Abstract
In Azotobacter vinelandii, activation of nif gene expression by the transcriptional regulatory enhancer binding protein NIFA is controlled by the sensor protein NIFL in response to changes in levels of oxygen and fixed nitrogen in vivo. NIFL is a novel redox-sensing flavoprotein which is also responsive to adenosine nucleotides in vitro. Inhibition of NIFA activity by NIFL requires stoichiometric amounts of the two proteins, implying that the mechanism of inhibition is by direct protein-protein interaction rather than by catalytic modification of the NIFA protein. The formation of the inhibitory complex between NIFL and NIFA may be regulated by the intracellular ATP/ADP ratio. We show that adenosine nucleotides promote complex formation between purified NIFA and NIFL in vitro, allowing isolation of the NIFL-NIFA complex. The complex can also be isolated from cell extracts containing coexpressed NIFL and NIFA in the presence of MgADP. Removal of the nucleotide causes dissociation of the complex. Experiments with truncated proteins demonstrate that the amino-terminal domain of NIFA and the C-terminal region of NIFL potentiate the ADP-dependent stimulation of NIFL-NIFA complex formation.
The enhancer binding protein NIFA and the sensor protein NIFL comprise an atypical two-component regulatory system which regulates the expression of genes involved in nitrogen fixation in the free-living diazotrophs Klebsiella pneumoniae and Azotobacter vinelandii. The NIFA protein activates transcription from ςN-dependent nif promoters in combination with ςN RNA polymerase holoenzyme, and its activity is repressed by the NIFL protein in response to increases in the levels of fixed nitrogen and extracellular oxygen (6). We have shown previously that A. vinelandii NIFL is a flavoprotein with flavin adenine dinucleotide (FAD) as the prosthetic group (13), and this has now been demonstrated for the K. pneumoniae protein (21). The oxidized form of NIFL is competent to inhibit NIFA activity, but reduction of the flavin moiety in NIFL abolishes its ability to inhibit NIFA. Thus, NIFL acts as a redox-sensitive molecular switch to regulate NIFA activity. The inhibitory activity of NIFL is also stimulated by adenosine nucleotides in vitro, suggesting that it may sense energy charge in vivo (8). The response to nucleotides overrides the redox switch. Forms of NIFL which lack the flavin moiety are still able to inhibit NIFA activity in response to ADP (13). We have shown recently that the C-terminal domain of NIFL binds nucleotides, and it is possible that this domain has evolved from a classical histidine protein kinase to a nucleotide-responsive domain (23). This domain may also contain sequences required for interaction with NIFA, and it is possible that the formation of the inhibitory complex between NIFL and NIFA may be regulated by the ATP/ADP ratio.
The NIFL protein is comprised of two domains tethered by a Q linker (5, 7, 26). The proposed amino-terminal domain contains the flavin binding site and shows some homology to other oxygen- and redox-sensing proteins (5). A motif consisting of two conserved regions termed S boxes has recently been identified in the amino-terminal domain of NIFL. This motif is found in the PAS domains of a wide range of sensory proteins in both prokaryotic and eukaryotic organisms and may be involved in transducing environmental signals to other domains of the protein (24, 27). In the case of NIFL, the PAS domains may contain the ligands for flavin binding.
The C-terminal domain of A. vinelandii NIFL has significant homology to the histidine protein kinase transmitter domains, including the region containing the conserved histidine residue. However, there is no evidence for any phosphotransfer occurring between NIFA and NIFL, and signal transduction is believed to occur via protein-protein interaction (2, 15). We have shown previously with cell extracts from K. pneumoniae that both proteins can be immunoprecipitated by antisera to either NIFA or NIFL, implying the formation of a complex (12). This is consistent with the requirement for a stoichiometric concentration of each protein for effective inhibition of NIFA activity in vivo (9–11) and in vitro (2). We have now demonstrated the existence of the A. vinelandii NIFL-NIFA complex by cochromatography experiments with histidine-tagged forms of the proteins. We show here that the presence of adenosine nucleotides promotes the formation of the NIFL-NIFA complex. We have characterized the isolated complex and assessed the ability of truncated fragments and domains of NIFA and NIFL to undergo complex formation in response to ADP.
MATERIALS AND METHODS
Protein purification.
The native forms of NIFL and NIFA were purified as described previously (2). The C-terminal hexahistidine-tagged forms of NIFL and NIFA and the truncated derivatives were purified by nickel affinity chromatography on Hi-trap chelating columns (Pharmacia) as recommended by the manufacturer. For the native and histidine-tagged NIFA proteins, 50 mM potassium thiocyanate was routinely added to the chromatography buffers to inhibit precipitation. Integration host factor (IHF) and K. pneumoniae ςN were purified as described previously (23). Escherichia coli core RNA polymerase was purchased from Epicentre Technologies.
For molecular mass estimation, the preformed NIFL-NIFA complex or the individual proteins were chromatographed on a Superose 12 gel filtration column (Pharmacia) which had previously been calibrated with molecular weight standards as described in the legend to Fig. 7.
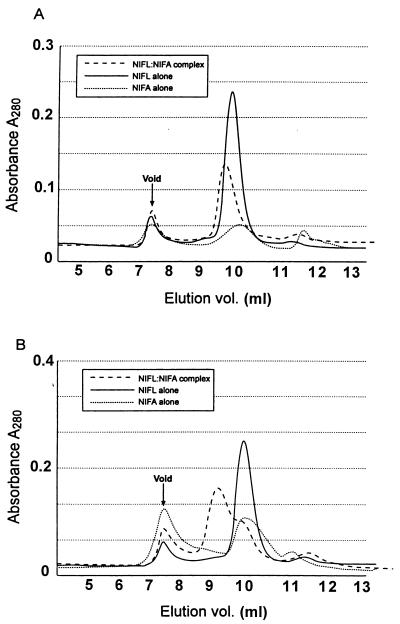
FIG. 7.
Absorbance traces of NIFL-NIFA complexes and individual proteins chromatographed on Superose 12. The column was calibrated with the following standards: thyroglobulin (669 kDa); β-amylase (200 kDa); alcohol dehydrogenase (150 kDa); bovine serum albumin (66 kDa); carbonic anhydrase (29 kDa); cytochrome c (12.4 kDa). The absorbance traces at 280 nm are shown for the NIFL-NIFA complex isolated from cell extract and chromatographed as described in Table 1 in the absence (A) or the presence (B) of 500 μM MgADP. The individual NIFL and NIFA proteins are those which had been dissociated from each other on the nickel column by removal of nucleotide and then chromatographed under the same conditions as the NIFL-NIFA complex in the presence and absence of 500 μM MgADP.
Transcription assays.
Single-round transcription assays were performed with purified proteins as described previously (2) except that 4 mM GTP was added in the absence of the other nucleotides to allow open complexes to form prior to heparin challenge. The template DNA (10 nM) was pNH8, which carries the nifH promoter and upstream binding sites for NIFA and IHF. NIFA was used at 150 nM, IHF at 50 nM, core RNA polymerase at 50 nM, and ςN at 200 nM.
NIFL-NIFA complex formation assay.
Reactions were carried out in Tris acetate buffer containing containing 50 mM Tris acetate (pH 7.9), 100 mM potassium acetate, and 8 mM magnesium acetate. NIFA and NIFL and their truncated derivatives were used at concentrations between 2 and 8 μM as stated in the figure legends. Nucleotides were used at 1 mM unless stated otherwise. Reaction mixtures (final volume, 230 μl) were preincubated for 5 min at 30°C and then loaded onto a 1-ml Hi Trap chelating column (Pharmacia) which had been charged with NiCl2 and equilibrated in buffer containing 50 mM Tris acetate (pH 7.9), 300 mM NaCl, 20 mM imidazole, and 5% glycerol. Where nucleotides were present in the reaction mixtures, they were added at the same concentrations to the chromatography buffers to prevent dissociation of NIFL-NIFA complexes. Nonbinding protein was washed from the column with the equilibration buffer, and bound material was then eluted with equilibration buffer containing 500 mM imidazole. Aliquots of the fractions were either mixed directly with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer or pooled and concentrated before electrophoresis. Electrophoresis was carried out with 10% polyacrylamide–SDS gels unless stated otherwise in the text.
Isolation of the NIFL-NIFA complex from cell extracts.
Expression of nifL and nifA was induced in 1-liter cultures of aerobically grown E. coli BL21(DE3)(pLysS) carrying plasmid pPR38 (23). Addition of 1 mM IPTG (isopropyl-β-d-galactopyranoside) for 3 h at 28°C resulted in synthesis of approximately stoichiometric amounts of NIFL and NIFA. Crude cell extract was obtained by French pressure cell disruption in a buffer containing 50 mM Tris-Cl (pH 8), 300 mM NaCl, 1 mM MgADP, 50 mM potassium thiocyanate, 5% (wt/vol) glycerol, and 1 mM Pefabloc, followed by low-speed centrifugation of the resultant lysate. Under these conditions, at least 50% of the overexpressed protein stayed in the supernatant fraction. This was loaded onto a 1-ml Hitrap chelating affinity column which had been charged with NiCl2 and equilibrated in start buffer containing 50 mM Tris-Cl (pH 8), 300 mM NaCl, 20 mM imidazole, 5% (wt/vol) glycerol, 50 mM potassium thiocyanate, and 1 mM MgADP. The nonbinding protein was washed from the column with start buffer, and the bound fractions were eluted with start buffer containing 500 mM imidazole. The fractions containing the NIFL-NIFA complex were pooled and dialyzed into storage buffer containing 10 mM Tris-Cl (pH 8), 50% glycerol, 0.1 mM EDTA, 0.1 mM dithiothreitol, 50 mM NaCl, 500 μM MgADP, and 50 mM potassium thiocyanate. The dialyzed protein was stored in small aliquots in liquid N2.
Molecular mass estimation of the NIFL-NIFA complex.
Approximately 250 μg of either preformed NIFL-NIFA complex or purified NIFA or NIFL alone were chromatographed on a Superose 12 column equilibrated in 10 mM Tris-Cl (pH 8), 10% glycerol, 0.1 mM EDTA, 0.1 mM dithiothreitol, and 150 mM NaCl. MgADP was present in the buffers where indicated at 500 μM. The in vitro-formed complex was prepared by mixing equimolar amounts of purified NIFL and NIFA with 500 μM MgADP prior to loading it on the column. NIFA and NIFL were also mixed separately with 500 μM MgADP and chromatographed as controls. A duplicate set of samples were then chromatographed in the absence of nucleotide. The NIFL-NIFA complex isolated from cell extract in the presence of 1 mM MgADP was divided into two aliquots and briefly dialyzed in the presence and absence of 500 μM MgADP before application to the column. The chromatographic behavior of these samples was compared to that of NIFL and NIFA, which had been dissociated from each other on the nickel column by removal of the nucleotide.
RESULTS
The complex between NIFL and NIFA formed in vitro is stimulated by the presence of adenosine nucleotides.
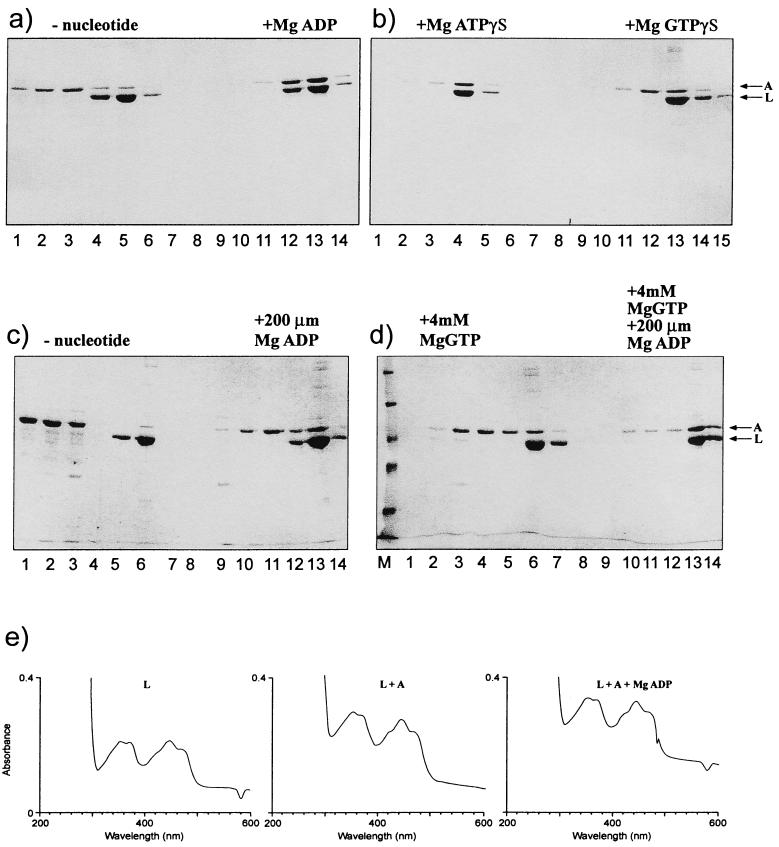
A binding assay with metal chelate affinity chromatography on nickel columns and histidine-tagged forms of purified NIFA or NIFL was employed to determine conditions which allowed coelution of the tagged version of one protein with its nontagged partner from the column. Equimolar concentrations of the proteins were used in the assays, assuming that both proteins are tetramers in solution (23) (Table 1). The proteins were incubated together in Tris acetate buffer consistent with the conditions used to measure inhibition of NIFA activity by NIFL. Reactions were carried out as described in Materials and Methods in the presence or absence of nucleotide with the native nontagged form of NIFA and C-terminal NIFL6his. In control experiments in the absence of NIFL6his, nontagged NIFA was eluted from the column in the wash fractions. However, the NIFA protein showed a tendency to bind nonspecifically to the column and required extensive washing with 20 mM imidazole buffer to eliminate it completely. The presence of nucleotides did not affect the chromatographic behavior of the individual proteins. In the absence of nucleotide, most of the NIFA did not form a stable complex with NIFL6his and was eluted in the wash fractions (Fig. 1a). A small amount of NIFA coeluted with NIFL in the absence of nucleotides in some experiments. This may be due to the tendency of nontagged NIFA to bind nonspecifically to the column as described above or may result from the formation of unstable NIFL-NIFA complex in the absence of nucleotides. The addition of 1 mM MgADP to the reaction mixture and column buffers resulted in NIFA binding to NIFL and the proteins coeluting at 500 mM imidazole (Fig. 1a). At 1 mM MgADP, almost all of the NIFA was retained by the NIFL on the column, with only trace amounts of NIFA appearing in the wash fractions. Concentrations of MgADP below 1 mM were also effective, and an increase in the level of complexes could be detected between 10 and 200 μM nucleotide concentration (Fig. 1c and data not shown). Complexes between NIFL and NIFA were also observed with 1 mM MgATPγS, the nonhydrolyzable analogue of ATP (Fig. 1b), but this was not as effective as ADP at lower concentrations. With MgGTPγS, the nonhydrolyzable analogue of GTP, only low levels of complexes were detected at 1 mM concentration of the analogue (Fig. 1b).
TABLE 1.
Gel filtration of NIFL-NIFA complexes on Superose 12
| Protein | MgADPa | In vitrob elution vol (ml) | Mass (kDa) | Cell extractc eolution vol (ml) | Mass (kDa) |
|---|---|---|---|---|---|
| NIFL | − | 10.22 | 250 | 10.12 | 260 |
| + | 10.14 | 260 | 10.13 | 260 | |
| NIFA | − | 10.43 | 210 | 10.8 | 160 |
| + | 10.38 | 220 | 10.22 | 250 | |
| NIFL-NIFA | − | 10.08 | 265 | 9.75 | 300 |
| + | 9.17 | 480 | 9.07 | 500 |
+, present; −, absent.
NIFL-NIFA complex formed by mixing purified proteins in vitro.
NIFL-NIFA complex isolated from cell extract containing coexpressed proteins.
FIG. 1.
NIFL-NIFA complex formed in the presence of nucleotides. Complexes were formed and chromatographed as described in Materials and Methods. Electrophoresis was carried out on SDS-polyacrylamide gels (10% polyacrylamide). Full-length NIFL6his and NIFA were used at 2.5 μM, and nucleotides were used at 1 mM. (a) Complex formation with 1 mM MgADP. Lanes 1 to 3 and 9 to 11, wash fractions; lanes 4 to 6 and 12 to 14, fractions containing bound protein which eluted with 0.5 M imidazole. (b) Complex formation with 1 mM MgATPγS or GTPγS. Lanes 1 to 3 and 10 to 12, wash fractions; lanes 4 to 6 and 13 to 15, fractions containing protein obtained after elution. (C) Complex formation with 200 μM MgADP. Lanes 1 to 3 and 9 to 11, wash fractions; lanes 5 to 6 and 12 to 13, fractions containing protein obtained after elution. (D) Complex formation with 4 mM GTP in the presence and absence of 200 μM MgADP. Lanes 3 to 5 and 10 to 12, wash fractions; lanes 6 to 7 and 13 to 14, fractions containing protein obtained after elution. (E) Absorbance spectra of oxidized NIFL in the presence and absence of NIFA and 1 mM MgADP were measured in a Hewlett-Packard diode array spectrophotometer.
We have shown previously that inhibition of NIFA activity by NIFL in assays for open promoter complex formation is observed in the presence of 4 mM GTP and that the inhibition is enhanced by the addition of low concentrations of ADP (8, 23). Under these conditions in our binding assay, the level of NIFL-NIFA complexes detected in the presence of 4 mM MgGTP was similar to that observed with 1 mM MgGTPγS. However, the presence of 200 μM MgADP in addition to 4 mM MgGTP resulted in nearly all of the NIFA binding to the NIFL (Fig. 1d). Although these experiments do not permit accurate quantitation of the amount of complex formed, qualitatively it would appear that more complex is formed when both nucleotides are present and that a correlation exists between the stimulation of the inhibitory effect of NIFL on NIFA activity by ADP and an increase in the level of NIFL-NIFA complexes. The presence of RNA polymerase and ςN in the initial incubation mix did not influence the amount of NIFA bound to NIFL in the presence of ADP (data not shown). The presence of NIFA did not alter the spectral features of NIFL either in the presence or absence of MgADP, implying that NIFA binding to NIFL does not change the environment of the bound flavin (Fig. 1e). Under the conditions of the assays, NIFL still displays the characteristic absorption maximum at 445 nm and shoulders at 420 and 470 nm indicative of protein-bound FAD.
The amino-terminal domain of NIFA influences the level of complex formation in the presence of ADP.
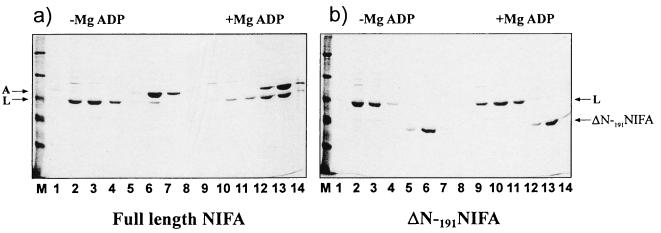
The putative regulatory function of the amino-terminal domain of NIFA makes it a likely target for interaction with NIFL, which may, by analogy with other ςN-dependent activators, result in modulation of the ATPase activity of the central domain of the protein in response to NIFL. We examined the ability of an amino-terminally deleted NIFA protein, ΔN-191NIFA, (Fig. 2), to form a complex with NIFL in the presence of ADP. ΔN-191NIFA has transcriptional activity in vitro which is inhibited by high concentrations of NIFL bound to ADP, although in contrast to full-length NIFA, the ATPase activity of the truncated NIFA protein is unaffected under these conditions (unpublished results). Reactions were performed with C-terminally tagged forms of NIFA, either the full-length protein or ΔN-191NIFA, with nontagged full-length NIFL as the partner. In the absence of MgADP, no binding of NIFL to either form of NIFA was observed (Fig. 3). In the presence of 1 mM MgADP, complexes were observed with the full-length NIFA and the proteins coeluted exactly as demonstrated in the previous experiments with tagged NIFL and the native form of NIFA (Fig. 3a). However, no binding to NIFL was detected with the truncated form of NIFA in the presence of 1 mM MgADP, and NIFL eluted from the column in the wash fraction (Fig. 3b). The same result was obtained when the experiment was repeated in the presence of 4 mM MgGTP in addition to ADP (data not shown). Thus, the amino-terminal domain of NIFA strongly influences complex formation between NIFL and NIFA in the presence of ADP.
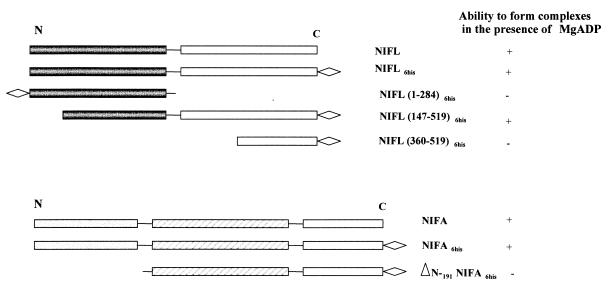
FIG. 2.
Comparison of the ability of truncated derivatives of NIFL and NIFA to form complexes in the presence of MgADP. N indicates the amino-terminal domain and C indicates the carboxy-terminal domain of each protein. ◊ indicates the histidine tag. +, able to form complex; −, unable to form complex.
FIG. 3.
Complex formation between NIFL and full-length NIFA or ΔN-191NIFA. Complexes were formed, chromatographed, and electrophoresed as for Fig. 1. Histidine-tagged forms of NIFA and ΔN-191NIFA were used at 2.9 μM, and NIFL was used at 2.3 μM. MgADP was used at 1 mM where indicated. (a) Full-length NIFA and NIFL. (b) ΔN-191NIFA and NIFL. Lanes 2 to 4 and 9 to 11, wash fractions; lanes 5 to 7 and 12 to 14, fractions containing protein obtained after elution. Lane M contains molecular weight markers.
The C-terminal region of NIFL is involved in the ADP-dependent stimulation of NIFL-NIFA complex formation.
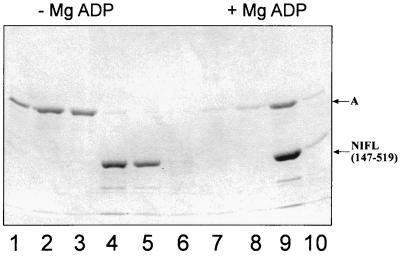
We have previously characterized the properties of various domains and fragments of the NIFL protein with respect to inhibition of NIFA activity and nucleotide binding (23). Two derivatives, NIFL(1–284), corresponding to the N-terminal domain, and the C-terminal subdomain, NIFL(360–519) (Fig. 2) do not function as inhibitors of the transcriptional activity of NIFA in vitro even in the presence of ADP, although NIFL(360–519) is competent to bind nucleotides (23). A longer fragment, consisting of the whole C-terminal region but lacking the first 147 amino acids of NIFL, including the flavin binding sites, NIFL(147–519), is competent to inhibit NIFA activity in the presence of ADP although it lacks the redox response (23). We have examined the ability of these truncated forms of NIFL to bind to NIFA in the presence of 1 mM MgADP. Experiments were performed with C-terminal His-tagged versions of NIFL and full-length NIFA as the nontagged partner. There was no ADP-dependent stimulation of complex formation between NIFA and NIFL(1–284) or NIFL(360–519) (data not shown). Presumably, these truncated proteins lack either regions required for NIFA interaction or, in the case of NIFL(1–284), the nucleotide binding domain. The NIFL(147–519) derivative was able to bind NIFA in the presence of nucleotide. However, this protein did not seem to bind NIFA with the same affinity as the wild-type NIFL. Assuming NIFL(147–519) is a dimer (23), a molar ratio of the truncated NIFL to NIFA of 3:1 was required before the majority of the NIFA was retained on the column and coeluted with NIFL in the presence of MgADP (Fig. 4). At lower protein ratios, less complex was detected (data not shown). Although the truncated NIFL must contain both the NIFA interaction and nucleotide binding sites, gel filtration experiments suggest that it is a dimer rather than a tetramer in solution, and it may not be able to bind to NIFA with the same affinity as the tetrameric form of the full-length protein.
FIG. 4.
Complex formation between NIFL(147–519)6his and NIFA. Complexes were formed and chromatographed as described in Materials and Methods. Full-length NIFA was present in the assays at 2.5 μM, and NIFL(147–519) was present at 8.25 μM (1:3 NIFA/NIFL ratio). Lanes 1 to 3 and 6 to 8, wash fractions; lanes 4, 5, 9, and 10, fractions containing protein which eluted with 0.5 M imidazole. The presence (+) or absence (−) of MgADP is indicated.
Isolation of the NIFL-NIFA complex from cell extracts in the presence of MgADP.
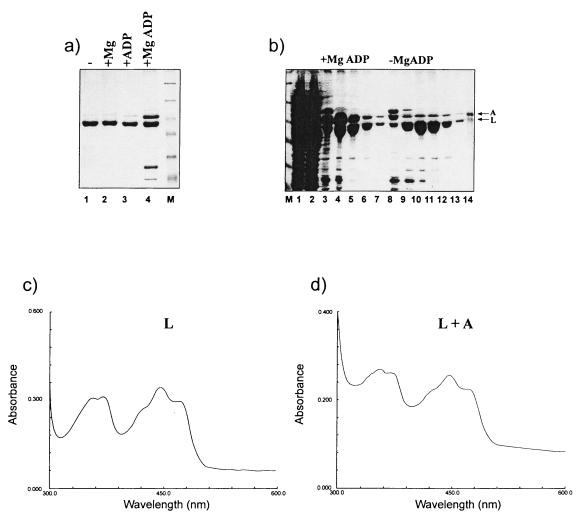
We purified coexpressed NIFL and NIFA proteins from an E. coli BL21(DE3)(pLysS) strain carrying plasmid pPR38 in which the A. vinelandii nifLA operon was expressed from the T7 promoter using the natural nifL ribosome binding site (23). In this construct the NIFL protein was expressed with a C-terminal histidine tag and NIFA was nontagged. Induction with IPTG gave rise to approximately stoichiometric amounts of NIFL and NIFA. Metal chelate affinity chromatography of the cell extract lysed and chromatographed in the presence or absence of 1 mM MgADP resulted in isolation of the NIFL-NIFA complex only in the presence of the magnesium-bound nucleotide (Fig. 5a). The presence of either magnesium or ADP alone was not able to stabilize the complex and resulted in chromatography of only NIFL6his, with NIFA eluting in the column wash fractions. The NIFL-NIFA complex could be dissociated on the column by removal of MgADP. Figure 5b shows the NIFL-NIFA-containing fractions eluted from the nickel column in the presence of 1 mM MgADP compared to those where NIFA had been dissociated from the bound complex by removal of nucleotide from the chromatography buffers. In this case, NIFA eluted from the column with 20 mM imidazole. After dialysis, this NIFA was essentially free of NIFL, as judged by inspection of Coomassie blue-stained gels (data not shown). The activity of this protein was compared to that of a control NIFA preparation which had been purified routinely in the absence of NIFL. Both NIFA preparations were active in transcriptional activation from the nifH promoter and displayed identical behavior in a single-round transcription assay in the presence and absence of IHF (Fig. 6).
FIG. 5.
Isolation of the NIFL-NIFA complex directly from lysed cells. BL21(DE3)(pLysS) cells carrying plasmid pPR38 were grown as described in Materials and Methods. (a) The cell paste was resuspended in lysis buffer and divided into four aliquots. These were lysed and chromatographed as described in Materials and Methods with the following additions: lane 1, no nucleotide; lane 2, with 1 mM magnesium acetate; lane 3, with 1 mM ADP; lane 4, with 1 mM MgADP. Lanes 1 to 4, fractions containing protein which eluted with 0.5 M imidazole. Lane M contains molecular weight markers. (b) Cell paste was resuspended in lysis buffer containing 1 mM MgADP and was divided into two aliquots. These were applied to the nickel chelating column and washed with equilibration buffer containing 1 mM MgADP as described in Materials and Methods. Lane 1, cell supernatant; lane 2, nonbound protein from cell supernatant; lanes 3 to 7, NIFL-NIFA-containing fractions eluted with 0.5 M imidazole in the presence of 1 mM MgADP; lanes 8 to 13, fractions eluted with 0.5 M imidazole after washing the column in equilibration buffer without nucleotide to dissociate NIFA. (c and d) Absorbance spectra of oxidized NIFL and isolated NIFL-NIFA complex in the presence of 500 μM MgADP. The spectra were recorded with a Shimadzu MP2000 spectrophotometer with a 1-cm light path and 1-nm slit width.
FIG. 6.
Transcriptional activity of NIFA dissociated from the NIFL-NIFA complex. A single-round transcription assay from the nifH promoter was carried out as described in Materials and Methods. NIFA was dissociated from the NIFL-NIFA complex as described in the legend to Fig. 5. +, present; −, absent.
The NIFL-NIFA complex could also be separated by heparin agarose chromatography performed in the absence of nucleotide where the NIFA bound normally and the NIFL eluted in the nonbound column fractions (data not shown). The spectral features of the oxidized NIFL-NIFA complex were similar to those of NIFL alone both in the presence and absence of MgADP, with a characteristic absorption maximum at 445 nm and shoulders at 420 and 470 nm, indicative of protein-bound FAD (Fig. 5c and d). The FAD moiety in NIFL complexed to NIFA in the presence of MgADP was reduced by sodium dithionite, as observed for the NIFL protein in isolation (data not shown).
Molecular mass estimation of the NIFL-NIFA complex.
The apparent molecular masses of the NIFL-NIFA complexes and isolated proteins were estimated by gel filtration on Superose 12 in the presence and absence of 500 μM MgADP (see Materials and Methods) (Table 1). In the absence of nucleotide, purified NIFL sieved as a tetramer and NIFL sieved as a species, which probably represents an equilibrium between the dimer and tetramer forms. The presence of nucleotide did not appear to change the association state of the NIFL protein significantly, whereas the apparent increase in molecular mass of NIFA with MgADP may represent a shift of the equilibrium to the tetramer form. The behavior of purified NIFA preparations on gel filtration was found to be somewhat variable among preparations, with a range of molecular masses being obtained (unpublished observation). However, this did not appear to influence the ability of the protein to bind to NIFL in the presence of MgADP, as all the NIFA preparations tested displayed similar behaviors in the binding assay. In the presence of MgADP, NIFL-NIFA complexes, which were either isolated from cell extracts or formed in vitro, sieved with an apparent molecular mass of approximately 500 kDa, which would be consistent with stoichiometry of a tetramer of NIFL bound to a tetramer of NIFA. In the absence of MgADP, the NIFL-NIFA complex isolated from cell extracts chromatographed with a lower apparent molecular mass, indicating dissociation of the complex upon removal of the nucleotide. The NIFL and NIFA proteins mixed in vitro without MgADP sieved as a single peak but with a molecular mass no greater than that of NIFL alone. The Superose 12 absorbance traces of the NIFL-NIFA complex isolated from cell extracts compared to each protein chromatographed alone are shown in Fig. 7.
DISCUSSION
In earlier work we have demonstrated that the inhibitory activity of NIFL on NIFA activity is stimulated by the presence of adenosine nucleotides, particularly ADP, in vitro (8). The presence of ADP overrides redox sensing by NIFL, since the ADP-bound form of the protein is inhibitory to NIFA activity even when the flavin moiety is in the reduced state (13). In the absence of ADP, the oxidized form of NIFL inhibits both the ATPase and GTPase activities of full-length NIFA, resulting in inhibition of open complex formation. We have now examined complex formation between oxidized NIFL and NIFA and found little interaction between the two proteins in the absence of nucleotide under the conditions of the binding assay. The complex may not form in the absence of nucleotides or may be too weak or transient to be detected by cochromatography. The inhibitory effect of NIFL on NIFA activity and, by definition, the formation of the inhibitory complex can be determined in vitro by measuring various functions of NIFA, including transcriptional activation and ATPase activity. Nucleotides are always present in these assays, and the situation is complicated by the potential interaction of the nucleotides with both NIFA and NIFL. Transcriptional activation by NIFA requires ATP or GTP hydrolysis to drive open complex formation (2, 3, 14). However, ATP hydrolysis by NIFA will result in the production of ADP, which increases the inhibitory effect of NIFL. Inhibition of NIFA activity by NIFL is also observed when GTP is used to make open complexes, but the inhibition is increased when low concentrations of ADP are added in addition to GTP (23). The presence of GTP in our binding assay results in a low level of NIFL-NIFA complexes, which is apparently sufficient for NIFL to inhibit the transcriptional activity of NIFA. The addition of ADP increases the amount of NIFL-NIFA complex detected when equimolar concentrations of the two proteins are used. This is consistent with the stimulation of the inhibitory effect of NIFL observed in the open-complex assays. Higher concentrations of NIFL may be required to shift the equilibrium towards complex formation in the absence of ADP.
The C-terminal domain of A. vinelandii NIFL shows strong similarity to the histidine protein kinase transmitter domains of two-component systems (5), including the region containing the highly conserved histidine residue, which is autophosphorylated in bona fide members of the family (19). However, there is no evidence that any phosphotransfer mechanism between NIFL and NIFA exists (2, 15, 20), and it seems likely that this pair of proteins has evolved from the classical sensor kinase response regulator system to one where protein-protein interaction replaces phosphotransfer as a mechanism for signal transduction. Although A. vinelandii NIFL possesses the conserved histidine residue, His305, no autophosphorylation of this residue has been detected (2). Our experiments with the truncated NIFL derivatives support the hypothesis that this region of the protein might be a candidate for interaction with NIFA (22, 25), as in orthodox systems the modified histidine is likely to approach the receiver domain of the response regulator to effect phosphotransfer (18). The lack of in vitro activity of the N-terminal and C-terminal subdomains of NIFL in the presence of MgADP can be attributed to lack of interaction with NIFA. The C-terminal subdomain, although competent to bind nucleotide, presumably does not possess the binding sites for NIFA. This is in contrast to the NIFL protein from K. pneumoniae, where a similar truncation of the C-terminal domain is capable of inhibiting NIFA activity both in vivo and in vitro and thus presumably contains the determinants for interaction with the activator (17). The longer NIFL derivative, NIFL(147–519), which does contain the region around the conserved histidine, can bind NIFA in the presence of ADP, implying that the site(s) of NIFA interaction is likely to be located between residues 147 and 360. The removal of the PAS domain in this NIFL derivative does not prevent the ADP-dependent stimulation of NIFL binding to NIFA (23). Experiments with a truncated form of NIFA, lacking the N-terminal domain, have indicated that there may be at least two mechanisms by which NIFL inhibits NIFA activity. Inhibition of the ATPase activity of NIFA by NIFL requires the presence of an intact NIFA N-terminal domain, while a second mechanism of inhibition still occurs with the truncated protein (unpublished observations). An amino-terminally deleted form of the NIFA protein from K. pneumoniae expressed as a maltose-binding fusion also has transcriptional activity in vitro which is inhibited by NIFL (4), consistent with our observations with the truncated A. vinelandii NIFA. We have now shown that the amino-terminal domain of NIFA also has a strong influence on the affinity of the NIFL-NIFA interaction observed in our binding assays. Interaction with the amino-terminal domain may provide a mechanism by which the ATPase activity of NIFA is inhibited by NIFL.
In aerobically grown cells under nitrogen-rich conditions, the constitutive activity of NIFA is repressed by NIFL, presumably by the formation of the inhibitory complex, to prevent synthesis of nitrogenase in physiologically unfavorable conditions. When we overexpressed NIFL and NIFA together under these conditions, we could isolate the NIFL-NIFA complex from the cell extract, but only when MgADP was present in the lysis and chromatography steps. Thus, in these experiments it is not possible to distinguish complexes which had been present in the cells from those which formed on cell lysis in the presence of MgADP. The NIFA which dissociated from NIFL when nucleotide was removed from the complex retained transcriptional activity, indicating that it is not irreversibly modified by NIFL binding. Whether NIFL-NIFA would normally be ADP bound under repressing conditions in vivo may depend on the ratio of ATP to ADP present in the cells. Changes in oxygen concentration and nitrogen status are likely to influence this ratio, and the physiological role of the nucleotide response may be to sense the energy status of the cells so that nitrogenase is not synthesized when the high energy demands of nitrogen fixation cannot be met. In an analogous system, the sporulation protein SpoIIAB from Bacillus subtilis is a serine protein kinase which has homology to the nucleotide binding pocket of the histidine protein kinase family. This protein interacts stoichiometrically with its partner, SpoIIAA, in response to adenosine nucleotide levels, with the ATP/ADP ratio influencing with which partner SpoIIAB interacts (1, 16). In the presence of ADP, SpoIIAB forms a complex with SpoIIAA, while with ATP it acts as an antisigma factor and forms a complex with ςF to inhibit its activity. In this system, however, phosphorylation is also involved, as SpoIIAB phosphorylates SpoIIAA in the presence of ATP, preventing its binding to SpoIIAB or reacting with SpoIIAB-ςF complexes. Although there is no evidence for any phosphotransfer occurring between NIFA and NIFL, it is possible that adenosine nucleotides influence NIFL and NIFA interactions similarly to the SpoIIAA-SpoIIAB system, with ADP promoting NIFL-NIFA complex formation while ATP stimulates NIFA and ςN RNA polymerase association.
ACKNOWLEDGMENTS
We are extremely grateful to Susan Hill for discussions and observations on the properties of the NIFL-NIFA complex. We also thank Andre Sobczyk for providing the plasmid construction used to obtain purification of the amino-terminally deleted form of A. vinelandii NIFA. We are grateful to Mike Merrick and Gary Sawers for comments on the manuscript.
This work was supported by a Grant-in-Aid from the BBSRC to the John Innes Centre.
REFERENCES
- 1.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in Bacillus subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 2.Austin S, Buck M, Cannon W, Eydmann T, Dixon R. Purification and in vitro activities of the native nitrogen fixation control proteins NIFA and NIFL. J Bacteriol. 1994;176:3460–3465. doi: 10.1128/jb.176.12.3460-3465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger D K, Narberhaus F, Kustu S. The isolated catalytic domain of NIFA, a bacterial enhancer-binding protein, activates transcription in-vitro. Activation is inhibited by NIFL. Proc Natl Acad Sci USA. 1994;91:103–107. doi: 10.1073/pnas.91.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger D K, Narberhaus F, Lee H S, Kustu S. In vitro studies of the domains of the nitrogen-fixation regulatory protein NIFA. J Bacteriol. 1995;177:191–199. doi: 10.1128/jb.177.1.191-199.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco G, Drummond M, Woodley P, Kennedy C. Sequence and molecular analysis of the nifL gene of Azotobacter vinelandii. Mol Microbiol. 1993;9:869–880. doi: 10.1111/j.1365-2958.1993.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 6.Dixon R. The oxygen-responsive NIFL-NIFA complex: a novel two-component regulatory system controlling nitrogenase synthesis in γ-Proteobacteria. Arch Microbiol. 1998;169:371–380. doi: 10.1007/s002030050585. [DOI] [PubMed] [Google Scholar]
- 7.Drummond M H, Wootton J C. Sequence of nifL from Klebsiella pneumoniae: mode of action and relationship to two families of regulatory proteins. Mol Microbiol. 1987;1:37–44. doi: 10.1111/j.1365-2958.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 8.Eydmann T, Söderbäck E, Jones T, Hill S, Austin S, Dixon R. Transcriptional activation of the nitrogenase promoter in vitro: adenosine nucleosides are required for inhibition of NIFA activity by NIFL. J Bacteriol. 1995;177:1186–1195. doi: 10.1128/jb.177.5.1186-1195.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govantes F, Andujar E, Santero E. Mechanism of translational coupling in the nifLA operon of Klebsiella pneumoniae. Embo J. 1998;17:2368–2377. doi: 10.1093/emboj/17.8.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govantes F, Molina-Lopez J A, Santero E. Mechanism of coordinated synthesis of the antogonistic regulatory proteins NifL and NifA of Klebsiella pneumoniae. J Bacteriol. 1996;178:6817–6823. doi: 10.1128/jb.178.23.6817-6823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govantes F, Santero E. Transcription termination within the regulatory nifLA operon of Klebsiella pneumoniae. Mol Gen Genet. 1996;250:447–454. [PubMed] [Google Scholar]
- 12.Henderson N, Austin S A, Dixon R A. Role of metal ions in negative regulation of nitrogen fixation by the nifL gene product from Klebsiella pneumoniae. Mol Gen Genet. 1989;216:484–491. [Google Scholar]
- 13.Hill S, Austin S, Eydmann T, Jones T, Dixon R. Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proc Natl Acad Sci USA. 1996;93:2143–2148. doi: 10.1073/pnas.93.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H-S, Berger D K, Kustu S. Activity of purified NIFA, a transcriptional activator of nitrogen fixation genes. Proc Natl Acad Sci USA. 1993;90:2266–2270. doi: 10.1073/pnas.90.6.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H-S, Narberhaus F, Kustu S. In vitro activity of NifL, a signal transduction protein for biological nitrogen fixation. J Bacteriol. 1993;175:7683–7688. doi: 10.1128/jb.175.23.7683-7688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min K T, Hilditch C M, Diederich B, Errington J, Yudkin M D. Sigma F, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-sigma factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 17.Narberhaus F, Lee H-S, Schmitz R A, He L, Kustu S. The C-terminal domain of NIFL is sufficient to inhibit NIFA activity. J Bacteriol. 1995;177:5078–5087. doi: 10.1128/jb.177.17.5078-5087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H, Saha S K, Inouye M. Two-domain reconstitution of a functional protein histidine kinase. Proc Natl Acad Sci USA. 1998;95:6728–6732. doi: 10.1073/pnas.95.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parkinson J S, Kofoid E C. Communication modules in bacterial signalling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz R, He L, Kustu S. Iron is required to relieve inhibitory effects of NifL on transcriptional activation by NifA in Klebsiella pneumoniae. J Bacteriol. 1996;178:4679–4687. doi: 10.1128/jb.178.15.4679-4687.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz R A. NifL of Klebsiella pneumoniae carries an N-terminally bound FAD cofactor, which is not directly required for the inhibitory function of NifL. FEMS Microbiol Lett. 1997;157:313–318. doi: 10.1111/j.1574-6968.1997.tb12791.x. [DOI] [PubMed] [Google Scholar]
- 22.Sidoti C, Harwood G, Ackerman R, Coppard J, Merrick M. Characterisation of mutations in the Klebsiella pneumoniae nitrogen fixation regulatory gene nifL which impair oxygen regulation. Arch Microbiol. 1993;159:276–281. doi: 10.1007/BF00248484. [DOI] [PubMed] [Google Scholar]
- 23.Söderbäck E, Reyes-Ramirez F, Eydmann T, Austin S, Hill S, Dixon R. The redox- and fixed nitrogen-responsive regulatory protein NIFL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding domains. Mol Microbiol. 1998;28:179–192. doi: 10.1046/j.1365-2958.1998.00788.x. [DOI] [PubMed] [Google Scholar]
- 24.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodley P, Drummond M. Redundancy of the conserved His residue in Azotobacter vinelandii NifL, a histidine protein kinase homologue which regulates transcription of nitrogen fixation genes. Mol Microbiol. 1994;13:619–626. doi: 10.1111/j.1365-2958.1994.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Wootton J C, Drummond M. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989;2:535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]
- 27.Zhulin I B, Taylor B L, Dixon R. PAS domain S-boxes in Archea, Bacteria and sensors for oxygen and redox. Trends Biochem Sci. 1997;22:331–333. doi: 10.1016/s0968-0004(97)01110-9. [DOI] [PubMed] [Google Scholar]