Abstract
During mammalian reproduction, germ cell chromatin packaging is key to prepare parental genomes for fertilization and to initiate embryonic development. While chromatin modifications such as DNA methylation and histone post-translational modifications are well known to carry regulatory information, histone variants have received less attention in this context. Histone variants alter the stability, structure and function of nucleosomes and, as such, contribute to chromatin organization in germ cells. Here, we review histone variants expression dynamics during the production of male and female germ cells, and what is currently known about their parent-of-origin effects during reproduction. Finally, we discuss the apparent conundrum behind these important functions and their recent evolutionary diversification.
Keywords: Histone, Variants, Reproduction, Mammals, Evolution, Parental-effect
Introduction: casting histone variants for the next blockbuster
In eukaryotes, genetic information is organized into chromatin. The basic unit of chromatin is the nucleosome composed of histone proteins wrapping ~ 147 bp of DNA. Most nucleosomes contain four types of replication dependent histone proteins H2A, H2B, H3, and H4 (thereafter referred to as core histones) (Phillips and Johns 1965; Kornberg 1974). They assemble as an octamer: one H3-H4 tetramer interacting with two H2A-H2B dimers (Arents et al. 1991; Luger 1997). An additional, structurally distinct, histone protein called H1 binds to the linker DNA located between nucleosomes and further contributes to genome packaging into “chromatosomes” (Simpson 1978; Thoma et al. 1979). Core histones are some of the slowest evolving proteins in eukaryotes, being nearly identical between distantly related species (Malik and Henikoff 2003; Molaro and Drinnenberg 2018; Talbert and Henikoff 2021). This is thought to be the result of strong purifying natural selection maintaining their essential functions (Rooney et al. 2002; Piontkivska et al. 2002; Eirín-López et al. 2004). Indeed, histones regulate access to genetic information by impeding protein interactions with DNA and by altering chromatin states through post-translational modifications (Kornberg and Lorch 2020; Millán-Zambrano et al. 2022).
Nevertheless, eukaryote histones did not remain evolutionary inert since their birth. In fact, stand-alone histone variants repeatedly arose from their core counterparts either by de novo gene duplication or via sub-functionalization of existing histone paralogs (Malik and Henikoff 2003; Talbert et al. 2012; Draizen et al. 2016; Osakabe and Molaro 2023). All extant histones have described variants differing by only a few amino-acids (a.a.), as seen for the pan-eukaryote H3 variant H3.3, to having gained entire additional domains as is the case for macroH2A in metazoans (reviewed in Malik and Henikoff 2003; Talbert and Henikoff 2021).
Unlike core histones, histone variants can be deposited into nucleosomes independently of DNA replication and influence genome regulation in post-mitotic cells (reviewed in Marzluff et al. 2008; Martire and Banaszynski 2020; Talbert and Henikoff 2021). Consequently, over the last 2 billion years, eukaryotic histone variants took on essential functions during DNA repair, transcription or chromatin remodeling and play major roles during both normal and disease development (reviewed in Maze et al. 2014; Zink and Hake 2016; Martire and Banaszynski 2020; Talbert and Henikoff 2021). For example, genetic ablation of many histone variants can have severe developmental consequences in both mice and human (e.g., H2A.Z: Faast et al. 2001; H3.3: Jang et al. 2015). In addition, a growing number of studies have linked somatic histone variant mutations, or ectopic induction, to cancer progression (Wu et al. 2012; Schwartzentruber et al. 2012; Kallappagoudar et al. 2015; Buschbeck and Hake 2017; Nacev et al. 2019; Gomes et al. 2019; Chew et al. 2021).
Beside these canonical roles in the soma, histone variant functions have most diversified in the germline. While we focus here on mammals, this observation holds true across eukaryotes (Orsi et al. 2009; Jiang et al. 2020a; Osakabe and Molaro 2023). Germline cells express histone variant families with unique sequence features or repurpose ubiquitous variants towards functions not seen in somatic cells (Kimmins and Sassone-Corsi 2005; Santenard and Torres-Padilla 2009; Hoghoughi et al. 2018). This is seen for H2A.X which carries out its DNA repair function in the context of meiotic cross-overs and colocalizes, together with macroH2A, to the sex-body during male meiosis (Hoyer-Fender et al. 2000; Turner et al. 2001; Celeste et al. 2002; Fernandez-Capetillo et al. 2003; Pasque et al. 2012). This diversification likely stems from the highly specialized functions of chromatin landscapes during reproduction. First, chromatin states established during gametogenesis are essential for proper genome packaging and contribute to the inheritance of parent-of-origin information to the zygote (Kimmins and Sassone-Corsi 2005; van der Heijden et al. 2006; Teperek et al. 2016; Hanna and Kelsey 2017). Then, following fertilization, chromatin remodeling can template developmental transitions relying on genome accessibility (Santos and Dean 2004; Saitou et al. 2012; Bošković et al. 2014). Finally, the specification of future germ cells requires coordinated chromatin reprogramming events safeguarding future generations (Surani et al. 2004; Hackett et al. 2012; Matsui and Mochizuki 2014; Kurimoto and Saitou 2019).
Here, we review histone variants with unique functions in the mammalian germline with a focus on their function setting up parental chromatin landscapes with putative effects on embryonic development. We then discuss their evolutionary trajectories highlighting conserved vs. species-specific functions.
Histone variants during male gametogenesis: stunt doubles steal the spotlight
Like their female counterparts, male germ cells arise from a population primordial germ cells (or PGCs) that colonize the gonads during embryonic development (Ginsburg et al. 1990). Major chromatin remodeling events occur during PGC development, including whole genome DNA methylation and histone modification reprogramming (Surani et al. 2004; Hackett et al. 2012; Matsui and Mochizuki 2014; Kurimoto and Saitou 2019). Probably owing to the technical difficulties of studying such a discrete cell population, we know surprisingly little about histone variants’ function in PGCs. For example, there have been no reports of PGC-specific mammalian histone variants and those investigated appear to carry out their somatic functions (Surani et al. 2004; Matsui and Mochizuki 2014; Kurimoto and Saitou 2019). For example, H2A.Z enrichment in PGC nuclei correlates with active transcription as seen in somatic cells (Hajkova et al. 2008) .
Nevertheless, once PGCs settle to establish spermatogonial stem cells (SSCs) and the process of spermatogenesis begins, histone variants take the center stage. This is due to the major chromatin remodeling events occurring both during meiosis (Turner 2015; Wang et al. 2017) and in post-meiotic germ cells (reviewed in Rathke et al. 2014). The highlight of this play during spermiogenesis is the replacement of histone-based nucleosomes with transition proteins and then protamines (Oliva and Dixon 1991). Yet, histone-to-protamine replacement is incomplete and a few nucleosomes are retained in mature sperm over a few genes and repeats (Erkek et al. 2013; Hammoud et al. 2009; Carone et al. 2014; Samans et al. 2014; Yamaguchi et al. 2018). Retained nucleosomes contain histone variants and can be post-translationally modified (Brunner et al. 2014; Luense et al. 2016). While there is much species-specific variation, these nucleosomes have the potential to contribute to chromatin-based paternal effects post-fertilization.
Considering the vast amount of histone variants identified in males, we separated them into their respective families. We also mostly cover those with specific expression in germ cells and/or carrying parental effects. Other recent reviews cover the function of ubiquitously expressed variants (Martire and Banaszynski 2020; Talbert and Henikoff 2021).
H3 variants
H3.3 is one of the main H3 variants present during spermatogenesis, and it is found in retained nucleosomes in mature sperm (Fig. 1) (Bramlage et al. 1997; Hammoud et al. 2009; Erkek et al. 2013). In this context, it has been mapped to CpG-rich unmethylated promoters and shown to carry post-translational modifications, such as trimethylation of Lysine 4, 9, 27, and 36 in mice humans or bulls (Samans et al. 2014; Brunner et al. 2014; Luense et al. 2016; Jung et al. 2017). H3.3 is encoded by two genes — h3fb3a and h3fb3b — coding for identical proteins that differ from core H3s by only a few amino acids, most notably in their chaperone interaction domain (Talbert and Henikoff 2021). While complete H3.3 KO are embryonic lethal, single KOs are viable and have non-overlapping expression and function during spermatogenesis (Couldrey et al. 1999; Yuen et al. 2014; Tang et al. 2015). H3.3A loss leads to mild sperm abnormality and sub-fertility suggesting that H3.3B alone is sufficient to complete spermatogenesis (Couldrey et al. 1999). On the other hand, H3.3B KO males are infertile and arrest after meiosis is completed (Fontaine et al. 2022). Although the precise molecular mechanisms behind this arrest are not fully understood, a recent study suggested that H3.3B might play a role in the chromatin remodeling of sex chromosomes, repeats, and piRNA clusters induced after meiosis (Fontaine et al. 2022). Yet, how these germ cell specific functions are tied to H3.3 post-translational modifications in mature sperm remains largely unknown.
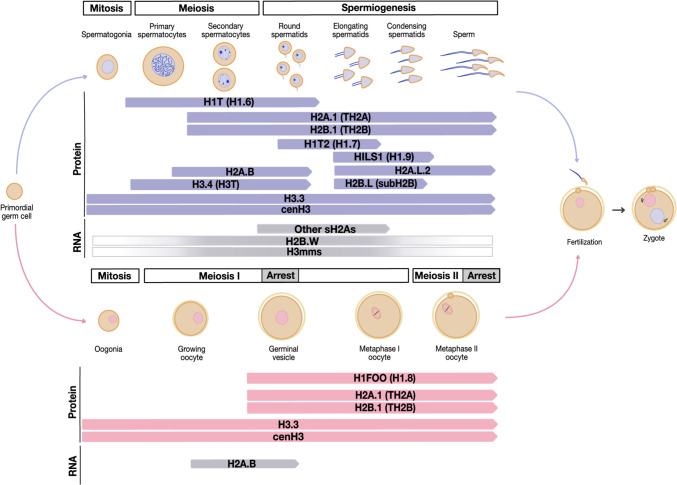
Fig. 1.
Expression timing of mouse histone variants during gametogenesis. The window of expression (RNA) or chromatin association (protein) is shown for histone variants discussed in this review. Stages of male (blue) or female (pink) germ cell differentiation are indicated above. H3mms and H2B.W expression has not been staged during gametogenesis. H3.3 and cenH3 are also included although their expression is not restricted to germ cells.
H3mm variants were identified through phylogenetic analyses suggesting a recent origin in mice via repeated amplifications of H3.3 coding genes (Maehara et al. 2015). The mouse genome encodes 14 H3mms, and four of these — H3mm7, 8, 13, and 15 — are expressed in the testis, albeit not exclusively (Maehara et al. 2015). Mouse KO for H3mm7 have altered muscle differentiation but defects during spermatogenesis were not reported (Harada et al. 2018). H3mms close sequence similarity to H3.3 suggest they might share similar deposition pathways with related functions during spermatogenesis. However, it remains to be shown if H3mms also contribute to mature sperm nucleosomes.
H3.5 arose in the last common ancestor of great-apes (Hominoids) likely via retroposition of H3.3B (Schenk et al. 2011). Compared to H3.3, H3.5 differ by 5 a.a. and one of these sites, Leucine 103, is responsible for weakened hydrophobic interactions with H4 which decreases the stability of H3.5 nucleosomes (Urahama et al. 2016). In human, H3.5 is highly expressed in spermatogonia and is lost during meiosis I suggesting it might accumulate during cell division (Urahama et al. 2016; Shiraishi et al. 2018; Ding et al. 2021). So far, one study investigated H3.5 genomic localization in human testes, and found preferential accumulation at the 5′ end of genes. This indicates that H3.5 loading might occur over transcribed genes or contributes to chromatin opening during spermatogonial differentiation (Urahama et al. 2016).
H3.X and H3.Y seem also restricted to primates and are detected in human testes, but not exclusively (Wiedemann et al. 2010). They share most similarities with H3.3, from which they differ by 26 a.a. for H3.Y and 35 a.a. for H3.X; these concentrate at sites interacting with nucelosomal DNA (Wiedemann et al. 2010; Kujirai et al. 2017). Although their localization in the germline has never been investigated, H3.X/Y deposition in somatic cells relies on the chaperone HIRA and is associated with active transcription (Kujirai et al. 2016; Zink et al. 2017; Resnick et al. 2019). Interestingly, they are regulated by the transcription factor DUX4 that plays an important role in testis and zygote transcriptional regulation (Young et al. 2013; Resnick et al. 2019; Vuoristo et al. 2022). This suggests that H3.X/Y might play key roles in the germline developmental program that remains to be discovered.
H3T (or H3.4). In addition to H3.3 variants, mammalian genomes also encode a testis-specific H3, known as H3T (or H3.4). This variant shares more similarity with core H3 (e.g., H3.1) than H3.3 (Witt et al. 1996; Ueda et al. 2017), and its deposition is limited to cells entering meiosis (spermatocytes). Mice KO for H3t are infertile, and structural analyses suggested that H3t-containing nucleosomes favor chromatin loosening required for meiotic progression (Ueda et al. 2017). As discussed above, this function is also suggested for human H3.5, but unlike this variant, H3T is found in most mammals (see below). Thus, it would be interesting to understand whether H3T and H3.3 variants interact to regulate chromatin conformation during meiosis. cenH3 defines chromosome centromeres in most eukaryotes (McKinley and Cheeseman 2016; Mellone and Fachinetti 2021). Although it is ubiquitously expressed, it performs specialized functions in the germline. Indeed, in male post-meiotic cells, cenH3 is retained at centromeres and carries-out paternal chromosome centromere inheritance through fertilization (reviewed by Das et al. 2017). As such, it is one of the few histone variants with clearly identified parental-effect molecular function. Interestingly, this function in sperm could help organize peri-centromeric chromatin into chromocenters which influence species-specific paternal genome remodeling following fertilization (Zalensky et al. 1993; Probst et al. 2007, 2009; van de Werken et al. 2014; Burton et al. 2020).
H2A variants
TH2A (or H2A.1) is considered to be the major histone variant replacing core H2A in post-replicative germ cells (Trostle-Weige et al. 1982) (Fig. 1). In this context, TH2A shares some of the interacting partners and post-translational modifications of core H2A (e.g., H2AK119Ub Chen et al. 1998; Baarends et al. 1999; Hasegawa et al. 2015). However, TH2A is also uniquely phosphorylated at Thr127 concomitant to its deposition around centromeres during male and female meiosis (Shinagawa et al. 2014; Hada et al. 2017; Talbert and Henikoff 2021). Th2a KO have no reported phenotypes, but TH2A loss combined with its partner TH2B (H2B.1) causes improper meiotic exit and histone-to-protamine replacement (Shinagawa et al. 2015). While maternal contribution of TH2A and TH2B to the embryo is required for both paternal genome reprogramming and zygotic gene activation, whether they also contribute to paternal chromatin-based inheritance remains currently unknown (Shinagawa et al. 2014, 2015).
Short H2As probably win the award for most unusual variants in the male germline. These histones arose in placental mammals and are subject to unprecedented levels of evolutionary diversification (Molaro et al. 2018). They exist in four flavors — H2A.B, H2A.L, H2A.P, and H2A.Q — and are expressed sequentially during spermatogenesis. In mice, this begins with H2A.B during meiosis, then H2A.P and finally H2A.Ls in differentiating spermatids, H2A.Q being lost in mice (Govin et al. 2007; Ferguson et al. 2009; Soboleva et al. 2012; Molaro et al. 2018) (Fig. 1). Compared to core H2A, all four short H2As have highly divergent tails, histone fold domains, and are truncated at their C-terminal docking domain. These alterations greatly reduce the DNA wrapping, stability, and interactions of short H2A containing nucleosomes (Bao et al. 2004; Doyen et al. 2006; Syed et al. 2009; Arimura et al. 2013; Molaro et al. 2018; Kohestani and Wereszczynski 2021). How these structural features affect chromatin remodeling likely depends on the developmental context, e.g., induced in somatic cells vs. endogenous expression in germ cells (also reviewed in Jiang et al. 2020b).
H2A.B accumulates in meiotic spermatocytes but disappears from chromatin when haploid spermatids begin their differentiation. In mice, H2A.B appears to associate with actively transcribed genes where it has been proposed to contribute to RNA processing, most notably via its interaction with RNA and the splicing machinery (Soboleva et al. 2012, 2017). While H2a.b KO males display mild transcriptional and spermatogenesis defects, they show decreased nucleosome wrapping in post-meiotic cells, are sub-fertile, and sire litters with increased mortality (Anuar et al. 2019; Molaro et al. 2020). Embryonic development is altered upon paternal and maternal loss of H2A.B indicating a parental-effect function during mouse reproduction (Molaro et al. 2020).
H2A.L.2 is broadly deposited in differentiating mouse spermatids concomitant with histone-to-protamine replacement (Govin et al. 2007; Barral et al. 2017). In this context, H2A.L.2 directly interacts with transition proteins helping unwrap DNA during protamine deposition. Despite the presence of 19 additional coding H2a.l paralogs in the mouse genome, H2A.L.2 function is essential and H2a.l.2 KO mice are infertile (Barral et al. 2017).
Aside for these two examples, the function of short H2As remains mostly unexplored. Further molecular studies will be required to understand their partners, modifications, and whether they interact with one another during germ cell development.
Special mentions: H2A.X, macroH2A, and H2A.Z. These three H2As are some of the most studied variants owing to their essential functions during development and recent reviews thoroughly cover these histones (Herchenröther et al. 2023; Oberdoerffer and Miller 2023). Yet, beside their canonical roles, it is worth mentioning that all three histones are known to regulate the packaging and transcription of sex chromosomes during male meiosis (Mahadevaiah et al. 2001; Fernandez-Capetillo et al. 2003; Greaves et al. 2006). Akin to its somatic function, H2A.X is also directly involved in the signaling and repair of double strand breaks induced during meiosis I (Lichten 2001). While the molecular details of these meiosis-specific functions are complex and beyond the scope of this overview, they are well documented examples of histone variants functional repurposing in the unique context of germ cell development.
H2B variants
TH2B (or H2B.1) is the main H2B variant present in both male and female germ cells and is likely present in nucleosomes retained in mature sperm (Mahadevaiah et al. 2001; Fernandez-Capetillo et al. 2003; Brock et al. 1980; Patankar et al. 2021; Singh and Parte 2021). TH2B shares ~ 85% identity with core H2B, with differences concentrated over the tail region thereby weakening DNA interactions (Pentakota et al. 2014). As mentioned above, when paired with Th2a, Th2b mouse KO display severe fertility defects. Yet, single Th2b KO showed that this variant played a key role in destabilizing nucleosomes during histone-to-protamine transition (Montellier et al. 2013). This function could be dependent on TH2B specific interaction with germ cell chromatin remodelers, as a tagged version of the histone display severe dominant negative sterility (Montellier et al. 2013). Which remodeling factor or nucleosome modifying enzyme might carry this function remains unknown.
H2B.L (or subH2B) is an unusual H2B variants that was first identified as part of the acrosomal region of bull sperm (Aul and Oko 2001). H2B.L is 4 amino acids shorter than core H2B and is subject to rapid evolutionary diversification in mammals (Aul and Oko 2001; Raman et al. 2022). Which function during reproduction might be driving this accelerated evolution is still unknown.
H2B.W is also unusual as it is the only H2B variant with an extended N-terminal tail (Churikov et al. 2004; Raman et al. 2022). It is most abundant in the testis and binds to telomeric chromatin when expressed in somatic cells; however, this function has not been tested in vivo (Churikov et al. 2004; Boulard et al. 2006). In most mammals, H2B.W is encoded by multiple paralogs — including a paralog previously known as H2B.M — and are the most rapidly diverging H2B genes identified to date (Raman et al. 2022).
H1 variants
In mammals, there are six somatic variants (H1.1 to 5 and H1.10) and four that are exclusively found in the germline (H1.6 to 9) (Fan et al. 2003, 2005; Eirín-López et al. 2004; Ponte et al. 2017; Talbert and Henikoff 2021). While they have received less attention overall, these variants contribute nonetheless to the functional diversity of germ cell chromatin.
H1T (or H1.6) was first identified during rat spermatogenesis where its abundance exceeds 50% of all H1s (Bucci et al. 1982). In mice, H1T expression is restricted to meiotic and post-meiotic cells (Drabent et al. 1996, 1998). Recent studies have mapped H1T to transposable elements coated with DNA methylation, H3K9me3 and H4K20me3 suggesting it might be associated with their transcriptional repression (Mahadevan et al. 2020). Surprisingly, H1t KO male mice display no abnormalities during spermatogenesis (Drabent et al. 1996). Thus, it would be interesting to investigate how other testis-restricted H1s might functionally replace H1T (such as H1.9, see below), as observed for somatic H1s (Fan et al. 2003, 2005), and if species-specific features drive H1T functions.
H1T2 (or H1.7) is expressed following meiosis during spermatogenesis in round and elongating spermatids (Shalini et al. 2021). Albeit with some divergence, H1.7 and H1.9 share an extended C-terminal tail not found in other H1s (Tanaka et al. 2005). Unlike H1T, male mice with homozygous deletion of H1.7 are infertile with sub-optimal histone to protamine replacement (Martianov et al. 2005; Tanaka et al. 2005). So far, H1.7 has not been found in mature sperm chromatin (Tanaka et al. 2005).
HILS1 (or H1.9) is the final testis-specific H1 variant expressed in elongating spermatids (Yan et al. 2003). In addition to being highly divergent compared to other H1s, H1.9 appears to be rapidly evolving in mammals (Su et al. 2013). A recent study showed that it induces relaxed chromatin states and is enriched over LINE-1 elements in spermatids (Mishra et al. 2018). How this function relates to species-specific histone-to-protamine exchange remains currently unknown (Mishra et al. 2018). However, since H1s are detected in mature sperm (Luense et al. 2016), which of these variants makes the final cut remains to be found.
Female germ cell specific histone variants: maternal breakthrough roles
Unlike continuous meiosis in males, female gametes are produced through one wave of meiosis from PGCs and arrest twice during this process: first, at the end of meiotic prophase I at the germinal vesicle, or GV, stage (Mehlmann 2005); second, meiosis resumes at each ovulation cycle after puberty but stops at metaphase of meiosis II to produce fertilization competent oocytes (Mehlmann 2005).
These meiotic arrests are coupled to broad transcriptional quiescence and the establishment of non-canonical patterns of histone modifications (Moore et al. 1974; Kageyama et al. 2007; Dahl et al. 2016). In mice, H3K4me3 becomes distributed over broad domains at non-transcribed genic and intergenic regions through the action of MLL2 and contributes to transcriptional silencing (Dahl et al. 2016; Zhang et al. 2016; Hanna et al. 2018). H3K27me3 and H2AK119Ub also expand to intergenic regions (Zheng et al. 2016; Chen et al. 2021; Mei et al. 2021). Together, these post translational modification patterns contribute to a novel form of maternal non-canonical imprint regulating embryonic growth (Inoue et al. 2017; Hanna and Kelsey 2017; Mei et al. 2021). Yet, these non-canonical imprints seem to diverge between species. Most notably, human oocytes are devoid of such noncanonical patterns of H3K27me3 and H3K4me3 (Lu et al. 2021).
Both core and variant histones have been detected in the oocyte, either at the RNA or protein level (Wassarman and Mrozak 1981; Aoki et al. 1997; Torres-Padilla et al. 2006; Nashun et al. 2010; Shinagawa et al. 2014, 2015; Kong et al. 2018; Raman et al. 2022). Maternal histones will contribute to the repackaging of parental genomes shortly after fertilization towards zygotic genome activation (reviewed in Yang et al. 2015). Thus, histone function in female gametes can rarely be decoupled from their maternal-effect on the zygote. Core H2A, H2A.Z and macroH2A have all been detected in oocyte chromatin (Nashun et al. 2010; Liu et al. 2022). These histones delocalize from the maternal chromatin following fertilization and only reassociate with embryonic chromatin after zygotic genome activation (Nashun et al. 2010; Liu et al. 2022). Only H2A.X is loaded onto parental genomes around fertilization, and this activity depends on its unique C-terminal tail involved in DNA damage sensing (Nashun et al. 2010).
Likely due to the difficulties of studying such a discrete cell type, most female specific histone variants have not been functionally characterized besides their expression patterns. This includes the only H4 variant described in mammals, H4.G. It is detected in human ovaries and various tumors (Wassarman and Mrozak 1981; Long et al. 2019). Another example are the recently identified H2B variants H2B.K and H2B.N (Raman et al. 2022). In humans, their expression peaks during meiosis I and again following fertilization (Raman et al. 2022). Finally, the short H2A variant H2A.B is also found during female meiosis but its chromatin function in females remains completely unknown (Molaro et al. 2020). While these variants have yet to reveal their female breakthrough roles, we specifically discuss established maternal-effect variants in the following sections (Fig. 1).
H3.3 is deposited in growing oocytes and becomes the main H3 variant up to their maturation (Torres-Padilla et al. 2006; Akiyama et al. 2011). As such it is the major carrier of post-translational modifications unique to the mouse oocyte (Akiyama et al. 2011). In this context, H3.3 accumulation gradually shifts from its canonical euchromatic pattern in growing oocytes to an enrichment at heterochromatic regions in the mature oocytes (Ishiuchi et al. 2021). H3.3 knockdown in mature oocytes leads to suboptimal development of early zygotes suggesting that maternal H3.3 deposition plays a critical role post-fertilization (Kong et al. 2018). This function is tied to paternal genome remodeling and activation (van der Heijden et al. 2005; Torres-Padilla et al. 2006; Santenard and Torres-Padilla 2009; Akiyama et al. 2011; Kong et al. 2018). Thus, H3.3 temporal function during gametogenesis and early embryonic development finely orchestrates parental chromatin remodeling around fertilization.
CenH3 is also found in the chromatin of mature oocytes. There, it performs its unique germline function of centromere identification and inheritance as discussed in the previous section (Das et al. 2017). However, in females, cenH3 binding to centromeres has been proposed to contribute to the suppression of selfish chromosomes drive during asymmetric meiosis (Henikoff et al. 2001; Kursel and Malik 2018). This crucial function has profound consequences on cenH3 evolutionary trajectory discussed in the next section.
TH2A (H2A.1) and TH2B (H2B.1) variants are highly expressed and favored over their core counterparts in the oocyte. They are maternally deposited in the mouse zygote, and briefly induced upon genome activation. Maternal TH2A/TH2B contribute to the activation of the paternal genome after fertilization, possibly by inducing a more open chromatin structure compared to the core histones (Tanaka et al. 2001; Shinagawa et al. 2014).
H1FOO (or H1.8) is specifically induced at the GV stage where it almost entirely replaces core H1 up to the mature MII oocyte stage in mice (Tanaka et al. 2001; Gao et al. 2004). Upon fertilization, some H1FOOs are loaded onto the paternal genome (Tanaka et al. 2001). Given that H1FOO has a greater chromatosome mobility than H1, it could contribute to chromatin remodeling leading-up to zygotic genome activation (Teranishi et al. 2004; Hayakawa et al. 2012). However, considering that mature oocytes are transcriptionally inert, this remodeling function must be coupled to specific chromatin features of the zygote.
Evolutionary trajectories of mammalian germline histone variants: spin-offs and sequels
From this overview, it is clear that when germline histone variants are investigated in details, all are found to be crucial for reproductive fitness. While this might be expected for variants sharing long evolutionary histories with our genomes, it is perhaps more surprising for recently evolved ones. Indeed, it is generally assumed that essential processes involve universally conserved players under strong purifying selection. However, reproduction is also a place of intense evolutionary tensions driving functional diversification (Moore and Haig 1991; Partridge and Hurst 1998; Martin and Hosken 2003; Crespi and Semeniuk 2004) (Fig. 2).
Fig. 2.
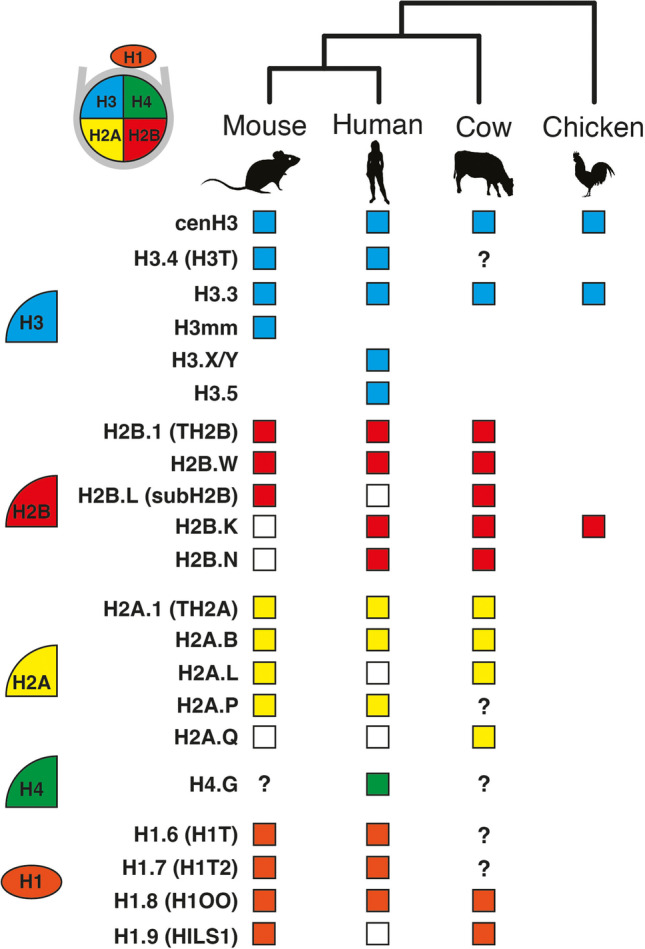
Germline histone variants orthologs identified in mammals. Histone variants with germline specific functions or expression are classified by type (H3, H2A, H2B, H4 and H1). Filled boxes indicate identified orthologous genes in the mouse, human, and cow genomes, with chicken used an outgroup. Empty boxes indicate pseudogenization or other secondary loss event. “?” denotes unresolved orthology. In the following paragraphs, we briefly discuss these evolutionary scenarios for germline histone variants.
To begin, there are those ubiquitously expressed histone variants showing unique functions in germ cells (e.g., H2A.X, macroH2A). All are deeply conserved and have maintained steady evolutionary trajectories in mammalian genomes (Malik and Henikoff 2003; Talbert et al. 2012; Molaro and Drinnenberg 2018; Talbert and Henikoff 2021). One might anticipate their function in gametes or zygote genome packaging to also be conserved between mammals. The only exception is cenH3 which, despite its ancient birth in eukaryotes, displays accelerated rates of evolution between closely related species. Part of this diversification might be the direct consequence of cenH3's role in chromosome drive suppression during female meiosis (Chmátal et al. 2014; also reviewed in Lampson and Black 2017; Kursel and Malik 2018). In this context, cenH3 centromere function is hypothesized to re-establish fair chromosome segregation when “cheating chromosomes” take advantage of female asymmetrical meiosis for inclusion in the oocyte. As such, cenH3 evolutionary trajectory is directly linked to its germline function. Nevertheless, future research will probably uncover novel species-specific layers of chromatin regulation involving other ancient variants in the germline.
The vast majority of variants have recent origins in mammals, some carrying out functions unique to the germline (Fig. 2). Some of these newcomers arose multiple times from the same parental histone during evolution, as seen for H3mms in mouse, or H3.5 and H3.Y/X in primates. In such cases, it is tempting to speculate that recurring gene duplications helped resolve incompatible ancestral functions carried by a single parental histone, as previously observed in flies (Kursel and Malik 2017). Perhaps also as a result of conflicting selective forces during reproduction, many variants got lost along specific mammalian lineages — e.g., H2B.K and H2B.N in mouse, or H2A.Ls and H1.9 in primates — (Su et al. 2013; Molaro et al. 2018; Raman et al. 2022) (Fig. 2).
Finally, novel mammalian germline histones are particularly prone to positive selection. These include recently evolved sex-specific H2Bs, short H2As, H1T, and H1.9 (Ponte et al. 1998; Su et al. 2013; Molaro et al. 2018; Raman et al. 2022). While their evolutionary trajectories differ from cenH3, these signatures might also reveal ongoing germline genetic conflicts. In the case of the short H2A variant H2A.B, there is functional evidence supporting that parental antagonism or sexual conflict could drive its rapid evolution (Soboleva et al. 2017; Moretti et al. 2017; Molaro et al. 2018, 2020). It is interesting to note that these rapidly evolving histone families also display high gene turnover, perhaps further supporting the evolutionary arms races hypothesis (Fig. 2). However, the nature of these rapidly evolving functions and selective forces remains unknown for most variants.
In conclusion, with ongoing efforts to study histone-based parental-effects during reproduction, and the increasing interest in mapping their evolutionary histories, we can only predict that germline histones still have many spin-offs and sequels ready to hit the screen.
Acknowledgements
We would like to thank Dr. Ana Boskovic, Dr. Ines Drinnenberg, and members of the Molaro laboratory for critically reading this manuscript.
Author contribution
G. K. and A. M. wrote the manuscript and prepared the figures.
Funding
This work was supported by the Fondation pour la Recherche Médicale (FRM: AJE201912009932), the French Agence Nationale de la Recherche (ANR) (ANR-22-CE12-0025–01), and the Genetics, Reproduction and Development Institute (iGReD).
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Additional declaration of consent
All the authors consent to participating and to the publication of the work presented here.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akiyama T, Suzuki O, Matsuda J, Aoki F. Dynamic replacement of histone H3 variants reprograms epigenetic marks in early mouse embryos. PLOS Genet. 2011;7:e1002279. doi: 10.1371/journal.pgen.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuar ND, Kurscheid S, Field M, et al. Gene editing of the multi-copy H2A.B gene and its importance for fertility. Genome Biol. 2019;20:23. doi: 10.1186/s13059-019-1633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol. 1997;181:296–307. doi: 10.1006/dbio.1996.8466. [DOI] [PubMed] [Google Scholar]
- Arents G, Burlingame RW, Wang BC, et al. The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci. 1991;88:10148–10152. doi: 10.1073/pnas.88.22.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura Y, Kimura H, Oda T, et al. Structural basis of a nucleosome containing histone H2A.B/H2A.Bbd that transiently associates with reorganized chromatin. Sci Rep. 2013;3:3510. doi: 10.1038/srep03510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aul RB, Oko RJ. The major subacrosomal occupant of bull spermatozoa is a novel histone H2B variant associated with the forming acrosome during spermiogenesis. Dev Biol. 2001;239:376–387. doi: 10.1006/dbio.2001.0427. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Hoogerbrugge JW, Roest HP, et al. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol. 1999;207:322–333. doi: 10.1006/dbio.1998.9155. [DOI] [PubMed] [Google Scholar]
- Bao Y, Konesky K, Park YJ, et al. Nucleosomes containing the histone variant H2A.Bbd organize only 118 base pairs of DNA. EMBO J. 2004;23:3314–3324. doi: 10.1038/sj.emboj.7600316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral S, Morozumi Y, Tanaka H, et al. Histone variant H2A.L.2 guides transition protein-dependent protamine assembly in male germ cells. Mol Cell. 2017;66:89–101.e8. doi: 10.1016/j.molcel.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Bošković A, Eid A, Pontabry J, et al. Higher chromatin mobility supports totipotency and precedes pluripotency in vivo. Genes Dev. 2014;28:1042–1047. doi: 10.1101/gad.238881.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulard M, Gautier T, Mbele GO, et al. The NH2 Tail of the novel histone variant H2BFWT exhibits properties distinct from conventional H2B with respect to the assembly of mitotic chromosomes. Mol Cell Biol. 2006;26:1518–1526. doi: 10.1128/MCB.26.4.1518-1526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlage B, Kosciessa U, Doenecke D. Differential expression of the murine histone genes H3.3A and H3.3B. Differ Res Biol Divers. 1997;62:13–20. doi: 10.1046/j.1432-0436.1997.6210013.x. [DOI] [PubMed] [Google Scholar]
- Brock WA, Trostle PK, Meistrich ML. Meiotic synthesis of testis histones in the rat. Proc Natl Acad Sci U S A. 1980;77:371–375. doi: 10.1073/pnas.77.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner AM, Nanni P, Mansuy IM. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin. 2014;7:1–12. doi: 10.1186/1756-8935-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci L, Brock W, Meistrich M. Distribution and synthesis of histone 1 subfractions during spermatogenesis in the rat. Exp Cell Res. 1982;140:111–118. doi: 10.1016/0014-4827(82)90162-8. [DOI] [PubMed] [Google Scholar]
- Burton A, Brochard V, Galan C, et al. Heterochromatin establishment during early mammalian development is regulated by pericentromeric RNA and characterized by non-repressive H3K9me3. Nat Cell Biol. 2020;22:767–778. doi: 10.1038/s41556-020-0536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschbeck M, Hake SB. Variants of core histones and their roles in cell fate decisions, development and cancer. Nat Rev Mol Cell Biol. 2017;18:299–314. doi: 10.1038/nrm.2016.166. [DOI] [PubMed] [Google Scholar]
- Carone BR, Hung J-H, Hainer SJ, et al. High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm. Dev Cell. 2014;30:11–22. doi: 10.1016/j.devcel.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Sun J-M, Zhang Y, et al. Ubiquitination of histone H3 in elongating spermatids of rat testes *. J Biol Chem. 1998;273:13165–13169. doi: 10.1074/jbc.273.21.13165. [DOI] [PubMed] [Google Scholar]
- Chen Z, Djekidel MN, Zhang Y. Distinct dynamics and functions of H2AK119ub1 and H3K27me3 in mouse preimplantation embryos. Nat Genet. 2021;53:551–563. doi: 10.1038/s41588-021-00821-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GL, Bleakley M, Bradley RK, et al. Short H2A histone variants are expressed in cancer. Nat Commun. 2021;12:490. doi: 10.1038/s41467-020-20707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmátal L, Gabriel SI, Mitsainas GP, et al. Centromere strength provides the cell biological basis for meiotic drive and karyotype evolution in mice. Curr Biol CB. 2014;24:2295–2300. doi: 10.1016/j.cub.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churikov D, Siino J, Svetlova M, et al. Novel human testis-specific histone H2B encoded by the interrupted gene on the X chromosome. Genomics. 2004;84:745–756. doi: 10.1016/j.ygeno.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Couldrey C, Carlton MB, Nolan PM, et al. A retroviral gene trap insertion into the histone 3.3A gene causes partial neonatal lethality, stunted growth, neuromuscular deficits and male sub-fertility in transgenic mice. Hum Mol Genet. 1999;8:2489–2495. doi: 10.1093/hmg/8.13.2489. [DOI] [PubMed] [Google Scholar]
- Crespi B, Semeniuk C. Parent-offspring conflict in the evolution of vertebrate reproductive mode. Am Nat. 2004;163:635–653. doi: 10.1086/382734. [DOI] [PubMed] [Google Scholar]
- Dahl JA, Jung I, Aanes H, et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature. 2016;537:548–552. doi: 10.1038/nature19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Smoak EM, Linares-Saldana R, et al. Centromere inheritance through the germline. Chromosoma. 2017;126:595–604. doi: 10.1007/s00412-017-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, Nguyen TT, Pang MYH, Ishibashi T. Primate-specific histone variants. Genome. 2021;64:337–346. doi: 10.1139/gen-2020-0094. [DOI] [PubMed] [Google Scholar]
- Doyen CM, Montel F, Gautier T, et al. Dissection of the unusual structural and functional properties of the variant H2A.Bbd nucleosome. EMBO J. 2006;25:4234–4244. doi: 10.1038/sj.emboj.7601310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabent B, Bode C, Bramlage B, Doenecke D. Expression of the mouse testicular histone gene H1t during spermatogenesis. Histochem Cell Biol. 1996;106:247–251. doi: 10.1007/BF02484408. [DOI] [PubMed] [Google Scholar]
- Drabent B, Bode C, Miosge N, et al. Expression of the mouse histone gene H1t begins at premeiotic stages of spermatogenesis. Cell Tissue Res. 1998;291:127–132. doi: 10.1007/s004410050986. [DOI] [PubMed] [Google Scholar]
- Draizen EJ, Shaytan AK, Mariño-Ramírez L, et al. HistoneDB 2.0: a histone database with variants—an integrated resource to explore histones and their variants. Database. 2016;2016:baw014. doi: 10.1093/database/baw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirín-López JM, González-Tizón AM, Martínez A, Méndez J. Birth-and-death evolution with strong purifying selection in the histone H1 multigene family and the origin of orphon H1 genes. Mol Biol Evol. 2004;21:1992–2003. doi: 10.1093/molbev/msh213. [DOI] [PubMed] [Google Scholar]
- Erkek S, Hisano M, Liang CY, et al. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat Struct Mol Biol. 2013;20:868–875. doi: 10.1038/nsmb.2599. [DOI] [PubMed] [Google Scholar]
- Evolutionary epigenomic analyses in mammalian early embryos reveal species-specific innovations and conserved principles of imprinting. 10.1126/sciadv.abi6178. Accessed 28 Feb 2023 [DOI] [PMC free article] [PubMed]
- Faast R, Thonglairoam V, Schulz TC, et al. Histone variant H2A.Z is required for early mammalian development. Curr Biol CB. 2001;11:1183–1187. doi: 10.1016/s0960-9822(01)00329-3. [DOI] [PubMed] [Google Scholar]
- Fan Y, Nikitina T, Morin-Kensicki EM, et al. H1 linker histones are essential for mouse development and affect nucleosome spacing in vivo. Mol Cell Biol. 2003;23:4559–4572. doi: 10.1128/MCB.23.13.4559-4572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Nikitina T, Zhao J, et al. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 2005;123:1199–1212. doi: 10.1016/j.cell.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Ferguson L, Ellis PJI, Affara NA. Two novel mouse genes mapped to chromosome Yp are expressed specifically in spermatids. Mamm Genome off J Int Mamm Genome Soc. 2009;20:193–206. doi: 10.1007/s00335-009-9175-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, et al. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell. 2003;4:497–508. doi: 10.1016/s1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Fontaine E, Papin C, Martinez G, et al. Dual role of histone variant H3.3B in spermatogenesis: positive regulation of piRNA transcription and implication in X-chromosome inactivation. Nucleic Acids Res. 2022;50:7350–7366. doi: 10.1093/nar/gkac541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Chung YG, Parseghian MH, et al. Rapid H1 linker histone transitions following fertilization or somatic cell nuclear transfer: evidence for a uniform developmental program in mice. Dev Biol. 2004;266:62–75. doi: 10.1016/j.ydbio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Dev Camb Engl. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Ilter D, Low V, et al. Dynamic incorporation of histone H3 variants into chromatin is essential for acquisition of aggressive traits and metastatic colonization. Cancer Cell. 2019;36:402–417.e13. doi: 10.1016/j.ccell.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govin J, Escoffier E, Rousseaux S, et al. Pericentric heterochromatin reprogramming by new histone variants during mouse spermiogenesis. J Cell Biol. 2007;176:283–294. doi: 10.1083/jcb.200604141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves IK, Rangasamy D, Devoy M, et al. The X and Y chromosomes assemble into H2A.Z, containing facultative heterochromatin, following meiosis. Mol Cell Biol. 2006;26:5394–5405. doi: 10.1128/MCB.00519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett JA, Zylicz JJ, Surani MA. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet TIG. 2012;28:164–174. doi: 10.1016/j.tig.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Hada M, Masuda K, Yamaguchi K, et al. Identification of a variant-specific phosphorylation of TH2A during spermiogenesis. Sci Rep. 2017;7:46228. doi: 10.1038/srep46228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajkova P, Ancelin K, Waldmann T, et al. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature. 2008;452:877–881. doi: 10.1038/nature06714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud SS, Nix DA, Zhang H, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna CW, Kelsey G. Genomic imprinting beyond DNA methylation: a role for maternal histones. Genome Biol. 2017;18:177. doi: 10.1186/s13059-017-1317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna CW, Taudt A, Huang J, et al. MLL2 conveys transcription-independent H3K4 trimethylation in oocytes. Nat Struct Mol Biol. 2018;25:73–82. doi: 10.1038/s41594-017-0013-5. [DOI] [PubMed] [Google Scholar]
- Harada A, Maehara K, Ono Y, et al. Histone H3.3 sub-variant H3mm7 is required for normal skeletal muscle regeneration. Nat Commun. 2018;9:1400. doi: 10.1038/s41467-018-03845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Sin H-S, Maezawa S, et al. SCML2 establishes the male germline epigenome through regulation of histone H2A ubiquitination. Dev Cell. 2015;32:574–588. doi: 10.1016/j.devcel.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Ohgane J, Tanaka S, et al. Oocyte-specific linker histone H1foo is an epigenomic modulator that decondenses chromatin and impairs pluripotency. Epigenetics. 2012;7:1029–1036. doi: 10.4161/epi.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K, Malik HS. The Centromere Paradox: Stable Inheritance with Rapidly Evolving DNA. Science. 2001;293:1098–1102. doi: 10.1126/science.1062939. [DOI] [PubMed] [Google Scholar]
- Herchenröther A, Wunderlich TM, Lan J, Hake SB. Spotlight on histone H2A variants: From B to X to Z. Semin Cell Dev Biol. 2023;135:3–12. doi: 10.1016/j.semcdb.2022.03.025. [DOI] [PubMed] [Google Scholar]
- Hoghoughi N, Barral S, Vargas A, et al. Histone variants: essential actors in male genome programming. J Biochem. 2018;163:97–103. doi: 10.1093/jb/mvx079. [DOI] [PubMed] [Google Scholar]
- Hoyer-Fender S, Costanzi C, Pehrson JR. Histone macroH2A1.2 is concentrated in the XY-body by the early pachytene stage of spermatogenesis. Exp Cell Res. 2000;258:254–260. doi: 10.1006/excr.2000.4951. [DOI] [PubMed] [Google Scholar]
- Inoue A, Jiang L, Lu F, et al. Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature. 2017;547:419–424. doi: 10.1038/nature23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiuchi T, Abe S, Inoue K, et al. Reprogramming of the histone H3.3 landscape in the early mouse embryo. Nat Struct Mol Biol. 2021;28:38–49. doi: 10.1038/s41594-020-00521-1. [DOI] [PubMed] [Google Scholar]
- Jang CW, Shibata Y, Starmer J, et al. Histone H3.3 maintains genome integrity during mammalian development. Genes Dev. 2015;29:1377–1392. doi: 10.1101/gad.264150.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Borg M, Lorković ZJ, et al. The evolution and functional divergence of the histone H2B family in plants. PLOS Genet. 2020;16:e1008964. doi: 10.1371/journal.pgen.1008964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Soboleva TA, Tremethick DJ. Short histone H2A variants: small in stature but not in function. Cells. 2020;9:867. doi: 10.3390/cells9040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YH, Sauria MEG, Lyu X, et al. Chromatin states in mouse sperm correlate with embryonic and adult regulatory landscapes. Cell Rep. 2017;18:1366–1382. doi: 10.1016/j.celrep.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama S, Liu H, Kaneko N, et al. Alterations in epigenetic modifications during oocyte growth in mice. Reprod Camb Engl. 2007;133:85–94. doi: 10.1530/REP-06-0025. [DOI] [PubMed] [Google Scholar]
- Kallappagoudar S, Yadav RK, Lowe BR, Partridge JF. Histone H3 mutations—a special role for H3.3 in tumorigenesis? Chromosoma. 2015;124:177–189. doi: 10.1007/s00412-015-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmins S, Sassone-Corsi P. Chromatin remodelling and epigenetic features of germ cells. Nature. 2005;434:583–589. doi: 10.1038/nature03368. [DOI] [PubMed] [Google Scholar]
- Kohestani H, Wereszczynski J. Effects of H2A.B incorporation on nucleosome structures and dynamics. Biophys J. 2021;120:1498–1509. doi: 10.1016/j.bpj.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Banaszynski LA, Geng F, et al. Histone variant H3.3–mediated chromatin remodeling is essential for paternal genome activation in mouse preimplantation embryos. J Biol Chem. 2018;293:3829–3838. doi: 10.1074/jbc.RA117.001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg RD, Lorch Y. Primary role of the nucleosome. Mol Cell. 2020;79:371–375. doi: 10.1016/j.molcel.2020.07.020. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Horikoshi N, Sato K, et al. Structure and function of human histone H3.Y nucleosome. Nucleic Acids Res. 2016;44:6127–6141. doi: 10.1093/nar/gkw202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Horikoshi N, Xie Y, et al. Identification of the amino acid residues responsible for stable nucleosome formation by histone H3.Y. Nucl Austin Tex. 2017;8:239–248. doi: 10.1080/19491034.2016.1277303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto K, Saitou M. Germ cell reprogramming. Curr Top Dev Biol. 2019;135:91–125. doi: 10.1016/bs.ctdb.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Kursel LE, Malik HS. Recurrent gene duplication leads to diverse repertoires of centromeric histones in Drosophila species. Mol Biol Evol. 2017;34:1445–1462. doi: 10.1093/molbev/msx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursel LE, Malik HS. The cellular mechanisms and consequences of centromere drive. Curr Opin Cell Biol. 2018;52:58–65. doi: 10.1016/j.ceb.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Black BE. Cellular and molecular mechanisms of centromere drive. Cold Spring Harb Symp Quant Biol. 2017;82:249–257. doi: 10.1101/sqb.2017.82.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M. Meiotic recombination: breaking the genome to save it. Curr Biol CB. 2001;11:R253–256. doi: 10.1016/s0960-9822(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang J, Zhou J, et al. Hierarchical accumulation of histone variant H2A.Z regulates transcriptional states and histone modifications in early mammalian embryos. Adv Sci Weinh Baden-Wurtt Ger. 2022;9:e2200057. doi: 10.1002/advs.202200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Sun X, Shi W, et al. A novel histone H4 variant H4G regulates rDNA transcription in breast cancer. Nucleic Acids Res. 2019;47:8399–8409. doi: 10.1093/nar/gkz547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zhang Y, Wang L, et al. Evolutionary epigenomic analyses in mammalian early embryos reveal species-specific innovations and conserved principles of imprinting. Sci Adv. 2021;7:eabi6178. doi: 10.1126/sciadv.abi6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luense LJ, Wang X, Schon SB, et al. Comprehensive analysis of histone post-translational modifications in mouse and human male germ cells. Epigenetics Chromatin. 2016;9:24. doi: 10.1186/s13072-016-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nat 389(6648):251–260. 10.1038/38444 [DOI] [PubMed]
- Maehara K, Harada A, Sato Y, et al. Tissue-specific expression of histone H3 variants diversified after species separation. Epigenetics Chromatin. 2015;8:35. doi: 10.1186/s13072-015-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JMA, Baudat F, et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Mahadevan IA, Kumar S, Rao MRS. Linker histone variant H1t is closely associated with repressed repeat-element chromatin domains in pachytene spermatocytes. Epigenetics Chromatin. 2020;13:9. doi: 10.1186/s13072-020-00335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nat Struct Mol Biol. 2003;10:882–891. doi: 10.1038/nsb996. [DOI] [PubMed] [Google Scholar]
- Martianov I, Brancorsini S, Catena R, et al. Polar nuclear localization of H1T2, a histone H1 variant, required for spermatid elongation and DNA condensation during spermiogenesis. Proc Natl Acad Sci. 2005;102:2808–2813. doi: 10.1073/pnas.0406060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin OY, Hosken DJ. The evolution of reproductive isolation through sexual conflict. Nature. 2003;423:979–982. doi: 10.1038/nature01752. [DOI] [PubMed] [Google Scholar]
- Martire S, Banaszynski LA. The roles of histone variants in fine-tuning chromatin organization and function. Nat Rev Mol Cell Biol. 2020;21:522–541. doi: 10.1038/s41580-020-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff WF, Wagner EJ, Duronio RJ. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet. 2008;9:843–854. doi: 10.1038/nrg2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Mochizuki K. A current view of the epigenome in mouse primordial germ cells. Mol Reprod Dev. 2014;81:160–170. doi: 10.1002/mrd.22214. [DOI] [PubMed] [Google Scholar]
- Maze I, Noh KM, Soshnev AA, Allis CD. Every amino acid matters: essential contributions of histone variants to mammalian development and disease. Nat Rev Genet. 2014;15:259–271. doi: 10.1038/nrg3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley KL, Cheeseman IM. The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol. 2016;17:16–29. doi: 10.1038/nrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- Mei H, Kozuka C, Hayashi R, et al. H2AK119ub1 guides maternal inheritance and zygotic deposition of H3K27me3 in mouse embryos. Nat Genet. 2021;53:539–550. doi: 10.1038/s41588-021-00820-3. [DOI] [PubMed] [Google Scholar]
- Mellone BG, Fachinetti D. Diverse mechanisms of centromere specification. Curr Biol CB. 2021;31:R1491–R1504. doi: 10.1016/j.cub.2021.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán-Zambrano G, Burton A, Bannister AJ, Schneider R. Histone post-translational modifications — cause and consequence of genome function. Nat Rev Genet. 2022;23:563–580. doi: 10.1038/s41576-022-00468-7. [DOI] [PubMed] [Google Scholar]
- Mishra LN, Shalini V, Gupta N, et al. Spermatid-specific linker histone HILS1 is a poor condenser of DNA and chromatin and preferentially associates with LINE-1 elements. Epigenetics Chromatin. 2018;11:43. doi: 10.1186/s13072-018-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaro A, Drinnenberg IA. Studying the evolution of histone variants using phylogeny. Methods Mol Biol Clifton NJ. 2018;1832:273–291. doi: 10.1007/978-1-4939-8663-7_15. [DOI] [PubMed] [Google Scholar]
- Molaro A, Young JM, Malik HS. Evolutionary origins and diversification of testis-specific short histone H2A variants in mammals. Genome Res. 2018;28:460–473. doi: 10.1101/gr.229799.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molaro A, Wood AJ, Janssens D, et al. Biparental contributions of the H2A.B histone variant control embryonic development in mice. PLOS Biol. 2020;18:e3001001. doi: 10.1371/journal.pbio.3001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montellier E, Boussouar F, Rousseaux S, et al. Chromatin-to-nucleoprotamine transition is controlled by the histone H2B variant TH2B. Genes Dev. 2013;27:1680–1692. doi: 10.1101/gad.220095.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 1991;7:45–49. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- Moore GPM, Lintern-Moore S, Peters H, Faber M. RNA synthesis in the mouse oocyte. J Cell Biol. 1974;60:416–422. doi: 10.1083/jcb.60.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti C, Serrentino M-E, Ialy-Radio C, et al. SLY regulates genes involved in chromatin remodeling and interacts with TBL1XR1 during sperm differentiation. Cell Death Differ. 2017;24:1029–1044. doi: 10.1038/cdd.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacev BA, Feng L, Bagert JD, et al. The expanding landscape of “oncohistone” mutations in human cancers. Nature. 2019;567:473–478. doi: 10.1038/s41586-019-1038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashun B, Yukawa M, Liu H, et al. Changes in the nuclear deposition of histone H2A variants during pre-implantation development in mice. Development. 2010;137:3785–3794. doi: 10.1242/dev.051805. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Miller KM. Histone H2A variants: Diversifying chromatin to ensure genome integrity. Semin Cell Dev Biol. 2023;135:59–72. doi: 10.1016/j.semcdb.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva R, Dixon GH. Vertebrate protamine genes and the histone-to-protamine replacement reaction. Prog Nucleic Acid Res Mol Biol. 1991;40:25–94. doi: 10.1016/s0079-6603(08)60839-9. [DOI] [PubMed] [Google Scholar]
- Orsi GA, Couble P, Loppin B. Epigenetic and replacement roles of histone variant H3.3 in reproduction and development. Int J Dev Biol. 2009;53:231–243. doi: 10.1387/ijdb.082653go. [DOI] [PubMed] [Google Scholar]
- Osakabe A, Molaro A. Histone renegades: unusual H2A histone variants in plants and animals. Semin Cell Dev Biol. 2023;135:35–42. doi: 10.1016/j.semcdb.2022.05.001. [DOI] [PubMed] [Google Scholar]
- Partridge L, Hurst LD. Sex and conflict. Science. 1998;281:2003–2008. doi: 10.1126/science.281.5385.2003. [DOI] [PubMed] [Google Scholar]
- Pasque V, Radzisheuskaya A, Gillich A, et al. Histone variant macroH2A marks embryonic differentiation in vivo and acts as an epigenetic barrier to induced pluripotency. J Cell Sci. 2012;125:6094. doi: 10.1242/jcs.113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patankar A, Gajbhiye R, Surve S, Parte P. Epigenetic landscape of testis specific histone H2B variant and its influence on sperm function. Clin Epigenetics. 2021;13:101. doi: 10.1186/s13148-021-01088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentakota SK, Sandhya S, Sikarwar P, A, , et al. Mapping post-translational modifications of mammalian testicular specific histone variant TH2B in tetraploid and haploid germ cells and their implications on the dynamics of nucleosome structure. J Proteome Res. 2014;13:5603–5617. doi: 10.1021/pr500597a. [DOI] [PubMed] [Google Scholar]
- Phillips DMP, Johns EW. A fractionation of the histones of group F2a from calf thymus. Biochem J. 1965;94:127–130. doi: 10.1042/bj0940127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkivska H, Rooney AP, Nei M. Purifying selection and birth-and-death evolution in the histone H4 gene family. Mol Biol Evol. 2002;19:689–697. doi: 10.1093/oxfordjournals.molbev.a004127. [DOI] [PubMed] [Google Scholar]
- Ponte I, Vidal-Taboada JM, Suau P. Evolution of the vertebrate H1 histone class: evidence for the functional differentiation of the subtypes. Mol Biol Evol. 1998;15:702–708. doi: 10.1093/oxfordjournals.molbev.a025973. [DOI] [PubMed] [Google Scholar]
- Ponte I, Romero D, Yero D, et al. Complex evolutionary history of the mammalian histone H1.1–H1.5 gene family. Mol Biol Evol. 2017;34:545–558. doi: 10.1093/molbev/msw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst AV, Santos F, Reik W, et al. Structural differences in centromeric heterochromatin are spatially reconciled on fertilisation in the mouse zygote. Chromosoma. 2007;116:403–415. doi: 10.1007/s00412-007-0106-8. [DOI] [PubMed] [Google Scholar]
- Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- Raman P, Rominger MC, Young JM, et al. Novel classes and evolutionary turnover of histone H2B variants in the mammalian germline. Mol Biol Evol. 2022;39:msac019. doi: 10.1093/molbev/msac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathke C, Baarends WM, Awe S, Renkawitz-Pohl R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta BBA - Gene Regul Mech. 2014;1839:155–168. doi: 10.1016/j.bbagrm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Resnick R, Wong C-J, Hamm DC, et al. DUX4-induced histone variants H3.X and H3.Y mark DUX4 target genes for expression. Cell Rep. 2019;29:1812–1820.e5. doi: 10.1016/j.celrep.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AP, Piontkivska H, Nei M. Molecular evolution of the nontandemly repeated genes of the histone 3 multigene family. Mol Biol Evol. 2002;19:68–75. doi: 10.1093/oxfordjournals.molbev.a003983. [DOI] [PubMed] [Google Scholar]
- Saitou M, Kagiwada S, Kurimoto K. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Dev Camb Engl. 2012;139:15–31. doi: 10.1242/dev.050849. [DOI] [PubMed] [Google Scholar]
- Samans B, Yang Y, Krebs S, et al. Uniformity of nucleosome preservation pattern in mammalian sperm and its connection to repetitive DNA elements. Dev Cell. 2014;30:23–35. doi: 10.1016/j.devcel.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Santenard A, Torres-Padilla M-E. Epigenetic reprogramming in mammalian reproduction: contribution from histone variants. Epigenetics. 2009;4:80–84. doi: 10.4161/epi.4.2.7838. [DOI] [PubMed] [Google Scholar]
- Santos F, Dean W. Epigenetic reprogramming during early development in mammals. Reprod Camb Engl. 2004;127:643–651. doi: 10.1530/rep.1.00221. [DOI] [PubMed] [Google Scholar]
- Schenk R, Jenke A, Zilbauer M, et al. H3.5 is a novel hominid-specific histone H3 variant that is specifically expressed in the seminiferous tubules of human testes. Chromosoma. 2011;120:275–285. doi: 10.1007/s00412-011-0310-4. [DOI] [PubMed] [Google Scholar]
- Schwartzentruber J, Korshunov A, Liu X-Y, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- Shalini V, Bhaduri U, Ravikkumar AC, et al. Genome-wide occupancy reveals the localization of H1T2 (H1fnt) to repeat regions and a subset of transcriptionally active chromatin domains in rat spermatids. Epigenetics Chromatin. 2021;14:3. doi: 10.1186/s13072-020-00376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa T, Takagi T, Tsukamoto D, et al. Histone variants enriched in oocytes enhance reprogramming to induced pluripotent stem cells. Cell Stem Cell. 2014;14:217–227. doi: 10.1016/j.stem.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Shinagawa T, Huynh LM, Takagi T, et al (2015) Disruption of Th2a and Th2b genes causes defects in spermatogenesis. Development dev.121830. 10.1242/dev.121830 [DOI] [PubMed]
- Shiraishi K, Shindo A, Harada A, et al. Roles of histone H3.5 in human spermatogenesis and spermatogenic disorders. Andrology. 2018;6:158–165. doi: 10.1111/andr.12438. [DOI] [PubMed] [Google Scholar]
- Simpson RT. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978;17:5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Singh I, Parte P (2021) Heterogeneity in the epigenetic landscape of murine testis-specific histone variants TH2A and TH2B sharing the same bi-directional promoter. Front Cell Dev Biol 3222. 10.3389/fcell.2021.755751 [DOI] [PMC free article] [PubMed]
- Soboleva TA, Nekrasov M, Pahwa A, et al. A unique H2A histone variant occupies the transcriptional start site of active genes. Nat Struct Mol Biol. 2012;19:25–30. doi: 10.1038/nsmb.2161. [DOI] [PubMed] [Google Scholar]
- Soboleva TA, Parker BJ, Nekrasov M, et al. A new link between transcriptional initiation and pre-mRNA splicing: The RNA binding histone variant H2A.B. PLoS Genet. 2017;13:1006633. doi: 10.1371/journal.pgen.1006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Wu D, Zhou W, et al. Rapid evolution of the mammalian HILS1 gene and the nuclear condensation process during mammalian spermiogenesis. J Genet Genomics. 2013;40:55–59. doi: 10.1016/j.jgg.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Surani MA, Ancelin K, Hajkova P, et al. Mechanism of mouse germ cell specification: a genetic program regulating epigenetic reprogramming. Cold Spring Harb Symp Quant Biol. 2004;69:1–9. doi: 10.1101/sqb.2004.69.1. [DOI] [PubMed] [Google Scholar]
- Syed SH, Boulard M, Shukla MS, et al. The incorporation of the novel histone variant H2AL2 confers unusual structural and functional properties of the nucleosome. Nucleic Acids Res. 2009;37:4684–4695. doi: 10.1093/nar/gkp473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Henikoff S. Histone variants at a glance. J Cell Sci. 2021;134:244749. doi: 10.1242/jcs.244749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Ahmad K, Almouzni G, et al. A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin. 2012;5:7. doi: 10.1186/1756-8935-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Hennebold JD, Macfarlane J, Adashi EY. A mammalian oocyte-specific linker histone gene H1oo: homology with the genes for the oocyte-specific cleavage stage histone (cs-H1) of sea urchin and the B4/H1M histone of the frog. Dev Camb Engl. 2001;128:655–664. doi: 10.1242/dev.128.5.655. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Iguchi N, Isotani A, et al. HANP1/H1T2, a novel histone H1-like protein involved in nuclear formation and sperm fertility. Mol Cell Biol. 2005;25:7107–7119. doi: 10.1128/MCB.25.16.7107-7119.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MCW, Jacobs SA, Mattiske DM, et al. Contribution of the two genes encoding histone variant H3.3 to viability and fertility in mice. PLOS Genet. 2015;11:e1004964. doi: 10.1371/journal.pgen.1004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teperek M, Simeone A, Gaggioli V, et al. Sperm is epigenetically programmed to regulate gene transcription in embryos. Genome Res. 2016;26:1034–1046. doi: 10.1101/gr.201541.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi T, Tanaka M, Kimoto S, et al. Rapid replacement of somatic linker histones with the oocyte-specific linker histone H1foo in nuclear transfer. Dev Biol. 2004;266:76–86. doi: 10.1016/j.ydbio.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Thoma F, Koller T, Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Padilla M-E, Bannister AJ, Hurd PJ, et al (2006) Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol 50:. 10.1387/ijdb.052073mt [DOI] [PubMed]
- Trostle-Weige PK, Meistrich ML, Brock WA, et al. Isolation and characterization of TH2A, a germ cell-specific variant of histone 2A in rat testis. J Biol Chem. 1982;257:5560–5567. doi: 10.1016/S0021-9258(19)83813-9. [DOI] [PubMed] [Google Scholar]
- Turner JMA. Meiotic silencing in mammals. Annu Rev Genet. 2015;49:395–412. doi: 10.1146/annurev-genet-112414-055145. [DOI] [PubMed] [Google Scholar]
- Turner JM, Burgoyne PS, Singh PB. M31 and macroH2A1.2 colocalise at the pseudoautosomal region during mouse meiosis. J Cell Sci. 2001;114:3367–3375. doi: 10.1242/jcs.114.18.3367. [DOI] [PubMed] [Google Scholar]
- Ueda J, Harada A, Urahama T, et al. Testis-specific histone variant H3t gene is essential for entry into spermatogenesis. Cell Rep. 2017;18:593–600. doi: 10.1016/j.celrep.2016.12.065. [DOI] [PubMed] [Google Scholar]
- Urahama T, Harada A, Maehara K, et al. Histone H3.5 forms an unstable nucleosome and accumulates around transcription start sites in human testis. Epigenetics Chromatin. 2016;9:2. doi: 10.1186/s13072-016-0051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Werken C, van der Heijden GW, Eleveld C, et al. Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications. Nat Commun. 2014;5:5868. doi: 10.1038/ncomms6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden GW, Dieker JW, Derijck AAHA, et al. Asymmetry in Histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech Dev. 2005;122:1008–1022. doi: 10.1016/j.mod.2005.04.009. [DOI] [PubMed] [Google Scholar]
- van der Heijden GW, Derijck AAHA, Ramos L, et al. Transmission of modified nucleosomes from the mouse male germline to the zygote and subsequent remodeling of paternal chromatin. Dev Biol. 2006;298:458–469. doi: 10.1016/j.ydbio.2006.06.051. [DOI] [PubMed] [Google Scholar]
- Vuoristo S, Bhagat S, Hydén-Granskog C, et al. DUX4 is a multifunctional factor priming human embryonic genome activation. iScience. 2022;25:104137. doi: 10.1016/j.isci.2022.104137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xu Z, Khawar MB, et al. The histone codes for meiosis. Reproduction. 2017;154:R65–R79. doi: 10.1530/REP-17-0153. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Mrozak SC. Program of early development in the mammal: Synthesis and intracellular migration of histone H4 during oogenesis in the mouse. Dev Biol. 1981;84:364–371. doi: 10.1016/0012-1606(81)90405-X. [DOI] [PubMed] [Google Scholar]
- Wiedemann SM, Mildner SN, Bönisch C, et al. Identification and characterization of two novel primate-specific histone H3 variants, H3.X and H3.Y. J Cell Biol. 2010;190:777–791. doi: 10.1083/jcb.201002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt O, Albig W, Doenecke D. Testis-Specific Expression of a Novel Human H3 Histone Gene. Exp Cell Res. 1996;229:301–306. doi: 10.1006/excr.1996.0375. [DOI] [PubMed] [Google Scholar]
- Wu G, Broniscer A, McEachron TA, et al. Somatic Histone H3 Alterations in Paediatric Diffuse Intrinsic Pontine Gliomas and Non-Brainstem Glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Hada M, Fukuda Y, et al. Re-evaluating the Localization of Sperm-Retained Histones Revealed the Modification-Dependent Accumulation in Specific Genome Regions. Cell Rep. 2018;23:3920–3932. doi: 10.1016/j.celrep.2018.05.094. [DOI] [PubMed] [Google Scholar]
- Yan W, Ma L, Burns KH, Matzuk MM. HILS1 is a spermatid-specific linker histone H1-like protein implicated in chromatin remodeling during mammalian spermiogenesis. Proc Natl Acad Sci. 2003;100:10546–10551. doi: 10.1073/pnas.1837812100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wu W, Macfarlan TS. Maternal histone variants and their chaperones promote paternal genome activation and boost somatic cell reprogramming: Prospects & Overviews. BioEssays. 2015;37:52–59. doi: 10.1002/bies.201400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Whiddon JL, Yao Z, et al. DUX4 binding to retroelements creates promoters that are active in FSHD muscle and testis. PLoS Genet. 2013;9:e1003947. doi: 10.1371/journal.pgen.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen BTK, Bush KM, Barrilleaux BL, et al. Histone H3.3 regulates dynamic chromatin states during spermatogenesis. Development. 2014;141:3483–3494. doi: 10.1242/dev.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalensky AO, Breneman JW, Zalenskaya IA, et al. Organization of centromeres in the decondensed nuclei of mature human sperm. Chromosoma. 1993;102:509–518. doi: 10.1007/BF00368344. [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng H, Huang B, et al. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature. 2016;537:553–557. doi: 10.1038/nature19361. [DOI] [PubMed] [Google Scholar]
- Zheng H, Huang B, Zhang B, et al. Resetting epigenetic memory by reprogramming of histone modifications in mammals. Mol Cell. 2016;63:1066–1079. doi: 10.1016/j.molcel.2016.08.032. [DOI] [PubMed] [Google Scholar]
- Zink L-M, Hake SB. Histone variants: nuclear function and disease. Curr Opin Genet Dev. 2016;37:82–89. doi: 10.1016/j.gde.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Zink L-M, Delbarre E, Eberl HC, et al. H3.Y discriminates between HIRA and DAXX chaperone complexes and reveals unexpected insights into human DAXX-H3.3-H4 binding and deposition requirements. Nucleic Acids Res. 2017;45:5691–5706. doi: 10.1093/nar/gkx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.