Abstract
Background
Reflection spectroscopy, utilized by the Veggie Meter, is a less-expensive, noninvasive method to quantify skin carotenoids and is a valid approximation of fruit and vegetable (FV) intake. However, it is unknown to what degree Veggie Meter–assessed skin carotenoid score change is responsive to changes in carotenoid intake.
Objectives
This study aimed to evaluate Veggie Meter–assessed skin carotenoid score response in a 6-wk randomized controlled trial of a carotenoid-containing juice to determine whether the Veggie Meter can be used to detect nutritionally relevant changes in carotenoid intake; and to compare skin and plasma carotenoid responses with the 6-wk trial.
Methods
In this 6-wk trial, participants (n = 162) who self-identified as one of 4 US racial/ethnic groups (25% Black, 25% Asian, 27% non-Hispanic White, 23% Hispanic) were randomized to a control group, receiving negligible carotenoids (177 mL apple juice/d), moderate-dose group, receiving 4 mg total carotenoids/d (177 mL orange–carrot juice/d), or high-dose group, receiving 8 mg total carotenoids/d (355 mL orange–carrot juice/d). Skin carotenoid score and plasma total carotenoid concentrations (α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, zeaxanthin) were assessed at baseline, 3 wk, and 6 wk (n = 158 completed the trial). Repeated measures linear models were used to examine skin and plasma carotenoids over time and between groups.
Results
At 6 wk, participants in the high-dose and moderate-dose groups had significantly higher mean skin carotenoid scores [414.0 (SD = 100.6) and 369.7 (SD = 100.3), respectively] compared with those in the control group [305.2 (100.5)]. In the high-dose group, there was a 42% change in skin carotenoids from baseline (mean = 290.4) to a 6-wk follow-up (increase of 123, 123/290 = 42.4%). There was a 61% change in the plasma carotenoids in the high-dose group.
Conclusions
The Veggie Meter is sensitive to increases in daily carotenoid intake in diverse racial/ethnic groups over 6 wk.
Clinical Trials Registry number
This trial was registered at clinicaltrials.gov as ID: NCT04056624. Study URL: https://clinicaltrials.gov/ct2/show/NCT04056624
Keywords: skin carotenoids, Veggie Meter, fruit and vegetable intake, plasma carotenoids
Introduction
Adequate fruit and vegetable (FV) intake is important for optimal health, with higher FV intake being associated with lower risks of cardiovascular disease [1,2], diabetes [3,4], and some cancers [2,5]. Because it was found that Americans do not consume adequate amounts of FV [6,7]. Therefore, to evaluate the effectiveness of programs and policies designed to increase FV intake, there is need for objective, noninvasive FV intake measurement methods [8,9]. The current gold standard biomarker of FV intake is blood carotenoid concentration – a relatively expensive and invasive measurement [10]. Skin carotenoid measurement is emerging as a method to noninvasively, yet objectively, assess the intake of carotenoid-rich FV [11]. The Veggie Meter is a device that uses pressure-mediated reflection spectroscopy (RS) to assess skin carotenoids [12]. Our previous cross-sectional validation study showed a positive correlation of 0.71 (P < 0.001) between total plasma carotenoids and Veggie Meter–assessed skin carotenoids, and a correlation of 0.37 (P < 0.001) between food frequency–assessed FV intake and skin carotenoids among 213 adults with diverse racial/ethnic identities [13]. Additional cross-sectional studies have found positive correlations between total plasma carotenoids and skin carotenoids, ranging from 0.60, P < 0.01 [14] to 0.70, P < 0.001 [15] to r = 0.81 [12]. Additional cross-sectional studies also found positive associations between self-reported FV intake and Veggie Meter–assessed skin carotenoids [[16], [17], [18], [19]], although results have been mixed [20,21].
Although the Veggie Meter has shown promise as a tool for noninvasive outcome assessment in nutrition intervention studies, the interpretation of changes in Veggie Meter–assessed carotenoid levels is challenging because of the lack of robust data on the sensitivity of the Veggie Meter to controlled changes in carotenoid intake. It is not known if, and to what degree, changes in Veggie Meter–assessed skin carotenoids are associated with changes in true carotenoid intake. Given the striking racial/ethnic disparities in diet-related chronic disease in the United States, and key social determinants of health that present substantial barriers to FV access and intake, it is especially important to understand the potential utility of the Veggie Meter to assess FV interventions in racially and ethnically diverse groups. Dragsted et al. [22] outlined 8 characteristics that comprise validity for biomarkers of food intake, including plausibility, dose–response, time–response, robustness, reliability, stability, analytical performance, and reproducibility. Although research has been conducted to determine the plausibility and dose–response [[12], [13], [14], [15],23], more research is needed to further examine the validity and sensitivity of the measure to understand other components of validity such as time–response and robustness [22]. Therefore, the purpose of this study was to evaluate Veggie Meter–assessed skin carotenoid response in a 6-wk randomized controlled trial of a carotenoid-containing juice to determine whether the Veggie Meter can be used to sensitively detect change in carotenoid-rich FV intake, particularly among a racially and ethnically diverse US population by comparing skin carotenoid status at baseline to skin carotenoid status at 3 and 6 wk, modeling short-term dietary interventions. Secondary outcomes were changes in plasma carotenoids from baseline to 3 wk and baseline to 6 wk. The ultimate objective was to determine whether the Veggie Meter can be used to sensitively detect changes in carotenoid-rich FV intake.
Methods
Study design
Participants were randomly assigned to 1 of the 3 intervention groups in this 6-wk randomized controlled trial. Intervention groups were 1) control, negligible carotenoid group (Mott’s Apple Juice), 2) moderate-dose (V8 orange–carrot juice, providing 4 mg total carotenoids/d), and 3) high-dose (V8 orange–carrot juice/d, providing 8 mg total carotenoids/d). Participants attended 3 study visits during the 6-wk trial, first at baseline (wk 0), second at wk 3, and third at wk 6. The participants nor the researcher could be blinded to the study condition because the 2 juices were different in taste and color.
Participant recruitment
Participants were recruited between June 28, 2021, and April 14, 2022, by researchers at East Carolina University (Greenville, North Carolina), the University of Minnesota (Minneapolis/St. Paul, Minnesota metro area), and Baylor College of Medicine (Houston, Texas metro area). Participants were recruited in various ways including sending study information to previous research participants who agreed to be contacted for future studies, posting flyers at university and community locations, sharing information on social media, and including the study in searchable institutional research study databases. The study was approved by the East Carolina University Medical Center Institutional Review Board, in accordance with the Helsinki Declaration of 1975, as revised in 1983. Informed consent was obtained before participant enrollment. This trial was registered at clinicaltrials.gov as ID: NCT04056624.
Participants were eligible if they met the following criteria: self-identified as one of the following: African American/Black, Asian, White, or Hispanic/Latinx; able to speak, read, and understand English; BMI of 18.534.9 kg/m2; age 18–65 y; had no history of chronic disease, including cancer, cardiovascular disease, previous heart attack or stroke, diabetes, chronic kidney disease; not taking lipid-altering medication; not taking carotenoid-containing supplements providing > 2.0 mg carotenoids; not pregnant or lactating and not planning to become pregnant in the next 2 mo; not having fruit or vegetable allergies or intolerances; not dieting or planning to begin a diet; had not previously had or were planning to have weight-loss surgery within the next 24 mo; and weight stable. Participants also had to be willing and able to drink either juice (V8 orange–carrot juice or Mott’s Apple Juice) for the duration of the trial.
Final eligibility and enrollment
At the baseline visit, participants underwent an informed consent process before the study measures began. Next, height and weight were measured to objectively assess participant BMI and confirm eligibility. Height and weight were measured using a calibrated height board and scale (TANITA DC-430U Dual Frequency Total Body Composition Analyzer), respectively. Final eligibility included participants’ agreement to keep fingernail length ≤ 2 cm from the fingertip to the end of the nail (so the finger would fit in the Veggie Meter) for the study duration.
Randomization and intervention group
Participants were randomized into 1 of the 3 treatment groups stratified by self-identified racial/ethnic group for each study site. In total, 53 participants were randomized into the control group, 53 into the moderate-dose group, and 56 into the high-dose group (N = 162). Once randomized, participants were provided general nutrition education about the juice and advised on how to maintain an isocaloric diet while maintaining their normal physical activity.
Study retention
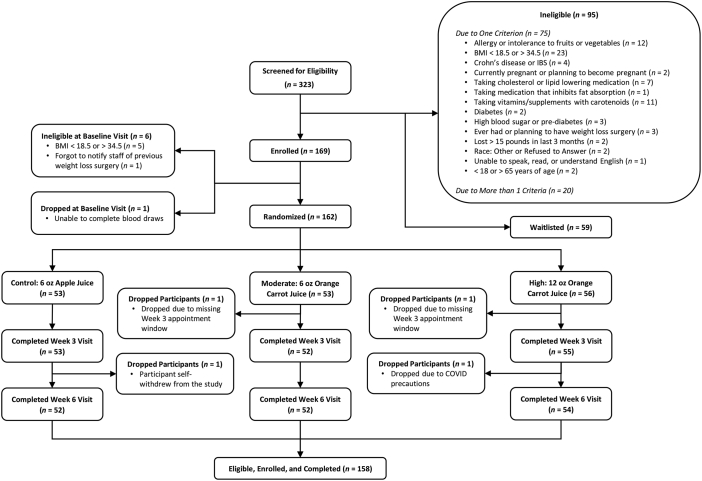
Across study sites, a total of 323 individuals were screened for eligibility, 95 were ineligible at screening and 59 were eligible but waitlisted and not enrolled in the study. Overall, 169 participants (52%) had a baseline visit and were enrolled (signed informed consent). Six participants were determined ineligible at the baseline visit (5 had an ineligible BMI, as determined via measured height and weight; 1 did not report previous weight-loss surgery during screening). An additional participant was removed from the study at baseline for their safety due to fainting during the blood draw. Ultimately, 162 participants were randomized, and 158 of 162 completed 6 wk of intervention (98%). Of the 4 participants lost to follow-up, 2 missed appointment windows (moved out of state or lost contact), 1 self-withdrew, and 1 tested positive for COVID-19 2 d before the wk 3 visit. See Figure 1 for the participant flow diagram.
FIGURE 1.
Participant flow diagram for a randomized controlled trial of a carotenoid-containing juice, among racially and ethnically diverse adults.
Overview of data collection measures
At each visit, participant weight and body fat percentage were measured (height was only measured at baseline). Participants were also assessed for 1) skin carotenoids using the Veggie Meter and 2) skin melanin index, hemoglobin index, and hemoglobin saturation index using the Konica Minolta CM-700d Spectrophotometer. Participants provided a 7- to 10-h fasted blood draw sample from which plasma carotenoids, cholesterol, and glucose were assessed. Participants completed the National Cancer Institute’s Diet History Questionnaire (past-month version III) and a questionnaire on demographics. Participants were provided with a financial incentive after each study visit.
Skin carotenoid measurement
The Veggie Meter uses pressure-mediated RS to quantify skin carotenoids on a scale from 0 to 800, with higher scores corresponding with greater skin carotenoids, generally suggesting greater carotenoid-rich FV intake [12,15,24]. The Veggie Meter was calibrated between participants following the manufacturer’s recommended protocol. Participants were instructed to first wash and dry their hands and place their right index finger into the device. This was repeated for a total of 3 readings with the average of the 3 used in the analyses. Participants removed their fingers between readings. Participants were not provided their skin carotenoid scores.
Skin melanin, hemoglobin, and hemoglobin saturation
These measurements were assessed as potential confounders or moderators of the association between plasma and skin carotenoids. Melanin index, hemoglobin index, and hemoglobin saturation index were measured using a portable spectrophotometer (CM-700d Spectrophotometer, Konica Minolta Sensing Americas, Inc) [[25], [26], [27], [28], [29]] with a skin analysis software package (CM-SA), which estimates skin pigment concentrations as previously described [30] on the participants’ right index finger pad and inner right forearm. Participants withdrew their fingers (or forearms) for each measurement, for a total of 3 measurements at each site. An average of the 3 readings was used in the analyses. The device was calibrated between participants following the manufacturer’s recommended protocol.
Plasma carotenoids, cholesterol, and glucose analyses
Plasma carotenoids were considered the criterion standard biomarker of FV intake; cholesterol and glucose were measured as potential confounders of the association between plasma and skin carotenoids. Each participant completed a fasting blood draw, with 6 mL whole blood collected by venipuncture. Blood was centrifuged within 15 min of collection at 2500 × g for 10 min at 4°C and plasma was aliquoted for downstream carotenoid, cholesterol, and glucose analyses. Samples were frozen at −80 °C within 3 h of processing and shipped to Eurofins Craft Technologies, Inc. in Wilson, NC, for analysis [13]. Carotenoid concentrations were measured by HPLC-photodiode array detection (PDA) and quantitated against authentic standard curves. Plasma HPLC quantified both E and Z isomers, which were summed to obtain total carotenoids.
Diet intake assessment
The Diet History Questionnaire (DHQ)-III was used to assess the background diet. The DHQ-III was developed by the National Cancer Institute and is a validated questionnaire including 135 foods/beverages and 26 dietary supplements [31]. Participants completed the past-month, with a portion size, version of the questionnaire online for each study visit. DHQ output was used to approximate self-reported FV intake by summing the total fruit and total vegetable variables created by the DHQ. Total carotenoid intake was estimated by summing the amounts of individual carotenoids available in the DHQ-III output (β-carotene, α-carotene, β-cryptoxanthin, lutein + zeaxanthin, lycopene).
Covariates
Participants completed surveys on demographics, sun exposure, tobacco use, and vitamin/supplement use. Demographic information was determined by verifying the date of birth, biological sex (male/female), and self-categorized race/ethnicity from the eligibility screener. Participants were also asked about marital status (single, living with partner/unmarried, married, separated, divorced, widowed), highest level of education, and annual household income. Because sun, UV, and tobacco exposure can affect skin carotenoid levels [32], self-reported UV light exposure from tanning beds/lamps and the sun was assessed using the previously validated survey items [33,34]. Tobacco use and exposure were assessed by a validated questionnaire [35,36], with items assessing whether the participant had smoked at least 100 cigarettes in their entire life; current smoking; use of chewing tobacco, snuff, or snus; and use of e-cigarettes, a vape pen, or an e-hookah. Finally, participants were asked about vitamin and supplement use. Vitamin and supplement types were documented at baseline, and their use was verified at subsequent visits. Participants also verified whether they began taking any new vitamins or supplements in the past wk at the wk 3 and wk 6 visits.
Juice intervention and compliance
Upon randomization (biostatistician generated racially/ethnically stratified randomization cards in sealed envelopes sent to each study site), and at the wk 3 visit, participants were given a 3-wk supply of juice, graduated plastic cups, a log for recording daily study juice intake, instructions, and an isocaloric handout. The juice intervention consisted of either 177 mL/d of apple juice (Mott’s Apple Juice, Mott’s LLP, Plano, Texas), 177 mL/d of orange–carrot juice (V8, Campbell Soup Company, Camden, New Jersey), or 355 mL/d of orange–carrot juice. These volumes of juices provided 0.0, 4.1, and 8.2 mg of total carotenoids, respectively. Participants in the high-dose group consumed 12 oz of V8 orange–carrot juice providing 8 mg of total carotenoids each day, an amount akin to consuming a cup of sweet potato, carrots, or cooked spinach. Likewise, those randomized to the moderate-dose group consumed 6 oz of V8 orange–carrot juice providing 4 mg total carotenoids each day, an amount similar to consuming a half cup of sweet potato, carrots, or cooked spinach. The carotenoid content and profile of the 3 juice treatments are presented in Table 1.
TABLE 1.
Carotenoid content of juice products given to the control (negligible), moderate-dose, and high-dose carotenoid intervention groups
| Carotenoid | Apple juice (mg/L) | Control, apple juice mg/6 fluid ounces (177 mL) | Orange–carrot juice (mg/L) | Moderate-dose, orange–carrot juice mg/6 fluid ounces (177 mL) | High-dose, orange–carrot juice mg/12 fluid ounces (355 mL) |
|---|---|---|---|---|---|
| α-Carotene | nd1 | nd | 7.899 | 1.398 | 2.804 |
| β-Carotene | 0.009 | 0.002 | 15.072 | 2.668 | 5.350 |
| Lutein | nd | nd | 0.032 | 0.006 | 0.011 |
| Zeaxanthin | nd | nd | 0.003 | 0.006 | 0.001 |
| Lycopene | nd | nd | 0.047 | 0.008 | 0.017 |
| Total carotenoids2 | 0.009 | 0.002 | 23.053 | 4.080 | 8.184 |
nd, not detected, means for triplicate analyses of 1 sample.
Sum of all listed carotenoid species.
The priorities when selecting the test juice included that it be a commercially available product that could be purchased in a minimal number of lots and provide a nutritionally relevant dose of carotenoids. The orange–carrot juice was selected after analyzing the carotenoid content of 9 commercially available, fruit and/or vegetable juice blends. These juices provided between <1 and 101 mg total carotenoids/L, with varying carotenoid profiles ranging from predominantly lycopene in tomato-based juices, to predominantly α- and β-carotene in carrot-containing juices. None of the juices had remarkable lutein, zeaxanthin, or β-cryptoxanthin content (all <0.5 mg/L). Given that previous cross-sectional findings of diet–skin carotenoid associations did not detect an association between dietary lycopene intake or plasma lycopene and RS-assessed skin carotenoid scores [13,37], an α- and β-carotene-rich orange–carrot juice was selected. The carotenoid content of the juices was monitored across the duration of enrollment and was not found to decrease over the course of storage.
During the intervention, participants were contacted weekly by their preferred method of contact with a compliance prompt. To determine compliance/adherence, participants completed juice logs. Participants were asked to record if they drank all their juice, some juice, or no juice every day on the log. If the participants only drank some juice, they were asked to record how much (in ounces) they consumed.
Juice carotenoid measurement and stability assessment
Each study site collected baseline juice samples and additional stability control samples at least every 6 mo, which were stored at −80°C until analysis. Juice carotenoid concentrations were measured by HPLC-PDA. Carotenoids were extracted as previously described [38] with the following modifications: Juice samples (500 μL) were vortexed with ethanol (500 μL), mixed with echinenone internal standard, then carotenoids were extracted 2 times with 2 mL of extraction solvent (hexanes/ethanol/acetone, 10:6:7). The upper organic phase was reserved, solvents were removed under reduced pressure, and samples were reconstituted in carrier solvent (methyl-tert-butyl ether:methanol, 1:1), and injected onto an HPLC-PDA detection system for carotenoid separation and quantitation as previously described [39]. The following carotenoids were quantified against authentic standard calibration curves: all trans β-carotene, all trans-/5-cis lycopene, all trans-lutein, all trans-zeaxanthin, and all trans-alpha-carotene (all standards purchased from Sigma–Aldrich).
Power calculation
Based on previous research [40], a sample size of 41 per intervention group was estimated to provide 90% power to detect 6-wk skin carotenoid change of 1.1 (SD = 0.2) vs. 1.3 (SD = 0.2) (the magnitude of change reported in Meinke et al. [40]) when a 1-sided 2-sample t-test was used with a significance level of 0.05. With an estimated 20% attrition, the goal was to enroll 52 per group, in each of the 3 conditions, for a total of 156 participants.
Statistics
An intent-to-treat analysis was conducted with a total sample of 162. Missing data at wk 3 and/or wk 6 follow-ups of 4 participants were automatically handled through maximum likelihood. Data were summarized in frequency (percentage) or mean (SD) for the whole sample and for each intervention group separately. Baseline differences in demographics and other biomarkers between the intervention groups were analyzed through either chi-squared tests or ANOVA tests. Changes in skin carotenoid status from baseline to wk 3 and baseline to wk 6 were used as an outcome. To correct the right-skewness in total plasma carotenoid concentrations, we applied a base-2 log transformation before computing the changes. The distributions of the variables were checked using graphical tools such as histograms and normal QQ-plots. The change in each of the skin carotenoid score and log2-transformed total plasma carotenoid concentrations (as a response) were analyzed using 2 general linear repeated measure models to test the effects of carotenoid dose with study site and season, and participant’s corresponding baseline carotenoid level, age, biological sex, race/ethnicity, education level, BMI, % body fat, total cholesterol level, arm melanin index, arm hemoglobin index, and daily sun exposure as potential covariates. The models treated observations from the same participant as repeated measures with an unstructured covariance matrix, which was assumed to be different across the intervention groups. Kenward–Roger degrees of freedom approximation method was implemented. We first tested the effects of carotenoid dose using Model 1 with the intervention group and follow-up time as 2 factors and their interaction term, and adjusted for the 2 design factors, study site, and participant’s race/ethnicity. In Model 2, we used a backward procedure for the selection of additional covariates for other possible confounding factors. Participants’ corresponding baseline skin or plasma carotenoid measurements and BMI achieved P values < 0.1 for either of the 2 responses so they were kept as additional covariates, whereas the other tested variables were not significant covariates. We also calculated sensitivity as the number of those classified as higher than median on both plasma and skin carotenoids, divided by the number of those classified as higher than median on plasma only, and specificity as the number of those classified as lower than median when on both plasma and skin carotenoids, divided the number of those classified as lower than median on plasma only. SAS version 9.4 (SAS Institute Inc.) was used for all analyses, and a statistical significance level of 0.05 was considered.
Results
Baseline participant characteristics
Table 2 provides summary statistics for demographics and other baseline measures both for the whole sample and for each intervention group. The sample had a mean (SD) age of 32.3 (11.1) y, BMI of 25.4 (3.8) kg/m2, Veggie Meter–assessed skin carotenoid score of 298.0 (97.1) units, and plasma total carotenoid concentration of 161.5 (69.8) μg/dL. Sex and education level were significantly different between the intervention groups.
TABLE 2.
Baseline characteristics of study participants (n = 162) overall and by the intervention group
| Variable | Category | Total sample (n = 162) |
Control group (n = 53) |
Moderate-dose group (n = 53) |
High-dose group (n = 56) |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Site | ECU | 61 (37.7) | 20 (37.7) | 20 (37.7) | 21 (37.5) |
| UMN | 41 (25.3) | 13 (24.5) | 13 (24.5) | 15 (26.8) | |
| BCM | 60 (37.0) | 20 (37.7) | 20 (37.7) | 20 (35.7) | |
| Season | 2021 Summer | 67 (41.4) | 24 (45.3) | 21 (39.6) | 22 (39.3) |
| 2021 Fall | 63 (38.9) | 17 (32.1) | 22 (41.5) | 24 (42.9) | |
| 2021 Winter – 2022 spring | 32 (19.7) | 12 (22.6) | 10 (18.9) | 10 (17.9) | |
| Sex∗ | Male | 79 (48.8) | 17 (32.1) | 33 (62.3) | 29 (51.8) |
| Female | 83 (51.2) | 36 (67.9) | 20 (37.7) | 27 (48.2) | |
| Race/ethnicity | Non-Hispanic African American/Black | 41 (25.3) | 13 (24.5) | 13 (24.5) | 15 (26.8) |
| Asian | 40 (24.7) | 13 (24.5) | 14 (26.4) | 13 (23.2) | |
| Non-Hispanic White | 44 (27.2) | 15 (28.3) | 14 (26.4) | 15 (26.8) | |
| Hispanic/Latinx | 37 (22.8) | 12 (22.6) | 12 (22.6) | 13 (23.2) | |
| Education∗ | Some college or less | 41 (25.3) | 9 (17.0) | 11 (20.8) | 21 (37.5) |
| College graduate | 121 (74.7) | 44 (83.0) | 42 (79.2) | 35 (62.5) | |
| Income | $39,999 or less | 43 (26.5) | 14 (26.4) | 12 (22.6) | 17 (30.4) |
| $40,000–$79,999 | 58 (35.8) | 21 (39.6) | 19 (35.9) | 18 (32.1) | |
| $80,000 or more | 48 (29.6) | 14 (26.4) | 18 (34.0) | 16 (28.6) | |
| Missing | 13 (8.0) | 4 (7.6) | 4 (7.6) | 5 (8.9) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (y) | 32.3 (11.1) | 31.3 (10.1) | 33.1 (11.8) | 32.45 (11.4) | |
| BMI (kg/m2) | 25.4 (3.8) | 24.6 (3.2) | 25.8 (4.2) | 25.9 (3.9) | |
| Skin carotenoid level | 298.0 (97.1) | 293.6 (100.9) | 310.8 (101.7) | 290.2 (89.1) | |
| Plasma carotenoid (μg/dL) | 161.5 (69.8) | 159.7 (66.7) | 162.8 (76.2) | 162.0 (67.8) | |
| Arm melanin index | 1.05 (0.50) | 1.02 (0.44) | 1.04 (0.54) | 1.08 (0.52) | |
| Arm hemoglobin index | 1.07 (0.25) | 1.03 (0.28) | 1.09 (0.23) | 1.11 (0.25) | |
| Arm hemoglobin saturation index | 42.8 (14.4) | 44.1 (12.6) | 42.7 (15.0) | 41.6 (15.6) | |
| Arm A | 6.43 (2.27) | 6.26 (2.12) | 6.37 (2.48) | 6.64 (2.21) | |
| Arm B | 16.8 (2.66) | 17.2 (2.43) | 16.7 (2.92) | 16.7 (2.63) | |
| Total sample (n = 162) | Control group (n = 53) | Moderate-dose group (n = 53) | High-dose group (n = 56) | ||
| Baseline nutrient intake | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Energy (kcal/d) | 1801 (1056) | 1640 (892) | 2016 (1232) | 1748 (1002) | |
| Protein (g/d) | 75.0 (51.1) | 69.3 (41.0) | 86.5 (68.1) | 69.4 (38.4) | |
| Total fat (g/d) | 70.5 (41.5) | 65.4 (37.5) | 78.6 (48.1) | 67.7 (37.8) | |
| Total fruit (cups/d) | 1.5 (2.0) | 1.5 (3.0) | 1.7 (1.6) | 1.2 (1.0) | |
| Total vegetables (cups/d) | 1.9 (1.5) | 2.0 (1.6) | 2.0 (1.6) | 1.82 (1.3) | |
| Total fruits and vegetables (cups/d) | 3.4 (2.9) | 3.5 (3.8) | 3.66 (2.6) | 3.02 (2.1) | |
| Cholesterol (mg/d)∗ | 270.6 (211.9) | 231.3 (151.4) | 327.6 (281.8) | 254.0 (172.5) | |
| Carbohydrates (g/d) | 210.9 (131.3) | 196.2 (128.1) | 238.4 (143.6) | 198.9 (119.9) | |
| Total sugars (g/d) | 89.3 (69.3) | 85.4 (90.3) | 99.2 (60.9) | 83.5 (51.8) | |
| Dietary fiber (g/d) | 19.6 (13.3) | 19.3 (13.9) | 21.4 (14.9) | 18.2 (10.8) | |
| Beta carotene (μg/d) | 4963 (5567) | 5127 (5905) | 4867 (5576) | 4898 (5322) | |
| Alpha carotene (μg/d) | 585.0 (809.7) | 561.7 (773.8) | 607.4 (697.4) | 585.8 (944.4) | |
| Beta cryptoxanthin (μg/d)∗ | 119.8 (157.5) | 88.9 (84.6) | 168.3 (232.1) | 103.0 (107.0) | |
| Lutein zeaxanthin (μg/d) | 5268 (7074) | 5946 (7997) | 5030 (7788) | 4852 (5294) | |
| Lycopene (μg/d) | 4951 (4621) | 4467 (3534) | 5849 (6291) | 4559 (3483) | |
| Total carotenoid intake (μg/d) | 15887 (15493) | 16191 (16268) | 16521 (18015) | 14998 (12011) |
Indicates statistically significant differences between the groups. ECU, East Carolina University; UMN, University of Minnesota; BCM, Baylor College of Medicine.
Intervention compliance
Based on compliance logs, those randomized to the control group consumed 98.3% (min: 85.7%, max: 100%) of the prescribed dose during the first 3 wk and 98.0% (min: 81.0%, max: 100%) during the second 3 wk, on average. Among those randomized to the moderate-dose group, 97.8% (min: 118 mL/d; max: 190 mL/d) of the prescribed dose was consumed during the first 3 wk, and 96.8% (min: 93 mL/d; max: 186 mL/d) was consumed in the second 3 wk, on average. Among those randomized to the high-dose group, 97.8% (min: 248 mL/d; max: 387 mL/d) of the prescribed dose was consumed during the first 3 wk and 95.9% (min: 229 mL/d: max: 355 mL/d) in the second 3 wk.
Change in skin and plasma carotenoids
Supplemental Table 1shows unadjusted means and SDs of skin carotenoid scores and plasma total carotenoid concentrations (in the original scale) for each intervention group and study timepoint. We present summaries for the whole sample and for each racial/ethnicity group. Both skin carotenoid score and plasma carotenoid concentration mean values increased between baseline and wk 3 and 6 in the moderate-dose and high-dose groups and stayed similar between baseline and wk 3 and 6 in the control group. These trends were seen in the total sample and in each of the 4 racial/ethnic groups.
Intervention effect on skin carotenoids
Table 3 shows the results for regression model-estimated mean changes in skin carotenoids at wk 3 and 6, by intervention groups. In Model 1 (adjusted for site and race/ethnicity, both design variables), there was a statistically significant (P < 0.001) time-by-dose interaction effect. The mean changes were significantly (P < 0.001) different among the 3 intervention groups as well as between wk 3 and 6 in both moderate-dose and high-dose groups. The participants’ mean skin carotenoids increased in a linear fashion and faster in the high-dose group than in the moderate-dose group (Figure 2A, B). In the moderate-dose group, there was a 20% increase from the baseline (mean = 290.4 skin carotenoid units) to a 6-wk follow-up (increase of 59 skin carotenoid units, 20.3%). In the high-dose group, there was a 42% increase from the baseline (mean = 290.4 skin carotenoid units) to a 6-wk follow-up (increase of 123 skin carotenoid units, 42.4%). Results from Model 2 (adjusted for site, race/ethnicity, baseline BMI, and baseline skin carotenoid level) were similar to those of Model 1. Study site and race/ethnicity group were significant covariates in Model 1 for the change in skin carotenoids but not for the change in plasma carotenoids. In Model 2, the study site and baseline skin carotenoid score were significant covariates of the change in skin carotenoid score, whereas BMI and baseline plasma carotenoid concentrations were significant covariates of the change in plasma carotenoid concentration. Other tested variables were not significant covariates. More details on the models are included in Supplemental Table 2.
TABLE 3.
Model-estimated mean change (95% CI) in skin carotenoids and model-estimated percentage change in geometric mean (95% CI) plasma carotenoids at wk 3 and wk 6 after the intervention
| Model | group/effects | Mean changes in skin carotenoid score |
% Change in geometric means of plasma total carotenoid concentration |
||||
|---|---|---|---|---|---|---|---|
| Wk 3 | Wk 6 | Between time P | Wk 3 | Wk 6 | Between time P | ||
| 1 | Control | 9.34 (-−1.14, 19.8) | 11.9 (0.16, 23.6) | 0.582 | 1% (−3%, 6%) | 1% (−5%, 7%) | 0.922 |
| 1 | Moderate dose | 28.0 (15.6, 40.5) | 59.0 (42.0, 76.1) | <0.001 | 26% (20%, 32%) | 30% (23%, 38%) | 0.136 |
| 1 | High dose | 62.3 (49.6, 74.9) | 123 (105, 141) | <0.001 | 51% (40%, 62%) | 61% (49%, 73%) | 0.015 |
| 1 | Between group P | <0.001 | <0.001 | <0.0011 | <0.001 | <0.001 | 0.1481 |
| 2 | Control | 8.24 (−2.59, 19.1) | 10.8 (−1.03, 22.6) | 0.580 | 1% (−3%, 6%) | 1% (−4%, 7%) | 0.923 |
| 2 | Moderate dose | 30.1 (18.1, 42.0) | 61.1 (44.8, 77.5) | <0.001 | 27% (21%, 32%) | 31% (24%, 39%) | 0.136 |
| 2 | High dose | 62.3 (50.0, 74.5) | 123 (106, 141) | <0.001 | 52% (42%, 63%) | 62% (51%, 75%) | 0.014 |
| 2 | Between group P | <0.001 | <0.001 | <0.0011 | <0.001 | <0.001 | 0.1431 |
Note: The estimated mean and % changes are computed by averaging across sites and race/ethnicities and at the means of the BMI and baseline level. Model 1: change in outcome = site + race + group + time + group×time; Model 2: change in outcome = site + race + baseline BMI + baseline outcome + group + time + group×time; 1: P values for the interaction effect of the group×time.
FIGURE 2.
Changes in skin carotenoid scores and total plasma carotenoid concentrations over 6 wk in 3 intervention groups. (A) Original skin carotenoids, (B) model-estimated mean change in skin carotenoids, (C) original plasma carotenoids, and (D) model-estimated mean change in plasma carotenoids.
Intervention effect on plasma carotenoids.
Table 3 shows regression model-estimated percentage of changes in the geometric mean of plasma total carotenoid concentrations at wk 3 and 6, by intervention groups. In Model 1 (adjusted for site and race/ethnicity), the interaction between time and dose was not statistically significant (P = 0.15). The percentage of changes differed significantly among the 3 intervention groups (P < 0.001) as well as between wk 3 and 6 in the high-dose group (P = 0.01). For plasma carotenoids, there was a plateau effect after 3 wk of intervention (Figure 2C, D). In the moderate-dose group, there was a 30% increase from baseline to the 6-wk follow-up. In the high-dose group, there was a 61% increase from baseline to 6-wk follow-up. Results from Model 2 (adjusted for site, race, baseline BMI, and baseline log2-transformed plasma total carotenoid level) were similar to those of Model 1. More details on the models are included in Supplemental Table 2.
Sensitivity and specificity
For those randomized to the moderate group, sensitivity at wk 6 was 0.50, and specificity was 0.50. In the high-dose group, sensitivity was 0.67 and specificity was 0.69. These numbers show a moderate-to-low agreement between the responses in skin and plasma carotenoids to the intervention, which echoes the 2 correlations in Supplemental Table 3.
Supplemental Table 3 shows the correlations between changes in skin and plasma carotenoids. There was a correlation of r = 0.44 (P < 0.001) between change in skin carotenoids and change in log2-transformed plasma carotenoids in the high-dose group at wk 6. This correlation was 0.08 in the moderate-dose group.
Discussion
The aim of this study was to examine skin carotenoid responses to nutritionally relevant increases in carotenoid intake in a sample of adults with racially and ethnically diverse identities. There was a statistically significant dose–response relationship between the intake of carotenoid-rich juice and skin carotenoids, comparable to that between the intake of carotenoid-rich juice and plasma carotenoids. Among those in the high-dose group consuming 8 mg of additional daily carotenoids for 6 wk, there was a corresponding 123 [95% CI = (105, 141)] unit increase in RS-assessed skin carotenoid score. The dose–response was evident at both moderate and high doses. This indicates that the skin carotenoid score, as assessed by the Veggie Meter, has potential as a noninvasive objective measure of change in carotenoid-rich FV intake.
Dragsted et al. [22] outlined plausibility, dose–response, time–response, robustness, reliability, stability, analytical performance, and reproducibility as 8 important features that make up validity of a biomarker of food intake. The current study addresses several of these including plausibility (it is plausible that skin carotenoids respond to carotenoid intake), dose–response (demonstrating a dose–response relationship between the control, moderate, and high-dose groups), and time–response (examining responses at both 3 and 6 wk). We also examined the biomarker’s robustness, as criterion-related validity of the biomarker was previously assessed in a cross-sectional study [13], and now has been shown in a dietary intervention study, as well as reliability (demonstrating comparability to the gold standard of plasma carotenoids). More work is needed to ensure the analytical performance and reproducibility of the measure in different conditions.
Another noteworthy finding is that plasma carotenoids increased more quickly than skin carotenoids, showing significant treatment effects by 3 wk and plateauing after 3 wk in both the moderate- and high-dose carotenoid treatment groups. In contrast, skin carotenoids did not plateau between wk 3 and 6 in the moderate- or high-dose treatment groups. Changes in skin and plasma were not strongly correlated, likely reflecting these timing differences in response, which may be a function of differences in distribution and clearance kinetics between the 2 tissue compartments. Whether the 2 biomarkers would be more strongly correlated after reaching steady-state conditions is unknown. Overall, this indicates that study duration and expected timeframe for increasing carotenoid intake are key variables to consider when using skin carotenoid measures to assess changes in dietary carotenoid intake.
One additional study has examined the sensitivity of the Veggie Meter to controlled fruit and vegetable intake changes, using a lycopene-rich juice [23]. The study findings of Casperson et al. [23] indicated that the Veggie Meter can sensitively detect change in carotenoid intake. Their study findings demonstrate that the high dose of 31.0 additional milligrams of carotenoid per day (1.5 mg β-carotene, 0.4 mg α-carotene, 0 β-cryptoxanthin, 28.1 mg lycopene, and 1.1 mg lutein/zeaxanthin) resulted in a significant time × dose interaction, and an increase of 114 units on the Veggie Meter, whereas in the current study, the high-dose group with 8.2 additional milligrams of carotenoid per day (5.4 mg β-carotene, 2.8 mg α-carotene, 0 β-cryptoxanthin, 0.02 mg lycopene, and 0.01 mg lutein/zeaxanthin) also resulted in a significant time × dose interaction, and an increase of 123 units on the Veggie Meter. The differential dose–response (31 mg additional carotenoid produced a change of 114 Veggie Meter units for Casperson et al., whereas 8 mg additional carotenoid produced a change of 123 Veggie Meter units for the current study) may be due to the fact that Casperson et al.’s study used a lycopene-rich juice [23], whereas the current study used an α- and β-carotene-rich juice. Future studies should further examine Veggie Meter sensitivity for the different carotenoid species.
Others have examined change in resonance Raman spectroscopy (RRS)-assessed skin carotenoids with supplementation over time. In an 8-wk randomized trial, Aguilar et al. [41] determined that daily increases of 30 mL of carotenoid-rich juice (2.8 mg carotenoid) resulted in an average increase of 56 Raman intensity counts. During an 8-wk controlled feeding trial where participants were provided 62 mg total carotenoids from an average of 6-cup equivalents of daily fruits and vegetables, Jahns et al. [42] found that RRS-measured skin carotenoids increased by 264% during the feeding phase. In a 12-wk study where women took Betatene (24 mg/d of β-carotene from an algal source), Stahl et al. [43] found increases in skin and plasma carotenoid levels, similar to what was found in our current study. These studies suggest that skin carotenoids are responsive to changing dietary carotenoid exposures, and as such, provide a noninvasive carotenoid concentration biomarker alternative to plasma carotenoids as an outcome measure in studies evaluating changes in FV intake.
Peer-reviewed public health nutrition studies are increasingly likely to use RS-assessed skin carotenoids over time to detect changes in carotenoid-rich FV intake within study participants: Bayles et al. [44] found that RS-assessed skin carotenoid scores decreased less in an intervention group compared with a control group in a preschool healthy eating intervention. DiNoia et al. [45] found an intervention versus control group difference of ∼24 RS-assessed skin carotenoid units after a farm-to-Special Supplemental Nutrition Program for Women, Infants, and Children intervention promoting vegetable intake. However, previously, it was unknown what a difference of 20–25 skin carotenoid units meant in terms of changes in FV intake. The current study provides more details on the magnitude of skin carotenoid response to 8 mg of additional carotenoid intake per day, with the high-dose intervention resulting in a mean skin carotenoid change of 123 skin carotenoid units over 6 wk.
This study is not without limitations. First, although we found increases in skin carotenoids in response to moderate and high doses of carotenoid-rich juices in each of the 4 racial/ethnic identity groups examined in this study, the study was not statistically powered to examine whether there were statistically significant differences in the magnitude of increase between racial/ethnic identity groups. In contrast, the study was adequately powered to detect an overall response in a racially/ethnically diverse sample, which is an important contribution to the literature. We excluded people with certain chronic health conditions (eg, cardiovascular disease, diabetes); this limits the generalizability of study results to the larger population. However, it is important to include such participants in future research, given the high rates of obesity, diabetes, and other diet-related diseases. Furthermore, consumption of the test juice does not fully represent carotenoid-rich fruit and vegetable intake. The study juice was rich in α- and β-carotene, and thus, we do not know how the Veggie Meter would perform in relation to the intake of other dietary carotenoids, and the Veggie Meter does not discriminate between different carotenoid species or geometric conformations; future research should investigate the kinetics of skin carotenoid responses to different dietary carotenoid species and amounts in participants of varying age groups and health conditions. The major strengths of the study were that the sample was racially and ethnically diverse, and compliance with the intervention juice intake was very high.
We found a significant response in RS (Veggie Meter)–assessed skin carotenoid scores to nutritionally relevant increases in carotenoid intake. Over a 6-wk study period, participants consuming 4 and 8 mg of carotenoids daily from a carotenoid-rich juice experienced 20% and 42% unit increases in skin carotenoid scores, respectively. Thus, Veggie Meter–assessed skin carotenoids are a valid, objective dietary exposure biomarker. Future studies can use the current study results to conduct power analyses for intervention studies using Veggie Meter–assessed skin carotenoids as an outcome measure, and to interpret observed changes in skin carotenoids over time.
Acknowledgments
We gratefully acknowledge assistance from Patty Brophy, Angela Clark, Gabriel Dubis, Brianna Alexander, Amanda Adam, Shivanki Juneja, and Varsha Varghese.
The authors’ responsibilities were as follows – SJP, NEM, MNL, QW, LH: conceptualized the study. PCM, EG: created data collection forms. SM, PCM, EG, JC, YZ, AG, LB: recruited study participants and collected and managed study data. NC: advised on plasma carotenoid interpretation and analysis. QW: completed all statistical analyses. SJP, EG: completed the initial manuscript draft. All authors interpreted data analyses, suggested formats for presentation of results, and revised the manuscript for critical intellectual content. All authors: read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.02.017.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending appropriate Institutional Review Board approval and review of and approval from the investigators on the current project.
Funding
This study was funded by the National Institutes of Health (R01 HL142544) and the USDA/ARS (cooperative agreement 3092-51000-059-NEW2S, to NEM). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or USDA.
Author disclosures
The authors report no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wang X., Ouyang Y., Liu J., Zhu M., Zhao G., Bao W., et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose–response meta-analysis of prospective cohort studies. BMJ. 2014:349. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aune D., Giovannucci E., Boffetta P., Fadnes L.T., Keum N., Norat T., et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality – a systematic review and dose–response meta-analysis of prospective studies. Int. J. Epidemiol. 2017;46(3):1029–1056. doi: 10.1093/ije/dyw319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partula V., Deschasaux M., Druesne-Pecollo N., Latino-Martel P., Desmetz E., Chazelas E., et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-santé cohort. Am. J. Clin. Nutr. 2020;112(1):195–207. doi: 10.1093/ajcn/nqaa063. [DOI] [PubMed] [Google Scholar]
- 4.Zheng J.S., Sharp S.J., Imamura F., Chowdhury R., Gundersen T.E., Steur M., et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case–cohort study in eight European countries. BMJ. 2020:370. doi: 10.1136/bmj.m2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Key T.J., Bradbury K.E., Perez-Cornago A., Sinha R., Tsillidis K.K., Tsugane S. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ. 2020:368. doi: 10.1136/bmj.m511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenther P.M., Dodd K.W., Reedy J., Krebs-Smith S.M. Most Americans eat much less than recommended amounts of fruits and vegetables. J. Am. Diet. Assoc. 2006;106(9):1371–1379. doi: 10.1016/j.jada.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Krebs-Smith S.M., Guenther P.M., Subar A.F., Kirkpatrick S.I., Dodd K.W. Americans do not meet federal dietary recommendations. J. Nutr. 2010;140(10):1832–1838. doi: 10.3945/jn.110.124826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedrick V.E., Dietrich A.M., Estabrooks P.A., Savla J., Serrano E., Davy B.M. Dietary biomarkers: advances, limitations and future directions. Nutr. J. 2012;11:109. doi: 10.1186/1475-2891-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson F.E., Subar A.F., Loria C.M., Reedy J.L., Baranowski T. Need for technological innovation in dietary assessment. J. Am. Diet. Assoc. 2010;110(1):48–51. doi: 10.1016/j.jada.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell D.R., Gross M.D., Martini M.C., Grandits G.A., Slavin J.L., Potter J.D. Plasma carotenoids as biomarkers of vegetable and fruit intake, Cancer Epidemiol. Biomarkers Prev. 1994;3(6):493–500. PMID: 8000300. [PubMed] [Google Scholar]
- 11.Radtke M.D., Poe M., Stookey J., Jilcott Pitts S., Moran N.E., Landry M.J., et al. Recommendations for the use of the Veggie Meter® for spectroscopy-based skin carotenoid measurements in the research setting. Curr. Dev. Nutr. 2021;5(8) doi: 10.1093/cdn/nzab104. nzab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermakov I.V., Ermakova M., Sharifzadeh M., Gorusupudi A., Farnswirth K., Bernstein P., et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018;646:46–54. doi: 10.1016/j.abb.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jilcott Pitts S.B., Moran N.E., Wu Q., Harnack L., Craft N.E., Hanchard N., et al. Pressure-mediated reflection spectroscopy criterion validity as a biomarker of fruit and vegetable intake: a 2-site cross-sectional study of 4 racial or ethnic groups. J. Nutr. 2022;152(1):107–116. doi: 10.1093/jn/nxab349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraji B., Bukowski M.R., Thompson-Johnson T., Krusinski L., Goldberg J.L., Brooks C.M., et al. Skin carotenoid status of Black/African American college students correlates with plasma carotenoids and fruit and vegetable intake independent of skin tone. Int. J. Clin. Nutr. Diet. 2022;8(161) doi: 10.15344/2456-8171/2022/161. 1–7. [DOI] [Google Scholar]
- 15.Jahns L., Johnson L.K., Conrad Z., Bukowski M., Raatz S.K., Jilcott Pitts S., et al. Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-h recalls and plasma carotenoid concentrations across one year: a cohort study. Nutr. J. 2019;18(1):78. doi: 10.1186/s12937-019-0500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fultz A.K., Rex S.M., Mazelin A., McGarry C., Brewer B., Patterson F., et al. Examining fruit and vegetable intake in low-income older adults using the Veggie Meter®. Nutr. Health. 2021;28(1):13–17. doi: 10.1177/02601060211022275. [DOI] [PubMed] [Google Scholar]
- 17.Nagao-Sato S., Baltaci A., Peralta Reyes A.O., Zhang Y., Hurtado Choque G.A., Reicks M. Skin carotenoid scores assessed with reflection spectroscopy are associated with self-reported fruit and vegetable intake among Latino early adolescents. J. Acad. Nutr. Diet. 2021;121(8):1507–1514. doi: 10.1016/j.jand.2021.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Di Noia J., Gellermann W. Use of the spectroscopy-based Veggie Meter® to objectively assess fruit and vegetable intake in low-income adults. Nutrients. 2021;13(7):2270. doi: 10.3390/nu13072270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinelli S., Acciai F., Tasevska N., Ohri-Vachaspati P. Using the Veggie Meter in elementary schools to objectively measure fruit and vegetable intake: a pilot study. Methods Protoc. 2021;4(2):33. doi: 10.3390/mps4020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill C.M., Paschall M.J., O'Brien D.M., Bersamin A. Characterizing vegetable and fruit intake in a remote Alaska native community using reflection spectroscopy and 24-hour recalls. J. Nutr. Educ. Behav. 2021;53(8):712–718. doi: 10.1016/j.jneb.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.May K., Jilcott Pitts S., Stage V.C., Kelley C.J., Burkholder S., Fang X., et al. Use of the Veggie Meter® as a tool to objectively approximate fruit and vegetable intake among youth for evaluation of preschool and school-based interventions. J. Hum. Nutr. Diet. 2020;33(6):869–875. doi: 10.1111/jhn.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dragsted L.O., Gao Q., Scalbert A., Vergeres G., Kolehmainen M., Manach C., et al. Validation of biomarkers of food intake—critical assessment of candidate biomarkers. Genes Nutr. 2018;13:14. doi: 10.1186/s12263-018-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casperson S.L., Roemmich J.N., Larson K.J., Hess J.M., Palmer D.G., Jahns L. Sensitivity of pressure-mediated reflection spectroscopy to detect changes in skin carotenoids in adults without obesity in response to increased carotenoid intake: a randomized controlled trial. J. Nutr. 2023 doi: 10.1016/j.tjnut.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Jilcott Pitts S.B., Wu Q., Truesdale K.P., Rafferty A.P., Haynes-Maslow L., Boys K.A., et al. A four-year observational study to examine the dietary impact of the North Carolina Healthy Food Small Retailer Program, 2017–2020. Int. J. Behav. Nutr. Phys. Act. 2021;18(1):44. doi: 10.1186/s12966-021-01109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezdirc K., Hutchesson M.J., Whitehead R., Ozakinci G., Perrett D., Collins C.E. Fruit, vegetable and dietary carotenoid intakes explain variation in skin-color in young Caucasian women: a cross-sectional study. Nutrients. 2015;7(7):5800–5815. doi: 10.3390/nu7075251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pezdirc K., Hutchesson M.J., Williams R.L., Rollo M.E., Burrows T.L., Wood L.G., et al. Consuming high-carotenoid fruit and vegetables influences skin yellowness and plasma carotenoids in young women: a single-blind randomized crossover trial. J. Acad. Nutr. Diet. 2016;116(8):1257–1265. doi: 10.1016/j.jand.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Yun I.S., Lee W.J., Rah D.K., Kim Y.O., Park B.K. Skin color analysis using a spectrophotometer in Asians. Skin Res. Technol. 2010;16(3):311–315. doi: 10.1111/j.1600-0846.2010.00434.x. [DOI] [PubMed] [Google Scholar]
- 28.Kikuchi K., Katagiri C., Yoshikawa H., Mizokami Y., Yaguchi H. Long-term changes in Japanese women’s facial skin color. Color Res. Appl. 2018;43(1):119–129. doi: 10.1002/col.22153. [DOI] [Google Scholar]
- 29.Walsh S., Chaitanya L., Breslin K., Muralidharan C., Bronikowska A., Pospiech E., et al. Global skin colour prediction from DNA. Hum. Genet. 2017;136(7):847–863. doi: 10.1007/s00439-017-1808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda Y., Yamashita T., Hirao T., Takahashi M. An innovative method to measure skin pigmentation. Skin Res. Technol. 2009;15(2):224–229. doi: 10.1111/j.1600-0846.2009.00359.x. [DOI] [PubMed] [Google Scholar]
- 31.Subar A.F., Thompson F.E., Kipnis V., Midthune D., Hurwitz P., McNutt S., et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 32.Moran N.E., Mohn E.S., Hason N., Erdman J.W., Jr., Johnson E.J. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 2018;9(4):465–492. doi: 10.1093/advances/nmy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glanz K., Yaroch A.L., Dancel M., Saraiya M., Crane L.A., Buller D.B., et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch. Dermatol. 2008;144(2):217–222. doi: 10.1001/archdermatol.2007.46. [DOI] [PubMed] [Google Scholar]
- 34.Lazovich D.A., Stryker J.E., Mayer J.A., Hillhouse J., Dennis L.K., Pichon L., et al. Measuring nonsolar tanning behavior: indoor and sunless tanning. Arch. Dermatol. 2008;144(2):225–230. doi: 10.1001/archdermatol.2007.45. [DOI] [PubMed] [Google Scholar]
- 35.Bondy S.J., Victor J.C., Diemert L.M. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob. Control. 2009;18(4):317–323. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- 36.National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. Behavioral Risk Factor Surveillance System. 2018 http://www.cdc.gov/brfss/questionnaires/pdf-ques/2014_BRFSS.pdf [Internet] c2018 [cited 2020 Jul 29]. Available from: [Google Scholar]
- 37.Moran N.E., Chang J., Stroh R., Zaidi Y., Hason N., Musaad S., et al. Non-invasive, reflection spectroscopy measurement of skin carotenoid score in infants is feasible and reliable. J. Nutr. 2022;152(12):2966–2977. doi: 10.1093/jn/nxac182. [DOI] [PubMed] [Google Scholar]
- 38.Kopec R.E., Schweiggert R.M., Riedl K.M., Reinhold C., Schwartz S.J. Comparison of high-performance liquid chromatography/tandem mass spectrometry and high-performance liquid chromatography/photo-diode array detection for the quantitation of carotenoids, retinyl esters, α-tocopherol and phylloquinone in chylomicron-rich fractions of human plasma. Rapid Commun. Mass Spectrom. 2013;27(12):1393–1402. doi: 10.1002/rcm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuniga K.E., Moran N.E. Low serum carotenoids are associated with self-reported cognitive dysfunction and inflammatory markers in breast cancer survivors. Nutrients. 2018;10(8):1111. doi: 10.3390/nu10081111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meinke M.C., Schanzer S., Lohan S.B., Shchatsinin I., Darvin M.E., Vollert H., et al. Comparison of different cutaneous carotenoid sensors and influence of age, skin type, and kinetic changes subsequent to intake of a vegetable extract. J. Biomed. Opt. 2016;21(10) doi: 10.1117/1.JBO.21.10.107002. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar S.S., Wengreen H.J., Dew J. Skin carotenoid response to a high-carotenoid juice in children: a randomized clinical trial. J. Acad. Nutr. Diet. 2015;115(11):1771–1778. doi: 10.1016/j.jand.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Jahns L., Johnson L.K., Mayne S.T., Cartmel B., Picklo M.J., Sr., Ermakov I.V., et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. Am. J. Clin. Nutr. 2014;100(3):930–937. doi: 10.3945/ajcn.114.086900. [DOI] [PubMed] [Google Scholar]
- 43.Stahl W., Heinrich U., Jungmann H., von Laar J., Schietzel M., Sies H., et al. Increased dermal carotenoid levels assessed by noninvasive reflection spectrophotometry correlate with serum levels in women ingesting Betatene. J. Nutr. 1998;128(5):903–907. doi: 10.1093/jn/128.5.903. [DOI] [PubMed] [Google Scholar]
- 44.Bayles J., Peterson A.D., Jilcott Pitts S., Bian H., Goodell L.S., Burkholder S., et al. Food-Based Science, Technology, Engineering, Arts, and Mathematics (STEAM) learning activities may reduce decline in preschoolers’ skin carotenoid status. J. Nutr. Educ. Behav. 2021;53(4):343–351. doi: 10.1016/j.jneb.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiNoia J., Monica D., Jensen H.H., Sikorskii A. Economic evaluation of a farm-to-Special Supplemental Nutrition Programme for Women, Infants and Children intervention promoting vegetable consumption. Public Health Nutr. 2021;24(12):3922–3928. doi: 10.1017/S1368980021001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending appropriate Institutional Review Board approval and review of and approval from the investigators on the current project.