Abstract
Objective
Intravenous thrombolytic therapy has become the standard of treatment for eligible patients with ischemic stroke. However, outcomes after receiving intravenous thrombolytic therapy vary widely. This study aims to investigate determinants of 1-year clinical outcomes of intravenous thrombolytic therapy for patients with acute ischemic stroke.
Methods
In a prospective, observational study, patients with acute ischemic stroke treated with intravenous thrombolysis were consecutively included, and clinical information and laboratory data were collected. The patients were followed up for 12 months after onset, and the 1-year clinical outcome was evaluated using modified Rankin Scale scores. A score ≥ 3 was defined as unfavorable functional outcome. Univariate and multivariate logistic regressions were used to assess the determinants of the 1-year clinical outcomes of intravenous thrombolysis for acute ischemic stroke.
Results
A total of 222 patients with intravenous thrombolysis were enrolled, and we identified 58 patients (26.1%) had unfavorable functional outcomes. Multivariate logistic regression analysis revealed that mean platelet volume-to-lymphocyte ratio (MPVLR) (odds ratio [OR] = 1.114, 95% confidence interval [CI]: 1.024–1.211, P = .012), atrial fibrillation (OR = 2.553, 95% CI: 1.086-6.002, P = .032), symptomatic stenosis occlusion (OR = 2.547, 95% CI: 1.269-5.110, P = .009), and baseline National Institutes of Health Stroke Scale (NIHSS) score (OR = 1.141, 95% CI: 1.074-1.212, P < .001) were independent predictors of unfavorable functional outcomes at 1 year.
Conclusions
In patients receiving intravenous thrombolysis, we found that MPVLR, atrial fibrillation, symptomatic stenosis occlusion, and baseline NIHSS score were significant predictors of unfavorable functional outcomes at 1 year.
Keywords: ischemic stroke, thrombolytic therapy, outcome, mean platelet volume-to-lymphocyte ratio, atrial fibrillation
Introduction
Acute ischemic stroke is the most common clinical cerebrovascular disease, with an increasing number of new cases each year, characterized by high disability, recurrence, and mortality rates.1,2 Although disability and mortality rates are decreasing with the advancement of medical technology and improvement in patients’ awareness of early consultation, most surviving patients have neurological deficits, which impose a considerable burden on patients and society.3,4 Recombinant tissue plasminogen activator (rtPA) attaches to fibrin on the surface of the clot and activates fibrin-bound plasminogen. Plasmin is subsequently cleaved from the plasminogen affiliated with the fibrin. The plasmin breaks down the fibrin molecules, and the clot dissolves, and thus improving the symptoms and prognosis of patients with thrombolysis; therefore, it is recommended by several guidelines.5,6 Currently, thrombolysis with alteplase within 4.5 h is the most effective pharmacological treatment for acute ischemic stroke.
However, clinical outcomes differed among patients, and a significant number of patients still have poor outcomes despite rtPA treatment due to individual factors and treatment complications. Several observational studies have demonstrated that intravenous thrombolysis significantly reduces 3 to 6 month disability and several factors affecting clinical outcomes have been extensively explored. The results of reported studies on predicting outcomes in patients treated with rtPA show wide variability.7–11 Recent studies on intravenous thrombolytic therapy with alteplase have mainly focused on the short-term outcome of patients, and few studies have investigated factors that influence the long-term outcomes of patients after intravenous thrombolysis. The prognosis of patients after intravenous thrombolytic therapy with rtPA is highly variable. Therefore, it is important to find prognostic predictors in patients with acute ischemic stroke treated with thrombolytic therapy. Blood cell variables have been reported to predict the risk and prognosis of ischemic stroke, particularly in patients receiving intravenous thrombolytic therapy.12,13 Relevant reports show that mean platelet volume (MPV) is an indicator of platelet function, reflecting platelet size and activity. Increased MPV is associated with poor prognosis in patients with acute ischemic stroke and lower lymphocyte count reduction was independently associated with an increased probability of poor function.14,15 Mean platelet volume-to-lymphocyte ratio (MPVLR) has been widely used as a composite index in prognostic studies of cardiovascular disease16,17 and there are few studies on cerebrovascular disease.
Therefore, this study is the first to explore the factors, that affecting the 1-year outcomes of patients with acute ischemic stroke after intravenous thrombolysis. In addition, we evaluated the effect of MPVLR on the 1-year unfavorable outcomes of intravenous thrombolysis in acute ischemic stroke, in order to provide clues for a timely follow-up treatment strategy for patients with intravenous thrombolysis.
Methods
Subjects
This is a prospective, observational study conducted during September 2017 to February 2022. Patients with acute ischemic stroke who were hospitalized in the Department of Neurology of the Affiliated Fuyang People's Hospital of Anhui Medical University and received rtPA intravenous thrombolysis were consecutively included in this study. The inclusion criteria for patients were as follows: (1) patients aged ≥18 years who met the indications for intravenous thrombolysis for acute ischemic stroke and completed intravenous thrombolysis and (2) patients or family members who signed the informed consent. The exclusion criteria for the patients were as follows: (1) patients treated with intra-arterial thrombolysis or embolization; (2) patients with modified Rankin Scale (mRS) scores >2 before onset; (3) patients with severe hepatic and renal insufficiency and heart failure; and (4) patients with incomplete clinical or imaging data. This study was approved by the Research Ethics Committee of the Affiliated Fuyang People's Hospital of Anhui Medical University and was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments. All patients or their family members signed an informed consent form prior to inclusion.
Clinical Data
We collected demographic information, including sex, age, smoking history, history of previous stroke or transient ischemic attack (TIA), hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, coronary artery disease, etc. Hypertension was identified by prior use of antihypertensive medication, systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg. Diabetes was determined by the prior use of antidiabetic drugs, fasting blood glucose ≥7.0 mmol/L, or 2h postprandial blood glucose ≥11.1 mmol/L. Hyperlipidemia was confirmed based on serum total cholesterol (≥5.2 mmol/L), triglycerides (≥1.7 mmol/L), low-density lipoprotein cholesterol (≥3.4 mmol/L), high-density lipoprotein (<1.0 mmol/L), or a previous diagnosis of dyslipidemia. Laboratory indicators were collected for all patients, such as neutrophil count, lymphocyte count, platelet count (PC), platelet distribution width, and MPV. MPVLR was calculated as the ratio of MPV to lymphocytes. Door-to-needle time was recorded in detail. Symptomatic stenosis occlusion was confirmed when magnetic resonance angiography (MRA) or computed tomography angiography (CTA) showed >50% stenosis or occlusion of the artery associated with an acute ischemic lesion, and symptomatic stenosis occlusion was defined according to Warfarin-Aspirin Symptomatic Intracranial Disease trial criteria. 18 The subtype classification of stroke was based on the Trial of ORG 10172 in Acute Stroke Treatment. 19 Subtypes of stroke were categorized by large-artery disease (LAD), small-artery disease (SAD), cardiac embolism (CE), or other determined and undetermined etiologies.
All patients underwent diffusion-weighted imaging and MRA or CTA within 48 h of admission. The type of lesion and the presence of arterial stenosis were studied. All imaging data were interpreted by experienced neurologists and neuroradiologists who were unaware of the clinical factors of the patients.
Evaluation of the Risk of Recurrent Stroke
The risk of stroke recurrence was assessed for each patient using the Essen Stroke Risk Score (ESRS). 20 The ESRS was calculated as a sum score (0-9 points): 2 points for age >75 years, 1 point each for age ≥65 to 75 years, arterial hypertension, diabetes mellitus, previous myocardial infarction, other cardiovascular diseases (except for myocardial infarction and atrial fibrillation), peripheral arterial disease, smoking, and previous TIA or ischemic stroke in addition to the qualifying event.
Stroke Severity and Clinical Outcome Assessment
The National Institutes of Health Stroke Scale (NIHSS) score was used to assess the severity of ischemic stroke at admission. 21 The clinical outcomes of all enrolled patients were followed by a qualified trained neurologist by telephone or by appointment at an outpatient clinic. The follow up was terminated 12 months after acute cerebral infarction or when death occurred. Outcomes were evaluated using mRS, and mRS scores ≥3 were classified as a unfavorable outcome. 22
Statistical Analyses
Continuous data were assessed for normality using the Kolmogorov-Smirnov test. Continuous variables with a normal distribution are expressed as the mean ± standard deviation. Other variables that did not follow normal distributions are presented as the median (M) and interquartile range (IQR). Categorical variables are reported as absolute numbers and percentages. Differences in continuous variables between groups were assessed using Student's t test or the Mann-Whitney U test. Differences in categorical variable distributions between groups were assessed using the χ2 test or Fisher's exact test, as appropriate. Spearman's correlation coefficient was used to determine the correlations of the MPVLR with mRS scores. More than 20 variables were included in the univariate model to estimate the factors affecting the 1-year clinical outcomes of patients. Variables with a potential association with unfavorable outcome (P < .1) from univariate analysis were used for multivariate analysis. After adjusting for age, dyslipidemia, and other conventional confounders, multivariate logistic regression was used to verify factors independently associated with the 1-year clinical outcomes. The results were analyzed for statistical significance using odds ratios (ORs) and 95% confidence intervals (CIs). All tests were 2-tailed, and P < 0.05 was considered to indicate statistical significance. Figures were generated using PowerPoint and GraphPad Prism software (version 8.0). All statistical analyses were performed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinical and Demographic Data
A total of 222 patients with intravenous thrombolysis for acute ischemic stroke were included in this study, including 147 (66.2%) males and 75 (33.8%) females. The median age was 64.5 years (IQR 54.0-71.0) years. In terms of etiological classification, 71 cases (32.0) had LAD, 29 cases (13.1%) had CE, 57 cases (25.7%) had SAD, and 65 cases (29.3%) had other causes and unknown causes. The ESRS was 2 (1-3) (median [IQR]), the baseline NIHSS score was 7 (4-12) (median [IQR]), and MPVLR was 6.43 (4.73-9.28) (median [IQR]). After 1 year of follow up, according to the mRS scores, 164 (73.9%) patients were in the favorable outcome group, and 58 (26.1%) were in the unfavorable outcome group, including 6 deaths, 2 patients died of sudden acute coronary syndrome, 1 died of multiple organ failure and 3 died of stroke (see Table 1).
Table 1.
The Clinical Characteristics of Patients With Acute Ischemic Stroke.
| Number | 222 |
|---|---|
| Age (years) | 64.5 (54.0-71.0) |
| Age grouped by median, n (%) | |
| Age <65 years | 111 (50.0) |
| Age ≥65 years | 111 (50.0) |
| Male, n (%) | 147 (66.2) |
| Vascular risk factors, n (%) | |
| Hypertension | 173 (77.9) |
| Diabetes | 54 (24.3) |
| Dyslipidemia | 95 (42.8) |
| Ischemic heart disease | 34 (15.3) |
| Stroke history | 49 (22.1) |
| Atrial fibrillation | 33 (14.9) |
| Smoker | 105 (47.3) |
| Alcohol user | 93 (41.9) |
| NIHSS score, median (IQR) | 7 (4-12) |
| ESRS, median (IQR) | 2 (1-3) |
| Laboratory indicators | |
| Neutrophil × 109/L, median (IQR) | 4.42 (3.51-5.88) |
| Lymphocyte × 109/L, median (IQR) | 1.56 (1.13-2.03) |
| PC ×109/L, median (IQR) | 202 ± 54 |
| PDW (fL), median (IQR) | 16.3 ± 0.4 |
| MPV (fL), median (IQR) | 10.1 ± 1.5 |
| MPVLR, median (IQR) | 6.43 (4.73-9.28) |
| DNT (min), median (IQR) | 49.5 (38.0-66.0) |
| Symptomatic stenosis occlusion | 98 (44.1) |
| Stroke etiology, n (%) | |
| LAD | 71 (32.0) |
| CE | 29 (13.1) |
| SAD | 57 (25.7) |
| Other and undetermined etiologies | 65 (29.3) |
Abbreviations: CE, cardiac embolism; DNT, door-to-needle time; ESRS, Essen Stroke Risk Score; IQR, interquartile range; LAD, large-artery disease; MPV, mean platelet volume; MPVLR, mean platelet volume-to-lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; PC, platelet count; PDW, platelet distribution width; SAD, small-artery disease.
Comparison of Clinical Data Between the 1-Year Favorable and Unfavorable Outcome Groups After Intravenous Thrombolysis for Acute Ischemic Stroke
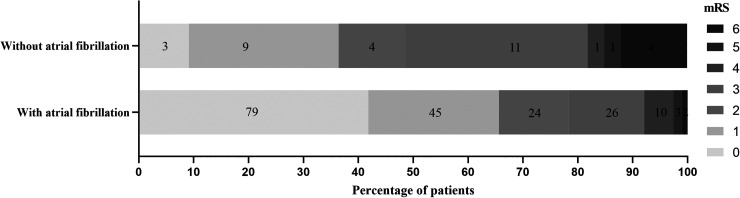
The clinical and demographic data for the 2 groups are shown in Table 2. Compared with the favorable outcome group, the unfavorable outcome group had significantly greater age (P = .047), atrial fibrillation (P < .001), baseline NIHSS scores (P < .001), symptomatic stenosis occlusion (P < .001), and MPV (P = .041), MPVLR (P = .009), and the differences were statistically significant. Dyslipidemia (P = .035) and lymphocyte (P = .036) were significantly greater in the favorable outcome group than in the unfavorable outcome group, with statistically significant differences. In terms of stroke etiology, the highest proportion of other and undetermined etiology and the lowest proportion of CE were found in the favorable outcome group, whereas the highest proportion of LAD and the lowest proportion of SAD were found in the unfavorable outcome group, so there was a significant difference in the distribution of stroke etiology between the 2 groups (P < .001), while the differences in other indicators were not statistically significant. Further analysis showed that mRS scores were significantly higher in patients with acute ischemic stroke with combined atrial fibrillation than in those without atrial fibrillation (P < .05; Figure 1).
Table 2.
Comparison of Clinical Data Between Patients With Favorable and Unfavorable Outcomes at 1 Year After Intravenous Thrombolysis for Acute Ischemic Stroke.
| Variable | Favorable outcome (n = 164) | Unfavorable outcome (n = 58) | t / Z or χ2 | P value |
|---|---|---|---|---|
| Age (years) | 63.5 (52.5-71.0) | 68.0 (59.0-73.0) | −1.989 | 0.047 |
| Age grouped by median, n (%) | 2.334 | 0.127 | ||
| Age <65 years | 87 (53.0) | 24 (41.4) | ||
| Age ≥65 years | 77 (47.0) | 34 (58.6) | ||
| Male, n (%) | 109 (66.5) | 38 (65.5) | 0.017 | 0.896 |
| Vascular risk factors, n (%) | ||||
| Hypertension | 127 (77.4) | 46 (79.3) | 0.087 | 0.768 |
| Diabetes | 38 (23.3) | 16 (27.6) | 0.454 | 0.501 |
| Dyslipidemia | 77 (47.0) | 18 (31.0) | 4.434 | 0.035 |
| Ischemic heart disease | 21 (12.8) | 13 (22.4) | 3.050 | 0.081 |
| Stroke history | 32 (19.5) | 17 (29.3) | 2.392 | 0.122 |
| Atrial fibrillation | 16 (9.8) | 17 (29.3) | 12.946 | <0.001 |
| Smoker | 76 (46.3) | 29 (50.0) | 0.230 | 0.631 |
| Alcohol user | 69 (42.1) | 24 (41.4) | 0.008 | 0.927 |
| NIHSS score, median (IQR) | 6 (3-9) | 12 (7-16) | −6.144 | <0.001 |
| ESRS, median (IQR) | 2 (1-3) | 3 (2-4) | −1.640 | 0.101 |
| Laboratory indicators | ||||
| Neutrophil × 109/L, median (IQR) | 4.38 (3.41-5.63) | 5.15 (3.74-7.23) | −11.740 | 0.111 |
| Lymphocyte × 109/L, median (IQR) | 1.65 (1.23-2.16) | 1.39 (1.09-1.88) | −2.103 | 0.036 |
| PC ×109/L, median (IQR) | 204.7 ± 54.7 | 195.0 ± 52.5 | 1.174 | 0.242 |
| PDW(fL), median (IQR) | 16.3 ± 0.4 | 16.2 ± 0.4 | 0.693 | 0.489 |
| MPV (fL), median (IQR) | 10.0 ± 1.4 | 10.5 ± 1.8 | −2.057 | 0.041 |
| MPVLR, median (IQR) | 6.05 (4.56-8.39) | 8.20 (4.91-11.63) | −2.581 | 0.009 |
| DNT (min), median (IQR) | 49.0 (38.5-65.0) | 53.5 (38.0-76.0) | −0.585 | 0.556 |
| Symptomatic stenosis occlusion | 60 (36.6) | 38 (65.5) | 14.546 | <0.001 |
| Stroke etiology, n (%) | 23.456 | <0.001 | ||
| LAD | 44 (26.8) | 27 (46.6) | ||
| CE | 15 (9.2) | 14 (24.1) | ||
| SAD | 52 (31.7) | 5 (8.6) | ||
| Other and undetermined etiologies | 53 (32.3) | 12 (20.7) |
Abbreviations: CE, cardiac embolism; DNT, door-to-needle time; ESRS, Essen Stroke Risk Score; IQR, interquartile range; LAD, large-artery disease; MPV, mean platelet volume; MPVLR, mean platelet volume-to-lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; PC, platelet count; PDW, platelet distribution width; SAD, small-artery disease.
Figure 1.
Distribution of mRS between acute ischemic stroke combined and noncombined atrial fibrillation. Abbreviation: mRS, modified Rankin Scale.
Associations of the MPVLR With mRS Scores and Rate of Unfavorable Outcome in Patients With Acute Ischemic Stroke
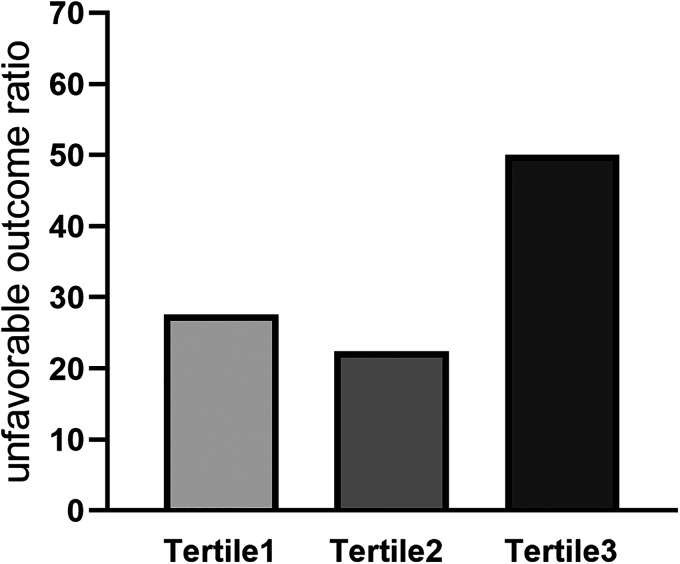
Spearman correlation analysis showed that the MPVLR was positively correlated with mRS scores (r = 0.194, P = .004). We divided the patients into tertile groups based on their MPVLRs on admission. In the first tertile (MPVLR ≤ 5.03) the unfavorable outcome rate was 27.6%; in the second tertile (5.03 < MPVLR ≤ 8.05) the unfavorable outcome rate was 22.4%; and in the third tertile (MPVLR > 8.05) the unfavorable outcome rate was 50.0% (Figure 2).
Figure 2.
Unfavorable outcome distribution of the tertile groups based on MPVLR. Abbreviation: MPVLR, mean platelet volume-to-lymphocyte ratio.
Independent Risk Factors for Unfavorable Outcomes at 1 Year After Intravenous Thrombolysis for Acute Ischemic Stroke
We used univariate and multivariate logistic regression models to analyze the associations between risk factors and unfavorable outcomes at 1 year after intravenous thrombolysis. The univariate logistic regression analysis indicated that the neutrophil, MPVLR, MPV, age, atrial fibrillation, NIHSS score, LAD, CE, and symptomatic stenosis occlusion were associated with unfavorable outcomes at 1 year. Multivariate logistic regression analysis indicated that MPVLR (OR = 1.114, 95% CI: 1.024-1.211), atrial fibrillation (OR = 2.553, 95% CI: 1.086-6.002), symptomatic stenosis occlusion (OR = 2.547, 95% CI: 1.269-5.110), and NIHSS (OR = 1.141, 95% CI: 1.074-1.212) were independent predictors of unfavorable outcomes at 1 year after intravenous thrombolysis (P < .05; see Table 3).
Table 3.
Univariate and Multivariate Logistic Regression Analysis of Outcome at 1 Year After Intravenous Thrombolysis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (years) | 1.030 (1.003-1.058) | 0.030 | ||
| Age grouped by median | ||||
| Age <65 years | Ref | |||
| Age ≥65 years | 1.601 (0.873-2.934) | 0.128 | ||
| Sex | 1.043 (0.555-1.961) | 0.896 | ||
| Vascular risk factors | ||||
| Hypertension | 1.117 (0.536-2.325) | 0.768 | ||
| Diabetes | 1.263 (0.640-2.495) | 0.501 | ||
| Dyslipidemia | 0.508 (0.269-0.960) | 0.037 | ||
| Ischemic heart disease | 1.967 (0.912-4.242) | 0.084 | ||
| Stroke history | 1.710 (0.862-3.392) | 0.124 | ||
| Atrial fibrillation | 3.835 (1.784-8.245) | 0.001 | 2.553 (1.086-6.002) | 0.032 |
| Smoker | 1.158 (0.636-2.108) | 0.632 | ||
| Alcohol user | 0.972 (0.529-1.784) | 0.927 | ||
| NIHSS score | 1.168 (1.102-1.238) | <0.001 | 1.141 (1.074-1.212) | <0.001 |
| ESRS | 1.227 (0.972-1.548) | 0.085 | ||
| Neutrophil×109/L | 1.120 (1.009-1.244) | 0.034 | ||
| Lymphocyte ×109/L | 0.641 (0.412-0.996) | 0.048 | ||
| PC ×109/L | 0.997 (0.991-1.002) | 0.242 | ||
| PDW | 0.776 (0.380-1.587) | 0.488 | ||
| MPV | 1.217 (1.006-1.472) | 0.043 | ||
| MPVLR | 1.118 (1.038-1.204) | 0.003 | 1.114 (1.024-1.211) | 0.012 |
| DNT | 1.003 (0.992-1.015) | 0.557 | ||
| Symptomatic stenosis occlusion | 3.292 (1.758-6.170) | <0.001 | 2.547 (1.269-5.110) | 0.009 |
| Stroke etiology | ||||
| LAD | 6.382 (2.226-17.970) | <0.001 | ||
| CE | 9.013 (2.768-29.350) | <0.001 | ||
| Other and undetermined etiologies | 2.355 (0.775-7.154) | 0.131 | ||
| SAD | Ref | |||
Abbreviations: 95% CI, 95% confidence interval; CE, cardiac embolism; DNT, door-to-needle time; ESRS, Essen Stroke Risk Score; MPV, mean platelet volume; MPVLR, mean platelet volume-to-lymphocyte ratio; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; PC, platelet count; PDW, platelet distribution width; SAD, small-artery disease.
Discussion
In this study, 222 patients with acute ischemic stroke who received intravenous thrombolysis were followed up for 1 year. We found that MVPLR, atrial fibrillation, symptomatic stenosis occlusion, and a high baseline NIHSS score were significantly correlated with an unfavorable 1-year outcome of acute ischemic stroke.
The mechanism underlying the effect of the MPVLR on the outcome of acute ischemic stroke can be explained from the following perspectives. First, in acute ischemic stroke, many platelets are consumed to form thrombi. When platelets are continuously activated and aggregated during thrombosis, platelets in the blood circulation are depleted, which may lead to a decrease in the PC. Meanwhile, compensatory hyperplasia of bone marrow megakaryocytes occurs, resulting in the production of oversized platelets rich in granular components, which manifests as an increased MPV. 23 Second, the MPV is closely related to inflammatory cytokines, and inflammatory cytokines such as interleukin (IL)-6 and IL-3 can promote the production of oversized platelets by increasing the number of megakaryocytes. Therefore, the pre-acute ischemic stroke inflammatory state may also be a cause of high MPV levels. 24 Compared with normally sized platelets, oversized platelets are more prone to aggregation, more responsive to adenosine diphosphate activation, and less responsive to the inhibitory effect of prostacyclin, and patients with an elevated MPV are at a higher risk of thrombosis. 25 In addition, the inflammatory response after stroke plays an important role in ischemic brain tissue injury, and lymphocytes are a good reflection of the ability of the human immune system to respond to external damage. When acute cerebral infarction occurs, the induced systemic immunosuppression can lead to a significant decrease in the number of lymphocytes; since lymphocytes have anti-inflammatory functions and provide endothelial protection, this decrease increases susceptibility to infection and adversely affects clinical outcomes impact on clinical outcomes.26,27 In the present study, lymphocytes were found to be significantly lower in patients in the unfavorable prognosis group than in the favorable prognosis group, which is consistent with these results.
MVPLR combined with the above pathophysiological characteristics of MPV and lymphocytes can provide more comprehensive information and better predict the outcome of related diseases. Previous studies have confirmed its correlation with various diseases such as acute coronary syndrome and diabetic nephropathy, indicating that MPVLR has a high clinical application.16,17,28 A recent study reported that MPVLR elevation was associated with an unfavorable 3-month outcome of acute ischemic stroke. 29 Our study also showed that MPVLR were higher in the unfavorable outcome group of acute ischemic stroke and that the MPVLR was an independent predictor of a unfavorable 1-year clinical outcome for intravenous thrombolysis in acute ischemic stroke.
Atrial fibrillation is a common risk factor for acute ischemic stroke and is significantly associated with the severity of ischemic stroke, high incidence of complications, and poor prognosis.30–33 Similar to previous studies, the results at 1 year after intravenous thrombolysis showed that the proportion of patients with combined atrial fibrillation was significantly higher in the poor outcome group than in the good outcome group (29.3% vs 9.8%), and further analysis showed that mRS scores were significantly higher in patients with acute ischemic stroke with combined atrial fibrillation than in those without atrial fibrillation, and that atrial fibrillation was a predictor of poor outcome at 1 year after intravenous thrombolysis in acute ischemic stroke. The results show that atrial fibrillation is a predictor of poor prognosis 1 year after intravenous thrombolysis in acute ischemic stroke. We speculate that the possible reasons for the poor outcome of thrombolysis in patients with ischemic stroke combined with atrial fibrillation are the large infarct size and high degree of disability. 34 A higher mRS score implies the occurrence of severe stroke, leading to a high rate of complications and poor outcome for the patient. Second, patients with atrial fibrillation form larger cardiogenic emboli that are not easily dissolved by thrombolytic drugs and obstruct early revascularization. 35 The results of this study suggest that, in addition to standardizing the treatment of patients with acute ischemic stroke with atrial fibrillation in the acute phase to effectively reduce the incidence of adverse outcome, primary prevention of stroke in patients with atrial fibrillation is more important for clinicians. The standardized assessment of stroke risk and bleeding risk in patients with atrial fibrillation, and the provision of relatively individualized and compliant anticoagulation strategies for patients with atrial fibrillation at high risk of stroke (eg, regular international standardized coagulation ratio monitoring or selection of new oral anticoagulants) are important.
The NIHSS score mainly reflects neurological impairment in patients with acute ischemic stroke and is an important indicator for assessing the severity of the disease. A higher NIHSS score indicates more severe neurological impairment, a larger infarct size, and more severe secondary brain injury. The NIHSS score has been widely used to assess the outcome of acute ischemic stroke. Studies have found that the NIHSS score at admission influences the 3-month outcome of acute ischemic stroke.36,37 This study also demonstrated that a high NIHSS score was an independent risk factor for a unfavorable 1-year outcome of acute ischemic stroke. In addition, our study found that symptomatic stenosis occlusion was an independent risk factor for a unfavorable 1-year outcome of acute ischemic stroke, which is consistent with the findings of previous studies and is likely because patients with symptomatic stenosis occlusion have a greatly increased probability of ipsilateral cerebral infarction, and brain tissue in the blood supply area is more prone to hypoperfusion and subsequent irreversible cerebral infarction if vascular stenosis is significant or blood pressure fluctuates widely.38,39
This study is the first to investigate predictors of the 1-year prognosis of patients with intravenous thrombolysis; however, this study also has some limitations. First, this study is a single-center observational study with a limited number of cases and geographic limitations, and some selection bias may exist; therefore, a multicenter study with a large sample is needed to further confirm the findings of this study. Second, this is an observational study in which some variables were excluded, such as the exclusion of COVID-19 patients and 12 months post-stroke due to missing data and missing outcomes at follow up. In addition, patients had very low mortality rates during a 1-year follow up after acute ischemic stroke may limit the findings to a low-risk population of acute ischemic stroke.
Conclusion
In this study, we found that MVPLR, atrial fibrillation, symptomatic stenosis occlusion, and a high baseline NIHSS score were significantly correlated with a unfavorable 1-year outcome of acute ischemic stroke.
Footnotes
Authors’ Contributions: MZ was involved in the design of the study, data collection, interpretation of the data, and manuscript writing and was a recipient of the obtained funding. HX and YL participated in the study design, data collection, and statistical analysis. JY and XC participated in the data analysis, interpretation of the data, and manuscript revision. YW is the guarantor. All authors read and approved the protocol.
Data Availability Statement: All data generated for this study are included in the article. The datasets generated during the current study are available from the corresponding author on reasonable request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: The studies obtained ethical approval from the Institutional Review Board of the Affiliated Fuyang People's Hospital of Anhui Medical University ([2019] 67). The current study was carried out according to the Declaration of Helsinki. All participants were above 18 years of age and all of them or their legal proxies signed the written informed consent.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Fuyang Health and Health Commission Project (grant number FY2019-056) and the National Natural Science Foundation of China (grant number 82071460).
ORCID iD: Mingfeng Zhai https://orcid.org/0000-0003-1934-2740
References
- 1.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barthels D, Das H. Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):165260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120(3):439-448. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38-360. [DOI] [PubMed] [Google Scholar]
- 5.Jilani TN, Siddiqui AH.Tissue Plasminogen Activator. In: StatPearls. StatPearls Publishing; February 20, 2023 [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Yan S, Wu C, et al. Characteristics and outcomes of intravenous thrombolysis in mild ischemic stroke patients. Front Neurol. 2021;12:744909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Çetiner M, Aydin HE, Güler M, et al. Predictive factors for functional outcomes after intravenous thrombolytic therapy in acute ischemic stroke. Clin Appl Thromb Hemost. 2018;24(9_suppl):171S-177S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono S, Deguchi K, Morimoto N, et al. Tissue plasminogen activator thrombolytic therapy for acute ischemic stroke in 4 hospital groups in Japan. J Stroke Cerebrovasc Dis. 2013;22(3):190-196. [DOI] [PubMed] [Google Scholar]
- 10.Herath HMMTB, Rodrigo C, Alahakoon AMBD, et al. Outcomes of stroke patients undergoing thrombolysis in Sri Lanka; an observational prospective study from a low-middle income country. BMC Neurol. 2021;21(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q, Li X, Dong W, et al. Factors associated with thrombolysis outcome in ischemic stroke patients with atrial fibrillation. Neurosci Bull. 2016;32(2):145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudzik B, Szkodziński J, Lekston A, Gierlotka M, Poloński L, Gąsior M. Mean platelet volume-to-lymphocyte ratio: a novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Complications. 2016;30(6):1097-1102. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Ying A, Lin Y, et al. Neutrophil-to-lymphocyte ratio, hyperglycemia, and outcomes in ischemic stroke patients treated with intravenous thrombolysis. Brain Behav. 2020;10(9):e01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dourado Sotero F, Calçada A, Aguiar de Sousa D, et al. Mean platelet volume is a prognostic marker in acute ischemic stroke patients treated with intravenous thrombolysis. J Stroke Cerebrovasc Dis. 2021;30(6):105718. [DOI] [PubMed] [Google Scholar]
- 15.Kömürcü HF, Gözke E, Doğan AP, Kalyoncu Aslan I, Salt I. Özgenç BEÇİ. Changes in neutrophil, lymphocyte, platelet ratios and their relationship with NIHSS after rtPA and/or thrombectomy in ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(8):105004. [DOI] [PubMed] [Google Scholar]
- 16.Ornek E, Kurtul A. Relationship of mean platelet volume to lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris. Coron Artery Dis. 2017;28(6):492-497. [DOI] [PubMed] [Google Scholar]
- 17.Besli F, Ilter A, Gungoren F. The link between mean platelet volume to lymphocyte ratio and complexity of coronary artery disease. Angiology. 2018;69(4):358-359. [DOI] [PubMed] [Google Scholar]
- 18.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643-646. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PH, Gao S, Wang YJ, et al. Classifying ischemic stroke, from TOAST to CISS. CNS Neurosci Ther. 2012;18(6):452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diener HC, Ringleb PA, Savi P. Clopidogrel for the secondary prevention of stroke. Expert Opin Pharmacother. 2005;6(5):755-764. [DOI] [PubMed] [Google Scholar]
- 21.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. [DOI] [PubMed] [Google Scholar]
- 22.Lima FO, Furie KL, Silva GS, et al. The pattern of leptomeningeal collaterals on angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010;41(10):2316-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TY, Lu WJ, Changou CA, et al. Platelet autophagic machinery involved in thrombosis through a novel linkage of AMPK-MTOR to sphingolipid metabolism. Autophagy. 2021;17(12):4141-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparyan AY, Ayvazyan L, Mikhailidis DP, et al. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47-58. [DOI] [PubMed] [Google Scholar]
- 25.Andrews RK, López JA, Berndt MC. Molecular mechanisms of platelet adhesion and activation. Int J Biochem Cell Biol. 1997;29(1):91-105. [DOI] [PubMed] [Google Scholar]
- 26.Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130(6):2777-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamorro Á, Meisel A, Planas AM, et al. The immunology of acute stroke. Nat Rev Neurol. 2012;8(7):401-410. [DOI] [PubMed] [Google Scholar]
- 28.Kocak MZ, Aktas G, Erkus E, et al. Mean platelet volume to lymphocyte ratio as a novel marker for diabetic nephropathy. J Coll Physicians Surg Pak. 2018;28(11):844-847. [DOI] [PubMed] [Google Scholar]
- 29.Chen SY, Lin YS, Cheng YF, et al. Mean platelet volume-to-lymphocyte ratio predicts poor functional outcomes among ischemic stroke patients treated with intravenous thrombolysis. Front Neurol. 2019;10:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789-797. [DOI] [PubMed] [Google Scholar]
- 31.Henninger N, Goddeau RP, Jr, Karmarkar A, et al. Atrial fibrillation is associated with a worse 90-day outcome than other cardioembolic stroke subtypes. Stroke. 2016;47(6):1486-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naganuma M, Inatomi Y, Yonehara T, et al. Underdosed direct oral anticoagulants in atrial fibrillation patients reduce stroke severity and improve outcome. Cerebrovasc Dis. 2022;51(4):473-480. [DOI] [PubMed] [Google Scholar]
- 33.Yiin GS, Howard DP, Paul NL, et al. Recent time trends in incidence, outcome and premorbid treatment of atrial fibrillation-related stroke and other embolic vascular events: a population-based study. J Neurol Neurosurg Psychiatry. 2017;88(1):12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu HT, Campbell BC, Christensen S, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. 2015;10(4):534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura K, Iguchi Y, Yamashita S, et al. Atrial fibrillation as an independent predictor for no early recanalization after IV-t-PA in acute ischemic stroke. J Neurol Sci. 2008;267(1-2):57-61. [DOI] [PubMed] [Google Scholar]
- 36.Mazya MV, Lees KR, Collas D, et al. IV Thrombolysis in very severe and severe ischemic stroke: results from the SITS-ISTR Registry. Neurology. 2015;85(24):2098-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satumanatpan N, Tonpho W, Thiraratananukulchai N, et al. Factors associated with unfavorable functional outcomes after intravenous thrombolysis in patients with acute ischemic stroke. Int J Gen Med. 2022;15:3363-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lung YJ, Weng WC, Wu CL, et al. Association between total cholesterol and 5 year mortality in patients with carotid artery stenosis and poststroke functional dependence. J Stroke Cerebrovasc Dis. 2019;28(4):1040-1047. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Zhang X, Chen H, et al. Prevalence and outcome of young stroke patients with middle cerebral artery stenosis. BMC Neurol. 2021;21(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]