Abstract
Background:
In patients with interstitial lung disease (ILD), decreased oxygen saturation (SpO2) reduces physical performance and causes exertional dyspnea. Portable oxygen concentrator (POC) and pursed-lip breathing (PLB) have the potential to improve these parameters in ILD patients.
Objective:
To evaluate the effects of PLB while using a POC during walking in ILD patients
Design:
Prospective, randomized crossover trial.
Methods:
We compared two breathing techniques. Participants not trained in PLB received a familiarization session before the first 6-min walking test (6MWT). During the first visit, patients performed the 6MWT under natural breathing (NB1) without oxygen (O2); during the second visit, they performed the 6MWT twice, once each with PLB (PLB1) and natural breathing (NB2) under O2 supplementation, to compare the effectiveness of NB and PLB.
Results:
Twenty participants were recruited; half had exercise-induced desaturation (EID) and half normal SpO2. In the normoxemia group (NG), the difference in the 6-min walking distance (6MWD) between NB1 and PLB1 was 28.8 ± 24.0 m, indicating reduced exercise capacity in PLB1. There were no significant differences in the quadriceps tissue saturation index (TSI), SpO2, and 6MWD between the PLB1 and NB2 in any patient or subgroup. All participants showed a significant increase in the SpO2 at rest, nadir SpO2, and mean SpO2 during the 6MWT with PLB and NB2 using a POC than with NB1. TSI showed a significant improvement at the beginning of 6MWT in ILD patients with EID in the PLB and NB2 condition.
Conclusion:
Acute exposure to PLB did not improve symptoms, muscle oxygenation, or SpO2; however, it decreased the walking distance in the normoxemia group. POC improved leg muscle oxygenation in ILD patients with EID. The use of PLB and POC should be prescribed according to disease characteristics and severity.
Keywords: 6-min walking test, breathing exercises, hypoxemia, interstitial lung diseases, oxygen saturation
Introduction
Interstitial lung disease (ILD) is associated with various clinical prognoses, and in some severe cases, lung transplantation is the only treatment option. 1 In ILD, lung ventilation/perfusion mismatch leads to a decrease in oxygen saturation (SpO2), restriction of oxygen (O2) diffusion, and muscle dysfunction, resulting in limited physical performance and exertional dyspnea. 2 Exercise-induced desaturation (EID), which frequently occurs in patients with ILD, has been reported to predict high mortality in idiopathic interstitial pneumonia. 3 However, SpO2 does not provide localized oxygenation information for muscles during exercises. Near-infrared spectroscopy (NIRS) is used to noninvasively measure leg muscle oxygenation and local blood flow of muscle tissues using wavelength-selective absorption patterns, which depend on whether hemoglobin and myoglobin are oxidized. 4
Pursed-lip breathing (PLB) stabilizes breathing patterns to reduce respiratory rates and dynamic hyperinflation. This strategy improves tidal volume and exercise capacity in patients with chronic obstructive pulmonary disease (COPD). 5 However, PLB can sometimes negatively affect exercise capacity or dyspnea, owing to increased metabolic demand in patients with ILD. 6
Long-term O2 therapy is commonly prescribed as it improves dyspnea, exercise capacity, and survival rates in patients with chronic hypoxemia. 7 Ambulatory O2 therapy is also commonly prescribed in patients with active COPD or ILD, but the evidence for its effectiveness in patients with ILD is still insufficient. 8 Compressed liquid O2 cylinders were mainly used in the past; however, various portable O2 concentrators (POCs) have now replaced them, and comparable effects have been reported. 9 The low adherence to ambulatory O2 therapy is expected to gradually improve because of the advantages of lightweight POCs and no requirement for O2 refills. POCs detect an inspiration trigger through a nasal cannula and supply O2 using a pulse delivery method. In one study, nasal breathing decreased during exercise to a greater extent in patients with chronic lung diseases than in healthy subjects. 10 Therefore, PLB that always induces inspiration through the nose would be able to increase oxygen delivery with a POC.
To date, no results have been reported on the use of POCs and PLB during exercise in patients with ILD. However, considering the mechanism of pulse delivery oxygenation via a nasal cannula, PLB and a POC applied simultaneously during walking can be expected to have a synergistic effect. Recently, a correlation between lower limb muscle function and functional capacity in patients with ILD has been reported.11,12 Muscle deoxygenation is associated with peripheral muscle dysfunction. 13 Peripheral muscle dysfunction is an essential contributor to reduced exercise capacity in ILD patients, and the resulting reduction in activity leads to a vicious cycle that exacerbates exercise capacity and dyspnea.14–16 In addition, it has been reported that oxygen supplementation in IPF patients improves cerebral and muscle oxygenation and alleviates dyspnea. 17 From this perspective, we considered oxygenation of the quadriceps, which is the most important muscle for walking, as the primary outcome. We aimed to evaluate the effect of PLB and a POC on the quadriceps tissue saturation index (TSI), walking distance, and SpO2 in patients with ILD.
Methods
Study design and participants
The diagnosis of ILD was based on clinical symptoms and signs, high-resolution computed tomography, pulmonary function tests, and a multidisciplinary team approach. Patients diagnosed with ILDs with a forced vital capacity (FVC) <80% of the predicted value or diffusing capacity of the lung for carbon monoxide (DLCO) <60% of the predicted value, based on pulmonary function tests conducted at the Pusan National University Hospital from November 2020 to December 2021, were recruited in this prospective, randomized crossover trial. Among them, ambulatory adults aged >19 years were defined as eligible. The exclusion criteria were severe resting hypoxemia (SpO2 <88%), obesity (thickness of the quadriceps adipose tissue >1 cm), and inability to perform 6-min walking test (6MWT) because of musculoskeletal, cognitive, and/or cardiovascular problems. Participants had never experienced 6MWT and PLB before recruitment. Data on participants’ age, sex, smoking history, type of ILDs, comorbidities, complete blood count, and body mass index were obtained from their medical records.
Protocol
Eligible participants underwent four 6MWTs at 1-week intervals in a 30-m straight corridor with distance marked according to standardized guidelines. 18 On the first day of the visit, an experienced respiratory rehabilitation therapist explained the PLB technique and 6MWT to the participants during the familiarization process. The familiarization process included the principles, clinical effects, and methods of PLB and was performed until the patient fully understood the technique. After a 30-min break, the participants performed the first 6MWT while wearing a POC without O2 supplementation. After the first 6MWT, 1:1 block randomization was performed with a block size of 4 using computer-generated random allocation on Excel 2016 (Microsoft Corp., Redmond, WA, USA). The random allocation was performed by a physician who did not participate in the study.
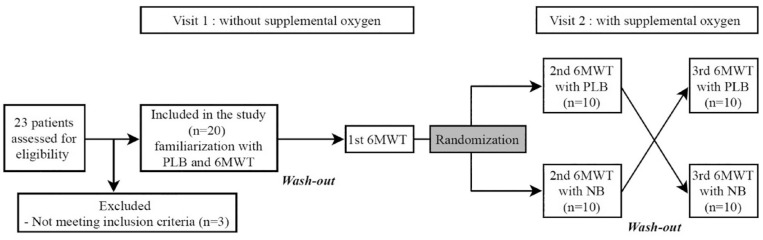
After 1 week, the participants were retrained on PLB at the second visit, and they performed the 6MWT twice with a 30-min wash-out time. All tests at the second visit were performed while participants received the maximal O2 flow (setting of 5) from the One G3 pulse-flow POC (Inogen, Goleta, CA, USA) with a nasal cannula. This setting could be converted to a maximum O2 supply of 1050 mL/min. According to the randomization order, one group performed PLB in the first test and natural breathing (NB) in the second test. During 6MWT with PLB, the evaluator supervised the continuation of PLB. Tests were conducted in the opposite way in the second group to prevent bias from learning effects. Figure 1 presents the Consolidated Standards for Reporting of Trials flowchart for this study.
Figure 1.
Schematic flowchart of the study.
6MWT, 6-min walking test; PLB, pursed-lip breathing.
Measurements and outcomes
In all 6MWTs, SpO2 and heart rate (HR) changes were measured in real time during 2 min of the resting period, walking, and 2 min of the recovery period using a wireless wrist oximeter (Nonin 3150, Nonin Medical, Plymouth, MN, USA). In addition, a portable NIRS device (PortaMon, Artinis Medical Systems, Gelderland, Netherlands) was attached with an adhesive tape to the participants’ left vastus lateralis muscle between the lateral epicondyle and greater trochanter of the femur to evaluate local muscle oxygenation and local blood flow. The wavelengths of the portable NIRS device were approximately 760 and 850 nm. Before attachment, the thickness of the thigh fatty tissue was measured with a skinfold caliber. The portable NIRS device was wrapped with a black elastic band to block the device from indoor light. The data collected from the two devices were transmitted at 10 Hz to a laptop via Bluetooth. Changes in the TSI, deoxyhemoglobin (HHb) level, and total hemoglobin (HHb + oxyhemoglobin [O2Hb], tHb) level were measured during the same period using NIRS. The following equation was used for measuring the TSI: 19
TSI(%) = oxyhemoglobin / (oxyhemoglobin + deoxyhemoglobin) × 100%
Dyspnea and fatigue were assessed before, during, and after 6MWT on the Borg CR-10 scale for measuring dyspnea and leg fatigue. 20
The primary outcome was the mean TSI during 6MWT for each test. Secondary outcomes included mean HHb, tHb, SpO2 during 6MWT, 6-min walking distance (6MWD), and Borg CR-10 dyspnea/fatigue scale scores.
Statistical analysis
An a priori sample size of 20 participants was calculated with α-value of 0.05 and a power of 0.8, based on data from a previous study. 9 All continuous data were reported as mean ± standard deviation, unless specified otherwise. Categorical data were presented as percentage and count. Normality was tested using the Shapiro–Wilk test. Baseline characteristics between subgroups were compared using the independent t-test or Mann–Whitney U test. Friedman and Wilcoxon signed-rank tests were used to compare the 6MWT results, including cardiorespiratory responses (HR and SpO2) and NIRS-derived variables (HHb, O2Hb, and TSI), between the groups throughout 6MWT and every 1 min of recovery time. Pearson correlation analysis was performed to evaluate the relationship between NIRS-derived variables and clinical variables, including 6MWD, SpO2 at rest and nadir, FVC, and DLCO.
A p-value < 0.05 indicated statistical significance. All statistical analyses were performed using SPSS Statistics for Windows (version 27, IBM Corp., Chicago, IL, USA).
Results
Participants
Twenty-three patients diagnosed with ILD were recruited, but 20 eligible participants were included in this study. The participants completed all assessments without dropping out. The demographic and clinical characteristics of the participants are presented in Table 1. The average patient age was 65 years, and the mean FVC was moderately reduced (67.9 ± 14.3% predicted). Most patients (85%) were diagnosed with idiopathic pulmonary fibrosis. No adverse events related to this evaluation were observed. EID was defined when the SpO2 decreased by >4% or to <90% during exercise. 21 In this study, EID was confirmed in 10 of 20 patients, and a subgroup analysis was conducted according to the presence of EID. Significant differences were found in FVC%, forced expiratory volume in 1 s (FEV1%), DLCO%, and nadir SpO2 during the 6MWT between the subgroups (see Table 1).
Table 1.
Participant characteristics.
| ILD with normoxemia | ILD with EID | All participants | |
|---|---|---|---|
| Participants (n) | 10 | 10 | 20 |
| Age (years) | 65.0 ± 5.5 | 66.4 ± 4.9 | 65.3 ± 5.6 |
| Males/females | 10/0 | 8/2 | 18/2 |
| BMI (kg/m2) | 24.4 ± 2.3 | 25.9 ± 1.2 | 25.4 ± 2.0 |
| Smoking history (pack/year) | 31.1 ± 11.9 | 35.7 ± 20.7 | 38.6 ± 20.2 |
| Hb (g/dL) | 14.5 ± 1.6 | 14.4 ± 1.8 | 14.2 ± 1.7 |
| Hct (%) | 43.6 ± 4.0 | 43.0 ± 4.9 | 42.9 ± 4.8 |
| FVC (% predicted) | 74.8 ± 15.5 | 59.5 ± 12.0* | 67.9 ± 14.3 |
| FEV1 (% predicted) | 80.0 ± 5.2 | 69.2 ± 16.6* | 74.7 ± 12.8 |
| DLCO (% predicted) | 79.4 ± 10.9 | 58.4 ± 17.9* | 66.6 ± 17.3 |
| 6MWD on room air (meter) | 543.0 ± 68.1 | 471.4 ± 101.7 | 508.5 ± 84.5 |
| 6MWD on room air (% predicted) | 85.5 ± 8.8 | 74.6 ± 15.7 | 78.5 ± 11.6 |
| Oxygen saturation at rest (%) | 94.8 ± 0.8 | 93.7 ± 1.9 | 94.6 ± 1.7 |
| Nadir oxygen saturation during 6MWT (%) | 93.5 ± 1.8 | 86.7 ± 3.2* | 90.1 ± 4.3 |
| SMI (kg/m2) | 8.6 ± 1.0 | 8.3 ± 1.3 | 8.5 ± 1.0 |
| Bioimpedance-derived 50 kHz whole body phase angle (°) | 6.3 ± 0.4 | 5.7 ± 1.0 | 6.1 ± 0.8 |
| ILD diagnosis | |||
| IPF | 9 | 8 | 17 |
| CTD-ILD | 2 | 2 | |
| NSIP | 1 | 1 | |
| Other |
6MWD, 6-min walking distance; 6MWT, 6-min walking test; BMI, body mass index; CTD, connective tissue disease; DLCO, diffusing capacity of the lung for carbon monoxide; EID, exercise-induced desaturation; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; Hb, hemoglobin; Hct, hematocrit; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; NSIP, nonspecific interstitial pneumonia; SMI, skeletal muscle mass index.
Data are presented as mean ± standard deviation unless otherwise noted.
p < 0.05 for differences between subgroups using an independent t-test or Mann–Whitney U test, depending on normality.
Effect of PLB on muscle oxygenation and SpO2 during 6MWT
During the second visit, differences in oxygenation in the left vastus lateralis muscle in PLB (PLB1) and NB (NB2) were compared under the same O2 supply conditions using a POC. There were no significant differences in the TSI, O2Hb level, or HHb level between the PLB1 and NB2 conditions in any of the patients and subgroups (mean TSI with PLB1: 62.1 ± 10.0 and mean TSI with NB2: 62.9 ± 8.3, p = 0.39; Table 2). There were also no significant differences in the mean SpO2 per min during 6MWT between the PLB1 and NB2 conditions (see Table 2, Figures 2(d) and 3(d)). In the normoxemia group, the difference in the 6MWD values was 28.8 ± 24.0 m [95% confidence interval (CI) = 11.6–46.0, p = 0.004, effect size = 1.2] and the 6MWD% was 4.5 ± 3.7% (95% CI = 1.8–7.1, p = 0.004, effect size = 1.2), which was significantly lower in the PLB1 than in the NB1 condition (see Table 3).
Table 2.
6MWT results of all participants (n = 20).
| NB with no POC (NB1) | PLB with POC (PLB1) | NB with POC (NB2) | |
|---|---|---|---|
| SpO2 (%) | |||
| At rest | 93.9 ± 2.4 | 96.3 ± 1.9* | 96.4 ± 1.4* |
| Nadir during 6MWT | 87.9 ± 5.1 | 91.5 ± 4.6* | 91.2 ± 5.1* |
| Mean during 6MWT | 90.0 ± 3.9 | 93.5 ± 3.4* | 93.3 ± 3.5* |
| Heart rate (beats/min) | |||
| At rest | 88.5 ± 18.1 | 83.8 ± 18.8 | 84.0 ± 18.8 |
| Maximum | 112.4 ± 16.8 | 109.6 ± 17.8 | 112.3 ± 18.4 |
| Mean during 6MWT | 103.6 ± 15.6 | 100.9 ± 17.1 | 102.1 ± 16.6 |
| TSI (%) | |||
| At rest | 60.8 ± 9.0 | 60.6 ± 5.4 | 61.3 ± 6.1 |
| Nadir during 6MWT | 58.8 ± 11.6 | 60.7 ± 10.4 | 61.1 ± 8.5 |
| Mean during 6MWT | 60.3 ± 11.2 | 62.1 ± 10.0 | 62.9 ± 8.3 |
| tHb (arbitrary units) | |||
| At rest | 4.2 ± 7.7 | 3.7 ± 5.1 | 4.7 ± 6.3 |
| Nadir during 6MWT | −6.2 ± 9.5 | −7.9 ± 6.2 | −5.8 ± 8.6 |
| Mean during 6MWT | −2.3 ± 9.3 | −5.0 ± 5.6 | −2.3 ± 9.4 |
| HHb (arbitrary units) | |||
| At rest | 1.1 ± 4.6 | 1.8 ± 3.2 | 1.8 ± 3.4 |
| Nadir during 6MWT | −2.8 ± 4.8 | −3.4 ± 2.8 | −2.8 ± 3.8 |
| Mean during 6MWT | −0.7 ± 4.9 | −2.0 ± 3.0 | −1.2 ± 4.2 |
| O2Hb (arbitrary units) | |||
| At rest | 3.0 ± 4.7 | 1.8 ± 2.8 | 2.9 ± 3.7 |
| Nadir during 6MWT | −3.4 ± 6.2 | −4.5 ± 4.2 | −3.0 ± 5.8 |
| Mean during 6MWT | −1.6 ± 6.2 | −3.0 ± 3.8 | −1.2 ± 6.2 |
| Post-test dyspnea (Borg scale) |
1.4 ± 1.6 | 1.0 ± 1.3 | 1.3 ± 1.5 |
| Post-test leg fatigue (Borg scale) |
0.4 ± 1.3 | 0.1 ± 0.5 | 0.5 ± 1.3 |
| 6MWD (m) | 497.1 ± 99.2 | 486.7 ± 86.5 | 495.1 ± 93.0 |
| 6MWD (% predicted) | 77.0 ± 12.2 | 75.5 ± 9.9 | 76.8 ± 10.8 |
| Patient preference (n) | – | 4 | 16 |
6MWD, 6-min walking distance; 6MWT, 6-min walking test; HHb, deoxyhemoglobin; NB, natural breathing; O2Hb, oxyhemoglobin; PLB, pursed-lip breathing; POC, portable oxygen concentrator; tHb, total hemoglobin; TSI, tissue saturation index.
Data are reported as mean ± standard deviation or numbers (%).
p < 0.05 for differences compared with the NB1 group using Freidman test and Wilcoxon signed-rank test.
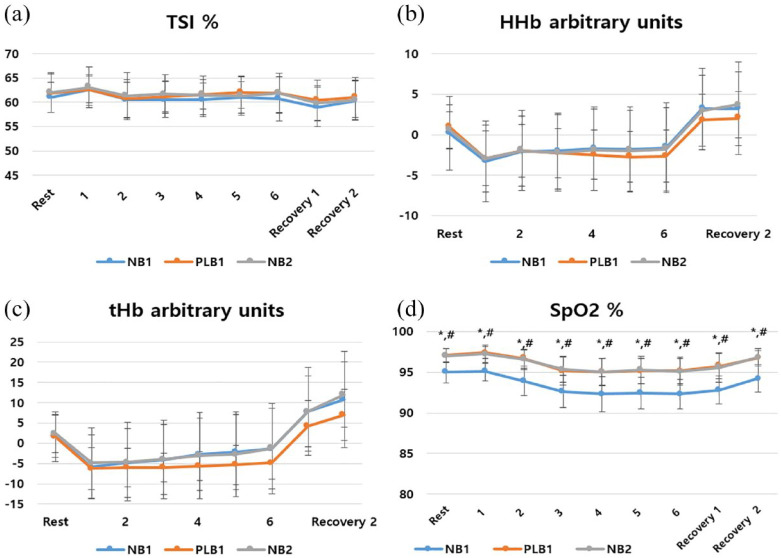
Figure 2.
Changes in the (a) TSI, (b) HHb level, (c) tHb level, and (d) SpO2 in the EID group.
Data points are mean values and error bars represent standard deviations.
EID, exercise-induced desaturation; HHb, deoxyhemoglobin; NB, natural breathing; PLB, pursed-lip breathing; POC, portable oxygen concentrator; SpO2, oxygen saturation; tHb, total hemoglobin; TSI, quadriceps tissue saturation index.
*p < 0.05 for differences compared with NB1 with PLB1.
#p < 0.05 for differences compared with NB1 with NB2.
Figure 3.
Changes in the (a) TSI, (b) HHb level, (c) tHb level, and (d) SpO2 in the normoxemia group.
Data points are mean values and error bars represent standard deviations.
HHb, deoxyhemoglobin; NB, natural breathing; PLB, pursed-lip breathing; POC, portable oxygen concentrator; SpO2,xygen saturation; tHb, total hemoglobin; TSI, quadriceps tissue saturation index.
*p < 0.05 for differences compared with NB1 with PLB1.
#p < 0.05 for differences compared with NB1 with NB2.
Table 3.
6MWT results in patients with ILD and normoxemia (n = 10).
| NB with no POC (NB1) | PLB with POC (PLB1) | NB with POC (NB2) | |
|---|---|---|---|
| SpO2 (%) | |||
| At rest | 95.0 ± 1.4 | 97.1 ± 0.9* | 97.0 ± 0.9* |
| Nadir during 6MWT | 91.9 ± 1.9 | 94.8 ± 1.8* | 94.8 ± 1.7* |
| Mean during 6MWT | 93.1 ± 1.8 | 95.8 ± 1.4* | 95.7 ± 1.5* |
| Heart rate (beats/min) | |||
| At rest | 87.9 ± 16.1 | 84.7 ± 16.9 | 83.6 ± 17.0 |
| Maximum | 115.6 ± 15.0 | 107.7 ± 18.2 | 109.9 ± 19.8 |
| Mean during 6MWT | 105.1 ± 15.6 | 98.6 ± 18.3 | 101.2 ± 18.3 |
| TSI (%) | |||
| At rest | 61.0 ± 3.3 | 61.8 ± 4.2 | 62.0 ± 4.3 |
| Nadir during 6MWT | 59.5 ± 4.5 | 60.4 ± 4.1 | 60.4 ± 4.9 |
| Mean during 6MWT | 61.0 ± 3.6 | 61.7 ± 3.4 | 61.8 ± 4.3 |
| tHb (arbitrary units) | |||
| At rest | 1.7 ± 5.5 | 1.6 ± 3.1 | 2.4 ± 4.9 |
| Nadir during 6MWT | −7.3 ± 9.4 | −7.7 ± 6.9 | −3.7 ± 10.5 |
| Mean during 6MWT | −3.5 ± 9.2 | −5.7 ± 6.9 | −3.4 ± 10.5 |
| HHb (arbitrary units) | |||
| At rest | 0.2 ± 4.8 | 1.0 ± 2.9 | 0.6 ± 2.4 |
| Nadir during 6MWT | −3.7 ± 5.5 | −3.4 ± 3.1 | −3.3 ± 4.7 |
| Mean during 6MWT | −2.1 ± 5.2 | −2.5 ± 3.3 | −2.1 ± 5.0 |
| O2Hb (arbitrary units) | |||
| At rest | 1.6 ± 3.4 | 0.6 ± 2.0 | 1.8 ± 2.9 |
| Nadir during 6MWT | −3.5 ± 5.5 | −4.3 ± 4.9 | −3.3 ± 6.3 |
| Mean during 6MWT | −1.4 ± 5.5 | −3.2 ± 4.7 | −1.3 ± 6.5 |
| Post-test dyspnea (Borg scale) |
1.5 ± 1.6 | 0.9 ± 0.9 | 1.1 ± 1.5 |
| Post-test leg fatigue (Borg scale) |
0.9 ± 1.7 | 0.2 ± 0.6 | 0.6 ± 1.6 |
| 6MWD (m) | 529.8 ± 71.9 | 501.0 ± 74.7* | 516.0 ± 79.1 |
| 6MWD (% predicted) | 80.6 ± 8.1 | 76.2 ± 8.2* | 78.4 ± 9.1 |
| Patient preference (n) | – | 1 | 9 |
6MWD, 6-min walking distance; 6MWT, 6-min walking test; HHb, deoxyhemoglobin; ILD, interstitial lung disease; NB, natural breathing; O2Hb, oxyhemoglobin; PLB, pursed-lip breathing; POC, portable oxygen concentrator; tHb, total hemoglobin; TSI, tissue saturation index.
Data are reported as mean ± standard deviation or numbers (%).
p < 0.05 for differences compared with the NB group using Freidman test and Wilcoxon signed-rank test.
Effect of a POC on muscle oxygenation and SpO2 during 6MWT
The effect of O2 supply using a POC on muscle oxygenation and SpO2 during walking was compared using the 6MWT under the same conditions on visits 1 and 2. When comparing NB1 and PLB1 in all patients, significant differences were confirmed in the SpO2 at rest (mean difference = 2.3 ± 1.6%, 95% CI = 1.6–3.1, p = 0.00, effect size = 1.4), nadir SpO2 (mean difference = 3.5 ± 2.6%, 95% CI = 2.6–4.7, p = 0.00, effect size = 1.3), and mean SpO2 (mean difference = 3.4 ± 2.3%, 95% CI = 2.3–4.5, p = 0.00, effect size = 1.5). When comparing NB1 and NB2 in all patients, significant differences in the SpO2 at rest (mean difference = 2.5 ± 1.6%, 95% CI = 1.7–3.2, p = 0.00, effect size = 1.5), nadir SpO2 (mean difference = 3.1 ± 2.0%, 95% CI = 2.2–4.0, p = 0.00, effect size = 1.6), and mean SpO2 (mean difference = 3.3 ± 2.0%, 95% CI = 2.3–4.2, p = 0.00, effect size = 1.6) were also confirmed (see Table 2). Subgroup analysis confirmed a significant increase in the SpO2 in all groups under the O2 supply condition (see Tables 3 and 4; Figures 2 and 3). There was no significant difference in the average TSI during the 6MWT according to the conditions. However, when analyzed in detail every minute, a significant increase was confirmed by O2 supply in the EID group at the beginning of 6MWT using the Freidman test and Wilcoxon signed-rank test (see Figure 2). In the other conditions, there was no significant difference in the NIRS-derived variables (see Figures 2 and 3).
Table 4.
6MWT results in patients with ILD and EID (n = 10).
| NB with no POC (NB1) | PLB with POC (PLB1) | NB with POC (NB2) | |
|---|---|---|---|
| SpO2 (%) | |||
| At rest | 92.9 ± 2.8 | 95.5 ± 2.3* | 95.8 ± 1.5* |
| Nadir during 6MWT | 83.7 ± 3.9 | 88.2 ± 4.3* | 87.5 ± 5.1* |
| Mean during 6MWT | 87.0 ± 3.0 | 91.2 ± 3.3* | 90.9 ± 3.2* |
| Heart rate (beats/min) | |||
| At rest | 88.9 ± 20.7 | 82.9 ± 21.4 | 84.3 ± 21.4 |
| Maximum | 109.0 ± 18.5 | 111.7 ± 17.8 | 114.7 ± 17.5 |
| Mean during 6MWT | 102.0 ± 16.2 | 103.2 ± 16.4 | 103.0 ± 15.8 |
| TSI (%) | |||
| At rest | 60.7 ± 12.7 | 59.4 ± 6.2 | 60.7 ± 7.7 |
| Nadir during 6MWT | 58.1 ± 16.2 | 61.0 ± 14.5 | 61.8 ± 11.2 |
| Mean during 6MWT | 59.7 ± 15.8 | 62.5 ± 14.1 | 64.1 ± 11.2 |
| tHb (arbitrary units) | |||
| At rest | 6.6 ± 9.4 | 5.7 ± 6.1 | 7.0 ± 7.1 |
| Nadir during 6MWT | −5.1 ± 10.1 | −8.0 ± 5.7 | −1.2 ± 8.6 |
| Mean during 6MWT | −1.1 ± 9.7 | −4.4 ± 4.1 | −1.3 ± 8.6 |
| HHb (arbitrary units) | |||
| At rest | 2.1 ± 4.8 | 2.7 ± 3.6 | 3.0 ± 4.1 |
| Nadir during 6MWT | −1.8 ± 4.3 | −3.4 ± 2.9 | −2.3 ± 3.1 |
| Mean during 6MWT | 0.6 ± 4.4 | −1.5 ± 2.8 | −0.2 ± 3.3 |
| O2Hb (arbitrary units) | |||
| At rest | 4.5 ± 5.5 | 3.0 ± 3.1 | 4.0 ± 4.3 |
| Nadir during 6MWT | −3.4 ± 7.2 | −4.6 ± 3.5 | −2.6 ± 5.5 |
| Mean during 6MWT | −1.7 ± 7.1 | −2.9 ± 2.8 | −1.0 ± 6.2 |
| Post-test dyspnea (Borg scale) |
1.3 ± 1.7 | 1.2 ± 1.6 | 1.4 ± 1.6 |
| Post-test leg fatigue (Borg scale) |
0 | 0.1 ± 0.2 | 0.3 ± 0.9 |
| 6MWD (m) | 464.4 ± 114.9 | 472.3 ± 98.9 | 474.1 ± 105.1 |
| 6MWD (% predicted) | 73.4 ± 14.8 | 74.8 ± 11.7 | 75.1 ± 12.6 |
| Patient preference (n) | – | 3 | 7 |
6MWD, 6-min walking distance; 6MWT, 6-min walking test; EID, exercise-induced desaturation; HHb, deoxyhemoglobin; ILD, interstitial lung disease; NB, natural breathing; O2Hb, oxyhemoglobin; PLB, pursed-lip breathing; POC, portable oxygen concentrator; tHb: total hemoglobin; TSI, tissue saturation index.
Data reported as mean ± standard deviation or numbers (%).
p < 0.05 for differences compared with the NB1 group using Freidman test and Wilcoxon signed-rank test.
Relationship between clinical variables and NIRS-derived variables
The FVC was positively correlated with the DLCO (r = 0.570, p = 0.014), 6MWD (r = 0.573, p = 0.008), and nadir SpO2 during 6MWT (r = 0.602, p = 0.005). The DLCO was positively correlated with nadir SpO2 during 6MWT (r = 0.530, p = 0.024). The 6MWD was negatively correlated with the O2Hb (r = –0.603, p = 0.005) and tHb (r = –0.486, p = 0.030). No significant correlation existed between other NIRS-derived variables (HHb level, TSI) and the SpO2, 6MWD, FVC, and DLCO (see Figure 4).
Figure 4.
Correlation between clinical variables and NIRS-derived variables. (a) FVC and 6MWD, (b) FVC and Nadir SpO2, (c) DLCO and nadir SpO2, and (d) 6MWD and O2Hb.
6MWD, 6-min walking distance; DLCO, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; O2Hb, oxyhemoglobin.
Borg CR-10 scale and participant preference
For each condition, leg muscle fatigue and dyspnea were investigated using the Borg CR-10 scale. Significant differences in the Borg leg fatigue and dyspnea scale scores immediately after exercise were not confirmed. On comparing the preference for NB and PLB breathing techniques, we found that 80% (n = 16) of the patients preferred NB. NB was selected by 70% of patients in the EID group and 90% in the normoxemia group.
Discussion
To the best of our knowledge, this prospective crossover trial is the first to investigate the effect of PLB on muscle oxygenation during walking in patients with ILDs. In addition, the effect of using a pulse-flow POC during walking on muscle oxygenation and SpO2 were confirmed. Our results can be summarized as follows: (1) Walking with PLB under the same conditions did not significantly improve leg muscle oxygenation or SpO2 in patients with ILD. Instead, a reduced 6MWD value was identified in the normoxemia group. (2) The use of a POC during walking significantly increased the SpO2 under both breathing conditions. Improvements in muscle oxygenation were only confirmed in the EID group under the NB condition. Overall, the PLB had no clinical effect during walking in patients with ILD and may have had a negative outcome (decreased walking distance) in patients with mild symptoms. Our results provide clinical evidence for the use of a POC in patients with severe ILD.
PLB is a breathing method in which the respiratory accessory muscles, such as the neck and shoulder muscles, are kept relaxed, air is inhaled slowly and deeply through the nose, and then air is slowly exhaled by rounding the lips. PLB transfers the positive pressure generated in the upper airway into the lower airway to prevent bronchial obstruction and accumulation of secretions. In addition, it is possible to stabilize breathing patterns to reduce respiratory rates, increase inspiratory volume during rest, and improve exercise capacity in patients with COPD.22,23 Based on these clinical effects in patients with COPD, PLB has been introduced to pulmonary rehabilitation education programs for patients, including those with ILD and rapid and shallow breathing, despite insufficient evidence. 24 In a recent study, the application of PLB during the 6MWT in 35 patients with ILDs showed high metabolic demands and worsened dyspnea and decreased 6MWD. 6 Herein, in the normoxemia group with good lung function, the 6MWD in the PLB1 condition was confirmed to be shorter than that in the NB1 condition. Moreover, 80% of the patients preferred NB to PLB during the 6MWT, and this ratio was higher in patients with mild symptoms. These results suggest that controlled breathing patterns impose excessive physiological stress that can decrease exercise capacity and offset the positive effect of an increased tidal volume caused by the PLB. In the EID group, there were no significant differences in the 6MWD and Borg leg fatigue and dyspnea scales. As the study population included patients with ILD who could walk independently as community ambulators, it cannot be concluded that PLB has no positive effect on all patients with ILD. Furthermore, it should be considered that one familiarization session alone is not enough to confirm that patients are sufficiently trained. However, our results suggest that further research is required to apply PLB during walking in patients with ILD, and decisions should be based according to disease severity.
Pulmonary function tests are widely used for the diagnosis and evaluation of the severity, prognosis, and treatment responses in patients with ILDs. Decreased SpO2 is common during maximal and submaximal exercises in patients with ILD. EID is caused by restriction of gas exchange due to the destruction of the pulmonary capillary bed. 18 EID is one of the significant factors limiting exercise capacity in patients with ILD. 19 However, pulmonary function and arterial blood tests conducted during the resting state have limitations in that the results of these tests cannot be correlated with the symptoms that occur during physical activity. Therefore, examining changes in the SpO2, HR, and muscle oxygenation during walking at the daily activity level is expected to provide more symptom-related physiological information. In addition, the reduced exercise capacity of patients with ILD cannot be explained only by reduced lung function, and changes in other organs, such as skeletal muscles used for walking, should be monitored. Peripheral muscle dysfunction is an essential contributor to reduced exercise capacity in patients with ILD. 20 In one study, the strength of the quadriceps muscle decreased by an average of 65% in patients with idiopathic pulmonary fibrosis. This decrease in muscle strength was represented as a predictor of peak O2 uptake at the end of the exercise. 25 As in other chronic lung diseases, dyspnea due to decreased activity results in a vicious cycle that causes worsening exercise capacity and loss of skeletal muscles. 18 From this viewpoint, we aimed to observe physiological changes in the quadriceps femoris muscle, which is one of the most essential skeletal muscles used during walking. There are various methods for monitoring physiological changes in skeletal muscles, but NIRS is a representative, noninvasive, and wireless method. 26
NIRS has been used to monitor changes in local blood flow, hemodynamic changes, tissue oxygenation, and consumption. 27 Regional blood flow is positively correlated with the tHb level measured using NIRS. 28 Muscle oxygenation (TSI), measured by NIRS, can decrease due to an increase in muscle oxygen extraction caused by increased workloads during exercise and reduction in oxygen supply or delivery. In other words, an increase in the HHb level during exercise means increased muscle O2 extraction. 12 Several studies have shown muscle oxygenation in patients with ILD.13,29 However, these studies revealed the physiological changes in the muscles during maximum load training or high-intensity exercise.
In this study, physiological changes in the vastus lateralis muscle during the 6MWT and submaximal test were analyzed using NIRS. In brief, differences in NIRS-derived variables in the two breathing techniques, PLB and NB, were not confirmed. However, a significant increase in TSI was observed in patients with ILD and EID under the condition of O2 supply with a POC (see Figure 2). This means that the decrease in muscle oxygenation due to reduced pulmonary O2 uptake in patients with ILD can be compensated by a POC. In addition, the negative correlation between 6MWD and O2Hb can be interpreted as exercise capacity being positively correlated with local oxygen consumption of the vastus lateralis. We expected controlled nasal inspiration through PLB to increase oxygen delivery efficiency of the POC under the assumption that the nasal breathing rate during ambulation would be decreased in ILD patients, according to a previous study. 10 However, the results of this study suggest that NB is a more effective breathing technique for leg muscle oxygenation than PLB. A systematic review indicated that O2 supply during exercising in patients with ILD improved exercise capacity but did not affect dyspnea. 30 Inconsistent results have been reported for dyspnea owing to differences in O2 supply, protocols, and patient severity. A recent study showed that O2 supply improved leg muscle oxygenation and fatigue in 15 patients with ILD and EID when a constant-load cycle was performed while supplying high-flow O2. 31 In this study, it was suggested that low-flow oxygen supply through POC did not significantly affect the improvement of exercise capacity and subjective dyspnea symptoms during walking. In summary, although there was no difference in the 6MWD, fatigue, or dyspnea in patients with ILD and EID, the use of POC with NB had a significant effect on the oxygenation of the vastus lateralis muscle. However, PLB did not significantly affect muscle oxygenation.
Because of the limitation of the study period, it was impossible to include only the EID group that needed O2 supply. Given this limitation, a crossover trial was designed, and additional analyses were conducted in the EID subgroup. Depending on the disease severity in the recruited patients, the effects of POC and PLB may differ. Future studies including only patients with severe EID are needed to determine whether PLB has only negative effects and whether POC use improves walking distance, dyspnea, and fatigue in patients with ILD. Moreover, the respiratory rate during walking may have affected the oxygen supply. However, it was difficult to analyze the respiratory rate during walking owing to the limitations of the research equipment. In this regard, the need for wireless respiratory rate monitor is recommended in future studies.
In conclusion, acute exposure to PLB during 6MWT did not show significant improvement in symptoms, walking distance, muscle oxygenation, and SpO2 compared with NB in patients with ILD; however, it reduced the walking distance in the normoxemia group. Moreover, it was confirmed that a POC improved SpO2 during walking in all patients with ILD and leg muscle oxygenation during walking in patients with ILD and EID. From a clinical standpoint, our findings serve as evidence of the negative effects of acute exposure to PLB in patients with mild ILD and the positive effect of a POC on leg muscle oxygenation during walking in patients with ILD and EID. Therefore, the prescription of PLB and a POC in pulmonary rehabilitation should only be considered in individualized treatment approaches, which should be designed according to disease character and severity.
Acknowledgments
The authors thank all the patients who participated in this study.
Footnotes
ORCID iDs: Sang Hun Kim  https://orcid.org/0000-0003-4849-5228
https://orcid.org/0000-0003-4849-5228
Yong Beom Shin  https://orcid.org/0000-0001-5026-1696
https://orcid.org/0000-0001-5026-1696
Myung-Jun Shin  https://orcid.org/0000-0003-4010-0383
https://orcid.org/0000-0003-4010-0383
Cho Hui Hong  https://orcid.org/0009-0008-1981-860X
https://orcid.org/0009-0008-1981-860X
Wanho Yoo  https://orcid.org/0000-0002-2344-0231
https://orcid.org/0000-0002-2344-0231
Kwangha Lee  https://orcid.org/0000-0001-9878-201X
https://orcid.org/0000-0001-9878-201X
Contributor Information
Sang Hun Kim, Department of Rehabilitation Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Republic of Korea.
Yong Beom Shin, Department of Rehabilitation Medicine and Biomedical Research Institute, Pusan National University Hospital and Pusan National University School of Medicine, Busan, Republic of Korea.
Myung-Jun Shin, Department of Rehabilitation Medicine and Biomedical Research Institute, Pusan National University Hospital and Pusan National University School of Medicine, Busan, Republic of Korea.
Cho Hui Hong, Biomedical Research Institute, Pusan National University Hospital, Busan, Republic of Korea; Department of Physical Therapy, Graduate School, Kyungsung University, Busan, Republic of Korea.
Sungchul Huh, Department of Rehabilitation Medicine and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea.
Wanho Yoo, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Pusan National University Hospital, Busan, Republic of Korea.
Kwangha Lee, Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Pusan National University Hospital, 179, Gudeok-ro, Seo-gu, Busan 49241, Republic of Korea. Department of Internal Medicine, Pusan National University School of Medicine, Busan, Republic of Korea.
Declarations
Ethics approval and consent to participate: This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2008-018-093) and registered in the Clinical Research Information Service (No. KCT0005440). Written informed consent was obtained from all the participants.
Consent for publication: Not applicable.
Author contributions: Sang Hun Kim: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Writing – original draft.
Yong Beom Shin: Conceptualization; Methodology; Supervision.
Myung-Jun Shin: Resources; Software; Supervision.
Cho Hui Hong: Data curation; Investigation.
Sungchul Huh: Formal analysis; Investigation; Visualization.
Wanho Yoo: Conceptualization; Supervision.
Kwangha Lee: Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Biomedical Research Institute Grant (grant no. 202002120001), Pusan National University Hospital.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data sets used and analyzed during this study are available from the corresponding author on reasonable request.
References
- 1.Kapnadak SG, Raghu G.Lung transplantation for interstitial lung disease. Eur Respir Rev 2021; 30: 210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakazawa A, Cox NS, Holland AE.Current best practice in rehabilitation in interstitial lung disease. Ther Adv Respir Dis 2017; 11: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 2003; 168: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari M, Muthalib M, Quaresima V.The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phy Eng Sci 2011; 369: 4577–4590. [DOI] [PubMed] [Google Scholar]
- 5.Cabral LF, D’Elia Tda C, Marins D S, et al. Pursed lip breathing improves exercise tolerance in COPD: a randomized crossover study. Eur J Phys Rehabil Med 2015; 51: 79–88. [PubMed] [Google Scholar]
- 6.Parisien-La Salle S, Abel Rivest E, Boucher VG, et al. Effects of pursed lip breathing on exercise capacity and dyspnea in patients with interstitial lung disease: a randomized, crossover study. J Cardiopulm Rehabil Prev 2019; 39: 112–117. [DOI] [PubMed] [Google Scholar]
- 7.Calverley PM.Supplementary oxygen therapy in COPD: is it really useful? Thorax 2000; 55: 537–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank RC, Hicks S, Duck AM, et al. Ambulatory oxygen in idiopathic pulmonary fibrosis: of what benefit. Eur Respir J 2012; 40: 269–270. [DOI] [PubMed] [Google Scholar]
- 9.Khor YH, McDonald CF, Hazard A, et al. Portable oxygen concentrators versus oxygen cylinder during walking in interstitial lung disease: a randomized crossover trial. Respirology 2017; 22: 1598–1603. [DOI] [PubMed] [Google Scholar]
- 10.Leiberman A, Ohki M, Cole P.The open mouth and oronasal breathing. Am J Rhinol 1991; 5: 1–5. [Google Scholar]
- 11.Mendes P, Wickerson L, Helm D, et al. Skeletal muscle atrophy in advanced interstitial lung disease. Respirology 2015; 20: 953–959. [DOI] [PubMed] [Google Scholar]
- 12.Panagiotou M, Polychronopoulos V, Strange C.Respiratory and lower limb muscle function in interstitial lung disease. Chron Respir Dis 2016; 13: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wickerson L, Mathur S, Brooks D, et al. Skeletal muscle oxygenation and regional blood volume during incremental limb loading in interstitial lung disease. ERJ Open Res 2020; 6: 00083–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonini M, Fiorenzano G.Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev 2017; 26: 160099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Someya F, Mugii N, Hasegawa M, et al. Predictors of exercise-induced oxygen desaturation in systemic sclerosis patients with interstitial lung disease. Respir Care 2014; 59: 75–80. [DOI] [PubMed] [Google Scholar]
- 16.Holland AE.Exercise limitation in interstitial lung disease-mechanisms, significance and therapeutic options. Chron Respir Dis 2010; 7: 101–111. [DOI] [PubMed] [Google Scholar]
- 17.Dipla K, Boutou AK, Markopoulou A, et al. Exertional desaturation in idiopathic pulmonary fibrosis: the role of oxygen supplementation in modifying cerebral-skeletal muscle oxygenation and systemic hemodynamics. Respiration 2021; 100: 463–475. [DOI] [PubMed] [Google Scholar]
- 18.Brown AW, Nathan SD.The value and application of the 6-minute-walk test in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2018; 15: 3–10. [DOI] [PubMed] [Google Scholar]
- 19.Niemeijer VM, Spee RF, Schoots T, et al. Limitations of skeletal muscle oxygen delivery and utilization during moderate-intensity exercise in moderately impaired patients with chronic heart failure. Am J Physiol Heart Circ Physiol 2016; 311: H1530–H1539. [DOI] [PubMed] [Google Scholar]
- 20.Williams N.The Borg Rating of Perceived Exertion (RPE) scale. Occup Med 2017; 67: 404–405. [Google Scholar]
- 21.van Gestel AJ, Clarenbach CF, Stöwhas AC, et al. Prevalence and prediction of exercise-induced oxygen desaturation in patients with chronic obstructive pulmonary disease. Respiration 2012; 84: 353–359. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi R, Gigliotti F, Romagnoli I, et al. Chest wall kinematics and breathlessness during pursed-lip breathing in patients with COPD. Chest 2004; 125: 459–465. [DOI] [PubMed] [Google Scholar]
- 23.Casciari RJ, Fairshter RD, Harrison A, et al. Effects of breathing retraining in patients with chronic obstructive pulmonary disease. Chest 1981; 79: 393–398. [DOI] [PubMed] [Google Scholar]
- 24.Makhdami N, Farooqi M, Thom-Fernandes C, et al. Pulmonary rehabilitation in interstitial lung diseases. Curr Opin Pulm Med 2020; 26: 470–476. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyama O, Taniguchi H, Kondoh Y, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest 2005; 127: 2028–2033. [DOI] [PubMed] [Google Scholar]
- 26.Jones S, Chiesa ST, Chaturvedi N, et al. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res 2016; 16: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macnab A, Shadgan B.Biomedical applications of wireless continuous wave near infrared spectroscopy. Biomed Spectrosc Imaging 2012; 1: 205–222. [Google Scholar]
- 28.Boushel R, Langberg H, Olesen J, et al. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 2001; 11: 213–222. [DOI] [PubMed] [Google Scholar]
- 29.McNarry MA, Harrison NK, Withers T, et al. Pulmonary oxygen uptake and muscle deoxygenation kinetics during heavy intensity cycling exercise in patients with emphysema and idiopathic pulmonary fibrosis. BMC Pulm Med 2017; 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell EC, Cox NS, Goh N, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev 2017; 26: 160080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marillier M, Bernard AC, Verges S, et al. Oxygen supplementation during exercise improves leg muscle fatigue in chronic fibrotic interstitial lung disease. Thorax 2021; 76: 672–680. [DOI] [PubMed] [Google Scholar]