Abstract
Carbonaceous nanomaterials (CNMs) have drawn tremendous biomedical research interest because of their unique structural features. Recently, CNMs, namely carbon dots, fullerenes, graphene, etc, have been successful in establishing them as considerable nanotherapeutics for phototherapy applications due to their electrical, thermal, and surface properties. This review aims to crosstalk the current understanding of CNMs as multimodal compounds in photothermal and photodynamic therapies as an integrated approach to treating cancer. It also expounds on phototherapy’s biomechanics and illustrates its relation to cancer biomodulation. Critical considerations related to the structural properties, fabrication approaches, surface functionalization strategies, and biosafety profiles of CNMs have been explained. This article provides an overview of the most recent developments in the study of CNMs used in phototherapy, emphasizing their usage as nanocarriers. To conquer the current challenges of CNMs, we can raise the standard of cancer therapy for patients. The review will be of interest to the researchers working in the area of photothermal and photodynamic therapies and aiming to explore CNMs and their conjugates in cancer therapy.
Keywords: photothermal therapy, nanomaterials, photosensitizer, NIR laser, reactive oxygen species, photodynamic therapy
Introduction
Despite significant advances over the last decade, accurate diagnosis, as well as effective cancer treatment, remains to be an enormous challenge. Surgery, radiation therapy, and chemotherapy are all frequently used cancer treatment options, but they cause side effects in healthy tissues and cells. 1 Phototherapy offers promising new avenues for use in a wide range of biological contexts to overcome such obstacles in cancer treatment. 2 In the last 10 years, phototherapy has sparked intense scientific attention as an innovative approach to cancer treatment. It is noninvasive, has low systemic toxicity, and has minimal side effects or drug resistance. 3 The 2 types of phototherapies most frequently utilized for cancer treatment are photodynamic therapy (PDT) and photothermal therapy (PTT). PTT uses photothermal agents (PTAs) to create heat by energy conversion absorbed from photons, which causes the cancer cells’ thermal ablation and subsequent cell death. PDT kills cancer cells by producing reactive oxygen species (ROS) under laser illumination using photosensitizer (PS) agents. 4
PDT focuses on utilizing the PS, which, when exposed to the correct wavelength of radiation, produces ROS. ROS molecules with extreme cytotoxicities, such as 1O2, superoxide, and OH, may be produced to kill cancer cells when they absorb the PS. The visible range, 350 to 700 nm, is typically used for irradiation in this method; however, some investigations also utilized near-infrared (NIR) (750-1700 nm).4,5 There are many PS, most of which contain porphyrin molecules, which accumulate and retain specifically in malignancies and allow targeted therapy following light irradiation. PS have been conjugated with various nanomaterials, including carbonaceous nanomaterials (CNMs), to boost their phototherapeutic effectiveness. In addition, fullerenes, carbon dots (CDs), and graphene quantum dots (GQDs) are examples of CNMs that can function as PS independently without including any external PS molecules. 6
The potential of PTAs, which generate heat, has piqued interest in PTT for its efficacy in killing cancer cells while sparing surrounding healthy tissues. A PTA should absorb NIR or visible light and convert it into heat, causing hyperthermia at the tumor location. 7 There are 2 distinct methods for determining PTT. Mild phototherapy is 1 option; this treatment raises the temperature to around 40 °C to 50 °C to disrupt typical cellular function. 5 Photothermal ablation is the second method, and it often involves temperatures exceeding 50 °C, which destroys cell membranes and causes necrosis. 8 The thermal stability and light absorption often displayed by CNMs make them promising candidates to be employed as PTAs in this scenario. 9
Research into PDT and PTT has shown promise as a minimally invasive and selective treatment approach. The synergistic effect of these 2 approaches can significantly improve the efficacy of cancer treatment.4,8 PTT can boost PDT effectiveness only after heat produced by irradiation increases cell permeability, which enhances PS uptake. 10 Enhanced PDT is also a result of the higher levels of ROS generated by raising the body temperature, which in turn enhances blood flow and causes tumor tissues to become significantly oxygenated. In addition, the formation of ROS during PDT hinders heat-shock proteins from shielding tumor cells from PTT's effects. 11 NIR irradiation is commonly used to activate therapeutic effects in PTT, while ultraviolet (UV) and visible light irradiations are more commonly used in PDT. CNMs can target and destroy tumor cells by loading PS/anticancer agents or conjugating them with macromolecules. 5
Photobiomodulation (PBM) is a form of alternative and traditional medicine that employs low-intensity light sources, such as lasers or light-emitting diodes (LEDs), on the site of illness for treating several diseases. 12 Medical professionals have tried PBM for various conditions, including chronic and acute pain, wound healing, Parkinson's disease, inflammatory disorders, musculoskeletal syndromes, dentistry, neurological diseases, cancers of the head and neck, colorectal cancer, and carpal tunnel syndrome. 13 When the light is delivered into the cells, PBM has both stimulating and inhibitory effects. 14 Most cells, including endothelial cells, fibroblasts, lymphocytes, and keratinocytes, respond to PBM by increasing their proliferation. This is achieved through the mitochondrial signaling process photostimulation and the transcription process regulation, which lead to enhanced growth factor production.15,16
Despite all this, the clinical utility of these methods is often showing some limitations, including limited selectivity as well as the depth of penetration and location as well as the size of tumors. The well-known restriction of these methods showed that they were beneficial for treating surface tumors but not deeper tumors like brain or liver tumors because of the restricted light penetration depth. 17 According to a study by Wang et al, 18 larger tumors needed greater gold nanoparticle (NP) dosages and higher irradiation times to completely disappear, even though some residual tumors were left behind. It was also observed by another group of researchers that both healthy and cancerous liver cells might be harmed by PTT utilizing gold nanorods (AuNRs). 19
The focus of this review is to expound on the recent progress made in cancer phototherapy with the utilization of CDs, fullerenes, graphene, etc. There will be a quick rundown regarding phototherapy and its biomechanism. The basic structural properties of each type of CNM, fabrication approaches, and methods of surface functionalization are hereby thoroughly discussed that render them more water-soluble and biocompatible. At the same time, it also gives them entirely new characteristics.
Biomechanics of Phototherapy
Due to the minimal invasiveness and site-directed treatment potential, PTT became popular in cancer studies. PTT typically employs a PTA, which converts the light energy into heat upon irradiation with a light source enhancing the surrounding tissue temperature and causing the death of cells. 20 A perfect PTA should be target-specific, especially tumor-specific, in case of cancer. It should also possess excellent photothermal conversion efficiency (PCE). 21 Attention has been focused on creating nanosized PTAs, which can accumulate in tumors via the enhanced permeability and retention (EPR) effect and active targeting. 22
A PTA works as an accelerator to heat the targeted tissue. In particular, the PTA absorbs energy and shows excitation from the ground to an excited state when irradiated with light of a particular wavelength. When the excited PTA collides with the surrounding molecules, the excited PTA experiences nonradiative vibrational relaxation and returns to the ground state. This excess kinetics leads to an increase in the heat in the tumor microenvironment (TME). 23 When tissues are subjected to temperatures exceeding 42 °C, 1 of 2 cell signaling pathways—necrosis or apoptosis—is typically associated with tissue death via the photothermal effect.
Additionally, recent research has revealed a novel mechanism of cell death in PTT called necroptosis, which resembles passive, uncontrolled necrotic death of cells but differs in being a strictly controlled cell death mechanism. 24 When the heat rises above 41 °C, a series of changes have been seen, such as the enhanced expression of genes that causes the activation of heat-shock proteins. 25 Above 42 °C, irreversible tissue damage happens, causing necroptosis. So, PTT generates high heat that leads to cellular death.
The combination of PTT with other techniques often results in clinical benefits that are cumulative or even synergistic in nature. 26 For instance, the combination of PTT and PDT produces local heat that enhances blood flow leading to the generation of a high concentration of oxygen in TME, improving the efficacy of PDT. 27
Phototherapy and Its Role in Cancer Biomodulation
Despite the significant advancements made over the past few years, early detection and effective cancer therapy are still challenging. Traditional treatments such as radiation, surgeries, and chemotherapies are widely used in cancer treatment, but they risk harming normal cells. 28 Phototherapy has been commonly used in different biomedical applications to solve this issue. Phototherapy comprises 2 main categories such as PTT and PDT. 29 This phototherapy could penetrate more effectively into the cells when using NIR, which can effectively target cancer cells with precision while sparing healthy cells. 11
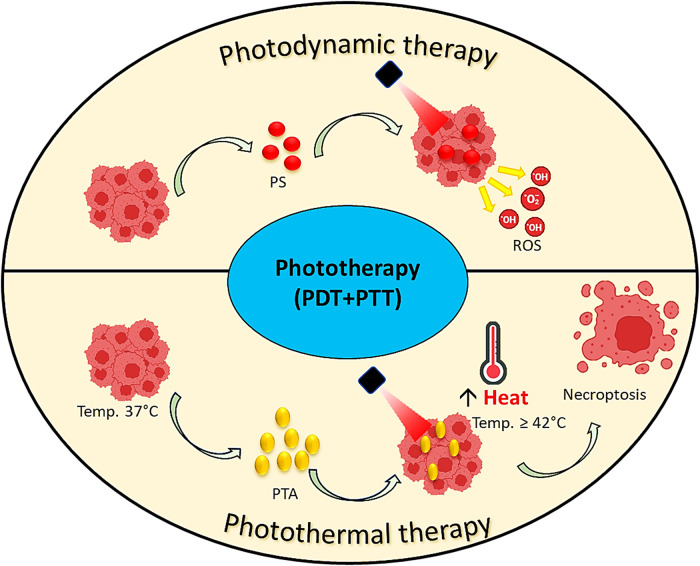
PTT is anticipated to achieve nontoxic and potent tumor cell targeting to improve clinical efficiency without harming healthy tissues. 30 Several CNMs exhibiting NIR absorption show remarkable clinical efficacy in numerous in vivo tests conducted in patient assessment. 2 Due to its ability to specifically destroy cancer cells utilizing heat radiation, PTT is currently attracting more and more attention in scientific circles. PTT leads to many biological and physiological alterations in the tumor cells that can maximize its therapeutic efficacy and boost the effectiveness of further treatments. For instance, localized heat release enhances the permeability of the tumor's cell membranes and blood vessels, which can boost the absorption of additional treatments like chemotherapy.31,32 In PDT, the PS is activated by a specific wavelength of light to kill tumors. PDT uses a light-activatable PS that generates ROS, such as peroxides and free radicals, after activation. 33 Compared to PDT or PTT alone, the integration of PDT and PTT may have a synergistic action. PTT can synergize PDT into the intracellular milieu and raise the O2 concentration in tumor tissues by enhancing the localized flow of blood, leading to the higher efficacy of PDT (Figure 1).
Figure 1.
Phototherapies used in cancer biomodulation.
Abbreviations: PDT, photodynamic therapy; PS, photosensitizer; PTA, photothermal agent; PTT, photothermal therapy; ROS, reactive oxygen species.
Phototherapy-Eliciting Materials: Understanding Dimensions of Carbonaceous Nanomaterials
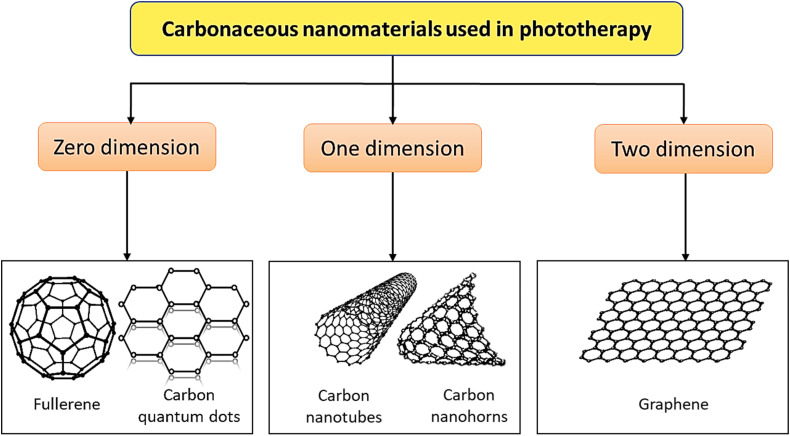
At the beginning of the study of CNMs, 0-dimensional (0D) fullerenes were discovered. Various CNMs such as 0D or dimensionless (eg, fullerenes and carbon quantum dots [CQDs]), 1-dimensional (1D) (eg, carbon nanotubes [CNTs] and carbon nanohorns [CNHs]), and 2-dimensional (2D) (eg, graphene) have been used as phototherapy-eliciting materials 13 (Figure 2). In the medical sciences, these CNMs possess distinctive physiochemical properties and structural features, which enable them to escape the hazardous side effects of existing chemotherapy and advance level efficient treatments.
Figure 2.
Different carbonaceous nanomaterials (CNMs) used in phototherapy.
Dimensionless or 0-Dimensional Carbonaceous Nanomaterials
Dimensionless or 0D CNMs exhibit exceptional qualities, including minimal cytotoxic effects, excellent photostability, outstanding cytocompatibility, and simplicity in conjugating with macromolecules. Examples of 0D CNMs are fullerenes and CQDs. 4 These materials are synthesized using various synthesis approaches such as chemical, biological, and physical. These synthetic methods have a significant impact on both properties and uses of CNMs. Chemical synthesis involves chemical vapor deposition, sol–gel techniques, and microemulsion. In contrast, in the biological approach, different biomolecules, such as enzymes, proteins, RNA, DNA, etc, have been used that make the nanomaterials less toxic and more biocompatible. The physical method involves thermal evaporation, pyrolysis, electrospraying, etc. Compared to chemical and physical methods, biological methods are less expensive and avoid toxic chemicals. The next sections discuss the 2 most crucial dimensionless CNMs, such as fullerenes and CQDs. 34
Fullerenes
Fullerenes have sp2-hybridized geometry organized in 12 pentagons and 20 hexagons within void spaces with diameters in the range of 7 to 10 Å. Different forms of fullerenes are available such as C72, C70, C78, C76, and C84. Among them, C60 has significantly less diameter, and it is a majorly used form of fullerene. 35 Moreover, the configuration of carbon atoms is in a pyramid shape, not a planar shape. The basic sp2 carbons must contain a sp3 pseudo-bonding element. The pentagon gives a curve-shaped structure that closes the cage. In the X-ray diffraction (XRD) pattern of fullerene C60, it was observed that 2 distinct bonds join 2 hexagons, and the other forms a hexagon–pentagon pair. The first bond, numbered 66, having 1.38 Å length, connects 2 hexagons. The second bond, numbered 56, has a size of 1.45 Å, which connects the pentagon–hexagon pair. A solid-state fullerene appears as a crystalline structure or aggregate showing a face-centered cubic (FCC) lattice. Solubility of both the aggregates and their crystalline structure was found in many organic solvents, especially carbon disulfide (8.00 mg/mL) and toluene (2.8 mg/mL). It also has good stability; moreover, cage degradation occurs above 1000 °C. The fullerenes can be characterized with the help of UV–visible, Fourier transform-infrared (FTIR), Raman, mass, and nuclear magnetic resonance (NMR) spectroscopy.
Carbon Quantum Dots
Other 0D CNMs that are CQDs are categorized as CDs and GQDs. The characteristics of GQDs and CDs that set them apart from other CNMs are their tiny size and fluorescence. 36 CDs mostly exist as quasi-spherical NPs having a size between 2 and 20 nm, with sp3 carbon geometry. GQDs are crystalline disk-shaped particles with diameters ranging from 2 to 10 nm that are primarily made up of sp2-hybridized carbon. 37
Researchers have synthesized CDs by the hydrothermal method using coronene derivatives with high PCE at 808 nm. CDs can primarily accumulate in lysosomes and have high biocompatibility, photostability, and cell penetration abilities. According to the findings of this investigation, CDs are a good choice for an effective NIR light-triggered PTA for an effective PTT against cancer. 38
1-Dimensional Carbonaceous Nanomaterials
CNTs and CNHs are examples of 1D CNMs widely used in PTT. In the next sections, we have briefly discussed CNTs and CNHs.
Carbon Nanotubes
CNTs exhibit light absorption in the NIR region. The 2 types of CNTs—single-walled CNTs (SWCNTs) and multiwalled CNTs (MWCNTs)—have unique characteristics and PTT effects. The only arrangement of carbon atoms in CNTs is in the form of the aromatic benzene ring. They resemble wrapped graphene layers in a hollow cylinder tube with open ends. Armchair, zigzag, and chiral are CNTs’ geometric representations, and sp2 planar and sp3 cubic are allotropic forms. CNT-based systems have received a considerable amount of interest in the field of biomedicine as sophisticated nanocarriers to transport diverse therapeutic compounds via interaction. CNTs may pass numerous biological barriers within the body to generate negligible immunogenic responses and damaging impacts. 39 As a result of inadequate targeting, low bioavailability, and organ damage, traditional cancer therapies, including radiotherapy surgery and chemotherapy, have several undesirable consequences and do not entirely treat the condition. 40 Researchers are now concentrating on combination therapies like nanomaterials and phototherapy to treat cancer effectively and precisely. To boost the bioavailability of PS and to target only cancer cells, the PS is combined with CNTs. 41 Researchers have studied the SWCNT for PTT for the treatment of metastatic breast cancer. 42
Carbon Nanohorns
CNHs (nanocones) mainly occur as a single-walled, horn-shaped structure. CNHs possess a diameter within the range of 2 to 5 nm, a length of around 40 to 50 nm, and an angle of ∼120°. 36 They often occur as circular clusters of 50 to 100 nm. A tumor site can be best accumulated by the EPR effect with a diameter in this size range. 43 Single-walled nanohorns are produced via arc discharge (AcD) or laser ablation (LA) utilizing pure graphite rods and catalysts devoid of metal, unlike single-walled nanotubes, which include metal impurities. 43 One more advantage of CNHs over CNTs is that they can be synthesized at room temperature. The remaining properties of CNHs show a resemblance to that of CNTs. NIR light can be absorbed by CNHs, which can have a significant photothermal impact. Their biological uses, particularly those in PTT, are improved by this trait. Whitney et al 44 have utilized the SWNHs as a temperature enhancer when irradiated with NIR light that causes the death of tumor cells. 45 CNHs have been used in conjunction with NIR irradiation to destroy cancer cells in vivo, proving the effectiveness of its PTT. 46 It is possible to eliminate tumors using PTT tumor ablation induced by CNHs without endangering healthy cells. Volunteers who received heat or CNH treatment experienced hair growth a week following treatment without tumor relapse. The safety of CNTs and CNHs in PTT and other medical applications was demonstrated by this investigation. 47
2-Dimensional Carbonaceous Nanomaterials
Due to their fragile structure, distinctive photoelectric capabilities, and larger surface area, 2D CNMs are currently being investigated as novel PTAs. 2D CNMs have shown substantial promise for photothermal-based synergistic strategies in addition to single PTT. 48 The benefits of 2D nanomaterials over other nanomaterials include the following. (i) The 2D nanomaterial's vast surface areas can accept guest molecules for synergetic treatment, such as PS, immunological adjuvants, and chemotherapeutic medicines, thanks to surface modification. (ii) The 2D nanomaterial's optical characteristics can be changed by altering the number of layers they contain or by integrating them with other metals showing surface plasmon resonance.49,50 These 2D nanomaterials are synthesized mainly by 2 major routes such as top-down and bottom-up. The top-down method involves breaking weak van der Waals forces between the layers of 2D nanomaterials. 51 In contrast, the bottom-up approach involves chemical reactions using proper starting materials to synthesize 2D CNMs. 48 One of the 2D CNMs widely used in PTT is graphene.
Graphene
Geim and Nosovelov first did isolation of graphene from graphite in 2004.52 It has a 2D carbon lattice with a sp2-hybridized hexagonal shape with unique electrical characteristics. It possesses a moderately high Young's modulus, high electric and thermal conductivity, and quicker electron mobility. Carboxyl graphene (GCOOH), graphene oxide (GO), and reduced graphene oxide (rGO) are the most common forms of graphene employed today.52,53 The Hummer technique is among the most widely used synthesis processes for producing graphene-derived products since it is highly affordable and easily scalable. GO can be reduced using hydrazine hydrate that gives rGO. Various techniques, such as XRD, FTIR spectroscopy, Raman scattering, scanning electron microscopy (SEM), etc, have been used to characterize the GO.
According to several studies, rGO has a high payload capacity and NIR absorption capability. To enhance the therapy of certain tumors, it could be chosen as a delivery mechanism to combine the PDT/PTT with chemotherapeutics. 54 In 1 such research study, scientists preloaded the rGO with doxorubicin (DOX). In addition to this, a PS (chlorin e6 [Ce6]) was added for the treatment of cancer. This study was performed on the 2D/3D cultured tumor cells (tumoroid/spheroid) and also in animal models. The graphene-based products were used with PTT/PDT and chemotherapy, and results showed that nanomaterials in combination with PTT are better than chemotherapeutics and PTT alone. 55
Approaches for Fabrication of Carbonaceous Nanomaterials
The recently found carbon nanodots (CNDs) are a type of CNMs that exhibits exceptional physical, chemical, and fluorescence properties, making them attractive for chemotherapeutics and diagnostics such as bioimaging, biosensing, and nanostructures for delivery systems pioneering bioactive molecules in PDT and PTT. For the objectives of synthesis, bottom-up and top-down methods may be used to classify the CNDs. Bottom-up approaches include thermal routes, microwave-assisted methods, and hydrothermal and aqueous-based methods. Top-down approaches include LA, electrochemical, and AcD methods. 56
Despite their similarities to quantum dots (QDs), CDs are formed from carbon rather than metal sulfides, metal oxides, etc, used to generate QDs.
The 2 prevalent synthetic techniques for CD synthesis are bottom-up and top-down. CNMs may be used in various top-down processes for cutting carbon materials, including plasma treatment, AcD, LA, and electrochemical procedures. Bottom-up approaches include reverse micelle processes, pyrolytic procedures, supported synthetic strategies, template techniques, chemical oxidation, microwave-based methods, and many others. Since green synthesis approaches will help reduce solid waste pollution, they are strongly recommended for synthesizing CDs. 57 To detect metronidazole, restricted access materials and molecularly imprinted polymers (RAM-MIP) were used to fabricate CDs by a custom-tailored approach. Advantages of the biomass CDs synthesized from longan peels with the help of high-pressure microwaves include their low toxicity, outstanding photostability, and minimal environmental impact. 58
Advanced magnetic CNMs have recently captured the attention of researchers due to their potential uses in various fields that benefited from the combination of nanoscale and magnetic features of CNMs. Drug delivery, carbon capture, adsorption, catalysis, cancer detection, biosensing, or energy and hydrogen storage are all possible applications for these materials. Ultrasonication, hydrothermal carbonization, chemical co-precipitation, and pyrolysis are all low-cost and time-efficient techniques for synthesizing magnetic CNMs. Each technique alters the final product differently and has distinct restrictions and drawbacks. 59
Researchers demonstrate a unique technique for producing graphene-encapsulated metal oxide (GE-MO) by co-assembly of positively charged oxide NPs and negatively charged GO. Electrostatic interaction between the 2 species triggers the reaction, which is further processed for chemical reduction. The resultant GE-MO has ultrathin graphene shells that are both flexible and able to encapsulate the oxide NPs completely. Using this novel hybrid architecture, the aggregation of oxide NPs can be avoided, which accounts for volume change during cycle processes, generates a high oxide content (∼91.5% by weight) in the composite, and keeps the electrode's overall electrical conductivity high. 60
Potential methodologies for producing functional nanoporous CNMs with required characteristics and morphologies include hard- and soft-templating procedures. Massive efforts have been made to comprehend the synthetic principles that profoundly affect material applications and design. All of these studies are important in encouraging the use of soft- and hard-templating techniques to produce nanoporous CNMs accurately. Various methods of fabrication for nanoporous CNMs are reviewed like a hard-templating synthesis of microporous carbon, fabrication of mesoporous carbon from mesoporous silica, fabrication of microporous carbon from zeolites, and soft-templating synthesis of mesoporous carbon. 61
Although LA and AcD are well-established procedures for producing practically flawless and high-quality nanotube structures, their manufacturing costs are prohibitively expensive due to scale-up restrictions. Impurities such as amorphous carbon, fullerenes, and catalyst particles are also formed during synthesis. Thus, purification is essential to remove the CNTs from contaminants. Some examples of CNT synthesis techniques include gas-phase catalytic growth from carbon monoxide, LA, AcD, and chemical vapor deposition from hydrocarbons. Traditional methods like in situ polymerization fabricated CNTs/polymer nanocomposites (PNCs), melt blending, and solution mixing. Advanced techniques for the fabrication of CNTs/PNCs were developed, such as the bulky paper-based approach and layer-by-layer route. 62
Covalently attaching medications or other therapeutic substances to the NPs can fabricate CNMs. DOX, a chemotherapeutic medication, was covalently attached to nanographene oxide (nGO), and this allowed the creation of a delivery system for drugs based on nGO. Compared to free DOX, DOX-nGO conjugates demonstrated improved medication delivery and cytotoxicity toward cancer cells. 63
Surface Modification of Carbonaceous Nanomaterials
Water dispersibility and biocompatibility must be considered while using nanomaterials for physiological purposes. Because of their interactions among CNMs and strong hydrophobic interactions, most CNMs (eg, CNTs, graphene, fullerenes, and CNHs) are insoluble in aqueous solutions, making them less attractive for biological applications. Relevant solutions for improving biocompatibility and water dispersibility must be devised to utilize these CNMs for biomedical applications. So far, 2 basic techniques for dispersing CNMs in an aqueous solution and making them physiologically friendly have been proposed, ie, surface modification by covalent and noncovalent methods. 4 Aligning and dispersing graphene and other CNMs in a polymer matrix is a formidable challenge because of the van der Waals force that causes the aggregation of CNMs. As a result, developing high-performance CNTs/graphene polymer composites with improved alignment and dispersion, as well as solid interfacial contacts, for improved load distribution all over the graphene/CNT polymer matrix interface is the primary concern. The surface modification of these CNMs is an excellent technique to prevent graphene and CNT aggregation, allowing them to stabilize and effectively distribute inside a polymer matrix. 64
Before selecting a suitable modification process for any CNMs, significant consideration must be given to the design and stability of the nanomaterial. Before any subsequent biological applications, equilibrium between structural integrity and stability must be maintained. Functionalization or modification methods are the most crucial aspects that might influence the eventual entrapment efficiency of imaging labels or genes or drugs. Surface modification by the covalent method can be chosen for materials not susceptible to structural changes, such as nGO, nanodiamonds, and CNTs. Still, other parameters like reagent availability, circulation half-life, and effective cargo release must be addressed. 65
Covalent-Based Surface Modification
Surface modification by the covalent functionalization method often entails including hydrophilic functional groups like amino groups, carboxyl groups, and hydroxyl groups to the gene or drug cargos, targeting ligands and protecting polymers like polyethylene glycol (PEG). Carboxyl groups can be generated through oxidation using agents like nitric acid. Aside from oxidation, another covalent method for functionalizing CNMs is the cycloaddition reaction, which uses the aromatic rings within the material structures as the reaction target. 66 The strong interactions ranging from infrared to terahertz frequencies between CNMs and low-frequency photons and extraordinary electronic properties are primarily attributable to the delocalized π–π system in CNMs. This endows them with exceptional NIR-mediated PCE. Covalent surface modification poses a risk to such decentralized networks of CNMs, which might reduce or eliminate their PCE and significantly impact their practical uses in phototherapy. CNMs functionalized by covalent modification methods are typically stable. Yet, this modification approach ultimately damages some material structures, resulting in the loss of certain inherent features like photothermal capacities. 65
Noncovalent-Based Surface Modification
Noncovalent modification, which includes coating the CNMs using amphiphilic compounds, has a milder reaction requirement than covalent modification. The amphiphilic molecules’ hydrophobic motifs may be attached to the surface of materials, with the hydrophilic ends protruding into the aqueous solution and preserving the materials' overall stability. van der Waals forces, hydrogen bonding, π–π interactions, and electrostatic forces are examples of noncovalent interactions. The biggest concern for this modification of noncovalent conjugates is reduced stability. Fast and simple direct absorption was observed via electrostatic interactions, but the generated conjugates exhibited less than ideal stability.65,67
Noncovalent surface modification of CNMs is possible by encapsulating proteins, DNA, polymers, surfactants, and viruses. π–π stacking to the surface of CNMs is a substantial property of water-soluble aromatic molecules, including porphyrin, pyrene, and phthalocyanine derivatives. CNMs have also been stabilized using biomacromolecules like proteins and DNA in addition to nonbiological components. Even noncovalent surface modification has certain shortcomings, like covalent surface modification. Even though noncovalent modifications do not disrupt the networks of CNMs, the resulting nanohybrids are not very stable.68,69
Because of their surface chemistry, size, and aggregation state, CNTs can cause fibrosis or inflammation. Coating these CNMs is 1 way to alter their surface characteristics and develop safer CNTs. For example, the surface of CNTs was coated with PF108, a nonionic triblock copolymer. Mitigated profibrogenic effects, decreased cellular absorption, decreased aggregation, and improved dispersion of CNTs were demonstrated due to the extended hydrophilic region included in this copolymer. Furthermore, these scientists showed that coating of PF108 mitigated CNT-induced toxicity in THP-1 cells and BEAS-2B and the lungs of mice. Protective coating with PF108 reduced CNT absorption by cells and prevented lysosomal membrane damage induced by CNTs in phagocytic THP-1 cells. Production of proinflammatory cytokines (IL-1) by THP-1 cells and production of profibrogenic transforming growth factor (TGF)-1 by BEAS-2B cells were also reduced. The protective role of the surface coating of PF108 against pulmonary fibrosis was further validated by in vivo tests, which showed that CNTs coated with PF108 decreased collagen synthesis and accumulation in the lungs compared to pristine CNTs.70,71
Carbonaceous Nanomaterials for Cancer Phototherapy
Carbon, with its 6 electrons, has unique qualities that permit it to establish covalent interactions with other carbon molecules, enhancing their complexity. 5 In the last 30 years, there has been a surge of interest in a low-dimensional class of carbon materials known as “CNMs” after the stunning introduction of fullerenes in 1985, followed by the creation of various CNMs with distinctive shapes, including graphene, CNTs, CNHs, and CQDs. 4 Their common feature is the presence of carbon atoms that are sp2-hybridized, which are often organized in hexagonal lattices. 72 The exceptional chemical and physical characteristics of CNMs have inspired their wide range of usage in biomedicine, including bioimaging, drug delivery, biosensing, tissue engineering, and phototherapy. CNMs provide many benefits because of their nanosize, hydrophilicity, and ideal diameters for maximum tumor uptake through the EPR effect. In cancer treatment, CNM-based phototherapy can be employed as a delivery mechanism. Several CNMs widely utilized in cancer therapy are summarized in Table 1.
Table 1.
Various Kinds of CNMs Utilized in the Cancer Phototherapy.
| Type of CNMs | Nanocarrier | Active constituent | Application | Inference | Ref. |
|---|---|---|---|---|---|
| Fullerenes | Nanodiamond composite | - | PDT | Enhanced survival time, cell apoptosis, and tumor shrinkage with no toxicity | Lee et al 97 |
| NPs | Antenna | PTT and PDT | Tumor growth inhibition via synergistic PTT and PDT | Shi et al 98 | |
| Micelles | DTX | Chemo-PDT | Increased antitumor effect with radiation as well as synergistic chemo-PDT | Guo et al 75 | |
| CNTs | Nanotubes | Annexin A5 | PTT | Tumor metastasis suppression with no toxicity | McKernan et al 83 |
| Nanotubes | Glycated chitosan | PTT | Highly efficient therapy for primary tumors | Li et al 99 | |
| Nanotubes | Spermine | PDT | Better photophysical properties as well as PDT shows lower cell viability | Ogbodu et al 100 | |
| CDs | Nanogel | Poly(N-isopropylacrylamide) | PTT and PDT | Efficient agent delivery with high therapeutic efficacy and lower adverse effects | Zhao et al 101 |
| NPs | DOX | Chemo-phototherapy | Delivery of DOX to tumor cells with radiation as well as synergistic chemo-phototherapy | Zhang et al 102 | |
| CNHs | - | IR808 | PTT and PDT | Cancer cells ablation and enzyme-responsive theranostic agents | Gao et al 45 |
| Zinc phthalocyanine | Photohyperthermia and PDT | Disappearance of tumors with laser radiation | Zhang et al 46 | ||
| GO | - | Folic acid, Ce6 | PTT and PDT | Excellent photothermal and photodynamic properties | Guo et al 103 |
| Nanocomposites | (4-Carboxybutyl)triphenylphosphonium bromide (TPP), ICG | PTT and PDT | Tumor progression inhibition with no toxicity as well as a higher anticancer effect | Zeng et al 104 | |
| Nanoflakes | DOX, folic acid | Chemo-PTT | Cost-efficient approach with excellent photothermal efficacy as well as targeted action | Mauro et al 105 | |
| Nanographene | NPs | PEG | PTT | Enhanced biocompatibility as well as physiochemical properties | Georgieva et al 106 |
| Nanoplatform | Hemin, Ce6 | PDT | High capability to suppress the tumor along with ROS production | Sahu et al 107 | |
| Nanoplatform | Methylene blue | PTT and PDT | More toxic to breast cancer cells as well as prevent metastasis | Dos Santos et al 108 | |
| Nanocomposite | PEG | PTT and PDT | Utilized as a theranostic medicine for cancer treatment | Kalluru et al 109 | |
| rGO | Nanocomposite | DOX | Chemo-PTT | Enhanced apoptosis of cells and decreased proliferation of cells | Dash et al 110 |
| Nanocomposite | Iron oxide | PTT | Lesser cell viability in cancer cells and antiapoptotic protein overexpression | Barrera et al 111 | |
| Nanoplatform | IR820, DOX | Chemo-PTT and PDT | Effective therapeutic effect with low DOX and high IR820 as well as higher cytotoxic effect with NIR | Zaharie-Butucel et al 112 | |
| GQDs | Liposomes | ICG | PTT | Excellent photothermal properties as well as reduced cell viability of cancer cells | Liu et al 113 |
| Nanocomplexes | DOX, siRNA | Chemo-photothermal gene therapy | Good therapeutic efficacy and low toxicity as well as controlled release of drugs and siRNA | Yang et al 114 | |
| QDs | Boron | PTT and PA agent | Tumor growth inhibition as well as outstanding PA agents | Guo et al 115 | |
| - | Bismuth sulfide | PTT and PDT | Utilized as a photoactive agent with outstanding biocompatibility | Yang et al 116 | |
| Nanomedicine | Copper(I) iodide, zinc stearate | PTT and PDT | Used as theranostic nanomedicine with good therapeutic efficiency as well as imaging potential | Lv et al 117 |
Abbreviations: CDs, carbon dots; Ce6, chlorin e6; CNHs, carbon nanohorns; CNMs, carbonaceous nanomaterials; CNTs, carbon nanotubes; DOX, doxorubicin; DTX, docetaxel; GO, graphene oxide; GQDs, graphene quantum dots; ICG, indocyanine green; NPs, nanoparticles; PA, photoacoustic; PDT, photodynamic therapy; PEG, polyethylene glycol; PTT, photothermal therapy; QDs, quantum dots; rGO, reduced graphene oxide; ROS, reactive oxygen species.
Fullerenes for Cancer Phototherapy
Fullerenes are spherical soccer ball–like structure that contains 60 atoms of carbon. They consist of fused rings as well as conjugated bonds with sp2 and sp3 bond hybridization. Due to their significant hydrophobicity, fullerenes have limited polar solvents and high solubility in organic solvents like chloroform, benzene, and toluene. 73 The small caged structure makes fullerene C60 highly reactive. Hydrophobicity, cohesion between fullerene molecules, photoactivity, reactivity, and the capacity to receive and release electrons are just some of the unique features that make fullerenes so versatile for biological applications. 74
Several researchers found that fullerenes show efficient results in cancer therapy. In 2014, 1 researcher group co-entrapped diadduct malonic acid-fullerene (DMA-C60) and docetaxel (DTX) in micelles for cancer photodynamic chemotherapy. The observed in vivo and in vitro characteristics of DTX-MC (Micelles) are greatly enhanced by DMA-C60 addition in terms of more excellent stability, reduced critical micellar concentration (CMC), long-lasting activity, and targeted dispersion. A promising prospect for the chemo-PDT combination for tumors is confirmed by the findings that the DMA-C60 addition under irradiation enhances cytotoxicity, influences cell cycle and death, and considerably enhances DTX-MC antitumor impact. 75
Guan et al 76 developed nanovesicles containing a trimalonate fullerene C70 derivative (TFC70) and a Photosensitizer (Chlorin e6) (FCNVs) for cancer therapy, which have proven to be both extremely effective and clearable phototheranostics, with improved tumor accumulation, in both imaging and treatment. Excellent loading efficacy of Ce6 (up to 57 wt%), effective absorption in the NIR light range, increased Ce6 cellular uptake efficacy in both in vitro and in vivo, high biocompatibility, and total clearance from the body are just some of the benefits of the created FCNVs. This discovery may prove helpful in advancing highly effective phototheranostic usage, utilizing nanostructures manufactured from fullerene derivatives.
Another group of researchers calculated the photothermal efficiency of polyhydroxy fullerenes based on the irradiation time of the laser and the concentration of the NPs. Polyhydroxy fullerenes were discovered to have a 69% PCE, and their photothermal reaction was observed to be stable with recurrent laser irradiation along with no alterations to the molecular structure. Polyhydroxy fullerenes are ideally suited for PTT because of their excellent photothermal efficacy and outstanding stability. 77
Carbon Nanotubes for Cancer Phototherapy
In 1991, Iijima 78 discovered CNTs. Because of the distinctive shapes and features of CNTs, such as higher aspect ratios, enormous surface areas, low density, nanometer-scale size stability, and extensive surface chemical functionality, they have attracted a lot of attention in biomedical sectors. They have a needlelike structure that helps them to enter the target cells. CNTs have shown great potential as nanocarriers for transporting bioactive molecules like proteins, drugs, and genes. 79 SWNTs and MWNTs are 2 types of CNTs that play essential roles in cancer research with distinct features based on the number of carbon atoms per sheet. 80
One research group developed hyaluronic acid–derived CNTs (HA-CNTs) for cancer therapy, which is also functionalized with hematoporphyrin monomethyl ether (HMME) for PDT. The therapeutic potential of cancer treatment was significantly increased by the capacity of HMME-HA-CNT nanostructures to merge localized selective PDT with exterior NIR PTT. This combination therapy proved that it has a synergistic impact and increased therapeutic efficacy with minimal disruption to healthy organs. 81
Xie et al 82 prepared a long-circulating drug delivery system based on SWCNTs, which can be used in conjunction with PDT and PTT to treat tumors effectively. ACEC is a multimodal Evan's blue (EB)/CNT-based theranostic system loaded with albumin/Ce6 with the potential to provide fluorescence and photoacoustic (PA) imaging of tumors to maximize the treatment window. This research introduces an SWCNT-based delivery system for combined PTT and PDT for treating tumors, which have shown tremendous promise in previous studies. When applied to other nanomaterials, EB’s functionalization of SWCNTs can increase their in vivo efficacy and circulation time.
In a similar line, researchers coupled SWCNTs with HA and underwent a coating of Ce6 on their walls to treat colon cancer. Due to their high surface area and strong interaction with various PS molecules, SWCNT-based nanobiocomposite systems demonstrate outstanding PS delivery. The current work revealed that exposure to SWCNT-HA-Ce6 in combination with PDT increased apoptosis of colorectal cancer.
Another research group named McKernan et al 83 introduced a new approach to treat metastatic breast cancer by combining PTT with targeting SWCNTs and checkpoint inhibitor immunostimulants. Low systemic doses of about 1.2 mg/kg of the SWCNT-ANXA5 (Annexin A5) nanoconjugate were administered to achieve tumor vasculature accumulation via ANXA5-dependent binding. Even though SWCNTs remained intact in organs for 4 months following the treatment, no adverse effects or apparent tissue toxicity were seen throughout the experiment.
Carbon Dots for Cancer Phototherapy
CNDs are 0D CNMs, ranging in size from 2 to 10 nm and exhibiting remarkable luminous capabilities. 84 In 2004, Xu et al, 85 while refining CNTs using electrophoresis, discovered CNDs by chance. Extremely considerable attention has been paid to CNDs in biomedical research because of their ease of production, rapid surface modification capacity, distinctive optical and physicochemical features, minimal cytotoxicity, and photoluminescence and fluorescence emission at specific excitation wavelengths. 86 Additionally, CNDs can serve as nanomaterials or PS in PDT due to their capacity to convert NIR energy into ROS. 87
One research group found that CDs could produce singlet oxygen (1O2), hydroxyl radical (OH . ), and heat using laser radiation of 635 nm with 73.5% PCE and 5.7% quantum yield for O2 generation. The lysosome, an excellent organelle for laser treatment due to its vital role in maintaining cellular stability and activity, can selectively accumulate CDs. Additionally, CDs have outstanding PA imaging capabilities as well as both one- and two-photon excited (OPE and TPE) fluorescence. The prepared CDs showed excellent biocompatibility and served as a multifunctional phototheranostic compound for PA/fluorescence imaging and PDT/PTT. 88 Lysine, sulfuric acid, and o-phenylenediamine were used as the starting materials for producing CDs doped with sulfur and nitrogen atoms (S, N-CDs) having absorption redshift effects. It was discovered that the therapeutic efficacy of S, N-CDs was higher than that of N-doped CDs, with 27% efficiency in creating 1O2 and 34.4% PCE. The research sheds new light on creating nanodrugs based on carbon for combinatorial phototherapy and precise tumor diagnostics. 89
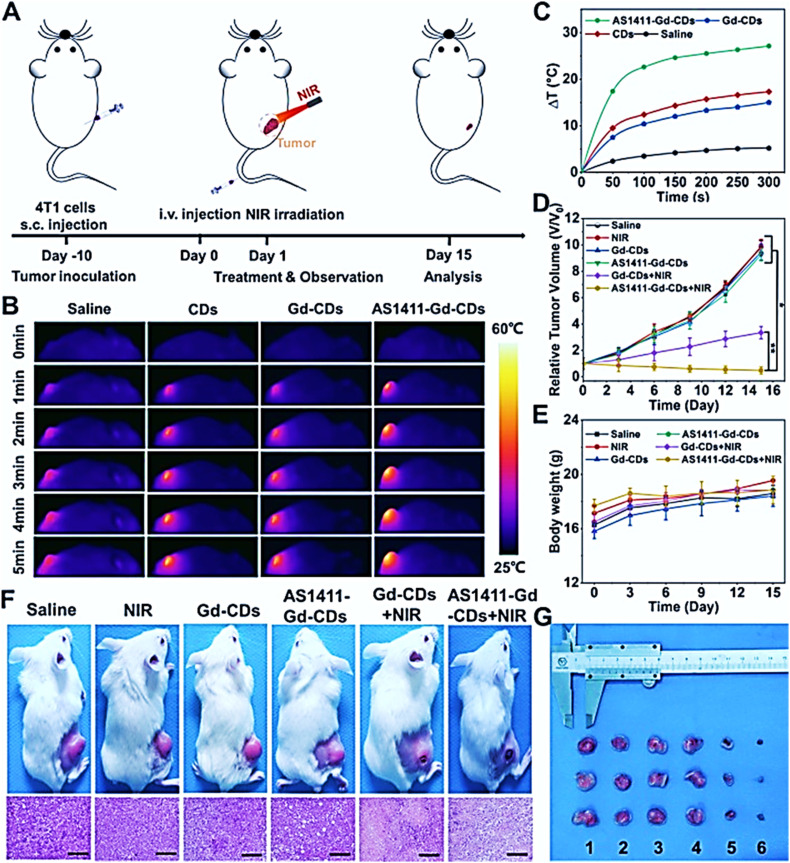
Further, Jiao et al 90 created CDs doped with gadolinium (Gd) using an easy solvothermal method conjugated with AS1411 aptamers for imaging-guided cancer phototherapy. AS1411-Gd-CDs displayed stable nanostructure, high biocompatibility, bright red fluorescence (FL) emission, notable magnetic resonance (MR) contrast, and high PCE. Moreover, AS1411-Gd-CDs showed substantial inhibition of tumors when imaged with FL/MR-guided imaging because of the powerful photothermal effect (Figure 3).
Figure 3.
Anticancer effect in vivo in 4T1 tumor–bearing mice. (A) Diagrammatic illustration of the design for animal experiments. (B) Thermal scans of the tumor-bearing nude mice after various injections under 808 nm NIR laser illumination for 5 min (808 nm, 1 W/cm2). (C) Temperature variations in the tumor region following 808 nm light irradiation at different injection rates. (D) Relative tumor sizes of the 4T1 tumor–bearing mice following different treatments for 14 days. (E) The change in body weight of mice over time in each group following various treatments. (F) Images of 4T1 tumor–bearing mice and the tumor's H&E staining at 14 days for different groups, including saline, NIR only, Gd-CDs, AS1411-Gd-CDs, Gd-CDs + NIR, and AS1411-Gd-CDs + NIR. (F) Images of tumors that were removed from each group on the 14th day following PTT. Saline is the first option, followed by NIR only, Gd-CDs, AS1411-Gd-CDs, Gd-CDs + NIR, and AS1411-Gd-CDs + NIR. Adapted with permission from Jiao et al 90 under a creative common license (https://creativecommons.org/licenses/by-nc/4.0/).
Abbreviations: CDs, carbon dots; Gd, gadolinium; NIR, near-infrared; PTT, photothermal therapy.
Carbon Nanohorns for Cancer Phototherapy
CNHs are nanostructures of horn shape having sp2-linked carbon atoms and are conceptually similar to the chemistry of CNTs. 91 Single-walled CNHs (SWNHs) are the most common type of CNHs, which have a tubular unit assembly with a diameter between 2 and 5 nm and a length between 40 and 50 nm. SWNHs’ aggregates can take on various shapes, including bud-like, dahlia-like, and seed-like forms. 65 Mainly, CNHs, which resemble dahlias, are widely utilized in nanooncology. These have a spherical architecture with an 80 to 100 nm diameter and are made up of almost 2000 tubular units. 92 Compared with SWNTs, SWNHs have various benefits, including better biocompatibility, a more uniform and predictable shape, simpler large-scale synthesis, and no metal contamination (often accepted as the leading cause of CNTs’ toxicity). 93
One study utilized a unique and straightforward 1-step technique to generate SWNH water-soluble and metal phthalocyanine (MPc) hybrids for PTT and PDT. The EPR study revealed that ROS are produced not only by the photoinduced electron transfer pathway from tetrasulfonic acid tetrasodium salt copper phthalocyanine (TSCuPc) to SWNHs but also via SWNHs that have not yet been aroused to their excited state by TSCuPc. The cell viability data from the in vitro study also showed that the combined PTT/PDT is noninvasive and considerably increased anticancer efficacy. They also suggest that the SWNH-TSCuPc nanohybrid is a promising and effective material for constructing an effective and biocompatible nanotool for biomedical use. 94
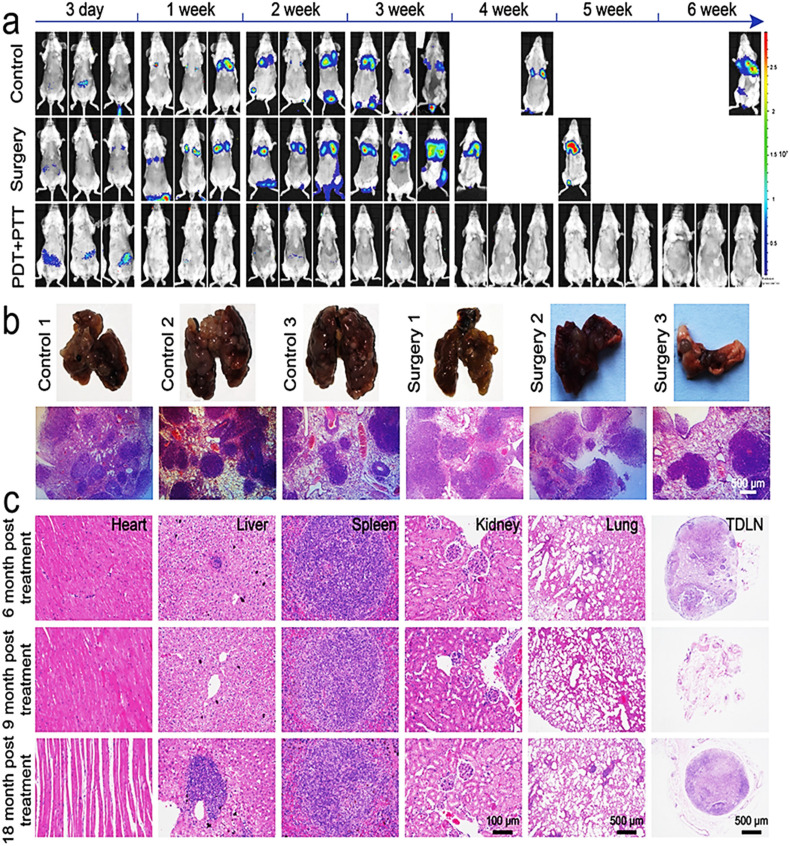
In a similar line, Yang et al 95 also developed SWNHs loaded with Gd3+ and Ce6 (Gd-Ce6@SWNHs), which were shown to be an effective immune adjuvant that can efficiently target and penetrate tumors. They established 3 in vivo cancer models of mice and discovered that sequential PDT and PTT using Gd-Ce6@SWNHs synergistically enhances antitumor immune responses where PDT stimulates CD80 and IFN-γ upregulation, and PTT activates dendritic cells (DCs) to secrete TNF-α and IL-6. This study shows that successive PDT and PTT utilizing Gd-Ce6@SWNHs in moderate circumstances produces synergistic and long-term antitumor immune responses, which is encouraging for patient treatment suffering from metastatic malignancies (Figure 4).
Figure 4.
The immunological memory actions created by Gd-Ce6@SWNHs in tumor-bearing mice after phototherapy. (A) Biofluorescence imaging with fluorescein-transfected 4T1 cells in cured mice (B) Analyzing pulmonary metastases in all categories. (C) In vivo H&E staining of primary organs as well as lymph nodes at 6, 9, and 18 months of fLuc-4T1 rechallenge to assess the long-term systemic toxicity of Gd-Ce6@SWNHs. Adapted with permission from Yang et al 95 under a creative common license (https://creativecommons.org/licenses/).
Abbreviations: Ce6, chlorin e6; Gd, gadolinium; SWNHs, single-walled CNHs.
Another research group developed a unique CNH/DNA/Pt NP nanoplatform relying on the clamped hybridization chain reaction (c-HCR) technique for Zn2+ imaging intracellularly and improved coordinated cancer cell phototherapy. The c-DNAzyme was fragmented after reaching living cells to trigger DNA in the presence of Zn2+ intracellularly and activate the c-HCR process for fluorescence amplification. Furthermore, this nanoprobe has significantly boosted the photodynamic effect of oxygen generation via Pt NPs in a TME. It enhances the CNH phototherapy effect by loading Pt NPs on it, suggesting that it is a potential biological technique for the diagnosis of cancer as well as cell therapy. 96
Graphene Oxide and Its Derivatized Compounds for Cancer Phototherapy
GO is a multilayered NP of graphene made from graphite that is structured irregularly or like a disk. 118 The majority of GO atoms are carbon, which provides them with outstanding biocompatibility as well as a nontoxic nature. 10 Carboxyl, carbonyl, epoxy, and hydroxyl are some of the functional groups with oxygen found on the edges and basal planes of GO, contributing to its high water solubility and adaptability to surface modification. 119 It is widely utilized in cancer treatment, and when exposed to NIR light, GO generates heat that can kill cancer cells. 120
To destroy cancerous cells with effective photothermal action, a 2D gold nanosheet hybrid templated with GO (GO@SiO2@AuNS) was developed. The GO@SiO2@AuNS hybrid demonstrated 30% PCE when irradiated with a low-power NIR laser, which resulted in a 16.4 °C rise in the temperature of the water. This GO@SiO2@AuNS hybrid can enhance the library of gold-based 2D nanomaterials for the PTT of cancer. 121 To increase GO's colloidal stability and phototherapeutic potential, it was first fabricated with an amphiphilic polymer named [2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide (SBMA) brushes and then IR780 was loaded into it. GO functionalized with SBMA was more colloidally stable in biological media, while GO not functionalized with SBMA quickly precipitated. SBMA-functionalized GO's NIR absorption was improved by adding IR780, contributing to 1.2 times more significant photothermal heating. The combination of NIR radiation and IR780-SPP/GO dropped 20% vitality of cancer cells, confirming the phototherapeutic potential of IR780-SPP/GOA. 120
A synergistic phototherapeutic agent composed of cationic porphyrin (TMePyP) with AuNRs embedded in a GO sheet is reported, which is focused on a blend of PDT and PTT. When exposed to light, the synthesized nanostructure generated more heat than the naked AuNRs while maintaining their stability in PBS and RPMI media. The nanostructure with good biocompatibility generated more 1O2 in the dark than TMePyP. Based on these findings, the synthesized nanostructure has tremendous potential as a dual photothermal and photodynamic agent for cancer treatment. 122
nGO is a graphene oxidative derivative with a 2D carbon layer as well as a large surface area, which is particularly gaining attraction due to its unique structure. 123 NIR absorption is a property of nGO due to its oxygen functional groups like epoxy, carboxyl, and hydroxyl. 124 Its capacity to adsorb various molecules via hydrophobic interactions or π–π stacking has led to its employment in cancer-related treatments, such as medication or delivery of protein. 125
PEGylated reduced nGO (rGOn-PEG) was synthesized from nGO via a single-step noncovalent chemical functionalization and thermal reduction method for its application in thermal ablation of nonmelanoma skin cancer. Compared with nGO, the absorbance of rGOn-PEG was ∼13 times greater in the NIR region. PEGylation of rGOn significantly increased its biocompatibility and its dispersibility in physiological solutions. The study found that irradiating rGOn-PEG with LEDs instead of high-power lasers offers significant advantages in terms of energy efficiency, cost, size, and safety for treating cancer by NIR mild hyperthermia. 126
Neelgund et al 123 developed a PTA by covalently conjugating folic acid with nGO, which was further coated with copper sulfide nanoflowers for cancer PTT. When exposed to light from a NIR laser, nGO-FA-CuS can rapidly raise their nucleus temperature, destroying genetic substances and ultimately leading to complete apoptosis. It also achieved a 46.2% photothermal efficacy when illuminated with a 980 nm laser and showed remarkable cytotoxicity in experiments with HeLa, KB, and SKOV3 cells. It was discovered that increasing the nGO-FA-CuS concentration and the incubation time increased its cytotoxicity toward cancer cells. One study discusses the eco-friendly strategy for creating laser-activatable nanosized colloids of graphene (nGC-CO-FA) for chemo-photothermal combination gene treatment of triple-negative breast cancer (TNBC). After being exposed to a NIR-808 laser, DOX was released from nGC-CO-FA/DOX/siRNA in a pH- and temperature-dependent manner. The NIR-808 nm laser was used in the combinatorial photo-chemo-gene therapy. It showed that it was more efficient than non-NIR-808 laser–treated counterparts, highlighting the critical significance of photothermal coupled gene therapy in TNBC treatment. 127
rGO is a 2D nanomaterial belonging to the graphene family, consisting of sp2-hybridized carbon atoms in thick layers organized in honeycomb-like structures. It is created by the reduction of GO via chemical, electrical, or thermal processes to remove the surface functional groups that contain oxygen. 128 rGO is a superb multifunctional nanostructure for PTT due to its simple synthesis, excellent water dispersibility, simple surface functionalization, and outstanding biocompatibility. 129
Polydopamine, rGO, and nano-zero-valent iron (nZVI) were combined to create a NP platform with targeting, imaging, and synergistic cancer phototherapy potential. When exposed to NIR irradiation (808 nm), even a minimal concentration of nZVI/rGO@pDA caused irreversible harm to MCF-7 cells without causing cytotoxic activity in normal cells. In MRI, nZVI/rGO@pDA showed high sensitivity comparable to nZVI@pDA at even low concentrations. The innovative material nZVI/rGO@pDA combines the benefits of pDA, nZVI, and rGO to provide unique targeting abilities, outstanding biocompatibility, phototherapeutics for cancer, and tumor imaging capabilities. 130 The use of iron hydroxide/oxide nanostructures anchored on rGO (FeOxH-rGO) as a treatment modality in the combination of PDT and PTT for cancer was also hypothesized. It was found that when the NIR wavelength of 808 nm is applied to FeOxH-rGO, high NIR absorption allows for a high rate of ROS production and light-to-heat conversion. In a mouse model with a breast tumor, NIR combined with FeOxH-rGO successfully ablated the tumor. Combining the benefits of FeOxH with rGO, the developed nanocomposites showed impressive tumoricidal ability. 131
To improve the PDT/PTT performance in vivo and in vitro, the rGO nanocomposite coated with mesoporous silica was synthesized that provides a high indocyanine green (ICG) encapsulation rate. After being exposed to NIR light, this ICG@MS-rGO-FA nanostructure selectively promoted CT-26 cell apoptosis by stimulating PTT and PDT actions, with no side effects on neighboring cells. In mice bearing the CT-26 tumor, the ICG@MS-rGO-FA nanocomposite was shown to have effective tumor targeting as well as biocompatibility, hence maximizing the therapeutic benefits of PDT and PTT in vivo. Phototherapy using this targeted ICG@MS-rGO-FA nanocomposite has a strong potential. 132
GQDs are tiny particles of GO with a size of <100 nm and a high concentration of O2-containing functional groups. 133 They are considered 0D nanomaterials due to their extremely small diameter and height of less than 10 graphene layers. 134 Lower toxicity, good chemical stability, and long-term resistance to photobleaching are just a few of the fascinating qualities demonstrated by GQDs.135,136 Several scientific disciplines have heavily emphasized GQDs because of their potential applications in catalysis, sensing, bioimaging, and PDT.137,138
A stable hybrid of GO and GQDs was produced using electrostatic layer-by-layer assembling via the bridge of polyethylene imine (GO-PEI-GQDs). When exposed to a NIR-808 nm laser (0.5 W/cm2) for 5 min at concentrations ∼50 µg/mL, GO-PEI-GQDs exhibited an outstanding photothermal response (44 °C-49 °C). Novel synergistic features of GO-PEI-GQDs are reported, including persistent fluorescence imaging and increased cytotoxic and photothermal actions on cancer cells. 139
Considering this, Liu et al 140 created a 1-step solvothermal approach employing phenol as a single precursor to synthesize GQDs with strong absorbance (1070 nm) in the NIR-II region by adjusting the breakdown of H2O2 in a 9 T magnetic field. It has been shown that 9T-GQDs, when exposed to NIR-II irradiation, may effectively cause ablation of tumor cells and suppress tumor growth in vitro and in vivo, respectively. The improved NIR imaging of tumors in living mice employing 9T-GQDs indicates a promising future for their use in NIR imaging–guided PTT in the NIR-II window. A novel method for PS production is described, in which GQDs are modified to form a core–shell structure on the surface of upconversion NPs (UCNPs) doped with rare earth elements. It efficiently generates ROS when excited by NIR light to initiate the PDT process. Exposure to PDT using UCNPs@GQDs revealed new insights into developing nanomaterials based on PDT, as they demonstrated both good biocompatibility and concentration-dependent PDT efficacy. 141
Quantum Dots for Cancer Phototherapy
Fluorescent NPs called QDs are renowned for their excellent optical characteristics, including photostability, strong fluorescence emission, a narrow spectrum of emission, and a broad excitation wavelength. 142 The advantages of QDs, including their ultrathin structure, large specified surface area, distinctive photoelectric properties, and excellent biological safety, have drawn much attention.143,144 Numerous unique QDs, including those made of GO, bismuth, black phosphorus, and antimonide, have been successfully synthesized and utilized in biomedical applications. 115
One group of researchers developed QDs in which they conjugated magneto-fluorescent FeN@CQDs with riboflavin and folic acid. Further, they incorporated an anticancer drug named DOX for the efficient treatment of cancer. It was found that GP-Rf-FA-FeN@CQD-DOX displayed NIR absorbance, strong photothermal efficiency, targeted accumulation at tumor regions, and great biocompatibility. Experiments show that the formulation, so designed, may transport anticancer medications to tumor cells, where they are released intracellularly in response to NIR irradiation, allowing for a combined chemo-phototherapy action that can successfully eradicate tumors. The synergistic therapeutic activity of GP-Rf-FA-FeN@CQD-DOX was confirmed by in vitro and in vivo studies, and the combination was also shown to destroy tumors without regrowth. 102
Xie et al 145 synthesized Bi2O2Se QDs via a top-down method for PTT of cancer. Bi2O2Se QDs are shown to be highly effective at both PA and PTT, as shown by in vivo and in vitro evaluations. Bi2O2Se QDs passively accumulate in tumors after systemic treatment, allowing for effective PA imaging of the whole tumors to provide imaging-guided PTT without apparent toxicity. The results demonstrate the promising future of Bi2O2Se QDs as a biodegradable multimodal agent in medicinal settings. Depending on dual-modality tumor imaging and combinatorial chemo-phototherapy, a straightforward yet potent nanoprobe (H-MnO2/DOX/BPQDs) was built for precise diagnosis and successful tumor treatment. It has been found that nanoprobes break down in the acidic microenvironment of tumors, releasing DOX/BPQDs. To achieve a synergistic therapeutic effect, DOX chemotherapy was coupled with PDT and PTT. In the end, the dual-modality imaging–supported coordinated treatment approach increased the therapeutic potential of cancer and the cancer patients’ rate of survival. 146
Biosafety and Toxicity of Carbonaceous Nanomaterials
Nanotechnology and nanomaterials hold great promise for enhancing the diagnosis, treatment, and monitoring of a wide range of diseases. According to various studies, using nanotechnology in the medical field improves the stability, solubility, biocompatibility, targeting, controllability, and permeability of drugs and vaccines. 147 Despite the growing attention and efforts being put into producing and utilizing nanomaterials, there are growing concerns regarding the possible toxicity of these materials. In fact, only a few nanomedicines are approved for clinical use.148,149
It is still not entirely clear how NPs behave after they enter the human body, including their interaction with biomacromolecules. More queries including the pattern of their accumulation, breakdown, and excretion from the body need detailed crosstalk. It is also a need of the hour to clearly delineate how various organs, tissues, and the circulatory system get affected by them. It has been hypothesized that nanomaterials can harm cells because they generate ROS, leading to DNA damage, protein denaturation, and lipid peroxidation.150–152
Additionally, there is a possibility that proteins and nucleic acids will bind to NPs when they enter the cells, which would disrupt metabolic processes and ultimately result in the death of the cells. This is an additional risk associated with the introduction of NPs.153–158 As interest in nanomaterials and concern over their biological effects and safety increase, more research is being performed in these fields. In the near future, it is anticipated that NP-relevant sectors will develop and adopt a systematic and precise procedure for assessing the safety of NPs.
Biosafety Assessment of Carbonaceous Nanoparticles
It is difficult to determine the toxicity of carbonaceous NPs. The behavior of these particles is significantly influenced by their surface quality, the presence of graphite metal catalysts, their dispersion characteristics, and their propensity to deposit as aggregates due to robust van der Waals forces. Additionally, variances between species make it difficult to generalize studies from 1 species to another. This is because some animals, such as rats, are more sensitive to particles, which makes it more challenging to apply the studies to humans. 158
Biosafety Assessment of Carbon Dots
It becomes clear that doing a CD-related biosafety assessment is the greatest priority. Even if carbon is believed to be non- or low-toxic, it is essential to undertake a comprehensive and rigorous study of nanomaterials’ biological safety. Many breakthrough investigations on the in vitro and in vivo behavior of CDs have been conducted. These studies took into account the aspects such as cell viability, biodegradability, pharmacokinetics, and biodistribution in the organs. These studies showed that CDs are biocompatible. In 2009, Yang et al performed trypan blue and methyl-thiazolyl diphenyltetrazolium bromide (MTT) experiments to examine the in vitro cytotoxicity of PEG1500N-passivated CDs. 159
In their investigation, human colorectal adenocarcinoma HT-29 cells and human breast cancer MCF-7 were co-cultured with CDs in which more than 80% cell viability was observed at the dosage of 0.1 mg/mL. Tao et al studied the in vivo cytotoxicity of CDs generated from SWCNTs, MWCNTs, and graphite in female BALB/c mice using mixed-acid treatment techniques in 2011. 160 They discovered that no mortality nor substantial loss in body weight was recorded in mice injected with CDs at a dosage of 20 mg/kg over 3 months, indicating that CDs had no apparent harmful effect on the animals.
Biosafety Assessment of Carbon Nanotubes
The cytotoxicity of CNTs is highly reliant on the method of their surface functionalization as well as the characteristics of capping molecules. Despite extensive sonication, SWCNTs and MWCNTs tend to form tight aggregates and bundles when used alone, and after only 6 h of incubation, alveolar macrophages exhibit considerable cytotoxicity. 161 An increase in ROS, which is an indicator of oxidative stress, and the activation of nuclear transcription factor-kB (NF-kB), which occurs in response to harmful cellular stimuli, have been observed after human keratinocytes have been treated with pristine SWCNTs dispersed in dimethylformamide without any surface functionalization. 162
Biosafety Assessment of Graphene and Its Derivatives
According to several in-depth research, pristine GO and rGO with fewer surface functional groups are more hazardous than GO. The number of studies published on graphene and its composites concerning toxicities is regrettably a lot lower than the number of studies published on CNTs. 163 The characteristics of graphene and its derivatives can vary greatly depending on the production method, starting material, and other variables. Numerous studies have been conducted with graphene and its derivatives, focusing on a wide range of properties, such as particle state, oxygen availability, defect, surface chemistry, and structure.
Zhang et al 164 demonstrated that graphene and SWCNTs had cytotoxic effects on neural pheochromocytoma-derived PC12 cells by examining their effects individually and in combination with their level of metabolic activity, the release of LDH, changes in morphology, levels of ROS, and the expression of caspase 3. They proved that the SWCNT in needle form damages membranes more than graphene's planar structure. The cytotoxic effects of graphene vary between standard and immortalized cells, as described by Lee et al. 165 However, they also demonstrated that graphene's cytotoxicity is induced by the production of intracellular ROS, which leads to oxidative stress and death and depends on its concentration, exposure period, size, shape, etc.
Current Regulatory and Market Status of Carbonaceous Nanomaterials
The following are some of the most recent factors that are driving the global market for nanomaterials: an increase in the market penetration of already existing materials; a decrease in the prices of nanomaterials; an improvement in the properties of materials; an expenditure of Research and Development (R&D) activities related to the development of new materials; an increase in the amount of money spent on nanotechnology research by both the public and private sectors; growing support from government institutions; and the rapid development of new materials and applications.166–168 As a consequence of the positive influence, the factors mentioned above have led to the development of the global nanomaterials industry. The primary market indices have been consistently growing over the past few years.
It was estimated that the global market for nanomaterials would be worth approximately US$4.1 billion in 2015, and it is expected to reach US$11.3 billion in the year 2020, growing at a compound annual growth rate (CAGR) of over 22% throughout 2017 to 2022. According to Allied Market Research, the global market for nanomaterials will rise at a CAGR of 20.7% from US$14.7 billion in 2015 to more than US$55.0 billion by 2022. However, Deloitte Touche Tohmatsu Limited specialists are far less optimistic, forecasting a CAGR of 15.5% for the global nanomaterials market between 2012 and 2019. In 2015, the European market for nanomaterials generated revenue greater than US$2.5 billion. It is anticipated that this figure will increase to US$9.1 billion by 2022 at a CAGR of 20.0% from 2016 to 2022. 169
The following organizations in the United States and the European Union are considered to be the most prominent regulatory authorities associated with nanomaterials:
The United States Environmental Protection Agency (EPA)
The European Chemicals Agency (ECHA)
Clinical Trials of Carbonaceous Nanomaterials
Research that studies and analyses the effects of novel medical procedures, tests, and medicines on human health outcomes is called a clinical trial. Clinical trials are conducted on volunteers to test a variety of medical interventions, such as medications, cells, and other biological products; surgical and radiological techniques; devices; behavioral therapies; and preventive care. The following Table 2 indicates clinical trials of different CNMs.
Table 2.
Clinical Trials on Various CNMs.
| Title of study | ID | Study type | Intervention | .Phase | Recruitment status | No. of participants | Sponsor |
|---|---|---|---|---|---|---|---|
| Clinical study on the harvesting lymph nodes with carbon nanoparticles for advanced gastric cancer | NCT02123407 | Interventional | Carbon NPs | Phase 3 | Unknown | 30 | Peking University |
| Potential role for carbon nanoparticles to guide central neck dissection in patients with papillary thyroid cancer | NCT02724176 | Interventional | Carbon NPs | Not applicable | Completed | 140 | Peking University Cancer Hospital & Institute |
| Topical fluorescent nanoparticles conjugated somatostatin analog for suppression and bioimaging breast cancer | NCT04138342 | Interventional | QDs coated with veldoreotide | Phase 1 | Recruiting | 30 | Al-Azhar University |
| Targeted biopsy of carbon nanoparticles labelled axillary node for cN+ breast cancer | NCT04482803 | Interventional | Carbon NP suspension injection | Not applicable | Recruiting | 100 | The First Affiliated Hospital with Nanjing Medical University |
| Carbon nanoparticles as lymph node tracer in rectal cancer after neoadjuvant radio chemotherapy (CALOR-NAT) | NCT03550001 | Interventional | Injection of CNP before NAT | Not applicable | Not yet recruiting | 252 | YE Yingjiang |
| Effect of CNSI vs. ICG in lymph node tracing during gastrectomy (FUTURE-01) | NCT05229874 | Interventional | Carbon NPs and ICG | Phase 2 | Recruiting | 96 | Hebei Medical University |
| Carbon nanoparticles and indocyanine green for sentinel lymph node biopsy in early-stage cervical cancer | NCT05167149 | Interventional | Carbon NPs | Not applicable | Not yet recruiting | 144 | Cancer Institute and Hospital, Chinese Academy of Medical Sciences |
Marketed Status of Carbonaceous Nanomaterials
The annual growth rate of CNTs was over 30% from 2004 to 2008; however, due to the global financial crisis, this rate fell in 2009 and 2010. On the other hand, around a 34% annual growth is anticipated. 170 The total value of the global CNT market was approximately US$100 million in 2009 and increased to approximately US$159 million in 2014. Despite this, the global market for CNTs continued to expand between 2004 and 2008, and from its current level of US$3.95 billion in 2017, it is expected to rise to around US$9.84 billion by the year 2023. This means that between 2018 and 2023, the CAGR will be about 16.7% of the CNT market in 2021.
Conclusion
Phototherapy has benefits over traditional treatments like radiotherapy and chemotherapy. It is economical, reduces the use of many peripheral lesions, and has high safety and selectivity. CDs, fullerenes, and GQDs have intense PS activity via ROS production, making them ideal for PDT. The generation of heat locally by NIR absorption in CNTs, CNHs, and graphene-based materials gives them great promise for PTT, which can then be used to ablate cancer cells thermally. Even though this sector has made numerous vital advancements, there are still several issues that need to be resolved to encourage their widespread clinical implementation. Before converting CNMs into clinical phototherapeutic uses, the biosafety of those compounds needs to be carefully assessed further. Most of the CNMs have certain limitations like toxicity, formation of agglomerates, stability as well as biodegradability issues, and lack of standardization, which can be overcome by surface modification, functionalization, and encapsulation. In the future, we can explore new functionalization and surface modification moiety, which will help these CNMs to reach the market without any toxicity issues. Future research should be done carefully to determine the surface modification effect of CNMs as well as phototherapies on the in vivo and in vitro immunity after phototherapy.
Most systems based on CNMs need to integrate components (such as PTT agents, PS agents, and hydrophilic moiety) to achieve combined phototherapies, which necessitates time-consuming synthetic processes but also runs the risk of unpredictable collaborative interference between the elements. The maximum depth of penetration for NIR laser irradiation is 0.5 cm. The need for deep tumor penetration cannot be adequately met by phototherapies mediated with NIR. It is essential to concentrate on alternative combination strategies. Resolving such obstacles will open up new possibilities for creating phototherapeutic systems based on CNMs. Due to the material's intrinsic benefits, including strong NIR absorption, broad surface area, and adequate size, it is expected that CNM-based phototherapies will play highly significant roles in the field of cancer theranostics in the coming years.
Acknowledgments
We acknowledge the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, India, for supporting our research on different ailments at NIPER Ahmedabad.
Abbreviations
- 0D
0-dimensional
- 1D
1-dimensional
- 2D
2-dimensional
- CDs
carbon dots
- CNHs
carbon nanohorns
- CNMs
carbonaceous nanomaterials
- CNTs
carbon nanotubes
- CQDs
carbon quantum dots
- EPR
enhanced permeability and retention
- GO
graphene oxide
- GQDs
graphene quantum dots
- LEDs
light-emitting diodes
- MWCNTs
multiwalled carbon nanotubes
- nGO
nanographene oxide
- NIR
near-infrared
- PBM
photobiomodulation
- PCE
photothermal conversion efficiency
- PDT
photodynamic therapy
- PS
photosensitizer
- PTA
photothermal agent
- PTT
photothermal therapy
- QDs
quantum dots
- ROS
reactive oxygen species
- rGO
reduced graphene oxide
- SWCNTs
single-walled carbon nanotubes.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RKT would like to acknowledge the Science and Engineering Research Board (Statutory Body Established through an Act of Parliament: SERB Act 2008), Department of Science and Technology, Government of India, for a grant (Grant no. ECR/2016/001964), and Core Research Grant funding (File Number: CRG/2021/005402) in Dr Tekade’s Laboratory. RKT would also like to acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for grant file ID: 2021-14161, as well as for the senior research fellowship grant (IRIS Cell No. 2019-4009; File No-5/3/8/33/ITR-F/2018-ITR) to support PhD work of Vishakha Tambe to develop cancer targeted nanoformulation in Dr Tekade’s Laboratory at National Institute of Pharmaceutical Education and Research-Ahmedabad (NIPER-A).
ORCID iD: Rakesh Kumar Tekade https://orcid.org/0000-0002-3024-3148
References
- 1.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751-760. doi: 10.1038/nnano.2007.387 [DOI] [PubMed] [Google Scholar]
- 2.Gürbüz B, Ayan S, Bozlar M, Üstündağ CB. Carbonaceous nanomaterials for phototherapy: a review. Emergent Materials. 2020;3(4):479-502. doi: 10.1007/s42247-020-00118-w [DOI] [Google Scholar]
- 3.Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chemical Reviews. 2014;114(21):10869-10939. doi: 10.1021/cr400532z [DOI] [PubMed] [Google Scholar]
- 4.Jiang B-P, Zhou B, Lin Z, Liang H, Shen X-C. Recent advances in carbon nanomaterials for cancer phototherapy. Chemistry – A European Journal. 2019;25(16):3993-4004. doi: 10.1002/chem.201804383 [DOI] [PubMed] [Google Scholar]
- 5.Amaral SI, Costa-Almeida R, Gonçalves IC, Magalhães FD, Pinto AM. Carbon nanomaterials for phototherapy of cancer and microbial infections. Carbon N Y. 2022;190:194-244. doi: 10.1016/j.carbon.2021.12.084 [DOI] [Google Scholar]
- 6.Hong G, Diao S, Antaris AL, Dai H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem Rev. 2015;115(19):10816-10906. doi: 10.1021/acs.chemrev.5b00008 [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Huang J, Song S, Chen H, Zhang Z. Cancer-targeted nanotheranostics: recent advances and perspectives. Small (Weinheim an der Bergstrasse, Germany). 2016;12(36):4936-4954. doi: 10.1002/smll.201600635 [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Wang Y, Liu J, Zhai G. Recent developments of phototherapy based on graphene family nanomaterials. Curr Med Chem. 2017;24(3):268-291. doi: 10.2174/0929867323666161019141817 [DOI] [PubMed] [Google Scholar]
- 9.Wagner WR, Sakiyama-Elbert SE, Zhang G, Yaszemski MJ. Biomaterials Science: An Introduction to Materials in Medicine. Academic Press; 2020. [Google Scholar]
- 10.Patel SC, Lee S, Lalwani G, Suhrland C, Chowdhury SM, Sitharaman B. Graphene-based platforms for cancer therapeutics. Ther Delivery. 2016;7(2):101-116. doi: 10.4155/tde.15.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657-674. [DOI] [PubMed] [Google Scholar]
- 12.Bjordal JM, Lopes-Martins RAB, Joensen J, et al. A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskelet Disord. 2008;9(1):75. doi: 10.1186/1471-2474-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundaram P, Abrahamse H. Phototherapy combined with carbon nanomaterials (1D and 2D) and their applications in cancer therapy. Materials (Basel, Switzerland). 2020;13(21):4830. doi: 10.3390/ma13214830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezende SB, Ribeiro MS, Núñez SC, Garcia VG, Maldonado EP. Effects of a single near-infrared laser treatment on cutaneous wound healing: biometrical and histological study in rats. J Photochem Photobiol, B. 2007;87(3):145-153. doi: 10.1016/j.jphotobiol.2007.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Frigo L, Fávero GM, Lima HJC, et al. Low-level laser irradiation (InGaAlP-660 nm) increases fibroblast cell proliferation and reduces cell death in a dose-dependent manner. Photomed Laser Surg. 2009;28(S1):S-151–S-156. doi: 10.1089/pho.2008.2475 [DOI] [PubMed] [Google Scholar]
- 16.Saygun I, Nizam N, Ural AU, Serdar MA, Avcu F, Tözüm TF. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed Laser Surg. 2012;30(3):149-154. doi: 10.1089/pho.2011.3079 [DOI] [PubMed] [Google Scholar]
- 17.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128(6):2115-2120. doi: 10.1021/ja057254a [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Black KC, Luehmann H, et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano. 2013;7(3):2068-2077. doi: 10.1021/nn304332s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou R, Zhang M, Xi J, et al. Gold nanorods-based photothermal therapy: interactions between biostructure, nanomaterial, and near-infrared irradiation. Nanoscale Res Lett. 2022;17(1):68. doi: 10.1186/s11671-022-03706-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Ning C, Zhou Z, et al. Nanomaterials as photothermal therapeutic agents. Prog Mater Sci. 2019;99:1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovell JF, Jin CS, Huynh E, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 2011;10(4):324-332. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto Y, Nichols JW, Toh K, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11(6):533-538. [DOI] [PubMed] [Google Scholar]
- 23.Han HS, Choi KY. Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines. 2021;9(3):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22(2):263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40(2):253-266. [DOI] [PubMed] [Google Scholar]
- 26.Wilson BC, Weersink RA. The yin and yang of PDT and PTT. Photochem Photobiol. 2020;96(2):219-231. [DOI] [PubMed] [Google Scholar]
- 27.Han HS, Choi KY, Lee H, et al. Gold-nanoclustered hyaluronan nano-assemblies for photothermally maneuvered photodynamic tumor ablation. ACS Nano. 2016;10(12):10858-10868. [DOI] [PubMed] [Google Scholar]
- 28.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2(12):751-760. [DOI] [PubMed] [Google Scholar]
- 29.Sundaram P, Abrahamse H. Phototherapy combined with carbon nanomaterials (1D and 2D) and their applications in cancer therapy. Materials (Basel). 2020;13(21):4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajani C, Patel V, Borisa P, et al. Photothermal therapy as emerging combinatorial therapeutic approach. In: The Future of Pharmaceutical Product Development and Research. Elsevier; 2020:297-339. [Google Scholar]
- 31.Gormley AJ, Greish K, Ray A, Robinson R, Gustafson JA, Ghandehari H. Gold nanorod mediated plasmonic photothermal therapy: a tool to enhance macromolecular delivery. Int J Pharm. 2011;415(1-2):315-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fay BL, Melamed JR, Day ES. Nanoshell-mediated photothermal therapy can enhance chemotherapy in inflammatory breast cancer cells. Int J Nanomed. 2015;10:6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.dos Santos A, De Almeida DRQ, Terra LF, Baptista M, Labriola L. Photodynamic therapy in cancer treatment-an update review. Journal of Cancer Metastasis and Treatment. 2019;5(25):25. [Google Scholar]
- 34.Hui YY, Chang H-C, Dong H, Zhang X. Carbon Nanomaterials for Bioimaging, Bioanalysis, and Therapy. John Wiley & Sons; 2019. [Google Scholar]
- 35.Georgakilas V, Perman JA, Tucek J, Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev. 2015;115(11):4744-4822. [DOI] [PubMed] [Google Scholar]
- 36.Jiang BP, Zhou B, Lin Z, Liang H, Shen XC. Recent advances in carbon nanomaterials for cancer phototherapy. Chemistry–A European Journal. 2019;25(16):3993-4004. [DOI] [PubMed] [Google Scholar]
- 37.Wo F, Xu R, Shao Y, et al. A multimodal system with synergistic effects of magneto-mechanical, photothermal, photodynamic and chemo therapies of cancer in graphene-quantum dot-coated hollow magnetic nanospheres. Theranostics. 2016;6(4):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao S, Yan L, Cao M, et al. Near-infrared light-triggered lysosome-targetable carbon dots for photothermal therapy of cancer. ACS Appl Mater Interfaces. 2021;13(45):53610-53617. [DOI] [PubMed] [Google Scholar]
- 39.Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43(3):744-764. [DOI] [PubMed] [Google Scholar]
- 40.Hou P, Bai S, Yang Q, Liu C, Cheng H. Multi-step purification of carbon nanotubes. Carbon N Y. 2002;40(1):81-85. [Google Scholar]
- 41.Wang L, Shi J, Liu R, et al. Photodynamic effect of functionalized single-walled carbon nanotubes: a potential sensitizer for photodynamic therapy. Nanoscale. 2014;6(9):4642-4651. [DOI] [PubMed] [Google Scholar]
- 42.McKernan P, Virani NA, Faria GN, et al. Targeted single-walled carbon nanotubes for photothermal therapy combined with immune checkpoint inhibition for the treatment of metastatic breast cancer. Nanoscale Res Lett. 2021;16(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karousis N, Suarez-Martinez I, Ewels CP, Tagmatarchis N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem Rev. 2016;116(8):4850-4883. [DOI] [PubMed] [Google Scholar]
- 44.Whitney JR, Sarkar S, Zhang J, et al. Single walled carbon nanohorns as photothermal cancer agents. Lasers Surg Med. 2011;43(1):43-51. [DOI] [PubMed] [Google Scholar]
- 45.Gao C, Jian J, Luo L, et al. Single-walled carbon nanohorns-based smart nanotheranostic: from phototherapy to enzyme-activated fluorescence imaging-guided photodynamic therapy. J Colloid Interface Sci. 2022;628(14):273-286. doi: 10.1016/j.jcis.2022.07.168 [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Murakami T, Ajima K, et al. Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proc Natl Acad Sci USA. 2008;105(39):14773-14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen D, Wang C, Nie X, et al. Photoacoustic imaging guided near-infrared photothermal therapy using highly water-dispersible single-walled carbon nanohorns as theranostic agents. Adv Funct Mater. 2014;24(42):6621-6628. [Google Scholar]
- 48.Liu S, Pan X, Liu H. Two-dimensional nanomaterials for photothermal therapy. Angew Chem. 2020;132(15):5943-5953. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Kim J, Jin C, et al. Direct observation of the layer-dependent electronic structure in phosphorene. Nat Nanotechnol. 2017;12(1):21-25. [DOI] [PubMed] [Google Scholar]
- 50.Peng B, Ang PK, Loh KP. Two-dimensional dichalcogenides for light-harvesting applications. Nano Today. 2015;10(2):128-137. [Google Scholar]
- 51.Zhang H. Ultrathin two-dimensional nanomaterials. ACS Nano. 2015;9(10):9451-9469. [DOI] [PubMed] [Google Scholar]
- 52.Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666-669. [DOI] [PubMed] [Google Scholar]
- 53.Gadeval A, Chaudhari S, Bollampally SP, et al. Integrated nanomaterials for non-invasive photothermal therapy of rheumatoid arthritis. Drug Discov Today. 2021;26(10):2315-2328. [DOI] [PubMed] [Google Scholar]
- 54.Gadeval A, Maheshwari R, Raval N, Kalyane D, Kalia K, Tekade RK. Green graphene nanoplates for combined photo-chemo-thermal therapy of triple-negative breast cancer. Nanomedicine : Nanotechnology, Biology, and Medicine. 2020;15(06):581-601. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Liu K, Feng L, Liu Z, Xu L. Comparison of nanomedicine-based chemotherapy, photodynamic therapy and photothermal therapy using reduced graphene oxide for the model system. Biomater Sci. 2017;5(2):331-340. [DOI] [PubMed] [Google Scholar]
- 56.Zaidi Z, Maiti N, Ali I, et al. Fabrication, characteristics, and therapeutic applications of carbon-based nanodots. J Nanomater. 2022;2022:1-12. doi: 10.1155/2022/8031495 [DOI] [Google Scholar]
- 57.Khayal A, Dawane V, Amin MA, et al. Advances in the methods for the synthesis of carbon dots and their emerging applications. Polymers (Basel). 2021;13(18):3190. doi: 10.3390/polym13183190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu H, Ding J, Zhang K, Ding L. Fabrication of carbon dots@restricted access molecularly imprinted polymers for selective detection of metronidazole in serum. Talanta. 2020;209:120508. doi: 10.1016/j.talanta.2019.120508 [DOI] [PubMed] [Google Scholar]
- 59.Siddiqui MTH, Nizamuddin S, Baloch HA, et al. Fabrication of advance magnetic carbon nano-materials and their potential applications: a review. Journal of Environmental Chemical Engineering. 2019;7(1):102812. doi: 10.1016/j.jece.2018.102812 [DOI] [Google Scholar]
- 60.Yang S, Feng X, Ivanovici S, Müllen K. Fabrication of graphene-encapsulated oxide nanoparticles: towards high-performance anode materials for lithium storage. Angew Chem, Int Ed. 2010;49(45):8408-8411. doi: 10.1002/anie.201003485 [DOI] [PubMed] [Google Scholar]
- 61.Malgras V, Tang J, Wang J, et al. Fabrication of nanoporous carbon materials with hard- and soft-templating approaches: a review. J Nanosci Nanotechnol. 2019;19(7):3673-3685. doi: 10.1166/jnn.2019.16745 [DOI] [PubMed] [Google Scholar]
- 62.Gupta TK, Kumar S. Fabrication of carbon nanotube/polymer nanocomposites. In: Rafiee R, ed. Carbon Nanotube-Reinforced Polymers. Elsevier; 2018:61-81. [Google Scholar]
- 63.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130(33):10876-10877. doi: 10.1021/ja803688x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karakoti M, Dhali S, et al. Surface modification of carbon-based nanomaterials for polymer nanocomposites. In: Ismail AF, Goh PS, eds. Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications. Elsevier; 2018:27-56. [Google Scholar]
- 65.Chen D, Dougherty CA, Zhu K, Hong H. Theranostic applications of carbon nanomaterials in cancer: focus on imaging and cargo delivery. J Controlled Release. 2015;210:230-245. doi: 10.1016/j.jconrel.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 66.Yang X, Ebrahimi A, Li J, Cui Q. Fullerene–biomolecule conjugates and their biomedicinal applications. Int J Nanomed. 2014;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krueger A. New carbon materials: biological applications of functionalized nanodiamond materials. Chemistry – A European Journal. 2008;14(5):1382-1390. doi: 10.1002/chem.200700987 [DOI] [PubMed] [Google Scholar]
- 68.Albert K, Hsu H-Y. Carbon-based materials for photo-triggered theranostic applications. Molecules. 2016;21(11):1585. doi: 10.3390/molecules21111585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyako E, Deguchi T, Nakajima Y, et al. Photothermic regulation of gene expression triggered by laser-induced carbon nanohorns. Proc Natl Acad Sci USA. 2012;109(19):7523-7528. doi: 10.1073/pnas.1204391109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Xia T, Duch MC, et al. Pluronic F108 coating decreases the lung fibrosis potential of multiwall carbon nanotubes by reducing lysosomal injury. Nano Lett. 2012;12(6):3050-3061. doi: 10.1021/nl300895y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang R, Mirshafiee V, Zhu Y, Xia T. Current approaches for safer design of engineered nanomaterials. Ecotoxicol Environ Saf. 2018;166:294-300. doi: 10.1016/j.ecoenv.2018.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angewandte Chemie (International ed in English). 2010;49(38):6726-6744. doi: 10.1002/anie.200906623 [DOI] [PubMed] [Google Scholar]
- 73.Cardin DJ, Smith P. Fullerenes: chemistry, physics and technology. Karl M. Kadish and Rodney S. Ruoff (eds) John Wiley & Sons Inc., New York, 2000 ix?+?968 pages. ⏢126 ISBN 0-471-29089-0. Applied Organometallic Chemistry - APPL ORGANOMETAL CHEM. 2001;15:646-647. doi: 10.1002/aoc.173 [DOI] [Google Scholar]
- 74.Fernandes N, Shenoy R, Kajampady M, et al. Fullerenes for the treatment of cancer: an emerging tool. Environmental Science and Pollution Research. 2022;29(39):1-21. doi: 10.1007/s11356-022-21449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo X, Ding R, Zhang Y, et al. Dual role of photosensitizer and carrier material of fullerene in micelles for chemo-photodynamic therapy of cancer. J Pharm Sci. 2014;103(10):3225-3234. doi: 10.1002/jps.24124 [DOI] [PubMed] [Google Scholar]
- 76.Guan M, Ge J, Wu J, et al. Fullerene/photosensitizer nanovesicles as highly efficient and clearable phototheranostics with enhanced tumor accumulation for cancer therapy. Biomaterials. 2016;103:75-85. doi: 10.1016/j.biomaterials.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 77.Chen A, Grobmyer SR, Krishna VB. Photothermal response of polyhydroxy fullerenes. ACS Omega. 2020;5(24):14444-14450. doi: 10.1021/acsomega.0c01018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56-58. doi: 10.1038/354056a0 [DOI] [Google Scholar]
- 79.Son KH, Hong JH, Lee JW. Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomed. 2016;11:5163-5185. doi: 10.2147/ijn.S112660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tang L, Xiao Q, Mei Y, et al. Insights on functionalized carbon nanotubes for cancer theranostics. J Nanobiotechnol. 2021;19(1):1-28. doi: 10.1186/s12951-021-01174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi J, Ma R, Wang L, et al. The application of hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin monomethyl ether-based photodynamic therapy for in vivo and in vitro cancer treatment. Int J Nanomed. 2013;8:2361-2373. doi: 10.2147/ijn.S45407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie L, Wang G, Zhou H, et al. Functional long circulating single walled carbon nanotubes for fluorescent/photoacoustic imaging-guided enhanced phototherapy. Biomaterials. 2016;103:219-228. doi: 10.1016/j.biomaterials.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 83.McKernan P, Virani NA, Faria GNF, et al. Targeted single-walled carbon nanotubes for photothermal therapy combined with immune checkpoint inhibition for the treatment of metastatic breast cancer. Nanoscale Research Letters. 2021;16(1):9. doi: 10.1186/s11671-020-03459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anwar S, Ding H, Xu M, et al. Recent advances in synthesis, optical properties, and biomedical applications of carbon dots. ACS Applied Bio Materials. 2019;2(6):2317-2338. doi: 10.1021/acsabm.9b00112 [DOI] [PubMed] [Google Scholar]
- 85.Xu X, Ray R, Gu Y, et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. Journal of the American Chemical Society. 2004;126(40):12736-7. doi: 10.1021/ja040082h [DOI] [PubMed] [Google Scholar]
- 86.Tejwan N, Saha SK, Das J. Multifaceted applications of green carbon dots synthesized from renewable sources. Adv Colloid Interface Sci. 2020;275:102046. doi: 10.1016/j.cis.2019.102046 [DOI] [PubMed] [Google Scholar]
- 87.Sekar R, Basavegowda N, Jena S, et al. Recent developments in heteroatom/metal-doped carbon dot-based image-guided photodynamic therapy for cancer. Pharmaceutics. 2022;14(9):1869. doi: 10.3390/pharmaceutics14091869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao S, Wu S, Jia Q, et al. Lysosome-targetable carbon dots for highly efficient photothermal/photodynamic synergistic cancer therapy and photoacoustic/two-photon excited fluorescence imaging. Chem Eng J. 2020;388:124212. doi: 10.1016/j.cej.2020.124212 [DOI] [Google Scholar]
- 89.Bai Y, Zhao J, Wang S, Lin T, Ye F, Zhao S. Carbon dots with absorption red-shifting for two-photon fluorescence imaging of tumor tissue pH and synergistic phototherapy. ACS Appl Mater Interfaces. 2021;13(30):35365-35375. doi: 10.1021/acsami.1c08076 [DOI] [PubMed] [Google Scholar]
- 90.Jiao M, Wang Y, Wang W, et al. Gadolinium doped red-emissive carbon dots as targeted theranostic agents for fluorescence and MR imaging guided cancer phototherapy. Chem Eng J. 2022;440:135965. doi: 10.1016/j.cej.2022.135965 [DOI] [Google Scholar]
- 91.Chechetka SA, Zhang M, Yudasaka M, Miyako E. Physicochemically functionalized carbon nanohorns for multi-dimensional cancer elimination. Carbon N Y. 2016;97:45-53. doi: 10.1016/j.carbon.2015.05.077 [DOI] [Google Scholar]
- 92.Curcio M, Cirillo G, Saletta F, et al. Carbon nanohorns as effective nanotherapeutics in cancer therapy. C – Journal of Carbon Research. 2021;7(1):3. doi: 10.3390/c7010003 [DOI] [Google Scholar]
- 93.Miyawaki J, Yudasaka M, Azami T, Kubo Y, Iijima S. Toxicity of single-walled carbon nanohorns. ACS Nano. 2008;2(2):213-226. doi: 10.1021/nn700185t [DOI] [PubMed] [Google Scholar]
- 94.Jiang B-P, Hu L-F, Shen X-C, et al. One-step preparation of a water-soluble carbon nanohorn/phthalocyanine hybrid for dual-modality photothermal and photodynamic therapy. ACS Appl Mater Interfaces. 2014;6(20):18008-18017. doi: 10.1021/am504860c [DOI] [PubMed] [Google Scholar]
- 95.Yang J, Hou M, Sun W, et al. Sequential PDT and PTT using dual-modal single-walled carbon nanohorns synergistically promote systemic immune responses against tumor metastasis and relapse. Advanced Science (Weinheim, Baden-Wurttemberg, Germany). 2020;7(16):2001088. doi: 10.1002/advs.202001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen F, Zhang C, Cai Z, et al. Carbon nanohorns/Pt nanoparticles/DNA nanoplatform for intracellular Zn2+ imaging and enhanced cooperative phototherapy of cancer cells. Anal Chem. 2020;92(24):16158-16169. doi: 10.1021/acs.analchem.0c03880 [DOI] [PubMed] [Google Scholar]
- 97.Lee H, Seok Lee J, Moor KJ, et al. Hand-ground fullerene-nanodiamond composite for photosensitized water treatment and photodynamic cancer therapy. J Colloid Interface Sci. 2021;587:101-109. doi: 10.1016/j.jcis.2020.12.020 [DOI] [PubMed] [Google Scholar]
- 98.Shi H, Gu R, Xu W, et al. Near-infrared light-harvesting fullerene-based nanoparticles for promoted synergetic tumor phototheranostics. ACS Appl Mater Interfaces. 2019;11(48):44970-44977. doi: 10.1021/acsami.9b17716 [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Li X, Doughty A, et al. Phototherapy using immunologically modified carbon nanotubes to potentiate checkpoint blockade for metastatic breast cancer. Nanomedicine : Nanotechnology, Biology, and Medicine. 2019;18:44-53. doi: 10.1016/j.nano.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogbodu RO, Limson JL, Prinsloo E, Nyokong T. Photophysical properties and photodynamic therapy effect of zinc phthalocyanine-spermine-single walled carbon nanotube conjugate on MCF-7 breast cancer cell line. Synth Met. 2015;204:122-132. doi: 10.1016/j.synthmet.2015.03.011 [DOI] [Google Scholar]
- 101.Zhao C, Sun S, Li S, et al. Programmed stimuli-responsive carbon dot-nanogel hybrids for imaging-guided enhanced tumor phototherapy. ACS Appl Mater Interfaces. 2022;14(8):10142-10153. doi: 10.1021/acsami.2c00174 [DOI] [PubMed] [Google Scholar]
- 102.Zhang M, Wang W, Zhou N, et al. Near-infrared light triggered photo-therapy, in combination with chemotherapy using magnetofluorescent carbon quantum dots for effective cancer treating. Carbon N Y. 2017;118:752-764. doi: 10.1016/j.carbon.2017.03.085 [DOI] [Google Scholar]
- 103.Guo S, Song Z, Ji DK, et al. Combined photothermal and photodynamic therapy for cancer treatment using a multifunctional graphene oxide. Pharmaceutics. 2022;14(7):1365. doi: 10.3390/pharmaceutics14071365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng WN, Yu QP, Wang D, et al. Mitochondria-targeting graphene oxide nanocomposites for fluorescence imaging-guided synergistic phototherapy of drug-resistant osteosarcoma. Journal of Nanobiotechnology. 2021;19(1):79. doi: 10.1186/s12951-021-00831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mauro N, Scialabba C, Agnello S, Cavallaro G, Giammona G. Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery. Materials Science and Engineering: C. 2020;107:110201. doi: 10.1016/j.msec.2019.110201 [DOI] [PubMed] [Google Scholar]
- 106.Georgieva M, Gospodinova Z, Keremidarska-Markova M, Kamenska T, Gencheva G, Krasteva N. PEGylated nanographene oxide in combination with near-infrared laser irradiation as a smart nanocarrier in colon cancer targeted therapy. Pharmaceutics. 2021;13(3):424. doi: 10.3390/pharmaceutics13030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sahu A, Min K, Jeon J, Yang HS, Tae G. Catalytic nanographene oxide with hemin for enhanced photodynamic therapy. Journal of Controlled Release : Official Journal of the Controlled Release Society. 2020;326:442-454. doi: 10.1016/j.jconrel.2020.07.023 [DOI] [PubMed] [Google Scholar]
- 108.Dos Santos MSC, Gouvêa AL, de Moura LD, et al. Nanographene oxide-methylene blue as phototherapies platform for breast tumor ablation and metastasis prevention in a syngeneic orthotopic murine model. J Nanobiotechnol. 2018;16(1):9-9. doi: 10.1186/s12951-018-0333-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalluru P, Vankayala R, Chiang C-S, Hwang KC. Nano-graphene oxide-mediated in vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. 2016;95:1-10. doi: 10.1016/j.biomaterials.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 110.Dash BS, Lu YJ, Chen HA, Chuang CC, Chen JP. Magnetic and GRPR-targeted reduced graphene oxide/doxorubicin nanocomposite for dual-targeted chemo-photothermal cancer therapy. Materials Science & Engineering C, Materials for Biological Applications. 2021;128:112311. doi: 10.1016/j.msec.2021.112311 [DOI] [PubMed] [Google Scholar]
- 111.Barrera CC, Groot H, Vargas WL, Narváez DM. Efficacy and molecular effects of a reduced graphene oxide/Fe(3)O(4) nanocomposite in photothermal therapy against cancer. Int J Nanomed. 2020;15:6421-6432. doi: 10.2147/ijn.S256760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaharie-Butucel D, Potara M, Suarasan S, Licarete E, Astilean S. Efficient combined near-infrared-triggered therapy: phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. J Colloid Interface Sci. 2019;552:218-229. doi: 10.1016/j.jcis.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 113.Liu C, Liu YY, Chang Q, et al. Pressure-controlled encapsulation of graphene quantum dots into liposomes by the reverse-phase evaporation method. Langmuir: The ACS Journal of Surfaces and Colloids. 2021;37(48):14096-14104. doi: 10.1021/acs.langmuir.1c02338 [DOI] [PubMed] [Google Scholar]
- 114.Yang C, Chan KK, Xu G, et al. Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS Appl Mater Interfaces. 2019;11(3):2768-2781. doi: 10.1021/acsami.8b16168 [DOI] [PubMed] [Google Scholar]
- 115.Guo T, Tang Q, Guo Y, et al. Boron quantum dots for photoacoustic imaging-guided photothermal therapy. ACS Appl Mater Interfaces. 2021;13(1):306-311. doi: 10.1021/acsami.0c21198 [DOI] [PubMed] [Google Scholar]
- 116.Yang H, Zhang Y, Song J, et al. Boosting phototherapeutic efficiency with single NIR laser-activated ultrasmall bismuth sulfide quantum dots. Chem Eng J. 2019;375:121941. doi: 10.1016/j.cej.2019.121941 [DOI] [Google Scholar]
- 117.Lv G, Guo W, Zhang W, et al. Near-infrared emission CuInS/ZnS quantum dots: all-in-one theranostic nanomedicines with intrinsic fluorescence/photoacoustic imaging for tumor phototherapy. ACS Nano. 2016;10(10):9637-9645. doi: 10.1021/acsnano.6b05419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel SC, Lee S, Lalwani G, Suhrland C, Chowdhury SM, Sitharaman B. Graphene-based platforms for cancer therapeutics. Ther Delivery. 2016;7(2):101-116. doi: 10.4155/tde.15.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yan H, Tao X, Yang Z, et al. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. Journal of Hazardous Materials. 2014;268:191-198. doi: 10.1016/j.jhazmat.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 120.Leitão MM, Alves CG, de Melo-Diogo D, Lima-Sousa R, Moreira AF, Correia IJ. Sulfobetaine methacrylate-functionalized graphene oxide-IR780 nanohybrids aimed at improving breast cancer phototherapy. RSC Adv. 2020;10(63):38621-38630. doi: 10.1039/d0ra07508f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He S, Li J, Chen M, et al. Graphene oxide-template gold nanosheets as highly efficient near-infrared hyperthermia agents for cancer therapy. Int J Nanomed. 2020;15:8451-8463. doi: 10.2147/ijn.S265134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lebepe TC, Parani S, Ncapayi V, et al. Graphene oxide-gold nanorods nanocomposite-porphyrin conjugate as promising tool for cancer phototherapy performance. Pharmaceuticals (Basel, Switzerland). 2021;14(12):1295. doi: 10.3390/ph14121295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neelgund GM, Oki A, Bandara S, Carson L. Photothermal effect and cytotoxicity of CuS nanoflowers deposited over folic acid conjugated nanographene oxide. Journal of Materials Chemistry B. 2021;9(7):1792-1803. doi: 10.1039/d0tb02366c [DOI] [PubMed] [Google Scholar]
- 124.Dreyer DR, Todd AD, Bielawski CW. Harnessing the chemistry of graphene oxide. Chemical Society Reviews. 2014;43(15):5288-5301. doi: 10.1039/c4cs00060a [DOI] [PubMed] [Google Scholar]
- 125.de Melo-Diogo D, Pais-Silva C, Costa EC, Louro RO, Correia IJ. D-α-tocopheryl polyethylene glycol 1000 succinate functionalized nanographene oxide for cancer therapy. Nanomedicine (London, England). 2017;12(5):443-456. doi: 10.2217/nnm-2016-0384 [DOI] [PubMed] [Google Scholar]
- 126.Costa-Almeida R, Bogas D, Fernandes JR, et al. Near-infrared radiation-based mild photohyperthermia therapy of non-melanoma skin cancer with PEGylated reduced nanographene oxide. Polymers (Basel). 2020;12(8):1840. doi: 10.3390/polym12081840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maheshwari R, Gadeval A, Raval N, Kalia K, Tekade RK. Laser activatable nanographene colloids for chemo-photothermal combined gene therapy of triple-negative breast cancer. Biomaterials Advances. 2022;133:112605. doi: 10.1016/j.msec.2021.112605 [DOI] [PubMed] [Google Scholar]
- 128.Dash BS, Jose G, Lu YJ, Chen JP. Functionalized reduced graphene oxide as a versatile tool for cancer therapy. Int J Mol Sci. 2021;22(6):2989. doi: 10.3390/ijms22062989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mun SG, Choi HW, Lee JM, et al. rGO nanomaterial-mediated cancer targeting and photothermal therapy in a microfluidic co-culture platform. Nano Convergence. 2020;7(1):10. doi: 10.1186/s40580-020-0220-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin CH, Chen YC, Huang PI. Preparation of multifunctional dopamine-coated zerovalent iron/reduced graphene oxide for targeted phototheragnosis in breast cancer. Nanomaterials (Basel, Switzerland). 2020;10(10):1957. doi: 10.3390/nano10101957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiu WJ, Chen YC, Huang CC, et al. Iron hydroxide/oxide-reduced graphene oxide nanocomposite for dual-modality photodynamic and photothermal therapy in vitro and in vivo. Nanomaterials (Basel, Switzerland). 2021;11(8):1947. doi: 10.3390/nano11081947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi HW, Lim JH, Kim CW, et al. Near-infrared light-triggered generation of reactive oxygen species and induction of local hyperthermia from indocyanine green encapsulated mesoporous silica-coated graphene oxide for colorectal cancer therapy. Antioxidants (Basel, Switzerland). 2022;11(1):174. doi: 10.3390/antiox11010174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jovanović SP, Syrgiannis Z, Budimir MD, et al. Graphene quantum dots as singlet oxygen producer or radical quencher—the matter of functionalization with urea/thiourea. Materials Science & Engineering C, Materials for Biological Applications. 2020;109:110539. doi: 10.1016/j.msec.2019.110539 [DOI] [PubMed] [Google Scholar]
- 134.Wang D, Chen J-F, Dai L. Recent advances in graphene quantum dots for fluorescence bioimaging from cells through tissues to animals. Part Part Syst Charact. 2015;32(5):515-523. doi: 10.1002/ppsc.201400219 [DOI] [Google Scholar]
- 135.Wang S, Cole IS, Li Q. The toxicity of graphene quantum dots. RSC Adv. 2016;6(92):89867-89878. doi: 10.1039/C6RA16516H [DOI] [Google Scholar]
- 136.Fan Z, Li S, Yuan F, Fan L. Fluorescent graphene quantum dots for biosensing and bioimaging. RSC Adv. 2015;5(25):19773-19789. doi: 10.1039/C4RA17131D [DOI] [Google Scholar]
- 137.Guo X, Zhu Y, Han W, et al. Nitrogen-doped graphene quantum dots decorated graphite foam as ultra-high active free-standing electrode for electrochemical hydrogen evolution and phenol degradation. Chem Eng Sci. 2019;194:54-57. doi: 10.1016/j.ces.2018.04.072 [DOI] [Google Scholar]
- 138.Du Y, Guo S. Chemically doped fluorescent carbon and graphene quantum dots for bioimaging, sensor, catalytic and photoelectronic applications. Nanoscale. 2016;8(5):2532-2543. doi: 10.1039/C5NR07579C [DOI] [PubMed] [Google Scholar]
- 139.Kumawat MK, Thakur M, Bahadur R, et al. Preparation of graphene oxide-graphene quantum dots hybrid and its application in cancer theranostics. Materials Science & Engineering C, Materials for Biological Applications. 2019;103:109774. doi: 10.1016/j.msec.2019.109774 [DOI] [PubMed] [Google Scholar]
- 140.Liu H, Li C, Qian Y, et al. Magnetic-induced graphene quantum dots for imaging-guided photothermal therapy in the second near-infrared window. Biomaterials. 2020;232:119700. doi: 10.1016/j.biomaterials.2019.119700 [DOI] [PubMed] [Google Scholar]
- 141.Li Y, Wang Y, Shang H, Wu J. Graphene quantum dots modified upconversion nanoparticles for photodynamic therapy. Int J Mol Sci. 2022;23(20):12558. doi: 10.3390/ijms232012558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dirheimer L, Pons T, Marchal F, Bezdetnaya L. Quantum dots mediated imaging and phototherapy in cancer spheroid models: state of the art and perspectives. Pharmaceutics. 2022;14(10):2136. doi: 10.3390/pharmaceutics14102136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Z, Zou H, Wang N, et al. Photoluminescence of carbon quantum dots: coarsely adjusted by quantum confinement effects and finely by surface trap states. Science China Chemistry. 2018;61(4):490-496. doi: 10.1007/s11426-017-9172-0 [DOI] [Google Scholar]
- 144.Xu Y, Wang X, Zhang WL, Lv F, Guo S. Recent progress in two-dimensional inorganic quantum dots. Chem Soc Rev. 2018;47(2):586-625. doi: 10.1039/C7CS00500H [DOI] [PubMed] [Google Scholar]
- 145.Xie H, Liu M, You B, et al. Biodegradable Bi(2) O(2) Se quantum dots for photoacoustic imaging-guided cancer photothermal therapy. Small (Weinheim an der Bergstrasse, Germany). 2020;16(1):e1905208. doi: 10.1002/smll.201905208 [DOI] [PubMed] [Google Scholar]
- 146.Wu Y, Chen Z, Yao Z, et al. Black phosphorus quantum dots encapsulated biodegradable hollow mesoporous MnO2: dual-modality cancer imaging and synergistic chemo-phototherapy. Adv Funct Mater. 2021;31(41):2104643. doi: 10.1002/adfm.202104643 [DOI] [Google Scholar]
- 147.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;7:5577-5591. doi: 10.2147/ijn.S36111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eifler AC, Thaxton CS. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol Biol. 2011;726:325-338. doi: 10.1007/978-1-61779-052-2_21 [DOI] [PubMed] [Google Scholar]
- 149.Ventola CL. The nanomedicine revolution: part 3: regulatory and safety challenges. P t. 2012;37(11):631-639. [PMC free article] [PubMed] [Google Scholar]
- 150.Hoshino A, Hanada S, Yamamoto K. Toxicity of nanocrystal quantum dots: the relevance of surface modifications. Arch Toxicol. 2011;85(7):707-720. doi: 10.1007/s00204-011-0695-0 [DOI] [PubMed] [Google Scholar]
- 151.Lanone S, Boczkowski J. Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr Mol Med. 2006;6(6):651-663. doi: 10.2174/156652406778195026 [DOI] [PubMed] [Google Scholar]
- 152.Zhang WX, Karn B. Nanoscale environmental science and technology: challenges and opportunities. Environ Sci Technol. 2005;39(5):94a-95a. [PubMed] [Google Scholar]
- 153.Arvizo RR, Giri K, Moyano D, et al. Identifying new therapeutic targets via modulation of protein corona formation by engineered nanoparticles. PLoS One. 2012;7(3):e33650. doi: 10.1371/journal.pone.0033650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lai ZW, Yan Y, Caruso F, Nice EC. Emerging techniques in proteomics for probing nano-bio interactions. ACS Nano. 2012;6(12):10438-10448. doi: 10.1021/nn3052499 [DOI] [PubMed] [Google Scholar]
- 155.Tenzer S, Docter D, Rosfa S, et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS Nano. 2011;5(9):7155-7167. doi: 10.1021/nn201950e [DOI] [PubMed] [Google Scholar]
- 156.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. Journal of the American Chemical Society. 2010;132(16):5761-5768. doi: 10.1021/ja910675v [DOI] [PubMed] [Google Scholar]
- 157.Zhang H, Burnum KE, Luna ML, et al. Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size. Proteomics. 2011;11(23):4569-4577. doi: 10.1002/pmic.201100037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ray S, Jana N. Toxicology and biosafety of carbon nanomaterials. 2017:205-229.
- 159.Yang S-T, Wang H, et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. The Journal of Physical Chemistry C. 2009;113(42):18110-18114 10.1021/jp9085969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tao H, Yang K, Ma Z, et al. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small. 2012;8(2):281-290. 10.1002/smll.201101706 [DOI] [PubMed] [Google Scholar]
- 161.Jia G, Wang H, Yan L, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39(5):1378-1383. doi: 10.1021/es048729l [DOI] [PubMed] [Google Scholar]
- 162.Manna SK, Sarkar S, Barr J, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5(9):1676-1684. doi: 10.1021/nl0507966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fisher C, Rider A, Han ZJ, Kumar S, Levchenko I, Ostrikov K. Applications and nanotoxicity of carbon nanotubes and graphene in biomedicine. J Nanomater. 2012;2012:315185. doi: 10.1155/2012/315185 [DOI] [Google Scholar]
- 164.Zhang Y, Ali SF, Dervishi E, et al. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano. 2010;4(6):3181-3186. doi: 10.1021/nn1007176 [DOI] [PubMed] [Google Scholar]
- 165.Lee J, Shin YC, Jin O, et al. Cytotoxicity evaluations of pristine graphene and carbon nanotubes in fibroblastic cells. J Korean Phys Soc. 2012;61:873-877 doi: 10.3938/jkps.61.873 [DOI] [Google Scholar]
- 166.Inshakova E, Inshakov O. World market for nanomaterials: structure and trends. EDP Sciences. 2017;129:02013. [Google Scholar]
- 167.Siddique JA, Numan A. Perspective future development of nanomaterials. In: Contemporary Nanomaterials in Material Engineering Applications. Springer; 2021:319-343. [Google Scholar]
- 168.Update N. US leads in government spending amidst increased spending across Asia. Lux Research Inc; 2015. [Google Scholar]
- 169.Market VF. Global opportunity analysis and industry forecast, 2017-2023. Allied Market Research; 2016. [Google Scholar]
- 170.Jensen K, Bøgelund J, Jackson P, et al. Carbon nanotubes—types, products, market, and provisional assessment of the associated risks to man and the environment. 2015.