Abstract
We used homologous and heterologous expression of the glycosyltransferase genes of the Lactococcus lactis NIZO B40 eps gene cluster to determine the activity and substrate specificities of the encoded enzymes and established the order of assembly of the trisaccharide backbone of the exopolysaccharide repeating unit. EpsD links glucose-1-phosphate from UDP-glucose to a lipid carrier, EpsE and EpsF link glucose from UDP-glucose to lipid-linked glucose, and EpsG links galactose from UDP-galactose to lipid-linked cellobiose. Furthermore, EpsJ appeared to be involved in EPS biosynthesis as a galactosyl phosphotransferase or an enzyme which releases the backbone oligosaccharide from the lipid carrier.
Many bacteria are known to produce polysaccharides, which can either be excreted into the environment as exopolysaccharides (EPSs), form a capsule around the cell as capsular polysaccharides, or be attached to the cell membrane as the O antigens of lipopolysaccharides. The biosynthesis of polysaccharides that consist of repeating units includes their assembly on a lipid carrier by sequential transfer of monosaccharides from nucleotide sugars by glycosyltransferases (GTFs) and the subsequent polymerization and export of these repeating units (15, 17).
Although numerous bacterial gene clusters involved in cell surface polysaccharide biosynthesis have been described, only a few of these have been analyzed for the function of their GTF genes. Homologous expression has been used to study the GTF genes involved in O-antigen synthesis from different serogroups of Salmonella enterica (8). Mutations in the different GTF genes involved in Rhizobium meliloti EPS biosynthesis have been generated, and the lipid-linked intermediates which accumulated in permeabilized cells of the mutant bacteria were analyzed by thin-layer chromatography (TLC) to infer the biosynthetic step catalyzed by each enzyme (12). Furthermore, heterologous complementation has been used for the functional analysis of GTF genes of Sphingomonas spp. and Rhizobium leguminosarum (10). The involvement of streptococcal GTF genes in capsule biosynthesis has been studied for type III of Streptococcus group B and Streptococcus pneumoniae serotype 14 (5, 6, 13). For serotype 14, these genes have been shown to be essential for the synthesis of the repeating unit by their expression in Escherichia coli (5, 6). Finally, the eps gene cluster of Streptococcus thermophilus coding for an unknown number of GTFs has been demonstrated to be involved in EPS biosynthesis by heterologous expression in a plasmid-free Lactococcus lactis strain (14).
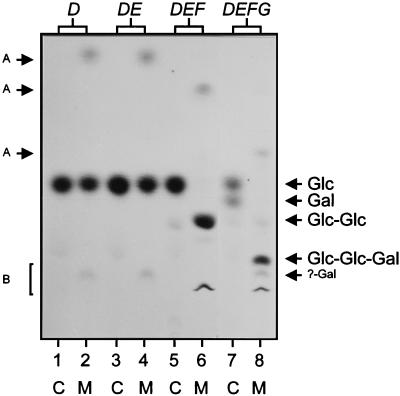
L. lactis NIZO B40 produces an extracellular phosphopolysaccharide with a repeating unit consisting of →4)[α-l-Rhap-(1→2)][α-d-Galp-1-PO4-3]-β-d-Galp-(1→4)-β-d-Glcp-(1→4)-β-d-Glcp-(1→ (9, 16). A plasmid-located eps gene cluster including 14 coordinately transcribed genes with the order epsRXABCDEFGHIJKL has been implicated in the biosynthesis of this EPS. A single gene disruption and heterologous expression of the epsD gene have been used to demonstrate that it is essential for the synthesis of the repeating unit and encodes a GTF transferring glucose from UDP-glucose to a lipid carrier (16). Because of its homology to GumD from Xanthomonas campestris, we assume that EpsD, like GumD, catalyzes the transfer of glucosyl-1-phosphate from UDP-glucose to undecaprenyl phosphate (4). To determine the function and substrate specificities of other NIZO B40 GTF gene products and to establish the order of assembly of the backbone of the EPS repeating unit, which could not be determined by our previous results, we expressed the relevant GTF genes in E. coli. Fragments containing the epsD, epsDE, epsDEF, and epsDEFG genes (Fig. 1) were cloned under control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lac promoter in pUC19 (18) and introduced into E. coli DH5α (3), which has no background GTF activity, as was shown previously (16). The fragment containing epsDE was generated by PCR with the primers 5′-CCGCGCGGATCCGGGTATAGATGATTATC-3′ and 5′-CCGCGCCGAATTCTTGAAACGCCCTTGCTATCTC-3′ by using the BamHI and EcoRI sites of the primers (underlined) for cloning. Since the backbone of the EPS repeating unit is known to contain glucose and galactose, permeabilized cells of IPTG-induced (0.1 ng ml−1) E. coli harboring these plasmids were incubated with UDP-[14C]glucose and UDP-[14C]galactose. The lipid fraction was extracted, subjected to complete and mild acid hydrolysis, and analyzed by TLC and autoradiography as described previously (16) to detect the 14C-labelled monosaccharides (complete acid hydrolysis) and oligosaccharides (mild acid hydrolysis), respectively (Fig. 2). Expression of epsDE showed the same sugar incorporation as expression of epsD alone. In contrast, expression of epsDEF resulted in the production of a lipid-linked oligosaccharide with the same mobility on TLC as cellobiose, which is the β-d-Glcp-(1→4)-β-d-Glcp part of the repeating unit. Expression of epsDEFG resulted in the production of lipid-linked oligosaccharides containing glucose and galactose. Mild acid hydrolysis of these products yielded two oligosaccharides with lower mobility on TLC than that of cellobiose that were retrieved from the TLC plate and subjected to complete acid hydrolysis, followed by a second TLC analysis. The oligosaccharide with the higher mobility consisted of glucose and galactose with a ratio of approximately 2:1, as judged from the intensity of the spots on the autoradiograph of the TLC, and is likely to be the β-d-Galp-(1→4)-β-d-Glcp-(1→4)-β-d-Glcp trisaccharide of the backbone of the repeating unit. The other oligosaccharide contained only 14C-labelled galactose, indicating that EpsG may act with slightly lower affinity on another lipid acceptor than lipid-linked cellobiose in the E. coli membrane. These results indicate that EpsF is the second GTF, linking glucose to lipid-linked glucose, and that EpsG is the third GTF, linking galactose to lipid-linked cellobiose. As EpsE and EpsF are homologous to pneumococcal Cps14F and Cps14G, respectively, we assume that, like Cps14F and Cps14G, both lactococcal gene products act together as one GTF and that EpsF contains the GTF activity while EpsE has an accessory function (5).
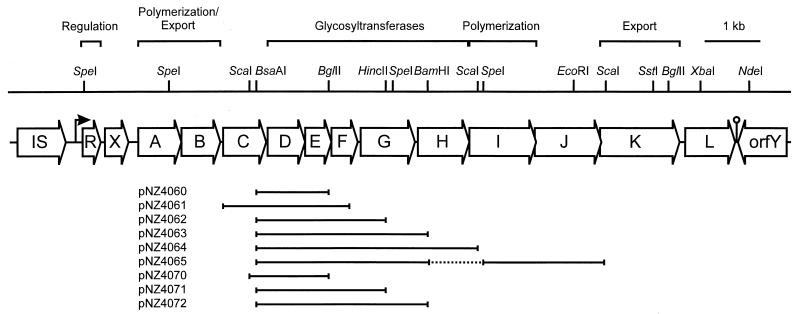
FIG. 1.
Physical and genetic map of the eps gene cluster of plasmid pNZ4000. For BsaAI, EcoRI, HincII, NdeI, ScaI, and SstI, only sites relevant for subcloning are indicated. The plasmids used for heterologous and homologous expression of the eps genes are listed. Plasmids pNZ4060 through pNZ4065 are pUC19 derivatives carrying the indicated fragments under control of the lac promoter. Plasmids pNZ4070, pNZ4071, and pNZ4072 are pNZ8020 derivatives carrying the indicated fragments under control of the nisA promoter.
FIG. 2.
TLC of 14C-labelled intermediates isolated from the lipid fraction of permeabilized E. coli cells. The positions of the standard sugars glucose (Glc), galactose (Gal), and cellobiose (Glc-Glc), as well as those of the predicted trisaccharide (Glc-Glc-Gal) and the unknown galactose-containing molecule (?-Gal), are indicated on the right. Additional products of incomplete mild hydrolysis, putative sugar phosphates (A) and putative lipid-linked sugars (B), are indicated on the left. Lanes: 1 and 2, E. coli carrying pNZ4060; 3 and 4, E. coli carrying pNZ4061; 5 and 6, E. coli carrying pNZ4062; 7 and 8, E. coli carrying pNZ4063. Complete (C) and mild (M) acid hydrolysis treatments are indicated.
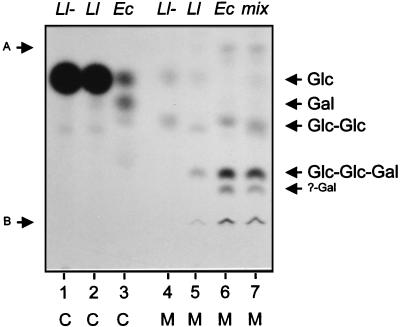
To test the substrate specificities of the epsDEFG gene products in L. lactis, fragments of pNZ4000 carrying epsD, epsDEF, or epsDEFG were cloned under control of the nisA promoter of pNZ8020, which, when introduced into L. lactis NZ3900 (1), allows the use of the NICE (nisin-controlled expression system) (2, 7). Cultures were induced with 0.1 ng of nisin A ml−1 at an optical density at 600 nm of 0.5, and cells were harvested 2 h after induction. After lysozyme treatment, permeabilized cells were prepared as described previously (16). Incubation of (uninduced) permeabilized, plasmid-free L. lactis NZ3900 cells with UDP-[14C]glucose or UDP-[14C]galactose resulted in a high level of incorporation of [14C]glucose in the lipid fraction by an unknown glucosyltransferase activity that may be involved in the biosynthesis of other cell surface polysaccharides (Fig. 3). Mild acid hydrolysis of the extracted lipid fractions yielded five species of labelled saccharides (Fig. 3): one with the same mobility on TLC as glucose, one migrating slightly slower than cellobiose, and three with higher mobility than glucose (the latter three are not shown in Fig. 3). This glucosyltransferase activity was not restricted to L. lactis subsp. cremoris MG1363 derivative NZ3900 but was also observed for L. lactis subsp. lactis IL1403 and L. lactis subsp. lactis biovar diacetylactis BU2-60 (data not shown). The background incorporation prevented detection of the activity of EpsD and EpsF, as no additional effect of expression of the epsD or epsDEF genes was observed (data not shown). However, expression of epsDEFG resulted in the formation of a new lipid-linked oligosaccharide. Its complete acid hydrolysis yielded an additional product with the same mobility as galactose. Its hydrolysis by mild acid resulted in a product with the same mobility as the putative trisaccharide detected in E. coli expressing epsDEFG (Fig. 3). The latter product was retrieved from the TLC plate and subjected to complete acid hydrolysis and a second TLC analysis. The oligosaccharide consisted of glucose and galactose, identical to the glucose-and-galactose-containing oligosaccharide found in E. coli expressing epsDEFG. Therefore, we conclude that the functions of the epsDEFG genes in E. coli and L. lactis are identical.
FIG. 3.
TLC of 14C-labelled intermediates isolated from the lipid fraction of permeabilized L. lactis and E. coli cells harboring plasmids with the epsDEFG genes. The positions of the standard sugars and the products of incomplete mild acid hydrolysis are indicated as in Fig. 2. Lanes: 1 and 4, plasmid-free L. lactis (L1−); 2 and 5, L. lactis carrying pNZ4072 (L1); 3 and 6, E. coli carrying pNZ4063 (Ec); 7, mixture of mild acid hydrolysates of L. lactis carrying pNZ4072 and E. coli carrying pNZ4063 (mix). Complete (C) and mild (M) acid hydrolysis treatments are indicated.
It is likely that the subsequent steps of the repeating unit synthesis include the coupling of the side chain sugars rhamnose and galactose-phosphate to the galactose of the backbone. Possible candidates for these activities are EpsH, which is homologous to several GTFs, and EpsJ, which is homologous to a CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase of Bacillus subtilis designated TagH (RodC) (11, 16). To test the function of epsH, a fragment containing epsDEFGH (Fig. 1) was cloned under control of the lac promoter in pUC19. Incubation of permeabilized E. coli cells expressing epsDEFGH with UDP-[14C]glucose and UDP-[14C]galactose resulted in the same products as those of cells expressing epsDEFG, indicating that EpsH is either not a galactosyltransferase, inactive in this assay, or not expressed. EpsH may be the rhamnosyltransferase, which could not be tested, as its substrate, dTDP-rhamnose, is unstable. The epsJ gene was cloned downstream of epsDEFG in pNZ4063 (Fig. 1). The resulting plasmid, pNZ4065, was readily obtained. Incubation of permeabilized E. coli cells expressing epsDEFGJ resulted in complete loss of incorporation of 14C-labelled sugars from the lipid fraction and no radioactivity on TLC. This strongly indicates that the epsJ gene is active and may encode either an enzyme linking galactosyl phosphate to galactose, after which E. coli enzymes can release the oligosaccharide from the membrane, or an enzyme releasing the trisaccharide backbone from the lipid carrier. Presently, we cannot distinguish between these two alternatives.
In conclusion, our report describes the heterologous and homologous expression of the lactococcal eps genes encoding the GTFs involved in the assembly of the EPS repeating unit. EpsD, EpsE, and EpsF, and EpsG link glucose-1-phosphate to a lipid carrier (presumably undecaprenyl phosphate), glucose to lipid-linked glucose, and galactose to lipid-linked cellobiose, respectively. The epsJ gene product is active and likely to be a galactosyl phosphotransferase or an enzyme releasing the trisaccharide backbone from the lipid carrier. Furthermore, to the best of our knowledge, this is the first report describing controlled homologous expression of GTF genes in gram-positive bacteria.
Acknowledgments
This work was partly supported by EC research grants 1116/92 1.6 and BIOT-CT96-0498.
We thank Roland Siezen and Ingeborg Boels for critically reading the manuscript.
REFERENCES
- 1.De Ruyter P G G A, Kuipers O P, Beerthuyzen M M, van Alen-Boerrigter I, de Vos W M. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178:3434–3439. doi: 10.1128/jb.178.12.3434-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Ruyter P G G A, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 4.Ielpi L, Couso R O, Dankert M A. Sequential assembly and polymerization of the polyprenol-linked pentasaccharide repeating unit of the xanthan polysaccharide in Xanthomonas campestris. J Bacteriol. 1993;175:2490–2500. doi: 10.1128/jb.175.9.2490-2500.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolkman M A B, van der Zeijst B A M, Nuijten P J M. Functional analysis of glycosyltransferases encoded by the capsular polysaccharide biosynthesis locus of Streptococcus pneumoniae serotype 14. J Biol Chem. 1997;272:19502–19508. doi: 10.1074/jbc.272.31.19502. [DOI] [PubMed] [Google Scholar]
- 6.Kolkman M A B, Wakarchuk W, Nuijten P J M, van der Zeijst B A M. Capsular polysaccharide synthesis in Streptococcus pneumoniae serotype 14: molecular analysis of the complete cps locus and identification of genes encoding glycosyltransferases required for the biosynthesis of the tetrasaccharide subunit. Mol Microbiol. 1997;26:197–208. doi: 10.1046/j.1365-2958.1997.5791940.x. [DOI] [PubMed] [Google Scholar]
- 7.Kuipers O P, de Ruyter P G G A, Kleerebezem M, de Vos W M. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64:15–21. [Google Scholar]
- 8.Liu D, Haase A M, Lindqvist L, Lindberg A A, Reeves P R. Glycosyltransferases of O-antigen biosynthesis in Salmonella enterica: identification and characterization of transferase genes of groups B, C2, and E1. J Bacteriol. 1993;175:3408–3413. doi: 10.1128/jb.175.11.3408-3413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakajima H, Hirota T, Toba T, Itoh T, Adachi S. Structure of the extracellular polysaccharide from slime-forming Lactococcus lactis subsp. cremoris SBT 0495. Carbohydr Res. 1992;224:245–253. doi: 10.1016/0008-6215(92)84110-e. [DOI] [PubMed] [Google Scholar]
- 10.Pollock T J, van Workum W A T, Thorne L, Mikolajczak M J, Yamazaki M, Kijne J W, Armentrout R W. Assignment of biochemical functions to glycosyl transferase genes which are essential for biosynthesis of exopolysaccharides in Sphingomonas strain S88 and Rhizobium leguminosarum. J Bacteriol. 1998;180:586–593. doi: 10.1128/jb.180.3.586-593.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pooley H M, Abellan F-X, Karamata D. CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, which is involved in the synthesis of the major wall teichoic acid in Bacillus subtilis 168, is encoded by tagF (rodC) J Bacteriol. 1992;174:646–649. doi: 10.1128/jb.174.2.646-649.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 13.Rubens C E, Heggen L M, Haft R F, Wessels M R. Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol Microbiol. 1993;8:843–855. doi: 10.1111/j.1365-2958.1993.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 14.Stingele F, Neeser J-R, Mollet B. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol. 1996;178:1680–1690. doi: 10.1128/jb.178.6.1680-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutherland I W. Biosynthesis and composition of Gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]
- 16.van Kranenburg R, Marugg J D, Willem N J, van Swam I I, de Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide production in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 17.Whitfield C, Valvano M A. Biosynthesis and expression of cell-surface polysaccharides in Gram-negative bacteria. Adv Microb Physiol. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 18.Yanish-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]