Abstract
A 13‐year‐old, male neutered domestic short‐haired cat was diagnosed with multiple biliary duct hamartomas after liver lobectomy for a suspected malignant hepatic mass. Distinguishing ultrasonographic findings included a lobular, mostly well‐defined, heterogeneous, predominantly hyperechoic, left hepatic mass. Computed tomography (CT) confirmed the presence of a lobular, well‐defined, fluid to soft tissue attenuating, heterogeneously hypoenhancing left divisional hepatic mass. Grossly, a large left sided multilobular pale pink gelatinous hepatic mass was surgically excised. Histopathologically, the mass was composed of irregular cystic spaces lined by cuboidal epithelium and separated by mature regular fibrous tissue. Three months following surgery there was no evidence of recurrence or progression of disease on repeat abdominal ultrasound (AUS).
Keywords: bile‐duct disease, diagnostic imaging, hamartoma, hepatectomy, pathology, surgical oncology
A 13‐year‐old, male neutered domestic short‐haired cat was diagnosed with multiple biliary duct hamartomas after liver lobectomy for a suspected malignant hepatic mass. Prior to surgery, comparative diagnostic imaging, including abdominal ultrasound and computed tomography of the abdomen both identified a lobular, well‐defined left hepatic mass suspected to be neoplastic in origin. Histopathologically, the mass was composed of irregular cystic spaces lined by cuboidal epithelium and separated by mature regular fibrous tissue consistent with multiple biliary duct hamartomas (Von Meyenberg Complexes).
1. INTRODUCTION
Hamartomas are rare tumours of focal disorganised mature mesenchymal or epithelial tissue found in their normal anatomic location (Sheikh et al., 2022). These tumours occur in both animals and humans. The majority of animal case reports are classified as vascular hamartomas (Starost, 2007). Additional types of hamartomas described in the veterinary literature include dermis, abomasal smooth muscle, ocular tissue, nasal cavity, retinal astrocytes, myocardium, pulmonary alveoli and ovarian interstitial cells (Bottero et al., 2018; Hwang et al., 2021; Starost, 2007).
Reports of hepatic and biliary duct hamartoma (BDHs) in veterinary medicine are scarce and have been described in dogs (Gualtieri et al., 2009; McGavin & Henry, 1972), in cattle (Ladds, 1983), in an equine fetus (Roperto & Galati, 1984), in a calf (Bosschere & Ducatelle, 1999), in snakes (Patania et al., 2021) and in a rabbit (Starost, 2007). Due to the few reports of BDHs in veterinary medicine, most of the information stems from the common findings within human literature. These benign tumours tend to present with little clinical signs or abnormalities and are often found incidentally on imaging or during laparotomies. In veterinary medicine, they have similarly been found incidentally after investigation for abdominal distension or on necropsy (Gualtieri et al., 2009; Patania et al., 2021; Roperto & Galati, 1984; Starost, 2007). Its rarity and varied clinical appearance make management and diagnosis of this illness difficult without histopathologic identification in both human and veterinary medicine; however, once a definitive diagnosis is reached they tend to have a generally positive outcome. Multiple biliary duct hamartomas have not yet been described in cats.
2. CASE PRESENTATION
A 13‐year‐old male neutered domestic short‐haired cat was presented for diagnostic evaluation of a large hepatic mass observed by the primary veterinarian upon exploratory laparotomy. The cat initially presented to the primary veterinarian with a 1‐day history of inappetence and a single episode of vomiting. On physical examination, the cat was very reactive to abdominal palpation, and no further abnormalities were noted. Complete blood count (CBC) and serum biochemistry (CHEM) abnormalities likely reflected a stress leukogram; mild neutropenia (2.13 K/μL; 2.30–10.29 K/μL) and lymphopenia (0.55 K/μL; 0.92–6.88 K/μL) with a mild hyperglycaemia (172 mg/dL; 71–159 mg/dL). Abdominal radiographs showed an obstructive gastrointestinal pattern with suspicion of linear foreign material. Upon exploratory laparotomy at the primary care veterinarian, 80–90% of the liver was effaced by a large mass. No foreign material was found in the gastrointestinal tract. Owners were presented with the options of referral or humane euthanasia based on differential diagnoses of neoplasia and the concern for nonresectability. The cat was recovered from surgery and directly transferred for specialised care. On presentation, the cat was alert and responsive, moderately reactive on abdominal palpation, and had a recent intact ventral midline abdominal incision. The cat was hospitalised for continued supportive care and further diagnostic work‐up. A CBC showed a mild anaemia (HCT 28.9%/ 30.3–52.3%) without reticulocytosis, suggesting a nonregenerative anaemia or preregenerative anaemia. CHEM analysis revealed no abnormalities. An abdominal ultrasound (AUS) (RS85, Samsung Healthcare, Danvers, MA, USA) was performed, and a large (at least 6.5 cm L × 7 cm W × 4.5 cm H), lobular, mostly well‐defined, heterogeneously hyperechoic left divisional hepatic mass that contained a centralised ill‐defined hypoechoic area was detected (Figure 1). Multiple, small, variably sized and shaped, predominantly anechoic, distally enhancing, thin‐walled, cyst‐like lesions were also noted at the periphery of the mass and throughout the hepatic parenchyma. Given the sonographic characteristics of the primary mass, the differential diagnoses considered were hepatic myelolipoma, hepatic adenoma and adenocarcinoma. On computed tomography (CT) (160 slice Aquilion Prime, Canon Medical Systems USA, Inc., Tustin, CA), a lobular, well‐defined, fluid to soft tissue attenuating, heterogeneously hypoenhancing left divisional hepatic mass measuring 7.7 cm L × 7 cm W × 4.5 cm H was identified, characterised by branching vasculature within (Figure 2). Throughout the remainder of the liver, there were numerous, well‐defined, fluid attenuating, mildly contrast‐enhancing lesions, which appeared loculated (Figure 2). Although a benign aetiology (e.g. biliary cystadenoma) was considered, different features including the pattern of contrast enhancement and size of the larger mass, suggested a malignant aetiology (e.g. biliary cystadenocarcinoma, hepatocellular carcinoma, or round cell neoplasm). Cytology of the left hepatic mass displayed a mild amount of reactive mesothelial cells with haemodilution and was considered inconclusive.
FIGURE 1.
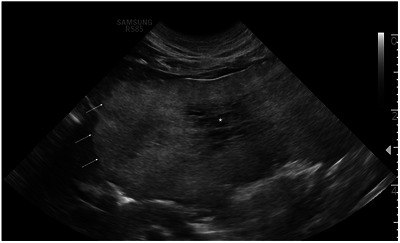
Transverse ultrasonographic image of the left divisional hepatic mass in a cat with multiple biliary hamartomas. The lateral most margin extended beyond the field of view (right side of the image). The mass displayed well‐defined margins and heterogeneously hyperechoic parenchyma (arrow), with a central ill‐defined hypoechoic area (asterisk).
FIGURE 2.
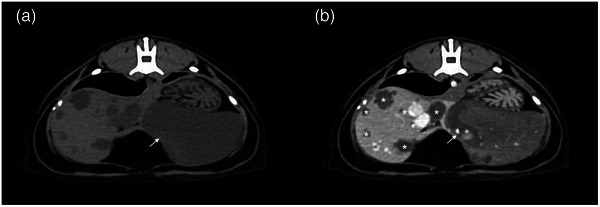
Transverse CT images in a cat with multiple biliary hamartomas precontrast (a) and postcontrast (b) of the lobular, well‐defined, fluid to soft tissue attenuating, heterogeneously hypoenhancing left divisional hepatic mass with branching vessels within (arrow). Multiple well‐defined, fluid attenuating, hypoenhancing, loculated lesions were noted in the remainder of the hepatic parenchyma (asterisk).
The cat underwent exploratory laparotomy, where a soft, multilobular mass measuring 7 cm × 7 cm × 3 cm was found in the left lateral liver lobe. The mass was composed predominantly of a central area of pale pink, cobblestoned, gelatinous, opaque tissue. An outer rim of dark brown wedges of soft tissue followed by presumptive normal liver tissue surrounded the mass. A TA v30 stapler (Covidien) was used to resect the lobe at its base, excising the mass completely. There were multifocal raised, partially translucent, pale‐tan nodules throughout the remaining liver lobes. A nodule on the papillary process of the caudate lobe was resected with a Ligasure (Coviden) along with several incisional punch biopsy samples taken from the right medial liver lobe. All tissue samples were submitted for histopathologic examination.
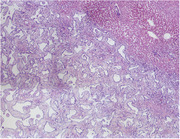
Histologically, the left hepatic mass consisted of a well‐demarcated, unencapsulated, expansile, moderately cellular mass (Figure 3). The mass was composed of cuboidal epithelial cells forming irregularly shaped cystic spaces separated and supported by fibrous tissue, resembling biliary ducts. The mass compressed the surrounding liver tissue. The surrounding hepatic tissue contained increased biliary cross sections (biliary ductular reaction), small lobules, dilated sinusoids, and increased golden‐brown cytoplasmic pigment in centrilobular hepatocytes. The mass extended to the sample margins. The remaining sections of liver biopsies were fairly similar microscopically to the main mass, but with smaller aggregates of cystic spaces. These smaller lesions were also enclosed by reactive hepatic tissue. Histopathologic findings were consistent with multiple BDH, or von Meyenburg complexes of the liver.
FIGURE 3.
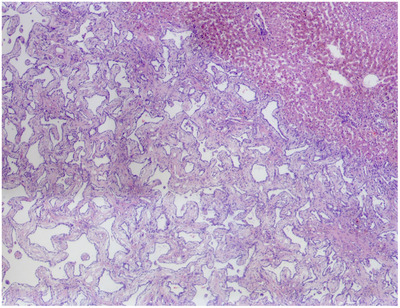
Histopathology of the mass (4× magnification) in a cat with multiple biliary hamartomas. The normal liver is in the upper right, with the mass occupying the remainder of the field of view.
The cat was restaged 3 months postoperatively, and there was no evidence of disease recurrence or progression on AUS. Throughout the hepatic parenchyma, there remained multifocal, variably shaped and sized (up to 0.6 cm diameter), well‐defined, smoothly marginated, anechoic cyst‐like lesions, similar to the preoperative imaging.
3. DISCUSSION
Multiple BDHs, commonly referred to as ‘Von Meyenberg complexes’, are unusual, benign abnormalities of the small interlobular bile ducts, first discovered in 1918 by by the Swiss Pathologist Professor Hanns von Meyenburg (von Meyenburg, 1918). The development of BDHs are associated with the late stage of bile‐duct embryogenesis from ductal‐plate abnormalities of the peripheral interlobular bile ducts (Starost, 2007). The majority of BDHs are asymptomatic and oftentimes are found incidentally during imaging or laparotomy for another procedure.
Rarely, cases can present with nonspecific clinical signs, such as pain on abdominal palpation, jaundice or fever. Although the majority of patients have normal laboratory results, some may develop minor haematologic abnormalities, most frequently increased liver enzymes (Carmona et al., 2022; Lanser & Puckett, 2023; Priadko et al., 2022; Sheikh et al., 2022).
These complexes can be diagnosed by imaging (either AUS and/or CT), as single or multiple small cyst‐like lesions < 1.5 cm in diameter that lack communication with the biliary tree (Lanser & Puckett, 2023; Sinakos et al., 2011; Song et al., 2008). According to a systematic review of 139 human cases, ultrasonography for BDHs is typically nonspecific, and lesions on CT scan are predominantly hypoattenuating (Sheikh et al., 2022; Torbenson, 2018). Several studies have found that the lesions, specifically the septae, become more distinct, rather than contrast enhancing on CT (Eisenberg et al., 1986; Ryu et al., 2010; Sheikh et al., 2022). This is a perplexing characteristic that could result in a malignancy diagnosis in humans (Sheikh et al., 2022). In the present case report, the differential diagnoses changed depending on the imaging modality used. These benign lesions may be difficult to differentiate between other ductal‐plate malformations (DPM), such as Caroli's disease and polycystic liver disease. BDHs can also be mistaken for more sinister diseases: biliary cystadenocarcinomas, cholangiocarcinoma, lymphangioma, hepatocellular carcinoma or cystic change of solid hepatic tumours (Priadko et al., 2022; Ryu et al., 2010; Soares et al., 2014; Strasberg & Chapman, 2021). In an animal with extrahepatic malignant neoplasia, establishing a diagnosis is of further importance due to BDHs bearing similar resemblance to metastatic lesions of the liver (Lanser & Puckett, 2023).
Human case reports frequently discuss the advantages of magnetic resonance imaging (MRI), particularly contrast‐enhanced cholangiopancreatography (MRCP), where BDHs manifest as numerous, small irregularly shaped cystic hepatic lesions (up to 1.5 cm) that lack communication with the biliary tree, resulting in a ‘starry sky’ or ‘honeycomb’ configuration (Priadko et al., 2022; Ryu et al., 2010). When paired with additional T1‐ and T2‐weighted imaging or the use of contrast medium, MRCP highlights fluid within the biliary tract and pancreatic ducts, helping to better identify both intra‐ and extrahepatic disorders, such as inflammation, obstruction, or fluid‐filled structures (Marolf, 2017; Vidal et al., 2020). In veterinary medicine, MRCP is feasible and has been performed in cats, however it is not widely available or investigated in clinical settings (Marolf et al., 2013). Imaging is typically limited to AUS or CT which lack the ability to highlight the distinctive features that are more obvious on MRCP. Therefore, biliary hamartomas are difficult to diagnose without histopathology in veterinary medicine (Carmona et al., 2022). Grossly, BDHs appear as multifocal pale discrete or irregular nodules with or without cystic components found on the surface of the hepatic parenchyma. The mass reported herein presented with similar characteristics to the BDHs seen in humans; with a large and irregular shape, pale discoloration and gelatinous or cystic changes (Sheikh et al., 2022; Starost, 2007).
Detection of the true incidence of BDH is difficult due to similarities with other benign neoplasms. Microscopically, BDHs consist of multiple irregularly shaped, dilated cystic spaces lined by a single layer of cuboidal epithelial cells free of atypia. These structures are supported and surrounded by abundant fibrous connective tissue (Kim & Jin, 2011; Torbenson, 2018). While these findings can also be seen in biliary adenoma or cystadenomas, a single compressing lesion and a lesion lacking hepatocytes are key microscopic features favouring an adenoma (Patania et al., 2021). In this case, the cat presented with multiple cyst‐like lesions of similar findings in addition to the main BDH. In addition, there was evidence of irregular, nonneoplastic, hepatic tissue throughout the histology sample, displaying biliary ductal reactions. This is likely secondary to hepatocyte entrapment within the stroma during the malformed bile ducts.
Although BDHs are clinically benign malformations, many patients have undergone surgery due to diagnostic uncertainty (Kakar et al., 2017; Priadko et al., 2022). Due to the low number of published cases and the lack of recognised guidelines, the management of such patients remains a clinical challenge (Lanser & Puckett, 2023; Priadko et al., 2022). Oftentimes, BDHs are treated conservatively; however, further investigation with biopsy may be required if progression is detected. Several studies in human literature have shown evidence of BDHs transitioning to intrahepatic cholangiocarcinoma or, less commonly, hepatocellular carcinomas (Carmona et al., 2022; Lanser & Puckett, 2023; Sheikh et al., 2022; Vijayan et al., 2022). Once diagnosed with BDHs, routine surveillance via diagnostic imaging is necessary, but there are no set guidelines or clinical recommendation on the frequency of rechecks in the human literature (Kakar et al., 2017; Priadko et al., 2022).
To the authors’ knowledge, this is the first report of multiple BDHs in a cat. Treatment of this condition is challenging due to its rarity, nonspecific clinical presentation and tendency to resemble more malignant disease processes, but once a definitive diagnosis is made, the prognosis is favourable.
AUTHOR CONTRIBUTIONS
Conception and design: Rachel Naghi and Judith Bertran. Acquisition of data: Rachel Naghi, Judith Bertran, Elisa Spoldi, Michael J. Dark, Helena Hauck de Oliveira. Analysis and interpretation of data: Rachel Naghi, Judith Bertran, Elisa Spoldi, Michael J. Dark, Helena Hauck de Oliveira. Drafting of article: Rachel Naghi. Revising article for intellectual content: Rachel Naghi, Judith Bertran, Elisa Spoldi, Michael J. Dark, Carlos Souza, Elizabeth Maxwell. Final approval of the completed article: Rachel Naghi, Judith Bertran, Elisa Spoldi, Michael J. Dark, Helena Hauck de Oliveira, Elizabeth A. Maxwell, Carlos Souza.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1175.
ACKNOWLEDGEMENTS
We would like to thank all coauthors for their support in the realisation of this case report.
Naghi, R. , Bertran, J. , Spoldi, E. , Dark, M. J. , de Oliveira, H. H. , Souza, C. , & Maxwell, E. A. (2023). Multiple biliary duct hamartomas in a cat resulting in a hepatic mass: A case report. Veterinary Medicine and Science, 9, 1441–1445. 10.1002/vms3.1175
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bosschere, H. D. , & Ducatelle, R. (1999). Bile duct hamartoma in a cal. The Veterinary Record, 144, 210–211. [DOI] [PubMed] [Google Scholar]
- Bottero, E. , Melega, M. , Dimartino, E. R. , Tricarico, M. , Lepri, E. , Lorenzi, D. D. , Miniscalco, B. , & Riondato, F. (2018). Diagnosis of feline mesenchymal nasal hamartoma by squash preparation cytology. Veterinary Clinical Pathology, 47, 629–633. [DOI] [PubMed] [Google Scholar]
- Carmona, V. , Justo, I. , Rodríguez‐Gil, Y. , Marcacuzco, A. , Loinaz, C. , & Jiménez, C. (2022). Multicystic biliary hamartoma. Cirugía Española (English Edition), 100(12), 800–802. [DOI] [PubMed] [Google Scholar]
- Eisenberg, D. , Hurwitz, L. , & Yu, A. (1986). CT and sonography of multiple bile‐duct hamartomas simulating malignant liver disease (case report). American Journal of Roentgenology, 147, 279–280. [DOI] [PubMed] [Google Scholar]
- Gualtieri, M. , Cocci, A. , Monti, S. , & Olivero, D. (2009). Surgical removal of a localised vascular hepatic hamartoma in a dog. Australian Veterinary Journal, 87, 360–362. [DOI] [PubMed] [Google Scholar]
- Hwang, H. , Kim, D. , Hillers, K. , & Kim, M. S. (2021). Ocular hamartoma in a five‐day‐old calf. Veterinary Record Case Reports, 9, e2. [Google Scholar]
- Vijayan, V. , VS, P. , & Vijayaraghavan, L. (2022). Mesenchymal hamartoma liver showing malignant transformation to undifferentiated embryonal sarcoma – A case report. International Journal of Medical Science and Clinical Research Studies, 2(5), 10.47191/ijmscrs/v2-i5-02 [DOI] [Google Scholar]
- Kakar, S. , Borhani, A. A. , & Malik, S. M. (2017). A liver full of stars: Hepatostellular! Gastroenterology, 153, e8–e9. [DOI] [PubMed] [Google Scholar]
- Kim, H. K. , & Jin, S.‐Y. (2011). Cholangiocarcinoma arising in von Meyenburg complexes. Korean Journal of Hepatology, 17, 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladds, P. W. (1983). Vascular hamartomas of the liver of cattle. Veterinary Pathology, 20, 764–767. [DOI] [PubMed] [Google Scholar]
- Lanser, H. C. , & Puckett, Y. (2023). Biliary Duct Hamartoma – StatPearls – NCBI Bookshelf. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557750/ [PubMed]
- Marolf, A. J. (2017). Diagnostic imaging of the hepatobiliary system an update. Veterinary Clinics of North America: Small Animal Practice, 47, 555–568. [DOI] [PubMed] [Google Scholar]
- Marolf, A. J. , Kraft, S. L. , Dunphy, T. R. , & Twedt, D. C. (2013). Magnetic resonance (MR) imaging and MR cholangiopancreatography findings in cats with cholangitis and pancreatitis. Journal of Feline Medicine and Surgery, 15, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin, M. D. , & Henry, J. (1972). Canine hepatic vascular hamartoma associated with ascites. Journal of the American Veterinary Medical Association, 160, 864–866. [PubMed] [Google Scholar]
- Patania, O. M. , Troan, B. V. , & Cullen, J. M. (2021). Ductal plate malformations in captive snakes. Veterinary Pathology, 58, 736–742. [DOI] [PubMed] [Google Scholar]
- Priadko, K. , Niosi, M. , Vitale, L. M. , Sio, C. D. , Romano, M. , & Sio, I. D. (2022). “Starry liver” –Von Meyenburg complex clinical case presentation and differential diagnosis discussion: A case report. World Journal of Hepatology, 14, 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roperto, F. , & Galati, P. (1984). Mixed hamartoma of the liver in an equine foetus. Equine Veterinary Journal, 16, 218–220. [DOI] [PubMed] [Google Scholar]
- Ryu, Y. , Matsui, O. , Zen, Y. , Ueda, K. , Abo, H. , Nakanuma, Y. , & Gabata, T. (2010). Multicystic biliary hamartoma: Imaging findings in four cases. Abdominal Imaging, 35, 543–547. [DOI] [PubMed] [Google Scholar]
- Sheikh, A. A. E. , Nguyen, A. P. , Leyba, K. , Javed, N. , Shah, S. , Deradke, A. , Cormier, C. , Shekhar, R. , & Sheikh, A. B. (2022). Biliary duct hamartomas: A systematic review. Cureus, 14, e25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinakos, E. , Papalavrentios, L. , Chourmouzi, D. , Dimopoulou, D. , Drevelegas, A. , & Akriviadis, E. (2011). The clinical presentation of Von Meyenburg complexes. Hippokratia, 15, 170–173. [PMC free article] [PubMed] [Google Scholar]
- Soares, K. C. , Arnaoutakis, D. J. , Kamel, I. , Anders, R. , Adams, R. B. , Bauer, T. W. , & Pawlik, T. M. (2014). Cystic neoplasms of the liver: Biliary cystadenoma and cystadenocarcinoma. Journal of the American College of Surgeons, 218, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. S. , Lee, Y. , Kim, K. W. , Huh, J. , Jang, S. J. , & Yu, E. (2008). Cholangiocarcinoma arising in von Meyenburg complexes: Report of four cases. Pathology International, 58, 503–512. [DOI] [PubMed] [Google Scholar]
- Starost, M. F. (2007). Solitary biliary hamartoma with cholelithiasis in a domestic rabbit (Oryctolagus cuniculus). Veterinary Pathology, 44, 92–95. [DOI] [PubMed] [Google Scholar]
- Strasberg, S. M. , & Chapman, W. C. (2021). Enucleation of biliary cystadenomas: A review. Journal of Gastrointestinal Surgery, 25, 2700–2706. [DOI] [PubMed] [Google Scholar]
- Torbenson, M. S. (2018). Hamartomas and malformations of the liver. Seminars in Diagnostic Pathology, 36, 39–47. [DOI] [PubMed] [Google Scholar]
- Vidal, B. P. C. , Lahan‐Martins, D. , Penachim, T. J. , Rodstein, M. A. M. , Cardia, P. P. , & Prando, A. (2020). MR cholangiopancreatography: What every radiology resident must know. Radiographics, 40, 1263–1264. [DOI] [PubMed] [Google Scholar]
- von Meyenburg, H. (1918). Uber die cystenleber. Beitrage Zur Pathologischen Anatomie, 64, 477–539. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


