Summary
The sleep disorder Narcolepsy, a hypocretin deficiency disorder thought to be due to degeneration of hypothalamic hypocretin/orexin neurons, is currently treated symptomatically. We evaluated the efficacy of two small molecule hypocretin/orexin receptor-2 (HCRTR2) agonists in narcoleptic male orexin/tTA; TetO-DTA mice. TAK-925 (1–10 mg/kg, s.c.) and ARN-776 (1–10 mg/kg, i.p.) were injected 15 min before dark onset in a repeated measures design. EEG, EMG, subcutaneous temperature (Tsc) and activity were recorded by telemetry; recordings for the first 6-h of the dark period were scored for sleep/wake and cataplexy. At all doses tested, TAK-925 and ARN-776 caused continuous wakefulness and eliminated sleep for the first hour. Both TAK-925 and ARN-776 caused dose-related delays in NREM sleep onset. All doses of TAK-925 and all but the lowest dose of ARN-776 eliminated cataplexy during the first hour after treatment; the anti-cataplectic effect of TAK-925 persisted into the 2nd hour for the highest dose. TAK-925 and ARN-776 also reduced the cumulative amount of cataplexy during the 6-h post-dosing period. The acute increase in wakefulness produced by both HCRTR2 agonists was characterized by increased spectral power in the gamma EEG band. Although neither compound provoked a NREM sleep rebound, both compounds affected NREM EEG during the 2nd hour post-dosing. TAK-925 and ARN-776 also increased gross motor activity, running wheel activity and Tsc, suggesting that the wake-promoting and sleep-suppressing activities of these compounds could be a consequence of hyperactivity. Nonetheless, the anti-cataplectic activity of TAK-925 and ARN-776 is encouraging for the development of HCRTR2 agonists.
Keywords: sleep, wake, cataplexy, neurodegeneration, EEG, body temperature
1 |. INTRODUCTION
Type 1 Narcolepsy (NT1) is characterized by excessive daytime sleepiness (EDS), rapid-eye-movement (REM) sleep abnormalities, and cataplexy, the sudden loss of muscle tone. NT1 is due to hypocretin/orexin (Hcrt) deficiency resulting from degeneration of the Hcrt neurons (Peyron et al., 2000; Thannickal et al., 2000) by an immune-related mechanism (Bassetti et al., 2019; Kornum, 2021; Latorre et al., 2018) or, in some cases, perhaps through epigenetic silencing of Hcrt expression (Seifinejad et al., 2021). The two Hcrt peptides have differential affinities for two receptors, HCRTR1 (OX1R) and HCRTR2 (OX2R).
In animal models, mutations in Hcrtr2 (Lin et al., 1999), knockout of the Hcrt precursor protein (Chemelli et al., 1999), Hcrt receptors (Kalogiannis et al., 2011; Mieda et al., 2011; Willie et al., 2003), or genetic ablation of Hcrt neurons (Beuckmann et al., 2004; Hara et al., 2001; Tabuchi et al., 2014) recapitulate aspects of human narcolepsy. Genetic and pharmacological rescue experiments (Blanco-Centurion et al., 2013; Liu et al., 2017; Mieda et al., 2004) established proof-of-concept for Hcrt replacement as the ideal therapy for narcolepsy. Indeed, intracerebroventricular and intrathecal administration of HCRT1/orexin-A increases wake and suppresses cataplexy in Hcrt neuron-ablated and Hcrt peptide-deficient mice (Kaushik et al., 2018; Mieda et al., 2004). This effect is absent in HCRTR2/OX2R-deficient narcoleptic canines (Fujiki et al., 2003), indicating therapeutic potential for selective HCRTR2/OX2R agonists since expression of HCRTR2 appears intact in humans with narcolepsy (Mishima et al., 2008).
The first selective HCRTR2/OX2R agonist, YNT-185 (Nagahara et al., 2015), reduced sleep onset REM periods (SOREMs) in Hcrt peptide-deficient and Hcrt neuron-ablated mice but not in Hcrt receptor-deficient mice (Irukayama-Tomobe et al., 2017). Peripheral administration of YNT-185 also promoted wakefulness in Hcrt-deficient, Hcrt neuron-ablated and wild-type mice. These and recent results (Yamamoto et al., 2022) established HCRTR2/OX2R agonism for treatment of NT1 and EDS.
Although YNT185 has limited in vivo efficacy, other HCRTR2/OX2R-selective agonists have been described, including TAK-925 (Yukitake et al., 2019) also known as danavorexton (Evans et al., 2022). Administered subcutaneously, TAK-925 was wake-promoting in wild-type but not in narcoleptic OX2R knockout (KO) mice. However, these preclinical TAK-925 studies left several questions unanswered, specifically: (1) What effects does TAK-925 have on sleep/wake beyond the first post-injection hour? (2) Since TAK-925 is wake-promoting, how does it affect activity levels and the EEG spectra? (3) Does TAK-925 affect cataplexy? (4) Does TAK-925 affect other aspects of physiology such as body temperature? To address these questions, we evaluated the efficacy of TAK-925 and a related analog, ARN-776 (Fujimoto et al., 2017), in a validated mouse model of NT1 (Black et al., 2014; Black et al., 2017; Sun et al., 2022; Tabuchi et al., 2014). In addition, we sought to determine whether ARN-776 might avoid the necessity for subcutaneous administration of TAK-925 in mouse studies (Ishikawa et al., 2022; Yukitake et al., 2019) and intravenous administration in humans. We find that both compounds acutely increase wakefulness and reduce cataplexy but also increase activity and subcutaneous temperature (Tsc) during the first post-dosing hour, suggesting the wake-promoting effects are a consequence of increased activity.
2 |. MATERIALS AND METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee at SRI International or UT Southwestern Medical Center and were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals.
Animals.
Male “DTA mice” used for EEG/EMG experiments were the double transgenic offspring of orexin/tTA mice (C57BL/6-Tg(hOX-tTA)1Ahky), which express the tetracycline transactivator (tTA) exclusively in Hcrt neurons (Tabuchi et al., 2014), and B6.Cg-Tg(tetO-DTA)1Gfi/J mice (JAX #008468), which express a diphtheria toxin A (DTA) fragment in the absence of dietary doxycycline. Both parental strains were from a C57BL/6J genetic background. Parental strains and offspring used for EEG/EMG recording were maintained on a diet (Envigo T-7012, 200 Doxycycline) containing doxycycline (DOX(+) condition) to repress transgene expression until neurodegeneration was desired. All mice were maintained on a LD12:12 light:dark cycle with food and water ad libitum; mice used for EEG/EMG recordings had access to running wheels in their home cage.
Surgical Procedures.
Male DTA mice (N = 7) were aged 11±1 weeks (23±2 g) at the time of surgery. Mice were anesthetized with isoflurane and sterile telemetry transmitters (HD-X02, DSI, St Paul, MN) were placed subcutaneously. Biopotential leads were routed subcutaneously to the head, and both EMG leads were positioned through the right nuchal muscles. Cranial holes were drilled through the skull at −2.0 mm AP from bregma and 2.0 mm ML and on the midline at −1 mm AP from lambda. The two biopotential leads used as EEG electrodes were inserted into these holes and affixed to the skull with dental acrylic. The incision was closed with absorbable suture. Analgesia was managed with meloxicam (5 mg/kg, s.c.) and buprenorphine (0.05 mg/kg, s.c.) upon emergence from anesthesia and for the first day post-surgery. Meloxicam (5 mg/kg, s.c., q.d.) was continued for 2 d post-surgery. Mice were monitored daily for 14 days post-surgery and any remaining suture material was removed at the end of this monitoring period. Three weeks after surgery, DTA mice were switched to normal chow (Dox(−) condition) to induce expression of the DTA transgene specifically in the Hcrt neurons and thereby initiate degeneration of these cells (Tabuchi et al., 2014). After a 42 d (6 wk) degeneration period, Dox(+) chow was reintroduced to minimize further degeneration and the experimental treatments began.
Drugs.
Methyl (2R,3S)-3-[(methylsulfonyl)amino]-2- {[(cis-4-phenylcyclohexyl)oxy]methyl}piperidine-1-carboxylate (TAK-925; Figure1a) and (2R,3S)-N-ethyl-2- {[(cis-4-isopropylcyclohexyl)oxy]methyl}−3-[(methylsulfonyl)amino]piperidine-1-carboxamide (ARN-776; Figure 1b) were synthesized at UT Southwestern Medical Center according to published procedures (Fujimoto et al., 2017). These compounds correspond to structures 5 and 2, respectively, from US Patent US 2017/0226137 A1 (Fujimoto et al., 2017). TAK-925 was dissolved in 10% DMSO / 90% 0.5% methycellulose (“TAK-925 vehicle”) and delivered s.c. and ARN-776 was dissolved in 10% DMSO / 10% Kolliphor EL / 20% PEG400 / 60% dH2O (“ARN-776 vehicle”) and delivered i.p. for the EEG/EMG studies. All solutions were prepared fresh each experimental day and administered at 10 mL/kg final volume.
Figure 1.
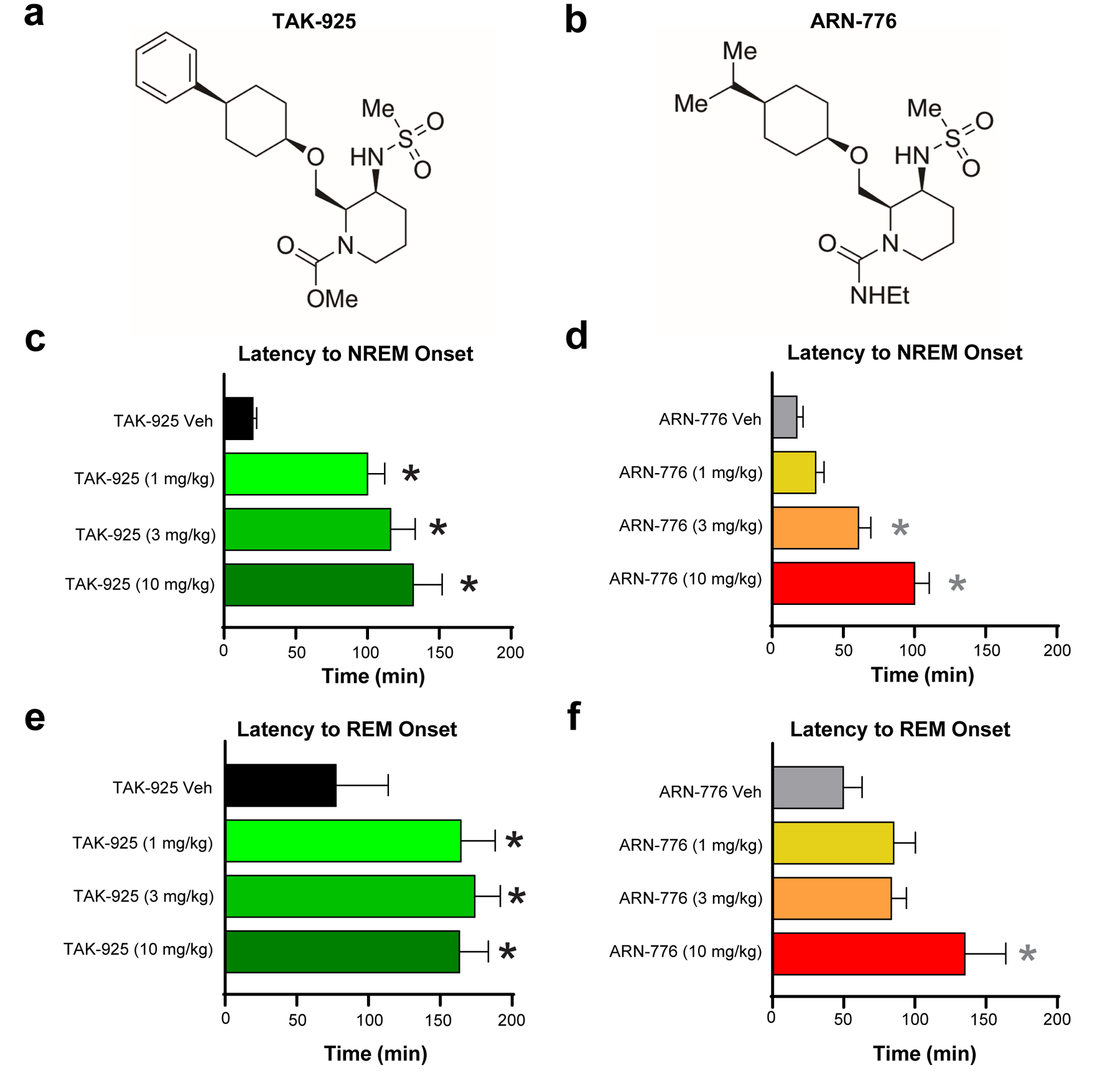
Chemical structures of (a) TAK-925 and (b) ARN-776. Latency to NREM (c & d) and REM (e & f) sleep in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 and ARN-776. Values are mean ± SEM. * p < 0.05 compared to Vehicle by 1-way ANOVA followed by the Holm-Sidak post hoc test. Abbreviations: Veh, vehicle.
Drug treatments.
We utilized a repeated measures design in which all animals received all treatments. Although DTA mice received 9 treatments in balanced order over a 4 week period with at least 3 d between dosings, only 8 treatments were analyzed for this study: TAK-925 vehicle, 1, 3 and 10 mg/kg, s.c. and ARN-776 vehicle 1, 3 and 10 mg/kg, i.p. Because cataplexy is the pathognomonic symptom of NT1 and cataplexy is much more prevalent in the dark period, all dosings started 15 min before light offset at ZT12 (ZT0 = 05:00; ZT12 = 17:00).
EEG, EMG, Tsc and activity recording and analysis.
Prior to data collection, DTA mice had at least 2 weeks post-surgical recovery and at least 2 weeks adaptation to running wheels, handling and dosing procedures. Throughout the study, mice were housed individually in home cages with access to food, water, nestlets and running wheels ad libitum. Room temperature (22 ± 2oC), humidity (50 ± 20% relative humidity), and lighting conditions (LD12:12) were monitored continuously. Animals were inspected daily in accordance with AAALAC and SRI guidelines. EEG, EMG, Tsc, and gross motor activity were recorded via telemetry using Ponemah (DSI, St Paul, MN). EEG and EMG were sampled at 500 Hz. Tsc reflects the temporal dynamics of relative changes in core body temperature (van der Vinne et al., 2020). Digital videos were recorded at 10 frames per second, 4CIF de-interlacing resolution.
Data analysis and statistics.
EEG/EMG data were classified in 10-s epochs by expert scorers as wakefulness (W), non-Rapid Eye Movement (NREM) sleep, REM sleep, or Cataplexy using NeuroScore (DSI, St. Paul, MN) as in our previous studies (Black et al., 2014; Black et al., 2017; Sun et al., 2022). Criteria for Cataplexy were >10 s of EMG atonia, theta-dominated EEG, and video-confirmed behavioral immobility preceded by ≥40 s of wakefulness (Scammell et al., 2009). Running wheel activity, determined from video recordings, was scored in 10-s epochs. Epochs containing non-physiological signals in the EEG (e.g., signal disconnection, artifacts due to movement, etc) were scored as artifact and excluded from subsequent spectral analysis. “Mixed epochs” containing more than one vigilance state were tagged similarly which also enabled exclusion from the spectral analysis, but such epochs were included in sleep architecture analyses as the majority state during the epoch. The latency to NREM sleep was defined as the time from dose administration until the first 6 consecutive epochs of NREM. The latency to REM sleep was from dose administration until the first 3 consecutive epochs of REM. Data were analyzed as time spent in state per hour and cumulative time spent in each state for 6-h post-dosing. Sleep/wake architecture measures included the duration and the number of bouts for each state. A “bout” of wake, NREM, REM, or Cataplexy was defined as 2 or more consecutive epochs of that state and ended with a single epoch of any other state. For running wheel activity, a bout was defined as 1 or more consecutive epochs ending in a single epoch of any other state.
The EEG power spectrum (0.3–100 Hz) during W, NREM, REM and Cataplexy was analyzed by fast Fourier transform algorithm on all artifact-free epochs. For spectral analyses, a minimum of 6 consecutive epochs of Wake or NREM sleep and a minimum of 3 consecutive epochs of REM sleep was required for inclusion in the analysis. All periods of Cataplexy, regardless of duration, were included in the spectral analysis. A minimum of 3 animals with enough epochs of each state was also required for inclusion in the analysis. EEG spectra were analyzed in 0.122 Hz bins and in standard frequency bands rounded to the nearest Hz (delta: 0.5–4 Hz, theta: 4–9 Hz, alpha: 9–12 Hz, beta: 12–30 Hz, low gamma: 30–60 Hz and high gamma: 60–100 Hz). For each mouse, power was normalized to the average power per bin during the 6-h vehicle recording. Hourly averages of Tsc and activity determined from the transmitters were also analyzed.
Latencies to NREM and REM sleep, REM/NREM ratios, and total time in state were analyzed using 1-way repeated-measures analysis of variance (ANOVA). When 1-way ANOVA indicated significance, the Holm-Sidak post hoc test was utilized to compare to the corresponding vehicle (i.e., TAK-925 doses were compared to the TAK-925 vehicle and ARN-776 doses were compared to the ARN-776 vehicle) to identify any doses that were significant. All other data analyses were by 2-way repeated-measures ANOVA with drug treatment and time as factors using data from all eight treatments including both vehicles. When 2-way ANOVA indicated a treatment x time interaction, paired two-tailed t-tests (treatment vs. vehicle) were performed to determine the specific hour during which the difference occurred. Statistics were calculated using functions provided in the MATLAB statistics and machine learning toolbox and using GraphPad Prism (ver. 8.4.2). For the EEG spectra data, statistical comparisons were calculated only on the standard frequency bands. The exact p values calculated from the F distribution are presented whenever possible but, in cases where the p values are extremely small, “p <” is used.
3 |. RESULTS
The chemical structures of TAK-925 and ARN-776 are presented in Figures 1a and 1b. The results of the pharmacokinetic studies conducted with each compound are presented in Figure S1 and Table S1 in Supporting Information. Analysis of these results by WinNonlin revealed brain:blood ratios of 0.07:1 for TAK-925 and 0.04:1 for ARN-776 (Table 1), indicating limited penetration of both molecules across the blood-brain barrier. Nonetheless, the quantities of TAK-925 and ARN-776 reaching the brain both produced significant physiological effects as described below.
Table 1.
WinNonlin Noncompartmental PK Parameters
| WinNonlin Noncompartmental PK Parameters (free druga) | |||||
|---|---|---|---|---|---|
| Parameter | Unit | Free TAK-925 (10 mpk SC) | Free ARN-776 (10 mpk IP) | ||
| Plasma | Brain | Plasma | Brain | ||
| Fraction unboundb | N/A | 0.107 | 0.077 | 0.176 | 0.121 |
| Terminal T1/2 | min | 36 | 53 | 125 | 38 |
| Tmax | min | 10 | 10 | 10 | 10 |
| Cmax | ng/ml or ng/g | 160 | 20 | 326 | 13 |
| AUClast | min*ng/mL or min*ng/g | 20,097 | 1433 | 11,482 | 497 |
| Brain:Blood Ratio | 0.07:1 | 0.04:1 | |||
Total drug concentrations determined by LC-MS/MS were converted to free drug after multiplying by the fraction unbound. PK parameters were determined based on the data presented in Fig. 1C and D using sparse sampling and noncompartmental analysis (NCA) with WinNonlin (Pharsight). Terminal T ½ was determined using best fit according to standard parameters provided for NCA in WinNonlin.
Fraction unbound was determined by incubation of 5 μM of each compound in either mouse plasma or brain homogenate using rapid equilibrium dialysis (RED).
3.1 |. Effects on Sleep Onset
Both TAK-925 and ARN-776 caused dose-related delays in the onset of NREM sleep (F(7, 42) = 18.67, p = 4.86 × 10−11). Post hoc tests revealed that all three s.c. doses of TAK-925 delayed NREM sleep onset (Figure 1c) whereas only the 3 mg/kg and 10 mg/kg i.p. doses of ARN-776 delayed NREM (Figure 1d). ANOVA also indicated that both compounds delayed REM sleep onset (F(7, 42) = 6.32, p = 4.29 × 10−5); post hoc tests determined that all doses of TAK-925 (Figure 1e) and the 10 mg/kg dose of ARN-776 (Figure 1f) delayed REM sleep.
3.2 |. Hourly Amounts of Sleep/Wake
Two-way ANOVA indicated significant treatment x time effects on the amounts of Wake (F(35, 210) = 2.79, p = 3.17 × 10−6), NREM (F(35, 210) = 2.11, p = 6.68 × 10−4) and REM sleep (F(35, 210) = 2.53, p = 2.54 × 10−5). TAK-925 caused continuous wakefulness (Figure 2a), eliminated both NREM (Figure 2d) and REM sleep (Figure 2g) as well as Cataplexy (Figure 2j) for the first hour at all doses tested (1–10 mg/kg, s.c.); the reduction of Cataplexy continued into the second post-dosing hour for the 10 mg/kg TAK-925 dose. However, the TAK-925 effects on arousal states were transitory (Figure 2c, 2f and 2i) and only the highest TAK-925 dose reduced Cataplexy across the entire 6-h recording period (Figure 2l). The suppression of REM sleep by TAK-925 early in the night was followed by a rebound that reached significance at ZT16, 4 hours post-treatment (Figure 2g).
Figure 2.
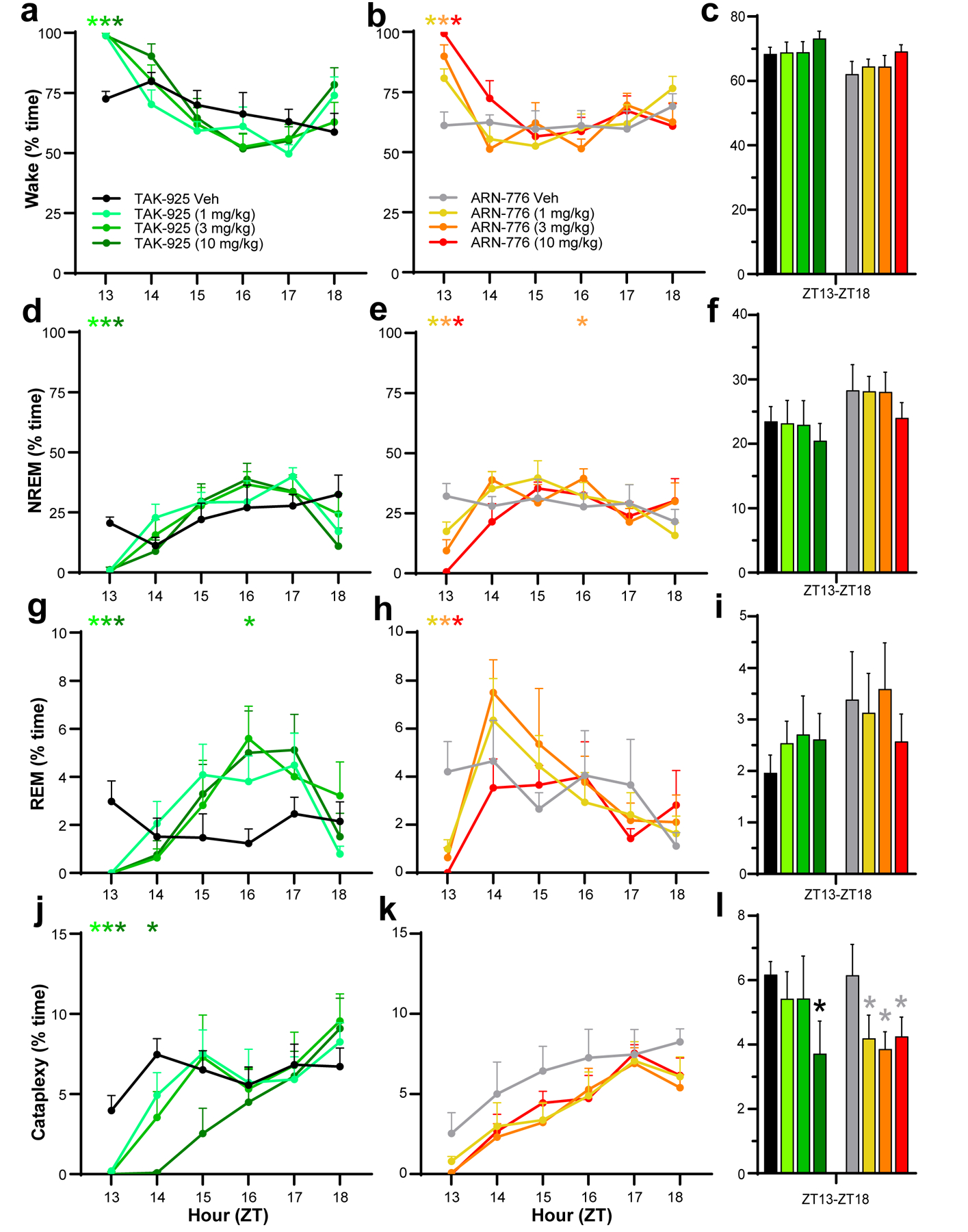
Hourly amounts of Wake (a, b), NREM sleep (d, e), REM sleep (g, h), and cataplexy (j, k) in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 and ARN-776. Percent time in Wake (c), NREM sleep (f), REM sleep (i) and Cataplexy (l) summed across the 6-h recording after treatment with TAK-925 and ARN-776. Values are mean ± SEM. Colored * above hourly graphs indicate significance (p < 0.05) during that hour for that dose relative to vehicle treatment. * in l denotes significance for that treatment relative to vehicle across the 6-h recording as determined by the Holm-Sidak post hoc test. Abbreviations: Veh, vehicle; ZT, Zeitgeber Time.
In contrast to TAK-925, ARN-776 (1–10 mg/kg, i.p.) produced dose-related changes in Wake, NREM and REM sleep during the first hour (Figure 2b, 2e, 2h) but the reduction in Cataplexy levels did not reach statistical significance (Figure 2k), likely due to the slightly lower levels of Cataplexy after treatment with the ARN-776 vehicle during that hour than with the TAK-925 vehicle. Across the entire 6-h recording, however, ANOVA revealed a significant treatment effect of ARN-776 on Cataplexy (F(3, 18) = 3.928 p = 0.0256); the post hoc Holm-Sidak multiple comparisons test indicated that all 3 doses of ARN-776 reduced Cataplexy across the 6-h period (Figure 2l). In further contrast to TAK-925, the suppression of REM sleep by ARN-776 was briefer and the rebound did not reach statistical significance (Figure 2g vs. 2h).
The REM/NREM ratio was unchanged by TAK-925 or ARN-776 when measured across the entire 6-h recording.
3.3 |. Cumulative Amounts of Sleep/Wake
Treatment effects.
The changes described above were reflected in the cumulative amounts of each state (Figure 3), for which two-way ANOVA revealed significant treatment effects on Wake (F(7, 42) = 5.03, p = 3.3 × 10−4), NREM (F(7, 42) = 3.26, p = 0.007), REM (F(7, 42) = 2.54, p = 0.03) and Cataplexy (F(7, 42) = 3.27, p = 0.007). The initial promotion of Wake (Figure 3a, 3b) and suppression of both NREM (Figure 3c, 3d) and REM sleep (Figure 3e, 3f) by both compounds are readily evident, as is the more sustained effect of the 10 mg/kg dose of ARN-776. Post hoc tests found significant effects of the 10 mg/kg ARN-776 dose on Wake (p = 0.005) and NREM (p = 0.028). Figure 3g confirms the suppression of Cataplexy by the TAK-925 10 mg/kg dose (p = 1.74 × 10−4) that is also shown in Figure 2l and Figure 3h shows the cumulative suppression of Cataplexy by all 3 doses of ARN-776 that is also apparent in Figure 2l.
Figure 3.
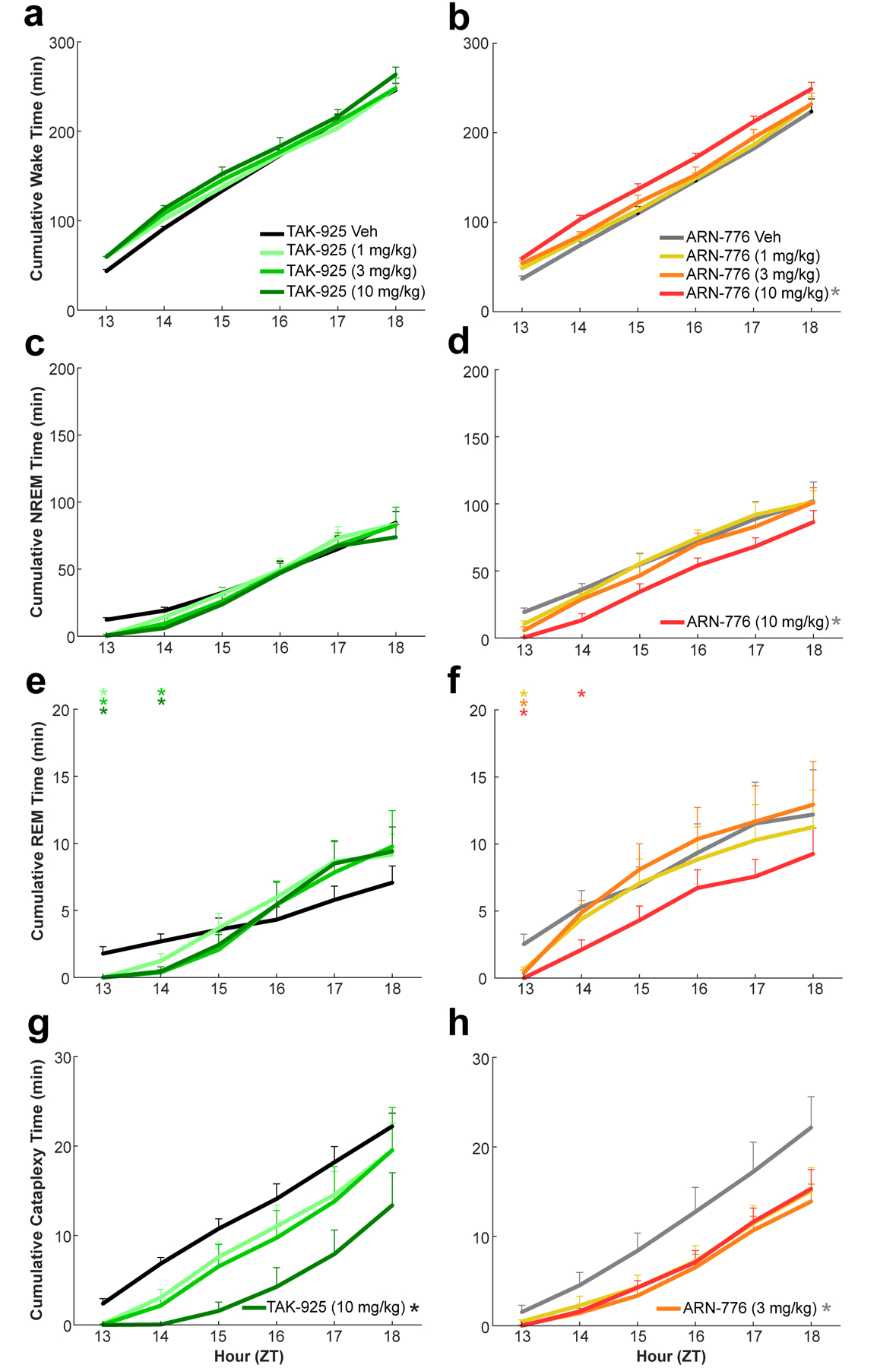
Cumulative time in Wake (a, b), NREM sleep (c, d), REM sleep (e, f), and Cataplexy (g, h) in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 (left) and ARN-776 (right). Values are mean ± SEM; * in legends denotes significant treatment effect by 2-way ANOVA; colored * above graph indicates significance (p < 0.05) during that hour for that dose relative to vehicle treatment. Abbreviations: Veh, vehicle; ZT, Zeitgeber Time.
Treatment x Time Effects.
Two-way ANOVA revealed a significant treatment x time effect only on the cumulative amount of REM sleep (F(35, 210) = 1.61, p = 0.02). Figure 3e clearly illustrates the significant suppression of REM sleep by TAK-925 during the first two hours after dosing that was followed by a later rebound; in contrast, the suppression of REM sleep by ARN-776 at the lower doses was limited to the first post-dosing hour (Figure 3f).
3.4 |. Sleep Architecture Measures
Bout Durations.
The increased wakefulness produced by both Hcrt agonists during the first dosing hour was due to very long Wake bout durations (Figure 4a, b). Two-way ANOVA confirmed overall treatment effects of TAK-925 and ARN-776 on bout durations for Wake (F(7, 42) = 14.48, p = 2.08 × 10−9), NREM (F(7, 42) = 4.85, p = 4.52 × 10−4), REM (F(7, 42) = 4.21, p = 1.36 × 10−3) and Cataplexy (F(7, 42) = 4.12, p = 1.58 × 10−3). Post hoc tests revealed significant treatment effects on Wake bout duration for all doses of both TAK-925 and ARN-776. In contrast, post hoc tests did not identify any specific dose of either TAK-925 or ARN-776 that contributed to the overall treatment effects on NREM or REM. The 3 mg/kg dose ARN-776 has a modest effect on Cataplexy bout duration (p = 0.046).
Figure 4.
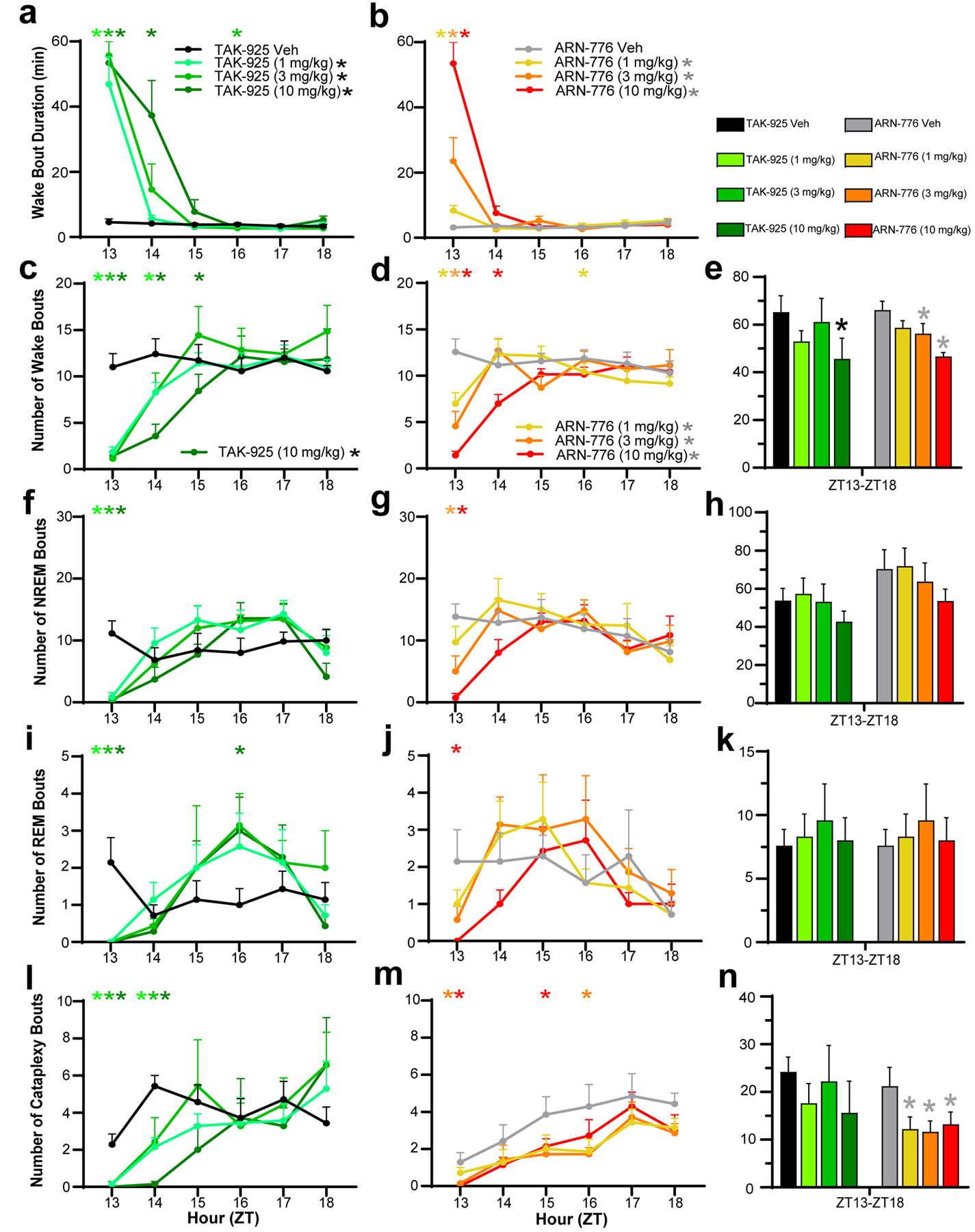
Mean Wake Bout Durations in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 (a) and ARN-776 (b). Hourly number of bouts of Wakefulness (c, d), NREM sleep (f, g), REM sleep (i, j), and Cataplexy (l, m) in narcoleptic mice treated with TAK-925 and ARN-776. Total number of bouts of Wake (e), NREM sleep (h), REM sleep (k) and Cataplexy (n) across the 6-h recording after treatment with TAK-925 and ARN-776. Values are mean ± SEM. Colored * above hourly graphs indicate significance (p < 0.05) during that hour relative to vehicle treatment. * in legend denotes significance for that treatment across the 6-h recording as determined by 2-way ANOVA. * in e and n denotes significance for that treatment relative to vehicle across the 6-h recording as determined by post hoc Holm-Sidak test. Abbreviations: Veh, vehicle; ZT, Zeitgeber Time.
Two-way ANOVA also revealed a treatment x time interaction on Wake bout duration (F(35, 210) = 12.74, p < 3.34 × 10−13). Post hoc tests revealed that all doses of both TAK-925 and ARN-776 increased Wake bout duration during the first post-dosing hour (ZT13), which continued into the second post-dosing hour (ZT14) for the highest dose of TAK-925 (Figure 4a).
Number of Bouts.
Two-way ANOVA determined significant treatment effects for TAK-925 and ARN-776 on the number of Wake bouts across the entire 6-h recording (F(7, 42) = 2.76, p = 0.02). The Holm-Sidak post hoc tests found that only the TAK-925 10 mg/kg dose reduced the number of Wake bouts across the entire 6-h recording (p = 0.013) whereas the 3 mg/kg (p = 0.03) and 10 mg/kg (p = 0.002) doses of ARN-776 significantly reduced the number of Wake bouts (Figure 4e).
Two-way ANOVA also revealed significant treatment x time interactions for TAK-925 and ARN-776 on the number of bouts of Wake (F(35, 210) = 4.78, p = 3.34 × 10−13), NREM (F(35, 210) = 2.81, p = 2.84 × 10−6), REM (F(35, 210) = 1.72, p = 0.011) and Cataplexy (F(35, 210) = 1.80, p = 6.32 × 10−3). During the first post-dosing hour when wakefulness was continuous, post hoc tests revealed that all doses of TAK-925 reduced the number of bouts of Wake (Figure 4c), NREM (Figure 4f), REM (Figure 4i) and Cataplexy (Figure 4l); the reduction of Wake bouts lasted up to 3h at 10 mg/kg. By contrast, ARN-776 had a dose-related effect on the number of Wake bouts during the first hour but only the 10 mg/kg dose reduced the number of Wake bouts during the second hour (Figure 4d). Overall, ARN-776 appeared to have weaker effects than TAK-925 at the doses tested, as only the middle and high doses reduced the number of NREM bouts during the first hour post-dosing (Figure 4g) and only the 10 mg/kg dose reduced the number of REM bouts (Figure 4j). Similarly, whereas all doses of TAK-925 reduced the number of Cataplexy bouts for the first two hours (Figure 4l), only the two highest concentrations of ARN-776 were effective during the first and later hours (Figure 4m). Nonetheless, the post hoc Holm-Sidak test revealed that all doses of ARN-776 (1 mg/kg: p = 0.0082; 3 mg/kg: p = 0.0078; 10 mg/kg: p = 0.0092) reduced the total number of Cataplexy bouts across the entire 6-h period (Figure 4n).
3.5 |. EEG Spectra Effects
Wakefulness.
The acute increase in wakefulness produced by both Hcrt agonists during the first hour post-dosing (ZT13) was characterized by increased spectral power in the alpha and both low and high gamma bands of the EEG (Figure 5a, 5b and Figure 6e, 6f, 6i, 6j, 6k and 6l). Two-way ANOVA found significant effects of treatment on the high gamma band (F(7, 42) = 4.91, p = 4.09 × 10−4) during Wake across the 6-h recording. Post hoc testing found that the 10 mg/kg doses of both TAK-925 (p = 0.02, Figure 6K) and ARN-776 (p = 0.04, Figure 6l) significantly increased high gamma during Wake across the entire recording period.
Figure 5.
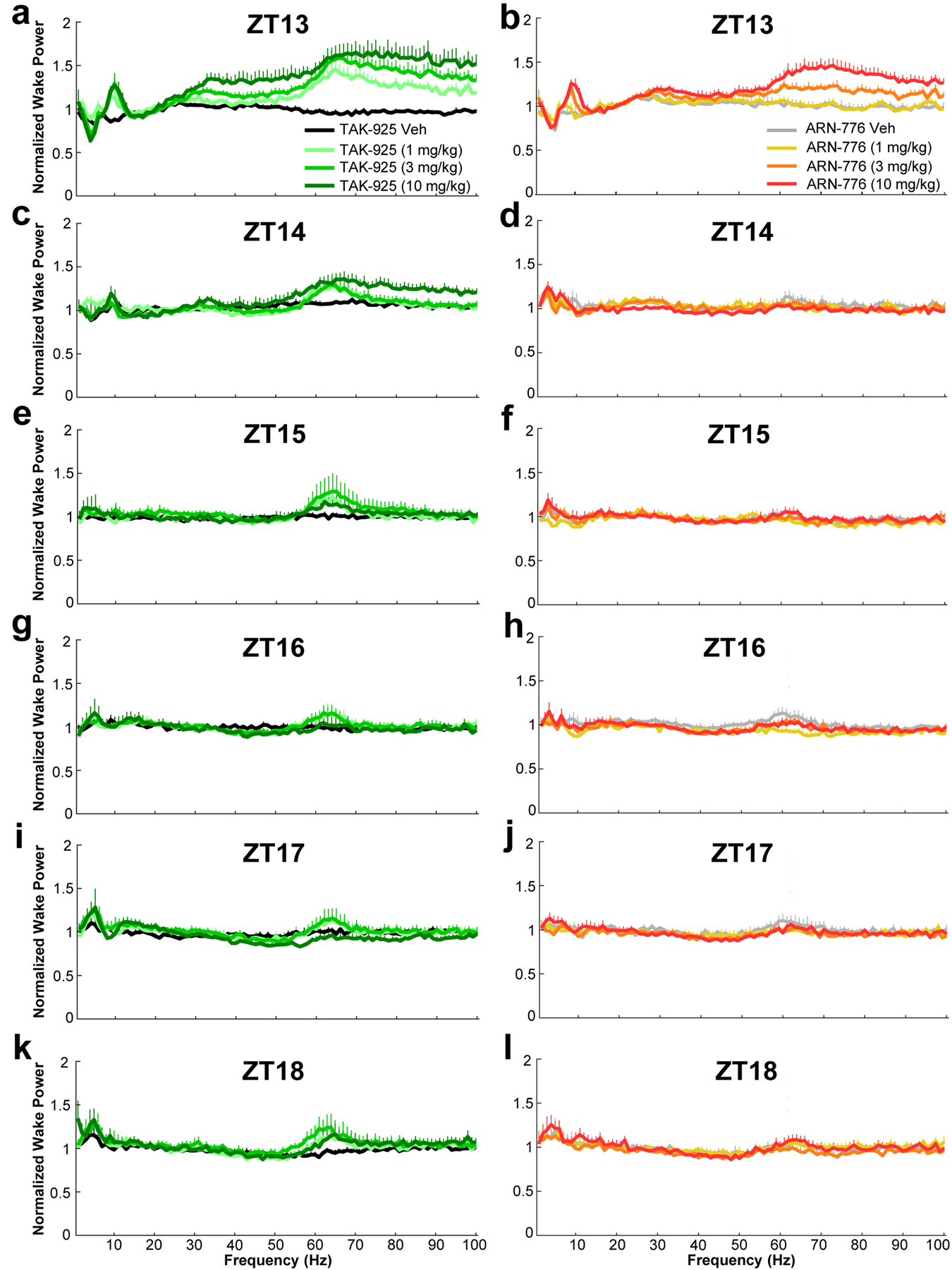
Normalized EEG spectral power (0 – 100 Hz) recorded during wakefulness for the 6-h post-dosing recording period in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 (left column) and ARN-776 (right column). Values are mean ± SEM. Abbreviations: Veh, vehicle; ZT, Zeitgeber Time; Hz, Hertz.
Figure 6.
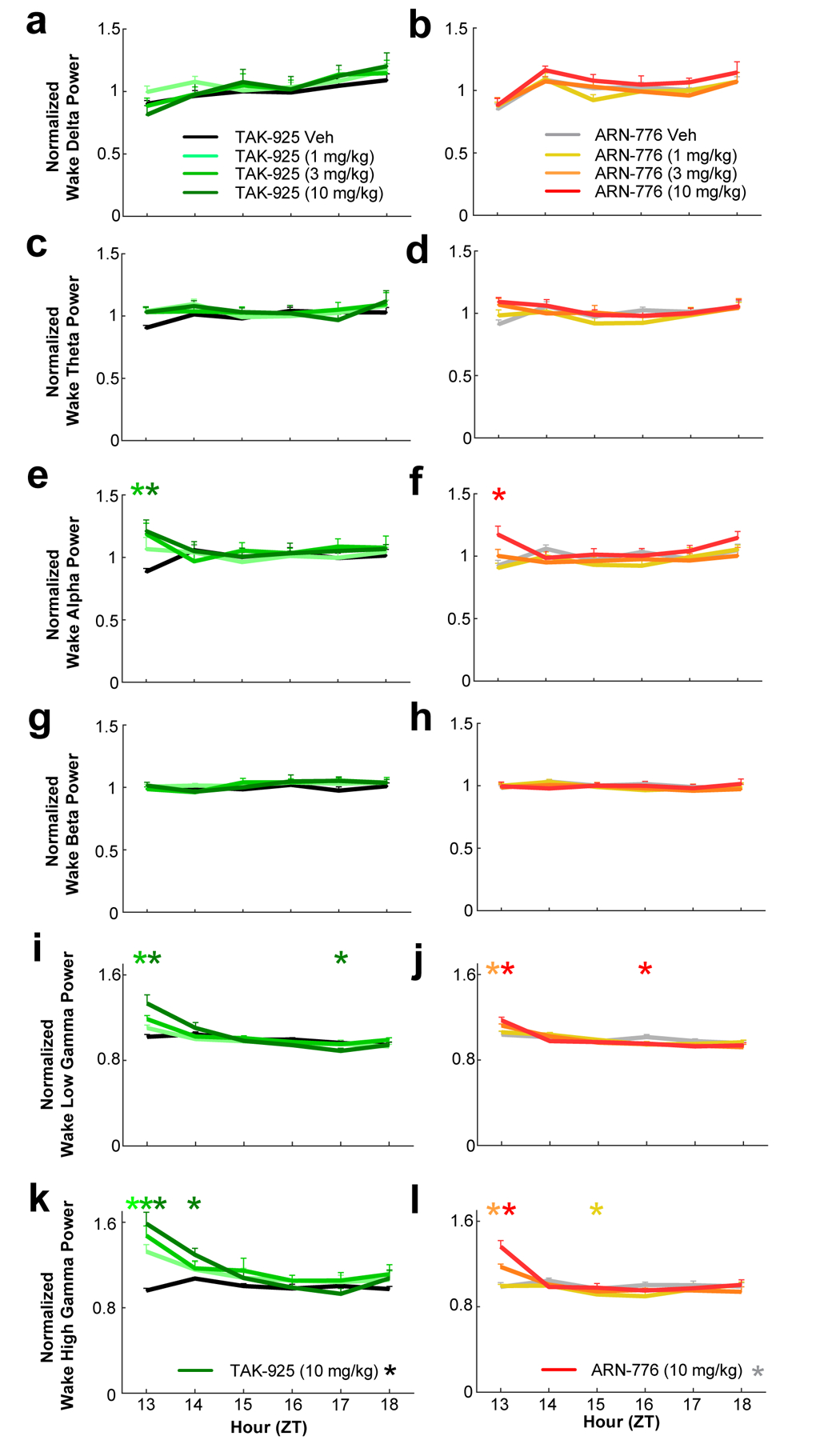
Waking EEG spectra for the conventional bandwidths in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 and ARN-776. a and b: delta (0.5–4.0 Hz); c and d: theta (4–9 Hz); e and f: alpha (9–12 Hz); g and h: beta (12–30 Hz); i and j: low gamma (30–60 Hz); and k and l: high gamma (60–100 Hz). Values are mean ± SEM. Colored * above hourly graphs indicate significance (p < 0.05) during that hour relative to vehicle treatment. * in legend denotes significance for that treatment across the 6-h recording as determined by 2-way ANOVA. Abbreviations: Veh, vehicle; ZT, Zeitgeber Time.
ANOVA also revealed significant treatment x time effects on the alpha (F(35, 210) = 1.99, p = 0.0016; Figure 6e, f), low gamma (F(35, 210) = 5.07, p = 3.41 × 10−14; Figure 6i, j) and high gamma F(35, 210) = 7.03, p < 3.34 × 10−13; Figure 6k, l) ranges of the waking EEG. Post hoc tests indicated that the 3 and 10 mg/kg doses of TAK-925 significantly increased spectral power in all three bandwidths during ZT13; the 1 mg/kg dose also significantly increased high gamma power during that hour. The effects of the 10 mg/kg dose continued into the second post-dosing hour (Figure 6k). Post hoc tests also revealed that the 10 mg/kg dose of ARN-776 significantly increased power in all three of these bands during the first post-dosing hour; the 3 mg/kg dose also significantly increased both low (Figure 6j) and high (Figure 6l) gamma during that hour (ZT13).
NREM Sleep.
The 10 mg/kg dose of TAK-925 increased spectral power during ZT14 for all EEG bands except for low gamma.; the 1 mg/kg dose also increased NREM delta during this hour (Figure S2 & S3). Post hoc tests indicated significant reductions in NREM alpha (Figure S3f) and high gamma (Figure S3l) during ZT14 after the 10 mg/kg ARN-776 dose; the reduction of high gamma continued into ZT15.
REM Sleep and Cataplexy.
The low levels of REM sleep and Cataplexy, and in the case of REM, the requirement for 3 consecutive epochs for spectral analysis precluded meaningful analysis of the EEG spectra during these states.
3.6 |. Activity Measures and Tsc
TAK-925 and ARN-776 increased both gross motor activity (Figure 7a and 7b) measured by the implanted telemeter and running wheel activity (Figure 7c and 7d) during the first post-dosing hour. Two-way ANOVA indicated significant treatment x time effects on gross motor activity (F(35, 210) = 2.49, p = 3.52 × 10−5); post hoc tests revealed significantly increased motor activity for all doses of TAK-925 as well as the 3 mg/kg and 10 mg/kg doses of ARN-776 during the first post-dosing hour (ZT13). Subsequent to this increase, reduced gross motor activity occurred later in the night for the highest doses of TAK-925 (ZT17; Figure 7a) and ARN-776 (ZT15; Figure 7b).
Figure 7.
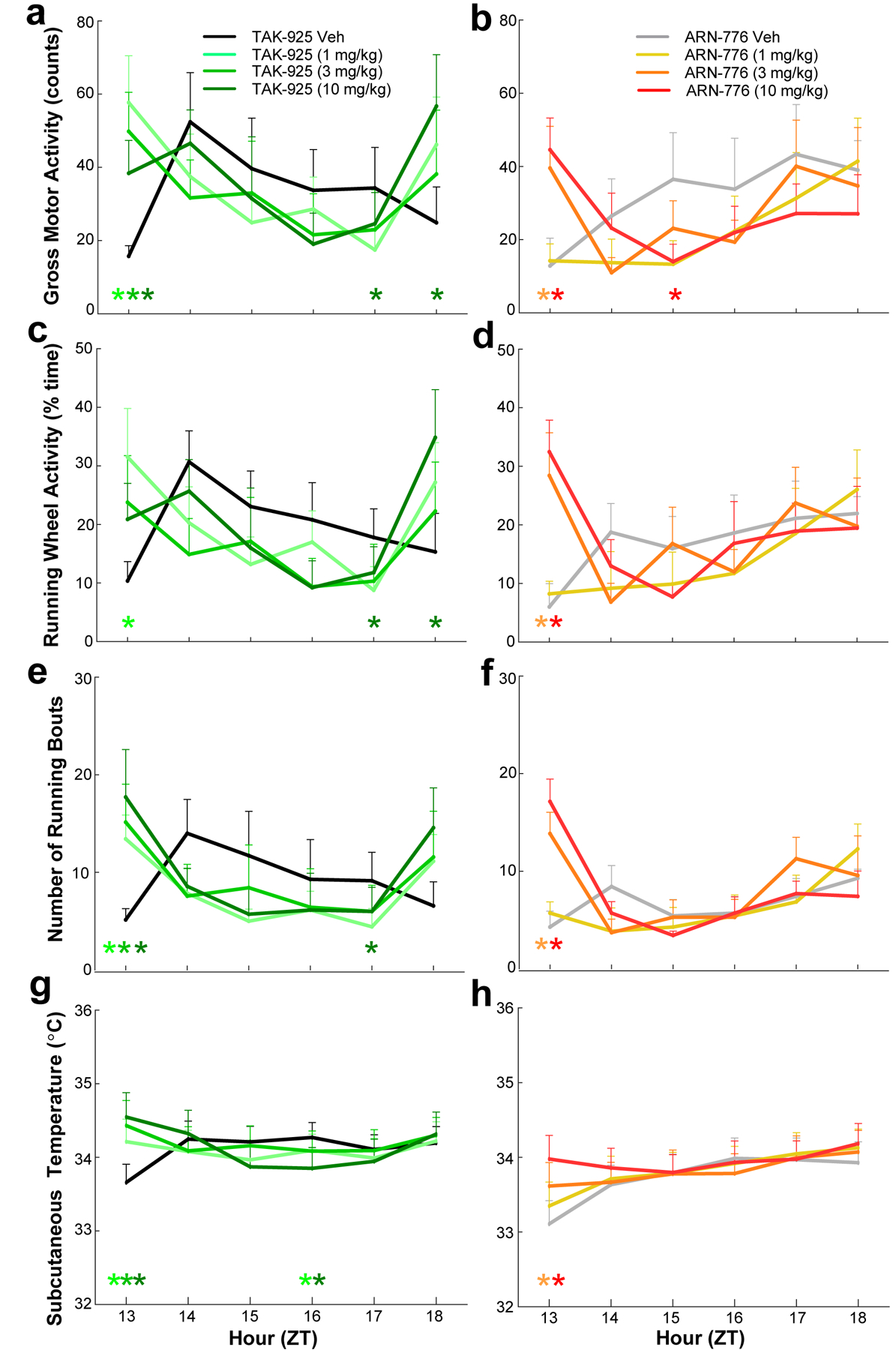
Activity (a, b), running wheel architecture (c, d, e, & f), and temperature (g, h) in narcoleptic male Orexin-tTA;TetO-DTA mice (n = 7) treated with the HCRTR2 agonists TAK-925 (left) and ARN-776 (right). Values are mean ± SEM. Colored * above hourly graphs indicate significance (p<0.05) during that hour relative to vehicle treatment. Abbreviations: Veh, vehicle; ZT, Zeitgeber Time.
Two-way ANOVA also indicated significant treatment x time effects on running wheel activity (F(35, 210) = 2.33, p = 1.32 × 10−4); post hoc tests revealed significantly increased running wheel activity during the first post-dosing hour (ZT13) for the 1 mg/kg dose of TAK-925 (Figure 7c) as well as for the 3 mg/kg and 10 mg/kg doses of ARN-776 (Figure 7d). Importantly, the increased running during ZT13 was due to an increased number of running bouts (F(35, 210) = 2.56, p = 2.02 × 10−5); post hoc tests confirmed more running bouts for all doses of TAK-925 (Figure 7e) and for the 3 mg/kg and 10 mg/kg doses of ARN-776 (Figure 7f). Reduced running wheel activity occurred later during ZT17 for TAK-925 (10 mg/kg) followed by an increase at ZT18.
The increases in activity early in the dark period were accompanied by elevated Tsc (Figure 7g and 7h). Two-way ANOVA indicated both significant treatment (F(7, 42) = 6.10, p = 5.97 × 10−5) and treatment x time (F(35, 210) = 6.78, p < 3.34 × 10−13) effects on Tsc. Post hoc tests revealed significantly increased Tsc for all doses of TAK-925 as well as for the 3 mg/kg and 10 mg/kg doses of ARN-776 during the first post-dosing hour (ZT13).
4 |. DISCUSSION
Current narcolepsy treatment is directed toward symptom management rather treatment of the underlying condition and often involves polypharmacy using, for example, wake-promoting agents to alleviate EDS (Barateau & Dauvilliers, 2019; Scammell, 2015; Szabo et al., 2019; Thorpy, 2020), or gamma hydroxybutyrate (GHB; aka sodium oxybate), a controlled substance with a difficult dosing regimen, to consolidate nocturnal sleep and thereby treat both cataplexy and EDS (Bosch et al., 2012; Boscolo-Berto et al., 2012). In contrast, genetic and pharmacological rescue experiments (Blanco-Centurion et al., 2013; Liu et al., 2011; Liu et al., 2016; Liu et al., 2017; Liu et al., 2008; Mieda et al., 2004) have established the proof-of-concept for Hcrt peptide replacement as the ideal therapy for narcolepsy. To date, however, only two small molecule HcrtR/OX2R agonists have been published, YNT-185 (Irukayama-Tomobe et al., 2017; Nagahara et al., 2015) and TAK-925 (Evans et al., 2022; Ishikawa et al., 2022; Yukitake et al., 2019). In the present study, we evaluated the efficacy of TAK-925 and a related Hcrt agonist, ARN-776.
4.1 |. Similarities between TAK-925 and ARN-776
Although a direct “head to head” comparison of these two compounds was not possible due to differences in vehicle and route of administration, TAK-925 and ARN-776 nonetheless produced remarkably similar results. Both compounds delayed sleep onset in a dose-related manner and, for at least the first hour after dosing, consolidated wakefulness into long wake bouts; reduced cataplexy, NREM and REM sleep; and increased activity, Tsc and Waking EEG spectral power in the alpha and gamma bands. All doses of TAK-925 and ARN-776 prolonged Wake Bout Duration across the entire 6-h recording. Furthermore, the reduction in cataplexy was sustained across the 6-h recording period at the highest dose of TAK-925 (10 mg/kg, s.c.) and all doses of ARN-776 (1–10 mg/kg, i.p.). Lastly, despite the increased wakefulness during the first post-dosing hour, there was no compensatory increase in NREM sleep in the subsequent hours. As such, both of these putative Hcrt receptor agonists appear to have beneficial effects on wakefulness and cataplexy and thus encourage further development of Hcrt agonists as potential narcolepsy therapeutics.
4.2 |. Differences between TAK-925 and ARN-776
Although it can be difficult to directly compare the efficacy of compounds, particularly since both the route of delivery (s.c. vs. i.p.) and the vehicles used to deliver these compounds differed, TAK-925 appeared to be more potent than ARN-776 on several measures. For example, although both compounds were tested at 1, 3 and 10 mg/kg, all doses of TAK-925 produced continuous wakefulness during the first hour after dosing whereas the wake-promoting effects of ARN-776 were dose-related. Furthermore, much longer sleep onset latencies were observed with the lower doses of TAK-925 than after comparable doses of ARN-776. Although neither compound provoked an increase in NREM sleep subsequent to the increased wakefulness during the first dosing hour, the suppression of REM sleep by TAK-925 was more sustained and resulted in a significant REM rebound later in the night (Figs. 2g and 3e). Despite the absence of a NREM sleep rebound, the two compounds had very different effects on the EEG spectra during NREM sleep in the hour after the increased wakefulness (ZT14): whereas the 10 mg/kg dose of TAK-925 increased normalized EEG spectral power in all but the low gamma range, the highest dose of ARN-776 reduced normalized EEG spectral power in the alpha and high gamma range (Figure S3). Although it is thus tempting to speculate that NREM sleep might thus be less restful after TAK-925, EEG delta power, a frequency band associated with deep sleep, was increased during ZT14 after TAK-925. Although several of these physiological responses suggest a more prolonged effect of TAK-925, the different effects could be due to differential rates of clearance of these compounds due to the route and/or vehicles used for administration rather than to differences in the intrinsic activities of the two molecules.
4.3 |. Perspective for Hcrt Agonist-based Therapeutics for Narcolepsy
Regarding potential clinical application, although both compounds effectively reduced cataplexy, the effects on wakefulness were primarily restricted to the first hour after dosing and may thus have limited utility to combat the EDS of narcolepsy patients. In addition, both agonists elevated gross motor and running wheel activity along with Tsc. The increased running wheel activity during the first hour after drug administration was due to an increased number of running bouts rather than fewer, prolonged bouts. These results suggest the possibility that the transient increase in wakefulness (and possibly alertness, as suggested by increased spectral power in the gamma bands of the EEG) were secondary consequences of the compound’s effects on activity levels. Nonetheless, the anti-cataplectic activity of TAK-925 and ARN-776 is encouraging for the development of Hcrt receptor agonists to treat this pathognomonic symptom of narcolepsy and suggests that these particular molecules may be useful as tool compounds rather than as clinical development candidates. A Phase 1 study conducted in healthy sleep-deprived adults demonstrated that TAK-925 was well-tolerated and improved individuals’ ability to maintain wakefulness (Evans et al., 2019). Ultimately further development of TAK-925 has not been pursued as it requires intravenous administration.
Recently, Takeda has described an orally available HCRTR2/OX2R agonist, TAK-994, with an EC50 on OX2R = 19 nM and >700-fold selectivity against OX1R (Kimura et al., 2019). Oral administration of TAK-994 at 5h into the light period promoted wakefulness in wild-type mice during their normal sleep phase (Ishikawa et al., 2019), and increased time spent in wake and improved wake maintenance while suppressing cataplectic episodes in Hcrt-neuron ablated mice (Kimura et al., 2019). TAK-994 had wake-promoting effects following chronic dosing for up to 14 days in orexin/ataxin-3 mice without causing a sleep rebound (Ishikawa et al., 2020). Unfortunately, a safety signal emerged in Phase 2 studies of TAK-994 which led Takeda to suspend dosing. Nevertheless, Takeda continues to advance other oral Hcrt receptor agonists, and other novel hypocretin agonists like ARN-776 are under development by other organizations as potential therapeutics for the treatment of narcolepsy, as well as for other disorders characterized by the presence of excessive daytime sleepiness.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by the NIH R21 NS106882 and R01 NS098813 to TSK. JKDB holds the Julie and Louis Beecherl, Jr., Chair in Medical Science and acknowledges support from the Robert A. Welch Foundation (grant I-1422). DMR acknowledges support from the Robert A. Welch Foundation (grant I-1770). We thank Akihiro Yamanaka of Nagoya University for the C57BL/6-Tg(orexin/tTA; TetO diphtheria toxin A fragment)/Yamanaka mice and Haley Courtney, Laure Alexandre, Francisco Ortiz, and the institutionally-supported Preclinical Pharmacology Core at UT Southwestern Medical Center for technical assistance.
Footnotes
CONFLICTS OF INTEREST
None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Supporting information is attached to this manuscript.
REFERENCES
- Barateau L, & Dauvilliers Y (2019). Recent advances in treatment for narcolepsy. Ther Adv Neurol Disord, 12, 1756286419875622. doi: 10.1177/1756286419875622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, & Yanagisawa M (2004). Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci, 24(18), 4469–4477. doi: 10.1523/JNEUROSCI.5560-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SW, Morairty SR, Chen TM, Leung AK, Wisor JP, Yamanaka A, & Kilduff TS (2014). GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy. J Neurosci, 34(19), 6485–6494. doi: 10.1523/JNEUROSCI.0080-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SW, Schwartz MD, Chen TM, Hoener MC, & Kilduff TS (2017). Trace Amine-Associated Receptor 1 Agonists as Narcolepsy Therapeutics. Biol Psychiatry, 82, 623–633. doi: 10.1016/j.biopsych.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C, Liu M, Konadhode R, Pelluru D, & Shiromani PJ (2013). Effects of orexin gene transfer in the dorsolateral pons in orexin knockout mice. Sleep, 36(1), 31–40. doi: 10.5665/sleep.2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OG, Quednow BB, Seifritz E, & Wetter TC (2012). Reconsidering GHB: orphan drug or new model antidepressant? J Psychopharmacol, 26(5), 618–628. doi: 10.1177/0269881111421975 [DOI] [PubMed] [Google Scholar]
- Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, & Dauvilliers Y (2012). Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev, 16(5), 431–443. doi: 10.1016/j.smrv.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, … Yanagisawa M (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell, 98(4), 437–451. doi: 10.1016/s0092-8674(00)81973-x [DOI] [PubMed] [Google Scholar]
- Evans R, Hazel J, Faessel H, Wu J, Hang Y, Alexande R, … Hartman D (2019). Results of a Phase 1, 4-Period Crossover, Placebo-Controlled, Randomized, Single Dose Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of TAK-925, a Novel Orexin 2 Receptor Agonist, in Sleep-Deprived, Healthy Adults, Utilizing Modafinil as an Active Comparator Sleep Med, 64, S106. [Google Scholar]
- Evans R, Kimura H, Alexander R, Davies CH, Faessel H, Hartman DS, … Mignot E (2022). Orexin 2 receptor-selective agonist danavorexton improves narcolepsy phenotype in a mouse model and in human patients. Proc Natl Acad Sci U S A, 119(35), e2207531119. doi: 10.1073/pnas.2207531119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Mignot E, & Nishino S (2003). Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep, 26(8), 953–959. doi: 10.1093/sleep/26.8.953 [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Rikimaru K, Fukuda K, Sugimoto H, Matsumoto T, Tokunaga N, & Hirozane M (2017). U.S. Patent No. US 2017/0226137 A1. US Publication No. 2017/0226137 A1: USPTO [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, … Sakurai T (2001). Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron, 30(2), 345–354. doi: 10.1016/s0896-6273(01)00293-8 [DOI] [PubMed] [Google Scholar]
- Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S,… Yanagisawa M (2017). Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A, 114(22), 5731–5736. doi: 10.1073/pnas.1700499114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Hara H, Kawano A, & Kimura H (2022). Danavorexton, a selective orexin 2 receptor agonist, provides a symptomatic improvement in a narcolepsy mouse model. Pharmacol Biochem Behav, 220, 173464. doi: 10.1016/j.pbb.2022.173464 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Suzuki M, Kajita Y, Miyanohana Y, Koike T, & Kimura H (2019). Discovery of a novel, orally available orexin 2 receptor-selective agonist, TAK-994, as a therapeutic drug for narcolepsy. Sleep Med, 64, S170. [Google Scholar]
- Ishikawa T, Suzuki M, & Kimura H (2020). A Novel, Orally Available Orexin 2 Receptor-Selective Agonist, TAK-994, Shows Wake-Promoting Effects Following Chronic Dosing in an Orexin-Deficient Narcolepsy Mouse Model. Sleep, 43, A56. doi: 10.1093/sleep/zsaa056.139 [DOI] [Google Scholar]
- Kalogiannis M, Hsu E, Willie JT, Chemelli RM, Kisanuki YY, Yanagisawa M, & Leonard CS (2011). Cholinergic modulation of narcoleptic attacks in double orexin receptor knockout mice. PLoS One, 6(4), e18697. doi: 10.1371/journal.pone.0018697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik MK, Aritake K, Imanishi A, Kanbayashi T, Ichikawa T, Shimizu T, … Yanagisawa M (2018). Continuous intrathecal orexin delivery inhibits cataplexy in a murine model of narcolepsy. Proc Natl Acad Sci U S A, 115(23), 6046–6051. doi: 10.1073/pnas.1722686115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Ishikawa T, & Suzuki M (2019). A novel, orally available orexin 2 receptor-selective agonist, TAK-994, ameliorates narcolepsy-like symptoms in narcolepsy mouse models. Sleep Med, 64, S199. [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, … Mignot E (1999). The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell, 98(3), 365–376. doi: 10.1016/s0092-8674(00)81965-0 [DOI] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, … Shiromani PJ (2011). Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci, 31(16), 6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, Konadhode RR, Luan L, & Shiromani PJ (2016). Orexin gene transfer into the amygdala suppresses both spontaneous and emotion-induced cataplexy in orexin-knockout mice. Eur J Neurosci, 43(5), 681–688. doi: 10.1111/ejn.13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, & Shiromani PJ (2017). Rewiring brain circuits to block cataplexy in murine models of narcolepsy. Curr Opin Neurobiol, 44, 110–115. doi: 10.1016/j.conb.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Thankachan S, Kaur S, Begum S, Blanco-Centurion C, Sakurai T, … Shiromani PJ (2008). Orexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in mice. Eur J Neurosci, 28(7), 1382–1393. doi: 10.1111/j.1460-9568.2008.06446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, & Sakurai T (2011). Differential roles of orexin receptor-1 and −2 in the regulation of non-REM and REM sleep. J Neurosci, 31(17), 6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, & Yanagisawa M (2004). Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A, 101(13), 4649–4654. doi: 10.1073/pnas.0400590101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Fujiki N, Yoshida Y, Sakurai T, Honda M, Mignot E, & Nishino S (2008). Hypocretin receptor expression in canine and murine narcolepsy models and in hypocretin-ligand deficient human narcolepsy. Sleep, 31(8), 1119–1126. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18714784 [PMC free article] [PubMed] [Google Scholar]
- Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, … Nagase H (2015). Design and Synthesis of Non-Peptide, Selective Orexin Receptor 2 Agonists. J Med Chem, 58(20), 7931–7937. doi: 10.1021/acs.jmedchem.5b00988 [DOI] [PubMed] [Google Scholar]
- Scammell TE (2015). Narcolepsy. N Engl J Med, 373(27), 2654–2662. doi: 10.1056/NEJMra1500587 [DOI] [PubMed] [Google Scholar]
- Scammell TE, Willie JT, Guilleminault C, Siegel JM, & International Working Group on Rodent Models of, N. (2009). A consensus definition of cataplexy in mouse models of narcolepsy. Sleep, 32(1), 111–116. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19189786 [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Tisdale R, Park S, Ma SC, Heu J, Haire M, … Kilduff TS (2022). The Development of Sleep/Wake Disruption and Cataplexy as Hypocretin/Orexin Neurons Degenerate in Male vs. Female Orexin/tTA; TetO-DTA Mice. Sleep. doi: 10.1093/sleep/zsac039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Thorpy MJ, Mayer G, Peever J, & Kilduff TS (2019). Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med Rev, 43, 23–36. doi: 10.1016/j.smrv.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi S, Tsunematsu T, Black SW, Tominaga M, Maruyama M, Takagi K, … Yamanaka A (2014). Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci, 34(19), 6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpy MJ (2020). Recently Approved and Upcoming Treatments for Narcolepsy. CNS Drugs, 34(1), 9–27. doi: 10.1007/s40263-019-00689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vinne V, Pothecary CA, Wilcox SL, McKillop LE, Benson LA, Kolpakova J, … Peirson SN (2020). Continuous and non-invasive thermography of mouse skin accurately describes core body temperature patterns, but not absolute core temperature. Sci Rep, 10(1), 20680. doi: 10.1038/s41598-020-77786-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, … Yanagisawa M (2003). Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron, 38(5), 715–730. doi: 10.1016/s0896-6273(03)00330-1 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Nagumo Y, Ishikawa Y, Irukayama-Tomobe Y, Namekawa Y, Nemoto T, … Yanagisawa M (2022). OX2R-selective orexin agonism is sufficient to ameliorate cataplexy and sleep/wake fragmentation without inducing drug-seeking behavior in mouse model of narcolepsy. PLoS One, 17(7), e0271901. doi: 10.1371/journal.pone.0271901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukitake H, Fujimoto T, Ishikawa T, Suzuki A, Shimizu Y, Rikimaru K, … Kimura H (2019). TAK-925, an orexin 2 receptor-selective agonist, shows robust wake-promoting effects in mice. Pharmacol Biochem Behav, 187, 172794. doi: 10.1016/j.pbb.2019.172794 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


