Abstract
Aim
Glaucoma is a group of degenerative diseases of the optic nerve whose predisposing factors may be genetic. The objective of this study was to estimate the frequency of the Glu323Lys mutation as a genetic risk factor for glaucoma.
Materials and methods
A cross-sectional study over 6 months from October 2020 to March 2021 in Ouagadougou, Burkina Faso. A total of 89 samples of patients with primary open-angle glaucoma (POAG) were collected. The frequency of the Glu323Lys mutation of the myocilin, trabecular meshwork inducible glucocorticoid response (TIGR/MYOC) gene by polymerase chain reaction (PCR)—restriction fragment length polymorphism.
Results
In glaucoma patients, only homozygous nonmutated guanine-guanine (GG) and heterozygous mutated adenine-guanine (AG) genotypes were found in 96.63 and 3.37% of cases, respectively. Around 69.66% of patients had a family history of glaucoma, 28.09% had a history of hypertension, and 7.86% had a history of diabetes.
Conclusion
The frequency of the Glu323Lys mutation of the TIGR/MYOC gene was 3.37% in the glaucoma population in Ouagadougou. A case-control study is necessary to know the contribution of the Glu323Lys mutation as a genetic risk factor for glaucoma in our study population.
Clinical significance
This study constituted the beginning of genetic investigations of glaucoma in our context and showed a low Glu323Lys mutation.
How to cite this article
Traoré L, Sanou J, Bakyono BS, et al. Prevalence of Glu323Lys Mutation of the TIGR/MYOC Gene and Risk Factors amongst Primary Open-angle Glaucoma Patients in Ouagadougou, Burkina Faso. J Curr Glaucoma Pract 2023;17(2):79-84.
Keywords: Burkina Faso, Glu323Lys mutation, Risk factors glaucoma
Introduction
Glaucoma is the second leading cause of blindness worldwide after cataracts.1 As the leading cause of irreversible blindness, glaucoma accounts for approximately 15% of all blindness.2,3 Glaucoma is, therefore, a public health problem. Older people pay the highest price. Indeed, the number of people aged 40–80 with glaucoma worldwide was estimated at 64.3 million.3 This number would be 76 million in 2020 and 111.8 million in 2040.3 Among the major forms of glaucoma, POAG is the most prevalent form.3 Its prevalence varies according to the geographical area, race, age, and ethnicity.3,4 POAG would be the most frequent form encountered in melanoderma subjects.3,5,6 In Africa, the overall prevalence of POAG is estimated to be between 1 and 8%, depending on the region.7 Glaucoma is a complex disease with an insidious course. Approximately 50% of patients with POAG are unaware of their condition.6–8 Several studies have shown that factors such as advanced age, high intraocular pressure (IOP), diabetes, and myopia would significantly increase the risk of developing glaucoma.4,5,9,10 Also, there would be predisposing genetic factors for glaucoma due to ethnic origin and family history of glaucoma.11 The various studies conducted on the genetics of glaucoma have provided strong evidence suggesting that the occurrence of glaucoma is strongly affected by genetic factors.12–17 Approximately 74 genes have been closely associated with glaucoma, 64% of which are associated with POAG, 16% with primary angle closure glaucoma, 4% with congenital glaucoma, and 4% with pseudoexfoliation glaucoma.11 Among the genes incriminated in the occurrence of glaucoma, only four of them [TIGR/MYOC, neurotrophin-4 (NTF4), OPTN, and WDR36] have been definitively associated with PAOG12 and one gene, CYP1B1, with guanine-cytosine.13 Three of these genes, TIGR/MYOC, OPTN, and CYP1B1, have been identified as the major genes responsible for the development of glaucoma in humans. Their involvement varies depending on the type of glaucoma.14 The myocilin gene (TIGR/MYOC) is the first-line gene associated with the occurrence of POAG.12 Autosomal dominant mutations in this gene are thought to be responsible for 33% of juvenile glaucoma cases and 3–5% of adult glaucoma cases.15 The Glu323Lys mutation, like most mutations in the TIGR/MYOC gene, is located in exon 3 and consists of a substitution of guanine for adenine in the gene sequence at position 967.17 The mutation has only been reported in cases of familial glaucoma.
In Burkina Faso, as in other African countries, the diagnosis of glaucoma is often late, and very few studies have attempted to explore the predisposing genetic factors involved in its occurrence. The aim of this study was to estimate the frequency of the genetic susceptibility factor of the Glu323Lys mutation of the TIGR/MYOC gene associated with the occurrence of glaucoma for the prevention of the pathology.
Materials and Methods
Type and Site of the Study
This was a cross-sectional study on the frequency of the Glu323Lys mutation of the TIGR/MYOC gene during a 6-month period (October 2020–March 2021) at the Centre Hospitalier Universitaire Yalgado Ouédraogo (CHU-YO) and the Centre Médical Don Orione (CMDO) in Ouagadougou, Burkina Faso.
Sampling and Inclusion of Patients
A total of 89 patients with POAG, most of whom resided in urban areas, were collected by accidental sampling. The estimated sample size was calculated using the Schwartz formula for an estimated glaucoma prevalence of 4.04% (which corresponds to the prevalence of glaucoma according to a cross-sectional study of retrospective data conducted in Ouagadougou between 2012 and 2019).
The study included people of all socioprofessional strata, regardless of sex or ethnicity, who were older than 3 years (excluding congenital glaucoma) and had POAG.
A patient with POAG was considered to be any patient with optic disc excavation and atrophy, visual field amputation characteristic of glaucoma, an open iridocorneal angle (degree of opening between 10 and 45°), an IOP at the time of diagnosis greater than 22 mm Hg, and follow-up by a specialist in the health centers involved in the study.
The age, family history, hypertension, and diabetes of the patients were collected through the medical file of each patient. Dietary and sports habits were collected by means of a questionnaire to which patients were asked to answer yes or no. Patients considered as practicing sports were those who practiced sports at least three times a week, and coffee or tea drinkers were those who had at least one cup of coffee or tea per day. All of this information was recorded, and the calculation of the proportions was performed by Microsoft Excel 2013.
A venous blood sample of about 5 mL in an ethylenediaminetetraacetic acid tube was taken from the patients, from which two aliquots were constituted—one of plasma and the other of the pellet after centrifugation of 3500 rpm for 15 minutes.
Extraction and Amplification of Deoxyribonucleic Acid (DNA)
Genomic DNA was extracted from the pellet using the ”rapid salting out” technique described by Miller et al. in 1988.
Amplification of the TIGR/MYOC gene was done by conventional PCR. Each well of a PCR plate contained a total reaction volume of 25 µL consisting of 10 µL of EmeraldAmp GT PCR master mix (2× Premix, containing taq polymerase, deoxynucleoside triphosphates, MgCl2, DNA bulking agent), 0.5 µL of each primer (0.1 µmol/µL), 9 µL of sterile water, and 5 µL of DNA (10 ng/µL). Amplification was performed using the GeneAmp* PCR System 9700. The sequence of the primer pair and the PCR program that was is are shown in Table 1.
Table 1.
Specific primer pair and PCR program for the amplification of the TIGR/MYOC gene23
| Primer sequences | Amplicon size in bp |
|---|---|
| Forward—CACCCAGGAGACCACGTGGAGAATC | 325 |
| Reverse—GAGGCCTGCTTCATCCACAGCCAAG | |
| Glu323Lys | Initial denaturation—94°C, 3 minutes |
| Denaturation—94°C, 30 seconds Hybridization—55°C, 30 seconds 35 cycles Elongation—72°C, 60 seconds | |
| Final extension—72°C, 5 minutes |
Visualization of PCR Products and Enzymatic Digestion
Polymerase chain reaction (PCR) products were subjected to electrophoresis on a 2% agarose gel containing 18 µL of 10 mg/mL ethidium bromide.
Polymerase chain reaction (PCR) products were then digested with Bsr I restriction endonuclease for 2 hours at 65°C in the GeneAmp* PCR System 9700 thermal cycler (Applied Biosystem, United States of America). The reaction volume was 20 µL consisting of 0.5 µL of Bsr I enzyme, 5 µL of buffer (1×), 9.5 µL of sterile water, and 5 µL of PCR product. Digestion was performed, and the digestion products were then subjected to electrophoresis on a 3.5% agarose gel containing 18 µL of ethidium bromide (10 mg/mL).
Visualization of DNA bands was done under UV light at 132 nm using the ”Vivilber” apparatus.
Ethical Considerations
The study was approved by the Pietro Annigoni Biomolecular Research Center/Laboratory of Molecular and Genetic Biology (CERBA/LABIOGENE) Institutional Ethics Committee and was conducted in strict compliance with the Declaration of Helsinki. Oral consent was obtained from each patient after reading the information sheet. The study was authorized by the Direction Regional health agency and the hospitals involved. The confidentiality of the patient's data was also respected.
Results
Sociodemographic Characteristics
Table 2 shows that the study population consisted of 89 patients with a confirmed diagnosis of glaucoma, most of whom lived in urban areas (93.26%). 69.66% of patients with glaucoma had a family history of glaucoma.
Table 2.
Demographics of our study population and family history of glaucoma
| Variables | Number (%) | |
|---|---|---|
| Sex | Man | 51 (57.30) |
| Women | 38 (42.70) | |
| Age | ≤ 40 years | 19 (21.35) |
| > 40 years | 70 (78.65) | |
| Residence area | Urban | 83 (93.26) |
| Rural | 6 (6.74) | |
| Profession | Public sector | 2 (24.72) |
| Private sector | 35 (39.32) | |
| Retirement | 24 (26.97) | |
| Pupil/student | 08 (8.99) | |
| Family history of glaucoma | ||
| Yes | 62 (69.66) | |
| No | 27 (30.34) | |
Fragments Obtained by PCR-RFLP of the TIGR/MYOC Gene
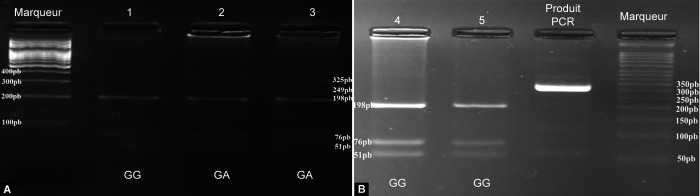
After conventional PCR, the amplicon obtained from the TIGR/MYOC gene had a size of 325 base pairs (bp). After digestion, the homozygous nonmutated genotype (GG) had three digest fragments, including 198, 76, and 51 bp. The homozygous mutated genotype (adenine-adenine) had two fragments, including 249 and 76 bp. The heterozygous mutated or heterozygous nonmutated genotype (AG) had four fragments, including 249, 198, 76, and 51 bp (Fig. 1).
Figs 1A and B.
Electrophoresis gels: (A) Electrophoretic profile of digestion products; (B) Electrophoretic profile of digestion products and amplification products; GG, unmutated homozygous; GA, mutated heterozygous A and B:
Genotypic and Allelic Frequencies in Our Study Population
Two genotypes were identified in glaucoma patients, with the wild-type (GG) genotype predominating (Fig. 2). The wild-type (G) allele was the most represented in our study population, with a frequency of 98.32%. The mean age at diagnosis of patients with the mutation was 41 years (age between 16 and 73).
Fig. 2.
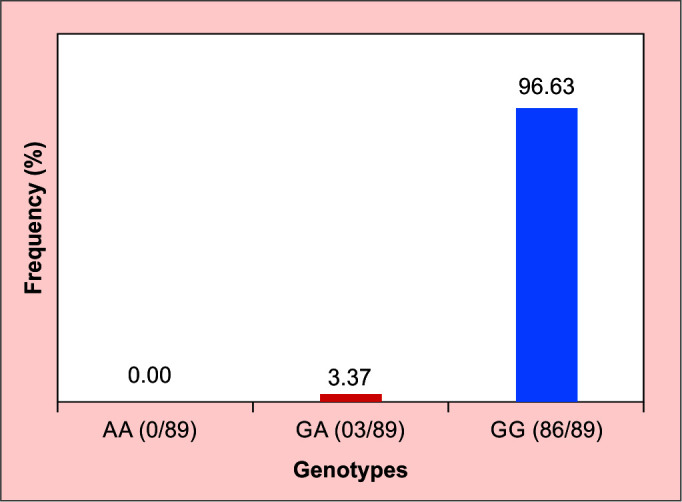
Genotypic frequencies; AA, homozygous mutated; GA, mutated heterozygous; GG, unmutated homozygous
Glaucoma and Other Risk Factors (Dietary Habits, Sports, History of Hypertension, and Diabetes)
Glaucoma patients who did not consume alcohol, did not smoke, or did not practice sports regularly were the most numerous. 28.09% of the glaucoma patients in our study had hypertension, and 7.86% had diabetes (Table 3).
Table 3.
Age at diagnosis according to the eating and sports habits of glaucomatous patients
| Number (%) | |
|---|---|
| Alcohol consumer | |
| Yes | 30 (33.7) |
| No | 59 (66.29) |
| Smoking | |
| Yes | 2 (2.25) |
| No | 87 (97.75) |
| Coffee or tea consumer | |
| Yes | 53 (59.55) |
| No | 36 (40.45) |
| Regular practice of sport | |
| Yes | 27 (30.34) |
| No | 62 (69.66) |
| HBP | |
| Oui | 25 (28.09) |
| Non | 64 (71.91) |
| Diabetes | |
| Oui | 7 (07.86) |
| Non | 82 (92.14) |
Discussion
Frequency of the Glu323Lys Mutation
The objective of our study was to determine the frequency of the Glu323Lys mutation. This choice is justified by the fact that, to our knowledge, the mutation had not been found in the melanoderma population. In addition, nearly 77 mutations in the TIGR/MYOC gene have been associated with glaucoma, including 71 mutations located on exon 3, five on exon 1, and one on exon 2.17 In our study, the frequency of the Glu323Lys mutation is 3.37% in the glaucoma population. In Panama, Central America, the study by Rozsa et al. estimated the frequency of the Glu323Lys mutation to be 100% in members of the same family with glaucoma. The frequency of the Glu323Lys mutation in our study is lower than in the study by Rozsa et al. This may be explained by the fact that our study included patients from different families in the great majority. The Glu323Lys mutation has been described as causing severe glaucoma with elevated IOP and age of onset of <30 years.17 In the present study, the mean age of the subjects with the Glu323Lys mutation was 41 years (the age of the patients ranged from 16 to 73); it is higher than the mean age found by Rozsa et al., which was 19 years. This could be explained by a late diagnosis of glaucoma in Burkina Faso. Indeed, a large number of studies suggest an early and sometimes very severe onset of glaucoma in melanoderma subjects compared with Caucasians5,18,19; in Burkina Faso, as in many sub-Saharan African countries, glaucoma is diagnosed at a fairly advanced stage of the disease.
Of the genes implicated in the development of glaucoma, only four (TIGR/MYOC, NTF4, OPTN, and WDR36) have been definitively associated with PAOG,13 and one gene, CYP1B1, with CG.13 Three of these genes, TIGR/MYOC, OPTN, and CYP1B1, have been identified as the major genes responsible for the development of glaucoma in humans, accounting for approximately 40% of cases. Their involvement varies according to the type of glaucoma.14
Prevalence of Glaucoma by Gender
The prevalence of glaucoma in men (57.30%) was slightly higher than in women (42.70%), with a sex ratio of 1.34. The TIGR/MYOC and OPTN genes are autosomal dominant,15 and the CYP1B1 gene has an autosomal recessive inheritance.20,21 Thus, individuals would be affected regardless of their sex. However, like our results, some authors have found a male or female predominance of the pathology.18,21
Age, history of glaucoma, diabetes, arterial hypertension, dietary, and sports habits of our study population
In this study, the aim was to estimate the proportion of glaucoma patients with a history of glaucoma, diabetes, high blood pressure (HBP) or with dietary habits (intake of stimulants such as coffee, tea, alcohol, or tobacco) or sports habits that, according to some studies, are risk factors for glaucoma.22–24
Glaucoma and Age
Age has been identified as a factor involved in the occurrence of glaucoma, with the incidence of glaucoma increasing with age 18. In our study, the mean age of patients at diagnosis was 47.33 ± 13.81 years. Atipo-Tsiba in Congo and de Ahnoux-Zabsonré and colleagues in Ivory Coast found a mean age of 47.9 ± 18.7 years and 47.47 ± 18.3 years, respectively.18,25 Boodhna and Crabb in England, Bron and collaborators in France, and Kooner and collaborators in Dallas (United States of America) found a mean age of 65.5, 65.6 ± 11.7, and 68.7 ± 13.8 years, respectively, in Caucasian subjects.4,19,26 According to some studies, the onset of glaucoma is earlier in melanoderma subjects than in Caucasians.5 These data support the idea of early onset of glaucoma in African melanoderma subjects.
Glaucoma and Alcohol, Tobacco, and Caffeine Use
The relationship between alcohol, smoking, and glaucoma remains less clear, and the results of studies associating them with the development of glaucoma are conflicting.4 In this study, among glaucoma patients, 33.7% had an alcohol consumption habit, and 2.25% smoked cigarettes; also, 59.55% had a daily coffee habit. The studies by Charliat et al. and Wilson et al. found no relationship between alcohol consumption and glaucoma and between smoking and glaucoma.27,28 On the other hand, Wu and Leske found a risk of developing glaucoma associated with alcohol and tobacco consumption.29 The contradictory effect could be explained by the variable effect depending on the type of alcohol and tobacco and the frequency of consumption of these products. Indeed, some alcoholic beverages are made up of mono alcohol and others of polyalcohol. The difference in chemical composition could, for example, according to the type of alcohol, induce different effects on the body.30
Caffeine would be involved in the increase of IOP in glaucoma patients and consequently would be associated with the progression of glaucoma. Indeed, the comparison of the effect of caffeine on IOP showed a significant increase in IOP in glaucoma subjects compared to normal subjects.31
Glaucoma and Sports Activities
Moderate and regular sports practice has been shown to be a protective factor not only in the occurrence but also in the progression of certain pathologies. For example, sport has a protective effect on the occurrence of cardiovascular disease.32 In our study, the proportion of patients who regularly practiced sports was 30.34% compared to 69.66% who did not practice sports.
Glaucoma, Diabetes, and High Blood Pressure
In this study, the glaucoma population consisted of 28.09% hypertensives and 7.86% diabetics. The prevalence of hypertension is estimated at 18.6% (Doulougou, 2015), and that of diabetes at 4.2% in the Burkinabè population.1 Several studies have evaluated the role of diabetes,4,10,33 and hypertension34,35 as risk factors associated with the occurrence of glaucoma. The information provided by these studies for the involvement of these pathologies in the occurrence of glaucoma is mostly contradictory.4 The frequency of hypertension and diabetes is higher in the glaucoma population than in the general population in Burkina Faso. However, the role of diabetes and hypertension as risk factors for glaucoma in the glaucoma population in Ouagadougou remains to be explored.
Family History of Glaucoma
In our study, 86.85% of patients had a family history of glaucoma. In general, our results are in agreement with those of other studies,36 which suggested that a family history of glaucoma constitutes a risk of developing glaucoma. Indeed, Wolfs et al. showed that first-degree relatives of individuals with glaucoma had a 10% increased risk of developing glaucoma. Furthermore, relatives of a person with glaucoma have a 22% lifetime risk of developing POAG, whereas this risk is 2–3% for relatives of people without glaucoma.5
With regard to family history of glaucoma, Wolfs et al., since 1998, have shown that first-degree relatives of people with glaucoma have a 10% increased risk of developing glaucoma. Also, according to the team of Racette et al., relatives of a person with glaucoma have a 22% lifetime risk of developing POAG, whereas this risk is 2–3% for relatives of people without glaucoma.5 A total of 69.66% (62/89) of the patients in our study had a history of glaucoma. As for the use of stimulants, the link with glaucoma remains less clear, and the results of studies associating them with the development of glaucoma are contradictory.4
Conclusion
This study is an initial investigation of the frequency of the Glu323Lys mutation of the TIGR/MYOC gene in patients with POAG in Ouagadougou, Burkina Faso. The heterozygous mutated genotype (guanine-adenine) with a frequency of 3.37%, and the homozygous wild-type genotype (GG) was the predominant genotype (96.63%). It would be interesting in our context to look for the Glu323Lys mutation in family members of patients with the mutation. In addition to the Glu323Lys mutation, it would be important to search for and estimate the frequency of other genetic susceptibility factors of the TIGR/MYOC gene in our study population. Patients with glaucoma consumed a minority of tobacco (2.25%), alcohol (33.7%), and a majority of coffee (59.55%). Sports were identified in a small proportion of the glaucoma patients in our study population (30.34%). A case-control and family study would allow us to better understand the involvement of the Glu323Lys mutation and to evaluate the role of certain risk factors for glaucoma in the occurrence of glaucoma in our study population.
Authors’ Contributions
BSB, JSi, and JSa designed this study. SBS, JSa, and GMH recruited patients and control cases. BSB, LT, AAZ, TMZ, HKS, ATY, and ZBT carried out the manipulations. BSB, AAZ, HKS, and LT carried out statistical analyses and wrote the manuscript. TMZ, HKS, ATY, ZBT, FWD, and JSi revised the manuscript. All authors have read and corrected the manuscript.
Acknowledgments
We thank the CHU-YO and CMDO for hosting the study, the participants for agreeing to be part of the study, and CERBA/LABIOGENE for the technical platform for molecular testing.
Footnotes
Source of support: This study was supported by CERBA/LABIOGENE (Pietro Annigoni Biomolecular Research Center/Laboratory of Molecular and Genetic Biology).
Conflict of interest: None
References
- 1.OMS. Maladies oculaires prioritaires. WHO. https://www.who.int/blindness/causes/priority/fr/index7.html https://www.who.int/blindness/causes/priority/fr/index7.html Published November 3, 2020. Accessed November 3, 2020.
- 2.Thylefors B, Négrel AD. Le glaucome dans le monde. Bull World Health Organ. 1994;72(4):539–542. [PMC free article] [PubMed] [Google Scholar]
- 3.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Bron A, Chaine G, Villain M, et al. Les facteurs de risque du glaucome primitif à angle ouvert. J Fr Ophtalmol. 2008;31(4):435–444. doi: 10.1016/S0181-5512(08)71443-8. [DOI] [PubMed] [Google Scholar]
- 5.Racette L, Wilson MR, Zangwill LM, et al. Primary open-angle glaucoma in blacks: a review. Surv Ophthalmol. 2003;48(3):295–313. doi: 10.1016/S0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DS, Wolfs RCW, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leske MC. Open-angle glaucoma—an epidemiologic overview. Ophthalmic Epidemiol. 2007;14(4):166–172. doi: 10.1080/09286580701501931. [DOI] [PubMed] [Google Scholar]
- 8.Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of hispanic subjects: proyecto VER. Arch Ophthalmol. 2001;119(12):1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 9.Le A, Mukesh BN, McCarty CA, et al. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44(9):3783–3789. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 11.Wang HW, Sun P, Chen Y, et al. Research progress on human genes involved in the pathogenesis of glaucoma (review). Mol Med Rep. 2018;18(1):656–674. doi: 10.3892/mmr.2018.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Malik MA, Goswami S, et al. Candidate genes involved in the susceptibility of primary open angle glaucoma. Gene. 2016;577(2):119–131. doi: 10.1016/j.gene.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Faiq M, Sharma R, Dada R, et al. Genetic, biochemical and clinical insights into primary congenital glaucoma. J Curr Glaucoma Pract. 2013;7(2):66–84. doi: 10.5005/jp-journals-10008-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresson-Dumont H, Zanlonghi X, Bodet S, et al. Le Glaucome: bases moléculaires et diagnostic génétique en France. J Fr Ophtalmol. 2005 doi: 10.1016/S0181-5512(05)73593-2. [DOI] [Google Scholar]
- 15.Belleau P. Identification des gènes modifiant l’âge d'apparition du glaucome primaire à angle-ouvert dans une famille canadienne-française fondatrice. Thèse de doctorat en medécine moléculaire. 2016 Québec; Accessed August 9, 2019. [Google Scholar]
- 16.Rozsa FW, Shimizu S, Lichter PR, et al. GLC1A mutations point to regions of potential functional importance on the TIGR/MYOC protein. Mol Vis. 1998;4:20. 9772276 [PubMed] [Google Scholar]
- 17.Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29(2):207–11. doi: 10.1002/humu.20634. Feb; [DOI] [PubMed] [Google Scholar]
- 18.Ahnoux-Zabsonre A, Keita C, Safede K, et al. Prévalence du glaucome chronique primitif à angle ouvert en Côte d’Ivoire. J Fr Ophtalmol. 1998;21(9):643–647. 9894202 [PubMed] [Google Scholar]
- 19.Boodhna T, Saunders LJ, Crabb DP. Are rates of vision loss in patients in English glaucoma clinics slowing down over time? Trends from a decade of data. Eye (Lond) 2015;29(12):1613–1619. doi: 10.1038/eye.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mookherjee S, Acharya M, Banerjee D, et al. Molecular basis for involvement of CYP1B1 in MYOC upregulation and its potential implication in glaucoma pathogenesis. PLoS ONE. 2012;7(9):e45077. doi: 10.1371/journal.pone.0045077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vittitow JL, Borrás T. Expression of optineurin, a glaucoma-linked gene, is influenced by elevated intraocular pressure. Biochem Biophys Res Commun. 2002;298(1):67–74. doi: 10.1016/S0006-291X(02)02395-1. [DOI] [PubMed] [Google Scholar]
- 22.Eballé AO, Owono D, Bella AL, et al. Caractéristiques cliniques et épidémiologiques du glaucome chronique à angle ouvert. Cah Santé. 2008;18(1):19–23. doi: 10.1684/san.2008.0095. [DOI] [PubMed] [Google Scholar]
- 23.Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma: the barbados eye study. Arch Ophthalmol. 1995;113(7):918–924. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 24.Wu SY, Nemesure B, Hennis A, et al. Nine-year changes in intraocular pressure: the barbados eye studies. Arch Ophthalmol. 2006;124(11):1631–1636. doi: 10.1001/archopht.124.11.1631. [DOI] [PubMed] [Google Scholar]
- 25.Atipo-Tsiba PW. Le glaucome primitif à angle ouvert : Evaluation du niveau de connaissance du médecin généraliste à Brazzaville sur cette maladie. Rwanda Med J. 2015;72(3):14–16. [Google Scholar]
- 26.Kooner KS, Joseph A, Shar A, et al. Dallas glaucoma registry: preliminary results. J Clin Exp Ophthalmol. 2011;2(6):164. doi: 10.4172/2155-9570.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charliat G, Jolly D, Blanchard F. Genetic risk factor in primary open-angle glaucoma: a case-control study. Ophthalmic Epidemiol. 1994;1(3):131–138. doi: 10.3109/09286589409047221. [DOI] [PubMed] [Google Scholar]
- 28.Wilson MR, Hertzmark E, Walker AM, et al. A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol. 1987;105(8):1066–1071. doi: 10.1001/archopht.1987.01060080068030. [DOI] [PubMed] [Google Scholar]
- 29.Wu SY, Leske MC. Associations with intraocular pressure in the barbados eye study. Arch Ophthalmol. 1997;115(12):1572–1576. doi: 10.1001/archopht.1997.01100160742012. [DOI] [PubMed] [Google Scholar]
- 30.Stuart KV, Madjedi K, Luben RN, et al. Alcohol, intraocular pressure, and open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2022;129(6):637–652. doi: 10.1016/j.ophtha.2022.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Wang M, Guo W, et al. The effect of caffeine on intraocular pressure: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2011;249(3):435–442. doi: 10.1007/s00417-010-1455-1. [DOI] [PubMed] [Google Scholar]
- 32.Charles-Edouard P. Les effets de l'activité physique et sportive sur les maladies cardiovasculaires. 2016 [Google Scholar]
- 33.Dielemans I, de Jong PT, Stolk R, et al. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. Ophthalmology. 1996;103(8):1271–1275. doi: 10.1016/S0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- 34.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt study. Ophthalmology. 2000;107(7):1287–1293. doi: 10.1016/S0161-6420(00)00138-X. [DOI] [PubMed] [Google Scholar]
- 35.Jonas JB, Gründler AE. Prevalence of diabetes mellitus and arterial hypertension in primary and secondary open-angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 1998;236(3):202–206. doi: 10.1007/s004170050065. [DOI] [PubMed] [Google Scholar]
- 36.Wolfs RC, Klaver CC, Ramrattan RS, et al. Genetic risk of primary open-angle glaucoma: population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1640–1645. doi: 10.1001/archopht.116.12.1640. [DOI] [PubMed] [Google Scholar]