Abstract
Background
Vitamin A deficiency (VAD) is an ongoing public health concern among children and pregnant women in Nepal despite robust national efforts to screen and treat this vision- and life-threatening condition.
Objectives
This study aimed to evaluate skin carotenoid scores measured using the Veggie Meter as a rapid, noninvasive screening tool for VAD in Nepali children and pregnant women.
Methods
This comparative cross-sectional study enrolled 164 pregnant women and 168 children (aged 8 to 12 y) from public hospitals in three distinct outlying ecological regions of Nepal (Terai, Hill, and Mountain). The primary outcome assessed whether skin carotenoid status could be a biomarker for VAD. We determined skin carotenoid scores using the Veggie Meter and compared them with serum retinol and total carotenoid concentrations assessed by HPLC. Correlation analysis was used to determine bivariate associations between serum retinol and total carotenoid concentrations, and the Veggie Meter assessed skin carotenoid status. Receiver operating characteristics curves were determined, and a P value <0.05 was considered statistically significant.
Results
We found that 8.5% of pregnant women and 13.0% of children in this study had severe VAD (serum retinol < 200 ng/mL). There were significant correlations between skin carotenoid scores with serum retinol and total carotenoid concentrations among pregnant women and children (r = 0.253–0.530, P ≤ 0.001). The Veggie Meter detected severe VAD with 57.1% sensitivity and 82.7% specificity in pregnant women and 61.9% sensitivity and 75.9% specificity in children.
Conclusions
Although sensitivity and specificity were moderate for detecting VAD with the Veggie Meter, skin carotenoid assessment using this rapid, noninvasive portable device could still be valuable for high-risk VAD screening in Nepal and similar developing countries with limited access to laboratory measurement of serum vitamin A concentrations.
Keywords: skin carotenoids, serum carotenoids, serum retinol, vitamin A, Veggie Meter, Nepal
Introduction
Vitamin A deficiency (VAD) is a persistent public health problem in developing countries, including Nepal [1,2]. In a survey of nutritional status conducted by the WHO between 1995 and 2005, 44%–50% of preschool children in South Asian regions had severe VAD [3]. Vitamin A (retinol) and its pro-vitamin A precursor carotenoids are crucial micronutrients for vision, immune function, cell growth, and differentiation, which are commonly found in the liver, fish oils, dairy products, leafy green vegetables, and orange/yellow fruits and vegetables [[4], [5], [6], [7]]. Despite global efforts to enhance local diets with more foods rich in vitamin A and its carotenoid precursors and to improve supplement access, studies continue to report high morbidity and mortality rates due to VAD, particularly in children, pregnant women, and women of childbearing age [[1], [2], [3],8,9].
If not diagnosed and treated in a timely manner, VAD may result in vision loss and blindness, especially in children, impacting the quality of life of affected individuals [2,8]. According to the 1998 Nepal Micronutrient Status Survey, 5% of nursing mothers with children aged 6–59 mo had night blindness. The extent of night blindness was more remarkable in rural areas (18%) relative to urban (6%) and differed across the various ecological regions [10]. As a result, the Nepal government launched an intervention program, which significantly decreased VAD incidence and its consequent comorbidities such as night blindness and mortality among the populace [11].
However, a recent study in 2016 revealed that VAD persists in most remote areas in Nepal [12]. The study reported 4% and 3% VAD prevalence in children and women, respectively. This prevalence is partly due to socioeconomic factors such as illiteracy, limited accessibility to health care facilities, and financial constraints as well as nutritionally poor local diets [1,4,9]. Of note, routine screening for VAD is not possible in Nepal, as there are no laboratory facilities for serum retinol analysis anywhere in this country of >29 million population. Hence, biochemical tests for VAD are usually sent to overseas laboratories, which are expensive and often subject to considerable delays. This challenge warrants the development and validation of alternative simple, rapid, and cost-effective approaches to assess VAD among the populace.
Although it is not feasible to measure systemic vitamin A concentrations noninvasively, it is possible to measure total skin carotenoids rapidly and noninvasively by resonance Raman spectroscopy [[13], [14], [15]] or by pressure-mediated reflectometry [[16], [17], [18]]. Previous studies have reported that skin carotenoid scores using either technique correlate well with serum total carotenoids (whose predominant carotenoid is typically pro-vitamin A β-carotene), and such measurements can be used as a biomarker of fruit and vegetable intake [[19], [20], [21]].
The Veggie Meter is a commercially available, portable, validated, noninvasive reflectometry device for skin carotenoid measurement that holds potential as a screening tool for identifying nutritionally compromised individuals [[16], [17], [18]]. However, to our knowledge, no study to date has assessed whether its skin carotenoid score could serve as a biomarker for VAD, especially in developing countries such as Nepal. Hence, our study sought to determine whether skin carotenoid status measured with the Veggie Meter could detect severe VAD in children and pregnant women from three outlying ecological regions of Nepal.
Methodology
Clinical study sites
This is a hospital-based cross-sectional study. The study was conducted in collaboration with three public hospitals located in three ecological regions of Nepal. The study sites were Narayani Hospital, Parsa, Birgunj, Province 2, Nepal (Terai); Trishuli Hospital, Bidur Municipality, Nuwakot district, Province 3, Nepal (Hill); and Rasuwa Hospital, Gosaikunda Rural Municipality, Rasuwa district, Province 3, Nepal (Mountain).
Inclusion criteria
Pregnant women in any trimester of their pregnancy and children (aged 8 to 12 y) were eligible for the study.
Exclusion criteria
Individuals were excluded from the study if they failed to provide written informed consent, had impairment/disease that affected study outcome measures, or were known to have blood-borne diseases (that is, diagnosed with human immune deficiency virus or hepatitis infection).
Sample size and power calculations
The use of the Veggie Meter as a screening tool for VAD has not been conducted previously. Therefore, based on previous studies, we assumed a prevalence of VAD of 10% to 20% for this study involving three distinct Nepali ecological regions. Allowing for ∼10% sample loss during shipment, handling, and analysis, the desired sample size was 165 pregnant women and 165 children. Thus, for each of the three participating public hospitals, we envisioned enrolling 55 pregnant women and 55 children.
Ethical approval
The Nepal Health Research Council provided ethical approval to conduct the study (Reference no. 899/2020). The study adhered strictly to the tenets of the Declaration of Helsinki, and all participants provided informed written consent before study enrolment. Parents/guardians consented for children. Informed assent was obtained from the children.
Demographic characteristics
We collected participants’ demographic data such as age, gender, occupation, educational level, height and weight for BMI calculation, dietary history (whether a vegetarian or not and frequency of vegetables and fruit ingestion), supplement use, and smoking history. In addition, the medical history, including diabetes, hypertension, and any other systemic problems, was examined and recorded by the pediatrician and gynecologist in children and pregnant women, respectively.
Skin carotenoid assessment
We used the Veggie Meter produced by the Longevity Link Corporation, Salt Lake City, UT, United States, to assess skin carotenoid status by pressure-mediated reflectometry. The Veggie Meter is a validated, rapid, noninvasive, and portable device that is a biomarker for fruit and vegetable intake, and its skin carotenoid score has a strong significant positive correlation with skin resonance Raman spectroscopy and serum total carotenoids [[16], [17], [18],22]. Briefly, the skin tissue of interest (that is, the index finger) is illuminated with broad-band white light spanning the spectral range from 350 to 850 nm, and the spectral composition of the diffusively reflected light is analyzed in real-time. Participants gently press their index fingers against the convex lens surface with the help of a spring-loaded cover, which momentarily squeezes blood out of the illuminated tissue volume, reducing oxyhemoglobin’s and other chromophores’ influence on the reflection spectra. A laptop computer connected to the Veggie Meter regulates the light exposure, data acquisition, processing, and display of the reflection spectra. The display provides the skin carotenoid score on a scale from 0 to 800. The single scan mode lasting ∼10 s was used in this study. Three single readings were taken, and the average score was recorded as the final skin carotenoid score. Before each measurement, participants had their hands sanitized or washed, and the device was calibrated daily with the dark and white reference sticks before starting measurements and at least every 2 h when doing multiple skin measurements over long periods.
Individuals with skin carotenoid scores <150 RU (classified as likely to be nutritionally compromised based on the device manufacturer’s recommendation) had a comprehensive ocular assessment to ascertain any VAD presentation in the eye. The comprehensive dilated eye examination included visual function measures (presenting visual acuity and best-corrected visual acuity), slit-lamp biomicroscopy, and direct or indirect ophthalmoscopy.
Blood collection
Five milliliters of venous blood samples were drawn for serum retinol and carotenoid analyses. After centrifugation at 2400 × g for 5 min, we extracted the serum samples into labeled and capped cryo tubes and stored them at −20 °C. In instances where immediate centrifugation was not possible, the blood sample was kept at +4 °C for a maximum of 2 d. The serum samples were then shipped on dry ice to the Bernstein Laboratory at the Moran Eye Center, University of Utah, Salt Lake City, Utah, United States for serum carotenoid and retinol measurements. Past studies show that retinol and carotenoids remain stable for ≥15 y when stored at −20 °C or −80 °C [23]. Therefore, conditions such as light, heat, and oxygen exposures, which could potentially degrade retinol and carotenoids, were kept at the barest minimum during collection, processing, shipment, and extraction.
Serum retinol and carotenoid extraction and analysis
Serum retinol and carotenoids were analyzed by reverse-phase HPLC using photodiode array detection as previously described with slight modifications [[24], [25], [26]]. In essence, 200 μL of human serum was extracted with ethanol containing 0.1% butylated hydroxytoluene, ethyl acetate, and hexane in succession in a dark room, and then the combined extracts were dried under inert gas. Subsequently, the dried film was cleaned with hexane/methanol/distilled water, and the supernatant was recovered and dried. The resulting dried extract was then dissolved in a 200 μL HPLC mobile phase [methanol:methyl tert-butyl ether (80:20, v/v)] and centrifuged at 9600 × g for 10 min. The clear supernatant phase was injected into an Agilent 1260 series HPLC system (Agilent Technologies Inc.). HPLC-diode array detection (DAD) method was developed to simultaneously determine serum retinol concentrations and the major carotenoids, including lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene from patients’ serum [27]. HPLC analysis was done on a C30 column (YMC Carotenoids; 25 cm length × 4.6 mm internal diameter; maintained at room temperature) using DAD monitored at 325 and 450 nm wavelengths, 100 μL injection volumes of standards and extracts, and 1 mL/min flow rate for 50 min. Diode array spectra, retention times, and co-elution with authentic standards identified and confirmed the various carotenoid peaks.
Standard solutions of retinol and each carotenoid of interest were prepared with known concentrations calculated spectroscopically using appropriate extinction coefficients and then injected in different volumes to achieve final injected amounts. Serum retinol and carotenoid concentrations were quantified based on standard curves of known quantities of the authentic carotenoid standards injected into the HPLC system compared with integrated ultraviolet/visible absorbance of their eluted peaks.
Validation of serum vitamin A measurements in the Bernstein Laboratory at the Moran Eye Center is given in Supplemental Methods. We found that our laboratory’s serum retinol concentrations had a strong positive correlation with local Clinical Laboratory Improvement Amendments–certified diagnostic laboratory’s serum retinol concentrations (Supplemental Figure 1; n = 14, r = 0.95, P < 0.0001). The favorable comparison between the two laboratories gives credence to the results and conclusions of this study.
Diagnostic criteria of VAD
We categorized VAD using the WHO serum retinol concentration diagnostic criteria. Thus, severe deficiency was <200 ng/mL (<0.70 μmol/L); marginal deficiency was ≥200 and <300 ng/mL (≥0.70 and <1.05 μmol/L) and normal, ≥300 ng/mL (≥1.05 μmol/L)[28].
Statistical analyses
We developed a special form for detailed data collection. All collected study data were entered, coded, and cleaned using Microsoft Excel 2007. We then imported the Excel data to Statistical Package for Service Solution (IBM SPSS Statistics for Windows, Version 27.0, IBM Corp) for thorough analysis. Descriptive statistics were used to summarize the sample data. Of note, we were underpowered to conduct a subgroup analysis in this study. Hence, we show only descriptive statistics for all study tables. Correlation analysis was used to determine bivariate associations between measures of serum retinol and total carotenoid concentrations, and Veggie Meter assessed skin carotenoid status. Sensitivity and specificity values of the Veggie Meter in detecting VAD were computed using skin carotenoid score cutoff obtained from ROC curve, and a P value <0.05 was considered statistically significant.
Results
Demographics
A total of 332 participants (164 pregnant women and 168 children) were enrolled in this study. Serum retinol and serum carotenoid concentrations were available for 326 participants (164 pregnant women and 162 children) because a few samples were lost or damaged during collection, processing, and shipment. The mean age of pregnant women was 24.4 ± 4.7 y (range: 15–42 y) and that for children was 10.4 ± 1.5 y (range: 8–12 y). Table 1 provides the demographic characteristics of all 332 participants enrolled in this study.
TABLE 1.
Demographic characteristics of participants enrolled in the study1
| Variable | Pregnant women (n = 164) | Children (n = 168) |
|---|---|---|
| Hospital | ||
| Terai | 53 (32.3) | 57 (33.9) |
| Hill | 55 (33.5) | 55 (32.7) |
| Mountain | 56 (34.1) | 56 (33.3) |
| Sex | ||
| Female | 164 (100.0) | 84 (50.0) |
| Male | 0 (0.0) | 84 (50.0) |
| Age, (y) | ||
| <20 | 20 (12.2) | 168 (100.0) |
| 20–29 | 119 (72.6) | 0 (0.0) |
| 30–42 | 25 (15.2) | 0 (0.0) |
| Literacy | ||
| Literate | 118 (72.0) | 168 (100.0) |
| Illiterate | 46 (28.0) | 0 (0.0) |
| Occupation | ||
| Agriculture | 10 (6.1) | 0 (0.0) |
| Business | 10 (6.1) | 0 (0.0) |
| Housewife | 132 (80.5) | 0 (0.0) |
| Service | 2 (1.2) | 0 (0.0) |
| Student | 7 (4.3) | 168 (100.0) |
| Tailor | 3 (1.8) | 0 (0.0) |
| Dietary pattern | ||
| Vegetarian | 2 (1.2) | 2 (1.2) |
| Nonvegetarian | 162 (98.8) | 166 (98.8) |
| Frequency of vegetable intake (d/wk) | ||
| <2 | 5 (3.0) | 25 (14.9) |
| 2–5 | 92 (56.1) | 102 (60.5) |
| >5 | 67 (40.9) | 41 (24.4) |
| Any multivitamin use | ||
| Yes | 10 (6.1) | 4 (2.4) |
| No | 154 (93.9) | 164 (97.6) |
| Vitamin A supplementation | ||
| Yes | 3 (1.8) | 18 (10.7) |
| No | 161 (98.2) | 150 (89.3) |
| Diabetes mellitus | ||
| Yes | 1 (0.6) | 1 (0.6) |
| No | 163 (99.4) | 167 (99.4) |
| BMI (kg/m2) | ||
| <18.5 | 9 (5.5) | 136 (81) |
| 18.5–25 | 87 (53.0) | 27 (16.1) |
| 25.1–30 | 47 (28.7) | 3 (1.8) |
| >30 | 21 (12.8) | 2 (1.2) |
| Number of previous pregnancies | ||
| First pregnancy | 62 (37.8) | |
| More than one pregnancy | 102 (62.2) | |
| Duration of current pregnancy | ||
| First trimester | 28 (17.0) | |
| Second trimester | 58 (35.4) | |
| Third trimester | 78 (47.6) | |
| Number of previous deliveries | ||
| 1 | 56 (54.9) | |
| 2 | 30 (29.3) | |
| 3 | 11 (10.8) | |
| 4 | 3 (2.9) | |
| 5 | 2 (2) | |
| Duration since last childbirth (y) | ||
| 1–2 | 46 (46.9) | |
| >2–3 | 18 (18.4) | |
| >3–4 | 6 (6.1) | |
| >4–5 | 17 (17.3) | |
| >5 | 11 (11.2) | |
Data are present as frequency (%) for categorical variables.
Serum vitamin A (retinol) analyses
Serum retinol concentrations in pregnant women and children
Considering serum retinol concentrations, 10.7% of all study participants had severe VAD and 25.8% had marginal VAD. In pregnant women, 8.5% had severe VAD and 19.5% had marginal VAD. Among the children, 13.0% had severe VAD and 32.1% had marginal VAD (Table 2).
TABLE 2.
Serum retinol concentrations among pregnant women, children, and all study participants1
| Pregnant women (n = 164) | Children (n = 162) | Total (n = 326) | |
|---|---|---|---|
| Retinol, ng/mL | |||
| <200 | 14 (8.5) | 21 (13.0) | 35 (10.7) |
| 200 to <300 | 32 (19.5) | 52 (32.1) | 84 (25.8) |
| ≥300 | 118 (72.0) | 89 (54.9) | 207 (63.5) |
Data are present as frequency (%) for categorical variables. Serum retinol < 200 ng/mL, severe vitamin A deficiency; 200 to <300 ng/mL, marginal vitamin A deficiency; ≥300 ng/mL, normal (that is, healthy with no vitamin A deficiency).
Vitamin A status in study participants by ecological regions
We found that Terai had the highest occurrence of severe and marginal VAD in both pregnant women and children, followed by Hill and Mountain. Table 3 provides vitamin A status for all study participants in these three ecological regions of Nepal.
TABLE 3.
Serum retinol concentrations among pregnant women, children, and all study participants in three ecological regions of Nepal1
| Serum retinol, ng/mL | Terai | Hill | Mountain |
|---|---|---|---|
| Pregnant women | (n = 52) | (n = 55) | (n = 55) |
| <200 | 11 (21.2) | 3 (5.5) | 0 (0.0) |
| 200 to <300 | 16 (30.8) | 8 (14.5) | 7 (12.7) |
| ≥300 | 25 (48.1) | 44 (80.0) | 48 (87.3) |
| Children | (n = 56) | (n = 55) | (n = 53) |
| <200 | 15 (26.8) | 4 (7.3) | 2 (3.8) |
| 200 to <300 | 23 (41.1) | 19 (34.5) | 11 (20.8) |
| ≥300 | 18 (32.1) | 32 (58.2) | 40 (75.5) |
| All study participants | (n = 108) | (n = 110) | (n = 108) |
| <200 | 26 (24.1) | 7 (6.4) | 2 (1.9) |
| 200 to <300 | 39 (36.1) | 27 (24.5) | 18 (16.7) |
| ≥300 | 43 (39.8) | 76 (69.1) | 88 (81.5) |
Data are present as frequency (%) for categorical variables. Serum retinol < 200 ng/mL, severe vitamin A deficiency; 200 to <300 ng/mL, marginal vitamin A deficiency; ≥300 ng/mL, normal (that is, healthy with no vitamin A deficiency).
Correlation of serum total carotenoid concentrations with serum retinol concentrations in study participants
Serum total carotenoid concentrations showed a positive statistically significant association with serum retinol concentrations among pregnant women (r = 0.447, P < 0.001; Figure 1A and in children (r = 0.530, P < 0.001; Figure 1B).
FIGURE 1.
Serum total carotenoid concentrations statistically correlated with serum retinol concentrations in pregnant women (n = 164) (A) and in children (n = 162) (B).
Skin carotenoid analyses
Skin carotenoid scores in all study participants
Of all study participants, 27.1% had skin carotenoid scores of <150 RU. Furthermore, skin carotenoid scores <150 RU among pregnant women and children were 23.2% and 31.0%, respectively (Table 4).
TABLE 4.
Skin carotenoid scores among pregnant women, children, and all study participants1
| A | Pregnant Women (n = 164) | Children (n = 168) | Total (n = 332) |
|---|---|---|---|
| Skin carotenoid score, RU | |||
| <150 | 38 (23.2) | 52 (31.0) | 90 (27.1) |
| ≥150 | 126 (76.8) | 116 (69.0) | 242 (72.9) |
1 Data are present as frequency (%) for categorical variables. Skin carotenoid score < 150 RU, substantial nutritional compromise; ≥150 RU, well-nourished healthy participant. RU, reflection units.
Skin carotenoid status in all study participants by ecological regions
TABLE 5, TABLE 6 show variability in skin carotenoid scores in the three ecological regions (Terai, Hill, and Mountain).
TABLE 5.
Skin carotenoid scores among pregnant women, children, and all study participants in three ecological regions of Nepal1
| Skin carotenoid score, RU | Terai | Hill | Mountain |
|---|---|---|---|
| Pregnant women | (n = 53) | (n = 55) | (n = 56) |
| <150 | 18 (34.0) | 14 (25.5) | 6 (10.7) |
| ≥150 | 35 (66.0) | 41 (74.5) | 50 (89.3) |
| Children | (n = 57) | (n = 55) | (n = 56) |
| <150 | 15 (26.3) | 27 (49.1) | 10 (17.9) |
| ≥150 | 42 (73.7) | 28 (50.9) | 46 (82.1) |
| All study participants | (n = 110) | (n = 110) | (n = 112) |
| <150 | 33 (30.0) | 41 (37.3) | 16 (14.3) |
| ≥150 | 77 (70.0) | 69 (62.7) | 96 (85.7) |
RU, reflection units.
Data are present as frequency (%) for categorical variables. Skin carotenoid score < 150, substantial nutritional compromise; ≥150, well-nourished healthy participant.
TABLE 6.
Mean skin carotenoid scores among pregnant women and children in three ecological regions of Nepal1
| Ecological region | n | Mean (SD) | Minimum | Maximum |
|---|---|---|---|---|
| Pregnant women | ||||
| Terai | 53 | 215 (116) | 5 | 524 |
| Hill | 55 | 220 (119) | 5 | 613 |
| Mountain | 56 | 278 (93) | 134 | 512 |
| Total | 164 | 238 (113) | 5 | 613 |
| Children | ||||
| Terai | 57 | 223 (89) | 37 | 384 |
| Hill | 55 | 164 (82) | 11 | 397 |
| Mountain | 56 | 234 (90) | 47 | 479 |
| Total | 168 | 208 (92) | 11 | 479 |
Data are present as mean (SD) for continuous variables.
Skin carotenoid status in children by age
The mean skin carotenoid score was the lowest (160 RU) in children aged 11 y, followed by 9-year-old children (192 RU), and the maximum recorded was for children aged 12 y (237 RU) (Table 7).
TABLE 7.
Mean skin carotenoid scores among children by age in the study1
| Children | n | Mean (SD) |
|---|---|---|
| Age (y) | ||
| 8 | 30 | 197 (83) |
| 9 | 19 | 192 (89) |
| 10 | 32 | 224 (94) |
| 11 | 32 | 160 (89) |
| 12 | 55 | 237 (88) |
Data are present as mean (SD) for continuous variables.
Correlation of serum total carotenoid concentrations with skin carotenoids in study participants
Consistent with previous studies [19,20,22], we show a statistically significant association between serum total carotenoid concentrations and skin carotenoids in this study cohort. Thus, we found a moderate positive significant association between serum total carotenoid concentrations and skin carotenoid scores in pregnant women (r = 0.314, P < 0.001), as shown in Figure 2A, and in children (r = 0.510, P < 0.001) depicted in Figure 2B.
FIGURE 2.
Serum total carotenoid concentrations statistically correlated with skin carotenoid score in pregnant women (n = 164) (A) and in children (n = 162) (B). RU, reflection units.
Correlation of serum retinol concentrations with skin carotenoids in study participants
Comparing skin carotenoid scores to serum retinol concentrations regardless of the ecological region, we found that serum retinol concentrations had a significant positive association with skin carotenoid scores in pregnant women, shown in Figure 3A, and children (r = 0.253, P = 0.001), illustrated in Figure 3B.
FIGURE 3.
Serum retinol concentrations and skin carotenoids score were statistically correlated in pregnant women (n = 164) (A) and in children (n = 162) (B). RU, reflection units; VAD, vitamin A deficiency.
Determining the cutoff level for VAD using skin carotenoid score
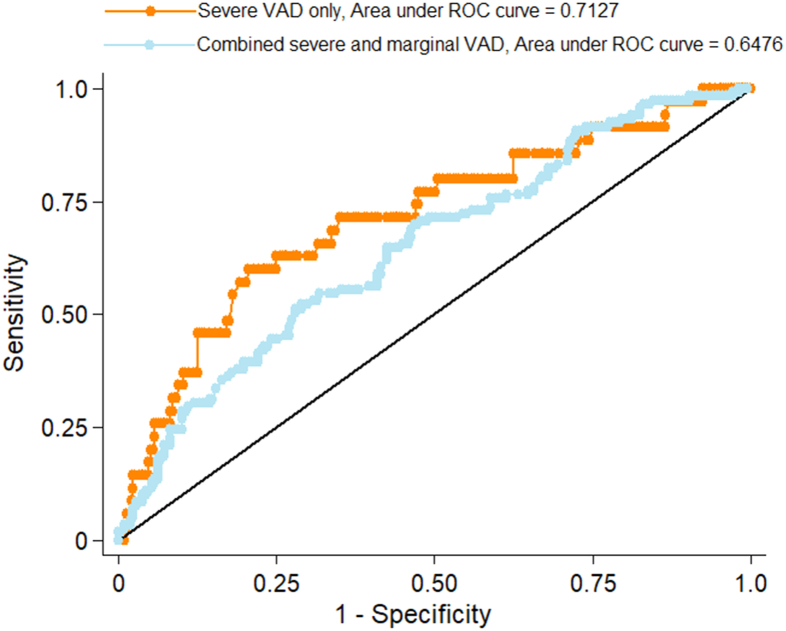
Presently, there is no standardized skin carotenoid cutoff score that suggests severe VAD, just the device manufacturer’s recommendation that a skin carotenoid score <150 RU is suggestive of substantial nutritional compromise. Hence, we performed an ROC analysis to determine the skin carotenoid score with the highest sum of sensitivity and specificity using combined pregnant women and children’s skin carotenoid scores. As shown in Figure 4, the orange plot gives a cutoff skin carotenoid score of 146 RU using only severe VAD (serum retinol concentrations < 200 ng/mL; n = 35) compared with combined marginal VAD and healthy participants (serum retinol concentrations ≥ 200 ng/mL; n = 291), whereas the light blue plot has a 185 RU-cutoff based on a combined severe and marginal VAD (serum retinol concentrations < 300 ng/mL; n = 119) and healthy participants (serum retinol concentrations ≥ 300 ng/mL; n = 207). The device manufacturer’s reference of 150 RU for probable nutritional compromise compared favorably with our calculated optimal cutoff of 146 RU for the detection of severe VAD.
FIGURE 4.
Receiver operating characteristic (ROC) analysis with sensitivity and specificity for vitamin A deficiency skin carotenoid cutoff score (n = 326). VAD, vitamin A deficiency.
Sensitivity and specificity of the Veggie Meter in detecting VAD
Among the pregnant women, the sensitivity and specificity of the Veggie Meter in detecting severe VAD were 57.1% and 82.7%, respectively. Likewise, 61.9% sensitivity and 75.9% specificity for severe VAD detection were found in children. Additionally, we found a sensitivity of 60.0% and specificity of 79.4% for the combined pregnant women and children (Table 8).
TABLE 8.
Sensitivity and specificity of the Veggie Meter in measuring skin carotenoid status using serum retinol concentrations as the reference standard in detecting severe vitamin A deficiency
| n | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
| Pregnant women | 164 | 57.1 | 82.7 | 23.5 | 95.4 |
| Children | 162 | 61.9 | 75.9 | 27.7 | 93.0 |
| Combined | 326 | 60.0 | 79.4 | 25.9 | 94.3 |
NPV, negative predictive value; PPV, positive predictive value.
Discussion
In this study, we sought to determine whether skin carotenoid assessment with the Veggie Meter could be a rapid, noninvasive screening tool for serum vitamin A status in children and pregnant women of Nepal. We found an overall prevalence of 10.7% VAD among the study participants. Further, the Veggie Meter detected VAD with sensitivity and specificity of 60.0% and 79.4%, respectively.
A finding worth highlighting is the 10.7% prevalence of VAD among the study participants, which is higher than that reported in the Nepal National Micronutrient Status Survey [12]. Despite the considerable efforts of the government and other policymakers in providing vitamin A supplement and other intervention programs to mitigate the burden of this life- and vision-threatening disease, VAD continues to be a public health concern in Nepal [12,29,30]. Although VAD is rarely present in developed countries, it is quite problematic in developing countries where resources are considerably constrained, and only limited facilities are available to access its biochemical status in the blood [2]. Our findings suggest the need for all authorities, stakeholders, and policymakers to re-examine and strategize the existing programs tailored to reduce VAD among the populace, so that sustainable and pragmatic measures may be implemented to effectively cover all areas/ecological regions.
Consistent with other studies, we found VAD prevalence to differ by ecological regions in Nepal, with Terai having the highest occurrence [10,12]. Differences in dietary patterns may account for the variation in VAD prevalence across the various ecological regions. For instance, most Mountain inhabitants usually cultivate and consume food (with good amount of vitamin A) grown on their jungle lands, whereas individuals in Terai have access to foods (such as potatoes, fried foods, etc.), which are relatively poor dietary sources of vitamin A. Three strategies—supplementation, food diversity, and biofortification—have been shown to be effective in augmenting the nutriture of the populace, thereby decreasing VAD [31]. However, in Nepal, supplementation has been the main method of confronting VAD with minimal progress toward food biofortification and diversity [29]. Biannual vitamin A supplementation programs with nationwide coverage may be challenging considering the workforce and the logistics involved. Therefore, increasing availability, and accessibility to affordable dietary vitamin A sources (such as fruits and vegetable) backed by appropriate education of the population on the importance of healthy nutrition could offer sustainable and meaningful lifestyle interventions and thereby decrease the prevalence of VAD. Moreover, as the wealth of every country lies in the health of its people as revealed by the Sustainable Development Goal 3: Good health and wellbeing [32], it is imperative that the Ministry of Health and Population in partnership with the Ministry of Agriculture and stakeholders implement policies that potentially could increase the production and consumption of vitamin A rich foods.
Again, it is quite difficult to improve and monitor vitamin A status when there is an absence of facilities or devices to biochemically analyze vitamin A status in a population of over 29 million, unless a symptomatic individual presents to an eye hospital for ophthalmological services [33]. By this time, visual impairment may be irreversible. Therefore, the use of the Veggie Meter to determine VAD holds potential, especially in resourced limited jurisdictions, in detecting and instituting treatment of VAD long before the onset of ocular problems. The Veggie Meter is advantageous in the sense that: 1) it is portable and can be used in remote areas; 2) provides rapid, repeatable, and consistent results that is suggestive of good or poor nutriture; and 3) it is easy to operate and maintain without sophisticated training [16,21,22]. Thus, in countries with no or limited availability of serum vitamin A assessment facilities, the use of the skin carotenoid assessment equipment as an indirect measure of serum retinol could aid in early detection and possible treatment of VAD, thereby preventing VAD-associated morbidities and mortalities. In agreement with other past studies in the United States and Japan, we found significant correlations between serum carotenoids and skin carotenoid status [19,22]. This finding indicates the wide use of the Veggie Meter in measuring skin carotenoids in diverse populations regardless of skin pigmentation.
The strength of our study lies in the fact that this first report of the use of the Veggie Meter as a VAD screening tool had a large sample size of over 300 participants, used high quality serum retinol and carotenoid analyses, and yielded consistent results from two distinct study populations (pregnant women and school-age children) known to be at risk of VAD in Nepal. An inherent limitation of our current study lies in the fact that skin carotenoid assessment with the Veggie Meter will always be an indirect biomarker of vitamin A status. In addition, we were underpowered to fully examine regional variations in VAD in a statistically significant manner. Because this is the first study to determine the use of the Veggie Meter as a VAD screening tool, this study should be replicated in other regions of the world to ensure consistency in the findings.
In conclusion, the VAD prevalence of 10.7% suggests that VAD is still a moderate public health problem in Nepal based on WHO standards [34]. Although sensitivity and specificity were moderate for detecting VAD with the Veggie Meter, skin carotenoid assessment using this rapid, noninvasive portable device could still be valuable for high-risk VAD screening in Nepal and similar developing countries with limited access to laboratory measurement of serum vitamin A concentrations.
Acknowledgments
We would like to thank the Thrasher Research Fund, Salt Lake City, Utah, United States for the financial support to carry out this study; Moran Eye Center, University of Utah for support of Veggie Meter and analysis of serum retinol and serum carotenoids; Tilganga Institute of Ophthalmology, Narayani Hospital, Birgunj, Parsa and Trishuli Hospital, Nuwakot and Rasuwa Hospital, Rasuwa for their supports in implementation of this study. We thank all the study participants for their co-operation in this study.
The authors’ responsibilities were as follow – RT and PSB: designed research; RT and SR: conducted the field research; EKA: performed HPLC and analyzed data; and RT, EKA, and PSB: wrote the article. PSB had primary responsibility for the final content. All authors: read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.02.005.
Contributor Information
Raba Thapa, Email: raba.thapa@tilganga.org.
Emmanuel K. Addo, Email: emmanuel.k.addo@utah.edu.
Sanduk Ruit, Email: sruit@tilganga.org.
Paul S. Bernstein, Email: paul.bernstein@hsc.utah.edu.
Data Availability
Data described in this manuscript will be made available from the corresponding author upon reasonable request.
Funding
This study was supported by the Thrasher Research Fund, Salt Lake City, UT, National Institutes of Health Grants (EY011600 and EY014800) and an Unrestricted Grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah. These supporting sources were not involved in the design, implementation, analysis, and interpretation of the data or had restrictions regarding this publication.
Author disclosures
The authors report no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Akhtar S., Ahmed A., Randhawa M.A., Atukorala S., Arlappa N., Ismail T., et al. Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. J. Health. Popul. Nutr. 2013;31:413–423. doi: 10.3329/jhpn.v31i4.19975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G.A., Bennett J.E., Hennocq Q., Lu Y., De-Regil L.M., Rogers L., et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet. Glob. Health. 2015;3:e528–e536. doi: 10.1016/S2214-109X(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization global database on vitamin A deficiency. World Health Organization; Geneva: 2009. https://apps.who.int/iris/bitstream/handle/10665/44110/9789241598019_eng.pdf [date cited: June 2, 2022]. Available from: [Google Scholar]
- 4.Blaner W.S. Elsevier; 2020. Vitamin A and provitamin A carotenoids, Present Knowledge in Nutrition; pp. 73–91. [Google Scholar]
- 5.Ross A.C., Caballero B.H., Cousins R.J., Tucker K.L., Ziegler T.R. In: Modern nutrition in health and disease, Wolters Kluwer Health. Ross A.C., editor. Lippincott Williams & Wilkins; Baltimore: 2014. Vitamin A; pp. 260–277. [Google Scholar]
- 6.Abdel-Aal el-S.M., Akhtar H., Zaheer K., Ali R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients. 2013;5:1169–1185. doi: 10.3390/nu5041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carazo A., Macáková K., Matoušová K., Krčmová L.K., Protti M., Mladěnka P. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. 2021;13 doi: 10.3390/nu13051703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey R.L., West K.P., Jr., Black R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015;66:22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 9.Wiseman E.M., Bar-El Dadon S., Reifen R. The vicious cycle of vitamin A deficiency: a review. Crit. Rev. Food Sci. Nutr. 2017;57:3703–3714. doi: 10.1080/10408398.2016.1160362. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health and Population (Nepal), United Nations Children’s Fund (UNICEF), World Health Organization (WHO), The Micronutrient Initiative, and New ERA. Ministry of Health and Population (Nepal) Nepal Micronutrient Status Survey 1997-1998. Ministry of Health and Population; Nepal: 1998. https://un.info.np/Net/NeoDocs/View/8446 [date cited: June 1, 2022]. Available from: [Google Scholar]
- 11.Gorstein J., Shreshtra R.K., Pandey S., Adhikari R.K., Pradhan A. Current status of vitamin A deficiency and the National Vitamin A Control Program in Nepal: results of the 1998 National Micronutrient Status Survey, Asia Pac. J. Clin. Nutr. 2003;12:96–103. [PubMed] [Google Scholar]
- 12.Ministry of Health and Population (Nepal), New ERA, UNICEF, EU, USAID, and CDC . Nepal National Micronutrient Status Survey 2016. Ministry of Health and Population; Nepal: 2018. https://www.unicef.org/nepal/media/1206/file/Nepal%20National%20Micronutrient%20Status%20Survey%20Report%202016.pdf [date cited: June 2, 2022]. Available from: [Google Scholar]
- 13.Ermakov I.V., Ermakova M.R., Bernstein P.S., Chan G.M., Gellermann W. Resonance Raman based skin carotenoid measurements in newborns and infants. J. Biophotonics. 2013;6:793–802. doi: 10.1002/jbio.201200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ermakov I.V., Gellermann W. Validation model for Raman based skin carotenoid detection. Arch. Biochem. Biophys. 2010;504:40–49. doi: 10.1016/j.abb.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Mayne S.T., Cartmel B., Scarmo S., Jahns L., Ermakov I.V., Gellermann W. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Arch. Biochem. Biophys. 2013;539:163–170. doi: 10.1016/j.abb.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ermakov I.V., Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics. 2012;5:559–570. doi: 10.1002/jbio.201100122. [DOI] [PubMed] [Google Scholar]
- 17.Ermakov I.V., Whigham L.D., Redelfs A.H., Jahns L., Stookey J., Bernstein P.S., et al. Skin carotenoids as biomarker for vegetable and fruit intake: validation of the reflection-spectroscopy based “veggie meter. F.A.S.E.B. J. 2016;30 doi: 10.1096/fasebj.30.1_supplement.409.3. 409.3. [DOI] [Google Scholar]
- 18.Radtke M.D., Poe M., Stookey J., Jilcott Pitts S., Moran N.E., Landry M.J., et al. Recommendations for the use of the Veggie Meter® for spectroscopy-based skin carotenoid measurements in the research setting. Curr. Dev. Nutr. 2021;5 doi: 10.1093/cdn/nzab104. nzab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrady C.D., Bell J.P., Besch B.M., Gorusupudi A., Farnsworth K., Ermakov I., et al. Correlations between macular, skin, and serum carotenoids. Invest. Ophthalmol. Vis. Sci. 2017;58:3616–3627. doi: 10.1167/iovs.17-21818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henriksen B.S., Chan G., Hoffman R.O., Sharifzadeh M., Ermakov I.V., Gellermann W., et al. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Invest. Ophthalmol. Vis. Sci. 2013;54:5568–5578. doi: 10.1167/iovs.13-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahns L., Johnson L.K., Mayne S.T., Cartmel B., Picklo M.J., Sr., Ermakov I.V., et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. Am. J. Clin. Nutr. 2014;100:930–937. doi: 10.3945/ajcn.114.086900. [DOI] [PubMed] [Google Scholar]
- 22.Ermakov I.V., Ermakova M., Sharifzadeh M., Gorusupudi A., Farnsworth K., Bernstein P.S., et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018;646:46–54. doi: 10.1016/j.abb.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comstock G.W., Alberg A.J., Helzlsouer K.J. Reported effects of long-term freezer storage on concentrations of retinol, beta-carotene, and alpha-tocopherol in serum or plasma summarized. Clin. Chem. 1993;39:1075–1078. doi: 10.1093/clinchem/39.6.1075. [DOI] [PubMed] [Google Scholar]
- 24.Addo E.K., Gorusupudi A., Allman S., Bernstein P.S. The Lutein and Zeaxanthin in Pregnancy (L-ZIP) study-carotenoid supplementation during pregnancy: ocular and systemic effects-study protocol for a randomized controlled trial. Trials. 2021;22:300. doi: 10.1186/s13063-021-05244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y.K., Quadro L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 2010;652:263–275. doi: 10.1007/978-1-60327-325-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Leenheer A.P., Nelis H.J., Lambert W.E., Bauwens R.M. Chromatography of fat-soluble vitamins in clinical chemistry. J. Chromatogr. 1988;429:3–58. doi: 10.1016/s0378-4347(00)83866-9. [DOI] [PubMed] [Google Scholar]
- 27.Gueguen S., Herbeth B., Siest G., Leroy P. An isocratic liquid chromatographic method with diode-array detection for the simultaneous determination of alpha-tocopherol, retinol, and five carotenoids in human serum. J. Chromatogr. Sci. 2002;40:69–76. doi: 10.1093/chromsci/40.2.69. [DOI] [PubMed] [Google Scholar]
- 28.WHO . Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. World Health Organization; Geneva: 2011. [date cited: June 2, 2022]. Available from: https://apps.who.int/iris/bitstream/handle/10665/85859/WHO_NMH_NHD_MNM_11.3_eng.pdf?sequence=4&isAllowed=y. [Google Scholar]
- 29.Thorne-Lyman A.L., Parajuli K., Paudyal N., Chitekwe S., Shrestha R., Manandhar D.L., et al. To see, hear, and live: 25 years of the vitamin A programme in Nepal. Matern Child Nutr. 2022;18 doi: 10.1111/mcn.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitekwe S., Parajuli K.R., Paudyal N., Haag K.C., Renzaho A., Issaka A., et al. Individual, household and national factors associated with iron, vitamin A and zinc deficiencies among children aged 6-59 months in Nepal. Matern Child Nutr. 2022;18(Suppl 1):e13305. doi: 10.1111/mcn.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guideline: vitamin A supplementation in infants and children 6-59 months of age. World Health Organization; Geneva: 2011. https://apps.who.int/iris/bitstream/handle/10665/44664/9789241501767_eng.pdf [date cited: June 2, 2022]. Available from: [PubMed] [Google Scholar]
- 32.Sachs J.D. From millennium development goals to sustainable development goals. Lancet. 2012;379:2206–2211. doi: 10.1016/S0140-6736(12)60685-0. [DOI] [PubMed] [Google Scholar]
- 33.Tanumihardjo S.A. World Health Organization, Report: priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. World Health Organization; Geneva: 2012. Biomarkers of vitamin A status: what do they mean? pp. 44–54. [Google Scholar]
- 34.Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. World Health Organization; Geneva: 1996. https://apps.who.int/iris/bitstream/handle/10665/63064/WHO_NUT_96.10_eng.pdf?sequence=1&isAllowed=y [date cited: June 2, 2022]. Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in this manuscript will be made available from the corresponding author upon reasonable request.