Abstract
Background
Patients with gastric cancer often encounter impaired quality of life and reduced tolerability to adjuvant treatments after surgery. Weight preservation is crucial for the overall prognosis of these patients, and exercise and supplemental nutrition play the main role. This study is the first randomized clinical trial to apply personalized, treatment stage-adjusted digital intervention with wearable devices in gastric cancer rehabilitation intervention for 12 months, commencing immediately after surgery.
Methods
This is a prospective, multicenter, two-armed, randomized controlled trial and aims to recruit 324 patients from two hospitals. Patients will be randomly allocated to two groups for 1 year of rehabilitation, starting immediately after the operation: a personalized digital therapeutic (intervention) group and a conventional education-based rehabilitation (control) group. The primary objective is to clarify the effect of mobile applications and wearable smart bands in reducing weight loss in patients with gastric cancer. The secondary outcomes are quality of life measured by the EORTC-QLQ-C30 and STO22; nutritional status by mini nutrition assessment; physical fitness level measured by grip strength test, 30-s chair stand test and 2-min walk test; physical activity measured by IPAQ-SF; pain intensity; skeletal muscle mass; and fat mass. These measurements will be performed on enrollment and at 1, 3, 6, and 12 months thereafter.
Conclusions
Digital therapeutic programs include exercise and nutritional interventions modified by age, body mass index, surgery type and postoperative days. Thus, expert intervention is pivotal for precise and safe calibration of this program.
Trial registration
Clinicaltrials.gov identifier: NCT04907591 (registration date: June 11, 2020; https://clinicaltrials.gov/ct2/show/NCT04907591).
Keywords: mHealth, digital therapeutics, randomized controlled trial, rehabilitation, gastric cancer, digital health, eHealth
Introduction
Patients with gastric cancer often become malnourished or sarcopenic during the cancer care continuum after surgery. Most patients have deteriorated quality of life and prognosis, including long-term oncological outcomes and reduced tolerability of adjuvant treatment.1,2 Preserving weight is known to be one of the most fundamental factors preventing postoperative complications and improving quality of life and cancer-specific survival. 3
Previous studies have shown that exercise and nutritional interventions are essential for maintaining weight and reducing postoperative impairments in patients with gastric cancer undergoing elective surgery. Sarcopenia is diagnosed in ∼21.2% of patients over 65 years of age with gastric cancer who undergo gastrectomy. 4 Previous studies have shown that exercise and nutritional support programs have the potential to reduce sarcopenia and improve postoperative outcomes in elderly patients with gastric cancer. 5 In contrast, two randomized controlled studies showed that elemental diet intervention significantly suppressed body weight loss, especially in patients with gastric cancer who underwent total gastrectomy, 6 and a positive effect was observed not only at 6–8 weeks postoperatively but also at 1 year postoperatively. 7 Thus, a comprehensive approach, including exercise and dietary intervention, is crucial for patients with gastric cancer who undergo gastrectomy.
Cancer survivors rarely use tools to report postoperative issues, such as pain, fatigue, and distress. 8 Many patients do not have adequate access routes to receive sufficient and precise information applicable to their exercise and dietary plan. 9 Hence, a comprehensive, easy-to-access platform for managing functional status and nutrition has been highlighted recently, as their alterations may influence the prognosis of the disease. 10
Digital therapeutics with wearable devices have emerged as a useful tool for managing the clinical course of cancer and improving the quality of life and physical performance of cancer survivors. However, most previous studies on mobile health applications focused on limited interventions, such as health programs only applied during chemotherapy or dealing mainly with only psychological or nutrition-related issues.11,12 To the best of our knowledge, no randomized clinical trial has applied personalized, treatment stage-adjusted digital health program to many patients with gastric cancer, especially starting immediately after the operation for a long period of time. This will be the first randomized clinical trial to perform personalized rehabilitation and dietary intervention with a mobile health program, flexibly modified according to the treatment phase and patient's condition, in a large number of patients with gastric cancer, starting within 1 week postoperatively. Digital therapeutic programs include exercise and nutritional support adjusted for age, body mass index (BMI), surgery type and postoperative days. Since aerobic and muscle strengthening exercises are adjusted by patient status as well as the onset of the operation, expert administration is pivotal for a fine-tuned personalized rehabilitation program. The primary objective of this protocol is to clarify the effect of mobile apps and wearable smart bands in preventing weight loss in patients with gastric cancer. The secondary outcomes were improvement in quality of life, enhanced physical fitness level, increased physical activity, and reduced pain intensity, muscle mass, and fat mass.
Methods and design
Study design
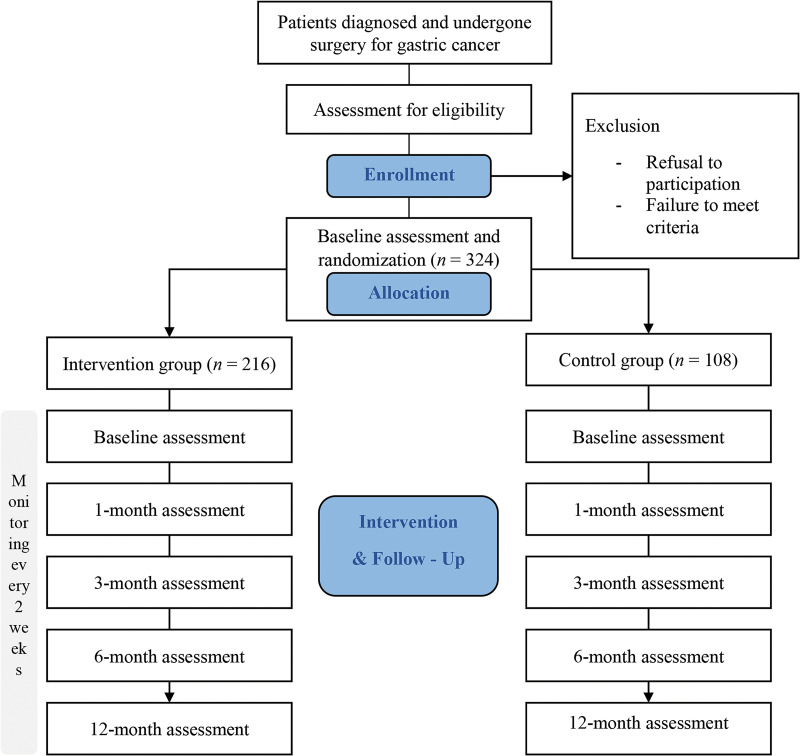
This is a prospective, multicenter, open, two-armed, randomized controlled trial. The trial protocol followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement. Supplemental Appendix 1 illustrates the checklist. Participants will be recruited from two university hospitals in South Korea (Samsung Medical Center and Korea University Anam Hospital). The enrolled participants will be randomly allocated to either the digital therapeutic or control group. Both groups will receive the standard care and conventional education on diet management, as well as training on preventing dumping syndrome while emphasizing the importance of muscle mass and weight maintenance. A flowchart of the study protocol is shown in Figure 1. The findings of this study are presented in concordance with the Consolidated Standards of Reporting Trials (CONSORT) checklist.
Figure 1.
Flowchart of the study protocol.
Participants
In this study, we will enroll 324 participants diagnosed with gastric cancer who underwent surgery. The inclusion criteria will be as follows: (a) aged 19–75 years, (b) underwent curative resection for gastric cancer as American Joint Committee on Cancer (AJCC) gastric cancer staging I–III, (c) use of an Android- or iOS-based smartphone, (d) able to use a mobile application and have regular follow-up assessment as outpatients, and (e) voluntary participation. The exclusion criteria will be as follows: (a) inability to perform exercise and diet management because of a severe underlying disease, neuromusculoskeletal disease, or cognitive or visual impairment, and (b) communication difficulties.
After consultation with the Department of Surgery and the Department of Rehabilitation Medicine to invite them to participate in the study, all eligible patients will be informed about the study procedures following surgery, and written informed consent will be obtained from all participants if they agree to participate voluntarily in the study.
Randomization, allocation, and blinding
Enrolled participants will be randomly assigned to either the digital therapeutic or control group in a 2:1 ratio using blocked randomization with randomly selected block sizes of three and six. There will be no blinding in the open study because the participant and evaluator will know which type of intervention has been applied to the participant. After randomly allocating patients to one of the two groups, a baseline assessment will be performed.
Interventions
Personalized digital therapeutic (intervention) group
For the personalized digital therapeutic group, gastric cancer by a second doctor program (Medi Plus Solution, Seoul, Korea) for health management after gastric cancer surgery and DoFit as a smart band on the wrist (NF-B20, Medi Plus Solution, Seoul, South Korea) will be offered. The expert team developed the app used on both Android and iOS platforms and fundamental content based on the evidence of app developers, designers, service programmers, cancer rehabilitation specialists, and researchers involved. The smart band measures the physical activity (step counts and energy expenditure) and heart rate through a built-in six-axis accelerometer, gyroscope, and photoplethysmography sensor, and via Bluetooth communication, collected data is transferred to the app. Additionally, diverse smart devices, such as blood pressure gauges, glucose monitors, and scales, can be connected to the app. In the trial, the participants in the intervention group will be encouraged to use smart bands as much as possible. Other smart devices can be used at any time depending on the participants’ needs and preferences.
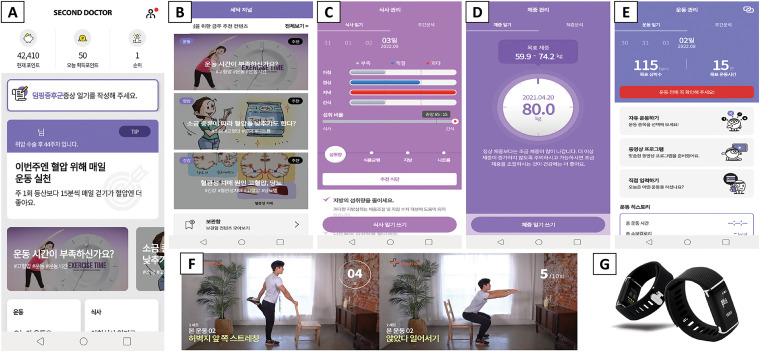
The main app functions are listed in Table 1, and screenshots of the representative functions are shown in Figure 2. User information for the algorithms of personalized content includes surgery type (total/subtotal gastrectomy), treatment type (chemotherapy), initial weight, age, perceived rating of exertion and comorbid diseases (hypertension, diabetes mellitus and hyperlipidemia). For a more personalized approach, the contents and goals of exercise management will be provided at each time point after surgery and modified by the type of treatment (chemotherapy). For example, if a patient starts chemotherapy, an exercise program is provided to manage the physical condition and maintain more than minimal physical activity. The target of performance time and heart rate in aerobic exercise and the intensity of muscle strengthening exercise program are lower for patients on chemotherapy than for those who did not receive chemotherapy. When patients are on chemotherapy, the prescribed exercises will be of a low-level intensity. In patients with completed chemotherapy or without chemotherapy, targets will be adjusted based on comorbidity (hypertension) and the user's subjective rating of perceived exertion. Starting from a low level, the intensity of exercise will gradually progress to moderate and high levels. The target exercise duration and heart rate for aerobic exercises will be gradually increased.
Table 1.
Functions of the mobile app and key characteristics.
| Functions | Key characteristics |
|---|---|
| Expert consultation |
|
| Second doctor journal |
|
| Exercise management |
|
| Diet management |
|
| Self-symptom tracking |
|
| Physical activity management |
|
| Comorbidity and weight management |
|
Figure 2.
Screenshots of the representative function and smart band: (A) home (today's to-do list and self-symptom tracking), (B) second doctor journal, (C) diet management, (D) weight management, (E) exercise management, (F) exercise video, and (G) DoFit.
In dietary management, initially recommended calories will be calculated by the standard weight, and then will undergo personalized modification according to the surgery type, BMI change, age, and comorbidities. After the primary analysis using this user information, an initial tailored guide will be provided, and the subsequent guide will be updated by analyzing the lifelog data collected from the individual. The individual usage and all lifelog data of participants can be monitored by clinical research coordinators, including nurses, physiotherapists, and occupational therapists, using a web-based open architecture management program. To manage adherence to the tailored digital therapeutic intervention, researchers will monitor the app usage rate every 2 weeks for 12 months and provide feedback to the participants during the initial 3 months. This monitoring period will focuse on postoperative recovery and the initial period of treatment. Based on previous research experience, adherence to self-management using a mobile application is expected to decrease after the start of adjuvant treatment in patients with postoperative breast cancer, 13 considering the limited resources of human support.
Education (usual care)
This group will receive regular diet management and dumping syndrome prevention training from the hospital.
Outcomes
Outcome measures will be evaluated by a clinical research coordinator face to face when patients visit the hospital, and a summary of baseline screening, assessment and follow-up during study visits is indicated in Table 2.
Table 2.
Summary of baseline screening, assessment and follow-up during study visits.
| Study Period | ||||||
|---|---|---|---|---|---|---|
| Post-allocation | ||||||
| Timepoint | Post op (+1 w) |
1 month (±2 w) |
3 months (±1 m) |
6 months (±2 m) |
12 months (±2 m) |
|
| Enrollment: | ||||||
| 1 | Eligibility screen | X | ||||
| 2 | Informed consent | X | ||||
| 3 | Allocation | X | ||||
| 4 | Demographic characteristics | X | ||||
| 5 | Medical history | X | ||||
| 6 | Health lifestyle | X | ||||
| 7 | eHealth Literacy Scale a | X | ||||
| Interventions: | ||||||
| Personalized digital therapeutic (intervention) group |

|
|||||
| Control group |

|
|||||
| Assessments: | ||||||
| 8 | Complication | X | X | X | X | |
| 9 | Hb, albumin | X b | X | X | ||
| 10 | Height and weight | X | X | X | X | X |
| 11 | Body composition | X | X | X | X | X |
| 12 | Mini Nutritional Assessment | X | X | X | X | X |
| 13 | IPAQ-SF | X | X | X | X | X |
| 14 | EORTC-QLQ-C30 | X | X | X | X | X |
| 15 | EORTC-QLQ-STO22 | X | X | X | X | |
| 16 | Numeric rating scale | X | X | X | X | X |
| 17 | Grip strength | X | X | X | X | X |
| 18 | 30-s chair stand test | X | X | X | X | |
| 19 | 2-min walk test | X | X | X | X | |
| 20 | Satisfaction questionnaire a | X c | ||||
IPAQ-SF: International Physical Activity Questionnaire-Short Form; EORTC-QLQ: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Personalized digital therapeutic (intervention) group only.
Tests analyzed within 6 months from the baseline can be collected.
Used it more than 3 times a week at least once.
Primary outcomes
The primary objective is to investigate the effect of personalized digital therapy in the management of factors affecting the prognosis of patients with gastric cancer. Therefore, the primary outcome is weight change in the intervention group compared with the control group at 12 months from baseline, as measured by the bioimpedance analysis machine.
Secondary outcomes
The secondary outcomes of the study protocol are quality of life, cancer-specific symptoms and function, physical activity, nutritional status, physical fitness, and pain intensity over time. Health-related quality of life will be assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30 and STO22). The EORTC-QLQ-C30 consists of 30 items, including functional, symptomatic and global quality of life domains. A high EORTC-QLQ-C30 score indicates good quality of life. The EORTC-QLQ-STO22 is a gastric cancer-specific questionnaire. It consists of 22 items and symptom domains related to gastric cancer.14,15 The nutritional status will be measured using the long form of the Korean Mini Nutritional Assessment (MNA), which is internationally used among patients with cancer 16 and found as a potential prognostic factor for not only treatment and health-related quality of life but also mortality, survival, and cancer progression. 17 A total score of <17 points indicates malnutrition, 17–23.5 points indicates a risk of malnutrition, and 24–30 points is within the normal range. 18 The International Physical Activity Questionnaire-Short Form (IPAQ-SF) will be used to evaluate self-reported physical activity in the last 7 days. 19 Pain intensity will be evaluated using an 11-point numeric rating scale for the past week, with 0 “no pain” and 10 “the worst possible pain.” Participants are asked about their average and worst perceived pain intensity. 20 The grip strength test, 21 30-s chair stand test (30CST)22,23 and 2-min walk test (2MWT) 24 will be used to measure strength generated by the forearm muscle, strength and endurance of the lower extremity and cardiorespiratory endurance, respectively. Grip strength of the dominant hand is assessed using a handheld dynamometer (microFET® digital HandGRIP dynamometer, Hoggan Scientific LLC, USA). All assessments of physical fitness will be performed according to standardized measurement methods.
The eHealth literacy of the intervention group will only be measured at baseline using the eHealth Literacy Scale (eHEALS), which consists of 10 items, and eight questions assess eHealth literacy on a 5-point Likert scale, with a higher score indicating higher literacy. 25 Additionally, the intervention group will complete a self-developed questionnaire on satisfaction with the mobile app and smart band after 6 months.
Sample size calculation
Using G-Power 3.1.9.2., the sample size was estimated at 195 for intervention group and 97 for control group with effect size of 0.35 (moderate level), an alpha level of 5%, and 80% of power based on a previous study. 26 The attrition rate of mobile-based intervention varied with the study design. With an expected dropout rate of 10%, there were a total of 324 participants (216 in the intervention group and 108 in the control group). (Note: approximately 6.39% of the participants dropped out for reasons other than changes in physical condition from our 12-week validation study involving 203 patients with advanced gastrointestinal cancer.) 27 Competitive participant enrollment will be conducted to achieve the target sample size.
Data management
Electronic data capture by Medicallogic Company (www.medicallogic.com) will be utilized for systematic and safe data management, and only permitted researchers can access the web-based platform. All data for individual participants will be completed in an electronic case report form (eCRF) and will be uploaded from the source documents into an eCRF by the involved researchers.
Statistical analysis
All statistical analyses will be conducted using IBM SPSS Statistics version 28.0.1 (Armonk, New York, USA) and a P-value <0.05, with a 95% confidence interval. The Kolmogorov–Smirnov test will be performed to assess the normality of the data. Student's t-test and chi-square test will be performed to examine the homogeneity of the baselines between the two groups. If randomization is not balanced, the baseline difference will be corrected using a multivariate logistic regression. To compare the mean difference, the primary outcome (weight) data in both groups will be analyzed using the independent t-test or Mann−Whitney test, depending on the normality of the data. To investigate the effects of the interventions and differences between groups on secondary outcomes, a mixed effects model or generalized estimating equations will be conducted, with one between-subject factor (group) and one within-subject factor (time). Missing data will be handled depending on the distribution of data after the study is completed.
Participant safety and withdrawal
The potential risk level that could be caused by this study was determined as the minimal level, based on a review of the institutional review board and principal investigator. During the study, all participants could call researchers and ask for help with study-related problems at any time. If they have unbearable pain or injury, they can be examined by the principal investigator. Depending on the cases, such as significant disease or not following the instruction of the doctor in charge, participants can be withdrawn and they can discontinue the study voluntarily.
Ethics and dissemination
All study procedures were approved by the institutional review boards of the two hospitals (approval numbers: SMC-2021-01-090 and 2021AN0104). The trial was registered at clinical trials.gov (approval ID: NCT04907591). The study protocol was reviewed by the institutional review board of Samsung Medical Center on 1 February 2021 and was approved on 23 February 2021, as the original protocol. If major protocol modifications are required, the principal investigator will deliver them to the coordinating investigators and study participants and report them to the institutional review board. The collected data will be stored for 3 years after the end of the study and then destroyed.
Discussion
Home-based exercises are expected to play a critical role in cancer rehabilitation. The program should have clear goals and include monitoring, follow-up visits, calls from health professionals, or self-monitoring diaries. 28 Digital rehabilitation can increase exercise adherence for musculoskeletal rehabilitation compared to non-digital rehabilitation. 29 Therefore, the role of digital therapeutics has become more fundamental in overcoming the limitations of traditional outpatient rehabilitation and non-guided home-based exercise. However, in most previous studies, digital health interventions were only applied during adjuvant therapy or focused on psychological or nutrition-related issues.11,12 Offering one-of-a-kind content to patients is inefficient because patient status varies according to diverse factors such as age, BMI, operation method, onset from the operation, and adjuvant therapeutic phases. Moreover, most of the previous studies enrolled a small number of patients and were not sufficient to apply to the general population.5,7,30 To the best of our knowledge, no study has applied a personalized exercise program according to the treatment stage or different dietary interventions according to the type of gastrectomy, especially starting immediately after the operation for 12 months.
To date, there has been no custom-made home-based exercise program for the participation of patients with gastric cancer after surgery or during adjuvant chemotherapy using a mobile app. Thus, the development of comprehensive personalized digital therapeutics with easy-to-access mobile apps that manage hardships in every postoperative phase, triggering dietary intake with enhanced physical activity starting immediately after the operation, is in demand.
This proposed study will have several strengths. This is a randomized controlled study with many patients with gastric cancer intervening for 12 months immediately after the operation. Furthermore, primary tailored digital health intervention will be modified according to the treatment phase and patient's medical information. In particular, exercise and nutritional support will be readjusted according to the patient's health status (comorbidity), lifelog data, and adjuvant therapy. Moreover, the target dietary intake would differ according to the type of surgery, considering that subtotal gastrectomy has an advantage over completion gastrectomy in terms of postoperative nutritional status. 31 For these reasons, expert intervention is fundamental to our tailored rehabilitation program. Finally, similar to other mobile health programs, reduction in financial and time burdens to patients and clinicians may cautiously be anticipated considering the conditions in Korea. 32
However, this study may have several limitations. First, this digital therapy may be daunting for patients who are unfamiliar with digital devices. Nevertheless, as the mobile industry expands and more user-friendly technologies are developed, this will gradually improve. Second, participants will be recruited from a single monoracial country. Therefore, global generalization may not be accurate. In future research, we hope to recruit samples that can incorporate the characteristics of each race. Third, both the intervention and control groups will have knowledge of which group they have been assigned to, potentially leading to bias. Despite these limitations, this study has potential to provide compelling evidence to develop similar digital health approaches for patients with cancer.
Conclusion
This study will clarify the effects of patient-centered digital therapeutics on gastric cancer rehabilitation. It will serve as a cornerstone for future digital therapeutics and telerehabilitation using hardware (Internet of Things) and software (monitoring platform), concentrating on personalized medicine.
Supplemental Material
Supplemental material, sj-doc-1-dhj-10.1177_20552076231187602 for Effectiveness of a personalized digital exercise and nutrition-based rehab program for patients with gastric cancer after surgery: Study protocol for a randomized controlled trial by Inah Kim, Ji Young Lim, Jong Kwang Kim, Jun Ho Lee, Tae Sung Sohn, Sungsoo Park, Seok Ho Kang, Ji Youl Lee and Ji Hye Hwang in DIGITAL HEALTH
Acknowledgments
We thank Medi Plus Solution for their technical support and all hospital members who devoted their efforts to the study.
Footnotes
Contributorship: JHH and JYLee conceptualized the project and study methodology, refined the manuscript, and acquired funding. IK and JYLim drafted the project proposal manuscript for publication. All the authors contributed to the design and coordination of the project. All authors reviewed and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: All study procedures were approved by the Institute Review Board of Samsung Medical Center and Korea University Anam Hospital (approval numbers: SMC-2021-01-090 and 2021AN0104, respectively). A written informed consent is obtained from the patient.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National IT Industry Promotion Agency (NIPA) funded by the Ministry of Science and ICT of Korea (project number: S2001-20-1006 and S2401-21-1001, Project Healthcare big data showcase utilization service support: Cancer patient treatment and prognosis management service development).
Guarantor: JHH and JYLee
ORCID iDs: Inah Kim https://orcid.org/0000-0001-7290-8745
Ji Young Lim https://orcid.org/0000-0002-8449-7637
Tae Sung Sohn https://orcid.org/0000-0002-5766-484X
Ji Hye Hwang https://orcid.org/0000-0002-8176-3354
Supplemental material: Supplemental material for this article is available online.
References
- 1.Wada Y, Nishi M, Yoshikawa K, et al. Preoperative nutrition and exercise intervention in frailty patients with gastric cancer undergoing gastrectomy. Int J Clin Oncol 2022; 27: 1421–1427. 2022/07/01. [DOI] [PubMed] [Google Scholar]
- 2.Ida S, Kumagai K, Nunobe S. Current status of perioperative nutritional intervention and exercise in gastric cancer surgery: a review. Ann Gastroenterol Surg 2022; 6: 197–203. 2022/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis JL, Selby LV, Chou JF, et al. Patterns and predictors of weight loss after gastrectomy for cancer. Ann Surg Oncol 2016; 23: 1639–1645. 2016/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda Y, Yamamoto K, Hirao M, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer 2016; 19: 986–993. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto K, Nagatsuma Y, Fukuda Y, et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017; 20: 913–918. 2016/12/30. [DOI] [PubMed] [Google Scholar]
- 6.Imamura H, Nishikawa K, Kishi K, et al. Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol 2016; 23: 2928–2935. 2016/04/17. [DOI] [PubMed] [Google Scholar]
- 7.Kimura Y, Nishikawa K, Kishi K, et al. Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann Gastroenterol Surg 2019; 3: 648–656. 2019/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soh JY, Cha WC, Chang DK, et al. Development and validation of a multidisciplinary mobile care system for patients with advanced gastrointestinal cancer: interventional observation study. JMIR Mhealth Uhealth 2018; 6: e115. 2018/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KS. Polarization of cancer patient management. J Korean Med Assoc 2017; 60: 223–227. [Google Scholar]
- 10.Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N. Sarcopenia in patients with colorectal cancer: a comprehensive review. World J Clin Cases 2020; 8: 1188–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maisto M, Diana B, Di Tella S, et al. Digital interventions for psychological comorbidities in chronic diseases-a systematic review. J Pers Med 2021; 11: 30. 20210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BJ M, Gutierrez-Osuna R. A review of digital innovations for diet monitoring and precision nutrition. J Diabetes Sci Technol 2021: 19322968211041356. 2021/09/02. DOI: 10.1177/19322968211041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim JY, Kim Y, Yeo SM, et al. Feasibility and usability of a personalized mHealth app for self-management in the first year following breast cancer surgery. Health Informatics J 2023; 29: 14604582231156476. [DOI] [PubMed] [Google Scholar]
- 14.Rausei S, Mangano A, Galli F, et al. Quality of life after gastrectomy for cancer evaluated via the EORTC QLQ-C30 and QLQ-STO22 questionnaires: surgical considerations from the analysis of 103 patients. Int J Surg 2013; 11: S104–S109. [DOI] [PubMed] [Google Scholar]
- 15.Vickery CW, Blazeby JM, Conroy T, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer 2001; 37: 966–971. [DOI] [PubMed] [Google Scholar]
- 16.Torbahn G, Strauß T, Sieber C, et al. Use of mini nutritional assessment (MNA)® in oncological patients–an evidence map. Clin Nutr 2018; 37: S122. [Google Scholar]
- 17.Torbahn G, Strauss T, Sieber C, et al. Nutritional status according to the mini nutritional assessment (MNA)® as potential prognostic factor for health and treatment outcomes in patients with cancer–a systematic review. BMC Cancer 2020; 20: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cereda E. Mini nutritional assessment. Curr Opin Clin Nutr Metab Care 2012; 15: 29–41. [DOI] [PubMed] [Google Scholar]
- 19.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- 20.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Pract 2003; 3: 310–316. [DOI] [PubMed] [Google Scholar]
- 21.Mathiowetz V, Weber K, Volland Get al. et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984; 9: 222–226. [DOI] [PubMed] [Google Scholar]
- 22.Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013; 53: 255–267. 20120520. [DOI] [PubMed] [Google Scholar]
- 23.Millor N, Lecumberri P, Gomez M, et al. An evaluation of the 30-s chair stand test in older adults: frailty detection based on kinematic parameters from a single inertial unit. J Neuroeng Rehabil 2013; 10: 86. 20130801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohannon RW, Wang YC, Gershon RC. Two-minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch Phys Med Rehab 2015; 96: 472–477. DOI: 10.1016/j.apmr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Norman CD, Skinner HA. eHEALS: the eHealth Literacy Scale. J Med Internet Res 2006; 8: e27. 20061114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkes AL, Chambers SK, Pakenham KI, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol 2013; 31: 2313–2321. 20130520. [DOI] [PubMed] [Google Scholar]
- 27.Soh JY, Cha WC, Chang DK, et al. Development and validation of a multidisciplinary mobile care system for patients with advanced gastrointestinal cancer: interventional observation study. JMIR Mhealth Uhealth 2018; 6: e9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solis-Navarro L, Gismero A, Fernandez-Jane C, et al. Effectiveness of home-based exercise delivered by digital health in older adults: a systematic review and meta-analysis. Age Ageing 2022; 51, 2022/11/09. DOI: 10.1093/ageing/afac243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang ZY, Tian L, He K, et al. Digital rehabilitation programs improve therapeutic exercise adherence for patients with musculoskeletal conditions: a systematic review with meta-analysis. J Orthop Sports Phys Ther 2022; 52: 726–739. 2022/08/13. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Chen J, Yuan X, et al. Feasibility of an individualized mHealth nutrition (iNutrition) intervention for post-discharged gastric cancer patients following gastrectomy: a randomized controlled pilot trial. Nutrients 2023; 15. 2023/04/28. DOI: 10.3390/nu15081883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goto H, Kanaji S, Otsubo D, et al. Comparison of total versus subtotal gastrectomy for remnant gastric cancer. Langenbecks Arch Surg 2019; 404: 753–760. 2019/09/06. [DOI] [PubMed] [Google Scholar]
- 32.Lee K, Seo L, Yoon D, et al. Digital health profile of South Korea: a cross sectional study. Int J Environ Res Public Health 2022; 19. 2022/05/29. DOI: 10.3390/ijerph19106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-dhj-10.1177_20552076231187602 for Effectiveness of a personalized digital exercise and nutrition-based rehab program for patients with gastric cancer after surgery: Study protocol for a randomized controlled trial by Inah Kim, Ji Young Lim, Jong Kwang Kim, Jun Ho Lee, Tae Sung Sohn, Sungsoo Park, Seok Ho Kang, Ji Youl Lee and Ji Hye Hwang in DIGITAL HEALTH