Keywords: GFR, creatinine, cystatin C, SCREAM
Abstract
Significance Statement
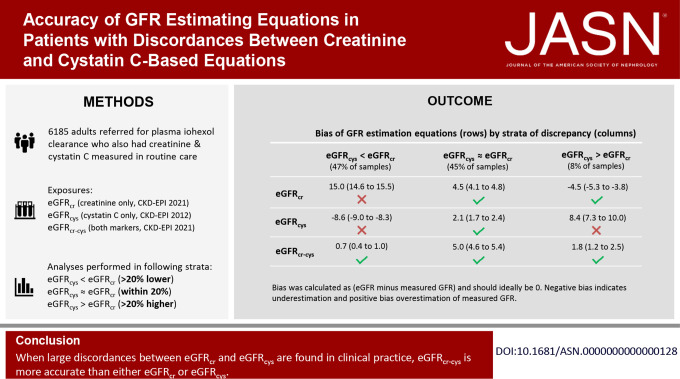
Large discordances between eGFR on the basis of creatinine (eGFRcr) or cystatin C (eGFRcys) are common in clinical practice. However, which GFR estimating equation (eGFRcr, eGFRcys, or eGFRcr-cys) is most accurate in these settings is not known. In this real-world study of 9404 concurrent measurements of creatinine, cystatin C, and iohexol clearance, all three equations performed similarly when eGFRcr and eGFRcys were similar (45% of cases). However, with large discordances (55% of cases), eGFRcr-cys was much more accurate than either alone. These findings were consistent among individuals with cardiovascular disease, heart failure, diabetes mellitus, liver disease, and cancer who have been underrepresented in research cohorts. Thus, when eGFRcr and eGFRcys are largely discordant in clinical practice, eGFRcr-cys is more accurate than eGFRcr or eGFRcys.
Background
Cystatin C is recommended as a confirmatory test to eGFR when more precise estimates are needed for clinical decision making. Although eGFR on the basis of both creatinine and cystatin (eGFRcr-cys) is the most accurate estimate in research studies, it is uncertain whether this is true in real-world settings, particularly when there are large discordances between eGFR based on creatinine (eGFRcr) and that based on cystatin C (eGFRcys)
Methods
We included 6185 adults referred for measured GFR (mGFR) using plasma clearance of iohexol in Stockholm, Sweden, who had 9404 concurrent measurements of creatinine, cystatin C, and iohexol clearance. The performance of eGFRcr, eGFRcys, and eGFRcr-cys was assessed against mGFR with median bias, P30, and correct classification of GFR categories. We stratified analyses within three categories: eGFRcys at least 20% lower than eGFRcr (eGFRcys<eGFRcr), eGFRcys within 20% of eGFRcr (eGFRcys≈eGFRcr), and eGFRcys at least 20% higher than eGFRcr (eGFRcys>eGFRcr).
Results
eGFRcr and eGFRcys were similar in 4226 (45%) samples, and among these samples all three estimating equations performed similarly. By contrast, eGFRcr-cys was much more accurate in cases of discordance. For example, when eGFRcys<eGFRcr (47% of samples), the median biases were 15.0 (overestimation), −8.5 (underestimation), and 0.8 ml/min per 1.73 m2 for eGFRcr, eGFRcys, and eGFRcr-cys, respectively; P30 was 50%, 73%, and 84%, respectively; and correct classification was 38%, 45%, and 62%, respectively. When eGFRcys>eGFRcr (8% of samples), the median biases were −4.5, 8.4, and 1.4 ml/min per 1.73m2. The findings were consistent among individuals with cardiovascular disease, heart failure, diabetes mellitus, liver disease, and cancer.
Conclusions
When eGFRcr and eGFRcys are highly discordant in clinical practice, eGFRcr-cys is more accurate than either eGFRcr or eGFRcys.
Introduction
eGFR is a key parameter to inform clinical decisions in medicine and plays a central role in the diagnosis, prognosis, and management of patients with chronic kidney disease. Currently, eGFR is most commonly estimated using serum creatinine (eGFRcr). However, recent statements from leading kidney organizations stress the need to facilitate increased, routine, and timely use of cystatin C in health care as an additional filtration marker.1,2 Research studies demonstrate that eGFR on the basis of both creatinine and cystatin C (eGFRcr-cys) is more accurate than eGFRcr when compared with measured GFR (mGFR) using plasma clearance of iohexol, an accepted reference method.3,4 However, less is known about the accuracy of the combined equation in real-world clinical practice because worldwide implementation of cystatin C testing has been slow, despite recommendations for use since 2012. Sweden is unique in this aspect: Since the early 2000s, measurements of serum cystatin C—as well as mGFR—have been widely available without subspecialty consultation.5,6
Both creatinine and cystatin C have non-GFR determinants which may result in inaccurate estimates in certain clinical settings. Recent evidence suggests that there are often large discrepancies between eGFR solely based on creatinine (eGFRcr) and that based on cystatin C (eGFRcys).7–10 Studies have suggested that intraindividual discrepancies between eGFRcys and eGFRcr are associated with adverse outcomes,7,9,11 with a worse prognosis for individuals who have an eGFRcys that is lower than eGFRcr. However, it is unknown whether this difference reflects better estimation of GFR or merely captures additional confounders (e.g., inflammation). Few studies have investigated which GFR estimating equation best approximates mGFR when eGFRcr and eGFRcys differ.12,13 Thus, there is uncertainty as to which estimating equation should guide clinical practice in situations of large discordances between eGFRcr and eGFRcys.
To address this question using real-world clinical data, we analyzed more than 9000 simultaneous assessments of serum creatinine, cystatin C, and mGFR in 6185 individuals from an independent cohort not involved in the development of the new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. We assessed the performance of eGFRcr, eGFRcys, and eGFRcr-cys against mGFR within three categories: eGFRcys at least 20% lower than eGFRcr (eGFRcys<eGFRcr), eGFRcys within 20% of eGFRcr (eGFRcys≈eGFRcr), and eGFRcys at least 20% higher than eGFRcr (eGFRcys>eGFRcr).
Methods
Data Source and Study Population
We used data from the Stockholm Creatinine Measurements (SCREAM) project,14 a health care utilization cohort from the region of Stockholm, Sweden, with data collected between 2006 and 2019. A single health care provider in the Stockholm region provides universal and tax-funded health care to 20%–25% of the population of Sweden. Using unique personal identification numbers,15 SCREAM linked regional and national administrative databases that hold complete information on demographics, health care utilization, dispensed drugs,16 diagnoses,17 vital status,18 kidney replacement therapy,19 and completed laboratory tests. The Regional Ethical Review Board in Stockholm approved the study (2017/793-31); informed consent was not deemed necessary because all data were deidentified at the Swedish Board of Health and Welfare.
We included all adult patients who received iohexol clearance testing between January 1, 2007, and December 31, 2018 (Supplemental Figure 1). Eligible individuals were required to have a serum creatinine and cystatin C test in the 30 days before or after the iohexol clearance measurement. All creatinine tests were standardized to isotope dilution mass spectrometry traceable methods and standardization of cystatin C occurred after 2010.20 Patients on dialysis and those who had implausible mGFR values (<0 or >150 ml/min per 1.73 m2) were excluded. When a patient had multiple concurrent iohexol-creatinine-cystatin C tests during follow-up, we included all measurements to increase statistical efficiency.
GFR Measurement
Iohexol clearance was analyzed at the central laboratory, Department of Clinical Chemistry, at Karolinska University Hospital in Stockholm, with clearance procedures performed by indication at specialist departments in the region of Stockholm following systematic protocols.21 In brief, iohexol clearance was measured using single-point plasma clearance of iohexol and expressed per 1.73 m2 body surface area. A total of 5 ml of iohexol (currently omnipaque 300 mg I/mL, GE Healthcare) was administered with an intravenous injection, followed by a 10 ml normal saline flush. Blood samples (5 ml) for plasma clearance measurement were obtained from the contralateral arm to the injection, with the timing based on the eGFR: approximately 4 hours for eGFR >40 ml/min per 1.73 m2, within approximately 6–8 hours for eGFR 15–40 ml/min per 1.73 m2 and after approximately 24 hours for eGFR <15 ml/min per 1.73 m2. The exact times of iohexol injection and blood sampling were recorded, and samples were centrifuged before transport if the transport to the central laboratories could not take place on the same day. Serum iohexol concentration was determined by ultra-high-performance liquid chromatography separation and ultraviolet detection. The performance of the iohexol method was monitored through internal controls and an external quality assurance program for iohexol standardization across the country by the Government-run monitoring company Equalis (Uppsala, Sweden). Bird et al.22 compared single-sample versus multisample GFR using both iohexol and 51Cr-EDTA as indicators (19). They found that single-sample iohexol at 3 and 4 hours was highly correlated with multisample iohexol (correlation coefficients of 0.97 and 0.99, respectively), with a mean difference (SD) of −3.0 (7.1) and 0.52 (4.3) and 95% limits of agreement of −17.2 to 11.2 and −8.1 to 9.1, respectively. Furthermore, compared with multisample 51Ct-EDTA, the mean difference (SD) was 1.1 (9.4) for single-sample iohexol at 3 hours, 4.5 (8.9) for single-sample iohexol at 4 hours, and 4.0 (7.9) for multisample iohexol.
GFR Estimating Equations, Discordance between eGFRcr and eGFRcys and Covariates
eGFRcr and eGFRcr-cys were calculated using the 2021 CKD-EPI equations and eGFRcys with the 2012 CKD-EPI equation.3,23 We assessed the performance of each equation in the overall population and according to the magnitude of discordance between eGFRcr and eGFRcys. Discordance was calculated as (eGFRcys−eGFRcr)/eGFRcr and categorized into eGFRcys<eGFRcr, eGFRcys≈eGFRcr, and eGFRcys>eGFRcr. A measurement fell within eGFRcys<eGFRcr when eGFRcys was more than 20% lower than eGFRcr; eGFRcys≈eGFRcr if the difference between eGFR values was within 20% of eGFRcr; and within eGFRcys>eGFRcr when eGFRcys was more than 20% higher than eGFRcr. We chose eGFRcr as denominator because it is currently the most commonly used eGFR measure worldwide. (eGFRcys−eGFRcr)/eGFRcr can thus be interpreted as the percentual difference from eGFRcr, with a negative number meaning lower eGFRcys than eGFRcr and positive number meaning a higher eGFRcys than eGFRcr. We chose 20% as threshold on the basis of previous analyses that show meaningfully elevated risks for outcomes10 and to allow for a significant proportion with eGFRcys<eGFRcr and eGFRcys>eGFRcr. In addition, we also assessed continuous percentage differences between eGFRcys and eGFRcr. For each individual, we extracted the following covariates: age, sex, body mass index (BMI), cardiovascular disease (CVD) (composite of myocardial infarction, other ischemic heart disease, heart failure, stroke, other cerebrovascular disease, arrhythmia, and peripheral vascular disease), hypertension, cancer, liver disease, and whether the individual had a kidney transplant or was a kidney donor (definitions in Supplemental Table 1).
Analysis
In our main analysis, we analyzed all measurements when patients had multiple concurrent iohexol-creatinine-cystatin C tests. The performance of all equations compared with mGFR was evaluated using the following metrics: bias, P30, interquartile range (IQR), and correct classification of GFR categories. Bias was defined as the median difference between eGFR and mGFR (eGFR−mGFR). P30 was defined as the proportion of eGFRs within 30% of mGFR (P30). A P30 value of 80%–90% is considered to be acceptable for GFR evaluation in many circumstances, and a P30 value of 90% or higher is preferred; these values correspond to approximately 60%–70% agreement and more than 70% agreement of eGFR with mGFR in GFR categories, respectively. We also reported P10, defined as the proportion of eGFRs within 10% of mGFR. IQR was defined as the magnitude of the IQR of the differences between mGFR and eGFR and is a measure of precision. Correct classification of GFR categories was defined as agreement of eGFR and mGFR categories using the Kidney Disease Improving Global Outcomes (KDIGO) GFR categories (<15, 15–29, 30–44, 45–59, 60–89, and ≥90 ml/min per 1.73 m2). Among patients with large discordances between eGFRcr and eGFRcys, we also assessed the proportion that would be correctly and incorrectly reclassified across KDIGO GFR categories when using eGFRcr-cys instead of eGFRcr or eGFRcys. The 95% confidence intervals (CIs) for each metric were calculated using the bootstrap method (10.000 bootstrap samples). All analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing).24
Subgroup Analyses and Sensitivity Analyses
We assessed performance within subgroups of interest by assessing median bias. A priori-defined strata included age (< or ≥65 years), sex, BMI (< or ≥25 kg/m2 and continuously), mGFR (< or ≥60 ml/min per 1.73 m2), and the presence of CVD, heart failure, diabetes mellitus, liver disease, and cancer. Furthermore, we performed the following sensitivity analyses: First, we redefined our exposure as the raw difference between eGFRcys and eGFRcr and used the categories eGFRcys<eGFRcr by more than 15 ml/min per 1.73 m2, eGFRcys≈eGFRcr (values do not differ by more than 15 ml/min per 1.73 m2) and eGFRcys>eGFRcr by more than 15 ml/min per 1.73 m2. The threshold of 15 ml/min per 1.73 m2 allows comparison with other studies that have used these thresholds.9,11 Second, we restricted our analysis to measurements of iohexol, creatinine, and cystatin C taken on the same day, instead of using a 30-day window. Third, we used the first measurement for each patient rather than all measurements. Fourth, to eliminate the possible interference of nonstandardized cystatin C tests, we restricted the analysis to measurements performed after 2011.20 Fifth, we combined sensitivity analyses two to four and restricted the analysis to the first measurement per patient using standardized cystatin C measurements and where iohexol, creatinine, and cystatin C were measured on the same day. Finally, we assessed the performance of the arithmetic mean of eGFRcr and eGFRcys (which is used in Sweden), rather than eGFRcr-cys.25
Results
Baseline Characteristics
We included 6185 individuals contributing 9404 mGFR measurements (Supplemental Figure 2). Of these 9404 measurements, 77.1% had mGFR, creatinine, and cystatin C measured on the same day. Of the 9404 measurements, 89.9% had creatinine or cystatin C measured within 7 days of mGFR, and 10.1% had creatinine or cystatin C measured between 7 and 30 days of mGFR. The mean (SD) age was 56 (17) years, with 38% aged 65 years or older and 40% female (Table 1). The median mGFR was 62 ml/min per 1.73 m2, eGFRcr 74 ml/min per 1.73 m2, eGFRcys 56 ml/min per 1.73 m2, and eGFRcr-cys 65 ml/min per 1.73 m2 (distributions shown in Supplemental Figure 3). The median (IQR) discordance between eGFRcys and eGFRcr was −18.3% (−35.3% to −0.6%), showing that most patients had eGFRcys that was lower than their eGFRcr (Supplemental Figure 4). In total, 47% of observations were in the category eGFRcys<eGFRcr by more than 20%, 45% had eGFRcys≈eGFRcr, and 8% had eGFRcys>eGFRcr by more than 20%. Individuals in the category eGFRcys<eGFRcr were older, had lower levels of mGFR, and were more likely to have CVD, heart failure, diabetes, cancer, and liver disease than individuals with eGFRcys≈eGFRcr or eGFRcys>eGFRcr (Table 1).
Table 1.
Baseline characteristics of 6185 persons (with 9404 observations) referred to iohexol clearance testing in Stockholm during 2007–2018, overall and stratified by discordance between eGFRcr and eGFRcys
| Baseline Characteristics | Overall | eGFRcys<eGFRcra | eGFRcys≈eGFRcra | eGFRcys>eGFRcra |
|---|---|---|---|---|
| eGFRcys >20% Lower Than eGFRcr | eGFRcys Within 20% of eGFRcr | eGFRcys >20% Higher Than eGFRcr | ||
| No. of measurements, n (%) | 9404 (100) | 4465 (47) | 4226 (45) | 713 (8) |
| No. of unique individuals, n (%) | 6185 (100) | 2927 (47) | 3174 (51) | 650 (11) |
| Mean age (SD), yr | 56 (17) | 60 (16) | 53 (18) | 50 (16) |
| Age ≥65 yr, n (%) | 3569 (38) | 2101 (47) | 1310 (31) | 158 (22) |
| Female sex, n (%) | 3751 (40) | 1681 (38) | 1760 (42) | 310 (43) |
| Mean BMI (SD), kg/m2 | 26 (8) | 26 (5) | 26 (8) | 26 (19) |
| BMI ≥25 kg/m2, n (%)b | 3887 (41) | 1938 (43) | 1665 (39) | 284 (40) |
| GFR evaluations, median (IQR) | ||||
| Cr, μmol/Lc | 94 (74–128) | 96 (75–130) | 89 (71–120) | 114 (93–239) |
| Cys, mg/L | 1 (1–2) | 2 (1–2) | 1 (1–1) | 1 (1–2) |
| eGFRcr, ml/min per 1.73 m2 | 74 (50–97) | 71 (48–94) | 80 (54–101) | 62 (25–79) |
| eGFRcys, ml/min per 1.73 m2 | 56 (35–84) | 42 (28–59) | 77 (50–99) | 84 (34–107) |
| eGFRcr-cys, ml/min per 1.73 m2 | 65 (42–90) | 54 (36–73) | 81 (53–103) | 74 (29–96) |
| mGFR, ml/min per 1.73 m2 | 62 (41–84) | 53 (36–71) | 74 (51–92) | 69 (27–89) |
| mGFR categories, n (%) | ||||
| ≥90 | 1755 (19) | 379 (8) | 1211 (29) | 165 (23) |
| 60 to <90 | 3245 (35) | 1412 (32) | 1579 (37) | 254 (36) |
| 45 to <60 | 1677 (18) | 1001 (22) | 602 (14) | 74 (10) |
| 30 to <45 | 1344 (14) | 897 (20) | 409 (10) | 38 (5) |
| 15 to <30 | 1020 (11) | 647 (14) | 308 (7) | 65 (9) |
| <15 | 363 (4) | 129 (3) | 117 (3) | 117 (16) |
| Medical history, n (%) | ||||
| CVDd | 2787 (30) | 1705 (38) | 942 (22) | 140 (20) |
| Heart failure | 974 (10) | 682 (15) | 253 (6) | 39 (5) |
| DM | 2490 (26) | 1549 (35) | 844 (20) | 97 (14) |
| Cancer | 2664 (28) | 1431 (32) | 1123 (27) | 110 (15) |
| Liver disease | 2521 (27) | 1694 (38) | 788 (19) | 39 (5) |
| Kidney transplantation | 350 (4) | 212 (5) | 132 (3) | 6 (1) |
| Kidney donor | 284 (3) | 14 (0) | 214 (5) | 56 (8) |
BMI, body mass index; IQR, interquartile range; Cr, creatinine; cys, cystatin C; mGFR, measured GFR; CVD, cardiovascular disease; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; RASi, renin-angiotensin system inhibition (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker); NSAIDs, nonsteroidal anti-inflammatory drugs.
Adds up to >100% because an individual can contribute multiple measured GFR measurements and therefore contribute to all three strata. Note that the numbers in Table 1 represent the number of measurements, not the number of unique individuals.
Body mass index was missing for 1640 (17%) in the overall group, 691 (15%) in the eGFRcys<eGFRcr group, 828 (20%) in the eGFRcys≈eGFRcr group, and 121 (17%) in the eGFRcys>eGFRcr group.
To convert plasma creatinine from μmol/L to mg/dl, multiply by 0.0113.
Cardiovascular disease was defined as a composite of myocardial infarction, other ischemic heart disease, heart failure, stroke, other cerebrovascular disease, arrhythmia, and peripheral vascular disease.
Performance of Equations Stratified by Discordance between eGFRcr and eGFRcys
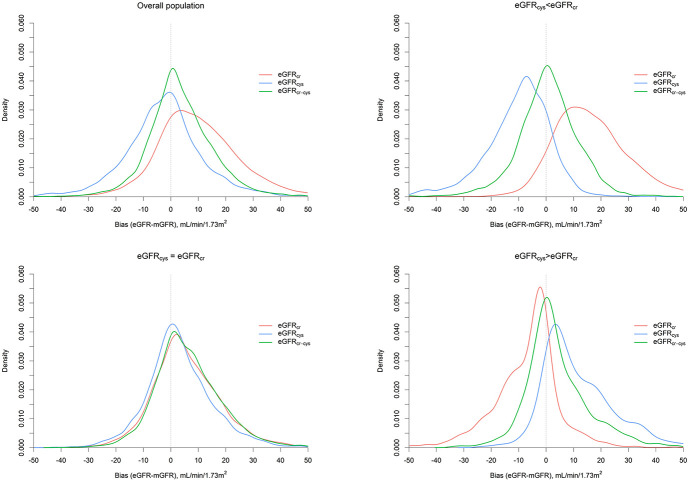
Overall, the median biases for eGFRcr, eGFRcys, and eGFRcr-cys were 8.7, −2.3, and 2.5 ml/min per 1.73 m2, respectively, with P30 of 68.8%, 80.7%, and 86.4% (Table 2). Furthermore, IQR was lowest for eGFRcr-cys (Table 2, Figure 1).
Table 2.
Bias, P30, interquartile range and correct classification of different Chronic Kidney Disease Epidemiology Collaboration eGFR equations, overall and stratified by the magnitude and direction of the discordance between eGFRcr and eGFRcys
| Metrics | Total Population | eGFRcys<eGFRcra | eGFRcys≈eGFRcra | eGFRcys>eGFRcra |
|---|---|---|---|---|
| eGFRcys >20% Lower Than eGFRcr | eGFRcys Within 20% of eGFRcr | eGFRcys >20% Higher Than eGFRcr | ||
| eGFRcrb | ||||
| Bias, median difference (ml/min per 1.73 m2)c | 8.7 (8.4–9.0) | 15.0 (14.6–15.5) | 4.5 (4.1–4.8) | −4.5 (−5.3 to −3.8) |
| P30 (%)b | 68.8 (67.8–69.7) | 49.7 (48.3–51.2) | 86.0 (84.9–87.0) | 85.9 (83.2–88.3) |
| IQR, ml/min per 1.73 m2d | 18.6 (0.2–18.8) | 17.5 (7.0–24.5) | 15.1 (−2.0 to 13.1) | 12.3 (−13.0 to −0.7) |
| Correct classification (%)e | 52.6 (51.6–53.6) | 38.1 (36.7–39.5) | 66.5 (65.1–67.9) | 61.9 (58.3–65.4) |
| eGFRcysb | ||||
| Bias, median difference (ml/min per 1.73 m2)c | −2.3 (−2.6 to −2.0) | −8.6 (−9.0 to −8.3) | 2.1 (1.7–2.4) | 8.4 (7.3–10.0) |
| P30f | 80.7 (79.9–81.5) | 72.9 (71.6–74.2) | 90.4 (89.5–91.3) | 71.8 (68.4–75.1) |
| IQR, ml/min per 1.73 m2d | 15.6 (−10.5 to 5.1) | 14.2 (−16.5 to −2.3) | 13.8 (−4.0 to 9.9) | 16.5 (2.5–19.0) |
| Correct classificatione | 57.4 (56.4–58.4) | 45.4 (43.9–46.8) | 69.2 (67.8–70.5) | 62.9 (59.4–66.6) |
| eGFRcr-cysb | ||||
| Bias, median difference (ml/min per 1.73 m2)c | 2.5 (2.2–2.7) | 0.7 (0.4–1.0) | 5.0 (4.6–5.4) | 1.8 (1.2–2.5) |
| P30b | 86.4 (85.7–87.1) | 84.3 (83.2–85.4) | 88.3 (87.3–89.3) | 87.8 (85.4–90.2) |
| IQR, ml/min per 1.73 m2d | 13.6 (−3.5–10.0) | 12.6 (−5.5–7.1) | 14.5 (−1.4–13.2) | 12.3 (−2.6–9.6) |
| Correct classificatione | 65.4 (64.5–66.4) | 61.6 (60.2–63) | 68.4 (67–69.7) | 71.9 (68.6–75.2) |
Cr, creatinine; cys, cystatin C; IQR, interquartile range; mGFR, measured GFR.
A measurement fell within eGFRcys<eGFRcr when eGFRcys was more than 20% lower than eGFRcr; eGFRcys≈eGFRcr if the difference between eGFR values was within 20% of eGFRcr; and within eGFRcys>eGFRcr when eGFRcys was more than 20% higher than eGFRcr.
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2012 and 2021 equations.
Bias was expressed as the median difference in eGFR minus measured GFR (95% confidence interval). A negative bias indicates underestimation of the measured GFR, and a positive bias indicates overestimation of the measured GFR.
Interquartile range is defined as the interquartile range and a measure of precision (the dispersion of individual errors around the median bias).
Correct classification of GFR categories was defined as agreement of eGFR and measured GFR categories using the Kidney Disease Improving Global Outcomes GFR categories (<15, 15–29, 30–44, 45–59, 60–89 and ≥90 ml/min per 1.73 m2).
P30 was defined as the percentage of individuals with eGFRs within 30% of measured GFR (95% confidence interval).
Figure 1.
Density plot of bias for three eGFR equations, overall and stratified by discordance between eGFRcr and eGFRcys. A measurement fell within eGFRcys<eGFRcr when eGFRcys was more than 20% lower than eGFRcr; eGFRcys≈eGFRcr if the difference between eGFR values was within 20% of eGFRcr and within eGFRcys>eGFRcr when eGFRcys was more than 20% higher than eGFRcr. Figure 1 can be viewed in color online at www.jasn.org.
When stratifying by discordance between eGFRcys and eGFRcr, all three equations displayed similar performance when eGFRcys≈eGFRcr, with little differences in bias, P30, and correct classification. For instance, the median biases were 4.5, 2.1, and 5.0 ml/min per 1.73 m2 for eGFRcr, eGFRcys, and eGFRcr-cys, respectively (Table 2, Figure 1). By contrast, when eGFRcys and eGFRcr were substantially different, eGFRcr-cys had better performance than eGFRcr and eGFRcys. Specifically, among observations with eGFRcys<eGFRcr, eGFRcr overestimated and eGFRcys underestimated mGFR (Figure 1, Supplemental Figure 5). The median biases were 15.0, −8.5, and 0.8 ml/min per 1.73 m2 for eGFRcr, eGFRcys, and eGFRcr-cys, respectively; P30 was 49.7%, 72.9%, and 84.3%, respectively; P10 was 15.8%, 24.0%, and 40.2%, respectively; and correct classification of GFR categories was 38.1%, 45.4%, and 61.6%, respectively (Table 2, Supplemental Table 2). By contrast, among observations with eGFRcys>eGFRcr, the pattern was reversed, with eGFRcr underestimating and eGFRcys overestimating mGFR (Supplemental Figure 5). The median biases were −4.5, 8.4, and 1.8 for eGFRcr, eGFRcys, and eGFRcr-cys, respectively; P30 was 85.9%, 71.8%, and 87.8%, respectively; P10 was 34.1%, 25.9%, and 43.9%, respectively; and correct classification of GFR categories was 61.9%, 62.9%, and 71.9%, respectively (Table 2, Supplemental Table 2).
The median bias for eGFRcr and eGFRcys was larger than the median bias for eGFRcr-cys whenever the discrepancy between eGFRcys and eGFRcr was >10% (Supplemental Figure 6). Correct classification of GFR categories for each GFR category is shown in Supplemental Tables 3 and 4. Consistent with the overall classification results, eGFRcr-cys showed better classification than eGFRcr or eGFRcys among observations with eGFRcys<eGFRcr or eGFRcys>eGFRcr. For instance, among those with eGFRcys<eGFRcr, correct classification for eGFRcr-cys was 50.5%, 56.5%, 66.5%, and 55.8% for mGFR categories of 45–59, 30–44, 15–29, and <15 ml/min per 1.73 m2, respectively.
Among patients with eGFRcys<eGFRcr, using eGFRcr-cys instead of eGFRcr reclassified 38.1% to a correct GFR category and incorrectly reclassified 25.5%, with a net difference of 12.6%. Furthermore, eGFRcr-cys reclassified more participants correctly than incorrectly than eGFRcys, with a net difference of 8.6% (Table 3, Supplemental Table 5). Similar findings were obtained in the stratum of patients with eGFRcys>eGFRcr.
Table 3.
Correct and incorrect reclassification from GFR categories based on eGFRcr or eGFRcys to categories based on eGFRcr-cys in patients with eGFRcys<eGFRcr and eGFRcys>eGFRcr
| Reclassification | Replacing GFR Categories Based on eGFRcr by eGFRcr-cys | Replacing GFR Categories Based on eGFRcys by eGFRcr-cys | ||
|---|---|---|---|---|
| eGFRcys<eGFRcr eGFRcys >20% Lower Than eGFRcr |
eGFRcys>eGFRcr eGFRcys >20% Higher Than eGFRcr |
eGFRcys<eGFRcr eGFRcys >20% Lower Than eGFRcr |
eGFRcys>eGFRcr eGFRcys >20% Higher Than eGFRcr |
|
| Participants, n | 4465 | 713 | 4465 | 713 |
| Total reclassified, n (%) | 2838 (63.6) | 284 (39.8) | 2407 (53.9) | 161 (22.6) |
| Correctly reclassified, n (%) | 1700 (38.1) | 174 (24.4) | 1396 (31.3) | 108 (15.1) |
| Incorrectly reclassified, n (%) | 1138 (25.5) | 110 (15.4) | 1011 (22.6) | 53 (7.4) |
| Net difference, % | 12.6 | 9.0 | 8.6 | 7.7 |
Performance of Equations in Subgroups
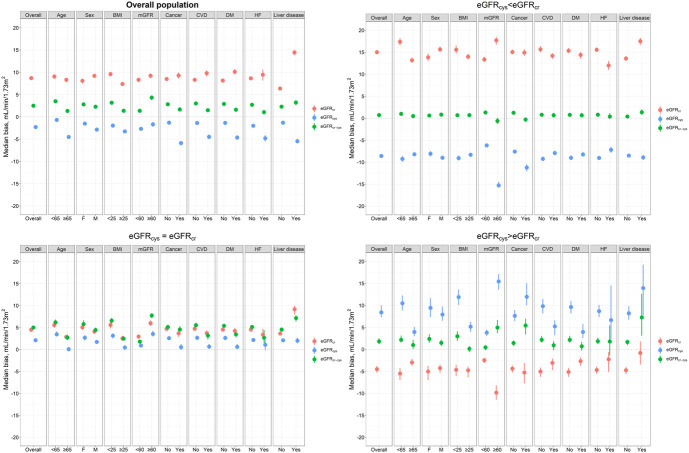
The findings were consistent in subgroups of age, sex, BMI, mGFR, CVD, diabetes, heart failure, liver disease and cancer, with eGFRcr-cys having the smallest bias across observations with eGFRcys<eGFRcr (Figure 2, Supplemental Table 6). Although the bias for eGFRcr-cys was small among the subgroup of patients with heart failure (N=974), P30 was low. For instance, P30 for eGFRcr-cys was 76.5% among heart failure patients who had eGFRcys<eGFRcr. P30 for eGFRcr-cys was acceptable among patients with liver disease (N=2521) or cancer (N=2664), being close to or >85%. Analysis by continuous BMI showed that when eGFRcys≈eGFRcr, eGFRcys had the smallest bias, particularly at low BMI. When eGFRcys<eGFRcr, eGFRcr-cys performed best, also at extreme values of BMI >40 (Supplemental Figure 7).
Figure 2.
Bias of eGFRcr, eGFRcys, and eGFRcr-cys across subgroups, overall and stratified by the extent of discordance between eGFRcr and eGFRcys. A measurement fell within eGFRcys<eGFRcr when eGFRcys was more than 20% lower than eGFRcr; eGFRcys≈eGFRcr if the difference between eGFR values was within 20% of eGFRcr and within eGFRcys>eGFRcr when eGFRcys was more than 20% higher than eGFRcr. BMI, body mass index; CVD, cardiovascular disease; DM, diabetes mellitus; HF, heart failure; mGFR, measured glomerular filtration rate. Figure 2 can be viewed in color online at www.jasn.org.
Sensitivity Analyses
The findings were consistent when using raw differences between eGFRcr and eGFRcys (Supplemental Tables 7 and 8, Supplemental Figures 8 and 9). Furthermore, we observed similar findings when restricting to same-day measurements (Supplemental Table 9), when restricting to one measurement per patient (Supplemental Table 10), when restricting to standardized cystatin C measurements (Supplemental Table 11), when combining the previous three sensitivity analyses (Supplemental Table 12), or when using the arithmetic mean of eGFRcr and eGFRcys instead of eGFRcr-cys (Supplemental Table 13).
Discussion
In this large study of >9000 closely spaced serum creatinine, cystatin C, and mGFR tests in a real-world clinical setting, we found that eGFR on the basis of both creatinine and cystatin C was the most accurate estimate of mGFR overall, similar to what has been established in research cohorts.3 Interestingly, this observation also held when there were large discordances between eGFRcr and eGFRcys. Similar to previous observations, we found that discordant eGFR values occurred commonly, with eGFRcys more than 20% lower than eGFRcr nearly half of the time. In situations of large discordances, eGFRcr and eGFRcys both showed substantial bias, albeit in opposite directions, as well as low P30 and low correct classification compared with mGFR. By contrast, the combined equation eGFRcr-cys performed well and would be acceptable for clinical decision making in many circumstances. Our findings were robust across multiple sensitivity analyses and consistent in subgroups of age, BMI, mGFR, and comorbidities known to affect the accuracy of serum creatinine and cystatin C levels. These findings have clinical implications, providing real-world evidence to guide GFR-based clinical decision making in situations of discordances between eGFRcr and eGFRcys.
Our observation that eGFRcys was more than 20% lower than eGFRcr in 47% of cases in routine clinical settings is novel. Previous observations have been made in research cohorts7,9,11 and generally show lower magnitude of discrepancies. This may be explained by the indications of iohexol clearance testing and the more common presence of comorbidities in routine clinical settings.26 We show that in situations of large discordances between eGFRcr and eGFRcys, both equations were similarly but oppositely biased and that eGFRcr-cys best approximated mGFR. A previous study similarly found that the bias for eGFRcr and eGFRcys progressively increased for larger discordances between eGFRcr and eGFRcys, whereas bias remained small when using the arithmetic mean of eGFRcr and eGFRcys.12. Both creatinine and cystatin C are affected by non-GFR determinants. Although serum creatinine may be influenced by muscle mass, diet, physical activity, and certain drugs, serum cystatin C is influenced by obesity, smoking, inflammation, and thyroid disorders.27–32 A large difference between eGFRcr and eGFRcys arises when serum creatinine, cystatin C, or both are influenced by their non-GFR determinants, leading to biased approximation of true GFR. Combining both markers improves precision by reducing errors that are due to variation in the non-GFR determinants of each marker.33 Grubb proposed an alternative hypothesis for the discrepancies between eGFRcys and eGFRcr called the shrunken pore syndrome, where an eGFRcys/eGFRcr ratio <0.6 or 0.7 reflects selective impairment of filtration of cystatin C and other middle molecular weight macromolecules (approximately 10k–30k daltons).34,35 Furthermore, the use of eGFRcr-cys instead of eGFRcr or eGFRcys reclassified more patients correctly than incorrectly. Such reclassification can have potential implications for drug dosing, initiation, and discontinuation which are based on GFR thresholds, including sodium-glucose cotransporter two inhibitors, renin-angiotensin system inhibitors, direct oral anticoagulants, and metformin.36,37
Our findings hold significant clinical implications that eGFRcr-cys best approximates mGFR in a variety of settings, including those when there are large discrepancies between eGFRcr and eGFRcys. A strength of our study is the evaluation of GFR equation's performance among patients with comorbid conditions known to affect serum creatinine or cystatin-C levels, including heart failure, liver disease, and cancer. These patients have been excluded or minimally included in preceding research cohorts. Among people with liver disease and cancer, eGFRcr-cys had acceptable performance. However, among heart failure patients, eGFRcr-cys had low P30 (approximately 75%), regardless of the magnitude of discrepancy between eGFRcr and eGFRcys. It is recommended to measure GFR through clearance of exogenous filtration markers rather than using GFR estimating equations when more accurate assessment of GFR is needed for decision making, such as in evaluating candidacy for living kidney donation and dosing for cancer chemotherapy.1,26 Other clinical scenarios where measuring GFR may be indicated because eGFRcr may not be valid are reviewed elsewhere.38
Previous studies have shown that on a population level, eGFRcys is more strongly associated with adverse outcomes than eGFRcr.39 Moreover, intraindividual differences in eGFR by creatinine vs. cystatin C predict adverse outcomes,7,9,11 with patients having a large discrepancy between eGFRcr and eGFRcys having a worse prognosis than individuals with no or small discrepancies. Our findings suggest that the stronger associations observed with eGFRcys do not reflect kidney function per se but rather non-GFR determinants of cystatin. Similarly, the strong associations between eGFR discrepancies and outcomes are likely to be explained by confounding of both eGFRcr and eGFRcys by non-GFR determinants.
Strengths of our study include its large sample size and unique setting, involving patients from a country with routine cystatin C testing and access to mGFR assessments. Inhabitants of Sweden enjoy universal tax-funded health care, which minimizes selection bias from disparate access to health care. Furthermore, this was an independent cohort not involved in the development of the novel CKD-EPI equations. Our study may better capture the performance of GFR estimating equations in routine clinical practice compared with research cohorts that include relatively healthy persons who are more likely to have predictable muscle mass and fewer comorbid conditions. Our study also has limitations. First, we lacked information on race. According to Swedish Government annual statistics,40 only approximately 2.5% of the included cohort were born in African countries; thus, our findings may be limited in terms of generalizability to other world regions. Second, the subgroups of comorbidity were defined according to International Classification of Diseases-10 codes. Although these have been shown to have good positive predictive value,17 they are not sensitive nor do they capture the severity of conditions. Third, we did not assess other developed eGFR equations, which should be the topic of future work. Fourth, certain specific subgroups may have been underrepresented in our cohort, including healthy individuals who are body builders or individuals with marked muscle wasting (e.g., spinal cord injury with paraplegia and advanced neuromuscular diseases). Fifth, we also included unstandardized cystatin C measurements. However, results were virtually identical in sensitivity analyses that only included standardized cystatin C measurements. Finally, we used single-sample iohexol as the gold standard, which has shown small bias but slightly lower precision than multisample iohexol. However, any inaccuracy in the gold standard would equally affect the different CKD-EPI eGFR equations.
In conclusion, eGFRcr-cys best approximates mGFR in routine clinical care, even when large discordances between eGFRcr and eGFRcys are found. When available, eGFRcr-cys should be used to guide clinical decisions.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the Swedish Research Council (2019-01059) and the Dutch Kidney Foundation (22OK2026). ELF is supported by a Rubicon Grant from the Netherlands Organization for Scientific Research (NWO) and an internal funding grant from Karolinska Institute.
Disclosures
P. Barany reports Research Funding: Astellas Pharma (observational anemia study); Honoraria: Fresenius (Life 2021 congress); and Other Interests or Relationships: Clinical trials with Alnylam, Bayer, GSK, and Omeros. J.-J. Carrero acknowledges consultancy for AstraZeneca, Bayer, Fresenius Kabi and research funding from Amgen, Astellas, and AstraZeneca, all outside the submitted work. J.-J. Carrero also reports Consultancy: Nestle; Research Funding: Swedish Heart and Lung Foundation, Swedish Research Council, and ViforPharma; Advisory or Leadership Role: Advisory Committee: AstraZeneca, Fresenius, and Nestle; Editorial board: American Journal of Kidney Diseases, European Heart Journal, Journal of Nephrology, and Nephrology, Dialysis Transplantation; Speakers Bureau: Abbott Laboratories, AstraZeneca, Baxter, Fresenius, and Viforpharma; and Other Interests or Relationships: European Renal Nutrition working group at the ERA-EDTA and Ïnternational Society of Renal Nutrition and Metabolism. J. Coresh is on the scientific advisory board for Healthy.io. F.W. Dekker reports Research Funding: Astellas, Chiesi, and Vifor. M.E. Grams reports Advisory or Leadership Role: American Journal of Kidney Diseases, CJASN, JASN Editorial board, ASN Publication Committee, KDIGO Executive Committee (co-chair elect), NKF Scientific Advisory Board, and USRDS Scientific Advisory Board; and Other Interests or Relationships: Grant funding from NKF—which receives funding from multiple pharmaceutical companies, grant funding from NIH, payment from academic institutions for grand rounds, and payment from NephSAP. L.A. Inker reports Consultancy: Diamtrix; Research Funding: to institute for research and contracts with the National Institutes of Health, National Kidney Foundation, Chinnocks, Omeros, and Reata Pharmaceuticals; and consulting agreements with Tricida Inc.; Advisory or Leadership Role: Alport Foundation—Medical Advisory Council and NKF—Scientific Advisory Board; and Other Interests or Relationships: American Society of Nephrology member and National Kidney Foundation member. A.S. Levey reports Research Funding: Grants and contracts paid to Tufts Medical Center: Contracts to paid from AstraZeneca (DSMB for dapagliflozin trials), NIH, and NKF; and Honoraria: Academic medical centers for visiting professorships. J.I. Rotmans reports Consultancy: Xeltis BV; Advisory or Leadership Role: Advisory Board Nextkidney; and Other Interests or Relationships: Chair Thematic Working Group Vascular Tissue Engineering at TERMIS, Member guideline committee Dutch Society of Nephrology, and presidentelect Vascular Access Society. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Juan-Jesus Carrero, Edouard L. Fu, Lesley A. Inker, Andrew S. Levey.
Data curation: Juan-Jesus Carrero.
Formal analysis: Edouard L. Fu.
Funding acquisition: Edouard L. Fu, Juan-Jesus Carrero.
Investigation: Juan-Jesus Carrero, Edouard L. Fu, Lesley A. Inker, Andrew S. Levey.
Methodology: Juan-Jesus Carrero, Edouard L. Fu, Lesley A. Inker, Andrew S. Levey.
Project administration: Juan-Jesus Carrero, Edouard L. Fu.
Resources: Juan-Jesus Carrero.
Software: Edouard L. Fu.
Supervision: Juan-Jesus Carrero, Lesley A. Inker, Andrew S. Levey.
Visualization: Edouard L. Fu, Lesley A. Inker, Andrew S. Levey.
Writing – original draft: Edouard L. Fu.
Writing – review & editing: Peter Barany, Juan-Jesus Carrero, Josef Coresh, Friedo W. Dekker, Carl-Gustaf Elinder, Edouard L. Fu, Morgan E. Grams, Lesley A. Inker, Andrew S. Levey, Julie M. Paik, Joris I. Rotmans.
Data Sharing Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data may be shared on reasonable request for academic research collaborations that fulfill GDPR as well as national and institutional ethics regulations and standards by contacting Juan-Jesus Carrero (juan.jesus.carrero@ki.se).
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E405.
Supplemental Table 1. Definition of study covariates.
Supplemental Table 2. P10 of different CKD-EPI eGFR equations stratified by the magnitude and direction of the discordance between eGFRcr and eGFRcys.
Supplemental Table 3. Agreement between mGFR and eGFR categories stratified by discordance between eGFRcr and eGFRcys: given a certain eGFR category, what is the probability an individual is in a certain mGFR category.
Supplemental Table 4. Agreement between mGFR and eGFR categories stratified by discordance between eGFRcr and eGFRcys: given a certain mGFR category, what is the probability an individual is in a certain eGFR category.
Supplemental Table 5. Correct and incorrect reclassification from GFR categories based on eGFRcr or eGFRcys to categories based on eGFRcr-cys, in patients with eGFRcys<eGFRcr and eGFRcys>eGFRcr.
Supplemental Table 6. Bias, P30, and correct classification of eGFRcr, eGFRcys, and eGFRcr-cys across subgroups stratified by discordance between eGFRcr and eGFRcys.
Supplemental Table 7. Baseline characteristics of included individuals (n=6185) with measured GFR measurements (n=9404) in Stockholm during 2007–2018 stratified by raw discordance between eGFRcr and eGFRcys.
Supplemental Table 8. Bias, P30, and correct classification of eGFRcr, eGFRcys, and eGFRcr-cys overall and stratified by raw discordance between eGFRcr and eGFRcys.
Supplemental Table 9. Sensitivity analysis restricting to same-day measurements of iohexol, creatinine, and cystatin C (N=7252).
Supplemental Table 10. Sensitivity analysis restricting to first measurement for each patient (N=6185).
Supplemental Table 11. Sensitivity analysis restricting to measurements taken after 2011 (i.e., after standardization of cystatin C) (N=7277).
Supplemental Table 12. Sensitivity analysis restricting to the first measurement per patient, with standardized cystatin C measurements and where iohexol, creatinine, and cystatin C were measured on the same day (N=3828).
Supplemental Table 13. Sensitivity analysis using the average of eGFRcr and eGFRcys instead of eGFRcr-cys.
Supplemental Figure 1. Study design diagram.
Supplemental Figure 2. Flow chart of included participants in the study.
Supplemental Figure 3. Density plot of mGFR, eGFRcr, eGFRcys, and eGFRcr-cys in the overall population and stratified by discordance between eGFRcr and eGFRcys.
Supplemental Figure 4. Density plot of discordance between eGFRcr and eGFRcys in the overall population.
Supplemental Figure 5. Comparison of mGFR and eGFR among patients with (A) eGFRcys<eGFRcr, (B) eGFRcys≈eGFRcr, and (C) eGFRcys>eGFRcr.
Supplemental Figure 6. Median bias for eGFRcr, eGFRcys, and eGFRcr-cys across the range of discordance between eGFRcr and eGFRcys.
Supplemental Figure 7. Density plot of bias for the three eGFR equations stratified by absolute discordance between eGFRcr and eGFRcys.
Supplemental Figure 8. Bias of different CKD-EPI eGFR equations across subgroups stratified by absolute discordance between eGFRcr and eGFRcys.
Supplemental Figure 9. Bias of different CKD-EPI eGFR equations across subgroups stratified by absolute discordance between eGFRcr and eGFRcys.
References
- 1.(KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. doi: 10.1038/kisup.2012.73 [DOI] [PubMed] [Google Scholar]
- 2.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 3.Inker LA Eneanya ND Coresh J et al.. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737–1749. doi: 10.1056/nejmoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soveri I Berg UB Bjork J et al.. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64(3):411–424. doi: 10.1053/j.ajkd.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Delgado C Baweja M Crews DC et al.. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32(12):2994–3015. doi: 10.1681/ASN.2021070988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert N, Shlipak MG. Cystatin C is ready for clinical use. Curr Opin Nephrol Hypertens. 2020;29(6):591–598. doi: 10.1097/mnh.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 7.Chen DC Shlipak MG Scherzer R et al.. Association of intraindividual difference in estimated glomerular filtration rate by creatinine vs cystatin C and end-stage kidney disease and mortality. JAMA Netw Open. 2022;5(2):e2148940. doi: 10.1001/jamanetworkopen.2021.48940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potok OA Ix JH Shlipak MG et al.. Cystatin C- and creatinine-based glomerular filtration rate estimation differences and muscle quantity and functional status in older adults: the Health, aging, and body composition study. Kidney Med. 2022;4(3):100416. doi: 10.1016/j.xkme.2022.100416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potok OA Ix JH Shlipak MG et al.. The difference between cystatin C- and creatinine-based estimated GFR and associations with frailty and adverse outcomes: a cohort analysis of the systolic blood pressure intervention trial (sprint). Am J Kidney Dis. 2020;76(6):765–774. doi: 10.1053/j.ajkd.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu EL Carrero JJ Sang Y et al.. Discordances between creatinine and cystatin C-based eGFR and adverse clinical outcomes in routine clinical practice [Abstract]. J Am Soc Nephrol. 2022;33:305.34607911 [Google Scholar]
- 11.Potok OA Katz R Bansal N et al.. The difference between cystatin C- and creatinine-based estimated GFR and incident frailty: an analysis of the cardiovascular health study (CHS). Am J Kidney Dis. 2020;76(6):896–898. doi: 10.1053/j.ajkd.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjork J Grubb A Larsson A et al.. Accuracy of GFR estimating equations combining standardized cystatin C and creatinine assays: a cross-sectional study in Sweden. Clin Chem Lab Med. 2015;53(3):403–414. doi: 10.1515/cclm-2014-0578 [DOI] [PubMed] [Google Scholar]
- 13.Grubb A, Nyman U, Bjork J. Improved estimation of glomerular filtration rate (GFR) by comparison of eGFRcystatin C and eGFRcreatinine. Scand J Clin Lab Invest. 2012;72(1):73–77. doi: 10.3109/00365513.2011.634023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrero JJ, Elinder CG. The Stockholm CREAtinine Measurements (SCREAM) project: fostering improvements in chronic kidney disease care. J Intern Med. 2022;291(3):254–268. doi: 10.1111/joim.13418 [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi: 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wettermark B Hammar N MichaelFored C et al.. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. doi: 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF Andersson E Ekbom A et al.. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooke HL Talback M Hornblad J et al.. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773. doi: 10.1007/s10654-017-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu EL Evans M Carrero JJ et al.. Timing of dialysis initiation to reduce mortality and cardiovascular events in advanced chronic kidney disease: nationwide cohort study. BMJ. 2021;375:e066306. doi: 10.1136/bmj-2021-066306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I; IFCC Working Group on Standardisation of Cystatin C WG-SCC. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–1621. doi: 10.1515/cclm.2010.318 [DOI] [PubMed] [Google Scholar]
- 21.Iohexol Clearance Protocol Stockholm. Accessed September 6, 2022. https://www.karolinska.se/49eb42/globalassets/global/2-funktioner/funktion-kul/klinisk-kemi/dokument-kopplade-till-pta/belastningar-anvisningar-och-protokoll/iohexolclerarance-pt-undersokning-for-externt-bruk.pdf. [Google Scholar]
- 22.Bird NJ, Peters C, Michell AR, Peters AM. Comparison of GFR measurements assessed from single versus multiple samples. Am J Kidney Dis. 2009;54(2):278–288. doi: 10.1056/nejmoa1114248 [DOI] [PubMed] [Google Scholar]
- 23.Inker LA Schmid CH Tighiouart H et al.. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/nejmoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. https://www.R-project.org/. [Google Scholar]
- 25.SBU, HTA. (2013). Methods to Estimate and Measure Renal Function (Glomerular Filtration Rate). A Systematic Review. Report no: 214. Stockholm, Swedish Council on Health Technology Assessment. [PubMed] [Google Scholar]
- 26.Shlipak MG, Mattes MD, Peralta CA. Update on cystatin C: incorporation into clinical practice. Am J Kidney Dis. 2013;62(3):595–603. doi: 10.1053/j.ajkd.2013.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inker LA, Titan S. Measurement and estimation of GFR for use in clinical practice: core curriculum 2021. Am J Kidney Dis. 2021;78(5):736–749. doi: 10.1053/j.ajkd.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 28.Stevens LA Schmid CH Greene T et al.. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003;14(4):1000–1005. doi: 10.1097/01.ASN.0000057856.88335.dd [DOI] [PubMed] [Google Scholar]
- 30.Nair S O'Brien SV Hayden K et al.. Effect of a cooked meat meal on serum creatinine and estimated glomerular filtration rate in diabetes-related kidney disease. Diabetes Care. 2014;37(2):483–487. doi: 10.2337/dc13-1770 [DOI] [PubMed] [Google Scholar]
- 31.Foster MC Levey AS Inker LA et al.. Non-GFR determinants of low-molecular-weight serum protein filtration markers in the elderly: AGES-kidney and MESA-kidney. Am J Kidney Dis. 2017;70(3):406–414. doi: 10.1053/j.ajkd.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X Foster MC Tighiouart H et al.. Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. doi: 10.1053/j.ajkd.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16(1):51–64. doi: 10.1038/s41581-019-0191-y [DOI] [PubMed] [Google Scholar]
- 34.Grubb A. Shrunken pore syndrome–a common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin Biochem. 2020;83:12–20. doi: 10.1016/j.clinbiochem.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 35.Grubb A. Glomerular filtration and shrunken pore syndrome in children and adults. Acta Paediatr. 2021;110(9):2503–2508. doi: 10.1111/apa.15846 [DOI] [PubMed] [Google Scholar]
- 36.de Boer IH Caramori ML Chan JCN et al.. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839–848. doi: 10.1016/j.kint.2020.06.024 [DOI] [PubMed] [Google Scholar]
- 37.Weir MR Lakkis JI Jaar B et al.. Use of renin-angiotensin system blockade in advanced CKD: an NKF-kdoqi controversies report. Am J Kidney Dis. 2018;72(6):873–884. doi: 10.1053/j.ajkd.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 38.Ebert N Bevc S Bokenkamp A et al.. Assessment of kidney function: clinical indications for measured GFR. Clin Kidney J. 2021;14(8):1861–1870. doi: 10.1093/ckj/sfab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlipak MG Matsushita K Arnlov J et al.. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/nejmoa1214234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Statistics Sweden. Statistikdatabasen. Population Statistics Web Site; 2021. http://www.statistikdatabasen.scb.se/sq/117607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data may be shared on reasonable request for academic research collaborations that fulfill GDPR as well as national and institutional ethics regulations and standards by contacting Juan-Jesus Carrero (juan.jesus.carrero@ki.se).