Abstract
Background
With the potential development of human pathogenic bacteria resistant to antibiotics, the use of antibiotics as growth promoter in poultry production was banned in different countries, and it has forced the poultry industry to consider ‘Biologically safer’ alternatives to antibiotics, among which the probiotics and microalgae can be mentioned.
Objective
Present study aimed to compare Spirulina platensis microalgae in combination with a native probiotic as an alternative to antibiotics.
Methods
336 male broiler chicks were allotted into 7 treatments and 4 repetitions in a completely randomised design to evaluate chick's performance and immune response to different treatment based on indexes as feed intake, weight gain, feed conversion ratio, humoral immunity, carcass characteristics, thigh and breast pH, intestinal morphology and microbial population. European production efficiency coefficient was also reported.
Results
No significant difference was appeared in the pH of thigh and breast meat (p > 0.05). Supplementation of diets with SP0.3 revealed better villi height, villi length to crypt depth ratio and villi surface. With significant difference (p < 0.05), the highest and lowest colonies of Lactobacillus and E. coli were recorded for PR0.5SP0.3 treatments.
Conclusions
Supplementation of broilers diets either with probiotic prepared from the microorganism isolated of native birds (1 g/kg) or S. platensis (0.2 g/kg) alone and their combination (0.3 g/kg of S. platensis in combination with 0.5 g/kg of native probiotic) are promising and can be a good alternative to antibiotics, lead to progress of broiler's performance.
Keywords: broilers, carcass characteristics, immune response, probiotic, Spirulina platensis
(1) Present study aimed to compare Spirulina platensis microalgae in combination with a native probiotic as an alternative to antibiotics, using 336 one‐day‐old broilers as 7 treatments and 4 replications. (2) Supplementation of broilers diets either with probiotic prepared from the microorganism isolated of native birds (1 g/kg) or S. platensis (0.2 g/kg) alone and their combination (0.3 g/kg of S. platensis in combination with 0.5 g/kg of native probiotic) is recommended. (3) Compared to commercial probiotics, recommended per cent of prepared probiotic, microalgae and their combination are promising and can be a good alternative to antibiotics and to improve immune system, intestinal morphology measures and gastrointestinal microbiota without any side effects and can lead to progress in the performance of broilers.
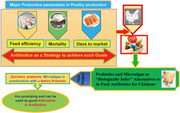
1. INTRODUCTION
In poultry production, feed efficiency, growth rate, days to market and mortality are major productive parameters. To achieve such goals, various strategies have been implemented, the most common of which is the use of antibiotics, due to its role to improve the growth performance and protect birds from the adverse effects of pathogenic and non‐pathogenic enteric microorganisms. But due to the potential development of human pathogenic bacteria resistant to antibiotics, this practice has been questioned in different countries, led to continuous efforts have been made and are being made to find natural alternatives to antibiotics in poultry nutrition for both the welfare of the birds and the confidence of consumers about the safe and hygienic products. On the other hand, it has forced the poultry industry to consider ‘biologically safer’ alternatives to in‐feed antibiotics for chickens, among which the probiotics and microalgae can be mentioned (Andrew et al., 2020; Mehdi et al., 2018).
With the aim of regulating the microbiota of the digestive tract of poultry, various therapeutic strategies have been proposed that can control several diseases that are closely related to inflammatory and metabolic disorders and lead to the improvement of animal health and performance. Among these solutions to overcome the problem are prebiotics, probiotics and herbal compounds (Shehata et al., 2022). In recent years, some live microorganism cultures have been described under the general term as ‘eubiotics’ (from the Greek word ‘eubiosis’), refers to the function of the microbiota in the digestive tract (Miniello et al., 2017; Yasar et al., 2017).
Possible modes of action of probiotics include competitive exclusion, increased digestive enzyme activity, production of substances that can inhibit the growth of pathogens or neutralise enterotoxins, modulation of the immune maturation of the host, and alteration of intestinal microbial activity (Sokale et al., 2019). To be used as an effective probiotic strain, the isolated microorganism must meet the following requirements: (1) Must be able to overcome the difficult conditions they face after entering the gastrointestinal tract. (2) Apart from the ability to survive in the host gastrointestinal tract, new strains must be correctly identified using biochemical and molecular methods. (3) Finally, new strains of probiotic bacteria should be able to inhibit the desired pathogens in vitro (EFSA, 2012).
Microalgae are natural feed ingredient with high nutritional value and efficiently used in poultry nutrition with different purposes such as to enhance the pigmentation and nutritional value (e.g., as partial replacement of conventional dietary protein sources) of meat and also been linked to an improvement in health and welfare (El‐Hady & El‐Ghalid, 2018). Microalgae classified as safe food sources by the US Food and Drug Administration include Spirulina, Chlorella, Danila, Hematococcus, Profiridium corinthum and Afanizomenon (Sathasivam et al., 2019). S. platensis is an edible microalga and a highly nutritious potential feed resource (e.g., 70–50% protein, amino acids, immune‐stimulants factors and antiviral activity) that can be used to improve biomass, chicken meat colour, chicken meat quality and egg quality. Yet, numerous factors may cause significant variation in S. platensis chemical composition, including climatic factors, light intensity, aeration, culture conditions and stress (Hassan et al., 2021; Soni et al., 2017).
Research findings have shown that the use of Spirulina and probiotics improves serum lipid concentrations and lipoprotein profiles by lowering total cholesterol, triglycerides, LDL and increasing HDL (De Jesus Raposo et al., 2016). Due to bioactive compounds in Spirulina such as flavonoids, carotenoids, linoleic acid, phenolic compounds, including phycocyanin, serum lipid profile in broilers can be improved (Lokapirnasari et al., 2016) and subsequently reduce serum cholesterol due to a decrease in the absorption, and synthesis of cholesterol is expected. Promotion of lactic acid bacteria and improvement of unpaired bile salts or inhibition of hydroxymethylglutaryl coenzyme A reductase activity by probiotics are other benefits that should be considered (Shokaiyan et al., 2019).
This research aims to investigate the effects of including microorganisms isolated from the digestive tract of native poultry (as a probiotic), and microalgae (as a phytogenic) in the diet to find a combination with synergetic effects on the modification of host metabolism, immune stimulation, inhibition and exclusion of pathogens in gut, enhanced nutrient absorption, lead to economic performance, and carcass components of broiler chicks.
2. MATERIALS AND METHODS
2.1. Animals and experimental design
A total of 336 one‐day‐old male broilers (Ross 308) were allotted into 7 treatments, 4 replications (12 chickens per replication) and reared for 42 days in a completely randomised design. Experimental treatments include (1) positive control 1 (basic diet + salinomycin antibiotic) (PC), (2) positive control 2 (basic diet + commercial probiotic) (COP), (3) basic diet + 0.2 g/kg Spirulina platensis powder (SP0.2), (4) basic diet + 0.3 g/kg S. platensis powder (SP0.3), (5) basic diet + 0.2 g/kg S. platensis powder + 0.5 g/kg native probiotic (PR0.5SP0.2), (6) basic diet + 0.3 g/kg S. platensis powder + 0.5 g/kg native probiotic (PR0.5SP0.3), (7) basic diet + 1 g/kg native probiotic (PR1).
The ingredient composition and chemical analysis of the basal diets (provided in the form of mash) formulated to meet nutrient requirements in different rearing periods (Table 1). Nutritional composition of Spirulina platensis microalgae powder used in the present study is shown in Table 2.
TABLE 1.
Components and chemical composition of the basal diets of broiler chicks in the starter, grower and finisher of rearing periods.
| Ingredients | Starter | Grower | Finisher |
|---|---|---|---|
| Corn | 52.1 | 54.6 | 59.4 |
| Soybean seed meal | 39.8 | 38.2 | 32.9 |
| Soybean oil | 1.62 | 3.18 | 3.9 |
| Corn gluten meal | 1.96 | 0.00 | 0.00 |
| Calcium carbonate | 0.980 | 0.90 | 0.830 |
| Dicalcium phosphate | 1.98 | 1.75 | 1.57 |
| Sodium chloride | 0.230 | 0.260 | 0.210 |
| Bicarbonate sodium | 0.140 | 0.110 | 0.180 |
| L‐threonine 98% | 0.100 | 0.0600 | 0.0500 |
| DL—methionine 99% | 0.310 | 0.280 | 0.250 |
| Lysine 78% | 0.200 | 0.100 | 0.110 |
| Vitamin premix a | 0.250 | 0.25 | 0.250 |
| Mineral premix b | 0.250 | 0.250 | 0.250 |
| Total | 100 | 100 | 100 |
| Chemical analysis | |||
| ME, kcal/kg | 2850 | 2950 | 3050 |
| Crude protein, % | 22.1 | 20.4 | 18.5 |
| Methionine, % | 0.640 | 0.580 | 0.530 |
| Methionine + cysteine, % | 0.990 | 0.910 | 0.840 |
| Lysine % | 1.34 | 1.20 | 1.08 |
| Threonine, % | 0.950 | 0.850 | 0.760 |
| Valine, % | 1.03 | 0.960 | 0.870 |
| Arginine, % | 1.44 | 1.36 | 1.22 |
| Isoleucine, % | 0.930 | 0.860 | 0.780 |
| Crude fibre, % | 0.830 | 0.780 | 0.700 |
| Crude fat, % | 4.49 | 6.01 | 6.80 |
| Linoleic acid, % | 2.13 | 2.98 | 3.43 |
| Calcium, % | 0.960 | 0.870 | 0.790 |
| Available phosphorus, % | 0.480 | 0.440 | 0.400 |
| Sodium, % | 0.150 | 0.150 | 0.150 |
| Potassium, % | 0.890 | 0.870 | 0.780 |
| Electrolyte balance (mEq/kg) | 0.230 | 0.230 | 0.200 |
Vitamin supplement per kg of diet contains vitamin A: 3,600,000 international units, vitamin D3: 800,000 international units, vitamin E: 7200 international units, vitamin K3: 0.8 mg, vitamin B1: 0.7 mg, vitamin B2: 64/2 mg, vitamin B3: 11.88 mg, vitamin B5: 2.92 mg, vitamin B6: 1.17 mg, vitamin B9: 0.4 mg, vitamin B12: 0.006 mg, choline: 100 mg.
Mineral supplement per kg of diet contains manganese: 39.68 mg, iron: 20 mg, copper: 4 mg, zinc: 33.88 mg, iodine: 0.39 mg, selenium: 0.08 mg.
TABLE 2.
Nutrient content in 100 grams of Spirulina platensis microalgae powder.
| Nutrients | Amount |
|---|---|
| Total protein | 55–70 g |
| total fat | 6–9 g |
| Total fibre | 2–10 g |
| Chlorophyll | 800–2000 mg |
| Ash | 662 g |
| Polysaccharides | 10–15 g |
| Humidity | 667 g |
| Beta‐carotene | 258 mg |
| Phosphorus | 914 mg |
| sodium | 186 mg |
| Calcium | 171 mg |
| Magnesium | 260 mg |
| Potassium | 1770 mg |
| Iron | 75 mg |
| Zinc | 5 mg |
Before the arrival of chickens and the rearing to be started, the rearing building and all the equipment were washed and disinfected. Temperature, humidity and lighting program were set up as it recommended in Ross 308 management manual.
2.2. Performance measures
Average body weight gain (BWG), feed intake (FI) and feed conversion ratio (FCR) were recorded periodically. Broiler's feed intake was calculated by subtracting the rejected feed from that offered in the rearing periods. The FCR for each treatment was done after correcting the weight of dead chickens. European production efficiency factor (EPEF) was also calculated based on the following equation (Marcu et al., 2013):
2.3. Immune response
Chicken antibody reactions were determined using SRBS suspension. On days 24 and 36 of the rearing period, two chickens were randomly selected from each cage and 0.1 cc of 25% SRBC solution was injected into the chick pectoralis muscle using an insulin syringe. Humoral immunity test was applied on days 30 and 42, using 1 cc of blood taken from the wing vein of chickens. The haemagglutination reaction was recorded based on the last two dilutions as an antibody for SRBC based on the logarithm as described previously (Dietert, 2009).
2.4. Carcass characteristics and internal organs weight
On day 42, 2 chicks from each cage whose weight was close to the average of the same cage were selected and after recording the live weight, slaughtered. Carcass weight (without viscera), weight of abdominal fat (fat around the cloaca, gizzard and proventriculus), weight of thymus, bursa fabricius, heart, gizzard, pancreas, liver, proventriculus, spleen, total intestinal and small intestinal weight were recorded. The percentage weight of each component was divided by the body weight and multiplied by 100 to obtain the relative values of specific organs
2.5. pH of meat
To measure the pH of the meat, 1 g of meat was isolated from the right side of breast and thigh of the bird on day of slaughter. After chopping the meat, the samples were poured into Falcon 15, and 5 cc of physiological saline was added and kept in refrigerator. Twenty‐four hours later, to reach ambient temperature, the samples were removed from the refrigerator and vortexed before pH measurement. In the next step, the pH of the meat of the thigh and breast was measured and recorded using a pH meter (Pestana et al., 2020).
2.6. Histomorphology of jejunum
At the end of the rearing period, 2 birds were randomly selected and slaughtered from each experimental replication. After removing the intestine from the bird's body, the jejunum sample was separated and, after rinsing with normal saline solution, was placed in a neutral 10% formalin buffer solution. In order to dehydrate the tissue, the samples were placed in 50%, 70%, 90% and absolute alcohol for 1 h, so that the tissue water was absorbed by alcohol. The samples were then placed in solid paraffin and 7 μm thick sections were prepared and transferred to a glass slide. Finally, these sections were stained with haematoxylin and eosin under a Leica microscope (Model DM 1000, USA) equipped with a digital camera with a 10× lens and the data were recorded. The average length of 4 healthy villi for each sample was calculated and reported in micrometres. The ratio of villi height (VH) to crypt depth was calculated in terms of micrometres (Ebrahimi et al., 2017), and villi surface area (VSA) was determined as in the formula below (Gangali et al., 2015).
2.7. Determination of bacterial population using real‐time PCR technique
To study the population of two types of gastrointestinal bacteria (Lactobacillus and E. coli), two chicks were selected from each cage on day of slaughter, slaughtered and DNA samples from isolated cecum were extracted as described in the instructions of Favorgen Biotech Corp. (Taiwanese product). Real‐time PCR was performed by BioRad real‐time PCR system CFX 96 (Applied Bio systems, Germany), using optical degree plates. The primers used to quantify different bacterial populations are shown in Table 3. The reaction was performed in a total volume of 15 μL using SYBR Green Master Mix. Each reaction consisted of 7.5 μL of 2 × SYBR Green Master Mix, 0.5 μL of 10 μM forward primer, 0.5 μL of 10 μM reverse primer, 2 μL of DNA sample and 4.5 μL of double distilled water. Each sample was assayed by duplicate reactions. Patternless control was included in real‐time PCR amplification to rule out any cross‐contamination. Real‐time PCR cycle conditions included initial denaturation at 94°C for 5 min, followed by 40 denaturation cycles at 94°C for 20 s, primer bonding at 58°C and 50°C for 30 s, respectively. Lactobacillus and E. coli were also propagated at 72°C for 20 s. As soon as the amplification was completed, the specificity of the amplified products was confirmed by melting curve analysis. Real‐time PCR products were incubated with increasing temperature from 70°C to 95°C with increasing of 0.5°C for 5 s interval. The results were expressed as log10 copies per gram of cecum content (Shokryazdan et al., 2017).
TABLE 3.
Primers used for Lactobacillus and E. coli populations using real‐time PCR technique.
2.8. Statistical analysis
Statistical analysis of data obtained from 336 one‐day‐old male broilers (Ross 308) was performed in a completely randomised design using the GLM procedure of SAS software package (Rastad et al., 2008). The mean of treatments for the studied traits were compared using Tukey's test at the level (p < 0.05). The statistical model with the components Yij (observation corresponding to repetition j of treatment i), μ (average attribute), ti (treatment effect), eij (test error) was as follows:
3. RESULTS
3.1. Growth performance
Data on feed intake, weight gain, feed conversion ratio and European production efficiency factor of different treatments are presented in Table 4. Based on these data, in the starter, grower and overall (days 1–42) periods, the highest daily feed intake was obtained with treatments PR0.5SP0.2, PC (except for overall period), PR1, and SP0.2, respectively, which showed a significant difference with the COP treatment (p < 0.05). The analysis of the data related to weight gain indicates that compared to COP, treatments PR0.5SP0.2 in starter and SP0.2 in overall periods resulted in a significant (p < 0.05) increase in average weight. Feed conversion ratio was not affected by the experimental treatments in entire rearing periods (p > 0.05). The lowest European production efficiency (EPEF) coefficient was related to treatment containing commercial probiotics (COP), which was significantly differ from PC (p > 0.05).
TABLE 4.
Effect of experimental treatments on growth performance in broilers (g /bird /day).*
| Item | PC | COP | SP0.2 | SP0.3 | PR0.5SP0.2 | PR0.5SP0.3 | PR1 | SEM | p Value |
|---|---|---|---|---|---|---|---|---|---|
| FI | |||||||||
| 1–10 | 22.3ab | 17.8c | 22.1ab | 20abc | 22.9a | 21.5ab | 22.3ab | 0.351 | 0.0001 |
| 11–24 | 84.3a | 72.7b | 85a | 82ab | 82.3ab | 85a | 86.3a | 1.02 | 0.0108 |
| 25–42 | 176 | 168 | 189 | 180 | 184 | 179 | 186 | 2.02 | 0.262 |
| 1–42 | 104a | 95b | 109 a | 101a | 106 a | 104 a | 103a | 1.08 | 0.0241 |
| BWG | |||||||||
| 1–10 | 14.3ab | 12b | 14.3a | 12.6b | 15.1a | 13.8ab | 14.7ab | 0.352 | 0.0262 |
| 11–24 | 48.9 | 41.6 | 51.8 | 47 | 44.5 | 48.5 | 51.2 | 0.911 | 0.125 |
| 25–42 | 105 | 95.6 | 102 | 103 | 103 | 101 | 107 | 0.971 | 0.0861 |
| 1–42 | 61.7a | 49.3b | 62.1a | 58.4a | 60.6ab | 59.5a | 60.4a | 0.955 | 0.0066 |
| FCR | |||||||||
| 1–10 | 1.56 | 1.49 | 1.55 | 1.59 | 1.51 | 1.58 | 1.53 | 0.0221 | 0.866 |
| 11–24 | 1.73 | 1.75 | 1.64 | 1.75 | 1.77 | 1.78 | 1.68 | 0.0292 | 0.966 |
| 25–42 | 1.66 | 1.76 | 1.85 | 1.74 | 1.78 | 1.77 | 1.75 | 0.0208 | 0.526 |
| 1–42 | 1.67 | 1.93 | 1.77 | 1.74 | 1.76 | 1.76 | 1.71 | 0.0233 | 0.278 |
| EPEF | 409a | 297b | 365ab | 385ab | 366ab | 382ab | 384ab | 8.03 | 0.0204 |
Note: Means in same row that do not have a common letter have a significant difference (p < 0.05).
FI, feed intake; BWG, body weight gain; FCR, feed conversion ratio; EPEF, European production efficiency factor.
PC: first positive control (with antibiotics), COP: second positive control (commercial probiotic), SP0.2: 0.2 g of Spirulina platensis powder, SP0.3: 0.3 g of Spirulina platensis powder, PR0.5SP0.2: 0.2 g of Spirulina platensis powder and 0.5 g of native probiotics PR0.5SP0.3: 0.3 g of Spirulina platensis powder and 0.5 g of native probiotics, PR1:1 g of native probiotics.
3.2. Antibody titres against SRBC
Total anti‐SRBC, IgG and IgM titres on days 30 and 42 are shown in Table 5. Experimental treatments on the 30th day of rearing period did not show any significant change in total antibody titre and IgM (p > 0.05). However, on the day 42, compared to PC, SP0.2 treatment significantly led to the higher IgG titre (p < 0.05) and also significant difference for IgM titre was obtained between PR0.5SP0.2 and COP treatment (p < 0.05).
TABLE 5.
Effects of experimental treatments on antibody titres against SRBC on days 30 and 42 of rearing periods.
| Item | PC | COP | SP0.2 | SP0.3 | PR0.5SP0.2 | PR0.5SP0.3 | PR1 | SEM | p Value |
|---|---|---|---|---|---|---|---|---|---|
| IgG30 | 0.370ab | 0.750ab | 0.121b | 0.620ab | 0.871ab | 0.850ab | 1a | 0.0801 | 0.0210 |
| IgM30 | 5.50 | 4.62 | 4.62 | 5.12 | 3.75 | 4.75 | 4.62 | 0.0211 | 0.0658 |
| Total30 | 5.62 | 5.62 | 4.62 | 5.12 | 4.62 | 5.87 | 5.75 | 0.1608 | 0.277 |
| IgG42 | 3b | 3b | 4.87a | 4.5ab | 3.37b | 3.12b | 3b | 0.1603 | 0.0060 |
| IgM42 | 5ab | 6.25a | 5.12ab | 5.37ab | 5.12ab | 4.37b | 5.12ab | 0.0211 | 0.0041 |
| Total42 | 8bc | 9.28ab | 10a | 9.87a | 8.5abc | 7.50c | 8.12bc | 0.180 | 0.0001 |
Note: Means in same row that do not have a common superscript have significant differences (p < 0.05).
PC: first positive control (with antibiotics), COP: second positive control (commercial probiotic), SP0.2: 0.2 g of Spirulina platensis powder, SP0.3: 0.3 g of Spirulina platensis powder, PR0.5SP0.2: 0.2 g of Spirulina platensis powder and 0.5 g of native probiotics PR0.5SP0.3: 0.3 g of Spirulina platensis powder and 0.5 g of native probiotics, PR1:1 g of native probiotics.
3.3. Carcass characteristics and meat pH
Results from data analysis of carcass characteristics and meat pH are presented in Table 6. According to these results, carcass yield, internal organs and pH of thigh and breast meat were not significantly affected by neither of the experimental treatments (p > 0.05).
TABLE 6.
Effect of experimental treatments on internal organ weight and pH of broiler meat and breast of broilers.
| Treatments | PC | COP | SP0.2 | SP0.3 | PR0.5SP02 | PR0.5SP0.3 | PR1 | SEM | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Heart | 0.520 | 0.569 | 0.497 | 0.521 | 0.520 | 0.540 | 0.531 | 0.0070 | 0.454 |
| The spleen | 0.111 | 0.111 | 0.100 | 0.100 | 0.100 | 0.111 | 0.0922 | 0.0031 | 0.199 |
| Liver | 2.13 | 1.98 | 2.03 | 2.14 | 1.95 | 2.10 | 2.10 | 0.0203 | 0.112 |
| gizzard | 1.36 | 1.52 | 1.4 | 1.41 | 1.46 | 1.47 | 1.43 | 0.0220 | 0.658 |
| Carcass weight | 64.8 | 64.4 | 65.3 | 66 | 65.4 | 65.9 | 66 | 0.295 | 0.121 |
| Intestine | 4.81 | 5.73 | 5.06 | 4.77 | 4.76 | 5.25 | 4.86 | 0.0911 | 0.246 |
| Abdominal fat | 0.955 | 0.880 | 0.891 | 1.07 | 1 | 0.821 | 0.930 | 0.0333 | 0.838 |
| Small intestine | 4.04 | 4.75 | 4.25 | 3.94 | 3.92 | 4.35 | 3.94 | 0.0800 | 0.266 |
| Pancreas | 0.221 | 0.280 | 0.201 | 0.211 | 0.226 | 0.210 | 0.225 | 0.0041 | 0.101 |
| Thymus | 0.244 | 0.215 | 0.254 | 0.238 | 0.221 | 0.250 | 0.223 | 0.0101 | 0.974 |
| Exchange | 0.180 | 0.141 | 0.158 | 0.132 | 0.140 | 0.0901 | 0.174 | 0.0080 | 0.153 |
| Proventriculus | 0.365 | 0.420 | 0.331 | 0.340 | 0.380 | 0.355 | 0.321 | 0.0070 | 0.0600 |
| Thigh pH | 6.45 | 6.43 | 6.45 | 6.44 | 6.41 | 6.43 | 6.45 | 0.0101 | 0.966 |
| Breast pH | 6.44 | 6.36 | 6.40 | 6.43 | 6.39 | 6.40 | 6.43 | 0.0080 | 0.233 |
Note: Means in same row that do not have a common superscript have significant differences (p < 0.05).
PC: first positive control (with antibiotics), COP: second positive control (commercial probiotic), SP0.2: 0.2 g of Spirulina platensis powder, SP0.3: 0.3 g of Spirulina platensis powder, PR0.5SP0.2: 0.2 g of Spirulina platensis powder and 0.5 g of native probiotics, PR0.5SP0.3: 0.3 g of Spirulina platensis powder and 0.5 g of native probiotics, PR1:1 g of native probiotics.
3.4. Histomorphology of jejunum
Histomorphometric examination of the chick jejunum fed various additives is shown in Table 7. The measures of jejunum morphometry, including villus length, crypt depth, villus length /crypt depth, and surface area, indicate that except to crypt depth, other measures significantly (p < 0.01) impacted by the supplementation of 0.3% Spirulina platensis powder, namely, SP0.3.
TABLE 7.
The effect of experimental treatments on the morphology of the small intestine jejunum of broilers.
| Treatments | PC | COP | SP0.2 | SP0.3 | PR0.5SP0.2 | PR0.5SP0.3 | PR1 | SEM | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Villus length (μm) | 896d | 943cd | 1268ab | 1446a | 1138bc | 1177bc | 976cd | 28.5 | 0.0001 |
| Crypt depth (μm) | 176 | 157 | 144 | 157 | 192 | 181 | 157 | 4.78 | 0.163 |
| Villus length/crypt depth (μm) | 5.55c | 6.11bc | 8.78ab | 9.20a | 6.25bc | 6.54abc | 6.13bc | 0.230 | 0.0184 |
| Surface area(μm) | 781b | 833b | 1003ab | 1076a | 882ab | 936ab | 852ab | 18 | 0.0040 |
Note: Means in same row that do not have a common superscript have significant differences (p < 0.05).
PC: first positive control (with antibiotics), COP: second positive control (commercial probiotic), SP0.2: 0.2 g of Spirulina platensis powder, SP0.3: 0.3 g of Spirulina platensis powder, PR0.5SP0.2: 0.2 g of Spirulina platensis powder and 0.5 g of native probiotics, PR0.5SP0.3: 0.3 g of Spirulina platensis powder and 0.5 g of native probiotics, PR1:1 g of native probiotics.
3.5. Bacterial population in cecum
Table 8 shows the influence of dietary additives inclusion on the Lactobacillus and Escherichia coli bacteria population in the experimental bird cecum. As data indicate, compared to PC treatment, a combination of probiotic and Spirulina Platensis powder (PR0.5SP0.3) resulted to significant increase in Lactobacillus bacteria and simultaneously significant reduction in Escherichia coli population (p < 0.05).
TABLE 8.
The effect of experimental treatments on the population of Lactobacillus and E. coli in broiler cecum (logcfu/g).
| Treatments | PC | COP | SP0.2 | SP0.3 | PR0.5SP0.2 | PR0.5SP0.3 | PR1 | SEM | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Lactobacillus | 5.86c | 6.59abc | 6.53bc | 6.71abc | 7.16ab | 7.45a | 6.44bc | 0.0700 | 0.0001 |
| E. coli | 1.28a | 1.05ab | 0.954ab | 0.750ab | 0.650ab | 0.351b | 0.960ab | 0.0804 | 0.0211 |
Note: Means in same row that do not have a common superscript have significant differences (p < 0.05).
PC: first positive control (with antibiotics), COP: second positive control (commercial probiotic), SP0.2: 0.2 g of Spirulina platensis powder, SP0.3: 0.3 g of Spirulina platensis powder, PR0.5SP0.2: 0.2 g of Spirulina platensis powder and 0.5 g of native probiotics, PR0.5SP0.3: 0.3 g of Spirulina platensis powder and 0.5 g of native probiotics, PR1: 1 g of native probiotics.
4. DISCUSSION
Microalgae are increasingly being studied as an emerging and promising feed additive to improve poultry productivity. Research findings show that Spirulina platensis (a filamentous blue‐green microalga) increased the digestibility of feed by improving the morphological structure of the digestive system, which results in an increase in chicken performance (El‐Hady & El‐Ghalid, 2018; Park et al., 2018). Moreover, probiotics are potentially known with various positive effects on the morphology of the digestive tract, gut microbiota improvement, intestinal barrier function, immune responses, integration of digestive tract, antioxidant capacity, nutrients absorption, and finally the health and growth performance of broiler chicken (He et al., 2019; Shehata et al., 2022).
This study highlights that inclusion of microalgae, probiotics (derived from local strains) and their combination to broiler diets showed an improvement of productive parameters and resulted in a significant increase in the feed consumption and weight gain; however, no significant difference was obtained in the feed conversion ratio, though, compared to control treatments, the calculated FCR was numerically improved or at least was similar. S. platensis is rich in nutrients such as amino acids, vitamins, zeaxanthin, vitamins and iron phycocyanin, carotenoid and protein, which can justify the reported feed intake and growth performance improvement (Ahmed et al., 2022; El‐Hady et al., 2022). In addition, development of favourable bacteria, probiotics reduce the breakdown of protein into nitrogen and optimal use of amino acids by preventing colibacilli and inhibiting enterotoxins in the digestive system (Ahmat et al., 2021).
S. platensis has also been linked to an improvement in broiler's immune system and health, due to β‐carotene and vitamin B12 content that appear to have immunomodulatory effects on the immune responses in broilers. Based on result in another study (Fathi et al., 2017), S. platensis has a direct effect on broiler's growth and lymphatic tissues stimulation. It has been reported that inclusion of Spirulina platensis microalgae in broiler diet can be result in immune positive response via increasing the serum level of IgM, IgG, phagocytic activity, and phagocytic index (Ahmed et al., 2022). On the other hand, probiotics may improve immune system via three mechanisms: (1) increasing the production of immunoglobulins M, G and interferons; (2) increasing the production of local antibodies in the mucous surfaces of the body, such as the intestinal wall, which are usually of the IgA type; and (3) the increase in the activity of macrophages, which is manifested through the increase in the phagocytosis of microorganisms (Jing et al., 2017). These findings are compatible with the results obtained in the present study and confirm them in most cases.
Jejunum morphometry measured in current study indicate that the villus length, crypt depth, villus length /crypt depth were significantly increase in broilers consumed diets containing S. platensis. These results are similar to that reported previously, in which stated that supplementing poultry diet with S. platensis powder leads to the homogeneous development of intestinal villi and epithelial cells (Shanmugapriya et al., 2015). Prebiotics, probiotics and phytogenic exert their beneficial effects indirectly through stabilising the natural intestinal microbiota which subsequently result in the intestinal epithelial barrier improvement (Shehata et al., 2022). In accordance with the results of other researchers, part of the interaction effect between S. platensis microalgae as a prebiotic in combination with probiotics in improving villus length or the ratio of villus length to crypt depth appeared in this study can be explained by the synergistic effect between these additives (Li et al., 2019; Tavaniello et al., 2018).
S. platensis contains growth stimulating nutrients such as exopolysaccharide, adenine, hypoxanthine, free amino acids, vitamins, minerals, phycocyanin (a powerful plant‐based protein) and beta‐carotene, which are effective on the survival of probiotic bacteria and increasing the Lactobacillus population (Finamore et al., 2017). If so, improved nutrient absorption, reduced movement of harmful bacteria and endotoxins secretion in the intestine will be expected, that resulting in improving the microbiome balance in the digestive system of the host bird. Combination of two important properties of probiotics, namely, the secretion of antimicrobial substances and competition for available nutrients, and at the same time providing good sources of available nutrients by S. platensis, leads to an increase in beneficial and reduce harmful bacteria (Awad et al., 2009; Mirzaie et al., 2018; Mookiah et al., 2014; Van zyl et al., 2020). Summarising the above findings and paying attention to the results reported in the present research indicate the compatibility between them, because first, the balance of microbiota in the digestive tract is in favour of the host bird; second, the improvement of their immune system through the increase of antibody titre; and finally, the improvement of absorptive cells in small intestine have been clearly shown in treatments containing probiotics, S. platensis or their combination (see Tables 5, 7, and 8).
5. CONCLUSIONS
The general conclusion is that compared to commercial probiotics, supplementation of broilers diets either with probiotic prepared from the microorganism isolated of native birds (1 g/kg) in current study or S. platensis (0.2 g/kg) alone and their combination (0.3 g/kg of S. platensis microalgae powder in combination with 0.5 g/kg of native probiotic) are promising and can be a good alternative to antibiotics and to improve immune system, intestinal morphology measures and gastrointestinal microbiota without any side effects and can lead to progress in the performance of experimental broilers, though more studies on microalgae are needed.
AUTHOR CONTRIBUTIONS
Khalilnia: Carried out investigation, data collection and curation during farm and lab works, statistical analysis, and writing – original draft of manuscript. Mottaghitalab: Responsible for conceptualising the project, investigation, description of project methodology, data curation, statistical analysis control, funding acquisition, project administration, resources, supervision, validation, writing, review and editing of manuscript. Mohiti: Participated in conceptualisation, data curation, methodology and visualisation. Seighalani: Provided laboratory method analysis, contribution in investigation, data curation, quality control of laboratory data, resources and visualisation. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that this article was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Every attempt has been made to ensure that the material in this article is accurate, true, correct, and relevant at the time of writing.
ETHICS STATEMENT
All experimental procedures were approved by the Research Ethics Committee on Animal Use in University of Guilan (ID:IR.GUILAN.REC.1400.015).
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1154.
ACKNOWLEDGEMENTS
The author gratefully acknowledges Animal Biotechnology Research Institute of the North of Iran, Parsian Microalgae Company, and Ramsar Tior Company for technical and partial financial support for this research.
khalilnia, F. , Mottaghitalab, M. , Mohiti, M. , & Seighalani, R. (2023). Effects of dietary supplementation of probiotic and Spirulina Platensis microalgae powder on growth performance immune response, carcass characteristics, gastrointestinal microflora and meat quality in broilers chick. Veterinary Medicine and Science, 9, 1666–1674. 10.1002/vms3.1154
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Ahmat, M. , Cheng, J. , Abbas, Z. , Cheng, Q. , Fan, Z. , Ahmad, B. , Hou, M. , Osman, G. , Guo, H. , Wang, J. , & Zhang, R. (2021). Effects of Bacillus amyloliquefaciens LFB112 on growth performance, carcass traits, immune, and serum biochemical response in broiler chickens. Journal Antibiotics, 10, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, M. M. , Said, I. , & Orabi, S. (2022). Enhancement effect of Spirulina platensis extract on broiler chicks’ growth performance and immunity. Journal of Current Veterinary Research, 4, 1. [Google Scholar]
- Andrew, S. L. , Hassan, Z. M. , Manyelo, T. G , & Mabelebele, M. (2020). The current status of the alternative use to antibiotics in poultry production: An African perspective. Antibiotics (Basel), 9(9), 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad, W. A. , Ghareeb, K. , Abdel‐Raheem, S. , & Bohm, J. (2009). Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poultry Science, 88, 49–55. [DOI] [PubMed] [Google Scholar]
- De Jesus Raposo, M. F. , de Morais, A. M. , & de Morais, R. M. (2016). Emergent sources of prebiotics: Seaweeds and microalgae. Marine Drugs, 14, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert, R. R. (2009). Immunotoxicity testing methods and protocols. Chapter, 25, 387–398. [Google Scholar]
- Ebrahimi, E. , Sobhani, R. S. , & Zarghi, H. (2017). Effect of triticale level and exogenous enzyme in the grower diet on performance, gastrointestinal tract relative weight, jejunal morphology and blood lipids of Japanese quail (Coturnix japonica). Journal of Agricultural Science and Technology, 19, 569–580. [Google Scholar]
- EFSA . (2012). Panel on additives and products or substances used in animal feed, guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. European Food Safety Authority Journal, 10, 1–10. [Google Scholar]
- El‐Hady, A. M. A. , & El‐Ghalid, O. A. H. (2018). Spirulina platensis algae (SPA): A novel poultry feed additive. Effect of SPA supplementation in broiler chicken diets on productive performance, lipid profile and calcium‐phosphorus metabolism. Mediterranean Poultry Summit, 74, 18–20. [Google Scholar]
- El‐Hady, A. M. A , El‐Ghalid, O. A. H. , El‐Naggar, A. S. H. , & El‐Khalek, E. A. (2022). Growth performance and physiological status evaluation of Spirulina platensis algae supplementation in broiler chicken diet. Science, 263, 105009. [Google Scholar]
- Fathi, M. , Abdelsalam, M. , Al‐Homidan, I. , Ebeid, T. , El‐Zarei, M. , & Abou‐Emera, O. (2017). Effect of probiotic supplementation and genotype on growth performance, carcass traits, hematological parameters and immunity of growing rabbits under hot environmental conditions. Animal Science Journal, 88, 1644–650. [DOI] [PubMed] [Google Scholar]
- Finamore, A. , Palmery, M. , Bensehaila, S. , & Peluso, I. (2017). Antioxidant, immunomodulating, and microbial‐modulating activities of the sustainable and ecofriendly Spirulina . Oxidative Medicine and Cellular Longevity, 28, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangali, H. , Raji, A. R. , & Zarghi, H. (2015). Effect of post hatch delayed access to feed on performance, GIT physical and histological development and yolk absorption in young broiler chicks. Biomedical and Pharmacology Journal, 8, 945–955. [Google Scholar]
- Hassan, F. , Samia, M. , Manal, M. , Attia, Y. A. , Mekawy, A. , & Mahrose, K. (2021). Zinc and/or selenium enriched spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals, 11, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, R. I. M. , Refaie, M. S. , El‐Shoukary, R. D. , Rehan, I. F. , Zigo, F. , Karaffová, V. , & Amer, H. Y (2022). Effect of dietary microalgae (Spirulina platensis) on growth performance, ingestive behavior, hemato‐biochemical parameters, and economic efficiency of fayoumi broilers. Life, 12, 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, T. , Long, S. , Mahfuz, S. , Wu, D. , Wang, X. , Wei, X. , & Piao, X. (2019). Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals, 9, 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, G. C. , Jing, G. , Sun, Z. , Wang, H. , Gong, Y. , Huang, S. , Ning, K. , Xu, J. , & Su, X. (2017). Parallel META 3: Comprehensive taxonomical and functional analysis platform for efficient comparison of microbial communities. Scientific Reports, 7, 40371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. L. , Wang, J. , Zhang, H. J. , Wu, S. G. , Hui, Q. R. , Yang, C. B. , Fang, R. J. , & Qi, G. H. (2019). Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Frontiers in Physiology, 9, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokapirnasari, W. P. , Yulianto, A. B. , & Legowo, D. (2016). The effect of Spirulina as feed additive to myocardial necrosis and leukocyte of chicken with avian influenza (H5N1) virus infection. Procedia Chem, 18, 213–217. [Google Scholar]
- Marcu, A. , Vacaru, I. , Gabi, D. , Liliana, P. C. , Marcu, A. , Marioara, N. , Ioan, P. , Dore, D. , Bartolomeu, K. , & Cosmin, M. (2013). The influence of genetics on economic efficiency of broiler chickens growth. Animal Science Biotech, 46, 339–346. [Google Scholar]
- Mehdi, Y. , Létourneau‐Montminy, M. P. , Gaucher, M. L. , Chorfi, Y. , Suresh, G. , Rouissi, T. , Brar, S. K. , Côté, C. , Ramirez, A. A. , & Godbout, S. (2018). Use of antibiotics in broiler production: Global impacts and alternatives. Animal Nutrition, 4, 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniello, V. , Diaferio, L. , Lassandro, C. , & Verduci, E. (2017). The importance of being eubiotic. Journal of Probiotics & Health, 5, 1–12. [Google Scholar]
- Mirzaie, S. , Zirak‐Khattab, F. , Hosseini, S. A. , & Donyaei‐Darian, H. (2018). Effects of dietary Spirulinaon antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian Australasian Journal of Animal Sciences, 31, 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookiah, S. , Sieo, C. C. , Ramasamy, K. , Abdullah, N. , & Ho, Y. W. (2014). Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. Journal of the Science of Food and Agriculture, 94, 341–348. [DOI] [PubMed] [Google Scholar]
- Park, J. H. , Lee, S. I. , & Kim1, I. H. (2018). Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poultry Science, 97, 2451–2459. [DOI] [PubMed] [Google Scholar]
- Pestana, J. M. , Puerta, B. , Santos, H. , Madeira, M. S. , Alfaia, C. M. , Lopes, P. A. , Pinto, R. M. A. , Lemos, J. P. C. , Fontes, C. M. G. A. , Lordelo, M. M. , & Prates, J. A. M. (2020). Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poultry Science, 99, 2519–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastad, A. H. , Samie, A. , & Daneshvar, F. (2008). Effect of Bactocell and dry whey on performance and carcass characteristics of broiler chickens. Journal of Crop Production and Processing, 12(43), 473–480. [Google Scholar]
- Sathasivam, R. , Radhakrishnan, R. , Hashem, A. , & Abd_Allah, E. F. (2019). Microalgae metabolites: A rich source for food and medicine. Saudi Journal of Biological Sciences, 26(4), 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugapriya, B. , Babu, S. S. , Hariharan, T. , Sivaneswaran, S. , Anusha, M. B. . , & Raja, P. U. (2015). Synergistic effect of Spirulina platensis on performance and gut microbial load of broiler chicks. Indo‐Asian Journal of Multidisciplnary Research, 1, 149–155. [Google Scholar]
- Shehata, A. A. S. , Yalçın, J. D. , Latorre, S. , Basiouni, Y. A. , Attia, A. A. , El‐Wahab, C. , Visscher, H. R. , El‐Seedi, C. , Huber, M. , Hafez, H. , Eisenreich, W. , & Tellez‐Isaias, G. (2022). Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms, 10(2), 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokaiyan, M. , Ashayerizadeh, O. , Shams Shargh, M. , & Dastar, B. (2019). Algal crude fucoidan alone or with Bacillus subtilis DSM 17299 in broiler chickens diet: Growth performance, carcass characteristics, blood metabolites, and morphology of intestine. Poultry Science Journal, 7, 87–94. [Google Scholar]
- Shokryazdan, P. , Faseleh Jahromi, M. , Liang, J. B. , Ramasamy, K. , Sieo, C. C. , & Ho, Y. W. (2017). Effects of a Lactobacillus salivarius mixture on performance, intestinal health and serum lipids of broiler chickens. PLoS ONE, 12(5), e0175959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokale, A. , Menconi, A. , Mathis, G. , Lumpkins, B. , Sims, M. , Whelan, R. , & Doranalli, K. (2019). Effect of Bacillus subtilis DSM 32315 on the intestinal structural integrity and growth performance of broiler chickens under necrotic enteritis challenge. Poultry Science, 98(11), 5392–5400. [DOI] [PubMed] [Google Scholar]
- Soni, R. A. , Sudhakar, K. , & Rana, R. S. (2017). Spirulina‐From growth to nutritional product: A review. Trends in Food Science & Technology, 69, 157–171. [Google Scholar]
- Tavaniello, S. , Maiorano, G. , Mucci, R. , Bogucka, J. , Stadnicka, K. , & Bednarczyk, M. (2018). Prebiotics offered to broiler chicken exert positive effect on meat quality traits irrespective of delivery route. Poultry Science, 97, 2979–2987. [DOI] [PubMed] [Google Scholar]
- Van Zyl, W. F. , Deane, S. M. , & Dicks, L. M. T (2020). Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes, 12(1), 1831339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar, S. , Okutan, I. , & Tosun, R. (2017). Testing novel eubiotic additives: Its health and performance effects in commercially raised farm animals. Journal of the Institute of Science and Technology, 7, 297–308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


