Abstract
Introduction
Postacute sequelae resulting from SARS-CoV-2 infections (LONG-COVID) have been reported. The resulting added economic burden from the perspective of healthcare organisations is not clear. Therefore, this study aims to evaluate the additive healthcare costs among COVID-19 recoverees, in a large community-dwelling general population, as incurred by an insurer-provider organisation over time.
Methods
In this historical cohort study, cost data from Clalit Health Services (CHS) were analysed. The primary endpoint was the direct cost incurred by CHS per month per person. Costs were measured for COVID-19 recoverees and matched controls, from January 2019 to January 2022. Difference in differences (DiDs) were calculated as the difference in mean monthly costs in cases and controls in the post-COVID-19 individual period, deducing their cost difference in a prepandemic 12 months baseline period.
Results
Among N=642 868 community-dwelling COVID-19 recoverees, 268 948 (40.8%) were 0–19 years old and 63 051 (9.6%) were 60 years or older. A total of 16 017 (2.5%) of recoverees had been hospitalised during the acute phase of the COVID-19 disease. Costs in cases and controls converged after 16 months from recovery. The mean monthly cost incurred by CHS per COVID-19 recoverees over up to 15 months (mean: 8.25) of post-COVID-19 follow-up was higher by 8.2% (US$8.2) compared with matched controls. The excess cost attributable to post-COVID-19 effects (DID) was 7.6% of the cost in controls (US$7.7 per patient per month). Both net and relative DIDs were substantially higher in patients who required hospitalisation during the acute phase of COVID-19 and in older adults. Excess in hospitalisations, primary care physicians and medical specialists’ visits-related costs were observed.
Conclusions
Long-term effects of SARS-CoV-2 infections translate into excess healthcare costs, months after recovery, hence requiring adjustments of funds allocation. These excess costs gradually diminish after recoveree, returning to baseline differences 16 months after recoveree.
Keywords: COVID-19, health economics, health services research
WHAT IS ALREADY KNOWN ON THIS TOPIC
A growing body of evidence demonstrates the clinical manifestations of long-Covid, reported to persist months after the acute phase. However, the resulting added economic burden from the perspective of healthcare organisations is not clear.
WHAT THIS STUDY ADDS
The mean cost attributable to long-Covid during 15 months of follow-up was estimated at an additional 7.7%. Most of the excess cost was attributed to hospital bills, though excess was also observed in healthcare services provided in community settings. Excess costs were substantially higher in patients who were hospitalised during the acute COVID-19 phase and in older patients. Costs in COVID-19 recoverees and controls converged 16 months after recovery.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Long-term effects of SARS-CoV-2 infections translate into excess healthcare costs, months after recovery, hence requiring adjustments of funds allocation.
Introduction
The COVID-19 pandemic has a major continuing effect on healthcare systems worldwide, including economic effects.1 2 Alongside the devastating effects of COVID-19-related deaths, the continuous effect it has on health must also be measured, and is essential for planning ahead adequate resources.3 One of the long-term effects of the pandemic, adding burden both at the level of health systems and of societies as a whole, is that of persistent symptoms after an acute SARS-CoV-2 infection (long-Covid) in a part of those infected.4 The burden added by the substantial clinical impact and high incidence of long-Covid, for example, as high as 2.4% of the UK population5 cannot be ignored.6
A growing body of evidence demonstrates the clinical manifestations of long-Covid, reported to persist months after the acute phase in a range of studies.7 8 However, some of the studies were limited to either inpatient9–11 or outpatient settings,12 while others were limited to specific population groups, mostly older adults.13 Despite a growing body of clinical evidence regarding long-Covid, the excess costs attributed to the condition are yet unclear.
This study provides information on the additive healthcare costs attributable to long-Covid over time in a large and diverse cohort from the perspective of an insurer-provider healthcare organisation.
Methods
Setting and study design
This observational, retrospective population-based cohort study was based on costs and healthcare utilisation data obtained from electronic administrative and medical records of Clalit Health Services (CHS) members. The Israeli healthcare system is characterised by universal mandatory healthcare coverage for all permanent residence. Four health funds function as integrated payers providers. All residents are free to choose which of the four health funds to join, and may transfer from one health fund to another several times a year. The health funds are not allowed to condition membership on any factor.14 CHS is the largest health fund in Israel, and insures 4.8 million individuals (52% of the population), and the geographical coverage of its services extends to the entire country. Therefore, CHS members constitutes a representative sample of the Israeli population.
Attributable costs were estimated as difference in differences (DiD), deducting the difference in costs between cases and controls in the baseline, prepandemic period, from the difference in costs between these two groups during the individual post-COVID-19 follow-up period. This approach permits to control for any baseline differences between the cases and the controls affecting their costs, beyond that expressed by the matching parameters.15 16
Study population and period
COVID-19 recoverees were defined as all CHS members who had a first-ever positive SARS-CoV-2 test result (either by reverse transcription-PCR or institutional antigen tests) from March 2020 to 31 December 2021.
The index date for each pair, signifying the start of a postrecoveree period was defined as 30 days after a first positive-SARS-CoV-2 test for individuals who were not hospitalised during their acute COVID-19 episode or 30 days after discharge from hospitalisation due to COVID-19. Each case was randomly matched 1:1 to a control, defined as an individual who did not have a positive SARS-CoV-2 test result up to the index date. Cases and controls were matched by parameters which reflect variability in healthcare services utilisation, and were found to differ in the general CHS population among persons infected and not infected with SARS-CoV-2 (online supplemental table S1). The matching parameters were age, sex, population sector, score for socioeconomic status and six adjusted clinical group (ACG) categories, expressing the overall burden of illness for each study participant.17
bmjgh-2023-012588supp001.pdf (98.6KB, pdf)
The study population included individuals who had at least 30 days of follow-up after the index date and a continuous membership in CHS at least a year prior to the beginning of the study, to ensure sufficient background information. To ensure the representation of costs in a general community-dwelling population, we have excluded CHS members not residing in the community, as well as those with diagnoses related to extreme costs (online supplemental figure S1). A full description of the clinical exclusion criteria is given in online supplemental file 1.
The study period commenced with a baseline prepandemic period that was defined uniformly for the entire study cohort as 12 consecutive months prior to the outbreak of the COVID-19 pandemic in Israel (March 2019–February 2020). The post-COVID-19 follow-up period was defined individually for each pair, starting at the individual index date and ending at the earliest of: CHS membership cessation or death of either the case or the control, the control having a positive SARS-CoV-2 test, or 31 January 2022 the end of follow-up.
Data extraction
The following data were extracted for each subject: direct monthly costs, age, sex, population sector, score for socioeconomic status, clinical ACG, dates of COVID-19 diagnoses and the severity of COVID-19 as reflected by hospitalisation due to COVID-19.
The outcome of interest was the direct monthly cost as incurred by CHS for each participant. Costs were extracted by pre-existing categories: (A) hospital bills (including emergency department visits, hospitalisation days, outpatients consultations, inpatient and outpatients procedures, imaging and laboratory tests), (B) Medical specialists’ visits in CHS community healthcare settings, (C) primary care physician visits, (D) nurse visits, (E) paramedical professions visits, (F) medications and (G) other costs, including imaging and laboratory tests in CHS outpatient settings, payments to private providers and patients’ copayments and refunds, excluding copayments for medications. The sum of the above categories of expenses encompasses the direct insurer-provider economic burden at the patient level.
Statistical analysis
Descriptive statistics were used to characterise the study participants, in participants who recovered from COVID-19 and in controls. Costs were expressed as average monthly rates.
The net differences between the costs of cases and controls were calculated by months of follow-up and for the entire post-COVID-19 follow-up period. Differences were evaluated using the Wilcoxon signed rank for paired difference test.
In the descriptive analysis, follow-up extended up to 20 months from the index date. However, as we observed a convergence of the costs in cases and controls after 16 months, overall DiDs were calculated for months 1–15 of follow-up.
In addition, we describe trends in mean monthly costs in controls, from January 2019 to January 2022, while excluding the individual acute COVID-19 periods for of both cases and matched controls.
Subgroup analysis
DIDs were examined separately among patients who were hospitalised due to COVID-19, and for COVID-19 outpatients, by age groups (<17, 18–40, 41–60 and >60 years) and by sex.
Patient and public involvement statement
No patients were involved in the current study.
Results
Study population
The study population consisted of N=642 868 pairs of cases and matched controls, who were followed for 1–20 months. Their selection process is described in online supplemental figure 1. A total of 355 229 (53.9%) of the study population were female; 268 948 (40.8%) were 0–19 years old, 199 311 (30.2%), were 20–39 years old, 128 302 (19.5%) were 40–59 years old and 63 051 (9.6%) were 60 years or older. A total of 16 017 (2.5%) of recoverees had been hospitalised during the acute phase of the COVID-19 disease. Cases had a higher prevalence of diabetes, hypertension and obesity, compared with controls (table 1).
Table 1.
Study population characteristics
| N (%) | Cases (COVID-19 Recoverees) | Controls | P value |
| 642 868 | 642 868 | ||
| Female | 346 048 (53.8%) | 346 048 (53.8%) | NS |
| Age groups | |||
| 0–19 | 260 293 (40.5%) | 260 293 (40.5%) | NS |
| 20–39 | 195 128 (30.4%) | 195 128 (30.4%) | |
| 40–59 | 125 565 (19.5%) | 125 565 (19.5%) | |
| 60–79 | 55 148 (8.6%) | 55 148 (8.6%) | |
| 80+ | 6734 (1%) | 6734 (1%) | |
| Ethnicity | |||
| Ultra-orthodox Jewish | 83 782 (13%) | 83 782 (13%) | NS |
| Israeli-Arab | 181 828 (28.3%) | 181 828 (28.3%) | |
| General Jewish or other | 377 258 (58.7%) | 377 258 (58.7%) | |
| Area of residence | NS | ||
| Central area of residence | 209 410 (32.6%) | 206 907 (32.2%) | |
| Proximate periphery | 305 386 (47.5%) | 296 181 (46.1%) | |
| Distant periphery | 124 536 (19.4%) | 133 581 (20.8%) | |
| Unknown | 3536 (0.6%) | 6199 (1%) | |
| Socioeconomic position score | NS | ||
| Low (1–3) | 194 789 (30%) | 194 789 (30%) | |
| Medium-low (4–5) | 207 646 (32%) | 207 646 (32%) | |
| Medium-high (6–7) | 171 003 (27%) | 171 003 (27%) | |
| High (8–10) | 69 430 (11%) | 69 430 (11%) | |
| ACG score | NS | ||
| 1–4 | 372 235 (57.9%) | 372 235 (57.9%) | |
| 5–6 | 28 336 (4.4%) | 28 336 (4.4%) | |
| Unknown | 242 297 (37.7%) | 242 297 (37.7%) | |
| Comorbidities and risk-factors | |||
| Diabetes | 39 021 (6.1%) | 37 543 (5.8%) | 0.000* |
| Hypertension | 51 463 (8.0%) | 47 599 (7.4%) | 0.000* |
| Hyperlipidaemia | 111 147 (17.3%) | 112 569 (17.5%) | 0.001* |
| Smoking | 113 813 (17.7%) | 143 092 (22.3%) | 0.000* |
| Obesity | 124 877 (19.4%) | 118 261 (18.4%) | 0.000* |
ACG, adjusted clinical group.
Overall cost DIDs
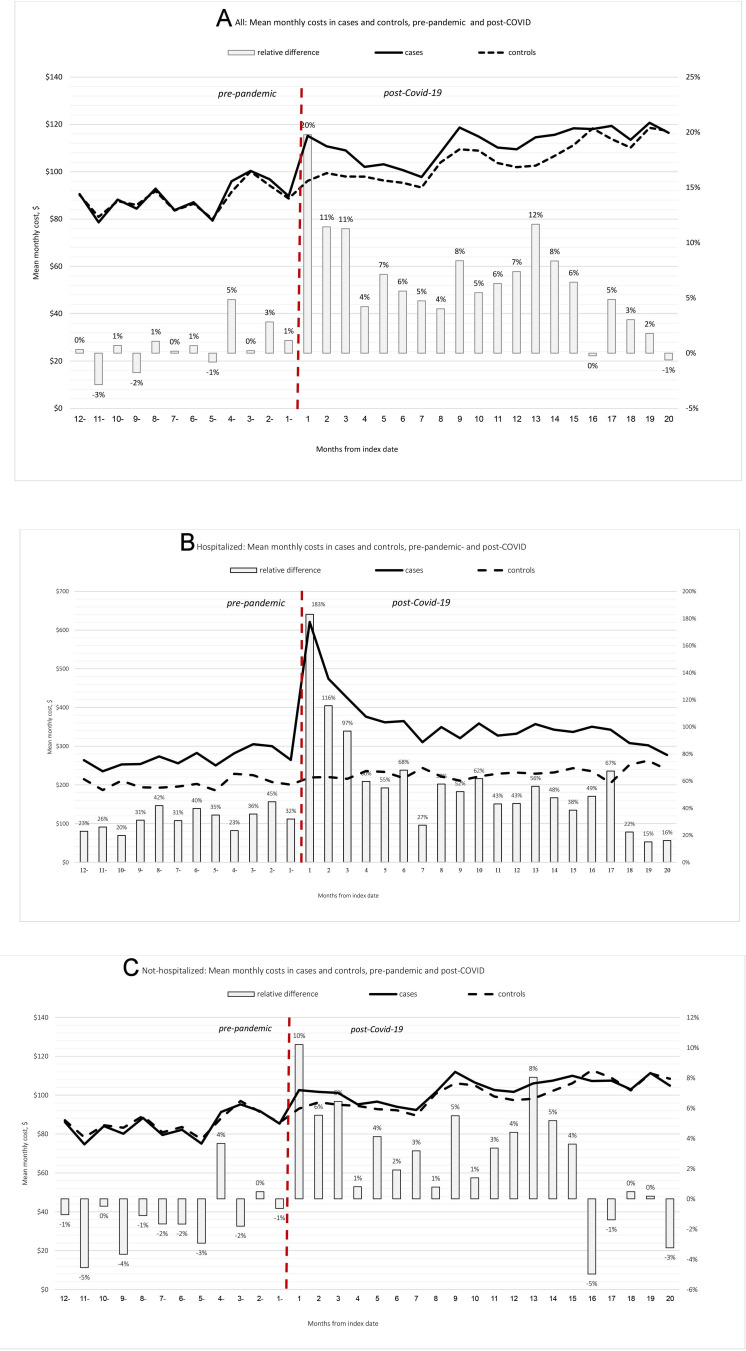
Minimal differences in mean costs between cases and controls were observed prior to the pandemic. However, the costs in cases were higher compared with controls during the first 15 months of the postrecoveree period (figure 1A), and converged subsequently. Due to this convergence, DiDs were calculated for a maximum postrecovery follow-up of 16 months (average: 8.25, median: 9 months), which consisted of more than 5.3 M person-months.
Figure 1.
Monthly costs of COVID-19 recoverees (cases) compared with controls and relative differences, prepandemic and postindividual COVID-19 recovery, by month. (A) in the entire study cohort, (B) in patients who were hospitalised during the acute COVID-19 phase and (C) in patients who were not hospitalised during the acute COVID-19 phase.
The mean monthly cost measured during the individual post-COVID-19 follow-up period (up to 15 months after recovery) in cases was US$108.8, compared with US$100.6 in individually matched controls. After deducing the net monthly difference between groups during the baseline period of 12 consecutive prepandemic months, the mean net excess cost in cases (DiD) was US$7.6 per patient per month, 7.6% of the mean cost in controls during the same period (table 2).
Table 2.
Mean monthly costs in cases and controls, net and relative differences, and DID over the first 15 months of follow-up
| Mean monthly costs, US$ | Net difference, US$ | Relative difference (%) | ||
| Cases | Controls | |||
| Prepandemic costs | ||||
| Not hospitalised during acute COVID-19, N=626 851 | 84.5 | 85.6 | −1.1 | −1.3 |
| Hospitalised during acute COVID-19, N=16 017 pairs | 268.2 | 203.6 | 64.6 | 31.7 |
| Total, N=642 868 | 89.0 | 88.4 | 0.6 | 0.7 |
| Individual post-COVID-19 costs: months 1–15 of follow-up | ||||
| Not hospitalised during acute COVID-19, N=626 851 | 100.9 | 97.0 | 3.9 | 4.0 |
| Hospitalised during acute COVID-19, N=16 017 pairs | 382.9 | 226.7 | 156.2 | 68.9 |
| Total, N=642 868 | 108.8 | 100.6 | 8.2 | 8.2 |
| DID: post-COVID-19 excess costs, after the subtraction of pre-COVID-19 differences (relative difference compared with post-COVID-19 costs in controls) | ||||
| Not hospitalised during acute COVID-19, N=626 851 | 5.0 | 5.2 | ||
| Hospitalised during acute COVID-19, N=16 017 pairs | 91.6 | 40.4 | ||
| Total, N=642 868 | 7.6 | 7.6 | ||
DID, difference in difference.
Subgroup analysis
In the subset of N=16 017 cases who were hospitalised during the acute phase of COVID-19, relative cost differences (RD) were observed in the baseline prepandemic period, ranging from RD=20% to 45% higher mean monthly costs in cases compared with controls. The excess cost in cases compared with control was substantially higher in the first postrecoveree months (RD=183%, 116% and 97% in the first 3 months) and decreased gradually thereafter, returning to prepandemic levels 18 months after the index date (defined as 30 days after discharge from the COVID-19-related hospitalisation) (figure 1B). During the entire study period, the monthly excess cost in cases was US$91.6 (RD=40.4%).
Among COVID-19 cases who were not hospitalised during their COVID-19 acute phase (N=626 838 pairs, 97.5% of the cohort), prepandemic costs were slightly lower in cases compared with controls. However, in the individual post-COVID-19 recoveree follow-up period, higher costs were observed in cases compared with controls, gradually decreasing in the first 4 months postrecoveree (figure 1C). During the entire study period, the monthly excess cost in cases was US$5.0 (RD=5.2%).
Excess costs by categories of expenses
The total net DiD ofUS$7.6 was mostly attributed to hospital bills, accounting for 89% of it. Excess costs were observed in most categories of expenses and were the highest for hospitalisation days (RD 20.3%), ambulatory care (RD 8.4%), paramedical professions visit (RD 8.0%) and primary care physician visits (RD 7.5%). CHS incurred lower DiD expenses for cases for primary care nurse visits (RD −9.6%) and for medications (RD −6.1%).
Although the net and relative additive costs were substantially higher in COVID-19 inpatients, categories of expenses contributed similarly to the overall excess costs regardless of hospitalisation status. The exception was the category of other costs, where no excess was observed in outpatients, but a 77.4% excess was observed in COVID-19 inpatients (table 3).
Table 3.
Relative DID, % by categories of expense and by severity of the COVID-19 episode over the first 15 months of follow-up
| Category of expense | Net DID, US$ | Relative DID | ||
| All patients | Hospital bills (overall) | 6.85 | 11.7% | |
| Hospital bills breakdown | Cost of hospitalisation days | 4.05 | 20.3% | |
| PRG costs | 1.08 | 6.3% | ||
| ED visits costs | 0.38 | 6.8% | ||
| Ambulatory care costs | 1.34 | 8.4% | ||
| Primary care physician visits | 0.64 | 7.5% | ||
| Other costs* | 0.33 | 2.7% | ||
| Medications (costs incomes) | −0.53 | −6.1% | ||
| Medical specialists’ visits | 0.58 | 6.9% | ||
| Primary care nurse visits | −0.29 | −9.6% | ||
| Paramedical professions visits† | 0.11 | 8.0% | ||
| Total DID | 7.67 | 7.6% | ||
| Hospitalisation during the COVID-19 episode | Category of expense | Net DID, $ | relative DID | |
| Not hospitalised | Hospital bills (overall) | 4.86 | 8.7% | |
| Hospital bills breakdown | Cost of hospitalisation days | 2.69 | 14.2% | |
| PRG costs | 0.74 | 4.6% | ||
| ED visits costs | 0.35 | 6.3% | ||
| Ambulatory care costs | 1.08 | 6.9% | ||
| Primary care physician visits | 0.58 | 7.0% | ||
| Other costs* | −0.23 | −1.9% | ||
| Medications (costs incomes) | −0.49 | −5.9% | ||
| Medical specialists’ visits | 0.52 | 6.3% | ||
| Primary care nurse visits | −0.30 | −9.9% | ||
| Paramedical professions visits† | 0.06 | 4.9% | ||
| Total DID | 5.01 | 5.2% | ||
| Category of expense | DID, $ | relative DID | ||
| Hospitalised | Hospital bills (overall) | 69.26 | 48.4% | |
| Hospital bills breakdown | Cost of hospitalisation days | 46.56 | 86.9% | |
| Procedure-related group (PRG) costs | 12.19 | 23.8% | ||
| Emergency department visits costs | 1.06 | 14.4% | ||
| Ambulatory care costs | 9.45 | 30.7% | ||
| Primary care physician visits | 2.19 | 15.4% | ||
| Other costs | 19.76 | 77.4% | ||
| Medications (costs incomes) | −3.58 | −14.8% | ||
| Medical specialists’ visits | 2.59 | 20.5% | ||
| Primary care nurse visits | −0.21 | −4.2% | ||
| Paramedical professions visits | 1.58 | 67.8% | ||
| Total DID | 91.59 | 40.4% | ||
P<0.0001 in Wilcoxon signed ranks test (for all tests).
*Other costs: imaging and laboratory tests in CHS outpatient settings, payments to private providers and patients’ copayments and refunds (excluding copayments for medications).
†Paramedical professions visits: physiotherapists, occupational therapists, speech-language-pathologist, dietitians, psychologists and social workers visits.
CHS, Clalit Health Service; DID, difference in difference.
Excess costs by age groups and sex
The lowest excess cost (both net and relative DiD) was observed in persons aged 20–39, in both COVID-19 outpatients and inpatients: US$1.4 (1.5%) and US$26.3 (24.3%), respectively. The highest excess costs were observed in persons 60 years or older in both subgroups: US$26.0 (9.2%) and US$128.7 (35.0%), respectively.
While the DiD was higher in outpatient female patients compared with males (US$4.9 vs US$4.7, respectively), among inpatients DiDs were lower in females (US$76.8 vs US$93.7, respectively) (online supplemental table S2).
Trends in costs: cases and controls
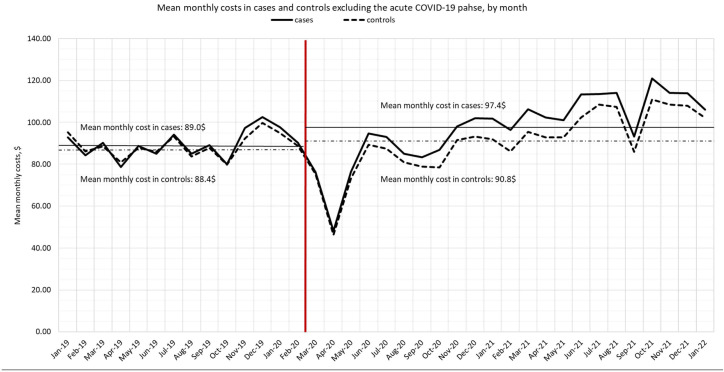
The overall expense of CHS per member of the study cohort had increased since the start of the pandemic, compared with 12 months before the pandemic commenced. Monthly costs in cases and controls are presented while excluding the individual acute COVID-19 phase, in order to reflect collateral and long-Covid effects, rather than the effects of an acute condition (figure 2). In both cases and control, we observed a sharp decline in costs as the COVID-19 pandemic surged in Israel in March–May 2020. After that period, costs had reached prepandemic levels and gradually increased over time. Despite the higher costs observed in cases, the trends in both groups are similar.
Figure 2.
Mean monthly costs in cases and controls, excluding the individual acute COVID-19 phase, January 2019–January 2022.
Discussion
Findings in context
This study evaluated the mean cost attributable to long-Covid, as incurred by CHS over a follow-up of up to 20 months after recovery from COVID-19 in the general, community-dwelling population. We found that over 15 months of follow-up after recovery from COVID-19, recoverees had significantly higher costs of healthcare services utilisation compared with matched controls. Mean costs converged in the 16th month from recovery and remained similar in both groups thereafter. As the measured costs largely represent healthcare services utilisation, the convergence of costs in cases after 15 months from recovery with those of controls is likely to indicate that most of the clinical manifestations of long-Covid disappear after that period. This is in accordance with a recent, large study which demonstrated that most health outcomes arising after mild COVID-19 remained for several months and returned to normal within the first year.18
The mean monthly cost attributable to long-Covid represented an addition of ~8% to the cost of controls in the same period. Excess costs were substantially higher in patients who were hospitalised during the acute COVID-19 phase compared with those who did not require hospitalisation, and this small group of patients (2.5% of the cohort) contributed most of the overall observed excess cost. This finding is in accordance with an increased risk for long-Covid diagnosis in patients hospitalised during the acute phase of the infection, recently reported,18 as well as with other studies which reported a proportion as high as 72.5% of patients experiencing at least one symptom in participants who were hospitalised due to COVID-19.7
However, age contributed independently to excess costs, as patients aged 60 years or older had higher excess costs after recoveree from COVID-19 both among those who were hospitalised during the acute phase of the infection and those who were not, compared with younger patients. Although long-Covid was reported to affect individuals of varied age groups,18 the higher cost observed in older persons is in accordance with other studies which found age to be a risk factor for long-Covid.8
Most of the excess cost was attributed to hospital bills, and the category of expense where the highest relative DiD was observed was hospitalisation days. However, a non-negligible DID of ~8% was also observed for primary care physician visits, medical specialists’ visits and paramedical professions visits. The lower costs observed in COVID-19 recoverees for primary nurse visits are attributable, at least in part, to a lower number of vaccinations uptake in this group. The lower costs related to medications in COVID-19 recoverees are explained by an excess dispensation of OTC drugs where the income for CHS is higher than the cost.
In addition, the overall expense of CHS per member since the start of the pandemic was higher compared with the previous year, regardless of care directly related to COVID-19 infections. Although this increase was more pronounced in cases, the costs of controls had also increased (by US$2.4 per month, 2.7% from baseline). It should be noted that the mean increase we report over March 2020–January 2022 is observed despite the fact that in the early stages of the pandemic, the cost was considerably low compared with prepandemic, in accordance with low use of healthcare services reported by numerous studies during the beginning of the pandemic.19–21 The overall increased costs in controls may reflect increases in healthcare services utilisation that may relate to the collateral effects of the pandemic on the general population over time.
The scientific literature includes reports of approximately 30% of COVID-19 recoverees who experience lasting symptoms, some typical of long-Covid.22 23 In some cases, these symptoms translate into healthcare seeking and increased healthcare services utilisation: Tartof et al had demonstrated elevated healthcare utilisation (as DiDs) in patients with positive SARS-CoV-2 results 6 months after the acute infection in infected persons, while referring to specific clinical outcomes (including alopecia, bronchitis, pulmonary embolism, venous thrombosis, dyspnoea and more.15 Another study showed that long-Covid (per diagnosis documented in medical records) is associated with a substantial increase in the utilisation of healthcare services and direct medical costs, including an almost twofold higher risk for post-acute hospitalisation.24
Strengths and limitations
Our study has several strengths. First, the results are based on the integrated administrative and medical records system of CHS, with detailed demographic, clinical and financial data, including information on SARS-CoV-2 tests results, regardless of where they were performed within the country. Additional important strengths are the large cohort of participants available for analysis, and the long period of follow-up available, compared with previous studies. This substantial follow-up allowed not only to evaluate the overall cost associated with long-Covid, but also to identify the time when excess costs in COVID-19 recoverees had returned to baseline of their controls. However, our study has some noteworthy limitations. First, the availability of data from a single healthcare provider acts as limitation, as the results may not be representative of other populations.
Second, our analyses were based on the comparison of mean costs, while individual costs within the population are not normally distributed. Hence, the comparison of means, highly affected by extreme costs, may not be representative of individual costs. However, means allows to easily deduct overall expenses and excess costs, moreover when calculated for an entire population rather than a sample.
Third, this study does not link excess costs to specific clinical manifestations, thus a clinical interpretation is not possible. Moreover, we include in the analyses patients who were infected with SARS-CoV-2 over a relatively long period of time (March 2020 to December 2021), during which different SARS-CoV-2 variants were prominent in Israel. Hence, it is not possible to compare the effect of different variants or pandemic waves on the additive costs of recoverees.
Fourth, we present trends in costs from January 2019 to January 2022 in both cases and controls (excluding the acute phase of COVID-19 for each pair). Changes in CHS expenses for controls may also reflect, additionally to changes in healthcare services utilisation, variability in pricing over time. However, changes in pricing during the study period were minor.
Also, recruitment of the study population ended (in 31 December 2021) before the Omicron variant (B.1.1.529) became dominant in Israel. Hence, the effects of the Omicron variant are not reflected in this study. This exclusion was necessary as SARS-CoV-2 testing in community settings dramatically diminished after that period, making the identification of persons affected by COVID-19 less certain. It is likely that the long-term effects of COVID-19 on health and health services utilisation following infection with the Omicron variant were milder, compared with infection with previous variants.25
It should be noted that this study only addressed the direct cost of healthcare services utilisation from the insurer-provider perspective. However, long-Covid has additional related costs, including higher personal spending on medical care, lost earnings (which is estimated to be particularly high) and lost quality of life. Moreover, the effects of long-Covid on the ability of working-age individuals' to perform their jobs were estimated based on CDC data at almost 1 percent of the US gross domestic product.26
Conclusions
Long-term effects of SARS-CoV-2 infections translate into excess healthcare costs months after recovery, hence requiring adjustments of funds allocation. These excess costs gradually diminish after recoveree, returning to baseline differences 16 months after recoveree.
Footnotes
Handling editor: Seye Abimbola
Contributors: GL conceptualised the study. YSW, GL and SB-G designed the study. IF extracted and analysed the data. YSW wrote the first draft of the paper. GL and SB-G reviewed the paper for critical content. GL act as guarantor for the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Due to the CHS data privacy regulations and per the institutional revive board and data utilisation committee approvals for the study, the patient-level data used for this study cannot be shared.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Clalit Health Services Institutional Ethics Committee and Clalit Health Services Institutional Data Transfer Committee. As this is a retrospective study, based on cost and clinical data from Clalit’s databases, the study was exempted from patients’ informed consent.
References
- 1.Chen S, Prettner K, Kuhn M, et al. The economic burden of COVID-19 in the United States: estimates and projections under an infection-based herd immunity approach. J Econ Ageing 2021;20:100328. 10.1016/j.jeoa.2021.100328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA 2020;324:1495–6. 10.1001/jama.2020.19759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briggs A, Vassall A. Count the cost of disability caused by COVID-19. Nature 2021;593:502–5. 10.1038/d41586-021-01392-2 [DOI] [PubMed] [Google Scholar]
- 4.Mikkelsen ME, Abramoff B. COVID-19: evaluation and management of adults with persistent symptoms following acute illness (long COVID). UpToDate 2022. Available: https://www.uptodate.com/contents/covid-19-evaluation-and-management-of-adults-with-persistent-symptoms-following-acute-illness-long-covid [Google Scholar]
- 5.Ayoubkhani D, Munro M. n.d. Prevalence of ongoing symptoms following Coronavirus (COVID-19) infection in the UK. data and analyses from census 2021: 3 March 2022. office of national statistics, UK. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronavirusCovid19infectionintheuk/3march2022
- 6.Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021;325:1525–34. 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: A systematic review. JAMA Netw Open 2021;4:e2111417. 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michelen M, Manoharan L, Elkheir N, et al. Characterizing long COVID: a living systematic review. BMJ Glob Health 2021;6:e005427. 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after Hospitalisation (PHOSP-COVID): a UK Multicentre, prospective cohort study. Lancet Respir Med 2021;9:1275–87. 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747–58. 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related Hospitalisation: a prospective study. Lancet Respir Med 2021;9:747–54. 10.1016/S2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 13.Cohen K, Ren S, Heath K, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-Cov-2 infection: retrospective cohort study BMJ. BMJ 2022:e068414. 10.1136/bmj-2021-068414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen B, Waitzberg R, Merkur S. Israel – health system review. Health Syst Transit 2015;17:1–212. [PubMed] [Google Scholar]
- 15.Tartof SY, Malden DE, Liu I-LA, et al. Health care utilization in the 6 months following SARS-Cov-2 infection. JAMA Netw Open 2022;5:e2225657. 10.1001/jamanetworkopen.2022.25657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abadie A. Semiparametric difference-in-differences Estimators. Rev Econ Stud 2005;72:1–19. 10.1111/0034-6527.00321 [DOI] [Google Scholar]
- 17.Taylor CO, Lemke KW, Richards TM, et al. Comorbidity characterization among eMERGE institutions: A pilot evaluation with the Johns Hopkins adjusted clinical groups® system. AMIA Jt Summits Transl Sci Proc 2019;2019:145–52. [PMC free article] [PubMed] [Google Scholar]
- 18.Mizrahi B, Sudry T, Flaks-Manov N, et al. Long Covid outcomes at one year after mild SARS-Cov-2 infection: nationwide cohort study. BMJ 2023;380:e072529. 10.1136/bmj-2022-072529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moynihan R, Sanders S, Michaleff ZA, et al. Impact of COVID-19 pandemic on utilisation of Healthcare services: a systematic review. BMJ Open 2021;11:e045343. 10.1136/bmjopen-2020-045343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howarth A, Munro M, Theodorou A, et al. Trends in Healthcare utilisation during COVID-19: a longitudinal study from the UK. BMJ Open 2021;11:e048151. 10.1136/bmjopen-2020-048151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagy YW, Cicurel A, Battat E, et al. The impact of COVID-19 pandemic on emergency Department visits and associated mortality during 14 months of the pandemic in Israel. Intern Emerg Med 2022;17:1699–710. 10.1007/s11739-022-02991-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bull-Otterson L, Baca S, Saydah S, et al. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States. MMWR Morb Mortal Wkly Rep 2020;71:713–7. 10.15585/mmwr.mm7121e1 [DOI] [Google Scholar]
- 23.Centers for Disease Control and Prevention . Long COVID: household pulse survey. 2022. Available: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm
- 24.Tene L, Bergroth T, Eisenberg A, et al. Risk factors, health outcomes, Healthcare services utilization, and direct medical costs of patients with long COVID. Int J Infect Dis 2023;128:3–10. 10.1016/j.ijid.2022.12.002 [DOI] [PubMed] [Google Scholar]
- 25.Antonelli M, Pujol JC, Spector TD, et al. Risk of long COVID associated with Delta versus Omicron variants of SARS-Cov-2. Lancet 2022;399:2263–4. 10.1016/S0140-6736(22)00941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho S. Long COVID could cost the economy trillions, experts predict. n.d. Available: https://www.webmd.com/covid/news/20220928/long-covid-could-cost-economy-trillions-experts
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2023-012588supp001.pdf (98.6KB, pdf)
Data Availability Statement
No data are available. Due to the CHS data privacy regulations and per the institutional revive board and data utilisation committee approvals for the study, the patient-level data used for this study cannot be shared.