Abstract
Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1, programmed cell death ligand 1, and cytotoxic T lymphocyte-associated antigen-4 have shown significantly durable clinical benefits and tolerable toxicities and have improved the survival of patients with various types of cancer. Since 2018, the National Medical Products Administration of China has approved 17 ICIs as the standard treatment for certain advanced or metastatic solid tumors. As ICIs represent a broad-spectrum antitumor strategy, the populations eligible for cancer immunotherapy are rapidly expanding. However, the clinical applications of ICIs in cancer patient populations with special issues, a term that refers to complex subgroups of patients with comorbidities, special clinical conditions, or concomitant medications who are routinely excluded from prospective clinical trials of ICIs or are underrepresented in these trials, represent a great real-world challenge. Although the Chinese Society of Clinical Oncology (CSCO) has provided recommendations for screening before the use of ICIs in special populations, the recommendations for full-course management remain insufficient. The CSCO Expert Committee on Immunotherapy organized leading medical oncology and multidisciplinary experts to develop a consensus that will serve as an important reference for clinicians to guide the proper application of ICIs in special patient populations. This article is a translation of a study first published in Chinese in The Chinese Clinical Oncology (ISSN 1009-0460, CN 32-1577/R) in May 2022 (27(5):442–454). The publisher of the original paper has provided written confirmation of permission to publish this translation in Therapeutic Advances in Medical Oncology.
Keywords: Chinese Society of Clinical Oncology, consensus, immune checkpoint inhibitor, recommendation, special population
Background
Immune checkpoint inhibitors (ICIs) have been shown to confer significant clinical benefits with tolerable toxicities to patients with malignant tumors and have improved overall survival (OS). Since 2018, based on a series of prospective clinical trials, the National Medical Products Administration of China has approved several types of programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors, including pembrolizumab, nivolumab, atezolizumab, durvalumab, sintilimab, camrelizumab, toripalimab, tislelizumab, envafolimab, and ipilimumab, for the treatment of advanced or metastatic solid tumors, including non-small-cell lung cancer (NSCLC), small-cell lung cancer, melanoma, classic Hodgkin’s lymphoma (cHL), hepatocellular carcinoma, esophageal cancer, gastric cancer, urothelial cancer, head and neck squamous cell carcinoma, pleural mesothelioma, and other malignant tumors. 1
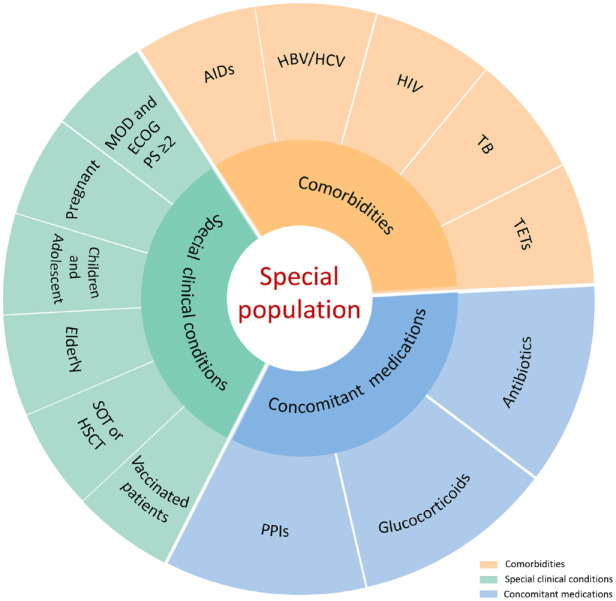
Compared with traditional chemotherapy and targeted therapy, ICIs have dramatically different mechanisms of action and toxicity profiles based on immune-related adverse events (irAEs), and diverse clinical manifestations of irAEs have also been noted. National cross-sectional surveys showed that 65.7% of Chinese cancer patients treated with ICIs report irAEs, and Chinese oncology prescribers are mostly concerned about ‘immunity-related adverse effects management’. Thus, cancer patients and oncologists in China have a preliminary understanding of ICIs.2,3 The Guidelines Working Committee of the Chinese Society of Clinical Oncology (CSCO) previously released multiple editions of ‘Guidelines for the Clinical Application of Immune Checkpoint Inhibitors’ and ‘Guidelines for the Management of Immune Checkpoint Inhibitor Related Toxicity’, aiming at guiding clinical oncologists to standardize the administration of ICIs and improve the management of irAEs.1,4 However, as ICIs are becoming a broad-spectrum antitumor strategy, the treatment populations have continued to expand to various special challenging cancer patient populations. At present, there is no clear definition of special cancer patient populations. Nevertheless, here, special populations refer to patients with special issues including comorbidities, special clinical conditions, or concomitant medications, such as those with autoimmune diseases, those with chronic viral infections or tuberculosis (TB), elderly patients, those receiving solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT), those with thymic epithelial tumors (TETs), those receiving concomitant medication, those with major organ dysfunction (MOD), Eastern Cooperative Oncology Group (ECOG) performance status (PS) ⩾ 2, pregnant individuals, children and adolescents, and those with specific vaccination statuses (Figure 1). As special cancer patient populations have been systematically excluded from prospective clinical trials of ICIs or underrepresented in these trials because of the strict limitation of inclusion criteria, data on the efficacy and safety associated with ICIs are extremely limited. 4 In fact, these special patients are usually encountered in daily clinical practice, indicating that every patient represents a unique situation. Although the CSCO Guidelines for the Management of Immune Checkpoint Inhibitor-Related Toxicity have provided preliminary suggestions for screening special populations prior to the initiation of immunotherapy, a comprehensive clinical consensus is urgently needed. 4
Figure 1.
Summary of special populations who are potentially administered ICIs.
AIDs, autoimmune diseases; ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; HSCT, hematopoietic stem cell transplantation; ICIs, immune checkpoint inhibitors; MOD, major organ dysfunction; PPIs, proton pump inhibitors; SOT, solid organ transplant; TB, tuberculosis; TETs, thymic epithelial tumors.
Based on the data from published case reports, case series, registering clinical trials, postmarketing clinical studies, and real-world medication experience of ICIs, the CSCO Immunotherapy Expert Committee organized a multidisciplinary expert panel for extensive discussion and developed these evidence-based consensus recommendations that will better guide the rational and safe application of ICIs in special challenging populations and serve as an important clinical decision-making reference for oncologists.1–6 To our knowledge, this is the first multidisciplinary expert consensus regarding the administration of ICIs to special cancer patient populations (Table 1).
Table 1.
Multidisciplinary recommendations for special cancer patient populations with potential ICI administration.
| Special populations | Recommendations based on the efficacy of ICIs | Recommendations based on the safety of ICIs | MDT involvement |
|---|---|---|---|
| Cancer patients with autoimmune diseases | Cancer patients with autoimmune diseases represent a population that could potentially benefit from ICI treatment | The occurrence of irAEs and autoimmune disease-related symptom flares should be considered. ICIs are not recommended if the patient has neurological autoimmune diseases or if the autoimmune diseases are moderate to severe or active and cannot be controlled by immunosuppressive agents or require high doses of immunosuppressive agents to control symptoms | Medical oncologist, rheumatologist, neurologist |
| Cancer patients with chronic viral infections | Cancer patients with HBV, HCV, or HIV infections are not contraindicated for the clinical application of ICIs | ICIs may have inhibitory effects on viral replication. It is recommended to perform an HBV/HCV/HIV serological examination before ICI treatment. In addition, the patients should receive active antiviral therapy throughout the course to prevent viral resurgence | Medical oncologist, hepatologist, epidemiologist |
| Cancer patients with TB infection | Rarely reported | A history of TB infection, advanced age, and the use of glucocorticoids may lead to TB occurrence or reactivation. Consideration should be given to the T-SPOT test or the tuberculin skin test for the assessment of TB infection. For those with active TB, it is necessary to stop ICI treatment and initiate anti-TB treatment in strict accordance with the guidelines/consensus until complete recovery | Medical oncologist, respiratory specialist, epidemiologist |
| Cancer patients with TETs | TET patients can benefit from ICI administration | TET patients treated with ICIs are at extremely high risk of developing life-threatening irAEs, especially patients with thymoma. Therefore, special caution must be exercised when using ICIs for TETs, and ICIs are generally not recommended for patients with thymoma | Medical oncologist, thoracic surgeon, pathologist |
| Cancer patients with MOD or ECOG PS ⩾ 2 | MOD is not an absolute contraindication to ICIs, and patients with mild to moderate organ dysfunction are potential candidates for ICIs | Patients with an ECOG PS of 2 may benefit from ICI treatment, but high-level evidence-based medical evidence to support this notion is limited. For patients with ECOG PS of 3, ICI treatment is not recommended | Medical oncologist, cardiologist |
| Pregnant cancer patients | Rarely reported | ICIs can cause miscarriage, preterm birth, and fetal death and are not recommended for pregnant women. An MDT should be involved in the care of pregnant women with cancer receiving ICIs to promote safe delivery, reduce the chance of transplacental metastasis, and systematically treating the mother after delivery | Medical oncologist, obstetrician, pediatricians |
| Children and adolescents with cancer | Children and adolescents with lymphoma could potentially benefit from ICI treatment, whereas ICI treatment is less effective in patients with solid tumors | The safety profile in children and adolescents is similar to that in adults, but it is necessary to closely follow growth, development, puberty, fertility, and mental health due to problems caused by endocrine toxicity in children | Medical oncologist, pediatrician |
| Elderly cancer patients | The efficacy of ICIs in elderly cancer patients (65–75 years old) are comparable to those in younger patients. However, elderly patients over 75 years should be especially evaluated before treatment when considering that chemotherapy will be added to immunotherapy in this population | The spectrum of irAEs in elderly patients may be distinct from that in younger patient, and the incidence of fatal irAEs is high. It is recommended that major organ functions, comorbidities, cognitive function, nutritional status, psychological status, social support, and concomitant medication should be comprehensively evaluated before ICI treatment | Medical oncologist, geriatrician |
| Cancer patients with SOT or HSCT | Cancer patients with SOT or HSCT could potentially benefit from ICI treatment | For cancer patients receiving SOT, the administration of ICIs may increase the risk of graft rejection. Therefore, special attention should be given to the risk of graft-versus-host disease in cancer patients treated with HSCT. If patients are eligible to receive ICI treatment, it is recommended to assess the benefit/risk comprehensively, and the patients should be fully informed before ICI treatment | Medical oncologist, hematologist, organ transplant specialist |
| Vaccinated cancer patients | It is recommended that patients do not receive vaccinations during chemoradiotherapy, the perioperative period, disease progression, or cachexia. In patients with stable disease and good immune status, vaccination may increase the efficacy of ICI treatment | Vaccination may have a potential impact on irAEs, especially in patients with dual ICI immunotherapy. In contrast, ICI treatment may enhance the viral protective effect of vaccination with minimal impact on the safety of vaccination | Medical oncologist, infection specialist |
| Cancer patients with antibiotics | Concomitant use of antibiotics may reduce the efficacy of ICIs | NA | Medical oncologist, infection specialist |
| Cancer patients with glucocorticoids | Concomitant use of glucocorticoids (under certain conditions, including pre-ICI and early application after immunotherapy) may reduce the efficacy of ICIs | An increased risk of acquired infection and peptic ulcer disease | Medical oncologist, immunologist |
| Cancer patients with PPIs | PPIs possess mechanisms that potentially affect the efficacy of ICIs. However, the conclusions of existing clinical studies differ, and more research is needed in the future | NA | Medical oncologist, gastroenterologist |
ECOG PS: Eastern Cooperative Oncology Group performance status; HBV: hepatitis B virus; HCV: hepatitis C virus; HSCT: hematopoietic stem cell transplantation; ICIs: immune checkpoint inhibitors; irAEs: immune-related adverse events; MDT: multidisciplinary team; MOD: major organ dysfunction; NA, no available or no applicable; PPIs: proton pump inhibitors; SOT: solid organ transplant; TB: tuberculosis; TETs: thymic epithelial tumors.
Development of the expert consensus
The development of this expert consensus was led by a multidisciplinary expert panel for which both funding sources and conflicts of interest were reported. This consensus aims to provide guidance and is not a substitute for the clinical judgment of physicians.
Conflict of interest management
Financial support for the development of these guidelines was provided exclusively by the CSCO. No commercial funding was received.
Expert consensus generation
The panel recommendations were based on literature evidence and clinical experiences. Expert consensus was generated by open and scientific discussion as well as formal voting in consensus meetings.
For transparency, a draft of this consensus was made publicly available for comment during the development process. All comments were evaluated and considered for inclusion.
Administration of ICIs to special populations
Cancer patients with autoimmune diseases
Autoimmune diseases refer to diseases caused by the body’s immune response to self-antigens, resulting in self-tissue damage. Currently, prospective clinical trials of ICIs generally exclude cancer patients with autoimmune diseases because of the important roles of PD-1/PD-L1 and CTLA-4 in maintaining self-tolerance and concerns about the potential worsening of autoimmune disease-related symptoms. In addition, patients with autoimmune diseases often require continuous immunosuppressive treatment, which may potentially affect the efficacy of ICIs. In clinical practice, cancer patients with a history of autoimmune diseases or immunosuppressive agent treatment for their primary disease may experience underlying autoimmune disease flares or newly developed irAEs after ICI treatment. Most of these symptoms and irAEs are mild and manageable, but occasionally, they can be life-threatening. Autoimmune diseases that are prone to exacerbated symptoms include rheumatic autoimmune diseases, psoriasis, and inflammatory bowel disease. Compared with PD-1/PD-L1 inhibitors, CTLA-4 inhibitors have a higher incidence of autoimmune disease flares and more severe symptoms. ICIs are not recommended if the patient has neurological autoimmune diseases or if the autoimmune diseases are moderate to severe, active, not controlled by immunosuppressants, or require treatment with high doses of immunosuppressants. 4
However, mild to moderate autoimmune diseases concurrent with cancer are not an absolute contraindication to ICI therapy. 6 Studies have shown that the objective response rate (ORR) of malignant melanoma patients with autoimmune diseases after receiving ICI treatment is 33%, the incidence of autoimmune disease flares is 38%, and the incidence of any-grade irAEs is approximately 29% (among which the incidence of grade 3 or higher irAEs is approximately 10%). For NSCLC patients with autoimmune diseases, the ORR of ICI treatment is 22–54%, the disease flare rate is 6–42%, and the incidence of any-grade irAEs is approximately 16–38%. In patients with urothelial carcinoma and autoimmune diseases who received ICIs, the ORR is 11%, the incidence of disease flares is 11%, and the incidence of any-grade irAEs is approximately 46% (of which the incidence of grade 3 or higher irAEs is approximately 14%). Based on the current data, 60–90% of patients have no autoimmune disease-related symptoms or only mild disease flares after ICI treatment. 7 At this stage, it is not necessary to stop ICIs and start glucocorticoid therapy. Even if irAEs and/or autoimmune diseases are exacerbated, most patients can be managed properly. 7 For these patients, the dose of prednisone should be lowered to the target range (<10 mg/day) before ICI treatment. During ICI treatment, it is necessary to closely monitor irAEs and/or autoimmune diseases. 4 Two ongoing prospective phase I studies are exploring the safety, tolerability, and activity of nivolumab in cohorts of patients with autoimmune disease and advanced NSCLC (ClinicalTrials.gov identifier: NCT03656627) or different tumor types (ClinicalTrials.gov identifier: NCT03816345).
Consensus recommendation 1
Cancer patients with autoimmune diseases represent a special population that could potentially benefit from ICI treatment, but the occurrence of irAEs and autoimmune disease-related symptom flares should be considered. Before ICI administration, it is recommended that benefits/risks are evaluated, and patients should be fully informed. ICIs are not recommended if the patient has neurological autoimmune diseases or if the autoimmune diseases are moderate to severe or active and cannot be controlled by immunosuppressive agents or require high doses of immunosuppressive agents to control symptoms. The dose of prednisone should be reduced to the target range (<10 mg, daily) before initiating ICIs. During ICI treatment, close monitoring for irAEs and/or autoimmune disease flares is needed.
Cancer patients with chronic viral infections or TB
Cancer patients with chronic hepatitis B virus or hepatitis C virus infection
Basic studies indicate that PD-1 is highly expressed in hepatitis B virus (HBV)-specific T cells, and PD-1 inhibitors may help restore T-cell function. CTLA-4 appears to act on other immune cells, such as follicular helper T cells.8–10 Studies of blood from HBV-infected mice and chronic HBV-infected patients show that follicular helper T-cell responses to HBsAg are required for HBV clearance, and this action can be blocked by regulatory T cells (Tregs). Inhibition of Treg activity with CTLA-4 monoclonal antibody restores the ability of follicular helper T cells to clear HBV infection, which may partially explain why the HBV viral load decreases following immunotherapy. 10
Currently, ICIs, including PD-1/PD-L1 and CTLA-4 inhibitors, are considered effective agents for the treatment of patients with advanced cancer and HBV/hepatitis C virus (HCV) infections. In the KEYNOTE-244 study of 104 pretreated hepatocellular carcinoma patients, most patients were HBV (78%) or HCV (75%) negative, and the ORR for patients treated with pembrolizumab was only 17%. 11 In the CheckMate 870 study involving 400 Chinese patients with advanced NSCLC, the ORRs for patients with HBV (n = 383) and without HBV (n = 17) were 17.6% and 15.4%, respectively. 12 A recently published systematic review of 186 cancer patients with preexisting chronic HBV/HCV infections reported that 18.6% of liver cancer patients, 32.4% of melanoma patients, and 16.7% of NSCLC patients showed objective responses to ICIs despite lines of therapy. 13 It seems that preexisting chronic HBV/HCV infections do not significantly impact the efficacy of immunotherapy in patients with various cancer types. In terms of safety, coexisting viral hepatitis does not increase the risk of irAEs. In general, immunotherapy was well tolerated in cancer patients with preexisting chronic HBV/HCV and no treatment-related deaths were reported. The most prevalent adverse events (AEs) were dermatic, liver function, and gastric abnormalities. 13 The CheckMate 870 study showed that the frequency of treatment-related AEs for patients with and without HBV was comparable (17.6% versus 12.5%). 12 However, a small number of patients exhibited hepatitis B reactivation. In addition to patients with liver cancer, antiviral treatment for HBV patients with other solid tumors may be neglected when receiving ICIs, leading to an increased incidence of HBV resurgence and/or immune-related hepatitis.14–17 In a retrospective study of ipilimumab for advanced melanoma, two of nine patients with HBV or HCV infections developed immune hepatitis, and the incidence was similar to that of patients without these infections. 18
A phase Ib clinical trial investigated the efficacy of nivolumab in 24 patients with chronic HBV infection who remained HBeAg positive despite nucleoside analoge (NA) antiviral therapy. 19 Patients received either a single dose of nivolumab at 0.1 mg/kg or 0.3 mg/kg or 40 yeast units of GS-4774 at baseline and 0.3 mg/kg of nivolumab at week 4. Patients receiving 0.3 mg/kg nivolumab with and without GS-4774 (GlobeImmune and Gilead) showed significant HBsAg declines from baseline, with the levels in three patients decreasing by more than half at the end of the study. In the 0.3 mg/kg monotherapy group, one patient was observed to be HBsAg negative. In another retrospective study, 35 patients with advanced hepatocellular carcinoma with HBV-DNA ⩽ 100 IU/mL were treated with PD-1 monoclonal antibody based on NA therapy. Of the six patients who did not receive NA therapy, three had a >1 log reduction in HBV viral load, and one had undetectable serum HBV-DNA during ICI treatment. 20
For HBsAg-positive patients, routine NA prophylaxis and dynamic detection of HBV-DNA are needed. For those with previous infection (HBsAg negative, HBcAb positive), whether routine NA prophylaxis and HBV-DNA detection are required remains inconclusive, but HBsAg should be monitored regularly. Unlike HBV patients, HCV patients are not excluded from clinical trials regardless of HCV-RNA load. In HCV patients, a transient decrease in HCV RNA has been observed after nivolumab treatment. 21 A significant decrease in HCV RNA has also been observed after tremelimumab treatment. 22
In conclusion, preexisting chronic HBV or HCV infections are not contraindications to the application of ICIs, but HBV/HCV serological tests (including HBsAg, HBsAb, HBcAb, HBV-DNA, and HCV-RNA) are required before ICI treatment. Patients with active HBV infection (HBsAg-positive) and negative or positive viral load, regardless of HBeAg status, need to be treated with NAs to prevent viral reactivation. Given that the activity of ICIs may vary after discontinuation, the duration of prophylactic antiviral therapy is difficult to determine, and it is currently considered preferable to continue antiviral therapy until 6 months after ICI discontinuation.14,23
Cancer patients with HIV
For HIV-positive cancer patients receiving antiretroviral therapy, PD-1 overexpression on the surface of T cells is detrimental to immune reconstitution and is negatively correlated with the number of CD4+ T cells, which may cause viral overload and disease progression. PD-1 expression on CD4+ T cells is partly responsible for viral latency. Therefore, ICIs should theoretically be able to reverse HIV latency and enhance the antiviral effect of T cells. 24 However, case report-based real-world evidence for treatment with PD-1/PD-L1 inhibitors in patients with concurrent HIV infection and advanced malignancies is limited. Recent clinical data indicated that PD-1 inhibitors are active in HIV-positive patients with melanoma and Merkel cell carcinoma. 25 No clinically meaningful increase in viral replication and opportunistic infections in patients receiving antiretroviral therapy during cancer treatment or new toxicity was observed. Patients with NSCLC or head and neck cancer and HIV infection also benefit from ICI treatment.26–29 A good safety profile has been reported, and no increase in viral load or changes in the number of CD4+ T cells have been observed. 29 Given that the number of cancer patients with HIV is relatively small and these patients are typically excluded from clinical trials, future studies should be conducted to determine the dose, toxicity, and feasibility of the application of PD-1/PD-L1 inhibitors in this population.
Cancer patients with TB
Screening for TB before ICI treatment is not routine in clinical practice, and TB infection during ICI treatment is recorded as an AE in clinical studies. However, clinicians should be aware that new TB infections and reactivation can occur during immunotherapy with serious consequences. In addition to typical symptoms, such as cough, fever, weight loss, and shortness of breath, patients may present with an asymptomatic infection. Imaging findings may represent real tumor progression or patterns that mimic tumor pseudoprogression and immune-related pulmonary toxicity. The infection/reactivation rate of TB in high-risk populations and the specific mechanisms remain unclear. A survey of 6335 NSCLC patients in Korea showed that 15 TB patients were identified among 899 patients treated with ICIs. Multivariate analysis showed that ICIs were not a risk factor for the occurrence of TB, whereas advanced age and the use of glucocorticoids may lead to the occurrence or reactivation of TB. 30 Another nationwide observational study with a large sample size in South Korea also showed that ICI exposure was not significantly associated with an increased risk of TB in cancer patients. 31 A T-SPOT test for TB infection or a tuberculin skin test should be considered and may be reviewed periodically during ICI treatment, especially in the setting of immunosuppressive therapy for irAEs. Another study reported 16 patients who developed TB during ICI treatment. 32 The median age was 61 years, and lung cancer was the most common type of cancer (n = 8). The median time to TB reactivation was 6.3 months after the initiation of ICI therapy, whereas no cases of TB reactivation occurred during anti-CTLA-4 therapy. Based on relevant domestic and foreign case reports or retrospective studies, the incidence of TB during ICI treatment was less than 2%, and the recurrence of latent TB cannot be excluded.33,34 For patients with active TB, it is recommended to strictly follow the guidelines/consensus for corresponding treatment, 35 and whether or when to restart ICIs is not currently defined. For cancer patients with TB, especially those suspected of TB infection after cancer diagnosis and treatment, it is recommended to perform a T-cell spot test for TB infection, as the sensitivity and specificity of this test are significantly better than those of the conventional tuberculin test and TB antibody test. 36
Consensus recommendation 2
Preexisting chronic HBV, HCV, or HIV infections are in cancer patients not contraindications to the clinical application of ICIs, and some studies have shown that ICIs have inhibitory effects on viral replication. Combined with the patient’s infection history, it is recommended to perform an HBV/HCV/HIV serological examination before ICI treatment. In addition, patients should receive active antiviral therapy throughout the course to prevent viral resurgence. A history of TB, advanced age, and the administration of glucocorticoids may lead to TB occurrence or reactivation. Consideration should be given to the T-SPOT test for the assessment of TB infection or the tuberculin skin test, which can also be reviewed regularly during the treatment process, especially in the case of TB infection. Adverse reactions are potentially related to immunosuppressive therapy. The probability of developing TB during ICI treatment is less than 2%. Clinical and imaging examinations are often insidious, and the T-SPOT test is recommended for the assessment of suspected TB infection after diagnosis. For those with active TB, it is necessary to stop ICI treatment and initiate anti-TB treatment in strict accordance with the guidelines/consensus until full recovery.
Elderly patients
Elderly patients often have more comorbidities than their younger counterparts. In addition, the functions of major organs decrease with increasing age. Basic studies have shown that elderly patients have a characteristic immune microenvironment, including increased tumor mutational burden (TMB), increased immune checkpoint gene expression, decreased promoter methylation, increased γ-interferon signaling, and low T-cell receptor diversity. These changes may alter the efficacy of ICIs. 37 However, there is limited information from prospective clinical trials regarding the efficacy and safety of ICIs for elderly cancer patients, especially those over 75 years old. Most evidence comes from subgroup analyses of prospective clinical trials with highly screened populations.
The existing evidence is not completely consistent regarding the efficacy of ICIs in elderly patients. A comprehensive meta-analysis published in 2019 included 34 studies involving more than 20,000 advanced patients with different tumor types. A statistically significant improvement in OS was noted in the ICI-treated group (in both the <65-year-old cohort and the ⩾65-year-old, and <75-year-old cohorts) compared with the control group, but the improvement in OS was less in patients ⩾75 years old. 38 A meta-analysis of nine randomized clinical trials (including four CTLA-4 inhibitor studies and five PD-1 inhibitor studies) showed OS benefits of ICIs in elderly (65–70 years old) as well as younger patients. 39 A meta-analysis of 2192 patients with NSCLC from four phase III clinical trials showed that PD-1 inhibitors (pembrolizumab or nivolumab) significantly prolonged the median OS for young (<65 years old) and older patients (⩾65 years old). In patients ⩾75 years old, no significantly prolonged OS was observed in the ICI group compared with the chemotherapy group. Compared with pembrolizumab, better OS was observed with nivolumab in elderly NSCLC patients (⩾65 years old). 40 For NSCLC patients with a PD-L1 score ⩾50%, an FDA pooled analysis of 12 randomized controlled trials of first-line immunotherapy or chemotherapy–immunotherapy combinations indicated that most subgroups of patients receiving FDA-approved chemotherapy–immunotherapy combination regimens may have OS and progression-free survival (PFS) outcomes that are comparable with or better than ICI-only regimens, but patients aged ⩾75 years receiving chemotherapy–immunotherapy may not have better survival outcomes over immunotherapy. 41 Similar outcomes were seen with first-line chemotherapy–immunotherapy and immunotherapy alone for advanced NSCLC with PD-L1 scores of 1–49%. 42
Elderly people receiving ICI treatment should be prepared for the higher occurrence of irAEs. An update of the FDA Adverse Event Reporting System (FAERS) in 2021 revealed an increased incidence of irAEs in patients over 65 years old compared with those 18–64 years old who received ICI monotherapy or combination therapy; however, substantial disagreement exists in retrospective analysis. 43 A retrospective analysis of patients with melanoma, renal clear cell carcinoma, and NSCLC showed no significant difference in the grade of irAEs across age groups. However, endocrine toxicity was more common in patients <65 years old, and dermatological toxicity was more common in patients ⩾75 years old. 44 Of note, a comprehensive meta-analysis exploring ICI-related fatal AEs reported that patients who died from irAEs were preferentially older. 45
Efforts to study ICI efficacy and toxicity among frail older adults in everyday clinical practice are important for expanding the evidence base to patients who were routinely excluded from landmark immunotherapy clinical trials. Immunotherapy trials designed specifically for older adults such as Alliance A171901 (ClinicalTrials.gov identifier: NCT04533451) are ongoing. Clinicians should comprehensively weigh the benefits and risks of immunotherapy based on an individualized approach aimed at improving goal-concordant care and outcomes among older adults with cancer. 46
Consensus recommendation 3
In general, the efficacy and safety of ICIs in elderly cancer patients (65–75 years old) are comparable to those in younger patients. However, elderly patients over 75 years should be especially evaluated before treatment when considering that chemotherapy will be added to immunotherapy in this population. The spectrum of irAEs in elderly patients may be distinct from that in younger patients. It is recommended that major organ functions, comorbidities, cognitive function, nutritional status, psychological status, social support, and concomitant medication should be comprehensively evaluated before ICI treatment.
Patients undergoing SOT or HSCT
Studies have shown that the PD-1/PD-L1 axis may play a key role in allograft rejection. 47 PD-L1 from donor tissue can interact with PD-1 expressed on recipient alloreactive T cells, thereby downregulating recipient alloreactive T-cell responses and limiting rejection. PD-1/PD-L1 inhibitors could disrupt the balance of the immune microenvironment through regulating graft-versus-host-reactive CD8 T cells, leading to potential allograft rejection. 48 Therefore, whether ICIs can destroy immune tolerance and lead to severe posttransplantation complications is a question that should not be ignored.
Cancer patients receiving SOT exhibited different clinical responses to ICIs. In terms of toxicity, some patients can tolerate ICIs, whereas others experience severe posttransplant complications. A retrospective study by Abdel-Wahab et al. 49 indicated that among 39 cancer patients who had undergone SOT, 16 patients experienced allograft rejection following ICI treatment, and 8 patients developed irAEs. Graft failure occurred in 81% of patients, and the mortality rate was 46%. The median OS of patients without rejection was 12 months, and the median OS of patients with rejection was 5 months (p = 0.03). Another analysis from the FAERS database showed that among 96 reports of transplant rejection after ICIs (including kidney, liver, cornea, and heart, etc.), 43.8% were patients with malignant melanoma. 50 Overall, rejection was more common in patients on PD-1/PD-L1 inhibitors than those on CTLA-4 inhibitors, with a mortality rate of 36.5%, and rejection was more common in liver transplant recipients than in recipients of other organs. Biopsy reports suggested that acute cellular rejection was the most common, and only 21.4% of patients showed antibody-mediated responses.
For HSCT, some patients who receive allogeneic hematopoietic stem cell transplantation (allo-HSCT) can benefit from PD-1 inhibitor therapy without serious AEs, whereas others with graft-versus-host disease (GVHD) have severe toxicity that is sometimes fatal. Herbaux et al. 51 reviewed the efficacy and toxicity of nivolumab in 20 patients with relapsed Hodgkin’s lymphoma (HL) after allo-HSCT. The ORR was 95%, with complete response (CR) and partial response (PR) rates of 42% and 52%, respectively. The 1-year PFS and OS rates were 58.2% and 78.7%, respectively. Six patients (30%) developed GVHD within 1 week of the first dose of nivolumab, but the disease was manageable with standard therapy. Notably, all six patients had previous acute GVHD. In another retrospective, multicenter study, 21 patients with hematological malignancies were analyzed, including 12 with multiple myeloma/acute myeloid leukemia, 5 with non-Hodgkin’s lymphoma (NHL), 2 with acute lymphoblastic leukemia, and 2 with myelofibrosis change. 52 These patients relapsed after allo-HSCT and subsequently received nivolumab, ipilimumab, nivolumab + ipilimumab, or nivolumab in combination with donor lymphocyte infusions (DLIs) and other treatments. The overall ORR was 43%. A higher ORR was observed in patients who received nivolumab in combination with DLIs than in those who received nivolumab or ipilimumab alone. However, grade 3–4 acute GVHD or moderate/severe chronic GVHD occurred in 29% of patients, 83% of whom were glucocorticoid refractory. There are currently no clinical factors that can help predict and prevent the risk of graft rejection. Thus, the implementation of ICIs in cancer patients with SOT or HSCT is very challenging.
Consensus recommendation 4
For cancer patients receiving SOT, the administration of ICIs may increase the risk of graft rejection. Therefore, special attention should be given to the risk of GVHD in cancer patients treated with HSCT. If patients are eligible to receive ICI treatment, it is recommended to assess the benefit/risk comprehensively, and the patients should be fully informed before ICI treatment.
Patients with TETs
TETs include thymoma and thymic carcinoma. Among them, thymoma is divided into five types: A, AB, B1, B2, and B3. Chemotherapy and chemoradiotherapy are the main treatments for advanced or inoperable TETs, but systemic treatments are limited. 53 Positive PD-L1 expression is commonly observed in TETs, and higher expression of PD-L1 is more often found in clinically aggressive tissue subgroups. 54 However, TETs have low TMB, and cases with microsatellite instability (MSI) are extremely rare.55,56 Currently, ICIs are not approved for the treatment of TETs, but theoretically, patients with TETs could benefit from PD-1/PD-L1 inhibitor therapy.
A clinical trial of avelumab in recurrent thymoma (n = 7) and thymic carcinoma (n = 2) showed an ORR of 29%. 57 In a phase II study of pembrolizumab in recurrent thymic carcinoma (n = 41), the ORR was 22.5%, and the median duration of response (DoR) was 22.4 months. In addition, the median PFS and median OS were 4.2 months and 24.9 months, respectively. Patients with high PD-L1 expression had a higher ORR than those with negative PD-L1 expression. 58 Another phase II study also showed the efficacy of pembrolizumab in recurrent thymoma (n = 26) and thymic carcinoma (n = 7) with a median DoR of not reached and 9.7 months, respectively. A median PFS of 6.1 months was obtained in both groups, with a median OS of not reached and 14.5 months, respectively. 59 In addition, the results of the PRIMER phase II study of nivolumab in recurrent thymic carcinoma (n = 15) showed that no patients had a response to immunotherapy with median PFS and OS values of 3.8 and 14.1 months, respectively. 60
The greatest challenges in the treatment of TETs with ICIs are some potential, complex, and life-threatening irAEs. Prospective clinical trials have shown that myotoxicity, neuromuscular toxicity, and cardiotoxicity are common, even in patients without a history of autoimmune symptoms prior to the administration of ICIs. Myotoxicity can occur in 8–57% of patients with TETs, whereas the incidences of myocarditis and myasthenia gravis are 5–57% and 3–14%, respectively. 61 The incidence is much higher in TETs than in other tumor types. There are many clinical case reports of fatal irAEs after ICI treatment.62,63 Other relatively rare irAEs, such as type 1 diabetes mellitus, Sjogren’s syndrome, and acquired coagulopathy, can also occur in patients with TETs receiving ICIs. 64 In addition to myasthenia gravis, neuromuscular disorders are common manifestations of paraneoplastic autoimmunity in patients with TETs. Myotoxicity and neuromuscular toxicity occur early and can be observed within 1–6 weeks after the initiation of ICI therapy, but sometimes symptom delays can also occur. irAEs can occur in all types of TETs. However, compared with those with thymic carcinoma, patients with thymoma are more likely to experience grade 3 or higher toxicity (71% versus 11.5–15%). 64
The cause of severe irAEs in patients with TETs is not fully understood, but it is presumed to be related to the deficiency in immune tolerance and the persistence of autoimmune T cells, especially autoimmune CD4+ or CD8+ T cells differentiated from immature CD4+CD8+ T cells. 65 Studies have shown that positive peripheral blood acetylcholine receptor autoantibodies, decreased B cells and Tregs, and increased T-cell receptor diversity in peripheral blood mononuclear cells are associated with polymyositis, but prospective evidence is lacking. In addition, the concurrent use of immunosuppressive agents during immunotherapy is a potential means to reduce the occurrence and severity of irAEs, but clinical trials in patients with TETs have not been conducted. 66
Consensus recommendation 5
TET patients treated with ICIs are at extremely high risk of developing life-threatening irAEs, especially patients with thymoma. Therefore, special caution must be exercised when using ICIs for TETs, and ICIs are generally not recommended for patients with thymomas.
Patients with concomitant medications
Long-term use of antibiotics reduces gut microbiota diversity and clears most immunogenic flora. The diversity and distribution abundance of the gut microbiota can affect the efficacy of ICIs. For example, in CTLA-4 inhibitor-induced enteritis, oral administration of bifidobacteria can modulate the activity of Tregs and increase the number of Tregs in the colonic mucosa, thereby suppressing autoimmune responses. 67 Therefore, in patients receiving CTLA-4 inhibitors, the use of vancomycin induces decreased intestinal bifidobacterial activity and increased irAEs. Bacteroides thetaiotaomicron and Bacteroides fragilis increase the activity of specific T cells and improve the efficacy of CTLA-4 inhibitors. Gut microbiota heterogeneity is associated with primary resistance to PD-1 and CTLA-4 inhibitors. A retrospective analysis found that the use of antibiotics within 2 months before or the first month of initial ICI treatment can significantly reduce PFS and OS, especially in patients with intravenous antibiotics and lower respiratory tract or urinary tract infections. 68 Among 109 advanced NSCLC patients with ICIs at Shanghai Pulmonary Hospital from 2016 to 2018, 18.3% received antibiotics before or within 1 month after the first immunotherapy. These patients had significantly lower PFS than those without antibiotics, and the proportion of patients with primary resistance to immunotherapy increased following the use of antibiotics. Given the ethnic and regional differences in the type and distribution of the gut microbiota, large-sample clinical studies are required for further verification in the future. 69
Glucocorticoids are commonly used to treat fatigue, dyspnea, decreased appetite, and symptomatic brain metastases in cancer patients. Glucocorticoids control the body’s autoimmune response by inhibiting self-antigen-specific CD8+ T cells and have a low impact on tumor neoantigen-specific CD8+ T cells. Glucocorticoids affect the function of low-affinity memory T cells by inhibiting fatty acid metabolism, thereby reducing the objective efficacy and clinical benefit of immunotherapy. 70 Cancer patients receiving daily prednisone doses of less than 10 mg or cumulative doses of less than 500 mg have a reduced risk of infection. The Memorial Sloan Kettering Cancer Center and Gustave Roussy Cancer Center study indicated that of 640 patients with advanced NSCLC who received PD-1/PD-L1 inhibitor monotherapy, 14% had previously received glucocorticoid daily doses of ⩾10 mg. In both independent cohorts, daily doses of glucocorticoids of ⩾10 mg were associated with reduced ORRs, PFS, and OS. The study also found that baseline glucocorticoid doses (10–19 mg versus ⩾20 mg, daily) had similar effects on the survival and efficacy of immunotherapy. The survival of patients who used glucocorticoids (⩾10 mg, daily) at baseline during immunotherapy was significantly lower than that of patients who did not use glucocorticoids in the previous 30 days and those who used glucocorticoids within 1–30 days. 71 In a study performed at the Dana-Farber and Harvard Cancer Center, advanced NSCLC patients receiving glucocorticoid therapy (⩾10 mg, daily) were divided into two groups: tumor-related glucocorticoid therapy (such as dyspnea, tumor-related fatigue, cancer-related pain, brain metastasis-related edema, etc.) and nontumor-related glucocorticoid therapy (such as chronic obstructive pulmonary disease (COPD), autoimmune disease, hypersensitivity preconditioning, etc.). For patients receiving nontumor-related glucocorticoids, there was no significant difference in median PFS and OS between ICIs and low-dose steroids (<10 mg, daily). This study retrospectively analyzed 650 patients with advanced NSCLC who received PD-1/PD-L1 and/or CTLA-4 inhibitors, of whom 93 received prednisone (⩾10 mg, daily) at baseline (within 24 h of starting ICIs). A total of 66 patients received cancer-related hormone therapy, and these patients had a higher proportion of ECOG PS ⩾ 2 and brain metastases. 72 Recently, Bai et al. 73 analyzed 947 melanoma patients who received ICIs as a single agent and found that early application of high-dose glucocorticoids (a daily dose of ⩾60 mg prednisone within 8 weeks after the initiation of immunotherapy) after the occurrence of irAEs decreased PFS and OS. Furthermore, metastatic cancer patients treated with glucocorticoids <2 months after starting immunotherapy had a significantly shorter PFS and OS than those who received glucocorticoids ⩾2 months after starting immunotherapy, indicating that the timing of steroid initiation is associated with response to ICIs. 74 Recently, a meta-analysis by Petrelli et al. 75 showed that the main negative effect on OS was associated with patients taking steroids for supportive care or brain metastases, but steroids used to mitigate irAEs did not negatively affect OS, indicating that caution is only limited in patients who were treated with steroids for symptom control.
Proton pump inhibitors (PPIs) increase the risk of intestinal infection and diarrhea by inhibiting gastric acid secretion, increasing gastric pH, and affecting the distribution of gastrointestinal microbiota (Clostridium and Campylobacter). Studies assessing whether PPIs in patients receiving ICIs affect the efficacy of ICIs have reported inconsistent conclusions. A pooled analysis of the POPLAR and OAK studies showed that 30.9% of patients receiving atezolizumab received concurrent PPIs, resulting in significantly shorter median PFS and OS. In another small retrospective analysis from Japan, PPIs did not significantly influence the efficacy of ICIs. In the future, large-scale, multicenter studies are needed to continue to explore the significance of PPIs in primary resistance to ICIs. 76
Consensus recommendation 6
Concomitant use of antibiotics and glucocorticoids (under certain conditions, including pre-immunotherapy and early application after immunotherapy) may reduce the efficacy of ICIs. PPIs possess mechanisms that potentially affect the efficacy of ICIs. However, the conclusions of existing clinical studies differ, and more research is needed in the future. Samples and multicenter clinical studies further verify the above conclusions.
Other populations
Patients with MOD and ECOG PS of ⩾2
Immune-related pneumonia is more common in patients with NSCLC; it has a low incidence (<5%) in those with renal cancer; and the real-world incidence seems to be slightly higher. 4 Retrospective data indicated a favorable safety profile for immunotherapy in elderly patients with underlying pulmonary disease. 77 However, patients with pulmonary fibrosis and COPD are at high risk of immune-related pneumonia. 78 Therefore, for patients with pulmonary fibrosis and COPD, it is recommended to comprehensively evaluate the tolerance of immunotherapy based on the patient’s physical situation, pulmonary function, and blood gas results. Immunotherapy can be considered for mildly symptomatic patients. In addition, immune-related cardiovascular AEs are rare but have a potential risk of death, accounting for approximately 6.3% of all irAEs, with a mortality rate up to 35%.
Immunotherapy with ICIs is associated with cardiovascular toxicities such as myocarditis, pericardial disease, and vasculitis. Preexisting cardiovascular risk factors may be associated with the development of ICI-associated myocarditis. A few risk factors have been identified to be associated with the development of ICI-induced cardiotoxicity. In a multicenter study, patients who developed myocarditis had a greater prevalence of hypertension and tobacco use and were more likely to be on statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. Preexisting coronary artery disease and atrial fibrillation were not identified as risk factors for ICI-mediated cardiotoxicity. 79 A retrospective study of patients with organ dysfunction (including cardiac left ventricular ejection fraction of 45%) reported worse organ function in patients treated with ICIs, but supportive care could improve these symptoms. 80 Thus, particular caution should be paid to patients with underlying acute cardiac disease when considering the use of ICIs. 81
Studies have shown that immune-related diabetes is a rare irAE but can be life-threatening if not promptly recognized and treated. A retrospective study based on the FAERS database in the United States showed that approximately 24.9% of patients with immune-related diabetes developed life-threatening fulminant type 1 diabetes, with a fatality rate of approximately 5.58%. 82 However, underlying type 2 diabetes did not appear to be a particular risk factor in a retrospective analysis, but patients with insulin-dependent type 2 diabetes can experience worsening glycemic control.83,84 In addition, a multicenter retrospective study of 1395 patients treated with ICIs showed that long-term poor glycemic control reduced PFS and OS. 85 Therefore, it is particularly important for diabetic patients to monitor and control blood glucose when receiving immunotherapy.
For patients with hepatic and renal insufficiency, the clearance rate was not affected in patients with mild to moderate hepatic and renal impairment, and data for patients with severe hepatic and renal impairment were limited. Two phase II studies of atezolizumab for urothelial carcinoma (creatinine clearance <60 ml/min or 30–60 ml/min) did not demonstrate worsening renal function or significant effect on median OS. 86 In patients with mild to moderate hepatic and renal insufficiency, hepatic and renal function should be comprehensively evaluated before ICI treatment, and the creatinine clearance rate and hepatic function should be closely monitored. ICIs are not recommended for patients with severe organ dysfunction.
Most prospective clinical studies of ICIs exclude patients with an ECOG PS of ⩾2. The results of the CheckMate 153 study showed that compared with the overall population, the treatment-related adverse events (TRAEs) of patients with an ECOG PS of 2 were not significantly increased, and their overall quality of life and symptoms were significantly improved. 87 Although the median OS was reduced in patients with an ECOG PS of 2 compared to the overall population, it was superior to that for conventional single-agent chemotherapy. 88 The CheckMate 171 study presented similar results. 89 In terms of TRAEs, patients with an ECOG PS of 2 were similar to the overall population, with a 6.8% incidence of grade 3–4 AE (13.9% in the overall population). In addition, Middleton et al. 90 conducted a prospective, single-arm, multicenter phase II clinical study (PePS2 study) to evaluate whether patients with an ECOG PS of 2 advanced NSCLC could benefit from PD-1 inhibitors. A total of 60 patients who received pembrolizumab as first-line or posterior treatment were included in the study, and the durable clinical benefit was similar in the two groups. PD-L1 TPS was stratified. Similar to previous studies applying ICIs in NSCLC patients with an ECOG PS of 0 or 1, higher PD-L1 expression was associated with better clinical benefit in patients with an ECOG PS of 2. Regarding safety, AEs occurred in 17 patients, but only 10% discontinued treatment due to toxicity, and the proportion of serious AEs was relatively low. In addition to ICI monotherapy, dual ICI therapy has also been explored. For patients with an ECOG PS of ⩾2, the safety of dual ICIs is tolerable, and the quality of life is also improved, but the efficacy needs to be supported by the data of prospective, randomized controlled studies. A systematic review and meta-analysis of interventional and observational studies showed that advanced NSCLC patients with an ECOG PS of ⩾2 are twice as likely to achieve a response when administered ICIs when compared with the population with an ECOG PS of ⩽1, while the safety profile is not affected. 91
In summary, ICIs should be used with caution. 4 In addition to safety and efficacy, some experts have also paid increasing attention to the negative economic effects of ICIs in recent years. Multiple studies have shown that administration of immunotherapy at the end of life (within 30 days before death) can lead to increased emergency room visits, hospitalization frequency, and treatment costs, especially for patients with an ECOG PS of 3 or 4.92,93 Therefore, immunotherapy is not recommended for this population with extremely poor PS. Prospective randomized trials are still indispensable for determining whether patients with impaired PS status derive benefit from ICIs.
Consensus recommendation 7
MOD is not an absolute contraindication to ICIs, and patients with mild to moderate organ dysfunction are potential candidates for ICIs. Clinicians should carefully weigh the benefits and risks and comprehensively assess patients with completely informed consent. Close monitoring is necessary during treatment, and timely management of irAEs is needed. For special populations, it is recommended to involve clinicians of multiple disciplines for individualized treatment. Patients with an ECOG PS of ⩾2 are highly heterogeneous because they are typically elderly with other comorbidities or organ dysfunction. Patients with an ECOG PS of 2 may benefit from ICI treatment, but high-level evidence-based medical evidence to support this notion is limited. For patients with an ECOG PS of ⩾3, ICI treatment is not recommended.
Pregnant patients
Melanoma is one of the most common malignancies during pregnancy and the most common tumor that metastasizes to the fetus. 94 Animal studies have shown that ipilimumab can cause miscarriage, stillbirth, premature birth, or low birthweight in the third trimester of pregnancy, resulting in a significant increase in mortality. However, preclinical studies of nivolumab have not suggested teratogenic effects on surviving offspring. 95 Based on the fact that ICIs may disrupt the immune tolerance of the fetus and the mother, resulting in spontaneous abortion, premature birth, and fetal death, the FDA classifies ICIs as Class D drugs.
The drug label of most ICIs calls for effective contraception during treatment and up to 5 months after the last dose, but human data are lacking. One study assessed five patients with metastatic melanoma administered ICIs during pregnancy. 96 One patient received ICIs in the ninth week of pregnancy, one in the second trimester, and three patients became pregnant while receiving ICIs. Obstetric complications occurred in three patients. Specifically, placental insufficiency and fetal bradycardia were noted in one case (ICIs started at the ninth week and discontinued in the second trimester), and intrauterine growth restriction was observed in two cases (one case was discontinued in the first trimester, and one case was treated with ICIs until delivery at 32 weeks). After follow-up, all infants met developmental requirements and were in good health. Some studies have also shown that pregnancy beyond 36 weeks may increase the risk of transplacental transmission of melanoma. Patients with melanoma during pregnancy should consider elective delivery at 34–36 weeks followed by systemic therapy. In addition, comprehensive follow-up should be performed after birth, and liver ultrasound, serum S100, and skin conditions should be reviewed every 3 months. Advanced melanoma is most likely to recur within 3 years of diagnosis, so patients are advised to delay pregnancy during this period.
Consensus recommendation 8
ICIs can cause miscarriage, preterm birth, and fetal death and are not recommended for pregnant women. A multidisciplinary team of obstetricians, pediatricians/neonatologists, and oncologists should be involved in the care of pregnant women with cancer receiving ICIs to promote safe delivery, reduce the chance of transplacental metastasis, and systematically treat the mother after delivery. In addition, newborns should be followed up closely.
Children and adolescents
The 2020 European Pediatric Strategy Forum meeting noted that except HL and some malignant tumors with high TMB, ICIs have limited efficacy in the treatment of childhood tumors (such as neuroblastoma, osteosarcoma, rhabdomyosarcoma, and Wilms tumor). 97 Currently, the FDA has approved pembrolizumab for the treatment of children with cHL, primary mediastinal large B-cell lymphoma, microsatellite instability-high (MSI-H)/deficient mismatch repair (dMMR) tumors, and Merkel cell carcinoma at a dose of 2 mg/kg (up to 200 mg, q3w), and no significant AEs on immune system development were found.
The multicenter phase I/II KEYNOTE-051 study included 155 children and adolescents from Europe and the United States aged 6 months to 17 years with malignant melanoma, PD-L1-positive relapsed or refractory solid tumors, and lymphomas treated with pembrolizumab. 98 The results showed that among 15 patients with relapsed or refractory HL, CR was achieved in two patients, and PR was achieved in seven patients. The ORR was 60%. Among 136 patients with other tumors, eight patients achieved PR (including two patients with adrenocortical carcinoma, two with mesothelioma, one with glioma, one with epithelioid sarcoma, one with lymphoepithelial carcinoma, and one with rhabdomyosarcoma), and the ORR was 5.9%. In addition, eight children with skin melanoma had no objective response to pembrolizumab. Grade 3–5 AEs occurred in 69 patients, and the most common AEs were anemia and decreased lymphocyte count. Of these patients, treatment was discontinued in 18 patients due to AEs. The multicenter, phase I/II iMATRIX study enrolled 90 patients with solid tumors (sarcomas, teratomas) and lymphomas (<30 years) receiving atezolizumab with a mean age of 14 years. 99 At 6 months of ICI treatment, PR was achieved in four patients, including two with HL, one with NHL, and one with rhabdoid tumor. The incidence of AEs was similar across different age groups. The most common AEs were fever and fatigue, and the most common grade 3–4 AE was anemia, with no fatal AEs identified. The multicenter phase I/II ADVL1412 study enrolled 85 pediatric patients treated with nivolumab. In total, 63 patients were evaluated for safety, of which five developed dose-limiting toxicity (anemia). Fatigue was reported as nonhematological toxicity. 100 In addition, there were 10 cases of hypothyroidism and 6 cases of hyperthyroidism. Among the 10 HL patients with evaluable efficacy, CR was achieved in one patient, PR was achieved in two patients, five patients had stable disease, and two patients had a mixed response. The best response for the remaining solid tumors was stable disease.
Consensus recommendation 9
Children and adolescents with lymphoma could potentially benefit from ICI treatment, whereas ICI treatment is less effective in patients with solid tumors. The safety profile in children and adolescents is similar to that in adults, but it is necessary to closely follow growth, development, puberty, fertility, and mental health due to problems caused by endocrine toxicity in children.
Vaccinated patients
Vaccines are active immunization preparations generated from pathogenic microorganisms and their metabolites that are artificially attenuated or inactivated or used with genetic technology to prevent infectious diseases. Vaccinations can stimulate the body to develop immune resistance to a certain pathogen, reducing the risk of specific diseases.
Given that viral antigens are more immunogenic than tumor antigens, vaccination can activate the immune system, which may lead to synergistic antitumor effects. Previous studies have suggested that the rotavirus vaccine reversed resistance to ICI treatment, and the combination of these two treatments generated durable tumor-specific immunity. 101 The INVIDIa study showed that the influenza vaccine in combination with ICI treatment improved OS by 10 months, and the 1-year OS rate of NSCLC patients increased by 20%. 102 Regarding safety, the CA184-004 study showed that after influenza vaccination in lung cancer patients, the incidence of irAEs in patients treated with ICIs was increased compared with historical data (⩾grade 1 52.2% versus 25.5%; ⩾grade 3 26.1% versus 9.8%). 103 Another study showed that the influenza vaccine did not increase the incidence of irAEs in patients treated with PD-1 inhibitor monotherapy but significantly increased the incidence of irAEs in those treated with dual ICIs (nivolumab combined with ipilimumab). 104
In contrast, ICI treatment may have an impact on viral protection and the AEs of vaccination. Cancer patients received ICIs and were then inoculated with trivalent inactivated influenza virus vaccine. The seropositivity rate was similar to that of healthy people. In addition, the IgM response was more pronounced, and seroconversion factor expression was higher. 105 On the one hand, the baseline level of immune status in cancer patients was lower, and the immune activation generated by vaccination after ICI treatment was greater. On the other hand, ICI treatment reversed the functional impairment of CD8+ cytotoxic T-lymphocytes induced by viral infection and reduced the viral infection titer, thereby enhancing the viral protective effect of vaccination and minimally affecting vaccine safety. 106
Similar conclusions regarding COVID-19 vaccination have been documented according to recent reports. 107 BNT162b2, an mRNA vaccine, elicited a sustained immune response against COVID-19 during the administration of ICIs. 108 Melanoma patients receiving ICI therapy showed comparable antibody responses after COVID-19 vaccination. 109 Conversely, with mRNA vaccination for COVID-19, patients with lung cancer who received ICI treatment did not have an increased incidence of irAEs.110,111
Consensus recommendation 10
For cancer patients, it is necessary to decide whether vaccination is appropriate based on various parameters, such as disease severity, treatment stage, and treatment methods. In patients with stable disease and good immune status, vaccination may increase the efficacy of ICI treatment, but there is a potential impact on irAEs, especially in patients with combined immunotherapy. In contrast, ICI treatment may enhance the viral protective effect of vaccination with minimal impact on the safety of vaccination.
Conclusions
As the standard treatment for some advanced or metastatic cancer patients, the emergence of ICIs has dramatically changed oncology clinical practice in a meaningful and positive manner. Special patient populations are routinely excluded from clinical trials or carefully selected due to concerns about clinical complications and other potential toxicity risks and not meeting the inclusion criteria for clinical trials. Available data on the efficacy and safety of ICIs for this population are extremely limited. However, special populations are unavoidable in real-world daily clinical practice, and the administration of ICIs to these patients is extremely challenging, representing a hot issue in the field of cancer immunotherapy. These various special patient populations should not easily be excluded from the clinical consideration of ICI usage. Instead, clinicians should carefully weigh the benefits and risks of immunotherapy and make a joint subjective and individual treatment decision based on the current best available evidence. These current expert consensus recommendations are based on the characteristics of special populations in China and previously published data and incorporate an individualized approach aimed at improving goal-concordant care and outcomes. Leading medical oncology and multidisciplinary experts have achieved relatively unanimous opinions, but consensus recommendations are not mandatory due to the lack of sufficient available evidence. Moreover, this consensus does not cover all special challenging patient populations, such as those with epidermal growth-factor receptor-positive NSCLC, those with ICI rechallenge or retreatment, and those with special organ metastases. We hope to provide objective multidisciplinary guidelines on the application of ICIs to each of these special challenging populations and improve oncologists’ ability to recognize and handle irAEs, improve patients’ quality of life, and maximize patient survival and clinical benefit. Considering the limitations of clinical data and evidence for special challenging populations, comprehensive patient evaluation before the application of ICIs, and close clinical monitoring during ICI administration should be considered for these patient populations. We look forward to conducting large-sample, prospective, and carefully designed clinical studies focusing on the investigation of ICI therapies in cancer patients with underlying special issues to obtain more evidence in the future.
Acknowledgments
This article was translated in its entirety by several coauthors, including Jun Wang, MD, PhD (Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital), Bicheng Zhang, MD, PhD (Cancer Center, Renmin Hospital of Wuhan University), and Ling Peng, MD, PhD (Department of Pulmonary and Critical Care Medicine, Zhejiang Provincial People’s Hospital). No company was involved in translation.
Footnotes
ORCID iDs: Jun Wang  https://orcid.org/0000-0003-3941-2507
https://orcid.org/0000-0003-3941-2507
Ling Peng  https://orcid.org/0000-0002-1359-4982
https://orcid.org/0000-0002-1359-4982
Contributor Information
Jun Wang, Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital; Shandong Key Laboratory of Rheumatic Disease and Translational Medicine; Shandong Lung Cancer Institute, Jinan 250014, China.
Bicheng Zhang, Cancer Center, Renmin Hospital of Wuhan University, Wuhan, China.
Ling Peng, Department of Pulmonary and Critical Care Medicine, Zhejiang Provincial People’s Hospital, Hangzhou, China.
Xiufeng Liu, Department of Hepatobiliary Oncology, Qinhuai Medical District, Eastern Theater Command General Hospital, Nanjing, China.
Jianguo Sun, Cancer Institute, Xinqiao Hospital, Army Medical University, Chongqing, China.
Chunxia Su, Department of Oncology, Shanghai Pulmonary Hospital, Thoracic Cancer Institute, School of Medicine, Tongji University, Shanghai, China.
Huijuan Wang, Department of Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China.
Zheng Zhao, Department of Oncology, Shannxi Cancer Hospital, Xi’an, China.
Lu Si, Department of Melanoma, Cancer Hospital and Institute, Peking University, Beijing, China.
Jianchun Duan, Department of Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
Hongmei Zhang, Department of Oncology, Xijing Hospital, Air Force Medical University, Xian, China.
Mengxia Li, Cancer Center, Daping Hospital and Research Institute of Surgery, Army Medical University, Chongqing, China.
Bo Zhu, Cancer Institute, Xinqiao Hospital, Army Medical University, Chongqing, China.
Li Zhang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Cancer Center, Sun Yat-sen University, Guangzhou, China.
Jin Li, Department of Oncology, Shanghai East Hospital, Tongji University, Shanghai, China.
Jun Guo, Department of Melanoma, Cancer Hospital and Institute, Peking University, Beijing, China.
Rongcheng Luo, Cancer Center, Jinshazhou Hospital, Guangzhou University of Chinese Medicine, Guangzhou, China.
Wensheng Qiu, Department of Oncology, The Affiliated Hospital of Qingdao University, Qingdao, China.
Dingwei Ye, Department of Urology, Shanghai Cancer Center, Fudan University, Shanghai, China.
Qian Chu, Department of Oncology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Jiuwei Cui, Department of Oncology, The First Hospital of Jilin University, Changchun, China.
Xiaorong Dong, Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Yun Fan, Department of Thoracic Oncology, Zhejiang Cancer Hospital, Hangzhou, China.
Quanli Gao, Department of Immunology, The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, China.
Ye Guo, Department of Oncology, Shanghai East Hospital, Tongji University, Shanghai, China.
Zhiyong He, Department of Thoracic Oncology, Fujian Cancer Hospital and Fujian Medical University Cancer Hospital, Fuzhou, China.
Wenfeng Li, Department of Oncology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
Gen Lin, Department of Thoracic Oncology, Fujian Cancer Hospital and Fujian Medical University Cancer Hospital, Fuzhou, China.
Lian Liu, Department of Oncology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China.
Yutao Liu, Department of Oncology, National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
Haifeng Qin, Department of Oncology, Chinese People’s Liberation Army General Hospital, Beijing, China.
Shengxiang Ren, Department of Oncology, Shanghai Pulmonary Hospital, Thoracic Cancer Institute, School of Medicine, Tongji University, Shanghai, China.
Xiubao Ren, Department of Immunology and Biotherapy, Cancer Institute and Hospital, Tianjin Medical University, Tianjin, China.
Yongsheng Wang, GCP Center/Institute of Clinical Pharmacology, West China Hospital, Sichuan University, Chengdu, China.
Junli Xue, Department of Oncology, Shanghai East Hospital, Tongji University, Shanghai, China.
Yunpeng Yang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Cancer Center, Sun Yat-sen University, Guangzhou, China.
Zhenzhou Yang, Department of Oncology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
Lu Yue, Department of Oncology, Qingdao Municipal Hospital, Qingdao, China.
Xianbao Zhan, Department of Oncology, Changhai Hospital, Navy Medical University, Shanghai, China.
Junping Zhang, Department of Cancer Biotherapy, Shanxi Bethune Hospital, Taiyuan, China.
Jun Ma, Harbin Institute of Hematology and Oncology, Harbin, China.
Shukui Qin, Department of Hepatobiliary Oncology, Qinhuai Medical District, Eastern Theater Command General Hospital, Nanjing 210008, China.
Baocheng Wang, Department of Oncology, The 960th Hospital, The People’s Liberation Army, Jinan 250031, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Jun Wang: Conceptualization; Formal analysis; Funding acquisition; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Bicheng Zhang: Conceptualization; Formal analysis; Funding acquisition; Project administration; Resources; Supervision; Writing – original draft; Writing – review & editing.
Ling Peng: Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Xiufeng Liu: Formal analysis; Writing – original draft.
Jianguo Sun: Formal analysis; Writing – original draft.
Chunxia Su: Investigation; Writing – original draft.
Huijuan Wang: Investigation; Writing – original draft.
Zheng Zhao: Formal analysis; Writing – original draft.
Lu Si: Investigation; Writing – original draft.
Jianchun Duan: Investigation; Writing – original draft.
Hongmei Zhang: Investigation; Writing – original draft; Writing – review & editing.
Mengxia Li: Investigation; Writing – original draft.
Bo Zhu: Conceptualization; Formal analysis; Supervision; Writing – original draft.
Li Zhang: Conceptualization; Supervision; Writing – review & editing.
Jin Li: Methodology; Writing – review & editing.
Jun Guo: Formal analysis; Writing – review & editing.
Rongcheng Luo: Methodology; Writing – review & editing.
Wensheng Qiu: Methodology; Writing – review & editing.
Dingwei Ye: Methodology; Writing – review & editing.
Qian Chu: Methodology; Writing – review & editing.
Jiuwei Cui: Investigation; Writing – review & editing.
Xiaorong Dong: Investigation; Writing – review & editing.
Yun Fan: Methodology; Writing – review & editing.
Quanli Gao: Investigation; Writing – review & editing.
Ye Guo: Methodology; Writing – review & editing.
Zhiyong He: Investigation; Writing – review & editing.
Wenfeng Li: Methodology; Writing – review & editing.
Gen Lin: Investigation; Writing – review & editing.
Lian Liu: Investigation; Writing – review & editing.
Yutao Liu: Methodology; Writing – review & editing.
Haifeng Qin: Investigation; Writing – review & editing.
Shengxiang Ren: Investigation; Writing – review & editing.
Xiubao Ren: Investigation; Writing – review & editing.
Yongsheng Wang: Investigation; Writing – review & editing.
Junli Xue: Investigation; Writing – review & editing.
Yunpeng Yang: Investigation; Writing – review & editing.
Zhenzhou Yang: Methodology; Writing – review & editing.
Lu Yue: Methodology; Writing – review & editing.
Xianbao Zhan: Methodology; Writing – review & editing.
Junping Zhang: Methodology; Writing – review & editing.
Jun Ma: Methodology; Writing – review & editing.
Shukui Qin: Conceptualization; Formal analysis; Supervision; Writing – review & editing.
Baocheng Wang: Conceptualization; Formal analysis; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Chinese Society of Clinical Oncology (CSCO).
The authors declare that there is no conflict of interest.
Availability of data and materials: All information in this consensus can be found in the references.
References
- 1.The Chinese society of clinical oncology (CSCO) Expert Committee of Immunotherapy. CSCO immune checkpoint inhibitor clinical practice. Beijing, China: People’s Medical Publishing House, 2021. [Google Scholar]
- 2.Zhang L, Wang J, Zhang B, et al. Attitudes and practices of immune checkpoint inhibitors in Chinese patients with cancer: a national cross-sectional survey. Front Pharmacol 2021; 12: 583126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B, Song Y, Fu Y, et al. Current status of the clinical use of PD-1/PD-L1 inhibitors: a questionnaire survey of oncologists in China. BMC Cancer 2020; 20: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Chinese society of clinical oncology (CSCO) Expert Committee of Immunotherapy. CSCO management of immune checkpoint inhibitor-related toxicity. Beijing, China: People’s Medical Publishing House, 2021. [Google Scholar]
- 5.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017; 123: 1904–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rzeniewicz K, Larkin J, Menzies AM, et al. Immunotherapy use outside clinical trial populations: never say never? Ann Oncol 2021; 32: 866–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson JA, Schneider BJ, Brahmer J, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, Version 1.2020. J Natl Compr Canc Netw 2020; 18: 230–241. [DOI] [PubMed] [Google Scholar]
- 8.Wong GL, Wong VW, Hui VW, et al. Hepatitis flare during immunotherapy in patients with current or past Hepatitis B virus infection. Am J Gastroenterol 2021; 116: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 9.De Keukeleire SJ, Vermassen T, Nezhad ZM, et al. Managing viral hepatitis in cancer patients under immune checkpoint inhibitors: should we take the risk? Immunotherapy 2021; 13: 409–418. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Dong Q, Li Q, et al. Dysregulated response of follicular Helper T Cells to Hepatitis B surface antigen promotes HBV persistence in mice and associates with outcomes of patients. Gastroenterology 2018; 154: 2222–2236. [DOI] [PubMed] [Google Scholar]
- 11.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 12.Lu S, Cheng Y, Zhou J, et al. Abstract CT218: flat-dose nivolumab (NIVO) as second-line (2L) treatment (tx) for Asian patients (pts) with advanced non-small cell lung cancer (NSCLC): CheckMate 870. Cancer Res 2020; 80: CT218–CT218. [Google Scholar]
- 13.Pu D, Yin L, Zhou Y, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: a systematic review. Medicine 2020; 99: e19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer 2019; 7: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z, Zhang X, Zhou Y, et al. Hepatotoxicity associated with PD-1 blockade antibodies in cancer patients co-infected with hepatitis B virus. Cancer Immunol Immunother 2022; 71: 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Tian D, Chen Y, et al. Association of hepatitis B virus infection status with outcomes of non-small cell lung cancer patients undergoing anti-PD-1/PD-L1 therapy. Transl Lung Cancer Res 2021; 10: 3191–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burns EA, Muhsen IN, Anand K, et al. Hepatitis B virus reactivation in cancer patients treated with immune checkpoint inhibitors. J Immunother 2021; 44: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravi S, Spencer K, Ruisi M, et al. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J Immunother Cancer 2014; 2: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gane E, Verdon DJ, Brooks AE, et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J Hepatol 2019; 71: 900–907. [DOI] [PubMed] [Google Scholar]
- 20.Lee PC, Chao Y, Chen MH, et al. Risk of HBV reactivation in patients with immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardi A, Mondelli MU. Review article: immune checkpoint inhibitors and the liver, from therapeutic efficacy to side effects. Aliment Pharmacol Ther 2019; 50: 872–884. [DOI] [PubMed] [Google Scholar]
- 23.Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 24.Guaitoli G, Baldessari C, Maur M, et al. Treating cancer with immunotherapy in HIV-positive patients: a challenging reality. Crit Rev Oncol Hematol 2020; 145: 102836. [DOI] [PubMed] [Google Scholar]
- 25.Heppt MV, Schlaak M, Eigentler TK, et al. Checkpoint blockade for metastatic melanoma and Merkel cell carcinoma in HIV-positive patients. Ann Oncol 2017; 28: 3104–3106. [DOI] [PubMed] [Google Scholar]
- 26.McCullar B, Alloway T, Martin M. Durable complete response to nivolumab in a patient with HIV and metastatic non-small cell lung cancer. J Thorac Dis 2017; 9: E540–E542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hentrich M, Schipek-Voigt K, Jager H, et al. Nivolumab in HIV-related non-small-cell lung cancer. Ann Oncol 2017; 28: 2890. [DOI] [PubMed] [Google Scholar]
- 28.Guihot A, Marcelin AG, Massiani MA, et al. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann Oncol 2018; 29: 517–518. [DOI] [PubMed] [Google Scholar]
- 29.Spano JP, Veyri M, Gobert A, et al. Immunotherapy for cancer in people living with HIV: safety with an efficacy signal from the series in real life experience. AIDS 2019; 33: F13–F19. [DOI] [PubMed] [Google Scholar]
- 30.Kim HW, Kim JS, Lee SH. Incidence of tuberculosis in advanced lung cancer patients treated with immune checkpoint inhibitors - a nationwide population-based cohort study. Lung Cancer 2021; 158: 107–114. [DOI] [PubMed] [Google Scholar]
- 31.Bae S, Kim YJ, Kim MJ, et al. Risk of tuberculosis in patients with cancer treated with immune checkpoint inhibitors: a nationwide observational study. J Immunother Cancer 2021; 9: e002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaemes J, Kim C. Immune checkpoint inhibitor use and tuberculosis: a systematic review of the literature. Eur J Cancer 2020; 132: 168–175. [DOI] [PubMed] [Google Scholar]
- 33.Fujita K, Yamamoto Y, Kanai O, et al. Incidence of active tuberculosis in lung cancer patients receiving immune checkpoint inhibitors. Open Forum Infect Dis 2020; 7: ofaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Im Y, Lee J, Kim SJ, et al. Development of tuberculosis in cancer patients receiving immune checkpoint inhibitors. Respir Med 2020; 161: 105853. [DOI] [PubMed] [Google Scholar]
- 35.Beijing Chest Hospital CMUBTaTTRI, Chinese Antituberculosis Association, Editorial Board of Chinese Journal of Antituberculosis. Chinese expert consensus on the all-oral treatment of drug-resistant pulmonary tuberculosis (2021 Edition). Chin J Antituberculosis 2021; 43: 859–866. [Google Scholar]
- 36.Lian JW, Dang LY, Xu JL, et al. The application value of T-SPOT.TB in patients with malignant tumor complicated with tuberculosis. Chin J Antituberculosis 2018; 40: 353. [Google Scholar]
- 37.Erbe R, Wang Z, Wu S, et al. Evaluating the impact of age on immune checkpoint therapy biomarkers. Cell Rep 2021; 37: 110033. [DOI] [PubMed] [Google Scholar]
- 38.Huang XZ, Gao P, Song YX, et al. Efficacy of immune checkpoint inhibitors and age in cancer patients. Immunotherapy 2020; 12: 587–603. [DOI] [PubMed] [Google Scholar]
- 39.Nishijima TF, Muss HB, Shachar SS, et al. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev 2016; 45: 30–37. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Ju Q, Qian B, et al. The effectiveness of PD-1 inhibitors in non-small cell lung cancer (NSCLC) patients of different ages. Oncotarget 2018; 9: 7942–7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akinboro O, Vallejo JJ, Nakajima EC, et al. Outcomes of anti–PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score⩾50%: FDA pooled analysis. J Clin Oncol 2022; 40: 9000–9000. [Google Scholar]
- 42.Akinboro O, Vallejo JJ, Mishra-Kalyani PS, et al. Outcomes of anti-PD-(L1) therapy in combination with chemotherapy versus immunotherapy (IO) alone for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC) with PD-L1 score 1-49%: FDA pooled analysis. J Clin Oncol 2021; 39: 9001–9001. [Google Scholar]
- 43.Chen C, Wu B, Zhang C, et al. Immune-related adverse events associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int Immunopharmacol 2021; 95: 107498. [DOI] [PubMed] [Google Scholar]
- 44.Samani A, Zhang S, Spiers L, et al. Impact of age on the toxicity of immune checkpoint inhibition. J Immunother Cancer 2020; 8: e000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Presley CJ, Gomes F, Burd CE, et al. Immunotherapy in older adults with cancer. J Clin Oncol 2021; 39: 2115–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassady K, Martin PJ, Zeng D. Regulation of GVHD and GVL activity via PD-L1 interaction with PD-1 and CD80. Front Immunol 2018; 9: 3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuchmann M, Meyer RG, Distler E, et al. The programmed death (PD)-1/PD-ligand 1 pathway regulates graft-versus-host-reactive CD8 T cells after liver transplantation. Am J Transplant 2008;8:2434–2444. [DOI] [PubMed] [Google Scholar]
- 49.Abdel-Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer 2019; 7: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen LS, Ortuno S, Lebrun-Vignes B, et al. Transplant rejections associated with immune checkpoint inhibitors: a pharmacovigilance study and systematic literature review. Eur J Cancer 2021; 148: 36–47. [DOI] [PubMed] [Google Scholar]
- 51.Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017; 129: 2471–2478. [DOI] [PubMed] [Google Scholar]
- 52.Holderried TAW, Fraccaroli A, Schumacher M, et al. The role of checkpoint blockade after allogeneic stem cell transplantation in diseases other than Hodgkin’s lymphoma. Bone Marrow Transplant 2019; 54: 1662–1667. [DOI] [PubMed] [Google Scholar]
- 53.Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26: v40–v55. [DOI] [PubMed] [Google Scholar]
- 54.Song JS, Kim D, Kwon JH, et al. Clinicopathologic significance and immunogenomic analysis of programmed death-ligand 1 (PD-L1) and programmed death 1 (PD-1) expression in thymic epithelial tumors. Front Oncol 2019; 9: 1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radovich M, Pickering CR, Felau I, et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell 2018; 33: 244–258 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanderwalde A, Spetzler D, Xiao N, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 2018; 7: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajan A, Heery CR, Thomas A, et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J Immunother Cancer 2019; 7: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018; 19: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol 2019; 37: 2162–2170. [DOI] [PubMed] [Google Scholar]
- 60.Katsuya Y, Horinouchi H, Seto T, et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019; 113: 78–86. [DOI] [PubMed] [Google Scholar]
- 61.Konstantina T, Konstantinos R, Anastasios K, et al. Fatal adverse events in two thymoma patients treated with anti-PD-1 immune check point inhibitor and literature review. Lung Cancer 2019; 135: 29–32. [DOI] [PubMed] [Google Scholar]
- 62.Portoles Hernandez A, Blanco Clemente M, Escribano Garcia D, et al. Checkpoint inhibitor-induced fulminant myocarditis, complete atrioventricular block and myasthenia gravis-a case report. Cardiovasc Diagn Ther 2021; 11: 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyun JW, Kim GS, Kim SH, et al. Fatal simultaneous multi-organ failure following pembrolizumab treatment for refractory thymoma. Clin Lung Cancer 2020; 21: e74–e77. [DOI] [PubMed] [Google Scholar]
- 64.Warner BM, Baer AN, Lipson EJ, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 2019; 24: 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019; 78: 150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Badran YR, Cohen JV, Brastianos PK, et al. Concurrent therapy with immune checkpoint inhibitors and TNFalpha blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 2019; 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossi G, Pezzuto A, Sini C, et al. Concomitant medications during immune checkpoint blockage in cancer patients: novel insights in this emerging clinical scenario. Crit Rev Oncol Hematol 2019; 142: 26–34. [DOI] [PubMed] [Google Scholar]
- 68.Mielgo-Rubio X, Chara L, Sotelo-Lezama M, et al. MA10.01 antibiotic use and PD-1 inhibitors: shorter survival in lung cancer, especially when given intravenously. Type of infection also matters. J Thorac Oncol 2018; 13: S389. [Google Scholar]
- 69.Zhao S, Gao G, Li W, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer 2019; 130: 10–17. [DOI] [PubMed] [Google Scholar]
- 70.Tokunaga A, Sugiyama D, Maeda Y, et al. Selective inhibition of low-affinity memory CD8(+) T cells by corticosteroids. J Exp Med 2019; 216: 2701–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018; 36: 2872–2878. [DOI] [PubMed] [Google Scholar]
- 72.Ricciuti B, Dahlberg SE, Adeni A, et al. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 2019; 37: 1927–1934. [DOI] [PubMed] [Google Scholar]
- 73.Bai X, Hu J, Betof Warner A, et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin Cancer Res 2021; 27: 5993–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maslov DV, Tawagi K, Kc M, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer 2021; 9: e002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers 2020; 12: 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol 2020; 31: 525–531. [DOI] [PubMed] [Google Scholar]
- 77.Soto-Perez-de-Celis E, Li D, Yuan Y, et al. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 2018; 19: e305–e316. [DOI] [PubMed] [Google Scholar]
- 78.Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018; 125: 150–156. [DOI] [PubMed] [Google Scholar]
- 79.Dal’bo N, Patel R, Parikh R, et al. Cardiotoxicity of contemporary anticancer immunotherapy. Curr Treat Options Cardiovasc Med 2020; 22: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanz BA, Pollack MH, Johnpulle R, et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J Immunother Cancer 2016; 4: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson JA, Schneider BJ, Brahmer J, et al. Management of immunotherapy-related toxicities, Version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022; 20: 387–405. [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Zhou H, Zhang Y, et al. Reporting of immune checkpoint inhibitor therapy-associated diabetes, 2015–2019. Diabetes Care 2020; 43: e79–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magis Q, Gaudy-Marqueste C, Basire A, et al. Diabetes and blood glucose disorders under anti-PD1. J Immunother 2018; 41: 232–240. [DOI] [PubMed] [Google Scholar]
- 84.Matsuura N, Koh G, Konishi C, et al. Fulminant onset of insulin-dependent diabetes with positive anti-GAD antibody titers during treatment with nivolumab in a patient with NSCLC. Cancer Immunol Immunother 2018; 67: 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cortellini A, Mallardo D, Cleary S, et al. 966P Diabetes therapy burden as proxy of impairment of immune checkpoint inhibitors efficacy. Ann Oncol 2021; 32: S834. [Google Scholar]
- 86.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153). J Thorac Oncol 2019; 14: 1628–1639. [DOI] [PubMed] [Google Scholar]
- 88.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 89.Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: a phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer 2020; 127: 160–172. [DOI] [PubMed] [Google Scholar]
- 90.Middleton G, Brock K, Savage J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med 2020; 8: 895–904. [DOI] [PubMed] [Google Scholar]
- 91.Tomasik B, Bieńkowski M, Braun M, et al. Effectiveness and safety of immunotherapy in NSCLC patients with ECOG PS score ⩾2 - systematic review and meta-analysis. Lung Cancer 2021; 158: 97–106. [DOI] [PubMed] [Google Scholar]
- 92.Khaki AR, Chennupati S, Fedorenko C, et al. Utilization of systemic therapy in patients with cancer near the end of life in the pre- versus postimmune checkpoint inhibitor eras. JCO Oncol Pract 2021; 17: e1728–e1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khaki AR, Li A, Diamantopoulos LN, et al. Impact of performance status on treatment outcomes: a real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer 2020; 126: 1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mendizabal E, De Leon-Luis J, Gomez-Hidalgo NR, et al. Maternal and perinatal outcomes in pregnancy-associated melanoma. Report of two cases and a systematic literature review. Eur J Obstet Gynecol Reprod Biol 2017; 214: 131–139. [DOI] [PubMed] [Google Scholar]
- 95.Driscoll MS, Grant-Kels JM. Hormones, nevi, and melanoma: an approach to the patient. J Am Acad Dermatol 2007; 57: 919–931; quiz 932–916. [DOI] [PubMed] [Google Scholar]
- 96.Pearson ADJ, Rossig C, Lesa G, et al. ACCELERATE and European medicines agency paediatric strategy forum for medicinal product development of checkpoint inhibitors for use in combination therapy in paediatric patients. Eur J Cancer 2020; 127: 52–66. [DOI] [PubMed] [Google Scholar]
- 97.Zhang YH, Sun HX. Immune checkpoint molecules in pregnancy: focus on regulatory T cells. Eur J Immunol 2020; 50: 160–169. [DOI] [PubMed] [Google Scholar]
- 98.Geoerger B, Kang HJ, Yalon-Oren M, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol 2020; 21: 121–133. [DOI] [PubMed] [Google Scholar]
- 99.Geoerger B, Zwaan CM, Marshall LV, et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study. Lancet Oncol 2020; 21: 134–144. [DOI] [PubMed] [Google Scholar]
- 100.Davis KL, Fox E, Merchant MS, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 2020; 21: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shekarian T, Sivado E, Jallas AC, et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci Transl Med 2019; 11: eaat5025. [DOI] [PubMed] [Google Scholar]
- 102.Bersanelli M, Giannarelli D, Castrignano P, et al. INfluenza vaccine indication during therapy with Immune checkpoint inhibitors: a transversal challenge. The INVIDIa study. Immunotherapy 2018; 10: 1229–1239. [DOI] [PubMed] [Google Scholar]
- 103.Weber JS, Hamid O, Chasalow SD, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 2012; 35: 89–97. [DOI] [PubMed] [Google Scholar]
- 104.Chong CR, Park VJ, Cohen B, et al. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis 2020; 70: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laubli H, Balmelli C, Kaufmann L, et al. Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J Immunother Cancer 2018; 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Erickson JJ, Gilchuk P, Hastings AK, et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest 2012; 122: 2967–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malek AE, Cornejo PP, Daoud N, et al. The mRNA COVID-19 vaccine in patients with cancer receiving checkpoint inhibitor therapy: what we know and what we don’t. Immunotherapy 2022; 14: 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lasagna A, Agustoni F, Percivalle E, et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open 2021; 6: 100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niewolik J, Mikuteit M, Cossmann A, et al. Immunogenicity of COVID-19 vaccination in melanoma patients under immune checkpoint blockade. Oncology 2022; 100: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hibino M, Uryu K, Takeda T, et al. Safety and immunogenicity of mRNA vaccines against severe acute respiratory syndrome coronavirus 2 in patients with lung cancer receiving immune checkpoint inhibitors: a multicenter observational study in Japan. J Thorac Oncol 2022; 17: 1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Widman AJ, Cohen B, Park V, et al. Immune-related adverse events among COVID-19-vaccinated patients with cancer receiving immune checkpoint blockade. J Natl Compr Canc Netw 2022; 20: 1134–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]