were taken at a Phillips EM-410 electron microscope using imaging plates (Ditabis, Pforzheim, Germany).
RNA Isolation and Quantitative Real-Time PCR
Total RNA isolation and quantitative real-time PCR (qRT-PCR) were performed as described previously.20,21 We used the Gen Elute Mammalian total RNA Miniprep Kit (Sigma-Aldrich) and Superscript III Reverse Transcriptase Kit (Invitrogen) according to the manufacturer's instructions. RT-qPCR was based on SYBR Green dye (Applied Biosystems, Foster City, CA) and was performed by the Core Facility Genomics (Medical Faculty, University Hospital Muenster, Germany) using CFX384 Touch (Bio-Rad, Hercules, CA). Relative gene expressions were normalized to glyceraldehyde 3-phosphate dehydrogenase and compared using the comparative ΔCT method.25 Used RT-qPCR primers are listed in Supplemental Table 1.
Cell Culture and Generation of Stable Cell Lines
Human immortalized podocytes (AB8/13) were cultivated as described earlier.20,26,27 For targeting the endogenous Rab7 gene in AB8 cells by CRISPR/Cas9, we used the pSpCas9(BB)-2A-Puro (PX459) V2.0 provided by Feng Zhang (Addgene plasmid #62988) with DNA encoding for the guide RNA in Exon 3 of the Rab7 sequence.28
Generation of AB8 Cell Lines Depleted for Rab7 by CRISPR/Cas9
Guide RNAs were ligated in a PCR following the conditions of Supplemental Table 3. The PCR run for 1 hour at 37°C followed by 5 minutes at 95°C and 10 minutes at 56°C for 70 cycles. Afterward, the PCR product and 50 ng of pSpCas9(BB)-2A-Puro (PX459) plasmid were digested with the restriction enzyme BbSI. A second PCR was run as described in Supplemental Table 4. Subsequently, E.coli DH5α bacteria were transformed with the construct for duplication. The construct was isolated with the PureLink Hi Pure Plasmid Filter Maxiprep Kit provided by Invitrogen. AB8 cells were transfected with Lipofectamine 2000 (Invitrogen) following the provider's protocol. Selection was done by puromycin (2 µg/ml) resistance. To confirm modification of the Rab7 gene on a genomic level, gDNA was isolated and sequenced with human Rab7 primers (Supplemental Table 1). Rab7 absence on the protein level was confirmed by using Western blot (WB) analysis.
Preparation of Cell and Kidney Lysates
Lysates were prepared as described before.20 In brief, cells were lysed in an appropriate volume of radioimmunoprecipitation assay buffer on ice (150 mM NaCl, 50 mM Tris-pH 8.0, 1% NP-40, 1% TritonX-100, 0.1% SDS, 0.5% Na-deoxycholate; supplemented with complete protease tablets [Roche] and phosphatase inhibitor cocktails [Sigma-Aldrich]). Cells were scratched off the plate into a reaction tube, which was vortexed every 5 minutes within 30 minutes. After incubation for 10 minutes in an ultrasonic bath, lysates were centrifuged 15 minutes at 14,000 rpm at 4°C. The supernatant was mixed with 2×Laemmli buffer before immunoblotting.
Kidney lysates were prepared by adding 150 µl radioimmunoprecipitation assay buffer on ice (150 mM NaCl, 50 mM Tris-pH 8.0, 1% NP-40, 1% TritonX-100, 0.1% SDS, 0.5% Na-deoxycholate; supplemented with complete protease tablets [Roche] and phosphatase inhibitor cocktails [Sigma-Aldrich]). After incubation for 20 min on ice, lysates were mechanically dissociated by a mortar (dstroy-s-15, Biozym). Lysates were centrifuged for 10 minutes at 14,000 rpm at 4°C. The supernatant was mixed with 2×Laemmli buffer before immunoblotting.
WB Analysis
WB analysis was performed as previously described.29,30 In brief, cell lysates were boiled for 5 minutes at 95°C, and equal volumes were separated through SDS-PAGE using 6%–10% gels (Bio-Rad System). Afterward, proteins were transferred to a polyvinylidene difluoride membrane (EMD Millipore) and incubated in blocking buffer (5% skim milk powder dissolved in tris-buffered saline (TBS) containing 0.05% Tween-20 [TBS-T]) for 1 hour at RT. Primary antibodies were diluted (Supplemental Table 2) in TBS-T with 5% BSA and incubated at 4°C overnight. Next, the membrane was washed three times with TBS-T and incubated with horseradish peroxidase–coupled secondary antibodies (Jackson Immunoresearch, Supplemental Table 2) diluted 1:1000 in blocking buffer for 45 minutes at RT. After three further wash steps with TBS-T, the signal was detected using a chemiluminescence detection reagent (Clarity, Bio-Rad) in an Azure biosystems imager (c600, BioRad).
Immunofluorescence Analysis of Cells
Cells were seeded and grown to 70% confluence. On fixation day, cells were washed three times with ice cold 1× PBS and fixed with 4% PFA for 20 minutes, then again washed three times with PBS, and quenched for 10 minutes with quenching solution (1× PBS, 100 mM NH4Cl). Afterward, cells were blocked for 60 min with blocking solution (1× PBS, 0.2% gelatin, 10% normal goat serum). Primary antibodies were diluted in antibody solution (1× PBS, 0.2% gelatin, 2% normal goat serum) over night at RT. After washing for 3 times with wash buffer (1× PBS, 0.2% gelatin), the secondary antibodies were diluted in antibody solution and applied for 30 minutes. Cells were mounted in Fluoromount (SigmaAldrich) and imaged with an Observer Z1 microscope with Apotome 2.0 (ZEISS) with a 63×1.4 oil immersion objective. Fiji and Zen Software were used to analyze images.
Live Cell Imaging of Cells
The live cell dye LysoTracker Green DND-26 (Thermo Fisher) was used to label acidic compartments within the cells. It was added to plated cells following the provider's protocol with a final concentration of 50 nM for 1.5 hours at 33°C.
Cells were washed twice with PBS and imaged in HBSS containing 30 mM HEPES by using an Observer Z1 microscope equipped with an Apotome 2.0, an Axiocam MRm camera (Zeiss), and Plan Apochromat 63×/1.40 Oil, or EC Plan-Neofluar 40×/1.30 Oil objectives. Images were processed with Fiji or Zen software.
Determination and Quantification of Lysosomal pH Values
To determine pH values inside the lumen of endosomes and lysosomes, a method established by Canton and Grinstein was adapted.31,32 Cells were grown in Ibidi 8-well chambers (Ibidi #80826), and OG-dextrane and TMR-dextran (both 10 kDa, Thermo Fisher) were added for 2 hours at 37°C (pulse). Cells were incubated at 37°C for another 2 hours in normal growth medium (chase) and were imaged with two Z-stack images from bottom to top (LSM780 equipped with a 63×/1.4 oil immersion objective, ZEISS). To determine the lysosomal pH, a standard curve was measured with standard pH solutions as previously described by Johnson et al.33 The signal intensity of OG-dextran and TMR-dextran was analyzed by using Fiji. The intensity ratio of OG-dextran to TMR-dextran was calculated and compared with the values of the standard curve.
GFP Uptake and LysoTracker Assays of Drosophila Nephrocytes
All fly lines were kept on standard food and under standard housing conditions. GFP uptake was measured as described previously.34,35 In brief, flies of the genotype UAS::Dcr; sns-Gal4, ubi::ANF-GFP-GFP/CyO were crossed with UAS::dsRNA strains (Rab7 VDRC #40337 (dsRNA1) and #40338 (sdRNA2) or control dsRNA targeting or83b #100825 which is an olfactory receptor that is not expressed in nephrocytes) at 25°C and transferred to 29°C 48 hours after egg laying. Garland cell nephrocytes of L3 larvae were dissected and stained with wheat germ agglutinin coupled to Alexa555 (Invitrogen) and 4’,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Images of nephrocytes were taken with a Zeiss LSM700 confocal microscope. The mean value of ANF-GFP-GFP fluorescence per nephrocyte area was measured with ImageJ. The unpaired, two-tailed Mann–Whitney test was applied. The results are presented as mean fluorescence/µm2 cell area normalized to control condition. At least five animals and five nephrocytes per animal were evaluated for each of four separate experiments. For evaluating the ANF-GFP-GFP signal together with LysoTracker staining, unfixed nephrocytes of the genotype detailed above were incubated with 5 mM LysoTracker Red (Invitrogen) for 5 minutes, washed with ice cold PBS, and imaged immediately.
Additional Immunofluorescence Analysis of Drosophila Nephrocytes
Garland cell nephrocytes of wandering third instar larvae were fixed in 4% PFA for 15 minutes, incubated in primary antibody at 4°C overnight, followed by washes in PBS-T. Secondary antibody was applied at room temperature, and samples were mounted in Mowiol. Anti-Sns (custom generated34,36) and anti-Pyd (Pyd2 from Developmental Studies Hybridoma Bank, USA) were used as primary antibodies. Anti-Sns antibodies were diluted 1:100; anti-Pyd antibodies were diluted 1:20 in PBS-T. Secondary antibodies and 4’,6-diamidino-2-phenylindole (DAPI) were from Invitrogen and diluted 1:1000 in 10% goat serum in PBS-T. Tangential and surface section images of the nephrocytes were obtained using a confocal microscope (Leica SP8 or Zeiss LSM700). Quantification of SDs visualized across 1 μm was performed manually. Slit diaphragms were quantified in more than 50 nephrocytes from seven different animals for each of three independent crosses per genotype. Slit diaphragms were counted perpendicularly across 5-µm cell membrane in five different, random areas of each cell.
TEM of Drosophila Nephrocytes
Wandering third instar larvae were dissected, and garland cell nephrocytes were prepared and fixed in 2.5% glutaraldehyde in Sørensen buffer overnight. Osmium tetroxide (1%) in Sørensen buffer was applied to nephrocytes for 1 hour, and samples were washed before and after application with Sørensen buffer. Samples were then dehydrated in an ascending alcohol series, infiltrated with epon using a series of mixtures of epon, intermedium propylene oxide, and pure epon. Polymerization was performed at 60°C for 36 hours; samples were cut in ultra-thin slices of 60 nm and contrasted with uranyl acetate for 20 minutes and lead citrate for 90 seconds. Images were taken with a transmission electron microscope (Phillips CM10 equipped with TVIPS CAM F416).
Statistical Analysis
Tests for statistical significance of normally distributed data were performed using GraphPad PRISM software with an unpaired two-tailed t test for comparison of two groups of data or one/two-way ANOVA with a Bonferroni correction for comparing three or more groups of data. If not otherwise indicated, all data are given as mean of at least three independent measurements (*P < 0.05, **P < 0.01, and ***P < 0.001).
Figure Illustrations
Parts of the figures were drawn by using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Results
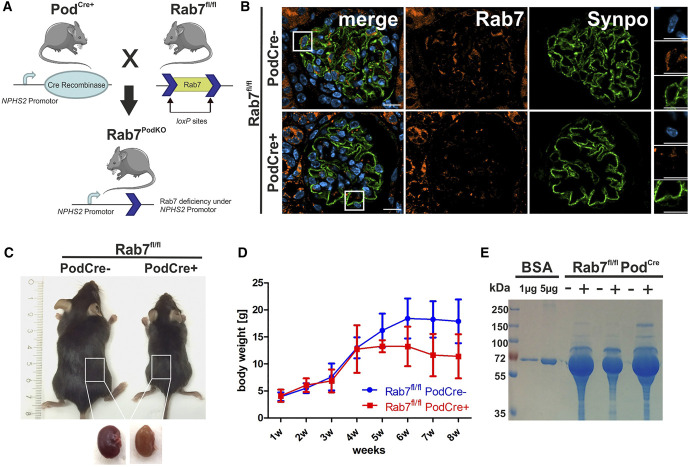
To investigate the function of Rab7 in podocytes in vivo, Rab7 was deleted specifically in podocytes by breeding a conditional Rab7 knockout mouse line with a Cre-driver line as described earlier.17,18 This resulted in Rab7fl/fl;PodCre+ mice (Figure 1A). Homozygous inactivation of the Rab7 gene led to loss of Rab7 in podocytes as shown by immunofluorescence staining (IF) of glomeruli from Rab7fl/fl;PodCre+ mice (Figure 1B). Rab7-depleted mice showed a severe phenotype. During the first 8 weeks, mice developed proteinuria and pathogenic alterations of the filtration barrier. We examined three time points: 2–3 week-old, 4-week-old, and 8-week-old mice. Heterozygous Rab7fl/+;PodCre+ mice did not exhibit an obvious phenotype.
Figure 1.
Loss of Rab7 in podocytes results in proteinuria. (A) Mating scheme of a conditional Rab7 knockout mouse (Rab7fl/fl) with the NPHS2-Cre line (PodCre+) that expresses Cre under the control of human podocin (NPHS2) promoter. (B) Confirmation of Rab7 depletion in the murine kidneys by immunofluorescence staining of the glomerulus with anti-Rab7 and anti-Synaptopodin (Synpo) antibodies on paraffin sections. Scale bar: 10 µm. (C) Representative image of Rab7fl/fl; PodCre+ and littermate control mice (Rab7fl/fl; PodCre−) eight weeks after birth. Kidneys of Rab7-deficient mice appear paler. (D) Body weight measurements in wildtype (Rab7fl/fl; PodCre−) and Rab7fl/fl; PodCre+ mice. N=3, P = 0.0032 (two-way ANOVA). (E) Eight-week-old Rab7fl/fl;PodCre+ mice were evaluated for proteinuria by Coomassie staining of spontaneous urine. Figure 1 can be viewed in color online at www.jasn.org.
Rab7fl/fl;PodCre+ mice were born at expected Mendelian frequencies. In comparison with their Cre-negative littermate controls, 8-week-old Rab7fl/fl;PodCre+ mice were significantly smaller in size and weight, and extracted kidneys appeared bloodless (Figure 1, C and D). In addition, Rab7fl/fl;PodCre+ showed severe proteinuria at age 8 weeks, suggesting an impairment of the GFB integrity (Figure 1E). Proteinuria and weight loss were sex-independent and started at 4 weeks after birth (Supplemental Figure 1A). For further investigation, we measured renal injury and inflammatory markers. We observed an upregulation of the mRNA expression levels of the inflammatory marker Ccl2 and the renal injury markers Kim1 (Havcr1), Lcn2, and Serpin1 (Supplemental Figure 1B).
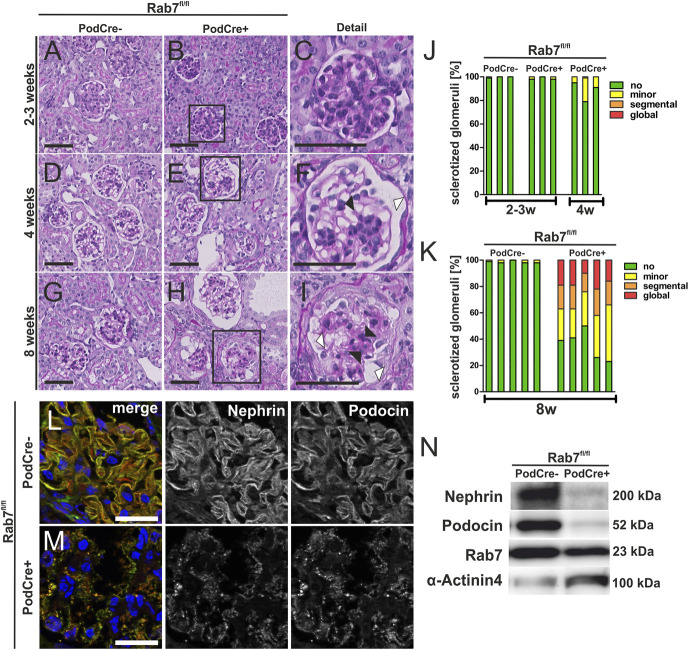
We did not observe proteinuria and histological alterations in 2–3-week-old control and Rab7fl/fl;PodCre+ mice (Figure 2, A–C and J). However, at age 4 weeks, Rab7fl/fl;PodCre+ mice exhibited a beginning FSGS-like phenotype with initial signs of glomerulosclerosis (black arrowheads, Figure 2, D–F and J) and dilated Bowman space (white arrowheads, Figure 2, D–F). In addition, 8-week-old mice further showed a more severe phenotype with globally sclerotic glomeruli, indicating a progressive aggravation of the phenotype over time (Figure 2, G–I and K). In addition, 8-week-old Rab7-depleted mice showed mild interstitial fibrosis (Supplemental Figure 2A, Supplemental Table 5) and moderate proteinaceous casts (Supplemental Figure 2B, Supplemental Table 5). We also detected podocyte depletion in relation to the progressive glomerulosclerosis as demonstrated in IF and PAS staining (Supplemental Figure 2C and D).
Figure 2.
Rab7 depletion of podocytes leads to FSGS. (A–I) PAS staining of Rab7fl/fl;PodCre+ mice at age 2–3, 4, and 8 weeks. Black arrowheads mark sclerosis of the glomerulus and Bowman capsule; white arrowheads mark the dilated Bowman space, scale bar: 50 nm. (J and K) Glomerulosclerosis score of 2–3-week-old and 4-week-old (J) and 8-week-old mice (K) (data are shown in %). Thresholds of sclerotization were defined as following: no alteration; minor, mesangial expansion and/or thickening of the basement membrane without sclerosis; segmental, ≤50%; and global, >50% tuft area of each glomerulus affected. Three (J) or five (K) animals per group and >50 glomeruli per animal were analyzed. An unpaired test with Welch correction was performed for 8-week-old control versus knock out (KO) mice with a significance of P = 0.0002. (L/M) Immunofluorescence staining of the SD proteins nephrin and podocin at age 4 weeks, scale bar: 50 µm. (N) Western blot analysis of glomerular lysates for the slit diaphragm proteins nephrin and podocin in 4-week-old mice show decreased nephrin and podocin levels. Rab7 is still detectable since Rab7 is only depleted in podocytes, and whole glomerular lysates were used. KO, knock out; SD, slit diaphragm. Figure 2 can be viewed in color online at www.jasn.org.
To further study the destruction of the glomeruli in the early stage, we evaluated the SD proteins nephrin and podocin by IF analyses in 4-week-old mice. We observed a reduced expression and an accumulation of nephrin and podocin in punctate granular structures, either representing the remaining signal for both proteins at the filtration barrier or internalized nephrin and podocin into the podocyte, indicating reduction or redistribution of SD structures (Figure 2, L and M). In addition, WB analysis confirmed severely reduced expression of nephrin and podocin in Rab7-depleted glomeruli (Figure 2N).
To determine early changes in podocyte morphology, we performed ultrastructural analysis at all three time points. Podocytes of 2–3-week-old Rab7fl/fl;PodCre+ mice, which were not proteinuric, did not show changes in the SD and FPE (Figure 3, A and B, Supplemental Figure 3, A and B). First signs of podocyte injury were detected at 4 weeks after birth and included partially disrupted SD and FPE (Figure 3, C and D, white arrowhead, Supplemental Figure 3, C and D). By 8 weeks, the morphology of glomeruli in Rab7fl/fl;PodCre+ mice was significantly altered, including extensive FPE (Figure 3, E–H). We did not perceive pathological changes in the fenestrated endothelium (Figure 3, D and F). Interestingly, 2–3-week-old Rab7fl/fl;PodCre+ mice exhibited accumulating electron-dense structures (Figure 3, I–K). We distinguished two main types of accumulating vesicles: double membrane-bound small vesicles (Figure 3 L+M, black arrowheads) and large vesicles bounded by a single membrane. The latter contained intraluminal vesicles similar to those found in multivesicular bodies (MVBs) (Figure 3 L+N, white arrowheads). For that reason, we named them unprocessed late endosomal-like vesicles (ULLVs). ULLVs represented most intraluminal accumulating vesicles and were not located to a specific cytoplasmic region but spread throughout the podocyte body and foot processes. ULLVs were also detected in 8-week-old mice and were similar in appearance and localization within the podocyte body (Supplemental Figure 3, E–G). The data indicate that ULLVs appear before morphological damage to the slit membrane and that proteinuria occurs at 4 weeks after birth concurrent with morphological slit membrane damage.
Figure 3.
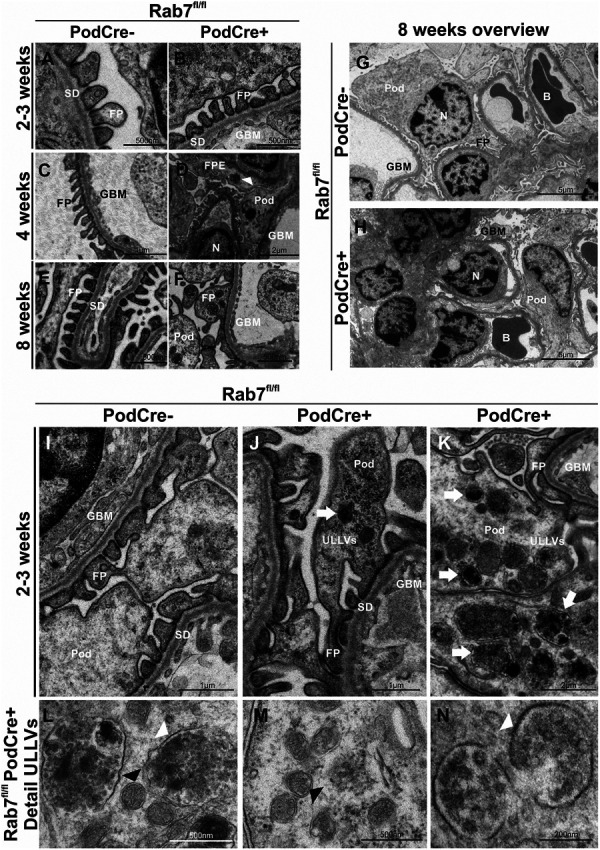
Rab7 KO mice podocytes accumulate unprocessed late endosome-like vesicles (ULLVSs). (A–F) Ultrastructural analyses (TEM close up) of 2–3-week-old, 4-week-old, and 8-week-old mice with a focus on FP and SD; 2–3-week-old Rab7fl/fl;PodCre+ mice seem healthy. Four-week-old Rab7fl/fl; PodCre+ mice show first signs of FPE and a slightly thickened GBM (white arrow). Eight-week-old mice show FPE and SD disruption. (G/H) TEM analyses of glomeruli of 8-week-old mice. Rab7-depleted podocytes show disrupted SDs and FPE. (I–K) TEM analyses of 2–3-week-old mice with a focus on accumulating vesicles within the podocyte (white arrow), named ULLVs (unprocessed late endosome-like vesicles). (L–N) TEM analyses: Detailed view on accumulating vesicles; accumulating double membrane structures are marked with black arrowheads, large single membrane structures with white arrowheads. B, blood vessel; FP, foot process; FPE, foot process effacement; GBM, glomerular basement membrane; N, nucleus; Pod, podocyte body; SD, slit diaphragm; TEM, Transmission Electron Microscopy; ULLVs, unprocessed late endosome-like vesicles.
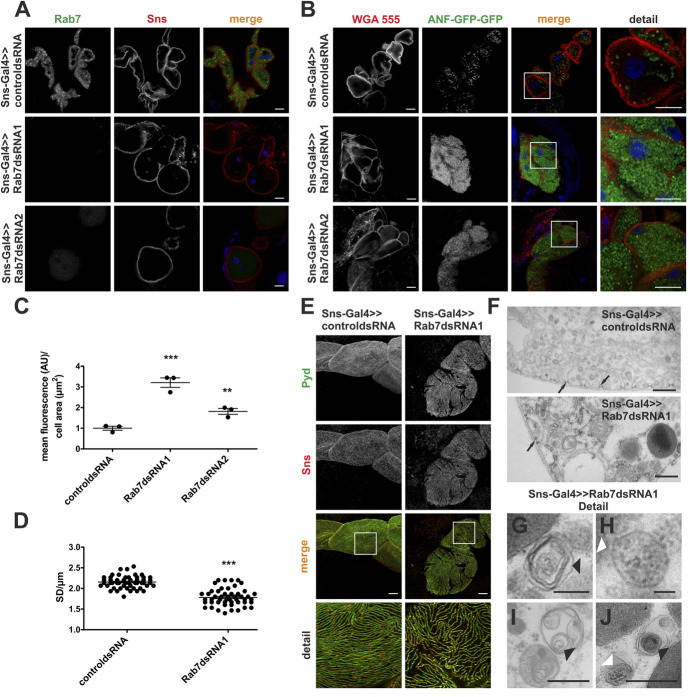
Rab7 is highly conserved from metazoans and yeast to mammals.37–40 To compare the effect of Rab7 depletion among different species and to investigate Rab7 function in a less complex renal system, we chose Drosophila as a second model organism. Filtration in Drosophila takes place in nephrocytes which enlarge their surface by invaginations.41–43 SD-like structures are built between those invaginations and comprised orthologs of the major mammalian SD proteins such as nephrin (in Drosophila, sticks-and-stones, Sns), ZO-1/2 (in Drosophila Pyd), and podocin (in Drosophila Mec2). Thus, the Drosophila nephrocyte is a genetically tractable in vivo SD model with short generation times. We generated two Rab7 knockdowns (KD) in Drosophila using RNA interference (Figure 4A). We then used an assay that evaluates protein filtration and uptake into nephrocytes, larvae ubiquitously expressing atrial natriuretic factor (ANF) fused to two GFP. ANF-GFP-GFP (MW of approximately 70 kDa) is secreted into the endolymph, filtered across the nephrocyte SD, and is then taken up by endocytosis.34,35 Thus, ANF-GFP-GFP uptake into nephrocytes is a surrogate of filtration and endocytic processes. For this experiment, we generated nephrocyte-specific Rab7 KDs with two different dsRNA on the ANF-GFP-GFP–expressing background using the UAS-Gal4 system by driving Gal4 expression with the Sns promoter (Sns>>Gal4). We observed a significant increase in ANF-GFP-GFP–containing vesicles within nephrocytes for both Rab7 KDs (Figure 4, B and C).
Figure 4.
Rab7 depletion in Drosophila nephrocytes leads to accumulating vesicles and a reduced SD number during larval development. (A) Immunofluorescence analysis of control (dsRNA for or83b) or Rab7 KD nephrocytes are shown (Rab7 dsRNA1 and 2). KD in nephrocytes was achieved by using Sns-Gal4. Nephrocytes were prepared, and immunofluorescence analysis was performed with antibodies specific for Rab7 (green) and Sns (red). The staining proves Rab7 KD. Scale bar represents 10 µm, N=3. (B) Uptake of secreted ANF-GFP-GFP into nephrocytes of prepared control (dsRNA for or83b) or Rab7 KD nephrocytes (dsRNA1 and 2) showing accumulation of ANF-GFP-GFP in Rab7 KD nephrocytes. KD in nephrocytes was achieved by using Sns-Gal4. Samples were stained with WGA-Alexa555 to visualize membranes. Merged images and zoom-in images are shown in the lower panel. Scale bar: 10 µm. (C) Accumulation of ANF-GFP-GFP in nephrocytes was statistically evaluated. Shown are means and SEM in arbitrary units per µm2 cell area. **P < 0.01, ***P < 0.001 by the unpaired two-tailed Mann–Whitney test, N=3. (D) Quantification of the number of SD per micrometer (SD/µm) from immunofluorescence analyses of surface sections of nephrocytes. Shown are the SD/µm from 3 independent experiments. ***P < 0.001 by the unpaired two-tailed Mann–Whitney test. (E) Immunofluorescence analysis of surface sections of control (Sns-Gal4>>control dsRNA, dsRNA for or83b) or Rab7 KD nephrocytes (Sns-Gal4>>Rab7 dsRNA) showing reduced SDs in Rab7 KD nephrocytes. KD in nephrocytes was achieved by using Sns-Gal4 and Rab7 dsRNA1. Nephrocytes of wandering third instar larvae were dissected, and immunofluorescence analysis was performed with antibodies specific for Pyd (green) and Sns (red). Merged images and higher magnifications of the marked area (detail) are shown to the right. Scale bars: 10 µm. (F) Ultrastructural analyses of dissected control and Rab7 KD nephrocytes. KD in nephrocytes was accomplished by using Sns-Gal4. Black arrows indicate SDs. Scale bars: 50 nm, N=3. (G–J) Zoom-in of different types of accumulating vesicles, such as single-membrane vesicles filled with membrane layers (indicated by black arrows) or single-membrane vesicles consisting of intraluminal vesicles (indicated by white arrows) detected in the ultrastructural analysis of Rab7 KD nephrocytes. Scale bar: 50 nm. KD, knockdowns; SD, slit diaphragm; WGA, wheat germ agglutinin. Figure 4 can be viewed in color online at www.jasn.org.
Given that Rab7 depletion disrupted SD structures in mice, we evaluated nephrocyte foot processes and SD frequency in Drosophila by analyzing confocal sections of nephrocytes stained with antibodies specific for the SD markers Sns and Pyd. This showed that Rab7 KD decreased SD frequency compared with Drosophila expressing a control dsRNA (Figure 4, D and E). To define the accumulating vesicles, we stained acidic compartments of prepared nephrocytes with LysoTracker Red in ANF-GFP-GFP expressing Drosophila with Rab7 KD or Drosophila expressing a control dsRNA (Supplemental Figure 4). ANF-GFP-GFP accumulated in both LysoTracker-positive and LysoTracker-negative compartments in Rab7 KD nephrocytes. ANF-positive structures only partially overlapped with LysoTracker-positive structures in the Rab7-depleted nephrocytes, indicating a maturation or fusion error in the endo-lysosomal pathway. To further characterize accumulating vesicles in Drosophila in Rab7 KD nephrocytes, we performed an ultrastructural analysis (Figure 4, F–J). We again observed two types of accumulating structures: vesicles surrounded by a single membrane containing multiple membrane layers (Figure 4, G, I, and J, black arrowheads) and large vesicles bound by a single membrane containing electron-dense structures, such as MVB (Figure 4, H and J, white arrowheads). Most accumulating structures were vesicles and comprised membrane layers (Figure 4, G, I, and J, black arrowheads).
To study the mechanisms of podocyte injury in vitro, we generated three independent Rab7-depleted human podocyte cultures by targeting the third exon of the human RAB7 gene with CRISPR/Cas9. In clones 1 and 2, a frame-shift mutation resulted in a premature stop codon in exon 3 and nonsense-mediated decay of the Rab7 protein (Supplemental Figure 5A). An in-frame deletion of nine nucleotides resulted in the loss of three amino acids in clone 3. Consistent with this sequencing data, we did not detect Rab7 in clone 1 or 2 in WB analysis, whereas clone 3 still expressed Rab7 (Supplemental Figure 5B). However, we observed similar deficiencies in the lysosomal degradation pathway in all three Rab7 CRISPR/Cas9–modified cell lines.
Ultrastructural analysis of RAB7 KO human podocytes revealed two major types of accumulating vesicles. We again observed that vesicles comprised a single membrane with intraluminal vesicles containing electron-dense punctate granular structures (Figure 5, A and B, black arrows). Furthermore, we found smaller vesicles containing membrane layers and resembling structures that have previously been described as endolysosomes44 (Figure 5B, white arrows). Both types of vesicles were spread throughout the cytosol and were not localized to a specific cellular region. Occasionally, we found double membrane-bounded structures within electron-dense compartments (Figure 5B, white arrowheads).
Figure 5.
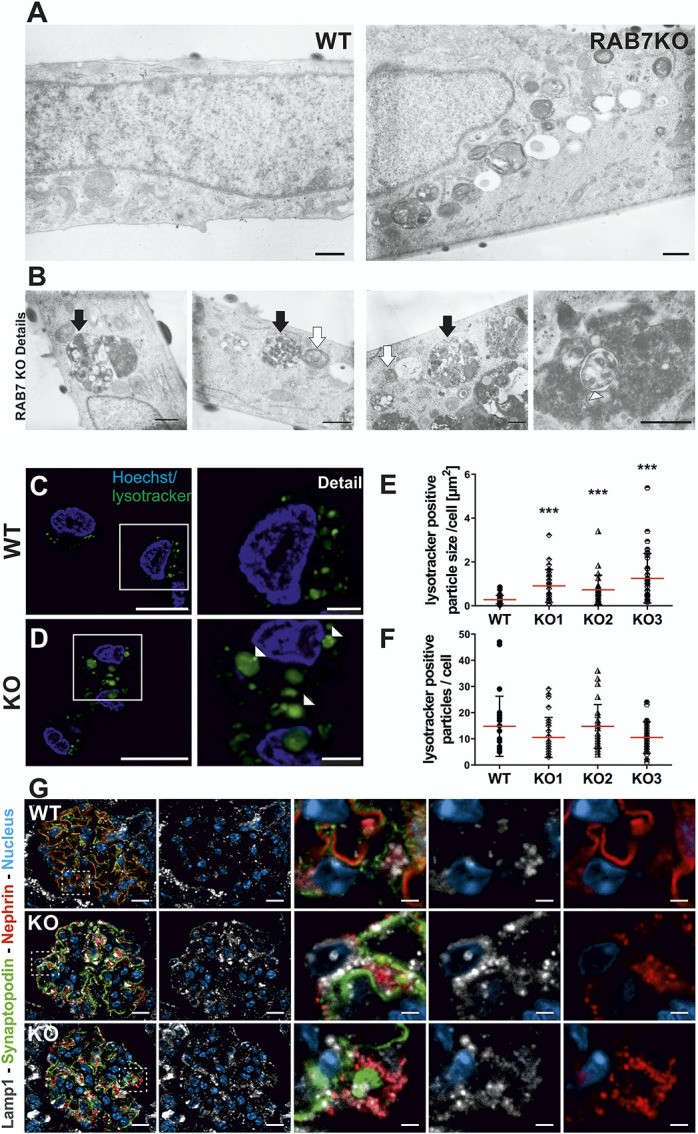
Rab7 depletion in podocytes results in accumulation of enlarged LAMP2-positive vesicles. (A) Ultrastructural analysis of Rab7-depleted podocytes shows different electron-dense structures accumulating compared with the wildtype (overview). Scale bar: 500 nm. (B) Detailed ultrastructural analysis of Rab7-depleted podocytes focusing on three accumulating vesicle types: black arrow, large single-membrane vesicle containing multiple intraluminal vesicles; white arrow, smaller, single-membrane vesicles containing membrane layers, and occasionally found double-membrane vesicles (white arrowhead). Scale bar: 500 nm. (C/D) Live cell staining with LysoTracker green revealed enlarged acidic compartments in Rab7-depleted cells (white arrowheads). (E/F) Quantification of the size and number of LysoTracker-positive particles in Rab7-depleted cell lines. ***P < 0.0001 by the unpaired two-tailed Mann–Whitney test, shown are mean with SD; cells analyzed: wildtype (wt): N=112, Rab7 knock out [knock out]: N=84. (G) Immunofluorescence analyses with control (wild type [WT], Rab7fl/fl; PodCre−) and Rab7fl/fl; PodCre+ (KO) mice. Images show representative IF-staining against Lamp1 (white) in glomeruli of WT and KO animals at 8 weeks. Nephrin (red), synaptopodin (green), and nuclei (Dapi, blue) were stained as comarkers. Nephrin-labeling shows a vesicular-like pattern and translocation toward the podocyte cell body apical of synaptopodin in KO animals. Synaptopodin shows intact localization to the basolateral podocyte compartment. Analysis of Lamp1 revealed enrichment of Lamp1-positive vesicles in the podocyte compartment of KO animals and colabeling of most but not all nephrin-positive structures. Interestingly, a subset of Lamp1-positive vesicles with perinuclear localization is negative for nephrin. Scale bar: overview=10 µm, details=2 µm. SD, slit diaphragm; WT, wild type. Figure 5 can be viewed in color online at www.jasn.org.
LysoTracker green staining of late endosomal and lysosomal vesicles revealed enlarged vesicles in all three clones compared with the control cell line (Figure 5, C–E, Supplemental Figure 5C), while the number of LysoTracker green particles per cell was not altered (Figure 5F). IF staining with an antibody against the lysosomal glycoprotein LAMP2 revealed enlarged LAMP2-positive vacuoles in cell lines with Rab7 editing (Supplemental Figure 5D).
Rab7 interacts with Rab interacting lysosomal protein which in turn stabilizes V1G1, a subunit of vacuolar-type ATPase (V-ATPase).45–47 We, therefore, hypothesized that acidification might be altered in Rab7-depleted cells. We used a method established by Canton and Grinstein31 to measure the pH of late endo-lysosomal compartments and detected alterations of the endo-lysosomal pH for Rab7-depleted cells. For cell line 1, lysosomal vesicles were more acidic on average than the control (Supplemental Figure 5E). On the other hand, lysosomal vesicles of cell line 2 showed no differences, and cell line 3 were more alkaline. Therefore, we conclude that Rab7 depletion disrupts the fine-tuning of intraluminal acidification, leading to variably increased or decreased pH levels in late endosomal/lysosomal vesicles.
We analyzed LAMP2, a highly glycosylated transmembrane protein of late endosomes and lysosomes and Rab5, a marker of early endosomes, by WB to detect possible changes in the endocytic pathway. Rab5 protein levels were slightly increased in the edited cell lines that did not express Rab7 (clones 1 and 2) but not in mutant 3 (Supplemental Figure 5, F–H). LAMP2 was not significantly regulated.
Because Rab7 is known to influence autophagic processes as well as the endocytic pathway,48 we also evaluated the autophagosome cargo protein, ubiquitin-binding protein, p62/SQSTM1, and LC3 by WB analysis (Supplemental Figure 5, I–L). P62 and the LC3II/I ratio were not significantly altered by loss of Rab7, indicating that autophagy is less affected in cultured human podocytes.
The accumulation of similar vesicles was the most prominent common feature of Rab7 depletion in three independent model systems. The vesicles in cultured podocytes likely represented unprocessed late endosomes, as we detected enlarged LysoTracker/LAMP2-positive vesicles, while the autophagic pathway was not significantly regulated. To determine whether nephrin accumulation in mice may be caused by trafficking disorders in the late endosome or autophagic pathway, we additionally performed a Lamp1/nephrin (Figure 5G) and p62/nephrin (Supplemental Figure 6) co-IF staining. This showed that Lamp1 was upregulated. Many but not all nephrin-positive structures colocalized with Lamp1, whereas p62 colocalized less with nephrin than Lamp1.
In summary, Rab7 depletion led to an accumulation of vesicles in three different model systems. Most accumulating vesicles in mice and human podocytes were large vesicles bounded by a single membrane containing intraluminal vesicles that we refer to as ULLVs. For Drosophila, we mostly found single-membraned vesicles containing membrane layers. In both mice and flies, the loss of Rab7 severely disrupted the SD structures.
Discussion
So far, little is known about the cell-specific and tissue-specific functions of Rab7. A complete knockout of Rab7 in mice is embryonically lethal by E7-8 accompanied by small endosome-like vesicles and isolated large apical vacuoles in the endoderm.49 Rab7 is a widely expressed gene expected to have similar functions in many cell types. Interestingly, it appears to mediate tissue-specific effects. For example, Rab7 appears to be dispensable for pancreatic development, as pancreas-specific knockout of Rab7 did not affect mouse development and did not lead to histological changes, although endosomal, autophagosomal, and lysosomal functions were disrupted.50
In humans, Rab7 has so far only been described in the context of autosomal dominant Charcot–Marie–Tooth diseases (CMT2B subtype).51 CMT2B particularly manifests with neurological phenotypes because of the defects in neurite outgrowth.52–54
Interestingly, mutations in some genes, such as INF2, can cause Charcot‐Marie-Tooth subtypes that involve podocytopathy in conjunction with neurological defects.55–57 However, this association has not yet been shown for Rab7 variants.
Our approach with Rab7 KO mice provides strong evidence that Rab7-dependent processes, such as endo-lysosomal maturation and autophagy, are of major importance for podocyte function. Remarkably, disruption of autophagy in podocytes by Atg5 depletion does not lead to a developmental phenotype in mice, while knockout of Rab7 results in early proteinuria and FSGS.15 This suggests that the common end route of endocytic and autophagic pathways is more important than the initiating steps of these pathways for podocyte survival.
Cell biologic studies by Yu et al. elucidate that the termination of autophagy as well as the autophagic lysosomal reformation from autolysosomes are controlled by mammalian target of rapamycin (mTOR) kinase.58,59
Interestingly, mTOR-dependent autophagic lysosome reformation (ALR) depends on Rab7, as ALR triggered by a re-activation of mTOR, requires the release of Rab7 from these compartments.60–63 In this context, it is interesting that the phenotype of mice lacking mTOR kinase in podocytes (MtorPodKO) is similar to the phenotype of mice lacking Rab7, suggesting that the mTOR-Rab7 axis is particularly important for podocytes. In support of this, Munson et al. observed that mTOR phosphorylates and thereby activates the VPS34–UVRAG complex to regulate ALR and cell survival under nutrient stress.64 Hence, the phosphoinositid-3-Kinase Vps34 could be an additional essential regulatory element for ALR. Therefore, it is tempting to speculate that the strong phenotype caused by the depletion of Vps34 in podocytes is not only due to an imbalanced endocytosis but also the result of disturbances of ALR.9,10,64,65 Our findings are consistent with this model.
We hypothesize that the large single membrane structures containing intraluminal vesicles that we detected and named ULLVs originate from late endosomes and are unable to mature and fuse with lysosomes due to the loss of Rab7 (Figure 6, A and B). In addition, we occasionally found double-membraned vesicles in mice that we assume to be autophagosomes or amphisomes (Figure 6B), although those vesicles occurred to a lesser extent than ULLVs.
Figure 6.
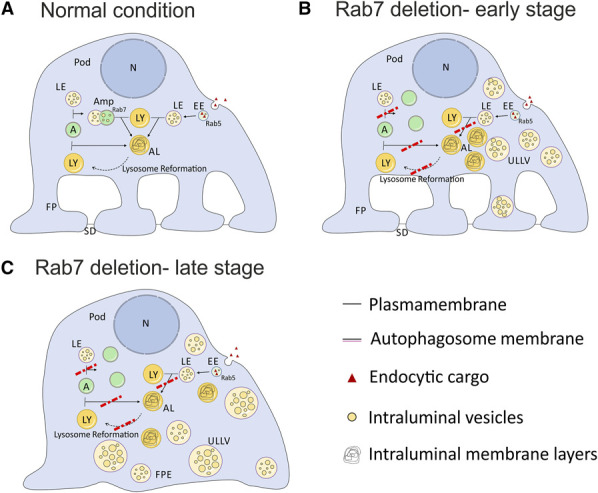
Schematic model on possible formation of unprocessed late endosomal-like vesicles and podocyte damage due to fusion and maturation defects in the autophagic and endo-lysosomal pathway. (A) Normal condition: Intracellular trafficking paths of the endocytic and autophagic pathway and their common end route—the lysosome. Late endosomes, autophagosomes, and amphisomes fuse with lysosomes and become autoendolysosomes. Autoendolysosomes are recycled by lysosome reformation. Podocyte is healthy and builds foot processes and SD. (B) Rab7 deletion early stage: Inhibited fusion of lysosomes with late endosomes leads to the accumulation of unprocessed late endosomal-like vesicles (ULLVs) as observed in mice and human podocytes. Autophagosomes do not fuse with lysosomes and, therefore, accumulate (double-membraned structures observed in mice). Lysosome maturation and reformation may be disturbed and result in an accumulation of autoendolysosomes, as especially seen in Drosophila but also in human podocytes. (C) Rab7 deletion late stage: The continuing accumulation of vesicles within the podocyte leads to the loss of foot processes and SDs due to (1) sterically hindrance in cell trafficking, (2) less recycled material/resources, and (3) the accumulation of toxic waste products from unprocessed vesicles. A, autophagosome; AL, autoendolysosome; Amp, amphisome; EE, early endosome; FP, foot process; FPE, foot process effacement; LE, late endosome; LY, lysosome; N, nucleus; Pod, podocyte; SD, slit diaphragm; ULLV, unprocessed late endosomal-like vesicle. Figure 6 can be viewed in color online at www.jasn.org.
The accumulation of both ULLVs and autophagosomes/amphisomes underlines the importance of Rab7 for endocytic pathways. However, common autophagy markers were not significantly altered by Rab7 depletion in cultured podocytes. While Rab7 appears to play a critical role in late endosome maturation and fusion as well as in lysosomal reformation, Rab7 depletion does not seem to cause obvious effects on autophagy.
Interestingly, we also find accumulating vesicles and defective SD-structures in Rab7-depleted nephrocytes in Drosophila. Thus, our data in the Drosophila nephrocyte model with Rab7 KD recapitulates the major results of defective SD and vesicle accumulation visible in our Rab7 knockout mice. Fu et al. previously performed a comprehensive functional analysis of Rab GTPases in nephrocytes, where they found that Rab7 KD in nephrocytes exhibited defective SDs and lacunae and an accumulation of large vacuoles by TEM.66 Most of the vesicles are bounded by a single membrane but contain multiple internal membrane layers, as previously described for autolysosomes.44 This might hint at a varying relevance of Rab7 in mammals and Drosophila considering the fine-tuning of the late endo-lysosomal pathway, as has been previously suggested.61 Overall, we can find many similarities between Rab7-depleted mice and Drosophila: both model organisms show accumulating vesicles that are consistent with a disruption of the late autoendolysosomal pathway. Accumulating ULLVs and autophagosomes/amphisomes in mice likely originate from disrupted maturation and fusion with lysosomes. Drosophila vesicles resemble autolysosomes and likely originate from inhibited depredatory processes and lysosome reformation.
We also detected a reduced SD frequency in nephrocytes and SD disruption in mice. We assume that the damage to the GFB is secondary to disruptions in late endocytic trafficking, as ULLVs were detected before the onset of proteinuria, glomerulosclerosis, and SD defects at 4 weeks after birth. The preceding appearance of ULLVs could cause the severe phenotype in mice by either (1) steric hindrance of cell traffic, (2) the accumulation of undegraded toxic waste products, or (3) limitation in substrate due to reduced recycling (Figure 6).
On a cellular level, the maturation of endo-lysosomal and autophagosomal compartments is directly linked to V-ATPase-dependent acidification, which is in turn connected to Rab7 through the Rab7-RILP-V-ATPase axis.45,47,67,68 Hence, the activation of lysosomal hydrolases for the degradation of lysosomal cargo may partly depend on Rab7. The podocyte-specific knockout of the V-ATPase subunit ATP6ap2 supports the central role of V-ATPase assembly for podocyte maintenance as ATP6ap2PodKO mice die early after birth and develop nephrotic syndrome within the first 3 weeks of life, accompanied by proteinuria, podocyte foot-process fusion, and cytoskeletal changes. Moreover, targeting the Atp6ap2 gene results in disturbed processing of late endosomal vesicles and lysosomal (Lamp2) markers, similar to the Rab7fl/fl;PodCre+ mice described in this study.69 However, our study shows that Rab7 depletion alters pH levels in both directions. Previous studies have shown that modulation of the lysosomal pH leads to degradation issues and pathological conditions.68,70–73 We assume that this eventually leads to the observed accumulation of unprocessed vesicles stuck in the late endosome, autophagosome, amphisome, or autoendolysosome stages (Figure 6).
Our data argue for the important role of lysosomes and lysosome-associated functions in podocytes and the importance of Rab7 in directly controlling late endosomal maturation, lysosomal fusion, and lysosomal reformation.
In postmitotic cells that develop complex asymmetric morphologies, including long and highly branched cell processes, lysosomes must travel long distances and, therefore, must be reformed or recycled locally. This might provide a partial explanation for why podocytes are particularly susceptible to disturbances of endo-lysosomal and autophagic processes.
Supplementary Material
Acknowledgments
We thank Truc Van Le, Karin Wacker, Karin Gäher, Alena Sammarco, and Antje Stöber for their excellent and longstanding technical support.
Footnotes
Kristin Vöing, Ulf Michgehl contributed equally (shared first authorship). Britta George, Thomas Weide, and Hermann Pavenstädt contributed equally (shared senior authorship).
K.V., U.M., B.G., T.W., and H.P. contributed equally to this work.
Disclosures
A.L. Edinger reports Consultancy: Alnylam Pharmaceuticals and Siege Pharmaceuticals; Ownership Interest: Siege Pharmaceuticals; Research Funding: Siege Pharmaceuticals; Patents or Royalties: patents issued and pending, unrelated to current publication; and Advisory or Leadership Role: Siege Pharmaceuticals Founder, President, BOD member (all unpaid). H. Pavenstädt reports Research Funding: Sanofi and Advisory or Leadership Role: Deutsche Forschungsgemeinschaft (DFG). B. Vollenbröker reports Employer: spouse: Klosterfrau Healthcare Group. All remaining authors have nothing to disclose.
Funding
This project was supported by the Deutsche Forschungsgemeinschaft (DFG), PA 483/18-1 to H. Pavenstädt. U. Rescher was supported by DFG, CRC 1348/A11; Interdisziplinäres Zentrum für Klinische Forschung (IZKF) Münster Re/022/20. N.D. Mertens and S. Waimann were supported by the Medical Faculty of the University of Muenster (Medizinerkolleg Münster, MedK Grant Nos. 20-0045 and 15-0048), T. Weide was supported by DFG (WE 2550/5-1), B. George was supported by DFG (GE 2158/3-3), and Innovative Medizinische Forschung Münster (GE112002). C. Schell was supported by the DFG (SCHE 2092/1-2, SCHE 2092/3-1, SCHE 2092/4-1 (RP9, CP2, CP3), project-IDs 241702976 and 438496892; CRU329, project-ID 386793560; SFB1453, project-ID 431984000; SFB1160, project-ID 256073931) and the MatrixCode research group by FRIAS, Freiburg.
Author Contributions
Conceptualization: Britta George, Ulf Michgehl, Hermann Pavenstaedt, Kristin Voeing, Thomas Weide.
Data curation: Kevin Gilhaus, Jonas Goretzko, Uwe Hansen, Mee-Ling Maywald, Nils Mertens, Ulf Michgehl, Cara Picciotto, Manuel Rogg, Christoph Schell, Kristin Voeing, Sofie Waimann.
Formal analysis: Kevin Gilhaus, Uwe Hansen, Manuel Rogg, Christoph Schell, Veerle Van Marck, Kristin Voeing.
Funding acquisition: Jürgen Klingauf, Hermann Pavenstaedt, Ursula Rescher, Beate Vollenbroeker.
Investigation: Kevin Gilhaus, Jonas Goretzko, Mee-Ling Maywald, Nils Mertens, Ulf Michgehl, Cara Picciotto, Manuel Rogg, Christoph Schell, Kristin Voeing, Sofie Waimann.
Methodology: Aimee Edinger, Britta George, Uwe Hansen, Jürgen Klingauf, Ursula Rescher, Manuel Rogg, Christoph Schell, Yaroslav Tsytsyura, Veerle Van Marck, Beate Vollenbroeker, Thomas Weide.
Resources: Aimee Edinger, Uwe Hansen, Jürgen Klingauf, Jürgen Klingauf, Veerle Van Marck.
Supervision: Daniela Braun, Britta George, Jürgen Klingauf, Ulf Michgehl, Hermann Pavenstaedt, Ursula Rescher, Thomas Weide.
Validation: Daniela Braun, Britta George, Kristin Voeing, Thomas Weide.
Writing – original draft: Ulf Michgehl, Hermann Pavenstaedt, Kristin Voeing, Thomas Weide.
Writing – review & editing: Daniela Braun, Aimee Edinger, Britta George, Jonas Goretzko, Mee-Ling Maywald, Nils Mertens, Hermann Pavenstaedt, Cara Picciotto, Ursula Rescher, Christoph Schell, Beate Vollenbroeker.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
Supplemental Table 1. Primers.
Supplemental Table 2. List of antibodies and imaging dyes used in this study.
Supplemental Table 3. Protocol for ligation of guide RNAs.
Supplemental Table 4. Protocol for digestion with BbSI for generating a CRISPR/Cas9 construct for Rab7 KO.
Supplemental Table 5. Average injury scores in Rab7-depleted 8-week-old mice.
Supplemental Figure 1. Rab7 depletion in podocytes in mice results in proteinuria and upregulation of renal injury markers.
Supplemental Figure 2. Rab7 depletion in podocytes in mice results in podocyte loss.
Supplemental Figure 3. Additional information: Rab7 KO mice podocytes accumulate unprocessed late endosome-like vesicles (ULLVS).
Supplemental Figure 4. Rab7 knockdown in Drosophila nephrocytes results in vesicle accumulation.
Supplemental Figure 5. Rab7 depletion in podocytes disrupts the fine-tuning of intraluminal acidification.
Supplemental Figure 6. Altered nephrin distribution in podocyte-specific Rab7-depleted mice only minor colocalizes with p62/Sqstm1-positive structures.
References
- 1.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002 [DOI] [PubMed] [Google Scholar]
- 2.Artelt N, Siegerist F, Ritter AM, Grisk O, Schlüter R, Endlich K. Comparative analysis of podocyte foot process morphology in three species by 3D super-resolution microscopy. Front Med (Lausanne). 2018;5:292–298. doi: 10.3389/fmed.2018.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon MC, Chuang PY, He CJ. The glomerular filtration barrier: Components and crosstalk. Int J Nephrol. 2012;2012:1–9. doi: 10.1155/2012/749010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott RP, Quaggin SE. The cell biology of renal filtration. J Cell Biol. 2015;209(2):199–210. doi: 10.1083/jcb.201410017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60(3):957–968. doi: 10.1046/j.1523-1755.2001.060003957.x [DOI] [PubMed] [Google Scholar]
- 6.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71(12):1205–1214. doi: 10.1038/sj.ki.5002222 [DOI] [PubMed] [Google Scholar]
- 7.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13(12):3005–3015. doi: 10.1097/01.ASN.0000039661.06947.fd [DOI] [PubMed] [Google Scholar]
- 8.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest. 2012;122(12):4401–4411. doi: 10.1172/jci65289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechtel W, Helmstädter M, Balica J, Hartleben B, Kiefer B, Hrnjic F. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol. 2013;24(5):727–743. doi: 10.1681/ASN.2012070700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Chen MX, Fogo AB, Harris RC, Chen JK. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J Am Soc Nephrol. 2013;24(2):198–207. doi: 10.1681/ASN.2012010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermle T, Schneider R, Schapiro D, Braun DA, Van Der Ven AT, Warejko JK. GAPVD1 and ANKFY1 mutations implicate RAB5 regulation in nephrotic syndrome. J Am Soc Nephrol. 2018;29(8):2123–2138. doi: 10.1681/ASN.2017121312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorval G, Kuzmuk V, Gribouval O, Welsh GI, Bierzynska A, Schmitt A. TBC1D8B loss-of-function mutations lead to X-linked nephrotic syndrome via defective trafficking pathways. Am J Hum Genet. 2019;104(2):348–355. doi: 10.1016/j.ajhg.2018.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bork T, Liang W, Yamahara K, Lee P, Tian Z, Liu S. Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy. 2020;16(11):1932–1948. doi: 10.1080/15548627.2019.1705007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang W, Yamahara K, Hernando-Erhard C, Lagies S, Wanner N, Liang H. A reciprocal regulation of spermidine and autophagy in podocytes maintains the filtration barrier. Kidney Int. 2020;98(6):1434–1448. doi: 10.1016/j.kint.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 15.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest. 2010;120(4):1084–1096. doi: 10.1172/jci39492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weide T, Huber TB. Implications of autophagy for glomerular aging and disease. Cell Tissue Res. 2011;343(3):467–473. doi: 10.1007/s00441-010-1115-0 [DOI] [PubMed] [Google Scholar]
- 17.Roy SG, Stevens MW, So L, Edinger AL. Reciprocal effects of rab7 deletion in activated and neglected T cells. Autophagy. 2013;9(7):1009–1023. doi: 10.4161/auto.24468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genesis—2002—Moeller—Podocyte-specific Expression of Cre Recombinase in Transgenic Mice.pdf. 2003; doi: 10.1002/gene.10164 [DOI] [PubMed]
- 19.Möller-Kerutt A Rodriguez-Gatica JE Wacker K, et al. . Crumbs2 Is an Essential Slit Diaphragm Protein of the Renal Filtration Barrier. 2021. 10.1681/ASN.2020040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Möller-Kerutt A, Rodriguez-Gatica JE, Wacker K, Bhatia R, Siebrasse JP, Boon N. Crumbs2 is an essential slit diaphragm protein of the renal filtration barrier. J Am Soc Nephrol. 2021;32(5):1053–1070. doi: 10.1681/ASN.2020040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weide T, Vollenbröker B, Schulze U, Djuric I, Edeling M, Bonse J. Pals1 haploinsufficiency results in proteinuria and cyst formation. J Am Soc Nephrol. 2017;28(7):2093–2107. doi: 10.1681/ASN.2016040474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogg M Maier JI Van Wymersch C, et al. . A-Parvin defines a specific integrin adhesome to maintain the glomerular filtration barrier. J Am Soc Nephrol. 2022;33(4):786–808. doi: 10.1681/ASN.2021101319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogg M, Maier JI, Dotzauer R, Artelt N, Kretz O, Helmstädter M. SRGAP1 controls small rho GTPases to regulate podocyte foot process maintenance. J Am Soc Nephrol. 2021;32(3):563–579. doi: 10.1681/ASN.2020081126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michgehl U, Skryabin BV, Bayraktar S, Vollenbröker B, Ciarimboli G, Heitplatz B. Nephron-specific knockin of the PIKfyve-binding-deficient Vac14L156R mutant results in albuminuria and mesangial expansion. Am J Physiol Ren Physiol. 2018;315(5):F1307–F1319. doi: 10.1152/ajprenal.00191.2018 [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 26.Vollenbröker B, George B, Wolfgart M, Saleem MA, Pavenstädt H, Weide T. mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. Am J Physiol Ren Physiol. 2009;296(2):418–426. doi: 10.1152/ajprenal.90319.2008 [DOI] [PubMed] [Google Scholar]
- 27.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630–638. doi: 10.1681/ASN.v133630 [DOI] [PubMed] [Google Scholar]
- 28.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weide T, Vollenbröker B, Schulze U, Djuric I, Edeling M, Bonse J. Pals1 haploinsufficiency results in proteinuria and cyst formation. J Am Soc Nephrol. 2017;28(7):2093–2107. doi: 10.1681/ASN.2016040474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granado D, Müller D, Krausel V, Kruzel-Davila E, Schuberth C, Eschborn M. Intracellular APOL1 risk variants cause cytotoxicity accompanied by energy depletion. J Am Soc Nephrol. 2017;28(11):3227–3238. doi: 10.1681/ASN.2016111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canton J, Grinstein S. Measuring Lysosomal pH by Fluorescence Microscopy. Elsevier Ltd; 2015. doi: 10.1016/bs.mcb.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 32.Kühnl A, Musiol A, Heitzig N, Johnson DE, Ehrhardt C, Grewal T. Late endosomal/lysosomal cholesterol accumulation is a host cell-protective mechanism inhibiting endosomal escape of influenza A virus. mBio. 2018;9(4):1–16. doi: 10.1128/mbio.01345-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212(6):677–692. doi: 10.1083/jcb.201507112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dlugos CP, Picciotto C, Lepa C, Krakow M, Stöber A, Eddy ML. Nephrin signaling results in integrin β1 activation. J Am Soc Nephrol. 2019;30(6):1006–1019. doi: 10.1681/ASN.2018040362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heiden S, Siwek R, Lotz ML, Borkowsky S, Schröter R, Nedvetsky P. Apical-basal polarity regulators are essential for slit diaphragm assembly and endocytosis in Drosophila nephrocytes. Cell Mol Life Sci. 2021;78(7):3657–3672. doi: 10.1007/s00018-021-03769-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maywald ML, Picciotto C, Lepa C, Bertgen L, Yousaf FS, Ricker A. Rap1 activity is essential for focal adhesion and slit diaphragm integrity. Front Cell Dev Biol. 2022;10:1–14. doi: 10.3389/fcell.2022.790365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bright LJ, Lynch M. The Rab7 subfamily across Paramecium aurelia species; evidence of high conservation in sequence and function. Small GTPases. 2020;11(6):421–429. doi: 10.1080/21541248.2018.1502056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashiwazaki J, Iwaki T, Takegawa K, Shimoda C, Nakamura T. Two fission yeast Rab7 homologs, Ypt7 and Ypt71, play antagonistic roles in the regulation of vacuolar morphology. Traffic. 2009;10(7):912–924. doi: 10.1111/j.1600-0854.2009.00907.x [DOI] [PubMed] [Google Scholar]
- 39.MacKiewicz P, Wyroba E. Phylogeny and evolution of Rab7 and Rab9 proteins. BMC Evol Biol. 2009;9(1):101–119. doi: 10.1186/1471-2148-9-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brighouse A, Dacks JB, Field MC. Rab protein evolution and the history of the eukaryotic endomembrane system. Cell Mol Life Sci. 2010;67(20):3449–3465. doi: 10.1007/s00018-010-0436-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457(7227):322–326. doi: 10.1038/nature07526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helmstädter M, Huber TB, Hermle T. Using the drosophila nephrocyte to model podocyte function and disease. Front Pediatr. 2017;5:262–269. doi: 10.3389/fped.2017.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyaki T, Kawasaki Y, Matsumoto A, Kakuta S, Sakai T, Ichimura K. Nephrocytes are part of the spectrum of filtration epithelial diversity. Cell Tissue Res. 2020;382(3):609–625. doi: 10.1007/s00441-020-03313-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klumperman J, Raposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb Perspect Biol. 2014;6(10):1–22. doi: 10.1101/cshperspect.a016857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Luca M, Bucci C. A new V-ATPase regulatory mechanism mediated by the rab interacting lysosomal protein (RILP). Commun Integr Biol. 2014;7(5):1–4. doi: 10.4161/cib.29616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu M, Wang T, Loh E, Hong W, Song H. Structural basis for recruitment of RILP by small GTPase Rab7. EMBO J. 2005;24(8):1491–1501. doi: 10.1038/sj.emboj.7600643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breton S, Brown D. Regulation of luminal acidification by the V-ATPase. Physiology. 2013;28(5):318–329. doi: 10.1152/physiol.00007.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerra F, Bucci C. Multiple roles of the small GTPase Rab7. Cells. 2016;5(3):34. doi: 10.3390/cells5030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamura N, Sun-Wada GH, Aoyama M, Harada A, Takasuga S, Sasaki T. Delivery of endosomes to lysosomes via microautophagy in the visceral endoderm of mouse embryos. Nat Commun. 2012;3(1):1071. doi: 10.1038/ncomms2069 [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Mashima H, Miura K, Maeda D, Goto A, Goto T. Disruption of small GTPase Rab7 exacerbates the severity of acute pancreatitis in experimental mouse models. Sci Rep. 2017;7(1):2817–2916. doi: 10.1038/s41598-017-02988-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucci C, de Luca M. Molecular basis of Charcot-Marie-Tooth type 2B disease. Biochem Soc Trans. 2012;40(6):1368–1372. doi: 10.1042/bst20120197 [DOI] [PubMed] [Google Scholar]
- 52.Cherry S, Jin EJ, Özel MN, Lu Z, Agi E, Wang D. Charcot-Marie-Tooth 2B mutations in rab7 cause dosage-dependent neurodegeneration due to partial loss of function. Elife. 2013;2:1–22. doi: 10.7554/elife.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano R, Rivellini C, de Luca M, Tonlorenzi R, Beli R, Manganelli F. Alteration of the late endocytic pathway in Charcot–Marie–Tooth type 2B disease. Cell Mol Life Sci. 2021;78(1):351–372. doi: 10.1007/s00018-020-03510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spinosa MR, Progida C, de Luca A, Colucci AMR, Alifano P, Bucci C. Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J Neurosci. 2008;28(7):1640–1648. doi: 10.1523/jneurosci.3677-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayraktar S, Nehrig J, Menis E, Karli K, Janning A, Struk T. A deregulated stress response underlies distinct INF2-associated disease profiles. J Am Soc Nephrol. 2020;31(6):1296–1313. doi: 10.1681/ASN.2019111174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G. INF2 mutations in Charcot–Marie–Tooth disease with glomerulopathy. New Engl J Med. 2011;365(25):2377–2388. doi: 10.1056/nejmoa1109122 [DOI] [PubMed] [Google Scholar]
- 57.Echaniz-Laguna A, Latour P. A cryptic splicing mutation in the INF2 gene causing Charcot-Marie-Tooth disease with minimal glomerular dysfunction. J Peripher Nerv Syst. 2019;24(1):120–124. doi: 10.1111/jns.12308 [DOI] [PubMed] [Google Scholar]
- 58.Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, Schapira AHV. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet. 2016;25(16):3432–3445. doi: 10.1093/hmg/ddw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rong Y, McPhee CK, Deng S, Huang L, Chen L, Liu M. Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation. Proc Natl Acad Sci U S A. 2011;108(19):7826–7831. doi: 10.1073/pnas.1013800108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Zhou W, Lin J, Wei P, Zhang Y, Jin P. Autophagic lysosomal reformation depends on mTOR reactivation in H2O2-induced autophagy. Int J Biochem Cell Biol. 2016;70:76–81. doi: 10.1016/j.biocel.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 61.Kuchitsu Y, Fukuda M. Revisiting Rab7 functions in mammalian autophagy: rab7 knockout studies. Cells. 2018;7(11):215. doi: 10.3390/cells7110215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465(7300):942–946. doi: 10.1038/nature09076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Yu L. Development of research into autophagic lysosome reformation. Mol Cells. 2018;41(1):45–49. doi: 10.14348/molcells.2018.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Munson MJ, Allen GF, Toth R, Campbell DG, Lucocq JM, Ganley IG. mTOR activates the VPS 34–UVRAG complex to regulate autolysosomal tubulation and cell survival. EMBO J. 2015;34(17):2272–2290. doi: 10.15252/embj.201590992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikonomov OC, Sbrissa D, Venkatareddy M, Tisdale E, Garg P, Shisheva A. Class III PI 3-kinase is the main source of PtdIns3P substrate and membrane recruitment signal for PIKfyve constitutive function in podocyte endomembrane homeostasis. Biochim Biophys Acta Mol Cell Res. 2015;1853(5):1240–1250. doi: 10.1016/j.bbamcr.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu Y, Zhu J-Y, Zhang F, Adam R, Zhanzheng Zhao ZH. HHS public access. Physiol Behav. 2018;176(1):139–148. doi: 10.1007/s00441-017-2575-2 [DOI] [Google Scholar]
- 67.de Luca M Cogli L Progida C, et al. . Correction to RILP regulates vacuolar ATPase through interaction with the V1G1 subunit [J. Cell Sci. 127, (2014) 2697–2708]. J Cell Sci. 2015;128(14):2565. doi: 10.1242/jcs.142604 [DOI] [PubMed] [Google Scholar]
- 68.Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74(1):69–86. doi: 10.1146/annurev-physiol-012110-142317 [DOI] [PubMed] [Google Scholar]
- 69.Riediger F, Quack I, Qadri F, Hartleben B, Park JK, Potthoff SA. Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol. 2011;22(12):2193–2202. doi: 10.1681/ASN.2011020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawrence RE, Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat Cell Biol. 2019;21(2):133–142. doi: 10.1038/s41556-018-0244-7 [DOI] [PubMed] [Google Scholar]
- 71.DiCiccio JE, Steinberg BE. Lysosomal pH and analysis of the counter ion pathways that support acidification. J Gen Physiol. 2011;137(4):385–390. doi: 10.1085/jgp.201110596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegenerating. 2015;4(1):1–10. doi: 10.1186/s40035-015-0041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jialiu Z, Orian SS, Mark WG. Modulating lysosomal pH: a molecular and nanoscale materials design perspective. HHS Public Access. 2017;176(10):139–148. doi: 10.36069/jols/20201204 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available in this article.