Abstract
Background
RBC folate concentrations are monitored at the population level, with a recommended threshold for optimal neural tube defect (NTD) prevention. A corresponding threshold for serum folate has not been established.
Objectives
This study aimed to estimate the serum folate insufficiency threshold corresponding to the RBC folate threshold for NTD prevention and examine how this threshold is modified by vitamin B12 status.
Methods
Participants were women (15–40 y; not pregnant or lactating; n = 977) from a population-based biomarker survey in Southern India. RBC folate and serum folate were measured via microbiologic assay. RBC folate deficiency (<305 nmol/L) and insufficiency (<748 nmol/L), serum vitamin B12 deficiency (<148 pmol/L) and vitamin B12 insufficiency (<221 pmol/L), elevated plasma MMA (>0.26 μmol/L), elevated plasma homocysteine (>10.0 μmol/L), and elevated HbA1c (≥6.5%) were evaluated. Bayesian linear models were used to estimate unadjusted and adjusted thresholds.
Results
Compared with adequate vitamin B12 status, the estimated serum folate threshold was higher in participants with serum vitamin B12 deficiency (72.5 vs. 28.1 nmol/L) or vitamin B12 insufficiency (48.7 vs. 24.3 nmol/L) and elevated MMA (55.6 vs. 25.9 nmol/L). The threshold was lower in participants with elevated HbA1c (HbA1c ≥6.5% vs. <6.5%; 21.0 vs. 40.5 nmol/L).
Conclusions
The estimated serum folate threshold for optimal NTD prevention was similar to previous reports (24.3 vs. 25.6 nmol/L) among participants with sufficient vitamin B12 status. However, this threshold was more than 2-fold higher in participants with vitamin B12 deficiency and substantially higher across all indicators of insufficient vitamin B12 status (<221 pmol/L, elevated MMA, combined B12, impaired vitamin B12 status), and lower in participants with elevated HbA1c. Findings suggest a serum folate threshold for NTD prevention may be possible in some settings; however, it may not be appropriate in populations with high prevalence of vitamin B12 insufficiency. Am J Clin Nutr 2023;xx:xx–xx.
This trial was registered at https://clinicaltrials.gov as NCT04048330.
Keywords: periconceptional, vitamin B12, folate, nutrition, India
Introduction
Neural tube defects (NTDs) are structural birth defects that result from failure of neural tube closure during embryonic development [1]. Over 260,000 NTDs occur globally each year (1–80 NTDs per 10,000 births), with the highest burdens in low and middle-income countries [2,3]. India is estimated to have among the highest burdens of NTDs [2,4,5].
Folate is required for DNA synthesis and cell division and is critical during periods of rapid growth and development [6]. The association of low maternal periconceptional folate status with NTD risk has been established [7,8]. Evidence from randomized trials [9,10] and a large community-based study [11] demonstrated periconceptional folic acid supplementation reduced NTD occurrence. These findings informed dietary recommendations for women of reproductive age in the United States [12] and folic acid fortification in over 70 countries [13]. Higher red blood cell (RBC) folate concentrations have been associated with lower risk of NTDs in observational studies [8,14] and Bayesian modeling analyses [15], and fortification of flour with folic acid has been consistently associated with reductions in NTDs at the population level [13,[16], [17], [18], [19], [20], [21]].
The World Health Organization (WHO) recommends monitoring RBC folate concentrations in women of reproductive age at the population level. A threshold of RBC folate ≥906 nmol/L, identified in an observational study and replicated through a Bayesian analysis, is recommended for optimal NTD prevention [15,22]. However, population-level RBC folate data is limited. Most national surveys evaluate serum or plasma folate, due in part to the reduced cost and complexity [6]. Of the 38 national surveys to date reporting folate status, 18 included RBC folate, of which 10 used the recommended microbiologic assay [23]. RBC folate reflects average folate intake during the past ∼90 to 120 d and has been consistently associated with NTD risk whereas serum/plasma folate reflects recent folate intake [6]. To our knowledge, no studies have evaluated associations between periconceptional serum/plasma folate and NTD risk.
A Bayesian analysis estimated a plasma folate threshold corresponding to the RBC folate threshold for optimal NTD prevention [24]. The findings identified a threshold of 27.2 nmol/L for optimal NTD prevention. This threshold was modified by vitamin B12 and overweight status; the authors concluded it would not be applicable in populations with a high burden of vitamin B12 deficiency [24]. Vitamin B12 is required for the conversion of 5-methyltetrahydrofolate (5-MTHF) to tetrahydrofolate (THF) and retention of folates in proliferating cells such as the developing RBC during erythropoiesis [6]. To our knowledge, no studies have estimated a serum or plasma folate threshold for NTD prevention in a population with high prevalence of vitamin B12 deficiency.
The objectives of this analysis were to estimate the serum folate threshold corresponding to the RBC folate threshold for optimal NTD prevention using data from a population-based biomarker survey in Southern India [25,26] and to evaluate how this threshold was modified by vitamin B12 status (e.g., vitamin B12 <148 pmol/L, <221 pmol/L), as well as other sociodemographic (e.g., age), nutritional (e.g., anemia, iron deficiency), and metabolic (e.g., BMI, HbA1c) characteristics.
Methods
Population
This analysis was conducted using data from a population-based biomarker survey in Southern India (NCT04048330). Participants were women 15 to 40 y, who were not pregnant or lactating. The design and findings from this study have been previously described [25,26]. Briefly, a census was conducted for all households in a 50 km2 catchment area; all rural households (n = 3124) and a random sample of urban households (n = 1000) were selected for screening and recruitment.
Ethics
The study protocol was reviewed and approved by the Institutional Review Board at Cornell University in the United States and the Institutional Ethics Committees at Arogyavaram Medical Centre and St. John’s Research Institute in India [25,26]. This study received clearance from the Indian Council of Medical Research Health Ministry Screening Committee and was registered at clincialtrials.gov (NCT04048330). The protocol for the biomarker survey was reviewed in accordance with the US Centers for Disease Control and Prevention human research protection procedures and was determined to be a nonresearch, routine surveillance activity. Written informed consent (≥18 y) or assent (15 to <18 y) was obtained from all participants before the start of data collection.
Data collection
Data was collected at Arogyavaram Medical Centre on electronic tablets, using standardized procedures [25,27]. Data collection included sociodemographic, dietary, anthropometric, and clinical information, and biological specimens (i.e., blood, saliva, urine). Anthropometric measurements included weight (via Seca 874 DR Doctor’s scale to the nearest 0.01 kg), height (via Shorr HeightLite stadiometer to the nearest 0.1 cm), and hip and waist circumference (WC) (via tape measure to the nearest 0.1 cm). BIA (BC-148 MA; Tanita Corporation; 8 electrode) was conducted among adults (≥18 y).
Laboratory analyses
Venous blood samples were collected by a trained phlebotomist, processed, and stored using standardized protocols [25]. Whole-blood samples were analyzed for hemoglobin (Hb) via automated Coulter counter (Coulter HMX) and HbA1c via nephelometry (Mispa i2 Specific Protein Analyzer, Agappe Diagnostics). Plasma, serum, and RBCs were processed and stored at -80ºC or less until batch analysis at the end of data collection. RBC folate and serum folate concentrations were measured using the WHO-recommended microbiologic assay [28]. Serum total vitamin B12 and serum ferritin concentrations were assessed via chemiluminescence on the Roche E411 analyzer (Roche Diagnostics). Soluble transferrin recepter (sTfR), serum C-reactive protein (CRP), and α-1 acid glycoprotein (AGP) concentrations were analyzed via immunoturbidimetry on the Roche COBAS Integra 400 plus analyzer (Roche Diagnostics).
Plasma methylmalonic acid (MMA) and plasma homocysteine (Hcy) were assessed by gas chromatography-mass spectrometry in a methylchloroformate derivatization (GC-MS, 5975, Agilent Technologies). The concentration ranges and mean coefficients of variation for quality controls measured in each assay were: serum folate (low: 5.6–11.3 nmol/L, 7.43%; high: 20.6–41.9 nmol/L, 7.12%), RBC folate (low: 127–300 nmol/L, 8.8%; high: 322–595 nmol/L, 7.5%), vitamin B12 (low: 296–445 pg/mL, 7.5%; medium: 322–595 pg/mL, 6.1%; high: 607–834 pg/mL, 4.5%), serum ferritin (low: 5.3–88.0 ng/mL, 2.4%; medium: 128–196 ng/mL, 3.7%; high: 276–424 ng/mL, 1.5%), CRP (low: 8.5–10.4 mg/L, 2.8%; high: 26.7–32.6 mg/L, 2.6%), sTfR (low: 2.0–2.5 mg/L, 3.5%; high: 6.6–8.1 mg/L, 3.1%), AGP (low: 0.4–0.6 g/L, 2.9%; high: 0.7–1.0 g/L, 2.2%), MMA (in-house pooled plasma: 0.4–0.5 μmol/L, 3.7%), Hcy (in-house pooled plasma: 6.8–8.1 μmol/L, 2.8%).
Definitions of variables
Folate deficiency was defined as RBC folate <305 nmol/L (i.e., risk of macrocytic anemia), and folate insufficiency was defined as RBC folate <748 nmol/L, the recommended calibrator-adjusted equivalent of the RBC folate threshold for optimal NTD prevention [22]. Anemia was defined as Hb <12.0 g/dL, and severe anemia was defined as Hb <8.0 g/dL [29]. Vitamin B12 deficiency and vitamin B12 insufficiency were defined as vitamin B12 <148 and <221 pmol/L, respectively [30]. Elevated MMA was defined as MMA >0.26 and >0.37 μmol/L [30,31]. Impaired vitamin B12 status was defined as total vitamin B12 <148 pmol/L and MMA >0.26 μmol/L. A composite indicator of vitamin B12 status (i.e., combined B12; cB12) using 3 biomarkers (i.e., vitamin B12, MMA, Hcy) was calculated and categorized (i.e., ≥1.5, -0.5 to <1.5, -1.5 to <-0.5, -2.5 to <-1.5, <-2.5) using methods developed by Fedosov et al [32]. Elevated Hcy was defined as >15.0 and >10.0 μmol/L [6]. Iron deficiency was defined as serum ferritin <15.0 μg/L; iron insufficiency was defined as serum ferritin <20.0 and <25.0 μg/L [33,34]. Body iron index was estimated using an equation proposed by Cook after converting the Roche sTfR data from this study to data equivalent to the original Flowers enzyme-linked immunosorbent assay used in the development of the body iron model (i.e., via the equation Flowers sTfR = 1.5 × Roche sTfR + 0.35 mg/L) [35]. Elevated inflammatory biomarkers were defined as CRP >5.0 mg/L or AGP >1.0 g/L [33]; additional definitions of CRP >3.0 mg/L and >1.0 mg/L [36] were also considered.
BMI was calculated as weight (kg) divided by height (m) squared and was categorized among adults (≥18 y) using WHO criteria (i.e., BMI <18.5, 18.5 to <25.0, 25.0 to <30.0, and ≥30.0 kg/m2) [37]. Additional anthropometric measurements (e.g., elevated WC, elevated WHR) were categorized among adults (≥18 y) using WHO criteria [38], and elevated whole body and trunk fat percent (%) were defined based on criteria suggested by the American Association of Clinical Endocrinology [39]. HbA1c levels were categorized as <5.7%, ≥5.7% to <6.5%, and ≥6.5%, using American Diabetes Association criteria [40] and also defined as <5.7% and ≥5.7%.
The primary outcomes for the population-based biomarker survey were Hb concentrations, anemia; RBC folate and serum folate concentrations, RBC folate deficiency and insufficiency; and vitamin B12 concentrations, vitamin B12 deficiency and insufficiency. The current analysis leverages paired RBC folate and serum folate biomarker data to estimate a population-based serum folate insufficiency threshold corresponding to the RBC folate threshold for optimal NTD prevention.
Statistical analyses
Descriptive analyses.
Continuous biomarker variables were natural logarithmically transformed prior to analyses. Biomarkers outside of the assay limits of detection (LOD) were set to half the LOD (below the lower LOD), or 2 times the LOD (above the upper LOD). Hb was adjusted for self-reported smoking status [29]. Serum ferritin concentrations were adjusted for inflammation using Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) methods [41]. Continuous variables were described using geometric means and 95% confidence intervals.
Bayesian linear models.
The Bayesian framework was used to develop population-specific Bayesian linear models, to estimate RBC folate concentrations as a function of serum folate concentrations using procedures developed by Crider et al. [15,24]. Briefly, the joint posterior probability distribution for parameters was calculated using Markov chain Monte Carlo simulations. Parameters were estimated using a posterior sample of 10,000, generated by selecting 500,000 samples (i.e., with a post burn-in of 10,000 samples), and thinning by retaining every 50th sample. Mildly informative priors of N (mean: 0, standard deviation:10) were used for all parameters. For each model, the 10,000 posterior samples of the estimated serum folate insufficiency threshold corresponding to the RBC folate threshold of <748 nmol/L were summarized by reporting the median (50th percentile) and 95% credible interval (i.e., the plausible range of values within which an unobserved parameter value falls with a 95% probability; 2.5th and 97.5th percentiles). The Bayesian credible interval describes which parameters lie in a given probability range. A 95% credible interval is a range of parameters/values that account for 95% of the posterior distribution.
Stratified analyses.
Sociodemographic (e.g., age, parity), nutritional (e.g., anemia, vitamin B12 deficiency), and metabolic (e.g., BMI categories, elevated HbA1c) characteristics were considered in stratified analyses, including variables identified in the previous analysis of the plasma folate insufficiency threshold for NTD prevention [24]. Variables were included in models as interaction terms to facilitate estimation of serum folate threshold by strata of interest, and strata were considered substantially different if the 95% Credible Interval of the difference did not include zero.
Multivariable analyses.
Associations between variables considered for adjustment (e.g., sociodemographic, nutritional, and metabolic characteristics) were examined using unsupervised hierarchical clustering methods. Both individual variables and identified clusters (Supplementary Figures 1–5) were considered for inclusion in adjusted models. Multivariable models from the previous Bayesian analysis [24] were also replicated to examine comparability of findings (Supplementary Table 1). Missing data for covariates (<2%) were assumed to be missing at random and estimated jointly with model parameters of interest. The findings for analyses with and without simulation of missing data were similar; for interpretation purposes, results from full-case models are reported.
Sensitivity analyses.
Sensitivity analyses were also conducted within strata, including vitamin B12 status (i.e., vitamin B12 <148, ≥148, <221, and ≥221 pmol/L).
Statistical analyses were conducted using SAS 9.4 (SAS Institute, Inc). All models were estimated using SAS MCMC. Analyses were reproduced by the Cornell Results Reproduction, R2 at the Cornell Institute for Social and Economic Research.
Results
Characteristics of the study population
Characteristics of participants in this study are presented in Table 1, and a flow chart of study participants is presented in Figure 1. Overall, 79.3% of participants had RBC folate <748 nmol/L, 41.5% had anemia (Hb <12.0 g/dL), 48.3% had vitamin B12 deficiency (<148 pmol/L), and 37.9% had impaired vitamin B12 status (vitamin B12 <148 pmol/L and MMA >0.26 μmol/L). A total of 16.1% had Hcy >10.0 μmol/L and 25.1% had HbA1c ≥5.7%. In adults (≥18 y), 19.2% were underweight (BMI <18.5 kg/m2), 48.0% were normal weight (≥18.5 to <25.0 kg/m2), 23.2% were overweight (≥25.0 to <30.0 kg/m2), and 9.6% were obese (≥30.0 kg/m2) based on WHO criteria (Table 1, Figure 2).
TABLE 1.
Characteristics of the study population (n = 977)1.
| Variables | n | Geometric Mean (95% Confidence Interval) or n (%) |
|---|---|---|
| Sociodemographic | ||
| Age, y | 977 | 28.8 (28.4, 29.3) |
| 15 to <18 | 49 (5.0) | |
| 18 to <26 | 228 (23.3) | |
| 26 to <36 | 452 (46.3) | |
| 36 to 40 | 248 (25.4) | |
| Biochemical | ||
| RBC folate2, nmol/L | 977 | 540.5 (526.2, 555.1) |
| <305 | 74 (7.6) | |
| <748 | 775 (79.3) | |
| Serum folate2, nmol/L | 977 | 16.5 (16.0, 17.0) |
| <7.0 | 34 (3.5) | |
| Hemoglobin3, g/dL | 977 | 11.9 (11.7, 12.0) |
| <12.0 | 405 (41.5) | |
| <8.0 | 29 (3.0) | |
| Serum ferritin4, μg/L | 975 | 10.6 (9.9, 11.2) |
| <15.0 | 599 (61.4) | |
| <20.0 | 709 (72.7) | |
| <25.0 | 790 (81.0) | |
| Body iron index5, mg/kg | 975 | -0.7 (-1.0, -0.4) |
| <0.0 | 506 (51.9) | |
| Serum CRP6, mg/L | 977 | 1.2 (1.1,1.4) |
| ≤1.0 | 425 (43.5) | |
| >1.0 to ≤3.0 | 261 (26.7) | |
| >3.0 to ≤5.0 | 123 (12.6) | |
| >5.0 | 168 (17.2) | |
| Serum α-1 acid glycoprotein7, g/L | 975 | 0.8 (0.8,0.8) |
| >1.0 | 216 (22.2) | |
| Serum vitamin B128, pmol/L | 977 | 155.9 (150.1, 161.8) |
| <148 | 472 (48.3) | |
| 148–221 | 255 (26.1) | |
| ≥221 | 250 (25.6) | |
| Plasma MMA8, μmol/L | 977 | 0.3 (0.3, 0.3) |
| >0.26 | 592 (60.6) | |
| >0.37 | 432 (44.2) | |
| Impaired vitamin B12status8 | 977 | |
| Vitamin B12 <148 pmol/L and MMA >0.26 μmol/L | 370 (37.9) | |
| Plasma homocysteine9, μmol/L | 977 | 6.6 (6.4, 6.8) |
| >10.0 | 157 (16.1) | |
| >15.0 | 65 (6.7) | |
| cB128 | 977 | -0.35 (-0.41, -0.30) |
| ≥1.5 | 20 (2.1) | |
| -0.5 to <1.5 | 517 (52.9) | |
| -1.5 to <-0.5 | 333 (34.1) | |
| -2.5 to <-1.5 | 100 (10.2) | |
| <-2.5 | 7 (0.7) | |
| ≥-0.5 | 537 (55.0) | |
| <-0.5 | 440 (45.0) | |
| Anthropometric | ||
| BMI, kg/m2 | 966 | 22.6 (22.2, 22.9) |
| BMI10,11 | 917 | |
| <18.5 | 176 (19.2) | |
| 18.5 to <25.0 | 440 (48.0) | |
| 25.0 to <30.0 | 213 (23.2) | |
| ≥30.0 | 88 (9.6) | |
| Waist circumference10,12, cm | 966 | 74.6 (73.9, 75.4) |
| >88.9 | 917 | 123 (13.4) |
| Waist-hip ratio10,12 | 917 | 0.8 (0.8, 0.8) |
| ≥0.85 | 191 (20.8) | |
| Metabolic characteristics | ||
| Whole body fat10,12, % | 919 | 30.6 (30.0, 31.2) |
| >35 | 360 (39.2) | |
| Trunk fat mass10,12, % | 919 | 27.8 (27.0, 28.7) |
| >35 | 332 (36.1) | |
| HbA1c13, % | 977 | 5.4 (5.4, 5.5) |
| <5.7 | 732 (74.9) | |
| ≥5.7 to <6.5 | 196 (20.1) | |
| ≥6.5 | 49 (5.0) | |
Geometric means and 95% Confidence Intervals were generated using Proc Genmod among all participants with RBC folate (n = 977). BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; cB12, combined B12.
Cutoffs for RBC folate deficiency (RBC folate: <305 nmol/L) and RBC folate insufficiency (RBC folate: <748 nmol/L)
Hemoglobin values from complete blood count; adjusted for smoking status [29]
Serum ferritin concentrations were adjusted for inflammation via BRINDA methods [41]; cutoffs for iron deficiency (serum ferritin <15.0 μg/L) and iron insufficiency (serum ferritin <20.0 and <25.0 μg/L) from [33,34]
Values are arithmetic mean (95% CI); body iron index (mg/kg) was estimated using Cook’s equation after adjusting serum ferritin for inflammation via BRINDA methods [41] and converting sTfR to Flowers Ramco equivalents (Ramco = 1.5 × Roche + 0.35) [35]
α-1 acid glycoprotein cutoff (>1.0 g/L) based on WHO criteria [33]
Cutoffs for vitamin B12 deficiency (<148 pmol/L) and vitamin B12 insufficiency (<221 pmol/L) from (30); cB12 was calculated using vitamin B12, MMA, and homocysteine, and categorized (i.e., ≥1.5, -0.5 to <1.5, -1.5 to <-0.5, -2.5 to <-1.5, <-2.5) using methods developed by Fedosov et al. [32]
Elevated vitamin B12 (≥1.5), Adequate vitamin B12 (-0.5 to <1.5), Decreased vitamin B12 (-1.5 to <-0.5) , Possibly deficient (-2.5 to <-1.5) , Probably deficient (<-2.5) Sufficient vitamin B12 (-0.5 to >1.5), Deficient vitamin B12 (<-0.5).
Homocysteine cutoffs (>15.0 and >10.0 μmol/L) from [6]
Among adult participants (>18 y; n = 928)
BMI categories (BMI <18.5, 18.5 to <25.0, 25.0 to <30.0, and ≥30.0 kg/m2) as defined by the WHO [37]
Anthropometric cutoffs (waist circumference >88.9 cm and waist-hip ratio ≥0.85) were defined based on WHO criteria [38]; elevated whole body (>35%) and trunk fat (>35%) were defined based on criteria suggested by the American Association of Clinical Endocrinology [39]
HbA1c cutoffs (<5.7%, ≥5.7% to <6.5%, and ≥6.5%) were defined using the American Diabetes Association criteria [40]
FIGURE 1.
Participant flow chart.
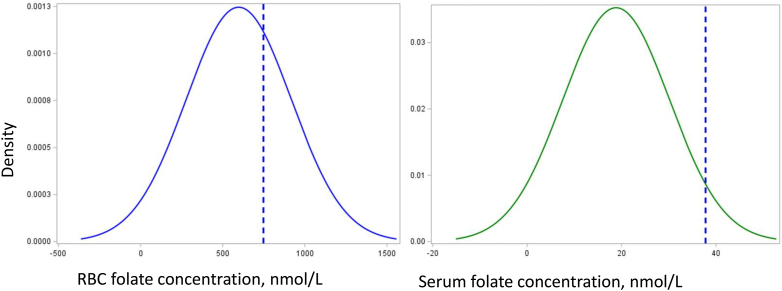
FIGURE 2.
Serum folate and RBC folate distributions in the population, with corresponding insufficiency thresholds for optimal NTD prevention.
RBC folate (n = 977, blue curve) and serum folate (n = 977, green curve) concentrations for this population. The blue dashed lines correspond to the RBC folate threshold for optimal NTD prevention and the corresponding estimated serum folate insufficiency threshold. Abbreviations: NTD, neural tube defect.
Estimated serum folate insufficiency threshold.
The overall estimated median serum folate insufficiency threshold corresponding to the RBC folate threshold for optimal NTD prevention in this population was 37.8 nmol/L (95% credible interval: 33.9, 43.3) (Table 2).
TABLE 2.
Estimated serum folate insufficiency threshold corresponding to the RBC folate threshold for optimal NTD prevention: Overall and by sociodemographic, biochemical, and metabolic characteristics1.
| Serum folate, nmol/L | Difference | ||
|---|---|---|---|
| Variables | n2 | Median (95% Credible Interval) | Median (95% Credible Interval) |
| Overall | 977 | 37.8 (33.9, 43.3) | n/a |
| Sociodemographic characteristics | |||
| Age, y | |||
| <26 | 277 | 54.0 (41.8, 81.5) | 21.9 (6.6, 49.8) |
| 26–36 | 452 | 33.9 (29.7, 40.5) | 2.0 (-7.9, 10.2) |
| ≥36 | 248 | 31.9 (26.9, 41.1) | Reference |
| Iron status | |||
| Hemoglobin3, g/dL | |||
| <12.0 | 405 | 32.8 (28.5, 40.2) | -9.1 (-19.7, 0.4) |
| ≥12.0 | 572 | 42.0 (36.0, 51.4) | Reference |
| Serum ferritin4, μg/L | |||
| <15.0 | 599 | 34.4 (30.7, 39.8) | -10.6 (-28.1, -0.5) |
| ≥15.0 | 376 | 45.2 (36.4, 62.1) | Reference |
| <20.0 | 709 | 36.4 (32.2, 42.6) | -4.4 (-19.1, 5.1) |
| ≥20.0 | 266 | 40.8 (33.4, 55.8) | Reference |
| <25.0 | 790 | 37.3 (33.0, 43.2) | -1.8 (-18.9, 7.8) |
| ≥25.0 | 185 | 39.1 (31.7, 55.9) | Reference |
| Body iron index5, mg/kg | |||
| <0.0 | 378 | 32.9 (29.2, 38.3) | -12.3 (-27.5, -3.0) |
| ≥0.0 | 597 | 45.3 (37.4, 59.9) | Reference |
| Specifically, for serum ferritin, CRP, serum B12, MMA, cB12, and HbA1c | |||
| Serum CRP 6, mg/L | |||
| ≤1.0 | 425 | 41.6 (35.6, 51.4) | Reference |
| >1.0 to ≤3.0 | 261 | 36.6 (30.7, 47.4) | -4.9 (-16.5, 7.3) |
| >3.0 to ≤5.0 | 123 | 28.8 (23.9, 38.4) | -12.7 (-23.7, -1.7) |
| >5.0 | 168 | 29.3 (23.4, 42.8) | -12.2 (-23.8, 1.8) |
| >1.0 | 552 | 33.4 (29.5, 39.4) | -8.1 (-19.2, 0.7) |
| ≤1.0 | 425 | 41.5 (35.4, 52.0) | Reference |
| >3.0 | 291 | 29.5 (25.2, 36.9) | -10.3 (-18.3, -1.6) |
| ≤3.0 | 686 | 39.8 (35.2, 46.8) | Reference |
| >5.0 | 168 | 29.3 (23.3, 43.7) | -8.8 (-17.0, 5.1) |
| ≤5.0 | 809 | 38.1 (34.2, 43.7) | Reference |
| Serum α-1 acid glycoprotein7, g/L | |||
| >1.0 | 216 | 33.5 (26.9, 48.1) | -4.7 (-13.6, 10.6) |
| ≤1.0 | 759 | 38.2 (34.1, 44.2) | Reference |
| Vitamin B12status | |||
| Serum vitamin B128, pmol/L | |||
| <148 | 472 | 72.5 (52.1, 125.1) | 44.2 (23.6, 97.3) |
| ≥148 | 505 | 28.1 (25.7, 31.5) | Reference |
| <221 | 727 | 48.7 (41.0, 61.3) | 24.3 (15.9, 37.5) |
| ≥221 | 250 | 24.3 (21.7, 27.8) | Reference |
| Plasma MMA8, μmol/L | |||
| >0.26 | 592 | 55.6 (44.8, 75.7) | 29.6 (18.3, 49.8) |
| ≤0.26 | 545 | 25.9 (23.5, 29.3) | Reference |
| >0.37 | 432 | 68.3 (50.1, 115.7) | 39.9 (21.5, 87.6) |
| ≤0.37 | 545 | 28.2 (25.7, 31.6) | Reference |
| Impaired vitamin B12status8 | |||
| Vitamin B12 <148 pmol/L and MMA >0.26 μmol/L | 370 | 78.0 (53.7, 154.9) | 48.5 (24.1, 125.3) |
| Not impaired vitamin B12 status | 607 | 29.3 (26.7, 32.8) | Reference |
| Plasma homocysteine9, μmol/L | |||
| >10.0 | 157 | 114.9 (60.3, 485.3) | 79.7 (25.3, 451.3) |
| ≤10.0 | 820 | 34.7 (31.2, 39.6) | Reference |
| cB128 | |||
| ≥1.5 | 20 | 14.6 (9.3, 22.4) | -14.7 (-21.5, -6.5) |
| -0.5 to <1.5 | 517 | 29.4 (26.6, 33.0) | Reference |
| -1.5 to <-0.5 | 333 | 54.2 (46.0, 66.5) | 24.9 (17.6, 35.5) |
| -2.5 to <-1.5 | 100 | 70.7 (55.3, 94.6) | 41.3 (26.9, 63.5) |
| <-2.5 | 7 | 123.2 (57.4, 280.1) | 93.8 (28.8, 250.0) |
| ≥-0.5 | 537 | 28.4 (25.8, 31.8) | Reference |
| <-0.5 | 440 | 57.4 (48.9, 70.0) | 28.9 (21.3, 40.1) |
| BMI10,11, kg/m2 | |||
| <18.5 | 176 | 43.3 (34.4, 63.3) | 9.5 (-1.5, 30.0) |
| 18.5 to <25.0 | 440 | 33.7 (29.6, 39.9) | Reference |
| 25.0 to <30.0 | 213 | 37.4 (29.4, 55.5) | 3.7 (-6.4, 22.3) |
| ≥30.0 | 88 | 25.3 (21.1, 32.9) | -8.4 (-16.0, 0.0) |
| Waist circumference10,12, cm | |||
| >88.9 | 123 | 26.6 (22.6, 33.7) | -10.5 (-17.6, -2.4) |
| ≤88.9 | 794 | 37.1 (33.1, 43.0) | Reference |
| Waist-hip ratio10,12 | |||
| ≥0.85 | 191 | 30.5 (25.2, 41.0) | -5.6 (-13.2, 5.4) |
| <0.85 | 726 | 36.0 (32.3, 41.4) | Reference |
| Whole body fat10,12, % | |||
| >35 | 360 | 31.0 (27.1, 37.4) | -7.1 (-15.4, 0.8) |
| ≤35 | 559 | 38.1 (33.3, 45.6) | Reference |
| Trunk fat10,12, % | |||
| >35 | 332 | 30.7 (26.7, 37.1) | -7.3 (-15.6, 0.7) |
| ≤35 | 587 | 38.1 (33.4, 45.4) | Reference |
| HbA1c13, % | |||
| <5.7 | 732 | 43.8 (37.5, 53.7) | Reference |
| ≥5.7 | 245 | 28.2 (24.9, 33.3) | -15.5 (-25.9, -7.5) |
| <6.5 | 928 | 40.5 (35.8, 47.3) | Reference |
| ≥6.5 | 49 | 21.0 (17.8, 25.6) | -19.5 (-27.2, -13.0) |
Results outside assay LOD were set to ½ LOD (if below LOD) or 2 ×LOD (if above LOD); Results outside assay LODs: serum folate (n = 1 below LOD), vitamin B12 (n = 4 below LOD, n = 6 above LOD). BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; cB12, combined B12; LOD, limit of detection; sTfR, soluble transferrin receptor; WHO, World Health Organization; 95% CI, 95% credible interval.
Individuals contributing data to the Bayesian simulations by strata; reported results are summarizing the posterior distributions of 10,000 iterations
Hemoglobin values from complete blood count; adjusted for smoking status [29]
Serum ferritin concentrations were adjusted for inflammation via BRINDA methods [41]; cutoffs for iron deficiency (serum ferritin <15.0 μg/L) and iron insufficiency (serum ferritin <20.0 and <25.0 μg/L) from [33,34]
Body iron index (mg/kg) was estimated using Cook’s equation after adjusting serum ferritin for inflammation via BRINDA methods [41] and converting sTfR to Flowers Ramco equivalents (Ramco = 1.5 × Roche + 0.35) [35].
CRP cutoffs for inflammation (>5.0 mg/L) and metabolic risk (>3.0 mg/L and >1.0 mg/L) from [33]
α-1 acid glycoprotein cutoff (>1.0 g/L) based on WHO criteria [33]
Cutoffs for vitamin B12 deficiency (<148 pmol/L) and vitamin B12 insufficiency (<221 pmol/L) from (30); cB12 was calculated using vitamin B12, MMA, and homocysteine and categorized (i.e., ≥1.5, -0.5 to <1.5, -1.5 to <-0.5, -2.5 to <-1.5, <-2.5) using methods developed by Fedosov et al. [32]
Elevated vitamin B12 (≥1.5), Adequate vitamin B12 (-0.5 to <1.5), Decreased vitamin B12 (-1.5 to <-0.5) , Possibly deficient (-2.5 to <-1.5) , Probably deficient (<-2.5) Sufficient vitamin B12 (-0.5 to >1.5), Deficient vitamin B12 (<-0.5).
Homocysteine cutoffs (>15.0 and >10.0 μmol/L) from [6]
Among adult participants (≥18 y; n = 928)
BMI categories (<18.5, 18.5 to <25.0, 25.0 to <30.0, and ≥30.0 kg/m2) as defined by the WHO [37]
Anthropometric cutoffs (waist circumference >88.9 cm and waist-hip ratio ≥ 0.85) were defined based on WHO criteria [38]; elevated whole body (>35%) and trunk fat (>35%) were defined based on criteria suggested by the American Association of Clinical Endocrinology [39]
HbA1c cutoffs (<5.7%, ≥5.7% to <6.5%, and ≥6.5%) were defined using the American Diabetes Association criteria [40]
Iron status.
The estimated serum folate insufficiency threshold did not differ substantially by anemia strata [Hb <12.0 g/dL vs. ≥12.0 g/dL; difference: -9.1 (-19.7, 0.4)]. However, the estimated threshold was lower in individuals with iron deficiency [BRINDA-adjusted serum ferritin <15.0 μg/L vs. ≥15.0 μg/L; difference: -10.6 (-28.1, -0.5) nmol/L] and in participants with low body iron index [body iron index <0.0 mg/kg vs. ≥0.0 mg/kg; difference: -12.3 (-27.5, -3.0) nmol/L] but did not substantially differ by iron insufficiency (i.e., serum ferritin <20.0 μg/L or <25.0 μg/L).
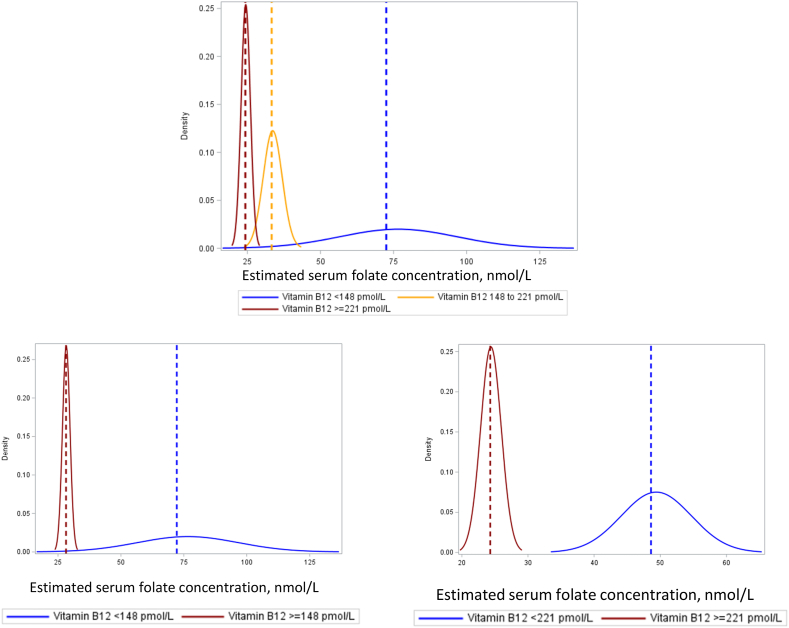
Vitamin B12status. The estimated serum folate insufficiency threshold was 72.5 (52.1, 125.1) nmol/L in participants with vitamin B12 deficiency (<148 pmol/L), compared with 28.1 (25.7, 31.5) nmol/L in individuals who were not vitamin B12 deficient (≥148 pmol/L), with a 44.2 (23.6, 97.3) nmol/L difference between vitamin B12 deficiency strata (Table 2, Figure 3). The estimated threshold was 48.7 (41.0, 61.3) nmol/L in participants with vitamin B12 <221 pmol/L, compared with 24.3 (21.7, 27.8) nmol/L in individuals with vitamin B12 ≥221 pmol/L [vitamin B12 <221 pmol/L vs. vitamin B12 ≥221 pmol/L; difference: 24.3 (15.9, 37.5) nmol/L] (Table 2, Figure 3). The estimated threshold was also higher in participants with MMA >0.26 μmol/L [difference: 29.6 (18.3, 49.8) nmol/L], impaired vitamin B12 status [vitamin B12 <148 pmol/L and MMA >0.26 μmol/L vs. not; difference: 48.5 (24.1, 125.3) nmol/L], or cB12 <-0.5 [cB12 <-0.5 vs. cB12 ≥-0.5; difference: 28.9 (21.3, 40.1) nmol/L].
FIGURE 3.
Estimated serum folate insufficiency threshold by vitamin B12 strata [n = 472 (<148 pmol/L), 255 (148–221 pmol/L), and 250 (≥221 pmol/L)].
Estimated serum folate concentrations corresponding to the RBC folate concentration of 748 nmol/L for optimal NTD prevention, by vitamin B12 status. The dashed lines correspond to the estimated median. Abbreviations: NTD, neural tube defect.
Elevated homocysteine. The estimated threshold was higher in participants with Hcy >10.0 μmol/L [difference: 79.7 (25.3, 451.3) nmol/L] (Table 2).
Metabolic characteristics.
The estimated serum folate insufficiency thresholds by metabolic risk factors are presented in Table 2.
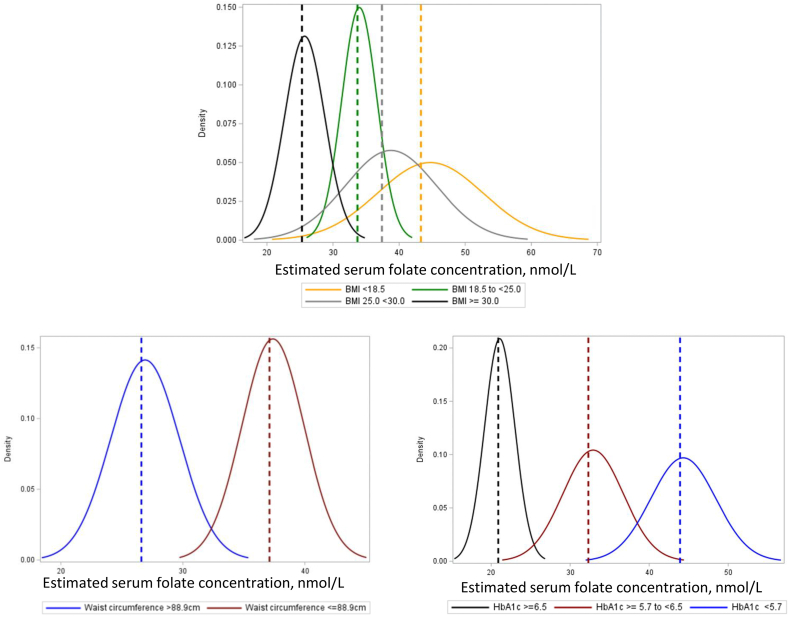
Body mass index. In analyses in adults (≥18 y), the estimated threshold did not substantially differ across BMI categories or by overweight strata (Table 2, Figure 4).
FIGURE 4.
Estimated serum folate insufficiency threshold by BMI, waist circumference, and elevated HbA1c strata Estimated serum folate concentrations corresponding to the RBC folate concentration of 748 nmol/L, by BMI [n =176 (<18.5 kg/m2), 440 (18.5 to <25.0 kg/m2), 213 (25.0 to <30.0 kg/m2), and 88 (≥30.0 kg/m2)], waist circumference [n = 123 (>88.9 cm) and 794 (≤88.9 cm)], and elevated HbA1c strata [(n = 732 (<5.7%), 196 (≥5.7 to <6.5%), and 49 (≥6.5%)]. The dashed lines correspond to the estimated median. Abbreviations: HbA1c, glycated hemoglobin; NTD: neural tube defect.
Central adiposity. In analyses in adults, the estimated serum folate insufficiency threshold was 10.5 nmol/L lower in participants with elevated WC (WC >88.9 cm vs. ≤88.9 cm; difference: -10.5 nmol/L (-17.6, -2.4) nmol/L] (Table 2, Figure 4). The serum folate insufficiency threshold did not substantially differ by elevated waist-hip ratio, elevated whole body fat mass, or elevated trunk fat mass (Table 2).
Elevated HbA1c. The estimated serum folate insufficiency threshold was lower in participants with elevated HbA1c, defined as HbA1c ≥6.5% [≥6.5% vs. <6.5%; difference: -19.5 (-27.2, -13.0) nmol/L] or HbA1c ≥5.7% [≥5.7% vs. <5.7%; difference: -15.5 (-25.9, -7.5) nmol/L] (Table 2, Figure 4).
Sensitivity analyses.
In analyses among participants with vitamin B12 ≥148 pmol/L, the estimated serum folate insufficiency threshold was 28.1 (25.6, 31.6) nmol/L. The estimated threshold was higher in participants with elevated MMA [MMA >0.26 μmol/L vs. ≤0.26 μmol/L; difference: 14.4 (6.8, 29.5) nmol/L; MMA >0.37 μmol/L vs. ≤0.37 μmol/L; difference: 17.7 (7.8, 41.8) nmol/L] or elevated Hcy [>10.0 μmol/L vs. ≤10.0 μmol/L; difference: 24.4 (2.5, 248.2) nmol/L], and lower in participants with elevated CRP [CRP >3.0 mg/L vs. ≤3.0 mg/L; difference: -7.8 (-13.3, -2.2) nmol/L] but did not vary substantially by age, anemia, iron deficiency, or other elevated inflammatory biomarkers (data not shown). In contrast, the serum folate insufficiency threshold was lower in participants with elevated WC [WC >88.9 vs. ≤88.9 cm; difference: -6.9 (-12.0, -1.6) nmol/L], elevated WHR [WHR ≥0.85 vs. <0.85; difference: -8.2 (-13.1, -3.1) nmol/L], elevated whole body fat [>35% vs. ≤35%; difference: -6.1 (-11.9, -0.8) nmol/L], and elevated trunk fat [>35% vs. ≤35%; difference: -6.5 (-12.1, -1.4) nmol/L]. The estimated threshold was also lower in participants with HbA1c ≥6.5% [≥6.5% vs. <6.5%; difference: -9.5 (-15.9, -2.4) nmol/L] (data not shown).
In analyses among participants with vitamin B12 ≥221 pmol/L, the estimated serum folate insufficiency threshold was 24.3 (21.5, 28.2) nmol/L. This threshold was lower among participants with low body iron index [<0.0 mg/kg vs. ≥0.0 mg/kg; difference: -9.6 (-21.7, -2.9) nmol/L] and in participants with elevated WHR [WHR ≥0.85 vs. <0.85; difference: -7.7 (-14.1, -1.5) nmol/L], but did not vary by other sociodemographic, nutritional, or metabolic characteristics.
Multivariable analyses.
The estimated serum folate insufficiency thresholds from multivariable analyses are summarized in Supplementary Tables 1–3. The overall serum folate insufficiency threshold was 36.7 (19.5, 68.6) nmol/L, after adjusting for Hb, CRP, and age. In multivariable analyses stratified by vitamin B12 status, the estimated serum folate insufficiency threshold was higher in participants with vitamin B12 <148 pmol/L [63.0 (26.3, 164.3) vs. 28.0 (17.0, 46.2) nmol/L] or vitamin B12 <221 pmol/L [46.1 (23.3, 89.9) vs. 24.2 (13.7, 44.3) nmol/L]. Findings were similar when models were adjusted for clusters identified through unsupervised hierarchical clustering methods.
Discussion
Monitoring RBC folate concentrations in women of reproductive age at the population level for optimal NTD prevention is recommended by the WHO. However, most countries monitor serum/plasma folate concentrations [23]; the WHO recommended that the relationship between serum folate and RBC folate concentrations be established at the population level to inform a corresponding serum folate threshold for optimal NTD prevention [22]. In this Bayesian analysis of population-based biomarker data from Southern India, the serum folate threshold was similar to the previously published threshold (24.3 vs. 25.6 nmol/L) among participants with sufficient vitamin B12 status. However, this threshold was more than 2-fold higher in individuals with vitamin B12 deficiency and substantially higher across other indicators of insufficient vitamin B12 status (<221 pmol/L, elevated MMA, cB12, impaired vitamin B12 status) and elevated Hcy but lower in participants with elevated HbA1c. These findings suggest that a serum folate threshold for NTD prevention may be possible in some settings; however, it may not be appropriate in populations with a high prevalence of vitamin B12 insufficiency.
In this study, the serum folate insufficiency threshold for optimal NTD prevention was 44.2 nmol/L higher in individuals with vitamin B12 deficiency (i.e., 72.5 vs. 28.1 nmol/L). This is consistent with previous Bayesian analyses in China [24], which identified a higher plasma folate insufficiency threshold in participants with vitamin B12 <148 pmol/L. The magnitude of the threshold observed here was higher (i.e., 72.5 vs. 34.6 nmol/L), likely driven by much lower vitamin B12 concentrations and the higher prevalence of vitamin B12 deficiency (48.3 vs. 17.9%) [24]. The findings here were also consistent across all indicators of inadequate vitamin B12 status and cutoffs evaluated (i.e., <221 pmol/L, elevated MMA, cB12, impaired vitamin B12 status). Vitamin B12 is required for the conversion of 5-MTHF to THF [6], maintenance of folates in RBCs, and retention of folates in proliferating cells such as developing RBCs during erythropoiesis [6]. Vitamin B12 deficiency can result in 5-MTHF accumulation, reduced THF availability, and impaired folate retention in RBCs [6]. Together, in the context of vitamin B12 deficiency, the estimated serum folate threshold for NTD prevention is above the physiological range of serum folate concentrations obtainable in the absence of high folic acid intake. Previous studies have found that folic acid intake does not impact the relationship between RBC folate and plasma folate thresholds [24], suggesting that this phenomenon (much higher serum threshold in vitamin B12 insufficiency) reflects differences in metabolism between folic acid and natural food folate and the importance of vitamin B12 status in folic acid naïve populations.
The estimated serum folate threshold did not vary substantially by overweight status or BMI strata. These findings contrast with previous analyses where the plasma folate threshold was lower in higher BMI strata [24]. Previous analyses were conducted in a population with lower prevalence of underweight and excluded women with vitamin B12 deficiency, constraining direct comparability of findings. Observational studies in nonpregnant women reported higher BMI was associated with lower serum folate but higher RBC folate concentrations [42]. The associations between higher BMI and lower serum folate concentrations may be due to increased blood volume or cellular folate uptake with increasing body size [43,44].
In the current study, the serum folate insufficiency threshold was lower in participants with elevated central adiposity or HbA1c (≥5.7% or ≥6.5%). HbA1c is an indicator used to screen for diabetes mellitus or to monitor glycemic control in individuals with diabetes. In randomized trials, folic acid supplementation was associated with lower insulin levels and insulin resistance [45,46], although it did not reduce HbA1c [45,46] or incidence of diabetes [47].
The serum folate threshold was also higher in individuals with insufficient (<221 pmol/L) or marginal (≥148 to <221 pmol/L) vitamin B12 status, suggesting that vitamin B12 may impact the serum folate threshold even in the absence of vitamin B12 deficiency. Evaluation of vitamin B12 status is important for estimation of the serum folate insufficiency threshold in populations with high prevalence of vitamin B12 deficiency and may be warranted in populations where a substantial proportion of the population has insufficient (<221 pmol/L) or marginal (≥148 to <221 pmol/L) vitamin B12 status. In the United States, although the prevalence of vitamin B12 deficiency in the overall population is 2.9% [48], it is estimated that 13.3% of nonpregnant women and 21.3% of pregnant women have vitamin B12 <221 pmol/L [49]. In other countries, nearly half of women have vitamin B12 <221 pmol/L, with ∼30% ≥148 to <221 pmol/L [[50], [51], [52]].
In this analysis, metabolic risk factors (e.g., elevated HbA1c) impacted the serum folate threshold; however, the magnitude was approximately 8 to 10 nmol/L compared with 40.0 nmol/L observed by vitamin B12 deficiency strata. The impact of metabolic characteristics on this threshold is of interest etiologically, and additional studies exploring these associations would enhance the findings here. The impact of vitamin B12 status is immediately important when considering a serum folate threshold to monitor optimal NTD prevention at the population level.
Folic acid fortification policy is based on evidence from randomized supplementation trials [[9], [10], [11]] and population-based fortification programs [[16], [17], [18], [19], [20], [21]] and is among the most efficacious and cost-effective public health interventions [53,54]. Folic acid fortification has been scaled up in over 70 countries and has been consistently associated with reduced NTDs, including populations with high prevalence of vitamin B12 deficiency [13]. The WHO recommends monitoring RBC folate at the population level; higher RBC folate concentrations have been associated with lower NTD risk in observational studies [8,14] and Bayesian analyses [15], and established an RBC threshold of ≥906 nmol/L for optimal NTD prevention [22]. The association between decreasing RBC folate concentrations and increasing NTD risk is independent of vitamin B12 [15].
The findings from the current study and Bayesian analyses of population data in China [24] suggest it may be possible to establish a serum/plasma folate threshold corresponding to the RBC threshold for NTD prevention. However, in both studies, the relationship between serum/plasma folate and RBC folate—and the corresponding thresholds for NTD prevention—were substantially modified by vitamin B12 deficiency. This impact of vitamin B12 deficiency on these folate biomarkers is consistent with the role of vitamin B12 in folate-mediated 1-carbon metabolism: vitamin B12 deficiency impairs conversion of folates to THF [6], retention of folates in RBCs during erythropoiesis, and maintenance of folates in RBCs [6]. Together, these findings suggest that although a serum folate threshold for NTD prevention may be estimated in the context of vitamin B12 sufficiency (>221 pmol/L), it may not be appropriate in populations with a high prevalence of vitamin B12 insuffieiency or deficiency, and serum/plasma folate concentrations should not be used to inform the design or monitoring of folic acid fortification programs. There is also emerging interest in vitamin B12 fortification, as vitamin B12 deficiency has been identified as an independent risk factor for NTDs [55] and can result in megaloblastic anemia [31] and pregnancy complications [56]. The efficacy of vitamin B12 fortification awaits appropriate randomized trials.
This study leverages a well-characterized population at high-risk for folate insufficiency and NTDs. This study applied previously developed Bayesian models [24] to estimate a serum folate threshold in a population with a high prevalence of vitamin B12 deficiency and included additional biomarkers of vitamin B12 status and metabolic outcomes.
This study has several important limitations. Its cross-sectional study design does not enable evaluation of the effects of risk factors on the estimated serum folate threshold. Although this study included participants who were not currently pregnant or lactating, the findings may not reflect the periconceptional period. The current study evaluated serum folate, whereas the previous Bayesian analysis evaluated plasma folate. Although these biomarkers are generally considered interchangeable, there may be unknown important differences. Assessment of the MTHFR genotype would improve comparability to the previous analysis, which identified a higher threshold with the MTHFR TT genotype [24]. However, previous studies have reported a low prevalence of MTHFR polymorphisms in South Indian populations [57], and modeling studies demonstrated that the RBC folate threshold is not influenced by the MTHFR genotype [15]. In this study, vitamin B12 deficiency and insufficiency were evaluated via total vitamin B12, and additional functional vitamin B12 biomarkers, elevated MMA acid and Hcy, were examined. Although total vitamin B12 and MMA are specific indicators of vitamin B12 status, Hcy is influenced by a number of factors within (e.g., folate) [56] and outside (e.g., renal disease) [58] 1-carbon metabolism. The different circulating (i.e., total vitamin B12, holo-transcobalamin) and functional (i.e., MMA, Hcy) biomarkers and cutoffs used pose challenges for evaluating vitamin B12 status and interpreting findings across different studies [30,31]. However, the findings here are consistent with the previous population-based study in China and were consistent across all vitamin B12 biomarkers and cutoffs evaluated. Inclusion of holo-transcobalamin in this study would improve assessment of vitamin B12 status and provide additional insights on the impact of vitamin B12 status on the estimated serum folate threshold. Inclusion of other metabolic risk factors (e.g., impaired kidney function [58]) also warrants consideration for inclusion in future analyses. The reported associations between serum folate and RBC folate are limited to the microbiologic assay and are not generalizable to other assays for folate assessment.
In our analysis of population-based biomarker data, the estimated serum folate threshold for optimal NTD prevention was consistent with the previous reports among participants with vitamin B12 >221 pmol/L. However, the serum folate threshold was substantially higher in individuals with vitamin B12 deficiency across all other indicators of insufficient vitamin B12 status and the cutoffs evaluated, suggesting a complex interaction between these two different measures of folate status in a folic acid naïve population. These findings suggest that although it may be possible to estimate a serum folate threshold for NTD prevention in some settings, it may not be appropriate in populations with high prevalence of vitamin B12 insufficiency.
Funding
This study was supported by the Centers for Disease Control and Prevention (CDC) and the University of South Carolina’s Disability Research and Dissemination Center (DRDC) through grant number 5U01DD001007 from the CDC. AF was supported by the National Institutes of Health (NIH) under award 5 T32HD087137, and HMG was supported by the NIH under award T32DK007158. The findings and conclusion in this report are those of the authors and do not necessarily represent the official positions of the CDC, DRDC, Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Diabetes and Digestive and Kidney Diseases, or the NIH.
Author disclosures
The authors have no conflicts of interest to disclose related to this manuscript. Findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention, the DRDC, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), or the National Institutes of Health.
Author Contributions
Analyses were replicated by Results Reproduction (R-squared), an independent statistical group at Cornell University. The authors’ responsibilities were as follows—JLF, KSC: designed the research; AF: wrote the initial draft manuscript; KSC, CER, JLF: provided expert guidance on the statistical analyses and revised the manuscript; WB, CBJ, AF: supervised data collection and field activities; BB: conducted the laboratory analyses with expert guidance from CMP, MZ, SJ; AF: conducted the statistical analyses; JLF, KSC: had primary responsibility for the final content; and all authors: contributed to the development of the manuscript, provided feedback, and read and approved the final manuscript.
Data Availability
Data described in this manuscript, code book, and analytic code will be made available upon request at: https://archive.ciser.cornell.edu.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.01.016.
Contributor Information
Krista S. Crider, Email: kcrider@cdc.gov.
Julia L. Finkelstein, Email: jfinkelstein@cornell.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Copp A.J., Stanier P., Greene N.D. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12(8):799–810. doi: 10.1016/s1474-4422(13)70110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaganjor I., Sekkarie A., Tsang B.L., Williams J., Razzaghi H., Mulinare J., et al. Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLOS ONE. 2016;11(4) doi: 10.1371/journal.pone.0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H., Kancherla V., Moorthie S., Darlison M.W., Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann. N. Y. Acad. Sci. 2018;1414(1):31–46. doi: 10.1111/nyas.13548. [DOI] [PubMed] [Google Scholar]
- 4.Allagh K.P., Shamanna B.R., Murthy G.V., Ness A.R., Doyle P., Neogi S.B., et al. Birth prevalence of neural tube defects and orofacial clefts in India: a systematic review and meta-analysis. PLOS ONE. 2015;10(3) doi: 10.1371/journal.pone.0118961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhide P., Sagoo G.S., Moorthie S., Burton H., Kar A. Systematic review of birth prevalence of neural tube defects in India. Birth Defects Res. A Clin. Mol. Teratol. 2013;97(7):437–443. doi: 10.1002/bdra.23153. [DOI] [PubMed] [Google Scholar]
- 6.Bailey L.B., Stover P.J., McNulty H., Fenech M.F., Gregory J.F., 3rd, Mills J.L., et al. Biomarkers of nutrition for development-folate review. J. Nutr. 2015;145(7) doi: 10.3945/jn.114.206599. 1636S–1680S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibbard E.D., Smithells R.W. Folic acid metabolism and human embryopathy. Lancet. 1965;285(7398):1254. doi: 10.1016/S0140-6736(65)91895-7. [DOI] [Google Scholar]
- 8.Daly L.E., Kirke P.N., Molloy A., Weir D.G., Scott J.M. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274(21):1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 9.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 10.Czeizel A.E., Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 11.Berry R.J., Li Z., Erickson J.D., Li S., Moore C.A., Wang H., et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine (US) Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification. US National Academies Press; Washington, DC: 2003. Committee on Use of Dietary Reference Intakes in Nutrition Labeling. [PubMed] [Google Scholar]
- 13.Crider K.S., Qi Y.P., Yeung L.F., Mai C.T., Head Zauche L., Wang A., et al. Folic acid and the prevention of birth defects: 30 years of opportunity and controversies. Annu. Rev. Nutr. 2022;42:423–452. doi: 10.1146/annurev-nutr-043020-091647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinker S.C., Hamner H.C., Qi Y.P., Crider K.S. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination survey 2007 to 2012. Birth Defects Res. A Clin. Mol. Teratol. 2015;103(6):517–526. doi: 10.1002/bdra.23378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crider K.S., Devine O., Hao L., Dowling N.F., Li S., Molloy A.M., et al. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ. 2014;349:g4554. doi: 10.1136/bmj.g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., De Steur H., Chen G., Zhang X., Pei L., Gellynck X., et al. Effectiveness of folic acid fortified flour for prevention of neural tube defects in a high risk region. Nutrients. 2016;8(3):152. doi: 10.3390/nu8030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams J.L., Mai C.T., Mulinare J., Isenburg J., Flood T.J., Ethen M., et al. Updated estimates of neural tube defects prevented by mandatory folic acid fortification — United States, 1995–2011. MMWR Morb. Mortal. Wkly. Rep. 2015;64(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 18.De Wals P., Tairou F., Van Allen M.I., Uh S.H., Lowry R.B., Sibbald B., et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007;357(2):135–142. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 19.Cortés F., Mellado C., Pardo R.A., Villarroel L.A., Hertrampf E. Wheat flour fortification with folic acid: changes in neural tube defects rates in Chile. Am. J. Med. Genet. A. 2012;158A(8):1885. doi: 10.1002/ajmg.a.35430. –1890. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.T., Rivera M.A. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr Rev. 2004;62(6 Pt 2) doi: 10.1111/j.1753-4887.2004.tb00073.x. S40–S43. [DOI] [PubMed] [Google Scholar]
- 21.Sayed A.R., Bourne D., Pattinson R., Nixon J., Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa, Birth Defects. Res. A Clin. Mol. Teratol. 2008;82(4):211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) World Health Organization; Geneva, Switzerland: 2015. Guideline: optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. [PubMed] [Google Scholar]
- 23.Rogers L.M., Cordero A.M., Pfeiffer C.M., Hausman D.B., Tsang B.L., De-Regil L.M., et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann. N. Y. Acad. Sci. 2018;1431(1):35–57. doi: 10.1111/nyas.13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M.Y., Rose C.E., Qi Y.P., Williams J.L., Yeung L.F., Berry R.J., et al. Defining the plasma folate concentration associated with the red blood cell folate concentration threshold for optimal neural tube defects prevention: a population-based, randomized trial of folic acid supplementation. Am. J. Clin. Nutr. 2019;109(5):1452–1461. doi: 10.1093/ajcn/nqz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkelstein J.L., Fothergill A., Johnson C.B., Guetterman H.M., Bose B., Jabbar S., et al. Periconceptional surveillance for prevention of anaemia and birth defects in Southern India: protocol for a biomarker survey in women of reproductive age. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-038305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelstein J.L., Fothergill A., Johnson C.B., Guetterman H.M., Bose B., Jabbar S., et al. Anemia and vitamin B-12 and folate status in women of reproductive age in Southern India: estimating population-based risk of neural tube defects. Curr. Dev. Nutr. 2021;5(5) doi: 10.1093/cdn/nzab069. nzab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruth C.J., Huey S.L., Krisher J.T., Fothergill A., Gannon B.M., Jones C.E., et al. An electronic data capture framework (ConnEDCt) for global and public health research: design and implementation. J. Med. Internet. Res. 2020;22(8) doi: 10.2196/18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiffer C.M., Zhang M., Lacher D.A., Molloy A.M., Tamura T., Yetley E.A., et al. Comparison of serum and red blood cell folate microbiologic assays for national population surveys. J. Nutr. 2011;141(7):1402–1409. doi: 10.3945/jn.111.141515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Casal M.N., Pasricha S.R., Sharma A.J., Peña-Rosas J.P. Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Ann. N. Y. Acad. Sci. 2019;1450(1):5–14. doi: 10.1111/nyas.14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen L.H., Miller J.W., de Groot L., Rosenberg I.H., Smith A.D., Refsum H., et al. Biomarkers of Nutrition for Development (BOND): vitamin B-12 review. J. Nutr. 2018;148(suppl_4):1995S. doi: 10.1093/jn/nxy201. –2027S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green R., Allen L.H., Bjørke-Monsen A.L., Brito A., Guéant J.L., Miller J.W., et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers. 2017;3 doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 32.Fedosov S.N., Brito A., Miller J.W., Green R., Allen L.H. Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin. Chem. Lab. Med. 2015;53(8):1215–1225. doi: 10.1515/cclm-2014-0818. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO) World Health Organization; Geneva, Switzerland: 2020. Guideline on use of ferritin concentrations to assess iron status in individuals and populations. [PubMed] [Google Scholar]
- 34.Mei Z., Addo O.Y., Jefferds M.E., Sharma A.J., Flores-Ayala R.C., Brittenham G.M. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8) doi: 10.1016/S2352-3026(21)00168-X. e572–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeiffer C.M., Cook J.D., Mei Z., Cogswell M.E., Looker A.C., Lacher D.A. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clin. Chim. Acta. 2007;382(1–2):112–116. doi: 10.1016/j.cca.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Dhingra R., Gona P., Nam B.H., D’Agostino R.B., Wilson P.W.F., Benjamin E.J., et al. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am. J. Med. 2007;120(12):1054–1062. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization (WHO) World Health Organization; Geneva, Switzerland: 2000. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. [PubMed] [Google Scholar]
- 38.World Health Organization (WHO) Report of a WHO expert consultation. World Health Organization; Geneva, Switzerland: 2008. Waist circumference and waist-hip ratio. [Google Scholar]
- 39.American Association of Clinical Endocrinologists/American College of Endocrinology AACE/ACE) Obesity Task Force, AACE/ACE position statement on the prevention, diagnosis, and treatment of obesity. Endocr. Pract. 1998;4:297–350. [Google Scholar]
- 40.American Diabetes Association . American Diabetes Association; Arlington, VA: 2020. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. 2019/12/22; pp. S14–S31. [DOI] [PubMed] [Google Scholar]
- 41.Namaste S.M., Rohner F., Huang J., Bhushan N.L., Flores-Ayala R., Kupka R., et al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017;106(Suppl 1) doi: 10.3945/ajcn.116.141762. 359S–371S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tinker S.C., Hamner H.C., Berry R.J., Bailey L.B., Pfeiffer C.M. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defects. Res. A Clin. Mol. Teratol. 2012;94(10):749–755. doi: 10.1002/bdra.23024. [DOI] [PubMed] [Google Scholar]
- 43.da Silva V.R., Hausman D.B., Kauwell G.P., Sokolow A., Tackett R.L., Rathbun S.L., et al. Obesity affects short-term folate pharmacokinetics in women of childbearing age. Int. J. Obes. (Lond) 2013;37(12):1608–1610. doi: 10.1038/ijo.2013.41. [DOI] [PubMed] [Google Scholar]
- 44.Stern S.J., Matok I., Kapur B., Koren G. A comparison of folic acid pharmacokinetics in obese and nonobese women of childbearing age. Ther. Drug Monit. 2011;33(3):336–340. doi: 10.1097/FTD.0b013e318219407a. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J.V., Schooling C.M., Zhao J.X. The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Ann. Epidemiol. 2018;28(4):249–257.e1. doi: 10.1016/j.annepidem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Asbaghi O., Ashtary-Larky D., Bagheri R., Moosavian S.P., Olyaei H.P., Nazarian B., et al. Folic acid supplementation improves glycemic control for diabetes prevention and management: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutrients. 2021;13(7):2355. doi: 10.3390/nu13072355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin X., Li J., Zhang Y., Chen D., Wang B., He M., et al. Effect of folic acid supplementation on risk of new-onset diabetes in adults with hypertension in China: findings from the China Stroke Primary Prevention Trial (CSPPT) J. Diabetes. 2016;8(2):286–294. doi: 10.1111/1753-0407.12346. [DOI] [PubMed] [Google Scholar]
- 48.Bailey R.L., Carmel R., Green R., Pfeiffer C.M., Cogswell M.E., Osterloh J.D., et al. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011;94(2):552–561. doi: 10.3945/ajcn.111.015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fothergill A., Finkelstein J. Vitamin B-12 status in women of reproductive age, NHANES 2013-2014. FASEB J. 2017;31(Suppl 1):lb439. [Google Scholar]
- 50.Rosenthal J., Largaespada N., Bailey L.B., Cannon M., Alverson C.J., Ortiz D., et al. Folate deficiency is prevalent in women of childbearing age in Belize and is negatively affected by coexisting vitamin B-12 deficiency: Belize national micronutrient survey 2011. J. Nutr. 2017;147(6):1183–1193. doi: 10.3945/jn.116.242628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenthal J., Lopez-Pazos E., Dowling N.F., Pfeiffer C.M., Mulinare J., Vellozzi C., et al. Folate and vitamin B12 deficiency among non-pregnant women of childbearing-age in Guatemala 2009–2010: prevalence and identification of vulnerable populations, Matern. Child Health J. 2015;19(10):2272–2285. doi: 10.1007/s10995-015-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrán O.F., Ward J.B., Villamor E. Vitamin B12 serostatus in Colombian children and adult women: results from a nationally representative survey. Public Health Nutr. 2015;18(5):836–843. doi: 10.1017/S1368980014001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoddinott J. The investment case for folic acid fortification in developing countries. Ann. N. Y. Acad. Sci. 2018;1414(1):72–81. doi: 10.1111/nyas.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grosse S.D., Berry R.J., Mick Tilford J., Kucik J.E., Waitzman N.J. Retrospective assessment of cost savings from prevention: folic acid fortification and spina bifida in the U.S. Am. J. Prev. Med. 2016;50(Suppl 1):S74–S80. doi: 10.1016/j.amepre.2015.10.012. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molloy A.M. Should vitamin B12 status be considered in assessing risk of neural tube defects? Ann. N. Y. Acad. Sci. 2018;1414(1):109–125. doi: 10.1111/nyas.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finkelstein J.L., Layden A.J., Stover P.J. Vitamin B-12 and perinatal health. Adv. Nutr. 2015;6(5):552–563. doi: 10.3945/an.115.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devi A.R.R., Govindaiah V., Ramakrishna G., Naushad S.M. Prevalence of methylene tetrahydrofolate reductase polymorphism in South Indian population. Curr. Sci. 2004;86(3):440–443. [Google Scholar]
- 58.Wang A., Yeung L.F., Ríos Burrows N., Rose C.E., Fazili Z., Pfeiffer C.M., et al. Reduced kidney function is associated with increasing red blood cell folate concentration and changes in folate form distributions (NHANES 2011–2018) Nutrients. 2022;14(5):1054. doi: 10.3390/nu14051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in this manuscript, code book, and analytic code will be made available upon request at: https://archive.ciser.cornell.edu.