Abstract
Microplastics are widely distributed in aquatic ecosystems along with other chemical pollutants. Therefore, it is vital to study the health-hazardous effects of MPs in combination with 4-nonylphenol (4-NP), which is a highly abundant industrial waste and a critical alkylphenol endocrine disruptor. We investigated the effects of the exposure to polyethylene microplastics (PE-MPs), 4-NP, and their combination on blood biomarkers in Cyprinus carpio juveniles. Four study groups were treated for 15 consecutive days: (1) control group, (2) 10 mg/L PE-MP group, (3) 10 mg/L PE-MPs + 200 µg/L 4-NP group, and (4) 200 µg/L 4-NP group, followed by 15 days of recovery. Biochemical analyses showed that creatine kinase, lactate dehydrogenase, glucose, liver enzymes, total protein, and A/G ratios were significantly increased after exposure to PE-MPs, 4-NP, and the combination. Hematological parameters (RBC's, Hb, Ht, neutrophil percentage, and WBC's) were significantly decreased in the three exposure groups, whereas mean corpuscular volume and lymphocyte percentages were significantly increased. The 15-day recovery period improved most hematobiochemical parameters and PE-MP accumulation indices. Taken together, we demonstrated the hazardous effects of PE-MP and 4-NP combinations on C. carpio blood parameters and highlighted their potential risk to human health.
Subject terms: Biochemistry, Physiology, Zoology
Introduction
Greater understanding of global pollution is warranted, especially in aquatic environments, and for animals, fish, and amphibians, where some industrial chemicals contaminate environments and cause serious damage during developmental and adult stages1. Chemical pollution is due to increased industrialization. Microplastics (MPs) and nonylphenols (NPs) are considered emerging pollutants and have attracted considerable environmental and research attention, but their combined toxicity toward aquatic organisms remains poorly researched. These chemicals are toxic when they persist in the environment and accumulate in different biota. Aside from their use in packaging, construction, transportation, electrical power, and medical products. Plastics are low cost, lightweight, and easy to process, which has made them popular in many industries2. Major MPs are categorized based on their monomer backbone structure and include polyethylenes (PEs), polypropylenes, polystyrenes, polyvinyl chlorides, and polyamides3,4. One of the most commonly used plastics is PE, which has the chemical formula (C2H4) n5,6. Many products are made from PE, including films, storage containers, toys, and packaging7. Plastics degrade during their life cycle via different mechanisms, including abrasion, mechanical wear, photooxidation, and biological destruction8. MPs are plastic pieces that vary across the size range from 5 to100 nm and are degraded from larger plastic pieces9, and NPs are < 100 nm in size10.
Despite differences in size, chemical content, and shape, MPs are heterogeneous groups of particles with varying toxicity11,12. Studies have shown that aquatic organisms ingest and accumulate MPs, concomitant contaminants13,14, and land invertebrates15–17. Although MPs come in many forms, the toxicity of each type is widely unknown18–20.
Certain species can be used to characterize the potential damage from MPs on aquatic health. Plastics may affect organisms in two ways: (i) physically by obstructing growth processes21 and reducing food and energy uptake22,23 and (ii) chemically by adsorbing contaminants such as polychlorinated biphenyls and polybrominated diphenyl ethers or releasing additives9,24,25. Freshwater is often the main source of such plastics, transporting medium, and the sink of MPs26. Little research has been conducted on MPs in freshwater when compared with marine water, but freshwater may accumulate numerous MPs27.
MPs also absorb other contaminants (organic and inorganic pollutants) from surrounding environments which is like the fact that these adhered pollutants are affected both spatially and biologically24,28 and warrants further study. Recently, this vector effect was summarized by Syberg et al.29, on base of the environment30, the organism22, and the cell (von Moos et al., 2012).
Persistent organic pollutants (POPs) are highly attracted to the hydrophobic surface of plastics, which facilitates the concentration of MPs21. However, it is highly unlikely that biota can absorb such chemicals from plastics31. Even though some POPs bioaccumulate and biomagnify within MPs, the risk of MPs-ingesting is generally the same as ingesting contaminants from prey or dispersed water contaminants32–34. The environmental endocrine disruptor nonylphenol (4-NP) is used in the manufacture of cleaning products, emulsifiers, and wetting agents and is found in paints, pesticides, and household products35.
Common carps (Cyprinus carpio) are a widely cultivated freshwater fish species because of their ability to withstand environmental changes and stresses. The species is widely used as a model species in ecotoxicological studies, so it was selected because of its importance in aquaculture36,37 using fish as biomarkers is vital for monitoring pollution. The hemato-biochemical parameters of fish are essential signs of water quality38.
Whereas, no previous studies have explored the synergistic effects of PE-MPs and 4-NP, especially in fauna from aquatic environments, we investigated the hematological and biochemical parameters in juvenile carp to determine the synergistic effects of PE-MPs and 4-NP using cytotoxicity biomarkers.
Materials and methods
Chemicals
PE-MP characterization
PE-MPs 5 mm > MPs > 100 nm were used in raw powdered form and were irregularly shaped particles (Toxemerge Pty Ltd., Australia). PE-MP particles were characterized using TEM (JEOL JEM-1200 EX II) at TEMU, Assiut University. PE-MPs were characterized using a protocol by Hamed et al.39.
4-NP
4-NP (99.3% purity) was supplied by Sigma–Aldrich (Schnelldorf, Germany).
Stock preparation
Approximately 1 g of PE-MPs and 6 mg of 4-NPs were individually dissolved into separate containers containing 1 L of distilled water and maintained in dark at 4 °C (stocks were shaken before use).
PE-MP detection
PE-MPs were observed in whole fish based on a method by Deng et al.40. A whole fish (5 ± 1 g) was put in 10 mL of hydrogen peroxide (30%, v: v) for 2 h at 70 °C, and 100 µL of the obtained mixture was microscopely-examined using 14 MP OMAX camera (A35140U3) according to Hamed et al.80.
Fish acclimation and exposure
Juveniles C. carpio (5 ± 1 g and 8.5 ± 1 cm) were acclimated in glass tanks (100 cm × 70 cm × 50 cm) under physicochemical conditions: Temperature 28.5 °C, pH 7.4, 6.9 mg/L DO, 12:12 h (light:dark), and 260.8 mM/cm conductivity. Four groups (36 fish/ 3 triplicate) were treated as follows: 1) control, 2) 10 mg/L PE-MPs, 3) 10 mg/L PE-MPs + 200 µg/L 4-NP, and 4) 200 µg/L 4-NP for 15 consecutive days and then 15 days of recovery. Doses were selected according to Hamed et al.39 and Sayed and Soliman41. Fish were fed each day with commercial pellets at 3% of their body weight, and water changed every day (50%) and MPs-redosed in water (immersion method of exposure) every day to prevent waste accumulation. Study procedures terminated with six fish in each group numbed on ice to eliminate stress caused by processing42 and fish was euthanized MS-222 (Millipore-Sigma-Aldrich, Oakville, ON, Canada; 0.5 g/L). After cutting the tail, blood collected in heparinized and non-heparinized tubes for hematological and biochemical assessments, respectively.
Hematological parameters
Blood samples (6/ group) were taken from the caudal vein into heparinzed tubes to measure Hematological parameters including counts comprising erythrocytes (RBC's), total white blood cells (WBC's), differential WBC's, hematocrit (Ht), hemoglobin concentrations (Hb) were performed using Auto Hematology Analyzer (Rayto RT-7600) according to Hamed et al.39 and Hamed et al.43. Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated using the formulae mentioned by Dacie and Lewis101
Biochemical parameters
Blood samples were taken from the caudal vein into non-heparinzed tubes to centrifugation at 5000 rpm for 5 min and then the serum was removed by subjecting the tubes, stored at − 20 °C until further analysis of the following blood parameters: albumin, globulin, total protein (TP), glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatine kinase (CK), and lactate dehydrogenase (LDH) were determined by kits of (SGMitalia Company U.S.A) using a spectrophotometer (T80 + UV/VIS, Bioanalytic Diagnostic Industry, Co.) according to Hamed et al.43.
Erythrocyte analysis
Blood smears fixed, air-dried, and stained with hematoxylin & eosin. Slides were selected and observed under a 40 × objective lens under a light microscope (VE-T2) attached to a 14 MP OMAX camera (A35140U3) according to Hamed et al.43 and Sayed et al.44.
Statistical analysis
The mean and standard error of the mean values were estimated. Statistical differences between groups were analyzed by one-way analysis of variance in SPSS45 at the 0.05 significance level (P < 0.05). Post hoc comparison was done using Tukey’s-b and Dunnett tests (SPSS V.16).
Ethics statement
Studies were approved by the Research Ethics Committee of the Molecular Biology Research and Studies Institute (MB-21–11-R), Assiut University, Assiut, Egypt. All methods were carried out following the relevant regulations and ARRIVE guidelines.
Results
PE-MP characterization
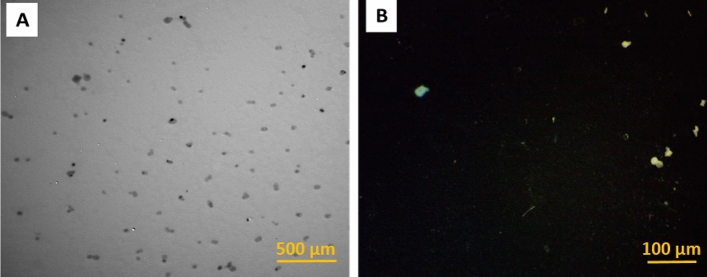
Light microscope and TEM images showed that PE-MP particles were irregularly shaped (Fig. 1).
Figure 1.
(a) Transmission electron microscope and (b) light microscope images of PE-MPs.
PE-MP detection
High PE-MP levels were observed in the PE-MP-exposed groups when compared with the control. After the recovery period, high PE-MP levels were still observed in the PE-MP-exposed groups compared with control (Fig. 2).
Figure 2.
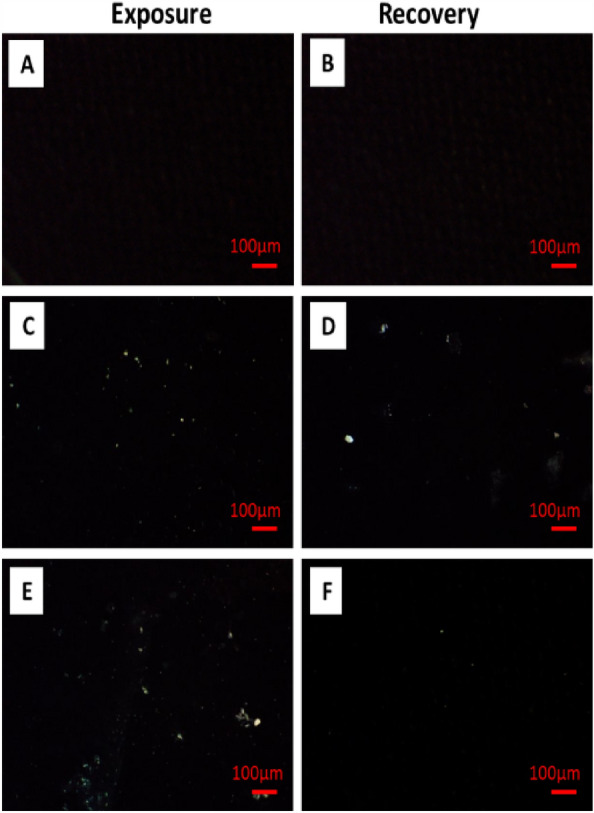
Light microscope images showing PE-MPs in fish, (a, b) Control, (c, d) 10 mg/L PE-MPs, and (e, f) 10 mg/L PE-MPs + 200 µg/L 4-NP.
The effects of combinations on hematological parameters
RBC's, Hb, and WBC's after exposure to 10 mg/L PE-MPs, 200 µg/L 4-NP, and 10 mg/L PE-MPs + 200 µg/L 4-NP; Ht after exposure to 200 µg/L 4-NP and 10 mg/L PE-MPs + 200 µg/L 4-NP; and neutrophil percentages after exposure to 200 µg/L 4-NP for 15 days showed significant (P < 0.05) decreases when compared with those of controls. MCV levels and lymphocyte percentages showed significant (P < 0.05) increases after exposure to 10 mg/L PE-MPs + 200 µg/L 4-NP for 15 days (Figs. 3 and 4).
Figure 3.
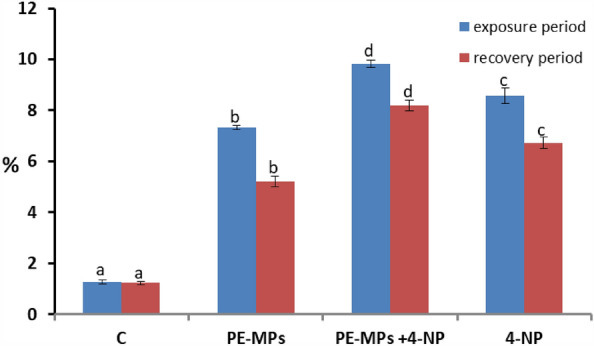
RBC's percentage alterations in juveniles Cyprinus carpio after exposure to 10 mg/L PE-MPs, 10 mg/L PE-MPs + 200 µg/L 4-NP, and 200 µg/L 4-NP for 15 days each and after 15 days of recovery. Different letters indicate significant differences (P < 0.05).
Figure 4.
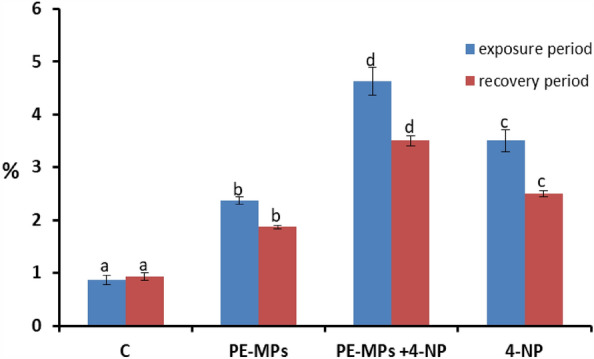
RBC's nuclear abnormality percentages in juveniles Cyprinus carpio after exposure to 10 mg/L PE-MPs, 10 mg/L PE-MPs + 200 µg/L 4-NP, and 200 µg/L 4-NP for 15 days each and after 15 days of recovery. Different letters indicate significant differences (P < 0.05).
Neutrophil and monocyte percentages and Ht levels after exposure to 10 mg/L PE-MPs, and monocyte and lymphocyte percentages and MCH levels after exposure to 10 mg/L PE-MPs + 200 µg/L 4-NP for 15 days showed nonsignificant decreases. MCHC levels after exposure to 10 mg/L PE-MPs, 200 µg/L 4-NP, and 10 mg/L PE-MPs + 200 µg/L 4-NP; neutrophil percentages and MCV levels after exposure to 10 mg/L PE-MPs + 200 µg/L 4-NP; monocyte percentages after exposure to 200 µg/L 4-NP; and MCH levels after exposure to 200 µg/L 4-NP and 10 mg/L PE-MPs for 15 days showed nonsignificant increases.
After the recovery period, the hematological parameters showed no change, except for the following: the RBC, MCV, and MCH levels in the PE-MP group, MCHC levels and neutrophil percentages in the PE-MPs + 4-NP group, and monocyte percentages in the 4-NP group; all showed nonsignificant decreases. The MCV levels in the 4-NP group showed a nonsignificant increase. The Ht levels in the PE-MP group showed a significant decrease. The lymphocyte percentages in the PE-MP + 4-NP group showed a significant increase (Table 1).
Table 1.
The effects of PE-MPs, 4-NP, and PE-MPs + 4-NP on hematological parameters (mean ± standard error) of juveniles Cyprinus carpio after 15 days of exposure, followed by 15 days of recovery.
| Exposure period | Recovery period | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | PE-MPs | PE-MPs + 4-NP | 4-NP | Control | PE-MPs | PE-MPs + 4-NP | 4-NP | |
| RBCs (million/µL) | 1.46 ± 0.02c | 1.37 ± 0.01b | 1.29 ± 0.01a | 1.34 ± 0.01b | 1.47 ± 0.02b | 1.43 ± 0.02b | 1.32 ± 0.02a | 1.33 ± 0.02a |
| Hemoglobin (Hb)(g/dL) | 9.58 ± 0.08c | 9.07 ± 0.06b | 8.34 ± 0.17a | 9.13 ± 0.01b | 9.63 ± 0.08c | 9.15 ± 0.10b | 8.33 ± 0.21a | 9.10 ± 0.00b |
| Ht (PCV) (%) | 39.24 ± 0.24c | 38.97 ± 0.30bc | 35.57 ± 0.44a | 37.88 ± 0.29b | 39.38 ± 0.41c | 37.13 ± 0.48b | 35.55 ± 0.53a | 37.52 ± 0.18b |
| MCV (µm3) | 268 ± 3.79a | 284. ± 2.66b | 276 ± 3.95ab | 284 ± 3.06b | 270 ± 4.27a | 262 ± 3.54a | 271 ± 5.96a | 277 ± 3.74a |
| MCH (µm3) | 65.47 ± 0.61a | 66.18 ± 0.48a | 64.80 ± 1.91a | 68.37 ± 0.35a | 65.90 ± 1.14a | 64.53 ± 0.93a | 63.48 ± 2.07a | 67.25 ± 0.82a |
| MCHC (%) | 20.75 ± 3.60a | 23.27 ± 0.15a | 23.47 ± 0.63a | 24.10 ± 0.20a | 24.47 ± 0.32a | 24.68 ± 0.37a | 23.45 ± 0.79a | 24.30 ± 0.13a |
| WBCs (thousand/µL) | 53.67 ± 0.33c | 51.67 ± 0.33b | 49.83 ± 0.54a | 52.09 ± 0.42b | 54.33 ± 0.42c | 51.50 ± 0.34ab | 50.50 ± 0.43a | 52.17 ± 0.17b |
| Neutrophils (%) | 15.33 ± 0.21bc | 14.50 ± 0.22ab | 16 ± 0.26c | 13.83 ± 0.31a | 15.67 ± 0.21c | 14.33 ± 0.21ab | 15 ± 0.26bc | 13.83 ± 0.17a |
| Lymphocytes (%) | 83 ± 0.26a | 84 ± 0.00b | 82.67 ± 0.42a | 84.33 ± 0.21b | 82.83 ± 0.17a | 84.17 ± 0.31b | 83.83 ± 0.40b | 84.83 ± 0.17b |
| Monocytes (%) | 1.67 ± 0.21a | 1.50 ± 0.22a | 1.33 ± 0.21a | 1.83 ± 0.17a | 1.67 ± 0.21a | 1.50 ± 0.22a | 1.17 ± 0.17a | 1.17 ± 0.17a |
Different superscript letters indicate significantly different (P < 0.05).
The effects of combinations on biochemical parameters
AST, ALT, ALP, LDH, CK, glucose, and TP after exposure to 10 mg/L PE-MPs, 200 µg/L 4-NP, and 10 mg/L PE-MPs + 200 µg/L 4-NP, and globulin and albumin after exposure to 10 mg/L PE-MPs and 10 mg/L PE-MPs + 200 µg/L 4-NP showed significant (P < 0.05) increases. By contrast, albumin and the A/G ratio after exposure to 200 µg/L 4-NP showed a nonsignificant increase. The A/G ratio after exposure to 10 mg/L PE-MPs + 200 µg/L 4-NP showed a nonsignificant decrease.
After the recovery period, the biochemical parameters did not change, except for the following: TP and AST in the PE-MPs, 4-NP, and PE-MPs + 4-NP groups; LDH and glucose in the 4-NP-exposed group; and albumin in the PE-MP-exposed group showed nonsignificant increases.
Additionally, the A/G ratio in the 4-NP group and globulin in the PE-MP group showed a nonsignificant decrease. However, the A/G ratio in the PE-MPs + 4-NP group and albumin in the 4-NP group showed a significant decrease (P < 0.05) (Table 2).
Table 2.
The effects of PE-MPs, 4-NP, and PE-MPs + 4-NP on biochemical parameters (mean ± standard error) of juveniles Cyprinus carpio after 15 days of exposure, followed by15 days of recovery.
| Exposure period | Recovery period | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | PE-MPs | PE-MPs + 4-NP | 4-NP | Control | PE-MPs | PE-MPs + 4-NP | 4-NP | |
| LDH (U/L) | 74.08 ± 0.66a | 79.80 ± 1.59b | 87.15 ± 0.76c | 78.62 ± 0.42b | 74.12 ± 0.56a | 84.68 ± 2.52b | 86.12 ± 0.69b | 77.62 ± 0.69a |
| Creatine kinase (CK) (U/L) | 82.85 ± 0.42a | 86.37 ± 0.46b | 86.75 ± 0.30b | 85.28 ± 0.65b | 81.80 ± 0.19a | 85.72 ± 0.45b | 85.82 ± 0.50b | 84.28 ± 0.55b |
| ALP (U/L) | 8.19 ± 0.02a | 8.59 ± 0.04b | 8.87 ± 0.05c | 8.53 ± 0.05b | 8.21 ± 0.05a | 8.67 ± 0.04b | 8.83 ± 0.04c | 8.53 ± 0.03b |
| ALT (U/L) | 48.42 ± 0.48a | 51.23 ± 0.31b | 53.05 ± 0.70b | 51.40 ± 0.35b | 48.35 ± 0.30a | 51.28 ± 0.25b | 52.90 ± 0.34c | 51.42 ± 0.14b |
| AST (U/L) | 82.83 ± 0.41a | 85.25 ± 0.30b | 86.30 ± 0.38b | 85.75 ± 0.50b | 84.30 ± 0.54a | 85.62 ± 0.44a | 85.30 ± 0.48a | 85.65 ± 0.31a |
| Glucose (mg/dL) | 55.73 ± 0.38a | 58.12 ± 0.35b | 58.68 ± 0.19b | 57.40 ± 0.49b | 55.27 ± 0.30a | 57.33 ± 0.64bc | 58.20 ± 0.36c | 55.85 ± 0.34ab |
| Total protein (g/dL) | 2.73 ± 0.04a | 2.93 ± 0.02b | 2.94 ± 0.02b | 2.88 ± 0.02b | 2.78 ± 0.03a | 2.87 ± 0.02a | 4.03 ± 1.17a | 2.83 ± 0.02a |
| A/G ratio (%) | 0.34 ± 0.01a | 0.34 ± 0.00a | 0.33 ± 0.00a | 0.35 ± 0.00a | 0.35 ± 0.00a | 0.35 ± 0.00a | 0.27 ± 0.04a | 0.32 ± 0.02a |
| Globulin (g/dL) | 2.15 ± 0.01a | 2.24 ± 0.03b | 2.41 ± 0.02c | 2.15 ± 0.02a | 2.13 ± 0.01a | 2.10 ± 0.03a | 3.35 ± 0.63a | 2.13 ± 0.03a |
| Albumin (g/dL) | 0.74 ± 0.01a | 0.77 ± 0.01a | 0.81 ± 0.01b | 0.76 ± 0.01a | 0.76 ± 0.01b | 0.77 ± 0.02b | 0.80 ± 0.00b | 0.70 ± 0.00a |
Different superscript letters indicate significantly different (P < 0.05).
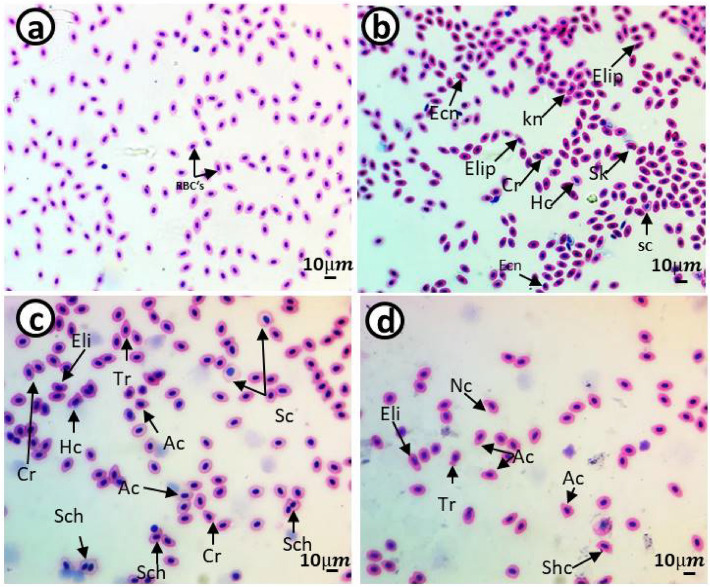
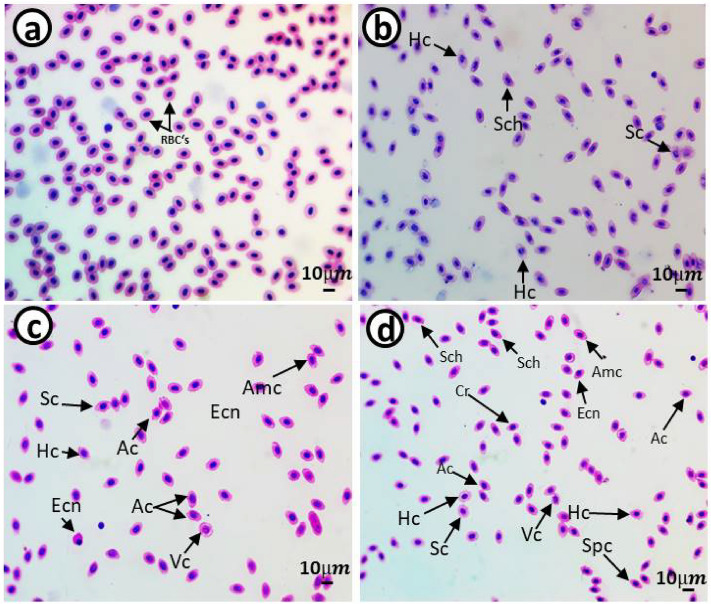
Erythrocyte morphological alterations and nuclear abnormalities
The control group showed a standard RBC's shape with a centrally located nucleus (Figs. 5a and 6a). Smears from the 10 mg/L PE-MP (Figs. 5b and 6b), 10 mg/L PE-MPs + 200 µg/L 4-NP (Figs. 5c and 6c), and 200 µg/L 4-NP groups displayed poikilocytosis in RBC's (Figs. 5d and 6d) after exposure, whereas after recovery, cells showed varied morphologies, including eliboat shapes, teardrops, schistocytic, swollen cells, eccentric nuclei, kidney shapes, eliptocytes, crenated shapes, sickle cells, acanthocytes, hemolyzed cells, vacuolated cells, ameboied cells, and spinocytes. The percentages of RBC alterations and nuclear abnormalities were significantly increased (P < 0.00001) after exposure to 10 mg/L PE-MPs, 200 µg/L 4-NP, and 10 mg/L PE-MPs + 200 µg/L 4-NP for 15 days when compared with controls. RBC's membrane and nuclei alterations remained significantly increased after the 15-day recovery period when compared with controls (Figs. 3 and 4). After both periods, percentage increments (for both types of alterations) followed the order: PE-MPs + 4-NP > 4-NP > PE-MPs.
Figure 5.
Blood smears from juveniles Cyprinus carpio after a 15-day exposure showing normal erythrocytes in the control group (a) and deformed erythrocytes in the 10 mg/L PE-MPs (b), 10 mg/L PE-MPs + 200 µg/L 4-NP (c), and 200 µg/L 4-NP groups (d). Eliboat shapes (Eli), teardrops (Tr), schistocytics (Sch), swollen cells (Sc), eccentric nucleus (Ecn), kidney shapes (Kn), crenated cells (Cr), sickle cells (Sk), acanthocytes (Ac), vacuolated cells (Vc), elliptocytes (Elip), spinocytes (Spc), and hemolyzed cells (Hc) (hematoxylin & eosin staining).
Figure 6.
Blood smears from juveniles Cyprinus carpio after a recovery period of 15 days showing normal erythrocytes in the control group (a) and deformed erythrocytes in the 10 mg/L PE-MPs (b), 10 mg/L PE-MPs + 200 µg/L 4-NP (c), and 200 µg/L 4-NP groups (d). Schistocytic (Sch), swollen cells (Sc), eccentric nucleus (Ecn), crenated cells (Cr), acanthocytes (Ac), vacuolated cells (Vc), ameboied cells (Amc), spinocytes (Spc), and hemolyzed cells (Hc) (hematoxylin & eosin staining).
Discussion
MP contamination in aquatic environments is a growing hazardous health problem as aquatic animals eat and digest MPs. Living organisms may be affected by MPs increasing their bioavailability and uptake of sorbed co-contaminates of different types. The combined effects of MPs and sorbed co-contaminants in aquatic organisms remain to be fully verified46. Therefore, in this study, hemato-biochemical parameters were used to investigate the effects of C. carpio exposure to 10 mg/L PE-MPs, 200 µg/L 4-NP, and 10 mg/L PE-MPs + 200 µg/L 4-NP for 15 days and then 15 days of recovery.
To assess fish health, hematological parameters must be measured47. We observed considerable variations in hematological parameters between groups. Our results were similar to the data reported by Hamed et al.39 and Hamed et al.43. Decreased RBC, Hb, Ht, and WBC levels with increased MCV levels and lymphocyte percentages were also reported by Hamed et al.39 in Oreochromis niloticus exposed to MPs and by43 in C. carpio exposed to PE-MPs. Additionally, in fish exposed to phosalone48, and copper oxide nanoparticles49. The catfish (Clarias gariepinus) was also affected by UVA exposure with respect to hematology and cell alterations50. Some of parameters with hydroxychloroquine in catfish (C. gariepinus), Sayed et al.44 and Mekkawy et al.51, and Abou Khalil et al.52 with African catfish (C. gariepinus) in the presence of 4-NP. The exposure of carp to PE-MPs, 4-NP, and PE-MPs + 4-NP caused anemia, which might have been attributed to hematopoietic tissue alterations53 and54. This possibly occurred because of increased mechanical fragility in cell membranes, which we observed in our erythrocyte morphology55. Under laboratory conditions and after exposure to different pollutants, peripheral RBCs, Hb, and Ht were decreased56, which might have been due to the heme-dilution of blood which resulted from tissue damage57. The negative effects of NPs on lymphoid tissues in exposed fish may reduce total WBC counts58. The bioaccumulation of pollutants in tissues may decrease WBCs. As blood oxygen levels decrease, toxicity caused by plastics could be enhanced by decreased hemoglobin levels. Similar results were reported by Mukherjee and Sinha59 as cadmium contamination response39 as effect of MPs and43 as an effect of PE-MPs. Damage to the immune system after MPs accordingly will cause damage to the defense system and animal health60.
Biochemical parameters are invaluable bioindicators41,56. Most biochemical parameters were significantly increased after exposure to PE-MPs, 4-NP, and PE-MPs + 4-NP when compared with controls and were consistent with Hamed et al.43. The rise in CK, LDH, ALP, ALT, AST, TP, glucose with variation by increase and decrease in albumin and globulin observed in our study were similarly studied by Hamed et al.39 with O. niloticus in the presence of MPs. Some parameters with hydroxychloroquine in catfish (C. gariepinus)44, 61 and41 with African catfish in the presence of 4-NP, and43 with common carp (C. carpio) in the presence of PE-MPs. Banaee et al.62 and Nematdoost Haghi and Banaee63 state that the higher levels of the enzymes (CK, AST, ALT, LDH, and ALP) in serum are regarded as biomarker for cell membranes damage and as a tool for diagnosing changes in the environment in ecotoxicological studies, these enzymes are indicative of lesions in the tissues. Furthermore, elevated glucose levels indicated that glycogen had disintegrated or that its absorption was restricted in the liver. By contrast, increased blood glucose levels may have been due to hepatic tissue glycogen disintegration or impaired glucose absorption64. Previously, damage to different organ membranes in fish was observed following increased enzyme activity (ALT, AST, and ALP) caused by paraquat and plastic particles65, and in Pomatoschistus microps, biochemical parameters were changed when exposed to MPs and pyrene or to MPs and/or nickel66. Body homeostasis is maintained by proteins that prevent fluid leakage throughout the body60.
Immune system diseases and other kidney and hepatic issues are assessed using TP, albumin, and globulin tests67. Stoyanova et al.68 reported that the intensification of anaerobic metabolism could be measured by LDH activity due to environmental changes, pollution, and energy depletion. Changes in LDH, AST, ALT, CK, and ALP activities were shown to indicate tissue lesions and to reflect environmental changes in ecotoxicology69. ALT and AST levels were increased following hepatocyte damage (Komatsu et al., 2002) or impaired carbohydrate and protein metabolism70. Peralta et al.71 and Ramos-Barron et al.72 reported that increased albumin levels indicated hepatorenal tissue alterations. Wiegertjes et al.73 demonstrated that increased globulin levels were viable immune responses. In Nile tilapia (O. niloticus) exposed to MPs, albumin, globulin, and TP levels were higher, potentially indicating a damaged liver39 and in C. carpio induced by PE-MPs43. Osman et al.50 showed that fish underwent hyperglycemia when exposed to UVA stress or heavy metals and other contaminants74.
High PE-MPs levels were observed in PE-MP-exposed fish when compared with controls. This may have been due to the entrance of plastic particles with the water flow to the fish's body. A significant concentration of MPs was reported in different zebrafish organs75. Additionally, significantly higher MPs were observed in O. niloticus after MP exposure for 15 days39. MP accumulation in zebrafish yolk sac and migration to other organs were observed during embryogenesis76. Furthermore, in mussels, MPs absorbed through the gut mucosa were transported through the bloodstream to different tissues77. Moreover, PS-MP bioaccumulation was dose- and time-dependent in O. niloticus78.
The percentage of erythrocytes with morphological alterations and nuclear abnormalities, when exposed to treatments, was significantly increased when compared with controls. Poikilocytosis may be affected by several factors, e.g., increased RBC membrane fluidity, declined ATP levels, and inhibited membrane-bound enzymes79. Our results were similar to other fish pollutant studies: Hamed et al.43, who studied C. carpio exposed to PE-MPs; Sayed et al.44, who studied C. gariepinus exposed to hydroxychloroquine; Hamed et al.80, who studied the protective role of Spirulina platensis against cytotoxicity and genotoxicity induced by lead nitrates in C. gariepinus; Soliman et al.49, who studied the damage caused by copper sulfate and copper oxide nanoparticles in O. niloticus; and Sayed et al.81, who investigated Oryzias latipes exposed to 4-NP. Several studies reported that morphological and nuclear abnormalities in erythrocytes and MN were genotoxicity biomarkers following exposure to radiation and chemicals51,82–89. These biomarkers are powerful assessment tools for genetic and cellular damage in eukaryotes as they reflect DNA damage, and are simple, reliable, and sensitive measurement tools51,84,86,87,90.
Our blood parameter data indicated that the damage caused by combined PE-MPs and 4-NP was higher than the damage caused by 4-NP alone, which may be due to MPs facilitating entrance of other contaminants into aquatic organisms24,29,91. Previous laboratory studies reported that MPs were ingested by aquatic organisms77,92. Besseling et al.22, Koelmans et al.93, and Chua et al.94 reported that MPs may carry other contaminants, including plasticizers and POPs, such as polychlorinated biphenyls and polycyclic aromatic hydrocarbons65,93,95,96, and could facilitate interactions with metals, such as PE-MP-mediated silver uptake in Danio rerio97. Some ecotoxicological studies have provided evidence of metal ion adsorption by plastic containers98,99. HOCs strongly and chemically sorb onto MPs than natural sediments according to a study comparing sorption rates onto natural and manufactured particulates30. Oliveira et al.65 reported that PE bead exposure significantly increased toxicant bioavailability in juveniles exposed to lethal pyrene concentrations.
After the 15-day recovery period, erythrocyte morphological alterations, nuclear abnormalities, PE-MPs, and hematobiochemical changes were apparent in all treated groups when compared with controls. Our results were supported by Martins and Guilhermino100, who reported that despite the depuration phase, MPs persisted in D. magna for many generations, whereas Hamed et al.39 indicated that MPs were detected and generated hematobiochemical effects in Nile tilapia after the recovery period. Notably, after recovery, a faint improvement in some parameters was observed but was not similar to controls. A possible reason for this could be that a 15-day recovery was not enough for fish to eliminate the toxic effects of pollutants or the tissue has bo ability to restore their functions as normal after MPs-exposure46.
Conclusions
The synergistic effect of PE-MPs and 4-NP induced a high degree of increase in creatine kinase, lactate dehydrogenase, glucose, liver enzymes, total protein, and A/G ratios after exposure to PE-MPs, 4-NP, and the combination. Also, hematological parameters (RBC's, Hb, Ht, neutrophil percentage, and WBC's) were significantly decreased in the three exposure groups. The 15-day recovery period improved most hematobiochemical parameters and PE-MP accumulation indices. Hematological and biochemical issues in carp when compared with individual exposures, our data showed that the synergistic effect of PE-MP and 4-NP caused more serious damage than each single chemical in dose dependent manner.
Author contributions
Experimental design: EA, AHS. Experiment and analysis: EA, AHS, Data interpretation: EA, MH, AHS, Writing and revision: EA, MSM, AHS. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analyzed during this study are included in the research article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Radhaiah V, Girija M, Rao KJ. Changes in selected biochemical parameters in the kidney and blood of the fish, Tilapia mossambica (Peters), exposed to heptachlor. Bull. Environ. Contam. Toxicol. (United States) 1987;39:6. doi: 10.1007/bf01689591. [DOI] [PubMed] [Google Scholar]
- 2.PlasticsEurope. Plastics – the Facts 2018 An analysis of European plastics production, demand and waste data. 60 (2018).
- 3.Bouwmeester H, Hollman PCH, Peters RJB. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: Experiences from nanotoxicology. Environ. Sci. Technol. 2015;49:8932–8947. doi: 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- 4.Li WC, Tse HF, Fok L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016;566–567:333–349. doi: 10.1016/j.scitotenv.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 5.da Costa JP, Santos PSM, Duarte AC, Rocha-Santos T. (Nano)plastics in the environment – Sources, fates and effects. Sci. Total Environ. 2016;566–567:15–26. doi: 10.1016/j.scitotenv.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 6.de Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018;645:1029–1039. doi: 10.1016/j.scitotenv.2018.07.207. [DOI] [PubMed] [Google Scholar]
- 7.Lusher, A., Welden, N., Sobral, P. & Cole, M. in Analysis of nanoplastics and microplastics in food 119–148 (CRC Press, 2020).
- 8.Kögel T, Bjorøy Ø, Toto B, Bienfait AM, Sanden M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020;709:136050. doi: 10.1016/j.scitotenv.2019.136050. [DOI] [PubMed] [Google Scholar]
- 9.Andrady AL. Microplastics in the marine environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Alimi OS, FarnerBudarz J, Hernandez LM, Tufenkji N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018;52:1704–1724. doi: 10.1021/acs.est.7b05559. [DOI] [PubMed] [Google Scholar]
- 11.Jeong C-B, et al. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus) Environ. Sci. Technol. 2016;50:8849–8857. doi: 10.1021/acs.est.6b01441. [DOI] [PubMed] [Google Scholar]
- 12.McDevitt JP, et al. Addressing the issue of microplastics in the wake of the microbead-free waters act—A new standard can facilitate improved policy. Environ. Sci. Technol. 2017;51:6611–6617. doi: 10.1021/acs.est.6b05812. [DOI] [PubMed] [Google Scholar]
- 13.Watts AJR, et al. Uptake and retention of microplastics by the shore crab carcinus maenas. Environ. Sci. Technol. 2014;48:8823–8830. doi: 10.1021/es501090e. [DOI] [PubMed] [Google Scholar]
- 14.Cole M, Galloway TS. Ingestion of nanoplastics and microplastics by pacific oyster larvae. Environ. Sci. Technol. 2015;49:14625–14632. doi: 10.1021/acs.est.5b04099. [DOI] [PubMed] [Google Scholar]
- 15.Wardrop P, et al. Chemical pollutants sorbed to ingested microbeads from personal care products accumulate in fish. Environ. Sci. Technol. 2016;50:4037–4044. doi: 10.1021/acs.est.5b06280. [DOI] [PubMed] [Google Scholar]
- 16.Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ. Sci. Technol. 2017;51:4714–4721. doi: 10.1021/acs.est.7b00635. [DOI] [PubMed] [Google Scholar]
- 17.Nelms SE, et al. Marine anthropogenic litter on British beaches: A 10-year nationwide assessment using citizen science data. Sci. Total Environ. 2017;579:1399–1409. doi: 10.1016/j.scitotenv.2016.11.137. [DOI] [PubMed] [Google Scholar]
- 18.Bakir A, Rowland SJ, Thompson RC. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014;185:16–23. doi: 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Hamlin HJ, Marciano K, Downs CA. Migration of nonylphenol from food-grade plastic is toxic to the coral reef fish species Pseudochromis fridmani. Chemosphere. 2015;139:223–228. doi: 10.1016/j.chemosphere.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Hu L, et al. Uptake, accumulation and elimination of polystyrene microspheres in tadpoles of Xenopus tropicalis. Chemosphere. 2016;164:611–617. doi: 10.1016/j.chemosphere.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Besseling E, Wegner A, Foekema EM, van den Heuvel-Greve MJ, Koelmans AA. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L) Environ. Sci. Technol. 2013;47:593–600. doi: 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- 23.Duis K, Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28:2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011;62:2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Ross PS, Morales-Caselles C. Out of sight, but no longer out of mind: microplastics as a global pollutant. Integr. Environ. Assess. Manag. 2015;11:721–722. doi: 10.1002/ieam.1681. [DOI] [Google Scholar]
- 26.Klein, S., Dimzon, I. K., Eubeler, J. & Knepper, T. P. in Freshwater Microplastics : Emerging Environmental Contaminants? (eds Martin Wagner & Scott Lambert) 51–67 (Springer International Publishing, 2018).
- 27.Li J, Liu H, PaulChen J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018;137:362–374. doi: 10.1016/j.watres.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 28.Arthur, C., Baker, J. & Bamford, H. Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris, September 9–11, 2008. (2009).
- 29.Syberg K, et al. Microplastics: addressing ecological risk through lessons learned. Environ. Toxicol. Chem. 2015;34:945–953. doi: 10.1002/etc.2914. [DOI] [PubMed] [Google Scholar]
- 30.Teuten EL, Rowland SJ, Galloway TS, Thompson RC. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007;41:7759–7764. doi: 10.1021/es071737s. [DOI] [PubMed] [Google Scholar]
- 31.Rochman CM, Hentschel BT, Teh SJ. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS One. 2014;9:e85433. doi: 10.1371/journal.pone.0085433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.EFSA. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA panel on contaminants in the food chain (CONTAM). EFSA J.14, 4501 (2016).
- 33.Koelmans AA, Bakir A, Burton GA, Janssen CR. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016;50:3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohmann R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—but should microplastics be considered POPs themselves? Integr. Environ. Assess. Manag. 2017;13:460–465. doi: 10.1002/ieam.1914. [DOI] [PubMed] [Google Scholar]
- 35.Jie Y, et al. Pollution by nonylphenol in river, tap water, and aquatic in an acid rain-plagued city in southwest China. Int. J. Environ. Health Res. 2017;27:179–190. doi: 10.1080/09603123.2017.1332345. [DOI] [PubMed] [Google Scholar]
- 36.Xu J-F, Zhang J-Y, Jie C, Tang L, Chou K-C. Measuring and modeling of density for selected CaO-MgO-Al2O3-SiO2 slag with low silica. J. Iron. Steel Res. Int. 2012;19:26–32. doi: 10.1016/S1006-706X(12)60109-5. [DOI] [Google Scholar]
- 37.Williams DR, et al. Genomic resources and microarrays for the common carp Cyprinus carpio L. J. Fish Biol. 2008;72:2095–2117. doi: 10.1111/j.1095-8649.2008.01875.x. [DOI] [Google Scholar]
- 38.Saravanan M, PrabhuKumar K, Ramesh M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pesticide Biochem. Physiol. 2011;100:206–211. doi: 10.1016/j.pestbp.2011.04.002. [DOI] [Google Scholar]
- 39.Hamed M, Soliman HAM, Osman AGM, Sayed AE-DH. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. Blood biomarkers. Chemosphere. 2019;228:345–350. doi: 10.1016/j.chemosphere.2019.04.153. [DOI] [PubMed] [Google Scholar]
- 40.Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sayed AE-DH, Soliman HA. Modulatory effects of green tea extract against the hepatotoxic effects of 4-nonylphenol in catfish (Clarias gariepinus) Ecotoxicol. Environ. Saf. 2018;149:159–165. doi: 10.1016/j.ecoenv.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Wilson MM, Peng J, Dabiri JO, Eldredge JD. Lagrangian coherent structures in low Reynolds number swimming. J. Phys. Condens. Matter. 2009;21:204105. doi: 10.1088/0953-8984/21/20/204105. [DOI] [PubMed] [Google Scholar]
- 43.Hamed M, Monteiro CE, Sayed AE-DH. Investigation of the impact caused by different sizes of polyethylene plastics (nano, micro, and macro) in common carp juveniles, Cyprinus carpio L., using multi-biomarkers. Sci. Total Environ. 2022;803:149921. doi: 10.1016/j.scitotenv.2021.149921. [DOI] [PubMed] [Google Scholar]
- 44.Sayed AE-DH, Hamed M, Soliman HAM. Spirulina platensis alleviated the hemotoxicity, oxidative damage and histopathological alterations of hydroxychloroquine in catfish (Clarias gariepinus) Front. Physiol. 2021 doi: 10.3389/fphys.2021.683669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SPSS. SPSS for windows step by step: A simple study guide and reference, 17.0 update, 10/e. (Pearson Education India, 1998).
- 46.Ammar E, Mohamed MS, Sayed AE-DH. Polyethylene microplastics increases the tissue damage caused by 4-nonylphenol in the common carp (Cyprinus carpio) juvenile. Front. Marine Sci. 2022 doi: 10.3389/fmars.2022.1041003. [DOI] [Google Scholar]
- 47.Thummabancha K, Onparn N, Srisapoome P. Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J. Immunotoxicol. 2016;13:909–917. doi: 10.1080/1547691x.2016.1242673. [DOI] [PubMed] [Google Scholar]
- 48.Ali HAJ, Rani VJ. Effect of phosalone on haematological indices in the tilapia, Oreochromis mossambicus. Turk. J. Vet. Anim. Sci. 2009;33:407–411. [Google Scholar]
- 49.Soliman HAM, Hamed M, Sayed AE-DH. Investigating the effects of copper sulfate and copper oxide nanoparticles in Nile tilapia (Oreochromis niloticus) using multiple biomarkers: The prophylactic role of Spirulina. Environ. Sci. Pollut. Res. 2021;28:30046–30057. doi: 10.1007/s11356-021-12859-0. [DOI] [PubMed] [Google Scholar]
- 50.Osman A, Hamed M, Sayed A. Protective role of Spirulina platensis against UVA-induced haemato-biochemical and cellular alterations in Clarias gariepinus. J. Photochem. Photobiol., B. 2019;191:59–64. doi: 10.1016/j.jphotobiol.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Mekkawy IA, Mahmoud UM, Sayed AE-DH. Effects of 4-nonylphenol on blood cells of the African catfish Clarias gariepinus (Burchell, 1822) Tissue Cell. 2011;43:223–229. doi: 10.1016/j.tice.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 52.AbouKhalil N, Abd-Elkareem M, Sayed A. Nigella sativa seed protects against 4-nonylphenol-induced haematotoxicity in Clarias gariepinus (Burchell, 1822): Oxidant/antioxidant rebalance. Aquacul. Nutrit. 2017;23:1467–1474. doi: 10.1111/anu.12522. [DOI] [Google Scholar]
- 53.Wintrobe, M. M. Clinical Haematology, London, H, 448 (1978).
- 54.Omoregie E. Changes in the haematology of the Nile tilapia Oreochromis niloticus Trewavas under the effect of crude oil. Acta Hydrobiol. 1998;40:287–292. [Google Scholar]
- 55.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osman AG, et al. Blood biomarkers in Nile tilapia Oreochromis niloticus niloticus and African catfish Clarias gariepinus to evaluate water quality of the river Nile. J. FisheriesSciences. com. 2018;12:1–15. doi: 10.21767/1307-234X.1000141. [DOI] [Google Scholar]
- 57.Tort L, Torres P, Flos R. Effects on dogfish haematology and liver composition after acute copper exposure. Compar. Biochem. Physiol. Part C Comp. Pharmacol. 1987;87:349–353. doi: 10.1016/0742-8413(87)90020-X. [DOI] [PubMed] [Google Scholar]
- 58.Alkaladi A, El-Deen NAMN, Afifi M, Zinadah OAA. Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J. Biol. Sci. 2015;22:556–563. doi: 10.1016/j.sjbs.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mukherjee J, Sinha G. Cadmium toxicity on haematological and biochemical aspects in an Indian freshwater major carp, Labeo rohita(Hamilton) J. Freshw. Biol. 1993;5:245–251. [Google Scholar]
- 60.Espinosa C, Cuesta A, Esteban MÁ. Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2017;68:251–259. doi: 10.1016/j.fsi.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Sayed AE-DH, Hamed HS. Induction of apoptosis and DNA damage by 4-nonylphenol in African catfish (Clarias gariepinus) and the antioxidant role of Cydonia oblonga. Ecotoxicol. Environ. Saf. 2017;139:97–101. doi: 10.1016/j.ecoenv.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 62.Banaee M, Sureda A, Zohiery F, Nematdoust Hagi B, Garanzini DS. Alterations in biochemical parameters of the freshwater fish, alburnusmossulensis, exposed to sub-lethal concentrations of fenpropathrin. Int. J. Aquat. Biol. 2014;2:58–68. [Google Scholar]
- 63.Nematdoost Haghi B, Banaee M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int. J. Environ. Sci. Technol. 2017;14:521–530. doi: 10.1007/s13762-016-1171-4. [DOI] [Google Scholar]
- 64.Banaee M, Haghi BN, Tahery S, Shahafve S, Vaziriyan M. Effects of sub-lethal toxicity of paraquat on blood biochemical parameters of common carp, Cyprinus carpio (Linnaeus, 1758) Iran. J. Toxicol. 2016;10:1–5. doi: 10.29252/arakmu.10.6.1. [DOI] [Google Scholar]
- 65.Oliveira M, Ribeiro A, Hylland K, Guilhermino L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Ecol. Ind. 2013;34:641–647. doi: 10.1016/j.ecolind.2013.06.019. [DOI] [Google Scholar]
- 66.Norberto, R. & Guilhermino, L. Toxicological interactions of microplastics and nickel in early juveniles of the common goby (Pomatoschistus microps), ECSA 54 conference – Coastal systems under change: tuning assessment and management tools. 12–16. (Portugal, 2014).
- 67.Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K. Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss) Pestic. Biochem. Physiol. 2011;99:1–6. doi: 10.1016/j.pestbp.2010.09.001. [DOI] [Google Scholar]
- 68.Stoyanova S, et al. Multi-biomarker assessment in common carp (Cyprinus carpio, Linnaeus 1758) liver after acute chlorpyrifos exposure. Water. 2020;12:1837. doi: 10.3390/w12061837. [DOI] [Google Scholar]
- 69.Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K. Biochemical and histological changes in the liver tissue of rainbow trout (Oncorhynchus mykiss) exposed to sub-lethal concentrations of diazinon. Fish Physiol. Biochem. 2013;39:489–501. doi: 10.1007/s10695-012-9714-1. [DOI] [PubMed] [Google Scholar]
- 70.Ramesh M, Anitha S, Poopal RK, Shobana C. Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol. Rep. 2018;5:18–27. doi: 10.1016/j.toxrep.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peralta CA, et al. Detection of chronic kidney disease With creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramos-Barron MA, et al. Assessment of kidney graft function evolution measured by creatinine and cystatin C. Transpl. Proc. 2016;48:2913–2916. doi: 10.1016/j.transproceed.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 73.Wiegertjes GF, Stet RM, Parmentier HK, van Muiswinkel WB. Immunogenetics of disease resistance in fish: A comparative approach. Dev. Comp. Immunol. 1996;20:365–381. doi: 10.1016/S0145-305X(96)00032-8. [DOI] [PubMed] [Google Scholar]
- 74.Rosety-Rodriguez M, Javier Ordonez F, Rosety I, Rosety JM, Rosety M. Erythrocyte antioxidant enzymes of gilthead as early-warning bio-indicators of oxidative stress induced by malathion. HAEMA. 2005;8:237–240. [Google Scholar]
- 75.Lu Y, et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- 76.Pitt JA, et al. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio) Aquat. Toxicol. 2018;194:185–194. doi: 10.1016/j.aquatox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L.) Environ. Sci. Technol. 2008;42:5026–5031. doi: 10.1021/es800249a. [DOI] [PubMed] [Google Scholar]
- 78.Ding J, et al. Gas phase dehydration of glycerol to acrolein over Cs2.5H0.5PW12O40/Zr-MCM-41 catalysts prepared by supercritical impregnation. Mol. Catal. 2018;461:1–9. doi: 10.1016/j.mcat.2018.09.021. [DOI] [Google Scholar]
- 79.Singh D, Nath K, Sharma Y, Trivedi S. Hepatotoxic effect of Cu (II) in freshwater fish, Channa punctatus: A histopathological study. Res. Environ. Life Sci. 2008;1:13–16. [Google Scholar]
- 80.Hamed M, Soliman HAM, Sayed AE-DH. Ameliorative effect of Spirulina platensis against lead nitrate–induced cytotoxicity and genotoxicity in catfish Clarias gariepinus. Environ. Sci. Pollut. Res. 2019;26:20610–20618. doi: 10.1007/s11356-019-05319-3. [DOI] [PubMed] [Google Scholar]
- 81.Sayed AE-DH, Kataoka C, Oda S, Kashiwada S, Mitani H. Sensitivity of medaka (Oryzias latipes) to 4-nonylphenol subacute exposure; erythrocyte alterations and apoptosis. Environ. Toxicol. Pharmacol. 2018;58:98–104. doi: 10.1016/j.etap.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 82.Ayllon F, Garcia-Vazquez E. Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: an assessment of the fish micronucleus test. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis. 2000;467:177–186. doi: 10.1016/S1383-5718(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 83.Ergene S, et al. Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicology. 2007;16:385–391. doi: 10.1007/s10646-007-0142-4. [DOI] [PubMed] [Google Scholar]
- 84.Gomes JMM, et al. What the erythrocytic nuclear alteration frequencies could tell us about genotoxicity and macrophage iron storage? PLoS One. 2015;10:e0143029. doi: 10.1371/journal.pone.0143029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sayed AH, Abdel-Tawab HS, Abdel Hakeem SS, Mekkawy IA. The protective role of quince leaf extract against the adverse impacts of ultraviolet-A radiation on some tissues of Clarias gariepinus (Burchell, 1822) J. Photochem. Photobiol. B Biol. 2013;119:9–14. doi: 10.1016/j.jphotobiol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Sayed AE-DH, El-Sayed YS, El-Far AH. Hepatoprotective efficacy of Spirulina platensis against lead-induced oxidative stress and genotoxicity in catfish; Clarias gariepinus. Ecotoxicol. Environ. Saf. 2017;143:344–350. doi: 10.1016/j.ecoenv.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 87.Sayed AE-DH, Watanabe-Asaka T, Oda S, Mitani H. Apoptosis and morphological alterations after UVA irradiation in red blood cells of p53 deficient Japanese medaka (Oryzias latipes) J. Photochem. Photobiol., B. 2016;161:1–8. doi: 10.1016/j.jphotobiol.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 88.Sayed AE-DH, Zaki RM, El-Dean AMK, Abdulrazzaq AY. The biological activity of new thieno[2,3-c]pyrazole compounds as anti-oxidants against toxicity of 4-nonylphenol in Clarias gariepinus. Toxicol. Rep. 2015;2:1445–1453. doi: 10.1016/j.toxrep.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sharma M, Chadha P, Sharma S. Acute and sub chronic exposure of 4-nonylphenol to freshwater fish channa punctatus to evaluate its cytotoxicity. Biochem. Cell. Arch. 2014;14:363–367. [Google Scholar]
- 90.Al-Sabti K, Metcalfe CD. Fish micronuclei for assessing genotoxicity in water. Mutation Res. Genet. Toxicol. 1995;343:121–135. doi: 10.1016/0165-1218(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 91.Mato Y, et al. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001;35:318–324. doi: 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- 92.Cole M, et al. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- 93.Koelmans AA, Besseling E, Wegner A, Foekema EM. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environ. Sci. Technol. 2013;47:7812–7820. doi: 10.1021/es401169n. [DOI] [PubMed] [Google Scholar]
- 94.Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO. Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes Compressa. Environ. Sci. Technol. 2014;48:8127–8134. doi: 10.1021/es405717z. [DOI] [PubMed] [Google Scholar]
- 95.Gouin T, Roche N, Lohmann R, Hodges G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environ. Sci. Technol. 2011;45:1466–1472. doi: 10.1021/es1032025. [DOI] [PubMed] [Google Scholar]
- 96.Fries E, Zarfl C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE) Environ. Sci. Pollut. Res. 2012;19:1296–1304. doi: 10.1007/s11356-011-0655-5. [DOI] [PubMed] [Google Scholar]
- 97.Khan FR, Syberg K, Shashoua Y, Bury NR. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio) Environ. Pollut. 2015;206:73–79. doi: 10.1016/j.envpol.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Giusti L, Hamilton Taylor J, Davison W, Hewitt CN. Artefacts in sorption experiments with trace metals. Sci. Total Environ. 1994;152:227–238. doi: 10.1016/0048-9697(94)90314-X. [DOI] [Google Scholar]
- 99.Fischer AC, Kroon JJ, Verburg TG, Teunissen T, Wolterbeek HT. On the relevance of iron adsorption to container materials in small-volume experiments on iron marine chemistry: 55Fe-aided assessment of capacity, affinity and kinetics. Mar. Chem. 2007;107:533–546. doi: 10.1016/j.marchem.2007.08.004. [DOI] [Google Scholar]
- 100.Martins A, Guilhermino L. Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci. Total Environ. 2018;631–632:421–428. doi: 10.1016/j.scitotenv.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 101.Dacie JV, Lewis SM. Practical haematology. London: ELBS with Churchill Livingston; 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the research article.