ABSTRACT
The multi-functional endoplasmic reticulum (ER) is exploited by viruses to cause infection. Morphologically, this organelle is a highly interconnected membranous network consisting of sheets and tubules whose levels are dynamic, changing in response to cellular conditions. Functionally, the ER is responsible for protein synthesis, folding, secretion and degradation, as well as Ca2+ homeostasis and lipid biosynthesis, with each event catalyzed by defined ER factors. Strikingly, these ER host factors are hijacked by viruses to support different infection steps, including entry, translation, replication, assembly and egress. Although the full repertoire of these ER factors that are hijacked is unknown, recent studies have uncovered several ER membrane machineries that are exploited by viruses – ranging from polyomavirus to flavivirus and coronavirus – to facilitate different steps of their life cycle. These discoveries should provide better understanding of virus infection mechanisms, potentially leading to the development of more effective anti-viral therapies.
Keywords: Endoplasmic reticulum, ER membrane complex, ER morphogenesis, Polyomavirus, Flavivirus, Coronavirus
Summary: We review recent advances revealing how diverse viruses exploit host endoplasmic reticulum membrane chaperones and morphogenic machinery to cause infection.
Introduction
The endoplasmic reticulum (ER) supports different steps of the virus life cycle, including entry, translation, replication, assembly and egress. Although a myriad of viruses – ranging from DNA viruses to RNA viruses, as well as from enveloped viruses to non-enveloped viruses – exploit the activities of many ER factors to facilitate these distinct infection steps, the full repertoire of ER factors hijacked by viruses to promote infection remains unknown. Nonetheless, the identities of these ER factors are slowly emerging, with recent reports identifying new ER membrane protein complexes that are exploited to facilitate the steps of viral entry and replication. This Review will focus on these exciting new findings, emphasizing their significance in the context of host ER–virus interactions.
Basic morphology and functions of the ER
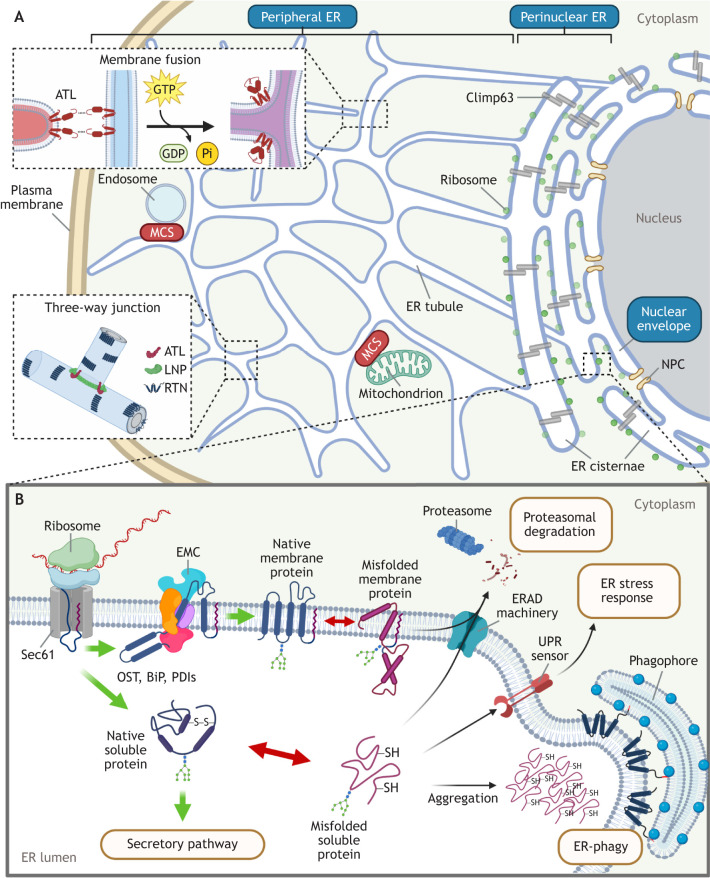
Morphologically, the ER is a highly interconnected membranous network that extends from the nuclear envelope to the plasma membrane (Fig. 1A). This organelle is divided into three main subdomains, with the perinuclear ER cisternae located proximal to the nucleus displaying a ribosome-decorated sheet-like morphology (referred to as rough ER), while the peripheral ER network extends from the perinuclear ER sheets, appearing as tubular structures devoid of ribosomes (referred to as smooth ER). The nuclear envelope is the final ER subdomain and is contiguous with the outer ER membrane (Perkins and Allan, 2021; Shibata et al., 2009; Voeltz et al., 2002).
Fig. 1.
Basic morphology and functions of the ER. (A) Morphologically, the ER is divided into three subdomains: the perinuclear ER, containing sheet-like membranes with associated ribosomes that support protein biosynthesis; the peripheral ER, which harbors tubules that create ER three-way junctions, giving rise to the web-like ER appearance; and the nuclear envelope, which is studded with nuclear pore complexes (NPCs) connecting the nucleoplasm to the cytoplasm. The ER transmembrane protein Climp63 acts as a luminal spacer to maintain the ER sheets of the perinuclear ER, while the ER morphogenesis machinery composed of ATL proteins, RTN proteins and LNP at the peripheral ER promotes formation of ER three-way junctions. ATL proteins trigger fusion between a tubular ER terminus and the side of another tubule via a GTP-dependent process to generate three-way junctions. The vast extent of the ER network within a cell enables it to engage other cellular organelles, including endosomes and mitochondria, via MCSs. (B) The biogenesis of luminal and membrane proteins by ER membrane-associated ribosomes is coupled to the co-translational translocation of the nascent polypeptide chain across the ER membrane and into the ER lumen by the Sec61 translocon. Folding of the imported nascent protein is assisted by several proteins that reside in the ER lumen, such as OST, BiP and PDIs. Membrane proteins are further incorporated into the lipid bilayer with the assistance of the EMC. When a client protein misfolds, sensors of the UPR transduce signals to activate the ER stress response. Misfolded proteins are retro-translocated and directed to the cytosolic proteasome for degradation via the ERAD pathway. In some circumstances, misfolded proteins form luminal aggregates that are targeted and engulfed by the cytosolic phagophore and directed further for lysosomal degradation via the ER-phagy pathway.
Although morphologically distinct, ER tubules and sheets are interchangeable, with several evolutionarily conserved ER-shaping factors playing key roles in rearranging the ER morphology (Chen et al., 2013; Wang and Rapoport, 2019). For instance, ER membrane proteins of the reticulon (RTN) family embed their wedge-like hydrophobic domains into the cytosolic leaflet of the lipid bilayer to generate positive ER membrane curvature, which is the key feature of ER tubules (Voeltz et al., 2006). In concert with RTN proteins, the ER membrane proteins lunapark (LNP) and members of the atlastin (ATL) family form a so-called ‘morphogenic protein machinery’, promoting the formation and stabilization of three-way ER junctions that give rise to the reticular tubular ER morphology (Wang et al., 2016). In this process, ATL proteins employ GTPase-dependent membrane fusion activity to promote homotypic fusion of ER tubules to generate the three-way ER junctional sites (Hu et al., 2009; Orso et al., 2009), whereas LNP acts to stabilize these junctions (Chen et al., 2015, 2012). This creates the reticular structure of the ER and helps to prevent ER fragmentation (Orso et al., 2009; Voeltz et al., 2006). By contrast, another ER membrane protein, Climp63 (also known as CKAP4), serves as a luminal ER spacer (Shibata et al., 2010) and is responsible for the formation of ER sheets. The relative levels of ER sheets and ER tubules are in constant flux in cells, changing in response to varying environmental conditions, with cytoskeleton dynamics playing a role (Puhka et al., 2012).
One defining characteristic of ER tubules is that they are highly branched, resulting in a sizable surface area that allows the ER to make direct physical contacts with many other organelles and lipid droplets (Elbaz and Schuldiner, 2011; Friedman and Voeltz, 2011; Hugenroth and Bohnert, 2020; Raiborg et al., 2015), called ER membrane contact sites (MCSs). Although the functions and the underlying mechanisms of ER MCSs remain largely elusive, the best characterized are the ER–mitochondria MCSs, which coordinate lipid transfer and Ca2+ flux between the two organelles (Rowland and Voeltz, 2012; Sassano et al., 2022; Wilson and Metzakopian, 2021).
Functionally, the ER executes many roles (Fig. 1B). One key ER function is the translation of the vast majority of cellular luminal and membrane proteins. Because translation on the outer surface of the ER is coupled with translocation of the nascent polypeptide chain across the ER membrane, this protein biosynthetic process is called co-translational translocation (Park and Rapoport, 2012). Co-translational translocation is mediated by docking of the translating ribosomes onto the Sec61 translocon, an ER membrane channel that provides the protein conduit for the translating polypeptide chain (Kalies et al., 1994). The Sec61 translocon is physically linked to an evolutionarily conserved multi-subunit ER membrane protein complex (EMC) (Lin et al., 2019), whose major function is to assist in the biosynthesis and insertion of client membrane proteins (Shurtleff et al., 2018). Once the nascent polypeptide chain is synthesized, it undergoes folding in a process mediated by several ER-resident oxidoreductases – including protein disulfide isomerases (PDIs), ER protein 57 (ERp57, also known as PDIA3), ER DNA J domain-containing protein 5 (ERDj5, also known as DNAJC10), ER oxidoreductase 1 alpha (Ero1α, also known as ERO1A) (Bulleid, 2012), oligosaccharyltransferase (OST) (Chavan and Lennarz, 2006), calnexin and calreticulin (Williams, 2006) – and ATP-dependent chaperones such as binding immunoglobulin protein (BiP, also known as HSPA5) (Behnke et al., 2015). Upon folding to its native state, the client protein exits the ER en route to the secretory pathway (Miller and Schekman, 2013).
Because approximately a third of the cellular proteome is synthesized in the ER, stringent protein quality control machineries must be in place in this compartment to rectify protein misfolding. A misfolded client protein in the ER triggers the unfolded protein response (UPR) (Gardner et al., 2013), a finely tuned process leading to either translational attenuation (to alleviate the overall ER burden) (Harding et al., 1999; Hollien and Weissman, 2006) or increased synthesis of ER-resident chaperones (to assist the folding of client proteins) (Kozutsumi et al., 1988). However, when the client (soluble or membrane) protein is terminally misfolded, it is redirected to the cytosol for proteasomal degradation via the ER-associated degradation (ERAD) pathway (Travers et al., 2000). In situations where misfolded clients form large insoluble protein aggregates, they are cleared from the ER by lysosomal degradation through the comparably less understood ER-coupled autophagy (ER-phagy), which involves recruitment of a pre-autophagosomal structure called the phagophore (Khaminets et al., 2015). It is important to note that in addition to promoting protein translation, folding and degradation, the ER is also responsible for the regulation of Ca2+ homeostasis through the control of many ER membrane-localized Ca2+ channels and pumps (Daverkausen-Fischer and Prols, 2022), as well as for lipid biosynthesis via activation of ER-associated enzymes that generate these lipid molecules (Jacquemyn et al., 2017).
Virus infection and the ER
Strikingly, many ER functions are exploited by viruses to support different steps of their life cycle. Briefly, in a typical infection cycle, an incoming virus enters the host cell, often via receptor-mediated endocytosis that delivers the virus to the endosome (Staring et al., 2018). To continue the entry process, the virus must navigate the complex endomembranous network, proceeding along a ‘productive’ route to establish infection, while avoiding a ‘non-productive’ fate that leads it to a degradative destination (Staring et al., 2018). The productive route often targets a DNA virus to the nucleus or an RNA virus to a protein biosynthesis hub to ensure proper transcription and/or translation of the viral genome – this in turn generates virus structural proteins and non-structural proteins (NSPs) that can assist in the subsequent replication of the viral genome. Assembly of the newly synthesized viral structural proteins and replicated genome then results in formation of a new viral progeny. Upon maturation, the viral progeny undergoes egress, exiting the host cell to prepare for the next round of infection.
Indeed, a wealth of evidence demonstrates that viruses hijack ER functions to promote several steps during infection, including virus membrane penetration, protein translation, genome replication, assembly and egress. As one example, after initial entry, the non-enveloped DNA polyomavirus simian virus 40 (SV40) is endocytosed and subsequently delivered to the ER lumen (Fig. 2A). Here it undergoes ER-dependent conformational changes so that the viral particle can penetrate the ER membrane in order to gain access to the cytosol, en route to the nucleus for infection (Chen et al., 2019). Specifically, in the ER lumen, the disulfide bonds of SV40 capsids are disrupted and re-shuffled by a network of ER-resident PDI redox-active chaperones, which normally mediate the oxidative folding of cellular client proteins (Magnuson et al., 2005; Schelhaas et al., 2007; Walczak and Tsai, 2011). This partially disassembles the virus, generating a membrane penetration-competent viral particle that crosses the ER membrane to gain entry to the cytosol (Chen et al., 2019). Importantly, regardless of which virus infection step is supported by the ER, numerous ER factors must be utilized to serve critical roles in these steps.
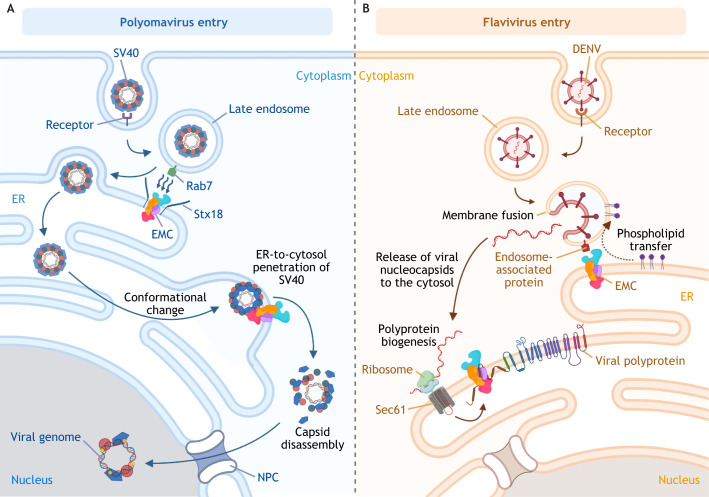
Fig. 2.
EMC-mediated ER–endosome MSCs support virus infection. (A) Upon entry, the non-enveloped DNA polyomavirus SV40 is endocytosed, reaching the early then late endosome. Through membrane-associated Rab7, the endosome membrane is tethered to the ER membrane via binding to the EMC (i.e. EMC4 and EMC7). Syntaxin18-mediated membrane fusion of a late endosome (harboring SV40) with the ER membrane delivers the virus into the ER lumen. Several ER-resident redox enzymes, including PDIs, reduce and isomerize the disulfide bonds of SV40 capsids inside the ER lumen; this results in conformational changes that expose the inner hydrophobic viral proteins, which support integration into the ER membrane. The membrane-embedded hydrophobic viral particle is stabilized by the chaperone activity of EMC1 of the EMC complex. This prevents premature virus disassembly, thereby enabling the successful penetration of the virus across the lipid bilayer of the ER membrane to reach the cytosol. Upon arrival in the cytosol, the SV40 capsid is disassembled, and the viral genome enters the nucleus via the nuclear pore complex (NPC) for replication and production of new virions. (B) The enveloped RNA flavivirus DENV also ends up in late endosomes after initial receptor-mediated endocytosis. An unknown endosome-associated protein tethers the late endosome to the ER via binding to the EMC. The resulting membrane contact between these two organelles allows for phospholipid transfer from the ER to the endosomal membrane, which facilitates the fusion between endosomal and viral membranes; this leads to release of the nucleocapsid containing the viral genome into the cytosol. The viral genome undergoes co-translational translocation on the ER membrane where the viral polyprotein is synthesized. The partially hydrophobic transmembrane segments of the polyprotein rely on EMC chaperone activity for proper insertion into the ER membrane, which is essential for polyprotein stability and subsequent replication of the viral genome, leading to productive infection.
Below, we focus on recent discoveries identifying how ER factors are hijacked to support the steps of virus entry and replication. Specifically, the EMC membrane chaperone complex mentioned above can act as a molecular tether to facilitate entry of DNA and RNA viruses. This unanticipated function is in addition to its well-documented conventional role in membrane protein biosynthesis and insertion (Hegde, 2022; Volkmar and Christianson, 2020). Furthermore, we will discuss how the protein machinery involved in ER morphogenesis – composed of RTN, ATL and LNP proteins – is co-opted by the non-enveloped DNA polyomavirus to promote membrane penetration during entry, as well as exploited by the enveloped RNA flavivirus and coronavirus to construct ER-derived organelles that are essential for genome replication.
The EMC facilitates ER–endosome membrane contacts to support virus entry
The multi-functional EMC, which is composed of ten different subunits (EMC1 to EMC10; EMC5 also known as MMGT1), normally acts as a chaperone complex. The core subunits EMC3 and EMC6 form a subcomplex that belongs to the Oxa1 membrane transporter superfamily, which is thought to share a common ancestor with the Sec61 translocon (Lewis and Hegde, 2021). The EMC promotes insertion of cellular tail-anchored or multi-pass transmembrane proteins into the ER lipid bilayer to ensure their proper transmembrane topology (Fig. 1B) (Guna et al., 2018; Jonikas et al., 2009; Pleiner et al., 2020). Intriguingly, this EMC chaperone activity is exploited by SV40 during ER-to-cytosol membrane transport, an essential infection step (Fig. 2A). In this case, EMC1 directly binds to SV40 in the ER. This engagement stabilizes the viral particle, preventing its premature disassembly as the virus penetrates through the lipid bilayer of the ER membrane to reach the cytosol (Bagchi et al., 2016). The ER-to-cytosol membrane transport process is further assisted by a cytosolic extraction machinery composed of heat shock cognate chaperone proteins Hsc70 (also known as HSPA8), Hsp105 (also known as HSPH1), Bag2 and small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA) (Dupzyk and Tsai, 2018; Ravindran et al., 2015; Walczak et al., 2014).
The EMC also promotes biosynthesis of partially hydrophobic cellular membrane proteins (Guna et al., 2018), and this feature is exploited by some RNA viruses. In the case of flaviviruses dengue virus (DENV) and Zika virus (ZIKV), the EMC is critical for biosynthesis of the viral polyprotein, which plays essential roles in downstream replication events (Barrows et al., 2019). Biosynthesis of the viral polyprotein is EMC-dependent because it encodes two viral muti-pass transmembrane proteins (called NS4A and NS4B) that contain partially hydrophobic transmembrane segments (Fig. 2B) (Lin et al., 2019; Ngo et al., 2019).
Beyond its established role in membrane protein insertion and biosynthesis, an unexpected function of the EMC has recently been reported and, importantly, shown to be co-opted by viruses during entry. Specifically, the EMC has been demonstrated to facilitate ER MCSs with other organelles. For instance, the EMC mediates ER–mitochondria MCSs by acting as a molecular tether, physically binding to Tom proteins that form the mitochondrial translocase of the outer membrane (TOM) complex, to facilitate phospholipid synthesis (Iyer et al., 2022) or phospholipid transfer from the ER to mitochondria (Lahiri et al., 2014). Likewise, the EMC – via EMC4 and EMC7 – mediates ER–endosome contacts by interacting with late endosome-localized Rab7 (Bagchi et al., 2020). This ER–endosome membrane contact similarly assists in transfer of phospholipids from the ER to the endosome (Bagchi et al., 2022).
Can viruses hijack EMC-dependent ER–endosome membrane contacts to support infection? Recent findings have provided at least two examples consistent with this possibility. After endocytosis, the non-enveloped DNA virus SV40 is targeted from the late endosome to the ER (instead of the degradative lysosome compartment), thereby directing the virus along a productive pathway (Fig. 2A). Importantly, it has been shown that delivery of SV40 from the late endosome to the ER relies on both EMC4 and EMC7 to tether the ER to the late endosome (Bagchi et al., 2020). When the late endosome harboring SV40 is in close proximity to the ER, SV40 likely buds off from this endosomal compartment to generate a vesicle (harboring the viral particle), which then fuses with the ER, a process mediated by the ER transmembrane soluble NSF-attachment protein receptor (SNARE) protein syntaxin18 (Stx18) (Bagchi et al., 2020). This membrane fusion event effectively delivers SV40 into the ER lumen. Hence, EMC-dependent juxtaposition between the late endosome and ER is critical for SV40 delivery into the ER, an essential step for virus infection (Qian et al., 2009; Schelhaas et al., 2007).
In addition to the example of a non-enveloped DNA virus, there is recent evidence that EMC-dependent ER–endosome membrane contact is also hijacked by the enveloped RNA virus DENV (Bagchi et al., 2022). During entry into the host cell, DENV is endocytosed to reach the endosome, where the viral envelope and endosome membrane fuse; this fusion event effectively releases the positive-strand RNA [(+)RNA] genome harbored inside the virus into the cytosol (Smit et al., 2011) (Fig. 2B). The released (+)RNA genome is then delivered to the cytosolic surface of the ER membrane (via a poorly characterized process) where co-translational translocation generates a polyprotein (encoding viral structural proteins and NSPs) that is essential for subsequent replication and assembly steps leading to the formation of new viral progeny (Nanaware et al., 2021; Neufeldt et al., 2018). Importantly, EMC4 (but not EMC7) plays a role in fusion between DENV and endosomal membranes by mediating the ER–endosome contact that enables ER-to-endosome transfer of phospholipids (phosphatidylserine in this case), whose presence in the endosome membrane then promotes fusion between DENV and endosomal membrane (Bagchi et al., 2022; Zaitseva et al., 2010) (Fig. 2B). Endosome-localized Rab7 proteins are not involved in this event (Bagchi et al., 2022), thus the identity of the corresponding endosomal protein that interacts with EMC4 to facilitate the ER–endosome membrane contact remains unknown. This suggests that different endosome-associated components contribute to EMC-dependent ER–endosome membrane contacts (Bagchi et al., 2022, 2020). Furthermore, this also demonstrates that multiple functions of the EMC can be exploited by a wide range of viruses during infection.
This raises the question of what are the mechanisms underlying the role of the EMC as a molecular tether that facilitates ER–endosome (or ER–mitochondria) membrane contacts? The EMC is a large protein complex (∼250–300 kDa) with ten distinct subunits (Pleiner et al., 2020), which potentially allows it to interact through its subunits with different partner proteins located on the opposing organelle membrane (for example, Rab7 on the endosome membrane). In-depth proteomic analyses on potential EMC binding partners should not only establish the identities of these corresponding partners, but might also reveal other organelles that can contact the ER via the EMC. It is worth noting that the EMC induces a local membrane thinning (from ∼35 Å to ∼25 Å) of the ER lipid bilayer (Pleiner et al., 2020). Bringing such a thin ER lipid bilayer in close proximity to the corresponding membrane of another organelle might enable a more efficient transfer of phospholipids between the two organelles, which appears to be critical for EMC-dependent membrane contacts (Lahiri et al., 2014).
ER morphogenesis machinery promotes cytosol arrival and replication of viruses
As mentioned above, a subset of the ER morphogenic machinery, composed of RTN, ATL and LNP membrane proteins, typically generates and maintains three-way ER junctions (Wang and Rapoport, 2019), giving rise to the web-like reticular ER morphology (Fig. 1A). Recent studies have shown that viruses can in fact exploit this machinery to facilitate the steps of entry and replication.
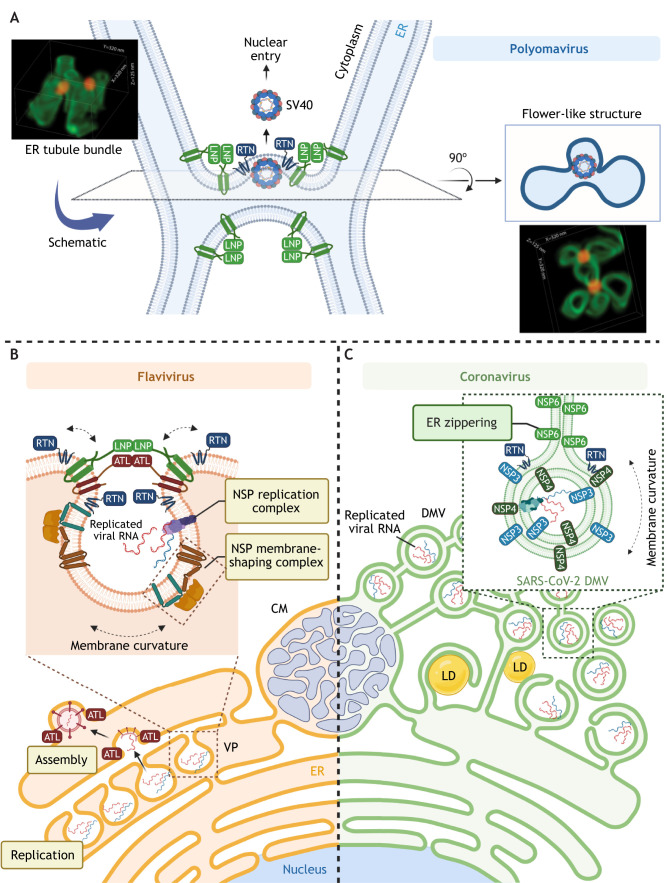
As part of its infection step, SV40 must be transported from the ER lumen to reach the cytosol en route to the nucleus to establish infection (Chen et al., 2019). How SV40 penetrates the ER membrane to enter the cytosol is not fully understood. In contrast to enveloped viruses, where fusion of the viral envelope with a host membrane delivers the viral particle into the cytosol, a non-enveloped virus such as SV40 must use a different mechanism to penetrate the host ER membrane. It has been shown that during SV40 infection, the virus induces formation of so-called ER foci, from which it escapes into the cytosol (Geiger et al., 2011). To construct these sites, SV40 reorganizes several ER membrane proteins (Bagchi et al., 2016, 2015; Geiger et al., 2011; Ravindran et al., 2015; Walczak et al., 2014), including RTN proteins and LNP (Bagchi et al., 2021; Chen et al., 2020), to form the ER foci (Fig. 3A). At the ER foci, RTN proteins induce membrane curvature to provide flexibility to the ER membrane as the virus undergoes membrane penetration, which is thought to protect the integrity of the ER membrane (Chen et al., 2020). Within the ER foci are numerous ER junctional sites that are formed by coalescing multi-ER tubules (Bagchi et al., 2021). These ER junctional sites are presumably generated by the membrane fusion activity of ATL proteins. LNP in turn acts to stabilize these multi-ER tubular junctions to preserve their structural integrity (Bagchi et al., 2021). Importantly, because ER junctional sites are considered the most destabilized region of the ER, they are therefore ideally suited to serve as sites of virus membrane penetration.
Fig. 3.
Exploiting of the ER morphogenic machinery to promote viral exit from the ER and construction of replication organelles. (A) In order to escape from the ER into the cytosol, polyomavirus SV40 triggers the mobilization of many ER membrane proteins to help establish multi-tubular ER junctions (which are stabilized by LNP proteins). Despite being stabilized, ER junctional sites remain more destabilized as compared to the rest of the ER and thus serve as ideal virus penetration sites. RTN proteins protect the membrane integrity by inducing membrane curvature, promoting membrane flexibility around the protruding virus. This membrane penetration site can be visualized using super-resolution focused ion beam scanning electron microscopy (FIB-SEM) and appears as a flower-like structure. Representative three-dimensional reconstruction images of the multi-tubular ER junctions are shown; images adapted from Bagchi et al. (2021) with permission from Elsevier. The green structures mark the ER, and the red structures depict the SV40 particle. (B) Some (+)RNA viruses, including flaviviruses, induce massive ER membrane rearrangement to form CMs, in which viral structural proteins and NSPs can be detected. The flaviviruses also trigger invagination of the ER membrane to form VPs, which support replication of the viral RNA genome (template RNA is indicated in blue, and the replicated RNA is shown in red). The ER morphogenesis machinery interacts with viral proteins to shape the ER membrane during infection. For example, RTN3 interacts with NS4A and can be recruited to VPs during infection with WNV, DENV and ZIKV. LNP can bind to TEBV NS4B and is recruited to VP-like structures (induced by expression of TBEV NS4A–NS4B fusion protein). Both ATL2 and ATL3 associate with the DENV NS2B–NS3–NS5 replication complex and localize to DENV-induced VPs. However, only ATL2 is required for VP formation, whereas ATL3 colocalizes with DENV envelope protein at assembly sites and might play a role in membrane trafficking or maturation of DENV. (C) Some (+)RNA viruses, including coronaviruses, induce formation of a DMV structure that also supports viral genome replication. The DMV is often found juxtaposed to a lipid droplet (LD) to facilitate lipid transfer to the DMV. It remains unclear how the host ER morphogenesis machinery is co-opted by coronaviruses to form the DMV. In the case of SARS-CoV-2, NSP3 and NSP4 are sufficient to induce DMV formation, but only RTN proteins of the host ER membrane-shaping machinery have been shown to play a functional role in this process. SARS-CoV-2 NSP6 induces ER zippering, forming a connector that allows localization of ER membrane proteins, but not ER luminal proteins, to the DMV.
The ER morphogenesis machinery is also hijacked to support replication of (+)RNA viruses, including flavivirus and coronavirus (Fig. 3B,C). For these viruses, replication occurs in virus-mediated, ER-derived organelles that are formed after rearrangement of the ER membrane (Neufeldt et al., 2018; Wolff et al., 2020). For instance, studies of infection with the flaviviruses DENV and ZIKV have revealed that this ER rearrangement (Fig. 3B) is induced by NSPs, which either create vesicles invaginated from the rough ER, forming vesicle packets (VPs), or orchestrate the bundling of smooth ER, forming convoluted membranes (CMs) (Cortese et al., 2017; Welsch et al., 2009). Because these ER-derived organelles physically harbor replication intermediates of the viral genome, which can be detected as double-stranded RNAs (dsRNAs), they are appropriately termed viral replication organelles (Neufeldt et al., 2018). Importantly, emerging evidence suggests that construction of these viral replication organelles is accomplished through the cooperation between viral NSPs and ER morphogenesis proteins of the RTN, ATL and LNP families. Specifically, RTN proteins have been shown to be essential for infection of DENV and ZIKV, as well as the flavivirus West Nile Virus (WNV) (Aktepe et al., 2017). For instance, in the case of WNV, RTN3 not only regulates the steady-state NSP levels, but also interacts with NS4A (Aktepe et al., 2017), a component of the membrane-shaping NSP complex known to mediate ER membrane rearrangements that give rise to VPs and CMs (Miller et al., 2007; Roosendaal et al., 2006). Of the three ATL proteins (ATL1, ATL2 and ATL3), both ATL2 and ATL3 associate with the DENV replication complex, which is composed of NS2B, NS3 and NS5 (Neufeldt et al., 2019). However, ATL2 and ATL3 support flavivirus infection by different means, owing in part to the different domains that flank their conserved cytosolic GTPase catalytic core (Neufeldt et al., 2019). ATL2 is required for maintaining VP morphology and viral genome replication of DENV, whereas ATL3 can additionally interact with other viral factors, such as NS1, the viral structural C (capsid) and E (envelope) proteins, as well as the host ADP-ribosylation factors ARF4 and ARF5, which are essential in the early secretory pathways (Neufeldt et al., 2019). ATL3 also interacts with ectopically expressed ZIKV NS2A and the NS2B–NS3 protease (a fusion protein known as NS2B3) (Monel et al., 2019), both of which are critical for ZIKV virion assembly. These findings suggest that post replication, ATL3 plays a further role in mediating viral particle maturation and release, steps that also require a reorganization of the ER membrane.
LNP has also been identified as a critical host factor for genome replication of WNV and of Langat virus, which is a member of the tick-borne encephalitis virus (TBEV) serocomplex (Tran et al., 2021). Mechanistically, TBEV NS4B interacts with LNP to potentially stabilize the VP structure, although it remains unclear whether three-way ER junctions are part of the replication organelle architecture. Integrated proteomics and gene-knockdown screening work has also identified LNP as a ZIKV NS4A interactor (Shah et al., 2018). Taken together, the ER morphogenic machinery composed of RTN, ATL and LNP proteins is exploited by different flaviviruses to support formation of the replication organelles to enable successful replication.
In addition to VPs and CMs, double-membrane vesicles (DMVs) are another type of ER-derived organelle used to support viral genome replication (Roingeard et al., 2022; Wolff et al., 2020). They are induced by (+)RNA viruses that are more distantly related to flaviviruses, including coronaviruses, hepaciviruses and picornaviruses (Fig. 3C). A DMV is generated by the protrusion of ER-derived membranes to enclose subcellular domains and partition them from the cytoplasm (Wolff et al., 2020). DMVs feature two closely apposed membranes and tend to cluster together, occupying a large area of the cytoplasm proximal to the nucleus (Snijder et al., 2020), and they frequently associate with the ER membrane and lipid droplets (Meyers et al., 2016; Ricciardi et al., 2022). Despite the importance of DMVs in cells infected by a wide range of (+)RNA viruses, the host factors responsible for DMV morphogenesis remain poorly understood. RTN proteins have been implicated in DMV formation (Diaz and Ahlquist, 2012), although both positive and negative roles have been proposed. For instance, RTN3 promotes viral RNA replication of the enterovirus EV71 (Tang et al., 2007; Wang et al., 2017), whereas it negatively controls viral RNA replication in hepatitis C virus (HCV) (Wu et al., 2014); in both cases, RTN3 is thought to directly interact with viral proteins that induce DMV formation. Additionally, confocal microscopy analyses have indicated that viral dsRNA localizes in close proximity to RTN3-enriched ER regions in cells infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (Cortese et al., 2020), the virus responsible for COVID-19. In fact, our most recent findings have revealed that RTN3 and RTN4 functionally play an important role in DMV formation during SARS-CoV-2 infection (Williams et al., 2023). Whether other ER morphogenesis factors such as ATL proteins and LNP have roles in the regulation of DMV formation remains unclear.
In contrast to the host components involved in DMV formation, the function of viral NSPs in this process is more well-established. Similar to other coronaviruses (Oudshoorn et al., 2017), ectopic expression of NSP3 and NSP4 of SARS-CoV-2 is sufficient to drive DMV formation in cells (Tabata et al., 2021; Twu et al., 2021). Not surprisingly, we have found that SARS-CoV-2 NSP3 and NSP4 directly interact with RTN3 and RTN4 (Williams et al., 2023). Furthermore, ectopic expression of NSP3 and NSP4 with NSP6 of SARS-CoV-2 leads to the formation of so-called ‘ER-zippering connectors’ that connect the ER membrane to the proximal DMV clusters (Ricciardi et al., 2022). Under this condition, only ER membrane proteins, but not ER luminal proteins, can translocate to NSP6-containing regions, suggesting a gatekeeping role of NSP6 at the ER-zippering connector (Ricciardi et al., 2022). Intriguingly, ATL2 is one of the ER membrane proteins that colocalizes with NSP6 when ectopically expressed (Ricciardi et al., 2022), although a functional role of this host protein in DMV formation has not been reported. Clearly, what is critically needed is further information regarding how viral NSPs cooperate with host ER factors – particularly the ER morphogenesis machinery – to shape and generate the DMVs required for supporting viral replication.
Conclusions and perspectives
Here, we have highlighted recent findings of how ER membrane chaperones and the ER morphogenesis machinery can be exploited to support viral entry and replication (summarized in Table 1). These findings suggest that during infection, the EMC promotes ER membrane contacts with other organelles, including the endosome, to facilitate the transfer of either cargos – namely, viral particles – or lipids essential for viral membrane fusion between the organelles. However, the precise mechanism by which EMC-dependent ER–endosome membrane contacts mediate virus and/or lipid transfer remains unknown. Beyond the EMC, questions remain regarding what other host ER proteins (required for virus infection) facilitate ER–endosome membrane contacts and what are the identities of the endosomal partner proteins. These outstanding questions deserve additional investigation. A combination of rigorous classical biochemical approaches coupled with the use of state-of-the-art proteomics strategies might begin to answer these questions.
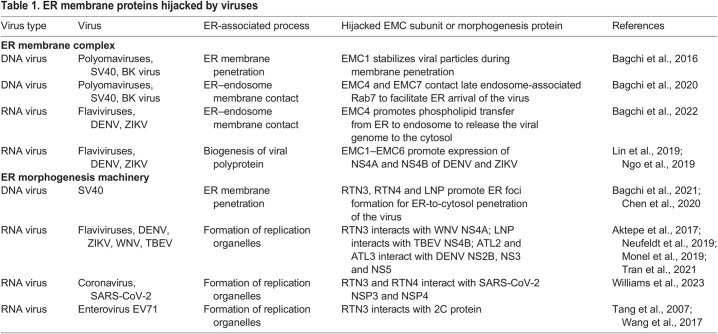
Table 1 .
. ER membrane proteins hijacked by viruses
In addition to the EMC, the ER morphogenesis machinery comprising RTN, ATL and LNP proteins has recently been reported to support not only the intracellular trafficking of viruses, but also the construction of replication organelles. Although a role in the formation of a membrane penetration site for the non-enveloped polyomavirus SV40 has been shown, it remains unknown whether the ER morphogenesis machinery also supports the escape of other non-enveloped viruses from the ER.
Furthermore, there is increasing evidence that RTN, ATL and LNP proteins also participate in formation of VPs and CMs, and possibly even in shaping of DMVs. If this is true, this raises the question of whether these replication organelles harbor ER three-way junctions, whose biogenesis and maintenance are known to depend on the RTN–ATL–LNP morphogenesis machinery. Loss-of-function analyses addressing the potential role of this machinery in the infection of other viruses, in concert with application of super-resolution imaging methods to visualize the molecular architecture of virus-induced replication organelles, should begin to elucidate these enigmas.
In summary, how the ER supports different steps in virus infection ultimately depends on the interplay between viral and host ER proteins. In general, the role of viral proteins in this interplay has been more intensely studied compared to that of the host factors. Therefore, the examples illustrated here, for which additional host ER factors have been identified and shown to promote important steps in the life cycle of different viruses, are of great significance in advancing our knowledge regarding the nature of the ER–virus interaction during infection. The discovery of additional host components obviously raises the exciting possibility of developing drugs targeting these cellular components in order to blunt virus infection.
Acknowledgements
We thank Riya Sarkar (University of Michigan, MI, USA) for critical reading of the manuscript. All figures in this paper were created with BioRender.com.
Footnotes
Funding
Our work is supported by research grants from the National Institutes of Health to B.T. (R01AI170514, R01AI150897 and R01AI064296). Deposited in PMC for release after 12 months.
References
- Aktepe, T. E., Liebscher, S., Prier, J. E., Simmons, C. P. and Mackenzie, J. M. (2017). The host protein reticulon 3.1A is utilized by flaviviruses to facilitate membrane remodelling. Cell Rep. 21, 1639-1654. 10.1016/j.celrep.2017.10.055 [DOI] [PubMed] [Google Scholar]
- Bagchi, P., Walczak, C. P. and Tsai, B. (2015). The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration. J. Virol. 89, 4058-4068. 10.1128/JVI.03574-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi, P., Inoue, T. and Tsai, B. (2016). EMC1-dependent stabilization drives membrane penetration of a partially destabilized non-enveloped virus. Elife 5, e21470. 10.7554/eLife.21470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi, P., Torres, M., Qi, L. and Tsai, B. (2020). Selective EMC subunits act as molecular tethers of intracellular organelles exploited during viral entry. Nat. Commun. 11, 1127. 10.1038/s41467-020-14967-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi, P., Liu, X., Cho, W. J. and Tsai, B. (2021). Lunapark-dependent formation of a virus-induced ER exit site contains multi-tubular ER junctions that promote viral ER-to-cytosol escape. Cell Rep. 37, 110077. 10.1016/j.celrep.2021.110077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi, P., Speckhart, K., Kennedy, A., Tai, A. W. and Tsai, B. (2022). A specific EMC subunit supports Dengue virus infection by promoting virus membrane fusion essential for cytosolic genome delivery. PLoS Pathog. 18, e1010717. 10.1371/journal.ppat.1010717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrows, N. J., Anglero-Rodriguez, Y., Kim, B., Jamison, S. F., Le Sommer, C., McGee, C. E., Pearson, J. L., Dimopoulos, G., Ascano, M., Bradrick, S. S.et al. (2019). Dual roles for the ER membrane protein complex in flavivirus infection: viral entry and protein biogenesis. Sci. Rep. 9, 9711. 10.1038/s41598-019-45910-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke, J., Feige, M. J. and Hendershot, L. M. (2015). BiP and its nucleotide exchange factors Grp170 and Sil1: mechanisms of action and biological functions. J. Mol. Biol. 427, 1589-1608. 10.1016/j.jmb.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleid, N. J. (2012). Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb Perspect Biol 4, a013219. 10.1101/cshperspect.a013219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan, M. and Lennarz, W. (2006). The molecular basis of coupling of translocation and N-glycosylation. Trends Biochem. Sci. 31, 17-20. 10.1016/j.tibs.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Chen, S., Novick, P. and Ferro-Novick, S. (2012). ER network formation requires a balance of the dynamin-like GTPase Sey1p and the Lunapark family member Lnp1p. Nat. Cell Biol. 14, 707-716. 10.1038/ncb2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., Novick, P. and Ferro-Novick, S. (2013). ER structure and function. Curr. Opin. Cell Biol. 25, 428-433. 10.1016/j.ceb.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S., Desai, T., McNew, J. A., Gerard, P., Novick, P. J. and Ferro-Novick, S. (2015). Lunapark stabilizes nascent three-way junctions in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 112, 418-423. 10.1073/pnas.1423026112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. J., Liu, X. and Tsai, B. (2019). SV40 Hijacks cellular transport, membrane penetration, and disassembly machineries to promote infection. Viruses 11, 917. 10.3390/v11100917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. J., Williams, J. M., Arvan, P. and Tsai, B. (2020). Reticulon protects the integrity of the ER membrane during ER escape of large macromolecular protein complexes. J. Cell Biol. 219, e201908182. 10.1083/jcb.201908182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, M., Goellner, S., Acosta, E. G., Neufeldt, C. J., Oleksiuk, O., Lampe, M., Haselmann, U., Funaya, C., Schieber, N., Ronchi, P.et al. (2017). Ultrastructural characterization of zika virus replication factories. Cell Rep. 18, 2113-2123. 10.1016/j.celrep.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, M., Lee, J. Y., Cerikan, B., Neufeldt, C. J., Oorschot, V. M. J., Kohrer, S., Hennies, J., Schieber, N. L., Ronchi, P., Mizzon, G.et al. (2020). Integrative imaging reveals SARS-CoV-2-induced reshaping of subcellular morphologies. Cell Host Microbe 28, 853-866.e5. 10.1016/j.chom.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daverkausen-Fischer, L. and Prols, F. (2022). Regulation of calcium homeostasis and flux between the endoplasmic reticulum and the cytosol. J. Biol. Chem. 298, 102061. 10.1016/j.jbc.2022.102061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, A. and Ahlquist, P. (2012). Role of host reticulon proteins in rearranging membranes for positive-strand RNA virus replication. Curr. Opin. Microbiol. 15, 519-524. 10.1016/j.mib.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupzyk, A. and Tsai, B. (2018). Bag2 is a component of a cytosolic extraction machinery that promotes membrane penetration of a nonenveloped virus. J. Virol. 92, e00607-e00618. 10.1128/JVI.00607-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz, Y. and Schuldiner, M. (2011). Staying in touch: the molecular era of organelle contact sites. Trends Biochem. Sci. 36, 616-623. 10.1016/j.tibs.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Friedman, J. R. and Voeltz, G. K. (2011). The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 21, 709-717. 10.1016/j.tcb.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, B. M., Pincus, D., Gotthardt, K., Gallagher, C. M. and Walter, P. (2013). Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect Biol. 5, a013169. 10.1101/cshperspect.a013169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger, R., Andritschke, D., Friebe, S., Herzog, F., Luisoni, S., Heger, T. and Helenius, A. (2011). BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol. Nat. Cell Biol. 13, 1305-1314. 10.1038/ncb2339 [DOI] [PubMed] [Google Scholar]
- Guna, A., Volkmar, N., Christianson, J. C. and Hegde, R. S. (2018). The ER membrane protein complex is a transmembrane domain insertase. Science 359, 470-473. 10.1126/science.aao3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, H. P., Zhang, Y. and Ron, D. (1999). Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271-274. 10.1038/16729 [DOI] [PubMed] [Google Scholar]
- Hegde, R. S. (2022). The function, structure, and origins of the ER membrane protein complex. Annu. Rev. Biochem. 91, 651-678. 10.1146/annurev-biochem-032620-104553 [DOI] [PubMed] [Google Scholar]
- Hollien, J. and Weissman, J. S. (2006). Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104-107. 10.1126/science.1129631 [DOI] [PubMed] [Google Scholar]
- Hu, J., Shibata, Y., Zhu, P. P., Voss, C., Rismanchi, N., Prinz, W. A., Rapoport, T. A. and Blackstone, C. (2009). A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell 138, 549-561. 10.1016/j.cell.2009.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenroth, M. and Bohnert, M. (2020). Come a little bit closer! Lipid droplet-ER contact sites are getting crowded. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118603. 10.1016/j.bbamcr.2019.118603 [DOI] [PubMed] [Google Scholar]
- Iyer, A., Niemann, M., Serricchio, M., Dewar, C. E., Oeljeklaus, S., Farine, L., Warscheid, B., Schneider, A. and Butikofer, P. (2022). The endoplasmic reticulum membrane protein complex localizes to the mitochondrial - endoplasmic reticulum interface and its subunits modulate phospholipid biosynthesis in Trypanosoma brucei. PLoS Pathog. 18, e1009717. 10.1371/journal.ppat.1009717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn, J., Cascalho, A. and Goodchild, R. E. (2017). The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep. 18, 1905-1921. 10.15252/embr.201643426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonikas, M. C., Collins, S. R., Denic, V., Oh, E., Quan, E. M., Schmid, V., Weibezahn, J., Schwappach, B., Walter, P., Weissman, J. S.et al. (2009). Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323, 1693-1697. 10.1126/science.1167983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalies, K. U., Gorlich, D. and Rapoport, T. A. (1994). Binding of ribosomes to the rough endoplasmic reticulum mediated by the Sec61p-complex. J. Cell Biol. 126, 925-934. 10.1083/jcb.126.4.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets, A., Heinrich, T., Mari, M., Grumati, P., Huebner, A. K., Akutsu, M., Liebmann, L., Stolz, A., Nietzsche, S., Koch, N.et al. (2015). Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354-358. 10.1038/nature14498 [DOI] [PubMed] [Google Scholar]
- Kozutsumi, Y., Segal, M., Normington, K., Gething, M. J. and Sambrook, J. (1988). The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332, 462-464. 10.1038/332462a0 [DOI] [PubMed] [Google Scholar]
- Lahiri, S., Chao, J. T., Tavassoli, S., Wong, A. K., Choudhary, V., Young, B. P., Loewen, C. J. and Prinz, W. A. (2014). A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria. PLoS Biol. 12, e1001969. 10.1371/journal.pbio.1001969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, A. J. O. and Hegde, R. S. (2021). A unified evolutionary origin for the ubiquitous protein transporters SecY and YidC. BMC Biol. 19, 266. 10.1186/s12915-021-01171-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, D. L., Inoue, T., Chen, Y. J., Chang, A., Tsai, B. and Tai, A. W. (2019). The ER membrane protein complex promotes biogenesis of dengue and zika virus non-structural multi-pass transmembrane proteins to support infection. Cell Rep. 27, 1666-1674.e4. 10.1016/j.celrep.2019.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson, B., Rainey, E. K., Benjamin, T., Baryshev, M., Mkrtchian, S. and Tsai, B. (2005). ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell 20, 289-300. 10.1016/j.molcel.2005.08.034 [DOI] [PubMed] [Google Scholar]
- Meyers, N. L., Fontaine, K. A., Kumar, G. R. and Ott, M. (2016). Entangled in a membranous web: ER and lipid droplet reorganization during hepatitis C virus infection. Curr. Opin. Cell Biol. 41, 117-124. 10.1016/j.ceb.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, E. A. and Schekman, R. (2013). COPII - a flexible vesicle formation system. Curr. Opin. Cell Biol. 25, 420-427. 10.1016/j.ceb.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S., Kastner, S., Krijnse-Locker, J., Buhler, S. and Bartenschlager, R. (2007). The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J. Biol. Chem. 282, 8873-8882. 10.1074/jbc.M609919200 [DOI] [PubMed] [Google Scholar]
- Monel, B., Rajah, M. M., Hafirassou, M. L., Sid Ahmed, S., Burlaud-Gaillard, J., Zhu, P. P., Nevers, Q., Buchrieser, J., Porrot, F., Meunier, C.et al. (2019). Atlastin endoplasmic reticulum-shaping proteins facilitate Zika Virus replication. J. Virol. 93, e01047-19. 10.1128/JVI.01047-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanaware, N., Banerjee, A., Mullick Bagchi, S., Bagchi, P. and Mukherjee, A. (2021). Dengue virus infection: a tale of viral exploitations and host responses. Viruses 13, 1967. 10.3390/v13101967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeldt, C. J., Cortese, M., Acosta, E. G. and Bartenschlager, R. (2018). Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 16, 125-142. 10.1038/nrmicro.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeldt, C. J., Cortese, M., Scaturro, P., Cerikan, B., Wideman, J. G., Tabata, K., Moraes, T., Oleksiuk, O., Pichlmair, A. and Bartenschlager, R. (2019). ER-shaping atlastin proteins act as central hubs to promote flavivirus replication and virion assembly. Nat Microbiol 4, 2416-2429. 10.1038/s41564-019-0586-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo, A. M., Shurtleff, M. J., Popova, K. D., Kulsuptrakul, J., Weissman, J. S. and Puschnik, A. S. (2019). The ER membrane protein complex is required to ensure correct topology and stable expression of flavivirus polyproteins. Elife 8, e48469. 10.7554/eLife.48469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso, G., Pendin, D., Liu, S., Tosetto, J., Moss, T. J., Faust, J. E., Micaroni, M., Egorova, A., Martinuzzi, A., McNew, J. A.et al. (2009). Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 460, 978-983. 10.1038/nature08280 [DOI] [PubMed] [Google Scholar]
- Oudshoorn, D., Rijs, K., Limpens, R., Groen, K., Koster, A. J., Snijder, E. J., Kikkert, M. and Barcena, M. (2017). Expression and cleavage of middle east respiratory syndrome coronavirus nsp3-4 polyprotein induce the formation of double-membrane vesicles that mimic those associated with coronaviral RNA replication. mBio 8, e01658-17. 10.1128/mBio.01658-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, E. and Rapoport, T. A. (2012). Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 41, 21-40. 10.1146/annurev-biophys-050511-102312 [DOI] [PubMed] [Google Scholar]
- Perkins, H. T. and Allan, V. (2021). Intertwined and finely balanced: endoplasmic reticulum morphology, dynamics, function, and diseases. Cells 10, 2341. 10.3390/cells10092341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiner, T., Tomaleri, G. P., Januszyk, K., Inglis, A. J., Hazu, M. and Voorhees, R. M. (2020). Structural basis for membrane insertion by the human ER membrane protein complex. Science 369, 433-436. 10.1126/science.abb5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhka, M., Joensuu, M., Vihinen, H., Belevich, I. and Jokitalo, E. (2012). Progressive sheet-to-tubule transformation is a general mechanism for endoplasmic reticulum partitioning in dividing mammalian cells. Mol. Biol. Cell 23, 2424-2432. 10.1091/mbc.e10-12-0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, M., Cai, D., Verhey, K. J. and Tsai, B. (2009). A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog. 5, e1000465. 10.1371/journal.ppat.1000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiborg, C., Wenzel, E. M. and Stenmark, H. (2015). ER-endosome contact sites: molecular compositions and functions. EMBO J. 34, 1848-1858. 10.15252/embj.201591481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran, M. S., Bagchi, P., Inoue, T. and Tsai, B. (2015). A non-enveloped virus hijacks host disaggregation machinery to translocate across the endoplasmic reticulum membrane. PLoS Pathog. 11, e1005086. 10.1371/journal.ppat.1005086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi, S., Guarino, A. M., Giaquinto, L., Polishchuk, E. V., Santoro, M., Di Tullio, G., Wilson, C., Panariello, F., Soares, V. C., Dias, S. S. G.et al. (2022). The role of NSP6 in the biogenesis of the SARS-CoV-2 replication organelle. Nature 606, 761-768. 10.1038/s41586-022-04835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roingeard, P., Eymieux, S., Burlaud-Gaillard, J., Hourioux, C., Patient, R. and Blanchard, E. (2022). The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses. Cell. Mol. Life Sci. 79, 425. 10.1007/s00018-022-04469-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal, J., Westaway, E. G., Khromykh, A. and Mackenzie, J. M. (2006). Regulated cleavages at the West Nile virus NS4A-2K-NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein. J. Virol. 80, 4623-4632. 10.1128/JVI.80.9.4623-4632.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, A. A. and Voeltz, G. K. (2012). Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 13, 607-625. 10.1038/nrm3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassano, M. L., Felipe-Abrio, B. and Agostinis, P. (2022). ER-mitochondria contact sites; a multifaceted factory for Ca(2+) signaling and lipid transport. Front. Cell Dev. Biol. 10, 988014. 10.3389/fcell.2022.988014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelhaas, M., Malmstrom, J., Pelkmans, L., Haugstetter, J., Ellgaard, L., Grunewald, K. and Helenius, A. (2007). Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell 131, 516-529. 10.1016/j.cell.2007.09.038 [DOI] [PubMed] [Google Scholar]
- Shah, P. S., Link, N., Jang, G. M., Sharp, P. P., Zhu, T., Swaney, D. L., Johnson, J. R., Von Dollen, J., Ramage, H. R., Satkamp, L.et al. (2018). Comparative flavivirus-host protein interaction mapping reveals mechanisms of dengue and Zika virus pathogenesis. Cell 175, 1931-1945.e18. 10.1016/j.cell.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, Y., Hu, J., Kozlov, M. M. and Rapoport, T. A. (2009). Mechanisms shaping the membranes of cellular organelles. Annu. Rev. Cell Dev. Biol. 25, 329-354. 10.1146/annurev.cellbio.042308.113324 [DOI] [PubMed] [Google Scholar]
- Shibata, Y., Shemesh, T., Prinz, W. A., Palazzo, A. F., Kozlov, M. M. and Rapoport, T. A. (2010). Mechanisms determining the morphology of the peripheral ER. Cell 143, 774-788. 10.1016/j.cell.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff, M. J., Itzhak, D. N., Hussmann, J. A., Schirle Oakdale, N. T., Costa, E. A., Jonikas, M., Weibezahn, J., Popova, K. D., Jan, C. H., Sinitcyn, P.et al. (2018). The ER membrane protein complex interacts cotranslationally to enable biogenesis of multipass membrane proteins. Elife 7, e37018. 10.7554/eLife.37018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit, J. M., Moesker, B., Rodenhuis-Zybert, I. and Wilschut, J. (2011). Flavivirus cell entry and membrane fusion. Viruses 3, 160-171. 10.3390/v3020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder, E. J., Limpens, R., de Wilde, A. H., de Jong, A. W. M., Zevenhoven-Dobbe, J. C., Maier, H. J., Faas, F., Koster, A. J. and Barcena, M. (2020). A unifying structural and functional model of the coronavirus replication organelle: Tracking down RNA synthesis. PLoS Biol. 18, e3000715. 10.1371/journal.pbio.3000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staring, J., Raaben, M. and Brummelkamp, T. R. (2018). Viral escape from endosomes and host detection at a glance. J. Cell Sci. 131, jcs216259. 10.1242/jcs.216259 [DOI] [PubMed] [Google Scholar]
- Tabata, K., Prasad, V., Paul, D., Lee, J. Y., Pham, M. T., Twu, W. I., Neufeldt, C. J., Cortese, M., Cerikan, B., Stahl, Y.et al. (2021). Convergent use of phosphatidic acid for hepatitis C virus and SARS-CoV-2 replication organelle formation. Nat. Commun. 12, 7276. 10.1038/s41467-021-27511-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. F., Yang, S. Y., Wu, B. W., Jheng, J. R., Chen, Y. L., Shih, C. H., Lin, K. H., Lai, H. C., Tang, P. and Horng, J. T. (2007). Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J. Biol. Chem. 282, 5888-5898. 10.1074/jbc.M611145200 [DOI] [PubMed] [Google Scholar]
- Tran, P. T., Asghar, N., Johansson, M. and Melik, W. (2021). Roles of the Endogenous Lunapark Protein during Flavivirus Replication. Viruses 13, 1198. 10.3390/v13071198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers, K. J., Patil, C. K., Wodicka, L., Lockhart, D. J., Weissman, J. S. and Walter, P. (2000). Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249-258. 10.1016/S0092-8674(00)80835-1 [DOI] [PubMed] [Google Scholar]
- Twu, W. I., Lee, J. Y., Kim, H., Prasad, V., Cerikan, B., Haselmann, U., Tabata, K. and Bartenschlager, R. (2021). Contribution of autophagy machinery factors to HCV and SARS-CoV-2 replication organelle formation. Cell Rep 37, 110049. 10.1016/j.celrep.2021.110049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz, G. K., Rolls, M. M. and Rapoport, T. A. (2002). Structural organization of the endoplasmic reticulum. EMBO Rep. 3, 944-950. 10.1093/embo-reports/kvf202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz, G. K., Prinz, W. A., Shibata, Y., Rist, J. M. and Rapoport, T. A. (2006). A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124, 573-586. 10.1016/j.cell.2005.11.047 [DOI] [PubMed] [Google Scholar]
- Volkmar, N. and Christianson, J. C. (2020). Squaring the EMC - how promoting membrane protein biogenesis impacts cellular functions and organismal homeostasis. J. Cell Sci. 133, jcs243519. 10.1242/jcs.243519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C. P. and Tsai, B. (2011). A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J. Virol. 85, 2386-2396. 10.1128/JVI.01855-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C. P., Ravindran, M. S., Inoue, T. and Tsai, B. (2014). A cytosolic chaperone complexes with dynamic membrane J-proteins and mobilizes a nonenveloped virus out of the endoplasmic reticulum. PLoS Pathog. 10, e1004007. 10.1371/journal.ppat.1004007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, N. and Rapoport, T. A. (2019). Reconstituting the reticular ER network - mechanistic implications and open questions. J. Cell Sci. 132, jcs227611. 10.1242/jcs.227611 [DOI] [PubMed] [Google Scholar]
- Wang, S., Tukachinsky, H., Romano, F. B. and Rapoport, T. A. (2016). Cooperation of the ER-shaping proteins atlastin, lunapark, and reticulons to generate a tubular membrane network. Elife 5, e18605. 10.7554/eLife.18605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T., Wang, B., Huang, H., Zhang, C., Zhu, Y., Pei, B., Cheng, C., Sun, L., Wang, J., Jin, Q.et al. (2017). Enterovirus 71 protease 2Apro and 3Cpro differentially inhibit the cellular endoplasmic reticulum-associated degradation (ERAD) pathway via distinct mechanisms, and enterovirus 71 hijacks ERAD component p97 to promote its replication. PLoS Pathog. 13, e1006674. 10.1371/journal.ppat.1006674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch, S., Miller, S., Romero-Brey, I., Merz, A., Bleck, C. K., Walther, P., Fuller, S. D., Antony, C., Krijnse-Locker, J. and Bartenschlager, R. (2009). Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5, 365-375. 10.1016/j.chom.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. B. (2006). Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 119, 615-623. 10.1242/jcs.02856 [DOI] [PubMed] [Google Scholar]
- Williams, J. M., Chen, Y. J., Cho, W. J., Tai, A. W. and Tsai, B. (2023). Reticulons promote formation of ER-derived double-membrane vesicles that facilitate SARS-CoV-2 replication. J. Cell Biol. 222, e202203060. 10.1083/jcb.202203060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, E. L. and Metzakopian, E. (2021). ER-mitochondria contact sites in neurodegeneration: genetic screening approaches to investigate novel disease mechanisms. Cell Death Differ. 28, 1804-1821. 10.1038/s41418-020-00705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, G., Melia, C. E., Snijder, E. J. and Barcena, M. (2020). Double-membrane vesicles as platforms for viral replication. Trends Microbiol. 28, 1022-1033. 10.1016/j.tim.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, M. J., Ke, P. Y., Hsu, J. T., Yeh, C. T. and Horng, J. T. (2014). Reticulon 3 interacts with NS4B of the hepatitis C virus and negatively regulates viral replication by disrupting NS4B self-interaction. Cell. Microbiol. 16, 1603-1618. 10.1111/cmi.12318 [DOI] [PubMed] [Google Scholar]
- Zaitseva, E., Yang, S. T., Melikov, K., Pourmal, S. and Chernomordik, L. V. (2010). Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 6, e1001131. 10.1371/journal.ppat.1001131 [DOI] [PMC free article] [PubMed] [Google Scholar]