Abstract
Background
This study investigates the effects of cryopreservation and supplementation of Azeri water buffalo's semen with proline (Lp) and fulvic acid (FA).
Objectives
Therefore, this study aimed to assess motility parameters, sperm viability, oxidative stress parameters, and DNA damage to detect the optimum concentrations of Lp and FA for buffalo semen cryopreservation.
Methods
Thirty semen samples of three buffalo bulls were diluted in Tris‐egg yolk extender and divided into 12 equal groups including control (C), Lp‐10, Lp‐20, Lp‐40, Lp‐60, Lp‐80 (containing 10, 20, 40, 60, 80 mM L‐proline, respectively), FA‐0.2, FA‐0.5, FA‐0.8, FA‐1.1, FA‐1.4 and FA‐1.7 (containing 0.2%, 0.5%, 0.8%, 1.1%, 1.4% and 1.7% fulvic acid, respectively).
Results
The velocity parameters, TM and PM were improved by FA‐1.7, FA‐1.4, Lp‐40 and Lp‐60 groups compared to the C group but no significant difference was found regarding the amplitude of lateral head displacement and straightness compared to the control groups. The percentage of sperm viability and PMF were increased by FA‐1.7, FA‐1.4, FA‐1.1, Lp‐40 and Lp‐60 groups compared to C group, while in terms of sperm DNA damage FA‐1.7, FA‐1.4, FA‐1.1, Lp‐10, Lp‐20, Lp‐40 and Lp‐60 groups showed better results compared to C group. The results also showed that FA‐1.7, FA‐1.4, FA‐1.1, Lp‐20, Lp‐40 and Lp‐60 groups could improve TAC, SOD, GSH and decrease MDA levels. Also, FA‐1.7, FA‐1.4, Lp‐20 and Lp‐40 groups could improve GPx levels but just FA‐1.7, and Lp‐40 groups could improve CAT levels compared to C group.
Conclusions
Thus, it can be concluded that L‐proline and fulvic acid supplementations can improve the quality parameters of post‐thawed buffalo bull semen.
Keywords: Azari water buffalo, fulvic acid, L‐proline, post‐thawed semen
1. INTRODUCTION
Genetic diversity and characteristics of native breeds are recognised as valuable resources that cannot be recovered if extinct (Antkowiak et al., 2012). In addition to the physical protection of native species and ecotypes, the protection of their genetic pool should also be considered (Aminafshar et al., 2008). Azari ecotype is small in size with black skin and high‐quality milk production rate as well as high fat and a long milking period per year (Borghese & Mazzi, 2005; Pour Azary et al., 2004). This native endangered ecotype may further proceed to extinction in the future due to climate change. Therefore, it necessitates preserving genetic reserves of the buffalo that is considered as a vital genetic source in the production of milk and other products. Therefore, sperm freezing of this valuable ecotype is very important (Tavakolian, 2000).
Moreover, cryopreservation and thawing of semen produces reactive oxygen species (ROS), thus exposing the sperm to oxidative stress. The physiological levels of ROS affect the fertilisation potency and capacitation and acrosomal reaction of spermatozoa; at extremely high levels, ROS lead to apoptosis, decreased motility, chromatin damage, and impaired fertilisation ability (Elsayed et al., 2019). According to previous evidence (Izanloo et al., 2022; Partyka et al., 2011), it also increases sperm susceptibility to lipid peroxidation (LPO). Glutathione peroxidase (GPx), glutathione reductase, superoxide dismutase (SOD), and catalase (CAT) as enzymatic or natural antioxidants, as well as glutathione (GSH), ascorbic acid, carotenoids, vitamin E, selenium, coenzyme Q, and zinc as nonenzymatic or synthetic antioxidants have a defence mechanism against free radicals and LPO (Kasimanickam et al., 2006; Tuncer et al., 2010; Partyka et al., 2015; Papas et al., 2020, 2019).
Buffalo semen has an anti‐oxidative stress system that includes enzymatic and nonenzymatic antioxidants (Turaja et al., 2019), but during cryopreservation, due to the low concentration of these antioxidants in semen, the endogenous defence system is insufficient for countering this stress (Ansari et al., 2012). Low temperatures during the buffalo semen freezing and thawing cycle cause excessive adenosine triphosphate consumption, cell membrane lesions, DNA apoptosis, increased osmotic pressure, early acrosome reactions, and oxidative stress, reducing sperm motility and fertilisation capacity (De Lamirande et al., 1997; Tariq et al., 2015; Ezz et al., 2017). Therefore, an improvement in sperm cryopreservation leads to increased productivity in the breeding program of this animal species (Topraggaleh et al., 2014). According to some studies, antioxidants protect cells against oxidative damage and are also essential for sperm integrity and motility. For this reason, adding antioxidants to buffalo semen is necessary to improve fertilisation capacity and semen quality (Beheshti et al., 2011; Ansari et al., 2012; Saeed et al., 2016; Soleimanzadeh et al., 2020).
L‐proline is one of the essential amino acids that has proteinogenic properties (Moradi et al., 2022); it also has various functions such as being an osmoprotectant, stabilising cellular structures and enzymes, scavenging ROS and keeping up redox balance in adverse situations (Meena et al., 2019). Some researchers have reported that L‐proline has antioxidant and natural osmoprotective properties in relation to the destructive effects of freezing on various species, including human, donkey and ram sperm as well as mice oocytes (Sangeeta et al., 2015; Zhang et al., 2016; Li et al., 2021; Moradi et al., 2022).
Fulvic acids, a type of humic acids, are organic substances obtained from peat. Fulvic acids are often found in soil and natural water systems and have greater biological activities and are soluble in both acid and alkali solutions (Islam et al., 2005; Bai et al., 2013). On the other hand, Fulvic acids are rich in many reactive functional groups (phenols, quinones and hydroxyl), and these groups cause anti‐oxidative activity (Chang et al., 2013; Jayasooriya et al., 2016). It has been showed that humic and fulvic acid could improve sexual desire and semen quality under heat‐stress conditions (Abdel‐Khalek et al., 2019). On the other hand, Xiao and coworkers observed that fulvic acid have a cryoprotective effect on freezing goat buck semen (Xiao et al., 2018).
Today, although the technology of freezing and preserving semen for artificial insemination, especially in bulls and buffalo, has advanced beyond other species, there are still prominent gaps in the foundation of this knowledge and technology. Still, sperm survival rates after thawing are low and vary considerably among breeding bulls. These weaknesses are significant because they prevent progress in the fundamental science of reproductive biotechnology. However, the protective effects of L‐proline and fulvic acids on buffalo semen are still unknown. Hence, this study aims to determine the effects of L‐proline and fulvic acid supplementations to the extender following the freezing of Azeri water buffalo, on sperm characteristics and oxidative stress parameters.
2. MATERIAL AND METHODS
2.1. Chemicals
All required chemical compounds were obtained from Sigma (St. Louis, MO, USA) and Merck (Darmstadt, Germany).
2.2. Collection and processing of the semen
All animals were healthy and under the same management conditions. The artificial vagina method was used for semen collection twice a week. Overall, 30 ejaculations from three Iranian water buffalo bulls (Bubalus bubalis, Azari ecotype), the fertility of which had already been confirmed, were obtained during five weeks. The samples were examined for sperm quality; only those with a concentration greater than 500 × 106 spermatozoa/mL, volume between 2–6 mL, total motility greater than 65% and abnormal morphology less than 20% per ejaculation were considered normal and investigated.
A standard Tris‐based extender (Tris: 2.66 g per 100 mL, citric acid: 1.47 g per 100 mL, glucose: 0.63 g per 100 mL, egg yolk: 20% (v/v), glycerol: 7% v/v, penicillin: 1 mg per 100 mL, streptomycin: 1 mg per 100 mL and pH: 6.8), according to previous research (Topraggaleh et al., 2014), was used as base extender in this study. In each replicate, semen samples (n = 3) were pooled and equally divided to 12 parts and each part was diluted to a final concentration of 15 × 106 spermatozoa/mL (Tahmasbian et al., 2022) with one of the following extenders including: Group 1: Control (Tris‐based extender without antioxidant); Lp‐10: Tris‐based extender + L‐proline (10 mM); Lp‐20: Tris‐based extender + L‐proline (20 mM); Lp‐40: Tris‐based extender + L‐proline (40 mM); Lp‐60: Tris‐based extender + L‐proline (60 mM); Lp‐80: Tris‐based extender + L‐proline (80 mM) (Li et al., 2021); FA‐0.2: Tris‐based extender + fulvic acid (0.2% w/w); FA‐0.5: Tris‐based extender + fulvic acid (0.5% w/w); FA‐0.8: Tris‐based extender + fulvic acid (0.8% w/w); FA‐1.1: Tris‐based extender + fulvic acid (1.1% w/w); FA‐1.4: Tris‐based extender + fulvic acid (1.4% w/w); FA‐1.7: Tris‐based extender + fulvic acid (1.7% w/w) (Xiao et al., 2018). The straws were put in a refrigerator set at 4˚C for 2 h and then semen was frozen according to the following protocol: from + 4°C to −12˚C at a rate of – 4˚C /min, from – 12˚C to – 40˚C at a rate of – 40˚C/min and from – 40˚C up to – 140˚C at a rate of – 50 ˚C/min. Then they were transferred into goblets floating in liquid nitrogen. After thawing at 38˚C for 30 s, three samples from each frozen pie were analysed for the quality parameters of semen (Soleimanzadeh et al., 2020).
2.3. Semen analysis
2.3.1. Motility and motion parameters
The motility characteristics of semen were evaluated by a CASA system (Test Sperm 3.2; Videotest, St. Petersburg, Russia), using 10 microliters of the thawed semen. Various parameters such as total motility (TM, %), progressive motility (PM, %), curvilinear velocity (VCL, µm/s), straight‐line velocity (VSL, µm/s), average path velocity (VAP, µm/s), straightness (STR, %), linearity (LIN, %), amplitude of lateral head displacement (ALH, µm/s) and beat‐cross frequency (BCF, Hz) were examined by studying at least 500 spermatozoa in five microscopic fields (Table 1).
TABLE 1.
Parameter settings for the CASA.
| Parameter | Setting |
|---|---|
| Frame rate | 60 Hz |
| Duration of capture | 1 s |
| Stage temperature rate | 37°C |
| Minimum cell size | 5 pixels |
| Cell size | 5 pixels |
| Minimum contrast | 80 |
| Cell intensity | 70 pixels |
| Chamber type | Slide coverslip (22 × 22 mm) |
| Volume per slide | 7 µL |
| Chamber depth | ≈ 20 µm |
| Minimum number of field analysis | 500 cells |
| Sample dilution | 20 × 106 |
| Image type | Phased contrast |
2.3.2. DNA damage evaluation
DNA fragmentation is one of the damages to sperm DNA and causes infertility. Acridine orange staining was performed to investigate DNA damage and identify denatured, double‐stranded DNA segments in the chromatin of the sperm at a low pH rate (Narayana et al., 2002). A thick smear was fixed for 2 h in a Carnoy's fixative consisting of methanol and acetic acid (1: 3). Then, it was extracted and dried at room temperature for 5 min. Next, it was placed in the stock solution containing 1 mg of orange acridine and 1000 mL of distilled water, and the resulting mixture was placed in a dark place at 4°C for 5 min. A fluorescent microscope (Model GS7, Nikon Co., Tokyo, Japan) in ×200 magnification was used to evaluate sperms at 490 nm. Among them, yellow and red sperms were considered damaged and abnormal (Talebi et al., 2011).
2.3.3. Sperm plasma membrane functionality
Hypoosmotic swelling test (HOST) was applied to identify spermatozoa with intact membranes. For this purpose, 10 µL of semen was diluted in a 100 µL of the hypoosmotic solution )fructose 1.35 g and sodium citrate 0.73 g) solution and the resulting mixture was incubated at 37°C (M. Khan & Ijaz, 2007). Subsequently, a contrast‐phase microscope (Olympus, BX41, Tokyo, Japan) with 200× magnification was employed to examine the PM of the sperm. In general, 200 curled or straight sperms were counted.
2.3.4. Sperm viability
Sperm viability was evaluated based on a WHO protocol by eosin‐nigrosine staining (WHO, 1999). Eosin (Merck, Darmstadt, Germany) and Nigrosine (Merck, Darmstadt, Germany) were prepared in distilled water. This was done by mixing two volumes of 1% eosin with one volume of sperm, and they were counted with a light microscope in ×200 magnification (Model CHT, Olympus optical Co. Ltd). The red stained spermatozoa were considered nonviable, while the viable ones were colourless.
2.4. Assessment of enzymatic antioxidants activity
Semen biochemical analyses were performed using six straws (one for each extender). After thawing, 120 µL of the semen was centrifuged at 1600 × g at 25°C for 5 min. The supernatant was removed, and 360 µL of 1% Triton X‐100 was precipitated for 20 min for extraction. The resulting mixture was centrifuged at 4000 × g at 25°C for 30 min. The suspending of the precipitate was repeated once more, and the supernatant was obtained as the crude extract of the enzymes present in the sperm.
2.4.1. Malondialdehyde (MDA)
An MDA test kit (Nalondi™ Lipid Peroxidation Assay Kit (NID), Navand Salamat Company, Urmia, Iran) was applied to evaluate oxidative stress by assessing MDA concentration. A spectrophotometer (Thermo Fisher Scientific; Waltham, MA, USA) and a standard curve were used to read the supernatant at 523 nm and to express the amount of MDA as nmol/mL, respectively.
2.4.2. Total antioxidant capacity (TAC)
A TAC kit was employed to measure TAC (Naxifer™ – Cat# NS‐15012; Total Antioxidant Capacity Assay Kit‐TAC, Navand Salamat Company, Urmia, Iran). The values of TAC were expressed as mmol/L.
2.4.3. Glutathione peroxidase (GPx)
A GPx kit (Nagpix™ – CAT# NS‐15083, Glutathione Peroxidase Activity Assay Kit; Navand Salamat Company, Urmia, Iran (was utilised to estimate GPx in semen after thawing according to the manufacturer's instructions. GPx was expressed as mU/mL.
2.4.4. Superoxide dismutase (SOD)
A SOD assay kit (Nasdox ™ Superoxide Dismutase Assay Kit; Navand Salamat Company, Urmia, Iran) was applied to evaluate SOD. The reduction of colour development at 405 nm was used to determine the SOD activity, which is considered an inhibitory activity. Plasma SOD activity was expressed as U/mL.
2.4.5. Catalase evaluation
A 2‐step method using a commercial CAT kit (Nactaz™ Catalase Activity Assay Kit; Navand Salamat Company, Urmia, Iran) was considered to evaluate the activity of CAT. An absorbance rate of 550 nm was taken into consideration for determining CAT after incubation for 10 min at room temperature. The CAT activity in the plasma was expressed as U/mL.
2.4.6. Glutathione evaluation
A GSH commercial kit (NarGul™ – Glutathione Assay Kit‐GSH; Navand Salamat Company, Urmia, Iran) was utilised for GSH activity, and its value was recorded as U/L.
2.5. Statistical analysis
The obtained data were analysed by SPSS (Version 26.0; IBM CO., Chicago, USA) using one‐way ANOVA and Tukey's post hoc test and p ≤ 0.05 was considered statistically significant.
3. RESULTS
According to the results obtained by CASA (Table 2), adding FA‐1.7, FA‐1.4, FA‐1.1, Lp‐20 and Lp‐40 could improve TM and PM compared to C group (p ≤ 0.05; Table 2). The lowest percentage of TM was observed in Lp‐80 and FA‐0.2 (p ≤ 0.05; Table 2).
TABLE 2.
Effects of different concentrations of L‐proline (Lp) and fulvic acid (FA) on buffalo sperm total and progressive at post‐thawed stage of cryopreservation. Values are expressed as mean ± SEM.
| Parameters | Control | Lp‐10 | Lp‐20 | Lp‐40 | Lp‐60 | Lp‐80 | FA‐0.2 | FA‐0.5 | FA‐0.8 | FA‐1.1 | FA‐1.4 | FA‐1.7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total motility (%) | 55.47±1.19de | 57.41±1.16d | 64.59±1.76bc | 68.45±1.69ab | 62.30±1.54c | 51.18±1.25f | 48.11±1.37g | 53.72±1.48ef | 58.24±1.56d | 64.07±1.89bc | 66.21±1.15b | 71.49±1.31a |
| Progressive motility (%) | 25.31±1.84d | 25.74±1.52d | 31.26±1.38c | 36.43±1.75b | 30.14±1.96c | 19.02±1.47e | 15.69±1.20f | 22.10±1.33d | 26.77±1.09d | 30.07±1.50c | 33.84±1.16bc | 40.23±1.47a |
Control (C): Tris‐based extender without antioxidant; Lp‐10: Tris‐based extender + L‐proline (10 mM); Lp‐20: Tris‐based extender + L‐proline (20 mM); Lp‐40: Tris‐based extender + L‐proline (40 mM); Lp‐60: Tris‐based extender + L‐proline (60 mM); Lp‐80: Tris‐based extender + L‐proline (80 mM); FA‐0.2: Tris‐based extender + fulvic acid (0.2%); FA‐0.5: Tris‐based extender + fulvic acid (0.5%); FA‐0.8: Tris‐based extender + fulvic acid (0.8%); FA‐1.1: Tris‐based extender + fulvic acid (1.1%); FA‐1.4: Tris‐based extender + fulvic acid (1.4%); FA‐1.7: Tris‐based extender + fulvic acid (1.7%). Different superscripts within the same row demonstrate significant differences (p ≤ 0.05).
Results presented in Table 3 showed motility characteristics (VCL, VSL, VAP, STR, LIN and BCF) in post‐thawed buffalo semen. The values of VCL, BCF and LIN in FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 groups showed an improvement compared to C group (p ≤ 0.05; Table 3), while Lp‐10 and FA‐0.8 groups showed no significant compared to C group (p > 0.05; Table 3). Also, the values of VCL and LIN in FA‐0.2 and Lp‐80 groups and BCF in FA‐0.5, FA‐0.2 and Lp‐80 groups showed decrease compared to C group (p ≤ 0.05; Table 3). The values of VAP in FA‐1.7, FA‐1.4, Lp‐40 and Lp‐20 groups and VSL in FA‐1.7, FA‐1.4, Lp‐60 and Lp‐40 groups showed an increase compared to C group (p ≤ 0.05; Table 3). According to the results, the values of ALH and STR showed no significant improvement in all treated groups compared to C group (p > 0.05; Table 3).
TABLE 3.
Mean (±SEM) sperm velocity characteristics of frozen‐thawed buffalo semen after addition of different concentrations of L‐proline (Lp) and fulvic acid (FA) in semen extender.
| Parameters | Control | Lp‐10 | Lp‐20 | Lp‐40 | Lp‐60 | Lp‐80 | FA‐0.2 | FA‐0.5 | FA‐0.8 | FA‐1.1 | FA‐1.4 | FA‐1.7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAP (µm/s) | 25.69±1.30de | 25.20±1.65de | 29.19±1.65bc | 32.28±1.54b | 28.57±1.31bcd | 22.02±1.12e | 22.35±1.47e | 23.78±1.34e | 26.17±1.79cd | 27.33±1.22cd | 31.96±1.39b | 36.43±1.40a |
| VCL (µm/s) | 39.41±1.65e | 41.05±1.01e | 46.20±1.12cd | 51.65±1.73b | 44.25±1.57d | 35.38±0.95f | 32.77±0.30g | 40.11±1.28e | 40.94±1.64e | 42.56±1.10d | 49.94±1.83bc | 55.69±1.19a |
| VSL (µm/s) | 20.93±1.03cd | 21.57±1.64bcd | 25.35±1.49bc | 27.08±1.10ab | 23.57±1.42bc | 16.50±1.18d | 17.35±1.61d | 19.89±1.84d | 21.33±1.17cd | 22.83±1.25c | 26.49±1.01b | 30.17±1.33a |
| LIN (%) | 43.20±1.75e | 44.79±1.62e | 51.72±1.56cd | 55.72±1.69b | 49.03±1.20cd | 39.17±1.58f | 37.93±1.05f | 41.65±1.19ef | 45.78±1.85de | 48.27±1.64d | 53.74±1.22bc | 59.20±1.94a |
| ALH (µm/s) | 2.45±0.11a | 2.31±0.14a | 2.46±0.18a | 2.34±0.07a | 2.23±0.18a | 2.32±0.15a | 2.25±0.73a | 2.24±0.46a | 2.33±0.70a | 2.37±0.25a | 2.30±0.19a | 2.34±0.72a |
| STR (%) | 72.73±2.28a | 72.99±2.55a | 72.53±1.41a | 73.45±1.41a | 72.67±1.22a | 72.24±1.66a | 72.68±1.50a | 72.30±1.85a | 72.55±1.57a | 72.48±1.82a | 72.64±1.15a | 73.18±1.09a |
| BCF (Hz) | 3.28±0.13e | 3.24±0.17e | 5.06±0.19d | 7.21±0.16b | 5.11±0.28d | 2.54±0.12f | 2.20±0.15f | 2.77±0.13f | 3.30±0.19e | 5.33±0.25d | 6.51±0.20c | 8.09±0.23a |
Control (C): Tris‐based extender without antioxidant; Lp‐10: Tris‐based extender + L‐proline (10 mM); Lp‐20: Tris‐based extender + L‐proline (20 mM); Lp‐40: Tris‐based extender + L‐proline (40 mM); Lp‐60: Tris‐based extender + L‐proline (60 mM); Lp‐80: Tris‐based extender + L‐proline (80 mM); FA‐0.2: Tris‐based extender + fulvic acid (0.2%); FA‐0.5: Tris‐based extender + fulvic acid (0.5%); FA‐0.8: Tris‐based extender + fulvic acid (0.8%); FA‐1.1: Tris‐based extender + fulvic acid (1.1%); FA‐1.4: Tris‐based extender + fulvic acid (1.4%); FA‐1.7: Tris‐based extender + fulvic acid (1.7%). Different superscripts within the same row demonstrate significant differences (p ≤ 0.05). VAP: average path velocity; VCL: curvilinear velocity; VSL: straight‐line velocity; LIN: linearity; ALH: amplitude of lateral Head displacement; BCF: beat‐cross frequency; STR: straightness.
The results showed that sperm viability in FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 groups and PMF in FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60 and Lp‐40 groups have been improved (p ≤ 0.05; Table 4; Figures 1 and 2). The results showed a decrease in DNA damage in FA‐1.7, FA‐1.4, FA‐1.1, FA‐0.8, Lp‐60, Lp‐40, Lp‐20 and Lp‐10 groups compared to C group (p ≤ 0.05; Table 4; Figure 3).
TABLE 4.
Effect of different concentrations of L‐proline (Lp) and fulvic acid (FA) supplementation to DNA damage, sperm viability and plasma membrane functionality (PMF) (mean ± SEM) in frozen‐thawed buffalo semen.
| Groups | Control | Lp‐10 | Lp‐20 | Lp‐40 | Lp‐60 | Lp‐80 | FA‐0.2 | FA‐0.5 | FA‐0.8 | FA‐1.1 | FA‐1.4 | FA‐1.7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sperm viability (%) | 66.83±2.15de | 67.90±2.53de | 74.58±2.46bc | 76.19±2.63b | 72.95±2.40c | 60.68±2.05f | 57.11±2.37f | 64.70±2.95e | 68.23±2.11d | 73.49±2.65bc | 74.10±2.03bc | 80.79±2.42a |
| DNA damage (%) | 9.30±0.18c | 8.73±0.31d | 6.65±0.70e | 3.27±0.94g | 6.09±0.27e | 11.35±0.41b | 12.47±0.21a | 10.84±0.27b | 8.10±0.19d | 5.84±0.21f | 3.31±0.25g | 3.05±0.11g |
| PMF (%) | 61.47±2.31c | 62.67±2.29c | 65.38±2.73bc | 70.42±2.67a | 66.74±2.12b | 56.83±1.95d | 55.74±1.43d | 59.02±1.69c | 63.79±2.15bc | 66.31±2.37b | 66.50±2.45b | 71.48±2.19a |
Control (C): Tris‐based extender without antioxidant; Lp‐10: Tris‐based extender + L‐proline (10 mM); Lp‐20: Tris‐based extender + L‐proline (20 mM); Lp‐40: Tris‐based extender + L‐proline (40 mM); Lp‐60: Tris‐based extender + L‐proline (60 mM); Lp‐80: Tris‐based extender + L‐proline (80 mM); FA‐0.2: Tris‐based extender + fulvic acid (0.2%); FA‐0.5: Tris‐based extender + fulvic acid (0.5%); FA‐0.8: Tris‐based extender + fulvic acid (0.8%); FA‐1.1: Tris‐based extender + fulvic acid (1.1%); FA‐1.4: Tris‐based extender + fulvic acid (1.4%); FA‐1.7: Tris‐based extender + fulvic acid (1.7%). Different superscripts within the same row demonstrate significant differences (p ≤ 0.05).
FIGURE 1.
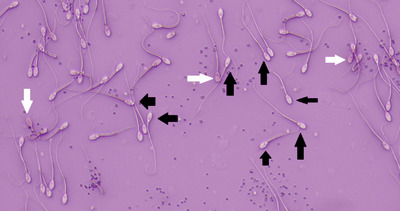
Sperm viability. White arrow – viable spermatozoa (colourless), black arrow – dead spermatozoa (red) (eosin/nigrosin, 200×).
FIGURE 2.
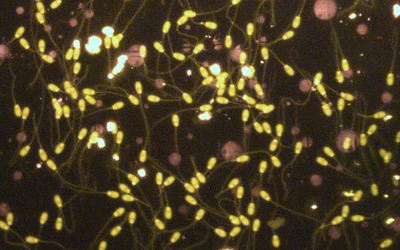
Buffalo spermatozoa DNA damage; normal spermatozoa (green) (acridine orange, 200×).
FIGURE 3.
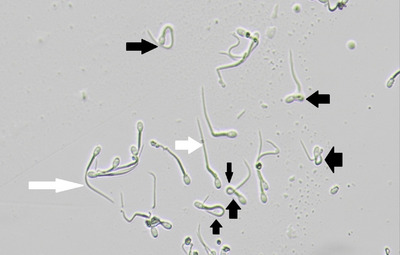
PM functionality. Black arrow – nonfunctional PM (spermatozoa with straight tails); white arrow – functional PM (spermatozoa with coiled tails) (200×).
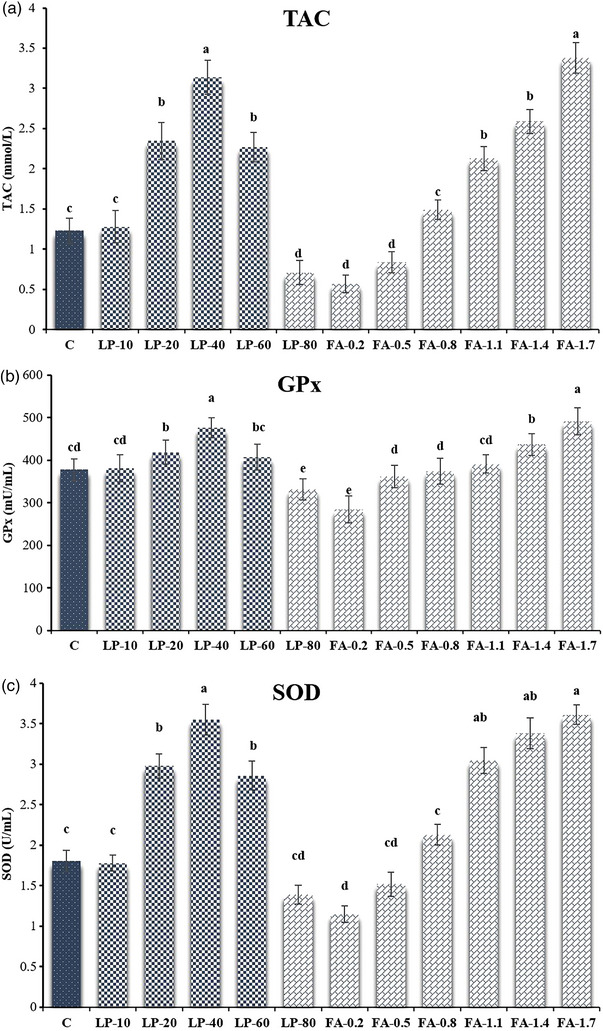
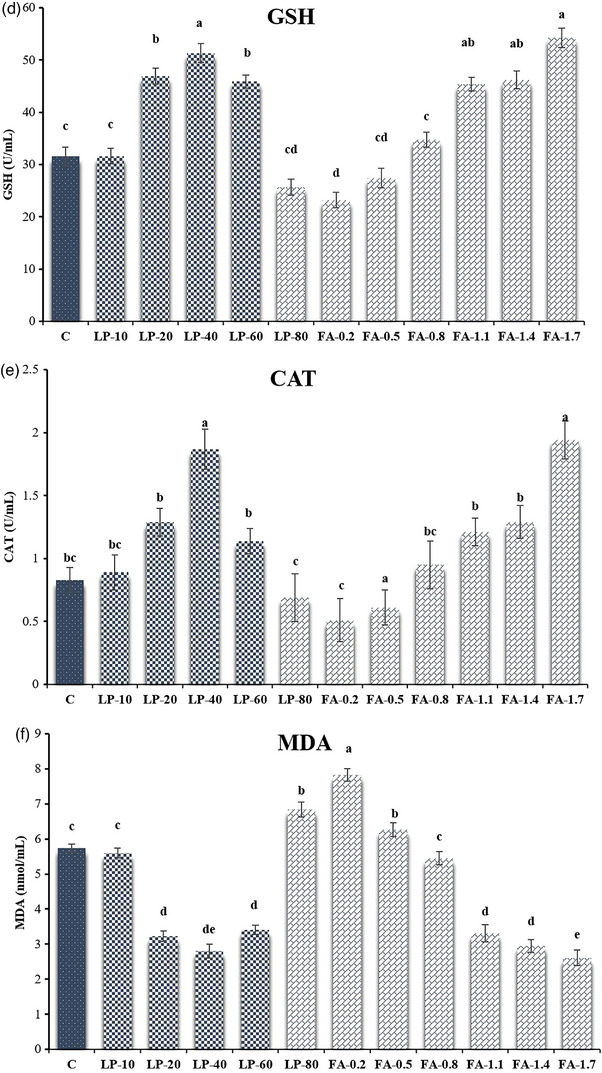
The TAC capacity was higher in the FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 compared to C group (p ≤ 0.05; Figure 4A). According to the results, GPx activities inFA‐1.7, FA‐1.4, Lp‐40 and Lp‐20 improved compared to C group (p ≤ 0.05; Figure 4B). The values of SOD and GSH improved in FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 groups compared to C group (p ≤ 0.05; Figure 4C and D). The values of CAT were higher in FA‐1.7 and Lp‐40 compared to C group (p ≤ 0.05). The values of MDA in FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 were lower than C groups (p ≤ 0.05; Figure 4F).
FIGURE 4.
(a) Total antioxidant capacity (TAC); (b) glutathione peroxidase (GPx); (c) superoxide dismutase (SOD); (d) glutathione (GSH) activities; (e) catalase (CAT); (f) lipid peroxidation (MDA) of frozen‐thawed buffalo semen after supplementation of different concentrations of L‐proline (Lp) and fulvic acid (FA) to the semen extender. Control (c): Tris‐based extender without antioxidant; Lp‐10: Tris‐based extender + L‐proline (10 mM); Lp‐20: Tris‐based extender + L‐proline (20 mM); Lp‐40: Tris‐based extender + L‐proline (40 mM); Lp‐60: Tris‐based extender + L‐proline (60 mM); Lp‐80: Tris‐based extender + L‐proline (80 mM); FA‐0.2: Tris‐based extender + fulvic acid (0.2%); FA‐0.5: Tris‐based extender + fulvic acid (0.5%); FA‐0.8: Tris‐based extender + fulvic acid (0.8%); FA‐1.1: Tris‐based extender + fulvic acid (1.1%); FA‐1.4: Tris‐based extender + fulvic acid (1.4%); FA‐1.7: Tris‐based extender + fulvic acid (1.7%). Different superscripts within the same row demonstrate significant differences (p ≤ 0.05; mean ± SEM).
4. DISCUSSION
The present study demonstrated that using Lp and FA supplementation could improve some sperm quality parameters (including sperm motility and velocity parameters, sperm viability and PMF, DNA integrity and antioxidant profile). Similar to our study, it was demonstrated that using various antioxidants also improved post‐thawed semen quality parameters (Longobardi et al., 2017; Lone et al., 2018; Ahmed et al., 2020b; Soleimanzadeh et al., 2020).
The cryopreservation process leads to the overproduction of ROS, and to cope with this issue, and maintain the balance of ROS, antioxidants are necessary. Some studies showed that the fertility rate of cryopreserved semen is lower than that of the fresh sperm due to reduced nucleus and mitochondrial membrane integrity, plasma loss, protein denaturation, DNA damage, and oxidative stress (Ejaz et al., 2014; Ahmed et al., 2016). Reactive oxygen species are produced during the cryopreservation process, which reduces motility, viability, intracellular enzyme activity and sperm fertility potential (Selvaraju et al., 2008). In addition, the antioxidant system of the buffalo semen is insufficient for protecting the sperm from the harmful effects of oxidative stress during the cryopreservation process (Bansal & Bilaspuri, 2011). The production of free radicals and ROS is directly related to protein dephosphorylation. Therefore, it is assumed that the compounds used in this study maybe inhibiting protein dephosphorylation through the cAMP‐PKA pathway in semen during storage (Fu et al., 2018). Different enzymes such as CAT, GPx and reductase, and SOD are the main defenders of the sperm plasma membrane against ROS and LPO (Selvaraju et al., 2008). Xiao et al. (2018) showed that FA addition to semen extender improved antioxidant profile of post‐thawed goat sperm. In the current study, FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 groups could improve TAC levels, SOD and GSH activities. Also, FA‐1.7, FA‐1.4, Lp‐40 and Lp‐20 could improve GPx activities and FA‐1.7 and Lp‐40 could improve CAT activities. While FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60, Lp‐40 and Lp‐20 could decrease MDA levels compared to the C group. Therefore, it is assumed that the Lp and FA used in this study maybe inhibiting protein dephosphorylation during cryopreservation (Fu et al., 2018). In another study on buffalo spermatozoa, Iqbal et al. (2016) found that the activity of these enzymes was significantly improved after thawing by adding 30 mM trehalose to the extender. Based on the reports of Soleimanzadeh et al. (2020) the caffeic acid (100 µM) supplemented extender improved the antioxidant profile of buffalo semen after thawing. Reddy et al. (2010) also demonstrated that adding 50 mM taurine to the semen extender could significantly improve the antioxidant status.
Sperm motility and velocity parameters have positive correlation with sperm fertility and pregnancy in buffalo and cattle (Kasai et al., 2002; Kefer et al., 2009; Soleimanzadeh et al., 2020). Also, Shahzad et al. (2016) suggested that in order to check the fertility rate in buffalo, sperm motility rate can be evaluated after thawing, and the reason for that can be the damage to the sperm membrane that occurs during the cryopreservation process. The results of the current study showed that the addition of Lp and FA to extender could improve TM, PM and velocity parameters, except for ALH and STR, especially at FA‐1.7, FA‐1.4 and Lp‐40, which had the highest yield. Similarly, Sangeeta et al. (2015) reported that Lp at a concentration of 25 to 50 mM improved TM and PM in ram semen. Additionally, other study showed that the proline can increased sperm motility after thawing in buck semen (Farshad & Hosseini, 2013). Also, Li et al. (2021) observed that adding 30 mM of Lp to semen extender had favourable results on the TM of post‐thawed donkey sperm. In addition, our results are in agreement with Xiao et al. (2018) and Abdel‐Khalek et al. (2019) in bucks, who reported a beneficial effect of FA on sperm motility. These results can be explained by the fact that these compounds may protect the sperm membrane against protein dephosphorylation and cause them to improve sperm motility after freeze‐thawing process (Fu et al., 2018).
The main cause of sperm cell damage during freezing is the impairment in plasma membrane (Ugur et al., 2019). Sperm cryo‐injury occurs during cryopreservation due to changes in ice crystal damage. These injuries of sperm lead to decreased sperm viability and functionality (Khan et al., 2021). Also, in the course of the cooling process, limitations of phospholipid lateral motion induce a liquid‐to‐gel phase shift, making the plasma membrane rigid and fragile. These changes lead to lipid phase separation, and thus, the proteins are irreversibly clustered (De Leeuw et al., 1990). El‐Sheshtawy et al. (2008) found that antioxidants supplements can increase buffalo sperm viability after thawing. In a previous study, it has been reported that FA can improve sperm viability of goat spermatozoa during preservation (Xiao et al., 2018). Similar above‐mentioned studies, our study showed that FA‐1.7, FA‐1.4, FA‐1.1, Lp‐60 and Lp‐40 groups could be improved sperm viability and PMF in buffalo semen after cryopreservation. Other studies demonstrated that sperm viability could be improved by cysteine and glutamine (Topraggaleh et al., 2014), Royal jelly (Shahzad et al., 2016) or green tea extract (Ahmed et al., 2020a).
According to previous evidence, infertile males have more DNA damage than fertile males, and this damage may have a negative effect on male fertility (Narayana et al., 2002; Talebi et al., 2011). Sperm freezing, as an adverse process, has been shown to destabilise chromatin and lead to sperm DNA fragmentation in boars and birds (Fraser & Strzeżek, 2007; Gliozzi et al., 2011). Double‐stranded DNA breaks are due to high levels of ROS production, disruption of DNA repair enzymes and mechanical stress of DNA molecular genomic regions where chromatin density increases due to cell contraction (Bogle et al., 2017). Some studies have shown that DNA damage can be a cause of infertility in males (Narayana et al., 2002; Talebi et al., 2011). The results of current study showed that FA‐1.7, FA‐1.4, FA‐1.1, FA‐0.8, Lp‐60, Lp‐40, Lp‐20 and Lp‐10 groups could reduce DNA damage in buffalo semen after cryopreservation. In the present study, the concentration of 40 mM Lp had the greatest effect in minimising the amount of DNA damage. The high level of sperm DNA damage at higher doses (80 mM) of LP may be due to the fact that increasing the amount of L‐proline creates a toxic state for sperm. This is confirmed by the MDA values shown in this study, which increase the amount of malondialdehyde in the cells as the dose of L‐proline increases. In this respect, Topraggaleh et al. (2014) found that the concentration of 7.5 mM cysteine supplementation in the extender reduced amount of DNA damage in buffalo semen. In the study by Ejaz et al. (2014) adding 20 ng/mL arachidic acid to the extender also improved sperm chromatin integrity in buffalo semen. Dorostkar et al. (2012) reported that the addition of 2 mg/mL sodium selenite to extender reduced damage to buffalo sperm DNA, which is in line with our findings.
5. CONCLUSION
In conclusion, the addition of Lp and FA to semen extender could improve sperm motility and had antioxidant properties against ROS and free radicals (especially at the Lp‐40 and FA‐1.7 groups). It also improved sperm antioxidant capacity, reduced damage to sperm viability, and improved sperm PMF. Therefore, the addition of Lp and FA can improve the quality of frozen‐thawed semen in buffaloes. Further studies are warranted to determine the effects of Lp and FA supplementations into the Tris‐based extender on fertility.
AUTHOR CONTRIBUTIONS
Negin Ramazani: data curation; methodology; software; supervision; writing – original draft; writing – review & editing. Farid Mahd Gharebagh: conceptualisation; data curation; methodology; software; writing – original draft; writing – review & editing. Ali Soleimanzadeh: conceptualisation; data curation; formal analysis; methodology; project administration; resources; software; writing – original draft; writing – review & editing. Esin Keles: methodology; writing – original draft; writing – review & editing. Desislava Georgieva Gradinarska‐Yanakieva: writing – original draft; writing – review & editing. Mahdi Zhandi: resources; software; writing – original draft; writing – review & editing. Alper Baran: methodology; resources; writing – original draft; writing – review & editing. Esmail Ayen: methodology; resources; supervision; writing – original draft; writing – review & editing. Dursun Ali Dinç: methodology; software; supervision; writing – original draft; writing – review & editing.
CONFLICT OF INTEREST STATEMENT
None of the authors have any conflict of interest to declare.
FUNDING
This research has not been financially supported.
ACKNOWLEDGEMENTS
The authors would like to sincerely thank the members of the Faculty of Veterinary Medicine, Urmia University Research Council, Bulgarian Academy of Sciences Research Council, Istanbul University‐Cerrahpasa Research Council and University of Selcuk Research Council, for the approval and support of this research.
Ramazani, N. , Mahd Gharebagh, F. , Soleimanzadeh, A. , Arslan, H. O. , Keles, E. , Gradinarska‐Yanakieva, D. G. , Arslan‐Acaröz, D. , Zhandi, M. , Baran, A. , Ayen, E. , & Dinç, D. A. (2023). The influence of L‐proline and fulvic acid on oxidative stress and semen quality of buffalo bull semen following cryopreservation. Veterinary Medicine and Science, 9, 1791–1802. 10.1002/vms3.1158
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Abdel‐Khalek, A. E. , El‐Gohary, E. S. , Gabr, S. A. , Hamad, M. E. , & Abdul Jabbar, N. B. (2019). Effect of humic and fulvic acids mixture treatment on productive and reproductive performance of Damascus goat bucks: 1. thermoregulation, body weight, sexual desire and semen quality under heat stress condition. Journal of Animal and Poultry Production, 10(5), 141–148. [Google Scholar]
- Ahmed, H. , Andrabi, S. M. H. , & Jahan, S. (2016). Semen quality parameters as fertility predictors of water buffalo bull spermatozoa during low‐breeding season. Theriogenology, 86(6), 1516–1522. [DOI] [PubMed] [Google Scholar]
- Ahmed, H. , Jahan, S. , Khan, A. , Khan, L. , Khan, B. T. , Ullah, H. , Riaz, M. , & Ullah, K. (2020a). Supplementation of green tea extract (GTE) in extender improves structural and functional characteristics, total antioxidant capacity and in vivo fertility of buffalo (Bubalus bubalis) bull spermatozoa. Theriogenology, 145, 190–197. [DOI] [PubMed] [Google Scholar]
- Ahmed, H. , Jahan, S. , Khan, A. , Khan, L. , Ullah, H. , Riaz, M. , Ullah, K. , & Ullah, F. (2020b). Supplementation of l‐tryptophan (an aromatic amino acid) in tris citric acid extender enhances post‐thaw progressive motility, plasmalemma, mitochondrial membrane potential, acrosome, and DNA integrities, and in vivo fertility rate of buffalo (Bubalus bubalis) bull spermatozoa. Cryobiology, 92, 117–123. [DOI] [PubMed] [Google Scholar]
- Aminafshar, M. , Amirinia, C. , & Torshizi, R. V. (2008). Genetic diversity in buffalo population of Guilan using microsatellite markers. Journal of Animal and Veterinary Advances, 7, 1499–1502. [Google Scholar]
- Ansari, M. S. , Rakha, B. A. , Andrabi, S. M. H. , Ullah, N. , Iqbal, R. , Holt, W. V , & Akhter, S. (2012). Glutathione‐supplemented tris‐citric acid extender improves the post‐thaw quality and in vivo fertility of buffalo (Bubalus bubalis) bull spermatozoa. Reproductive Biology, 12(3), 271–276. [DOI] [PubMed] [Google Scholar]
- Antkowiak, I. , Pytlewski, J. , Purczyńska, A. , & Skrzypek, R. (2012). A preliminary study of the behaviour of water buffaloes (Bubalus bubalis) imported to Poland. Archives Animal Breeding, 55(5), 415–419. [Google Scholar]
- Bai, H. X. , Chang, Q. F. , Shi, B. M. , & Shan, A. S. (2013). Effects of fulvic acid on growth performance and meat quality in growing‐finishing pigs. Livestock Science, 158(1–3), 118–123. [Google Scholar]
- Bansal, A. K. , & Bilaspuri, G. S. (2011). Impacts of oxidative stress and antioxidants on semen functions. Veterinary Medicine International, 2011, 686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beheshti, R. , Asadi, A. , Eshratkhah, B. , GhaleKandi, J. G. , & Ghorban, A. (2011). The effect of cysteine on post‐thawed buffalo bull (Bubalus bubalis) sperm parameters. Advances in Environmental Biology, 5(6), 1260–1263. [Google Scholar]
- Bogle, O. A. , Kumar, K. , Attardo‐Parrinello, C. , Lewis, S. E. M. , Estanyol, J. M. , Ballescà, J. L. , & Oliva, R. (2017). Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology, 5(1), 10–22. [DOI] [PubMed] [Google Scholar]
- Borghese, A. , & Mazzi, M. (2005). Buffalo population and strategies in the world. Buffalo Production and Research, 67, 1–39. [Google Scholar]
- Chang, Q. F. , Bai, H. X. , Shi, B. M. , Shan, A. S. , Wei, C. Y. , Yu, C. Q. , & Tong, B. S. (2013). Effects of dietary FA on the growth performance, serum biochemical indices, routine blood parameter and immunity of growing swine. Chinese Journal Of Animal Nutrition, 25, 1836–1842. [Google Scholar]
- De Lamirande, E. , Leclerc, P. , & Gagnon, C. (1997). Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Molecular Human Reproduction, 3(3), 175–194. [DOI] [PubMed] [Google Scholar]
- De Leeuw, F. E. , Chen, H.‐C. , Colenbrander, B. , & Verkleij, A. J. (1990). Cold‐induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology, 27(2), 171–183. [DOI] [PubMed] [Google Scholar]
- Dorostkar, K. , Alavi‐Shoushtari, S. M. , & Mokarizadeh, A. (2012). Effects of in vitro selenium addition to the semen extender on the spermatozoa characteristics before and after freezing in water buffaloes (Bubalus bubalis). Veterinary Research Forum, 3(4), 263. [PMC free article] [PubMed] [Google Scholar]
- Ejaz, R. , Ansari, M. S. , Rakha, B. A. , Ullah, N. , Husna, A. U. , Iqbal, R. , & Akhter, S. (2014). Arachidic acid in extender improves post‐thaw parameters of cryopreserved Nili‐Ravi buffalo bull semen. Reproduction in Domestic Animals, 49(1), 122–125. [DOI] [PubMed] [Google Scholar]
- Elsayed, D. H. , El‐Shamy, A. A. , Abdelrazek, H. M. A. , & El‐Badry, D. A. (2019). Effect of genistein on semen quality, antioxidant capacity, caspase‐3 expression and DNA integrity in cryopreserved ram spermatozoa. Small Ruminant Research, 177, 50–55. [Google Scholar]
- El‐Sheshtawy, R. I. , El‐Sisy, G. A. , & El‐Nattat, W. S. (2008). Use of selected amino acids to improve buffalo bull semen cryopreservation. Global Veterinaria, 2(4), 146–150. [Google Scholar]
- Ezz, M. A. , Montasser, A. E. , Hussein, M. , Eldesouky, A. , Badr, M. , Hegab, A. E. , Balboula, A. , & Zaabel, S. M. (2017). The effect of cholesterol loaded cyclodextrins on post‐thawing quality of buffalo semen in relation to sperm DNA damage and ultrastructure. Reproductive Biology, 17(1), 42–50. [DOI] [PubMed] [Google Scholar]
- Farshad, A. , & Hosseini, Y. (2013). The cryoprotective effects of amino acids supplementation on cooled and post‐thaw Markhoz bucks semen quality. Small Ruminant Research, 114(2–3), 258–263. [Google Scholar]
- Fraser, L. , & Strzeżek, J. (2007). Is there a relationship between the chromatin status and DNA fragmentation of boar spermatozoa following freezing–thawing? Theriogenology, 68(2), 248–257. [DOI] [PubMed] [Google Scholar]
- Fu, J. , Yang, Q. , Li, Y. , Li, P. , Wang, L. , & Li, X. (2018). A mechanism by which Astragalus polysaccharide protects against ROS toxicity through inhibiting the protein dephosphorylation of boar sperm preserved at 4 C. Journal of Cellular Physiology, 233(7), 5267–5280. [DOI] [PubMed] [Google Scholar]
- Gliozzi, T. M. , Zaniboni, L. , & Cerolini, S. (2011). DNA fragmentation in chicken spermatozoa during cryopreservation. Theriogenology, 75(9), 1613–1622. [DOI] [PubMed] [Google Scholar]
- Iqbal, S. , Andrabi, S. M. H. , Riaz, A. , Durrani, A. Z. , & Ahmad, N. (2016). Trehalose improves semen antioxidant enzymes activity, post‐thaw quality, and fertility in Nili Ravi buffaloes (Bubalus bubalis). Theriogenology, 85(5), 954–959. [DOI] [PubMed] [Google Scholar]
- Islam, K. M. S. , Schuhmacher, A. , & Gropp, J. M. (2005). Humic acid substances in animal agriculture. Pakistan Journal of Nutrition, 4(3), 126–134. [Google Scholar]
- Izanloo, H. , Soleimanzadeh, A. , Bucak, M. N. , Imani, M. , & Zhandi, M. (2022). The effects of glutathione supplementation on post‐thawed Turkey semen quality and oxidative stress parameters and fertilization, and hatching potential. Theriogenology, 179, 32–38. [DOI] [PubMed] [Google Scholar]
- Jayasooriya, R. G. P. T. , Dilshara, M. G. , Kang, C.‐H. , Lee, S. , Choi, Y. H. , Jeong, Y. K. , & Kim, G.‐Y. (2016). Fulvic acid promotes extracellular anti‐cancer mediators from RAW 264.7 cells, causing to cancer cell death in vitro. International Immunopharmacology, 36, 241–248. [DOI] [PubMed] [Google Scholar]
- Kasai, T. , Ogawa, K. , Mizuno, K. , Nagai, S. , Uchida, Y. , Ohta, S. , Fujie, M. , Suzuki, K. , Hirata, S. , & Hoshi, K. (2002). Relationship between sperm mitochondrial membrane potential, sperm motility, and fertility potential. Asian Journal of Andrology, 4(2), 97–104. [PubMed] [Google Scholar]
- Kasimanickam, R. , Pelzer, K. D. , Kasimanickam, V. , Swecker, W. S. , & Thatcher, C. D. (2006). Association of classical semen parameters, sperm DNA fragmentation index, lipid peroxidation and antioxidant enzymatic activity of semen in ram‐lambs. Theriogenology, 65(7), 1407–1421. [DOI] [PubMed] [Google Scholar]
- Kefer, J. C. , Agarwal, A. , & Sabanegh, E. (2009). Role of antioxidants in the treatment of male infertility. International Journal of Urology, 16(5), 449–457. [DOI] [PubMed] [Google Scholar]
- Khan, I. M. , Cao, Z. , Liu, H. , Khan, A. , Rahman, S. U. , Khan, M. Z. , Sathanawongs, A. , & Zhang, Y. (2021). Impact of cryopreservation on spermatozoa freeze‐thawed traits and relevance omics to assess sperm cryo‐tolerance in farm animals. Frontiers in Veterinary Science, 8, 609180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. , & Ijaz, A. (2007). Assessing undiluted, diluted and frozen‐thawed Nili‐Ravi buffalo bull sperm by using standard semen assays. Italian Journal of Animal Science, 6(sup2), 784–787. [Google Scholar]
- Li, N. , Yu, J. , Yang, F. , Shao, Y. , Wu, S. , Liu, B. , Li, M. , Wang, T. , Li, J. , & Zeng, S. (2021). L‐proline: An effective agent for frozen and post‐thawed donkey semen storage. Journal of Equine Veterinary Science, 101, 103393. [DOI] [PubMed] [Google Scholar]
- Lone, S. A. , Prasad, J. K. , Ghosh, S. K. , Das, G. K. , Balamurugan, B. , & Verma, M. R. (2018). Study on correlation of sperm quality parameters with antioxidant and oxidant status of buffalo bull semen during various stages of cryopreservation. Andrologia, 50(4), e12970. [DOI] [PubMed] [Google Scholar]
- Longobardi, V. , Zullo, G. , Salzano, A. , De Canditiis, C. , Cammarano, A. , De Luise, L. , Puzio, M. V. , Neglia, G. , & Gasparrini, B. (2017). Resveratrol prevents capacitation‐like changes and improves in vitro fertilizing capability of buffalo frozen‐thawed sperm. Theriogenology, 88, 1–8. 10.1016/j.theriogenology.2016.09.046 [DOI] [PubMed] [Google Scholar]
- Meena, M. , Divyanshu, K. , Kumar, S. , Swapnil, P. , Zehra, A. , Shukla, V. , Yadav, M. , & Upadhyay, R. S. (2019). Regulation of L‐proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon, 5(12), e02952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi, B. , Faramarzi, A. , Ghasemi‐Esmailabad, S. , Aghaz, F. , Hashemian, A. H. , & Khazaei, M. (2022). L‐proline as a novel additive to cryopreservation media improved post‐thaw quality of human spermatozoon via reducing oxidative stress. Andrologia, 54(1), e14301. [DOI] [PubMed] [Google Scholar]
- Narayana, K. , D'Souza, U. J. A. , & Rao, K. P. S. (2002). Ribavirin‐induced sperm shape abnormalities in Wistar rat. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 513(1), 193–196. [DOI] [PubMed] [Google Scholar]
- Papas, M. , Catalan, J. , Barranco, I. , Arroyo, L. , Bassols, A. , Yeste, M. , & Miró, J. (2020). Total and specific activities of superoxide dismutase (SOD) in seminal plasma are related with the cryotolerance of jackass spermatozoa. Cryobiology, 92, 109–116. [DOI] [PubMed] [Google Scholar]
- Papas, M. , Catalán, J. , Fernandez‐Fuertes, B. , Arroyo, L. , Bassols, A. , Miró, J. , & Yeste, M. (2019). Specific activity of superoxide dismutase in stallion seminal plasma is related to sperm cryotolerance. Antioxidants, 8(11), 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partyka, A. , Łukaszewicz, E. , Niżański, W. , & Twardoń, J. (2011). Detection of lipid peroxidation in frozen‐thawed avian spermatozoa using C11‐BODIPY581/591. Theriogenology, 75(9), 1623–1629. [DOI] [PubMed] [Google Scholar]
- Partyka, A. , Niżański, W. , Bratkowska, M. , & Maślikowski, P. (2015). Effects of N‐acetyl‐L‐cysteine and catalase on the viability and motility of chicken sperm during liquid storage. Reproductive Biology, 15(2), 126–129. [DOI] [PubMed] [Google Scholar]
- Pour Azary, M. A. , Pirmohammadi, R. , & Manafi Azar, Q. (2004). Breeding of buffaloes in West Azerbaijan of Iran. Proceedings of the Seventh World Buffalo Congress, Manila, Philippines, 20 to 23 October 2004, pp. 535–537. [Google Scholar]
- Reddy, N. S. S. , Mohanarao, G. J. , & Atreja, S. K. (2010). Effects of adding taurine and trehalose to a tris‐based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Animal Reproduction Science, 119(3–4), 183–190. [DOI] [PubMed] [Google Scholar]
- Saeed, A. M. , El‐Nagar, H. A. , Wafa, W. M. , & Hussein, Y. S. (2016). Effect of coenzyme Q10 as an antioxidant added to semen extender during cryopreservation of buffalo and cattle semen. Journal of Animal and Poultry Production, 7(11), 403–408. [Google Scholar]
- Sangeeta, S. , Arangasamy, A. , Kulkarni, S. , & Selvaraju, S. (2015). Role of amino acids as additives on sperm motility, plasma membrane integrity and lipid peroxidation levels at pre‐freeze and post‐thawed ram semen. Animal Reproduction Science, 161, 82–88. [DOI] [PubMed] [Google Scholar]
- Selvaraju, S. , Ravindra, J. P. , Ghosh, J. , Gupta, P. S. P. , & Suresh, K. P. (2008). Evaluation of sperm functional attributes in relation to in vitro sperm‐zona pellucida binding ability and cleavage rate in assessing frozen thawed buffalo (Bubalus bubalis) semen quality. Animal Reproduction Science, 106(3–4), 311–321. [DOI] [PubMed] [Google Scholar]
- Shahzad, Q. , Mehmood, M. U. , Khan, H. , ul Husna, A. , Qadeer, S. , Azam, A. , Naseer, Z. , Ahmad, E. , Safdar, M. , & Ahmad, M. (2016). Royal jelly supplementation in semen extender enhances post‐thaw quality and fertility of Nili‐Ravi buffalo bull sperm. Animal Reproduction Science, 167, 83–88. [DOI] [PubMed] [Google Scholar]
- Soleimanzadeh, A. , Talavi, N. , Yourdshahi, V. S. , & Bucak, M. N. (2020). Caffeic acid improves microscopic sperm parameters and antioxidant status of buffalo (Bubalus bubalis) bull semen following freeze‐thawing process. Cryobiology, 95, 29–35. [DOI] [PubMed] [Google Scholar]
- Tahmasbian, H. , Ayen, E. , & Khaki, A. (2022). Evaluation of the effects of hesperidin on fresh and frozen‐thawed semen quality using two different cryopreservation methods in Simmental bull. Animal Reproduction, 19, e20220042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi, A. R. , Sarcheshmeh, A. A. , Khalili, M. A. , & Tabibnejad, N. (2011). Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol, 45(4), 403–409. [DOI] [PubMed] [Google Scholar]
- Tariq, M. , Khan, M. S. , Shah, M. G. , Nisha, A. R. , Umer, M. , Hasan, S. M. , Rahman, A. , & Rabbani, I. (2015). Exogenous antioxidants inclusion during semen cryopreservation of farm animals. Journal of Chemical and Pharmaceutical Research, 7(3), 2273–2280. [Google Scholar]
- Tavakolian, J. (2000). An introduction to genetic resources of native farm animals in Iran. Tehran: Animal Science Genetic Research Institute Press. [Google Scholar]
- Topraggaleh, T. R. , Shahverdi, A. , Rastegarnia, A. , Ebrahimi, B. , Shafiepour, V. , Sharbatoghli, M. , Esmaeili, V. , & Janzamin, E. (2014). Effect of cysteine and glutamine added to extender on post‐thaw sperm functional parameters of buffalo bull. Andrologia, 46(7), 777–783. [DOI] [PubMed] [Google Scholar]
- Tuncer, P. B. , Bucak, M. N. , Büyükleblebici, S. , Sarıözkan, S. , Yeni, D. , Eken, A. , Akalın, P. P. , Kinet, H. , Avdatek, F. , Fidan, A. F. , & Gündoğan, M. (2010). The effect of cysteine and glutathione on sperm and oxidative stress parameters of post‐thawed bull semen. Cryobiology, 61(3), 303–307. 10.1016/j.cryobiol.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Turaja, K. I. B. , Vega, R. S. A. , Saludes, T. A. , Tandang, A. G. , Bautista, J. A. N. , Salces, A. J. , & Rebancos, C. M. (2019). Influence and total antioxidant capacity of non‐enzymatic antioxidants on the quality and integrity of extended and cryopreserved semen of murrah buffalo (Bubalus bubalis). Philippine Journal of Science, 148(4), 619–626. [Google Scholar]
- Ugur, M. R. , Saber Abdelrahman, A. , Evans, H. C. , Gilmore, A. A. , Hitit, M. , Arifiantini, R. I. , Purwantara, B. , Kaya, A. , & Memili, E. (2019). Advances in cryopreservation of bull sperm. Frontiers in Veterinary Science, 6, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1999). WHO laboratory manual for the examination of human semen and sperm‐cervical mucus interaction. Cambridge University Press. [Google Scholar]
- Xiao, Y. , Wu, Z. , & Wang, M. (2018). Effects of fulvic acids on goat sperm. Zygote, 26(3), 220–223. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Xue, X. , Yan, J. , Yan, L.‐Y. , Jin, X.‐H. , Zhu, X.‐H. , He, Z.‐Z. , Liu, J. , Li, R. , & Qiao, J. (2016). L‐proline: A highly effective cryoprotectant for mouse oocyte vitrification. Scientific Reports, 6(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


