Abstract
Background:
Patient perspectives on meaningful symptoms and impacts in early Parkinson’s disease (PD) are lacking and are urgently needed to clarify priority areas for monitoring, management, and new therapies.
Objective:
To examine experiences of people with early-stage PD, systematically describe meaningful symptoms and impacts, and determine which are most bothersome or important.
Methods:
Forty adults with early PD who participated in a study evaluating smartwatch and smartphone digital measures (WATCH-PD study) completed online interviews with symptom mapping to hierarchically delineate symptoms and impacts of disease from “Most bothersome” to “Not present,” and to identify which of these were viewed as most important and why. Individual symptom maps were coded for types, frequencies, and bothersomeness of symptoms and their impacts, with thematic analysis of narratives to explore perceptions.
Results:
The three most bothersome and important symptoms were tremor, fine motor difficulties, and slow movements. Symptoms had the greatest impact on sleep, job functioning, exercise, communication, relationships, and self-concept— commonly expressed as a sense of being limited by PD. Thematically, most bothersome symptoms were those that were personally limiting with broadest negative impact on well-being and activities. However, symptoms could be important to patients even when not present or limiting (e.g., speech, cognition).
Conclusion:
Meaningful symptoms of early PD can include symptoms that are present or anticipated future symptoms that are important to the individual. Systematic assessment of meaningful symptoms should aim to assess the extent to which symptoms are personally important, present, bothersome, and limiting
Keywords: Parkinson’s disease, digital health technology, qualitative, meaningfulness
INTRODUCTION
Parkinson’s disease (PD) is a devastating neurodegenerative condition and current therapies are unable to prevent or delay progression [1]. One major challenge in developing new treatments has been a lack of sensitive, patient-centric endpoints that can be used to evaluate treatment efficacy [2, 3]. Promising new digital measures are under development and could address this gap [3, 4]. However, there is limited understanding of the extent to which these technologies capture what is important to patients, which limits use in clinical trials [5, 6]. In light of recent FDA guidance highlighting the need for patient-focused drug development [7, 8], a better understanding of the symptoms and impacts of disease that are meaningful to people with early PD is needed to clarify priority areas for monitoring and management [9].
To date, research on symptoms and impacts of PD has focused on populations with more advanced symptoms, and there is limited data as to whether these differ in earlier stages of disease [10]. Recently published conceptual models in early PD have begun to clarify this [11, 12]; however, further evidence is needed to understand prevalence and bothersomeness, and to identify which symptoms are most important from the patient perspective. This knowledge can improve care, support the selection of appropriate outcomes measures for clinical trials, and guide development of future patient-centric measures [9]. Thus, the purpose of this exploratory study was to systematically identify and describe personally meaningful symptoms and impacts of disease, determine which were most bothersome and important, and explore experiences of early PD.
METHODS
Setting, Sample
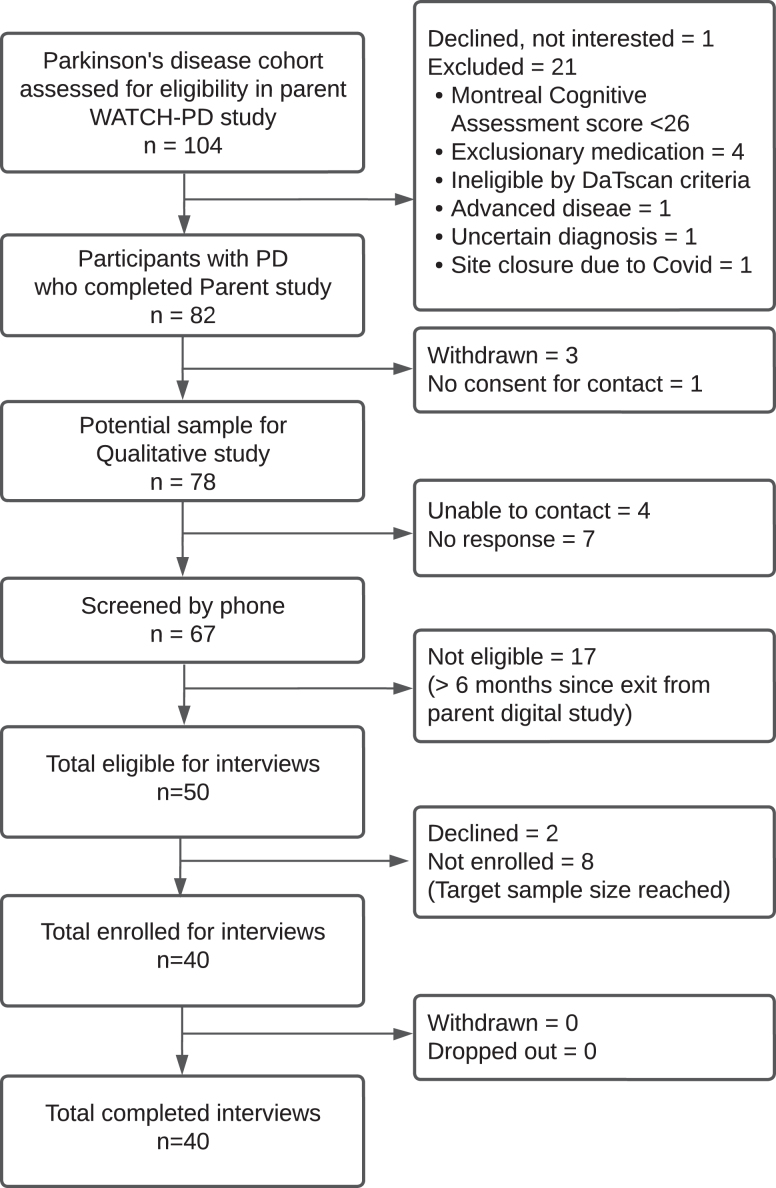
This study was conducted collaboratively with Critical Path Institute, US Food and Drug Administration, individuals with PD, and academic and industry partners. It was designed as follow up to the parent WATCH-PD study (NCT03681015), which was a 12-month multi-center observational study evaluating use of a smartwatch and smartphone applications to detect cognitive and motor progression over time in individuals with early, untreated PD [4]. This dual-purpose follow-up study was conducted for the purpose of 1) identifying meaningful symptoms and impacts (i.e., functional or psychosocial changes resulting from symptoms) of early untreated PD and 2) exploring the extent to which the digital measures in the parent study were perceived as relevant to monitoring meaningful aspects of disease by participants. This manuscript presents results from Aim 1. All individuals with early PD who completed their final visit of the WATCH-PD parent study [13] within 6 months were eligible to participate (N = 54). Main inclusion criteria for the parent study were: 1) diagnosed PD duration≤2 years; 2) Modified Hoehn & Yahr stage≤2; and 3) not taking any PD medications. Main exclusion criteria were: 1) confounding comorbidities; 2) history of PD related falls; and 3) Montreal Cognitive Assessment score (MoCA) < 24 [4, 14]. The enrollment diagram is shown in Fig. 1. Participants from the parent study were randomly selected for interviews, contacted via phone, and screened for interest and eligibility. Forty (50%) were enrolled to correspond with parent study gender distribution with inclusion of all participants from underrepresented groups (N = 4). Sample size was based on maximal ranges identified in prior qualitative descriptive studies [15]. Data saturation was assessed after completing all 40 interviews to confirm adequacy [16]. Saturation was defined as the point after which no new or additional symptoms/impacts were identified in succeeding interviews. IRB approval was obtained (IRB# 00006429) and participants provided digital informed consent.
Fig. 1.
Enrollment diagram.
Data collection
An integrated mixed-methods approach was used [15, 17] consisting of a preliminary survey followed by 1:1 online interviews that used symptom mapping in conjunction with a semi-structured interview protocol, as described below. All data collection procedures were developed in collaboration with people with Parkinson’s disease and the study advisory group, and were pretested (JC, JH).
Survey
Participants first completed a brief online survey (Supplement A) using Redcap, which is a secure, web-based software platform designed to support survey data capture [18, 19]. The purpose of the survey was to gather demographic data and preliminary qualitative information on personally important symptoms, which was used as a starting point for the symptom mapping interview. For the survey, participants were asked to describe all symptoms of PD that they experienced and explain which were most bothersome using open response items.
Online interview
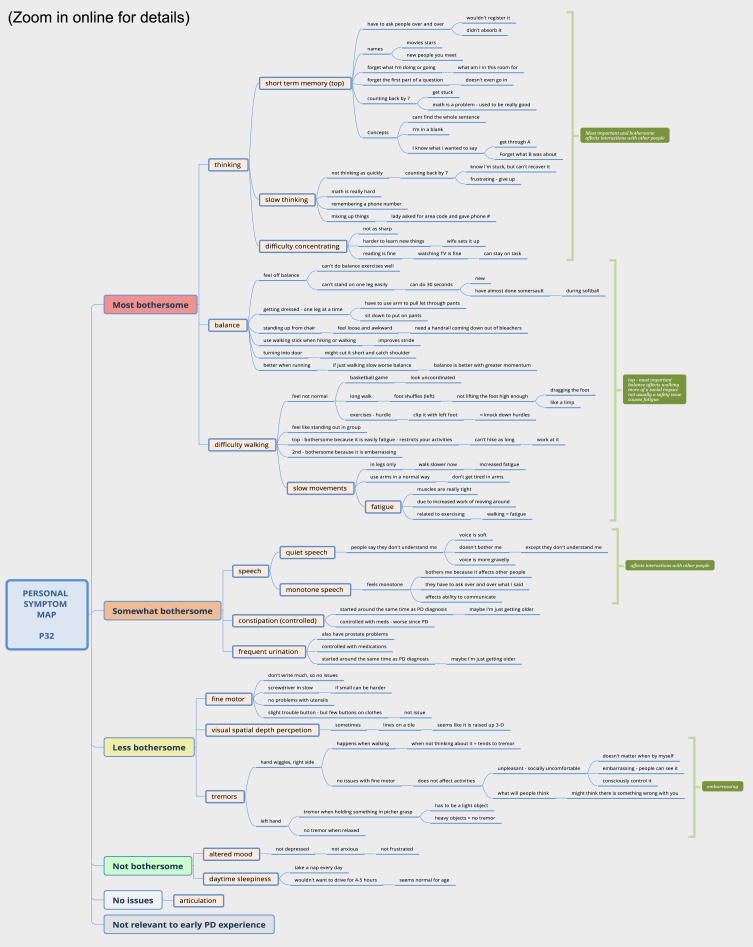
One-week later, online interviews were conducted via Zoom videoconferencing with participants at home (average 100 min). As shown in Supplement B, interview questions focused on understanding PD symptoms, experiences, characteristics and impacts of symptoms, and relative bothersomeness. Symptom mapping [20] was used to delineate all personal symptoms and impacts as shown in Fig. 2. Symptom mapping is a hybrid mixed-method technique that enables the collection of qualitative data in a quantifiable manner by active categorization of experiences inside of a structured framework [20]. Interviews were conducted by a qualitative researcher experienced in these methods (JM; white, female, PhD-prepared advanced practice nurse, unacquainted with participants). Interviews were audio-visually recorded with permission.
Fig. 2.
Sample participant symptom map.
Symptom mapping [20]
During the interview, a detailed concept map of the individual’s PD symptom experience was developed by the interviewer, as observed and directed by the participant via Zoom screen sharing [21]. Individual symptom maps were created using XmindTM software, with map levels organized hierarchically top to bottom from “Most bothersome” to “No current issues,” with an additional category “Not [personally] relevant to early PD”. This categorization of bothersomeness was intended to reflect the extent to which a symptom disturbed or distressed the individual.
Step 1. Prior to the interview, the researcher reviewed the survey data and entered reported symptoms into a preliminary map, with each symptom represented as a single yellow node.
Step 2. At the start of the interview, the participant was oriented to the mapping process and shown the preliminary map via screensharing. They were then asked to list and describe all symptoms of PD and how these impacted their life (past or present), while the researcher entered this information into the mutually viewable map.
Step 3. Next, the researcher probed for common symptoms of PD identified in prior literature (difficulties with tremor, walking, balance, fine motor, speech, thinking, mood, daytime sleepiness, fatigue, depth perception) if not spontaneously mentioned by the participant. Symptoms not experienced were categorized under “No current issues", or “Not relevant to early PD” based on the participants’ perspective.
Step 4 - Bothersomeness. Once symptoms and impacts were fully described, supporting details were collapsed (i.e., hidden) leaving only primary yellow “symptom” nodes visible. The participant was then asked to rank symptoms according to bothersomeness (i.e., how distressing the symptom was from most to least).
Step 5 - Importance. Lastly, participants identified which symptoms were most important to them overall and explained any differences between what was important vs. bothersome. A final opportunity was provided to review and edit the map, and copies of maps were given to participants at the end of the interview, if desired.
Data analysis
Content coding [22] was performed on symptom maps with thematic analysis of verbatim transcripts [23]. Maps were coded for type, frequency, and bothersomeness of symptoms and impacts. As shown in Supplement C, each hierarchical level in the map was associated with a Patient Reported Symptom Score (PRSS; range 0–4), where scores 1–4 indicated the symptom was present and degree of bothersomeness, 0 indicated the symptom was not present but still viewed as important to the participant, and “.” Indicated that the symptom was not present and not personally relevant.
Coding was performed in cycles by two coders (JM, PY) and differences resolved by consensus. In Cycle 1, open coding (i.e., no a priori coding schema) was performed on maps to develop a comprehensive list of symptom types using spreadsheets. Cycle 2, maps were coded again quantify frequencies and bothersomeness of each symptom by participant. In Cycle 3, maps were coded to derive a comprehensive list of all impacts with details on symptoms that contributed to the impact. Cycle 4, maps were re-coded to quantify frequencies of impacts by contributing symptoms for each participant. In Cycle 5, the frequencies of symptoms and impacts were compared to the Staunton conceptual model of early PD [11], with attention to divergence or alignment with conceptual domains and domain items [22]. Lastly, in Cycle 6, inductive thematic analysis was conducted on narratives using Nvivo12, starting with open coding to identify recurrent ideas within interviews, followed by pattern coding to identify dominant themes across interviews regarding how participants experienced and perceived early PD symptoms [24, 25]. Descriptive statistics were computed for demographic survey items. Independent T-tests were conducted in SPSS 28 to assess for any differences in symptom frequencies between those taking versus not taking PD medication.
Rigor
Procedures to ensure rigor included: co-development and pretesting of study procedures with people with PD (surveys, interview guide, mapping procedures), observing of interviews for consistency (RS), triangulated data collection approaches (preliminary survey, followed by mapping and cognitive interviewing), member-checking during interviews, peer-debriefing on thematic findings at weekly scheduled meetings, use of multiple coders, participant identifiers to show representativeness of quotes, and a formal audit trail [26]. Symptom maps were returned to participants who reviewed and confirmed validity of their personal data.
RESULTS
Sample and interview characteristics
Of 54 eligible participants from the parent study, one declined to participate, 5 could not be reached, and 8 were not solicited for interviews due to having achieved targeted sample size, as delineated in Fig. 1. Demographic data comparative to parent study demographics are displayed in Table 1. Participants were mostly white, male, and not taking PD medication at the time of interview. Data saturation for symptoms, impacts and themes was achieved by the 17th of 40 interviews, after which no new findings emerged.
Table 1.
Qualitative interview study demographics compared to parent study Parkinson’s cohort
| Sample | Parent study | |
| n = 40 | (n = 82) | |
| Age, y | 63.9 (SD 8.8) | 63.3 (SD 9.4) |
| Female, n (%) | 19 (47.5%) | 36 (43.9%) |
| Race/ethnicity, n (%) | ||
| White | 37 (92.5%) | 78 (95.1%) |
| Asian | 3 (7.5%) | 3 (3.7%) |
| Not specified | – | 1 (1.2%) |
| Hispanic or Latino, n (%) | 1 (2.5%) | 3 (3.7%) |
| Education > 12 y, n (%) | 40 (100.0%) | 78 (95.1%) |
| PD duration, y* | 2.1 (SD 0.9) | 0.8 (SD 0.6) |
| Taking medications for PD, n (%)* | 16 (40.0%) | – |
*Difference in Parkinson’s duration and medication use reflects qualitative study data collection that occurred approximately one year after the start of the parent study.
Symptoms and impacts
Symptoms frequencies in early PD (All bothersome symptoms; PRSS 1-4)
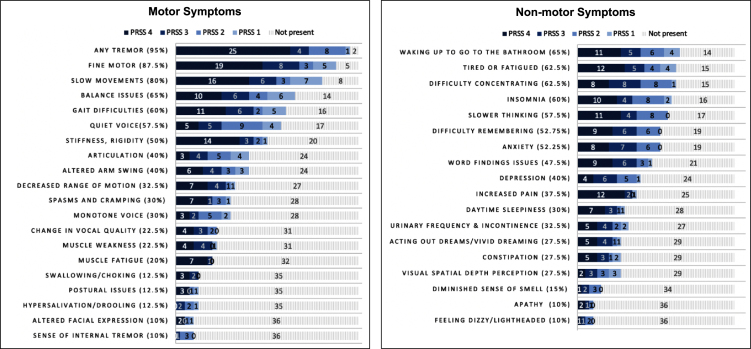
Motor and non-motor symptoms of early PD are displayed by frequency of bothersomeness in Fig. 3. There were no significant differences in symptoms between those taking PD medications (N = 16/40; 40%) and those not taking PD medications (p > 0.05; range 0.083— 0.986). For all, tremor was the most commonly reported motor symptom (95%), followed by fine motor difficulties (87%), and slow movements (80%). Over half of people reported gait changes, stiffness/rigidity, and quiet voice. For example:
Fig. 3.
Patient reported motor and non-motor symptoms of early Parkinson’s disease as shown in symptom maps (N = 40). PRSS 1 = Patient Reported Symptom Score – Likert scale rating of bothersomeness ranging from most bothersome to not bothersome. PRSS 4 = Symptoms that are present and most bothersome; PRSS 3 = Symptoms that are present and somewhat bothersome symptoms; PRSS 2 = Symptoms that are present and less bothersome; PRSS 1 = Symptoms that are present but not bothersome; Not present, Symptom not experienced. PRSS Scores are absolute. Figure includes both primary and contributing symptoms for each PRSS level. Percentage (%) represents the total percent of participants who experience the symptoms (encompassing 1–4). Tremor subcategories included: Hand tremor (85%) Leg/foot tremor (42.5%; Face/neck/Jaw tremor 12.5%). The following symptoms were reported by < 10% sample and are not represented in the graphs: dry mouth, diminished sensation, temperature dysregulation, sexual dysfunction, tearing of eyes, loss of appetite, double vision, right/left confusion, hemi-spatial neglect. Dyskinesias were not reported.
P14: I move in slow motion like I’m a sloth. It’s just so frustrating ... I can’t [ever] hurry. [It’s] like I’m turning into a stone.
The most common non-motor symptoms were nocturia (65%), feeling tired or fatigued (62.5%), difficulty concentrating (62.5%), and insomnia (60%), with more than half of participants reporting slow thinking, difficulty remembering, and anxiety.
“Most bothersome” symptoms (PRSS 4)
Participants identified an average of 10 “most bothersome” symptoms (range 0–28). Again, difficulties with tremor (63%), fine motor (48%), and slow movements (40%) were most reported. However, when evaluating symptoms categorically by area of impact (e.g., mobility & balance, speech, mood, cognitive changes) rather than individually, symptoms affecting mobility and balance (gait, posture, balance, slowness, stiffness/rigidity) were most bothersome to 57% (Supplement D). Other most bothersome categories included changes to speech (40%), disturbed sleep (30%), altered thinking (27%), and altered mood (22%).
“Most important” symptoms of early PD
Participants identified an average of 2 symptoms that they believed were “most important.” These were tremor (27%), fine motor (25%), slow movements (12.5%), and word finding difficulties (10%). As shown in Supplement E, when evaluated categorically rather than as individual symptoms, 32.5% identified issues of mobility as “most important” and 12.5% identified mood changes and cognitive difficulties.
Impacts of early PD symptoms
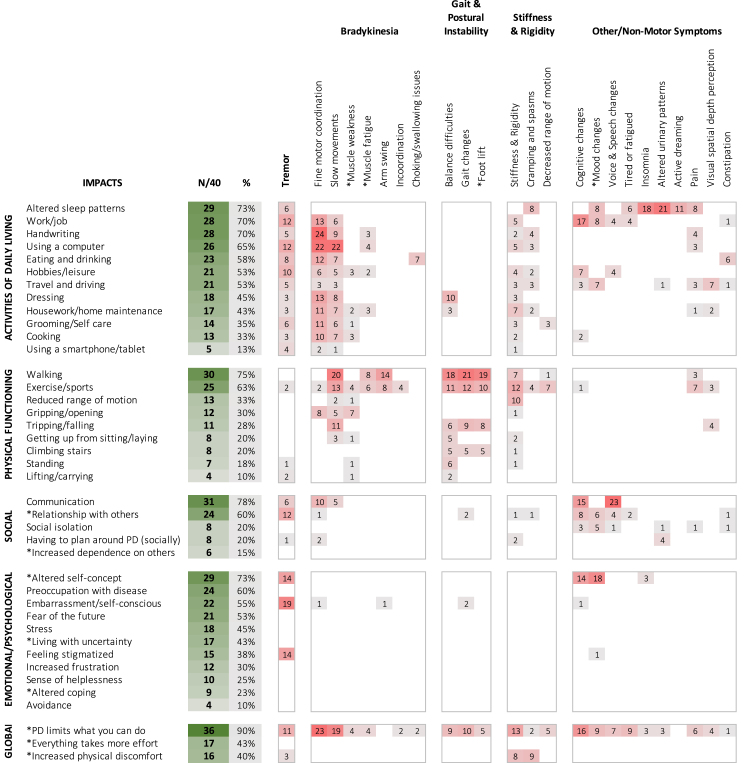
Figure 4 displays the frequencies of different impacts experienced in early PD, along with the symptom believed to have contributed to the impact. These were evaluated with reference to Staunton conceptual model, which included activities of daily living (ADL), physical, social, and emotional & psychological functioning, and fine motor domains [11]. In our analysis, “fine motor skills” (i.e., computer/smartphone use, handwriting) was subsumed under ADL based on descriptive patterns that indicated these impacts typically occurred within the context of ADLs, as described below.
Fig. 4.
Impacts of disease in people with early Parkinson’s disease. Table comparative to the Patient-Centered Conceptual Model of symptoms and impacts in early PD from Staunton et al. (2022) [11]. Items with * were modified from or not present in the original model. Impacts affecting < 10% of total sample (N = 4/40) are not reported. Green shaded boxes visually represent number of participants reporting a particular impact as present. Red shaded boxes visually represent number of participants reporting a specific symptom as contributing to or causing the listed impact. Relative bothersomeness of impacts was not quantified and occurred across multiple symptom levels. Impacts were counted only once per participant.
ADL
The most discussed ADL impacts were altered sleep patterns and increased difficulty performing one’s job (70%). Writing (70%) and using a computer mouse/keyboard (68%) contributed largely to job-related difficulties, with close to half referencing cognitive changes (42.5%), including slower thinking, increased difficulty following sequences, and multitasking.
P8: I am scared to death of failing and [doing] the wrong thing - If you give me five things to do, I will do three successfully and mess up two.
Difficulty using a computer appeared to have greatest impact on job function (e.g., sending emails, working in spread sheets), whereas handwriting affected both job and social interactions (ability to write notes/letters, holiday cards, or sign documents). Difficulties with the computer were most commonly due to slow fine motor movements (missing keys, double striking, holding down keys too long, difficulty manipulating the mouse, click/drag function). Other common ADLs impacts affecting more than half of participants included eating and drinking (choking, trouble using utensils due to tremor/fine motor) and increased difficulty driving (depth perception, anxiety, reaction times).
Physical functioning
Changes in walking, which affected exercise, were most common (75%; 63%, respectively). Less than one-third of participants experienced difficulties in other physical functioning areas, such as falling, getting up from sitting, standing, or climbing stairs. Most were physically active, exercised regularly, and had few physical limitations that were apparent to others.
Social
Many indicated PD affected their ability to communicate and interact as usual (78%), including energy to maintain social interactions (fatigue), ability to express ideas rapidly within a group context (cognitive), speak clearly (articulation) and be easily heard (quiet voice), or formulate written communications. PD also impacted personal relationships with others. This was most often reported with respect to immediate family, but also affected friends and co-workers. Impacts on relationships were due to increased dependence on others, tremor, cognitive issues affecting interactions, and mood changes.
P25: I’m a very social person, and I feel like I’m being muted. I used to be this flamboyant, happy, outgoing, vivacious person. I feel like I’m not that person anymore.
Emotional/psychological
Nearly three-quarters of participants (73%) reported altered self-concept— i.e., viewing oneself as less capable, less competent, and less healthy, translating to decreased sense of well-being. Many also described preoccupation with PD (60%) and hypervigilance towards monitoring for symptoms and impacts, or fear of the future and inevitable disease progression (53%).
P16: I’m clumsy. I’ve never been clumsy before ... when I see myself walking, I see myself as a sick person.
P13: Am I going to be in a wheelchair someday? That’s [something] I worry about now.
Approximately half (55%) reported embarrassment, most commonly with respect to tremor (47.5%) or other socially apparent symptoms (e.g., gait disturbances, flat affect) which sometimes led to feeling stigmatized (38%).
P11: [Tremor] is embarrassing– you appear weak or infirm.
P8: [My lack of expression is] interpreted as if I’m angry. I’m not angry. It’s just the way I look. It has a real negative impact on [relationships]
Global
In all, over 90% of participants reported a sense that PD limited what they could do, with many indicating doing things in general required substantially more effort than prior to having PD. A large number (40%) also reported increased personal discomfort ranging from pain to being unable to get comfortable and relax due to stiffness, cramping, or tremor.
P5: [I] have to slow down if I don’t want to make mistakes— it’s probably added 50% to the time it takes to answer emails or to write something.
Themes
Three key themes were identified with regards to what made symptoms meaningful from the perspective of people with early PD. Meaningful symptoms were those that were 1) personally important— whether actively present or not, with symptoms becoming more meaningful as 2) bothersomeness of an experienced symptom increased and 3) the symptom became more limiting. These themes are described below and supporting data are presented in Supplement F.
Theme 1. Symptoms can be important even when not present or present but not important
Personally important symptoms fell into two categories: 1) symptoms currently experienced that impacted physical and psychosocial functioning, and 2) symptoms not currently experienced that had potential to impact future physical and psychosocial functioning. For example, many people felt speaking and cognitive symptoms were very important (i.e., “staying me” [P28]) and they actively monitored for onset of cognitive and speech difficulties or engaged in activities to strengthen speech and cognition, even though currently without symptoms in that area. As one woman explained:
P6: I don’t experience [trouble speaking] but I want to be able to speak clearly. Speaking is important to me.
Theme 2. Symptoms can be bothersome even when not limiting or limiting but not bothersome.
Similar to theme 2, symptoms did not have to be limiting in order to be perceived as bothersome. For example, tremor was often perceived as bothersome, even though it did not actually limit ability to do things. For example:
P14: [Tremor] has less to do with my quality of life than slow movement. It’s annoying, but it doesn’t stop me from doing anything.
The more limiting a symptom was, the more bothersome it was generally perceived to be, as seen here:
P26: Anxiety is screwing up my life – it affects what you can do, where you can go, and who you can see.
However, in some instances, symptoms caused limitations that were not viewed as bothersome. One individual who experienced substantially slower walking speed explained it this way:
P3: It slows me down, but I’ve got too many other things that are more important, so I’d say it’s almost not bothersome. I can’t worry about every little thing.
Theme 3. Most bothersome symptoms are those which are limiting and have the greatest current negative impact on an individual’s sense of well-being and usual activities.
P2: [Tremor is] less of a concern. It makes me self-conscious, but I don’t let that get in the way of activities in my life. ... For the moment, I would say fatigue is more bothersome ... . although I’m anticipating that they will probably switch [in time].
When discussing personally meaningful symptoms, individuals prioritized aspects of bothersomeness based upon a hierarchy of needs similar to that described by Maslow [27]: 1) physiologic needs (e.g., eating, breathing, sleep, pain/injury prevention, and ability to perform ADLs that meet these basic needs), 2) safety & security needs (symptoms affecting security, including employment or fear of the future, anxiety), 3) love and belonging needs (symptoms adversely impacting ability to communicate with others or interpersonal relationships), and 4) self-esteem needs (symptoms causing social embarrassment or affecting sense of oneself as a healthy competent person). When higher risk symptoms were not present, lower risk symptoms were prioritized instead.
For example, choking (Maslow level 1) was a very bothersome symptom, as were other symptoms that threatened personal safety, as shown in this comment:
P9: Hyposmia is a safety issue for me ... I can’t trust my sense of smell ... I’m concerned I won’t know if something’s gone bad ... [or] if there’s a toxic odor. ... once, there was plastic melting in the ceiling ... and I wasn’t aware of it. (PRSS 4)
Similarly, tremor was less bothersome when lacking direct impact and more bothersome when it caused pain or discomfort (Maslow level 1), interfered with work (Maslow level 2), or was apparent in social situations, resulting in a sense of stigma and embarrassment (Maslow levels 3-4). When individuals were able to mitigate the impact of the symptom or find workarounds (i.e., protecting hierarchy of needs), the symptom was viewed as less bothersome. For instance, loss of fine motor skills and slower movements were more bothersome when they affected the individual’s job and less bothersome after retirement when extensive computer work was not required. Similarly, when symptoms improved with medication use and became less limiting, they were viewed as less bothersome but still important.
DISCUSSION
To our knowledge, this is the first study to systematically evaluate prevalence, personal importance, and relative bothersomeness of symptoms and impacts in people with early PD using in-depth interviews and symptom mapping approaches. We found the three most common motor symptoms were tremor, fine motor difficulties, and slow movements, whereas most common non-motor symptoms were nocturia, fatigue, insomnia, and cognitive changes. Notably, when clustering symptoms by functional impacts, those affecting mobility (e.g., slowness, stiffness/rigidity, and gait changes) and balance were cumulatively most important to a larger percentage of people than tremor alone (32.5% vs. 27.5% respectively), which is consistent with other literature [5, 12, 28]. Thus, our data support mobility and balance as high priority symptoms for people with early PD.
Our results corresponded well with prior studies that have investigated bothersomeness of PD symptoms [5, 11, 12, 28–30]. Similar to Staunton et al., Morel et al., and Port et al., tremor and mobility issues were the most prevalent symptoms in our sample [5, 11, 12]. However, our data point to a much higher rate of fine motor difficulties than observed previously (87.5%), with fine motor tasks of handwriting and computer use generally intertwined with ADLs. We also observed a high percentage of individuals (90%) who expressed broad-spectrum impacts not reflected in previous models— namely, feeling limited by PD such that activities and interactions which were once intuitive required more time, effort, and intent. Based on these findings, we would propose amending emerging domains of impact to: ADL (inclusive of fine motor skills), physical, social, and emotional/psychological functioning, and global impacts, to reflect the broader experience of PD across domains. Reevaluation of existing clinical tools and outcome measures might be warranted in light of these findings.
Lastly, this study revealed that the extent to which symptoms are viewed as bothersome is in fact contingent on impact, with symptoms prioritized based on the extent to which they affected or could affect physical safety, security, relationships, and self-esteem. These findings are consistent with Maslow’s hierarchy of need and other theories relating to perceptions of unpleasant symptoms and bear implications for measurement [27, 31]. Specifically, we found that the overall assessment of “meaningfulness” was based on whether the symptom was personally important (present or not) and the degree to which it was actively bothersome (e.g., distressing), which generally corresponded with physical and psychosocial impacts. “Important” symptoms often aligned with but were not entirely equivalent to “bothersome” symptoms, which is a key distinction. Bothersome symptoms were always personally important, and more bothersome symptoms were generally more important; however, symptoms that were not present (e.g., cognitive or speech difficulties) or bothersome were also considered important and “something to keep an eye on.” This led to hypervigilance towards future symptoms with self-monitoring for decline in speech or cognition in people who had not experienced these symptoms. Thus, pro-active monitoring for onset of future symptoms that are important to people with early PD may be warranted, even when not actively present.
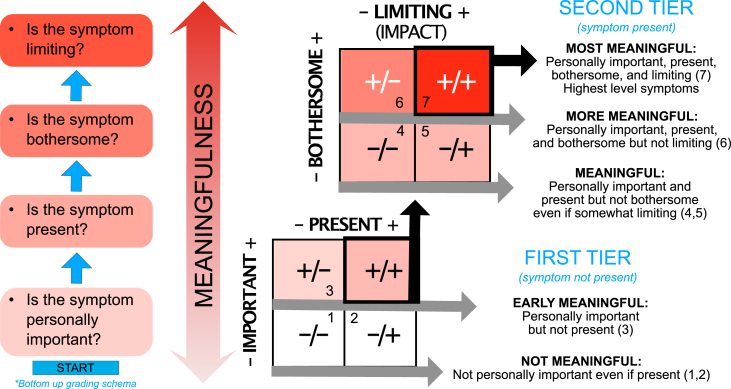
Cumulatively, our findings suggest that measuring “meaningfulness” of symptoms might require assessing four discrete components: personal importance, presence, bothersomeness, and the extent to which a symptom impacts or limits quality of life and usual activities. Figure 5 presents a proposed model and classification schema of meaningfulness that could enable better understanding of what matters to people with PD. Use of a systematic evaluation approach, such as proposed here, could improve understanding of the extent to which outcome measures target personally important and bothersome symptoms via a stepwise approach to meaningfulness.
Fig. 5.
Conceptual model of meaningful symptoms and stepwise classification schema.
Limitations and future directions
This study was conducted with people who were recruited from a prior study investigating the use of smartphones/smartwatches for PD symptom monitoring. Participants were predominantly higher socio-economic status, higher health/technologicallyliterate, white individuals, with qualitative data collected at a single time-point. While inclusion/exclusion criteria were compatible, the sampling approach affects generalizability and findings reported here may not reflect experiences of individuals from underrepresented groups or those with lower technological and health literacy. Sample size was also small and no significant differences in symptoms/impacts were seen between those taking and not taking PD medications. Lastly, in addition to a need for replication with larger and more demographically diverse samples, longitudinal data will be needed to support understanding of how meaningful symptoms change over time. Our data tentatively suggested that as life contexts change (e.g., transitions from working to retirement, change in living situations, or duration of disease) symptoms that are “most” bothersome may also change, which has been suggested elsewhere [5]. Thus, reevaluation of meaningful symptoms over time with the goal of developing population-based models to predict trends in PD symptom progression are warranted to guide long-term therapeutic objectives.
Conclusion
The findings and approaches described in this study can support rigorous, systematic identification and grading of meaningful symptoms and impacts of early PD, which is critical to selection of valid patient-centered endpoints for therapeutic trials. We believe the conceptual model and categorical classification of meaningfulness proposed here will be broadly relevant. Future work is needed to determine the extent to which this classification system can support interpretable evaluation of different outcomes assessments relevant to patients.
Supplementary Material
ACKNOWLEDGMENTS
The researchers thank the many individuals who contributed to this work. The content is based solely on the perspectives of the authors and do not necessarily represent the official views of the Critical Path Institute, the US FDA, or other sponsors. BrainBaseline application screenshots reprinted with permission from Clinical ink.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-225068.
FUNDING
This study was funded by the Critical Path for Parkinson’s (CPP) Consortium. The CPP 3DT initiative is funded by the CPP Consortium members including Biogen; GSK; Takeda; Lundbeck; UCB Pharma; Roche; AbbVie and Merck, Parkinson’s UK, and the Michael J Fox Foundation. Critical Path Institute is supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) and is 54.2% funded by the FDA/HHS, totaling $13,239,950, and 45.8% funded by non-government source(s), totaling $11,196,634. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government.
CONFLICT OF INTEREST
GTS is an employee of Rush University and has consulting and advisory board membership with honoraria for: Acadia Pharmaceuticals; Adamas Pharmaceuticals, Inc.; Biogen, Inc.; Ceregene, Inc.; CHDI Management, Inc.; the Cleveland Clinic Foundation; Ingenix Pharmaceutical Services (i3 Research); MedGenesis Therapeutix, Inc.; Neurocrine Biosciences, Inc.; Pfizer, Inc.; Tools-4-Patients; Ultragenyx, Inc.; and the Sunshine Care Foundation. He has received grants from and done research for: the National Institutes of Health, the Department of Defense, the Michael J. Fox Foundation for Parkinson’s Research, the Dystonia Coalition, CHDI, the Cleveland Clinic Foundation, the International Parkinson and Movement Disorder Society, and CBD Solutions, and has received honoraria from: the International Parkinson and Movement Disorder Society, the American Academy of Neurology, the Michael J. Fox Foundation for Parkinson’s Research, the FDA, the National Institutes of Health, and the Alzheimer’s Association. JC is Director of Digital Health Strategy at AbbVie and Industry Co-Director of CPP. TD is Executive Medical Director at Biogen. JH is Senior Scientist, Patient Insights at H. Lundbeck A/S, Valby, Denmark. TS has served as a consultant for Acadia, Blue Rock Therapeutics, Caraway Therapeutics, Critical Path for Parkinson’s Consortium (CPP), Denali, General Electric (GE), Neuroderm, Sanofi, Sinopia, Sunovion, Roche, Takeda, MJFF, Vanqua Bio and Voyager. She served on the ad board for Acadia, Denali, General Electric (GE), Sunovion, Roche. She has served as a member of the scientific advisory board of Caraway Therapeutics, Neuroderm, Sanofi and UCB. She has received research funding from Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Amneal, Prevail, UCB, NINDS, MJFF, Parkinson’s Foundation. ERD Has stock ownership in Grand Rounds, an online second opinion service, has received consultancy fees from 23andMe, Abbott, Abbvie, Amwell, Biogen, Clintrex, CuraSen, DeciBio, Denali Therapeutics, GlaxoSmithKline, Grand Rounds, Huntington Study Group, Informa Pharma Consulting, medical-legal services, Mednick Associates, Medopad, Olson Research Group, Origent Data Sciences, Inc., Pear Therapeutics, Prilenia, Roche, Sanofi, Shire, Spark Therapeutics, Sunovion Pharmaceuticals, Voyager Therapeutics, ZS Consulting, honoraria from Alzeimer’s Drug Discovery Foundation, American Academy of Neurology, American Neurological Association, California Pacific Medical Center, Excellus BlueCross BlueShield, Food and Drug Administration, MCM Education, The Michael J Fox Foundation, Stanford University, UC Irvine, University of Michigan, and research funding from Abbvie, Acadia Pharmaceuticals, AMC Health, BioSensics, Burroughs Wellcome Fund, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Nuredis, Inc., Patient-Centered Outcomes Research Institute, Pfizer, Photopharmics, Roche, Safra Foundation. JLA Has received honoraria from Huntington Study Group, research support from National Institutes of Health, The Michael J Fox Foundation, Biogen, Safra Foundation, Empire Clinical Research Investigator Program, and consultancy fees from VisualDx.
TS is an Editorial Board Member of this journal but was not involved in the peer review
process nor had access to any information regarding its peer-review.
The following authors (JRM, RMS, MLTMM, PY, MC, JEC, SJR, MK, KWB, DS) have no conflict of interest to disclose.
DATA AVAILABILITY
Data are available to Critical Path for Parkinson’s (CPP) Consortium 3DT Initiative Stage 2 members. Non-members may submit proposals for de-identified datasets to CPP 3DT via the corresponding author.
REFERENCES
- [1]. Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33, 1248–1266. [DOI] [PubMed] [Google Scholar]
- [2]. Mantri S, Wood S, Duda JE, Morley JF (2019) Comparing self-reported and objective monitoring of physical activity in Parkinson disease. Parkinsonism Relat Disord 67, 56–59. [DOI] [PubMed] [Google Scholar]
- [3]. Rovini E, Maremmani C, Cavallo F (2017) How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front Neurosci 11, 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Dorsey E, Wearable Assessments in the Clinic and Home in PD (WATCH-PD), https://clinicaltrials.gov/ct2/show/NCT03681015.
- [5]. Port RJ, Rumsby M, Brown G, Harrison IF, Amjad A, Bale CJ (2021) People with Parkinson’s disease: What symptoms do they most want to improve and how does this change with disease duration? J Parkinsons Dis 11, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Byrom B, Watson C, Doll H, Coons SJ, Eremenco S, Ballinger R, Mc Carthy M, Crescioni M, O’Donohoe P, Howry C, e PROC (2018) Selection of and evidentiary considerations for wearable devices and their measurements for use in regulatory decision making: Recommendations from the ePRO Consortium. Value Health 21, 631–639. [DOI] [PubMed] [Google Scholar]
- [7]. USDHHS (2018) Patient-focused drug development: Collecting comprehensive and representative input. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input.
- [8]. USDHHS (2022) Patient-focused drug development: Methods to identify what is important to patients (Guidance for industry, food and drug administration staff, and other stakeholders). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients.
- [9]. Espay AJ, Hausdorff JM, Sanchez-Ferro A, Klucken J, Merola A, Bonato P, Paul SS, Horak FB, Vizcarra JA, Mestre TA, Reilmann R, Nieuwboer A, Dorsey ER, Rochester L, Bloem BR, Maetzler W, Movement Disorder Society Task Force on Technology (2019) A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord 34, 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Tarolli CG, Zimmerman GA, Auinger P, McIntosh S, Horowitz RK, Kluger BM, Dorsey ER, Holloway RG (2020) Symptom burden among individuals with Parkinson disease: A national survey. Neurol Clin Pract 10, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Staunton H, Kelly K, Newton L, Leddin M, Rodriguez-Esteban R, Chaudhuri KR, Weintraub D, Postuma RB, Martinez-Martin P (2022) A patient-centered conceptual model of symptoms and their impact in early Parkinson’s disease: A qualitative study. J Parkinsons Dis 12, 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Morel T, Cleanthous S, Andrejack J, Barker RA, Blavat G, Brooks W, Burns P, Cano S, Gallagher C, Gosden L, Siu C, Slagle AF, Trenam K, Boroojerdi B, Ratcliffe N, Schroeder K (2022) Patient experience in early-stage Parkinson’s disease: Using a mixed methods analysis to identify which concepts are cardinal for clinical trial outcome assessment. Neurol Ther 11, 1319–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Adams J, Kangarloo T, Tracey B, O’Donnell P, Volfson D, Latzman R, Zach N, Alexander R, Bergethon P, Cosman J, Anderson D, Best A, Kostrzebski M, Auinger P, Wilmot P, Pohlson Y, Waddell E, Jensen-Roberts S, Gong Y, Kilambi KP, Herrero TR, Dorsey E (2022) A multicenter study using a smartwatch, smartphone, and wearable sensors to assess early Parkinson’s disease: Baseline results of the WATCH-PD Study. Research Square, 10.21203/rs.3.rs-2289246/v1. [DOI] [Google Scholar]
- [14]. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [15]. Flick U (2022) The SAGE Handbook of Qualitative Research Design, SAGE Publications, London. [Google Scholar]
- [16]. Guest G, Bunce A, Johnson L (2005) How many interviews are enought?: An experiment with data saturation and variability. Field Methods 18, 59–82. [Google Scholar]
- [17]. Sandelowski M (2014) Unmixing mixed-methods research. Res Nurs Health 37, 3–8. [DOI] [PubMed] [Google Scholar]
- [18]. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium (2019) The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95, 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Mammen J, Speck R, Stebbins G, Müller M, Yang P, Campbell M, Cosman J, Crawford J, Dam T, Hellsten J, Jensen-Roberts S, Kostrzebski M, Simuni T, Ward Barowicz K, Cedarbaum J, Dorsey E, Stephenson D, Adams J (2022) Mapping meaningful symptoms and impacts of disease to digital outcome measures, https://digitalcommons.uri.edu/nursing_facpubs/343/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Mammen J, Norton S, Rhee H, Butz A (2016) New approaches to qualitative interviewing: Development of a card sort technique to understand subjective patterns of symptoms and responses. Int J Nurs Stud 58, 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Hsieh HF, Shannon SE (2005) Three approaches to qualitative content analysis. Qual Health Res 15, 1277–1288. [DOI] [PubMed] [Google Scholar]
- [23]. Braun V, Clarke V, Hayfield N, Terry G (2019) Thematic analysis. In Handbook of Research Methods in Health Social Sciences, Liamputtong P, ed. Springer, Singapore. [Google Scholar]
- [24]. Ayres L, Kavanaugh K, Knafl KA (2003) Within-case and across-case approaches to qualitative data analysis. Qual Health Res 13, 871–883. [DOI] [PubMed] [Google Scholar]
- [25]. Saldaña J (2013) The Coding Manual for Qualitative Researchers, Sage, Washington, DC. [Google Scholar]
- [26]. Maxwell JA (2012) Qualitative Research Design: An Interactive Approach, Sage, Thousand Oaks, CA. [Google Scholar]
- [27]. Maslow AH (1943) A theory of human motivation. Psychol Rev 50, 430–437. [Google Scholar]
- [28]. Javidnia M, Arbatti L, Hosamath A, Eberly SW, Oakes D, Shoulson I (2021) Predictive value of verbatim Parkinson’s disease patient-reported symptoms of postural instability and falling. J Parkinsons Dis 11, 1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Muller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB (2013) Importance of motor vs. non-motor symptoms for health-related quality of life in early Parkinson’s disease. Parkinsonism Relat Disord 19, 1027–1032. [DOI] [PubMed] [Google Scholar]
- [30]. Duncan GW, Khoo TK, Yarnall AJ, O’Brien JT, Coleman SY, Brooks DJ, Barker RA, Burn DJ (2014) Health-related quality of life in early Parkinson’s disease: The impact of nonmotor symptoms. Mov Disord 29, 195–202. [DOI] [PubMed] [Google Scholar]
- [31]. Lenz ER, Suppe F, Gift AG, Pugh LC, Milligan RA (1995) Collaborative development of middle-range nursing theories: Toward a theory of unpleasant symptoms. ANS Adv Nurs Sci 17, 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available to Critical Path for Parkinson’s (CPP) Consortium 3DT Initiative Stage 2 members. Non-members may submit proposals for de-identified datasets to CPP 3DT via the corresponding author.