Abstract
Purpose:
Pediatric optic neuritis (ON) is a rare disease that has not been well characterized. The pediatric ON prospective outcomes study (PON1) was the first prospective study aiming to evaluate visual acuity (VA) outcomes, including VA, recurrence risk, and final diagnosis 2-years post-enrollment
Design:
Non-randomized observational study at 23 pediatric ophthalmology or neuro-ophthalmology clinics in US and Canada.
Participants:
28 (64%) of 44 children initially enrolled in PON1 (age 3<16 years) who completed their 2-year study visit.
Exposure(s):
Treatment at investigator discretion.
Main Outcomes Measures:
Age-normal monocular high contrast VA (HCVA). Secondary outcomes included low contrast VA (LCVA), neuroimaging findings, and final diagnoses.
Results:
28 participants completed the 2-year outcome with a median enrollment age of 10.3 years (range 5–15); 46% were female and 68% had unilateral ON at presentation. Final 2-year diagnoses included isolated ON (n=11, 40%), myelin oligodendrocyte glycoprotein (MOG)-associated demyelination (n=8, 29%), multiple sclerosis (MS) (n=4,14%), neuromyelitis optica spectrum disease (NMOSD) (n=3,11%), and acute disseminated encephalomyelitis (n=2, 7%). Two (7%; 95% CI=1–24%) participants had subsequent recurrent ON (plus one participant who did not complete the 2-year visit); all had MS. Two other participants (7%) had a new episode in their unaffected eye. Mean presenting HCVA was 0.81 logMAR (~20/125), improving to 0.14 logMAR (~20/25−2) at 6 months, 0.12 logMAR (~20/25−2) at 1 year, and 0.11 logMAR (20/25−1) at 2 years (95% CI=−0.08–0.3 (20/20+1-20/40−1)). Twenty-four (79%) had age-normal VA at 2 years (95% CI=60–90%);21 (66%) had 20/20 vision or better. The six participants without age-normal VA had 2-year diagnoses of NMOSD (n=2 participants, 3 eyes), MS (n=2 participants, 2 eyes), and isolated ON (n=2 participants, 3 eyes). Mean presenting LCVA was 1.45 logMAR (~20/500−2), improving to 0.78 logMAR (~20/125+2) at 6 months, 0.69 logMAR (~20/100+1) at 1 year, and 0.68 logMAR (~20/100+2) at 2 years (95% CI=0.48–0.88 (20/50+1-20/150−1)).
Conclusions:
Despite poor VA at presentation, most children had marked improvement in VA by 6 months which was maintained over two years. Associated neurologic autoimmune diagnoses were common. Additional episodes of ON occurred in 5 (18%) of the participants (3 relapses and 2 new episodes).
Keywords: Pediatric Optic Neuritis, Pediatric Ophthalmology, Neuro-ophthalmology
Precis:
Despite poor visual acuity at presentation, most children in the pediatric optic neuritis prospective outcomes study had marked improvement which was maintained over 2 years. Associated neurologic disease was common in children with optic neuritis.
Introduction
Although optic neuritis (ON) has been well-studied in adults,1 there are limited prospective data related to ON in children.2 The Pediatric Optic Neuritis Prospective Outcomes Study (PON1) was conceived to determine our network’s ability to enroll children with ON into a prospective observational study with follow-up at 1-month, 6-months, 1-year and 2-years.3,4 In 2020, we published 6-month visual acuity (VA) outcomes for this cohort.4 Despite poor VA at presentation, the majority of children with ON had marked improvement in VA six months after onset and associated neurologic autoimmune diagnoses were common, specifically acute disseminated encephalomyelitis (ADEM, 16%), myelin oligodendrocyte glycoprotein positive (MOG+) demyelinating disorder (18%), multiple sclerosis (MS, 11%), and neuromyelitis optica spectrum disorder (NMOSD, 7%).4
Herein we report 2-year VA outcomes, recurrence, and prevalence of associated neurologic autoimmune diseases at 2 years.
Methods
The study was supported through a cooperative agreement with the National Eye Institute, National Institutes of Health, Bethesda, Maryland, and was conducted according to the tenets of the Declaration of Helsinki by the Pediatric Eye Disease Investigator Group (PEDIG) and the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC) at 23 academic- and community-based clinical sites specializing in either pediatric or neuro-ophthalmology in North America. The study protocol and Health Insurance Portability and Accountability Act compliant informed consent forms were approved by each site’s institutional review boards (IRB). A parent or guardian of each participant gave written informed consent, and children also provided written assent when applicable as determined by the local IRB. The full study protocol is available on the PEDIG web site (www.pedig.net; accessed March 23, 2021).
Children and teenagers (3 to <16 years old at enrollment) who presented with a first episode of ON in one or both previously unaffected eyes (based upon clinical diagnosis) within two weeks of symptom(s) onset were enrolled. To be considered a study eye with ON, affected eyes had to have vision loss and/or pain on eye movements for ≤2 weeks and at least one of the following: distance high contrast visual acuity (HCVA) deficit at least 0.2 logMAR below age-based norms,5,6 diminished color vision, abnormal visual field, or optic disk swelling. Age- based normative values for VA were referenced from prior studies which demonstrated that normal visual acuity improves with age, and is approximately 0.4 logMAR for 3 to <4 year-olds, 0.3 logMAR for subjects 4 to <6 years, 0.2 logMAR for subjects 6 to <7 years, and 0.1 for children over 7 years old on HOTV testing. For children over 7 years of age, the ETDRS test was performed, and 0.16 LogMAR was considered age-normal.5,6 For unilateral cases, a relative afferent pupillary defect was required. ON was deemed bilateral and simultaneous if present in both eyes at enrollment or if the fellow eye developed ON within one month of enrollment. Not all participants classified as having bilateral ON contributed two study eyes because inclusion of study eyes was based on additional eye-level eligibility criteria (such as the presence of symptoms for longer than 2 weeks). Patients with a known history of demyelination were included if they did not have a reported or documented prior episode of ON in the affected eye.
At enrollment, monocular distance HCVA and low contrast visual acuity (LCVA; using 2.5% low contrast letters) were tested in the participant’s habitual refractive correction (right eye, then left eye) by a study-certified examiner using the electronic ATS-HOTV© protocol if <7 years,7 or E-ETDRS© protocol if ≥7 years of age.8 A cycloplegic refraction was performed if not done within the past month and HCVA testing was repeated in trial frames if the participant had uncorrected refractive error requiring optical correction or a significant change refractive error.
MRI of the brain with and without intravenous gadolinium was required within 2 weeks of symptom onset. If the MRI had not yet been performed at enrollment, the recommended technique included fat-saturation for orbital images and Short-T1 Inversion Recovery (STIR) sequences. MRI images were collected and sent for review by a masked examiner (AW) to confirm the presence of optic nerve enhancement and associated white matter lesions (WML). The masked assessment was performed without demographic or clinical information MRIs performed 2 years post-presentation were analyzed. If already completed by the enrolling site as part of standard care, the results of aquaporin-4 (AQP4) antibody testing for NMOSD were collected. If blood or serum had not been collected as part of standard care, the parent(s) (and child, if old enough for assent) were given the option at enrollment to participate in a study to measure AQP4 antibodies as well as MOG.9 A Clinical Laboratory Improvement Amendment (CLIA)-certified test for antibodies to MOG was not clinically available at the start of the study.
Follow-up visits were conducted at 1 month (1–75 days), 6 months (76–272 days), 1 year (273–544 days), and 2 years (545–907 days) post-enrollment. At each study visit, monocular HCVA and LCVA were tested and anterior and posterior segments were examined. At the 1-month visit, a Tanner puberty stage questionnaire10 and a neurologic symptom questionnaire11 were administered, and sites completed a neurological summary diagnosis form,4 detailing the investigator’s diagnosis utilizing standardized definitions12 (isolated ON, ADEM, NMOSD or MOG+ ON) at each visit. Cases initially classified as “clinically isolated syndrome” were combined for analysis with isolated ON. The etiology was considered an “associated neurologic autoimmune diagnosis” if the MRI showed WML. If the masked MRI reading or results of the AQP4 or MOG antibody tests (if done) yielded discrepant findings from the investigator’s initial diagnosis, the initial diagnosis and enrollment data were reviewed by the study chairs and the diagnoses were adjudicated to a final diagnosis at enrollment and again at the 2-year study visit.
Statistical Analyses
To evaluate the difference in baseline HCVA between 2-year visit completers and non-completers, a mixed model with an exchangeable correlation matrix to account for correlation between eyes at enrollment was performed. Due to concerns about missing not at random, the outcomes for later visits are calculated separately for the limited data from non-completers.
The proportion of eyes with age-normal HCVA eyes at 2 years were calculated using a binomial regression model that adjusted for between-eye correlation.13 A generalized linear model with robust variance estimation was used to calculate means and 95% confidence intervals (CIs) for HCVA at 6 months, 1 year, and 2 years; and change in HCVA from baseline at each visit. For this primary analyses, an exchangeable correlation matrix was used to account for correlation between study eyes from the same participant.
Monocular LCVA scores at 2 years was the secondary outcome. An exploratory analysis for percent and exact confidence interval of participants with age-normal VA by final diagnosis was also performed. No adjustment for multiple testing was performed because these analyses were considered exploratory. Analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC).
Results
Baseline Characteristics
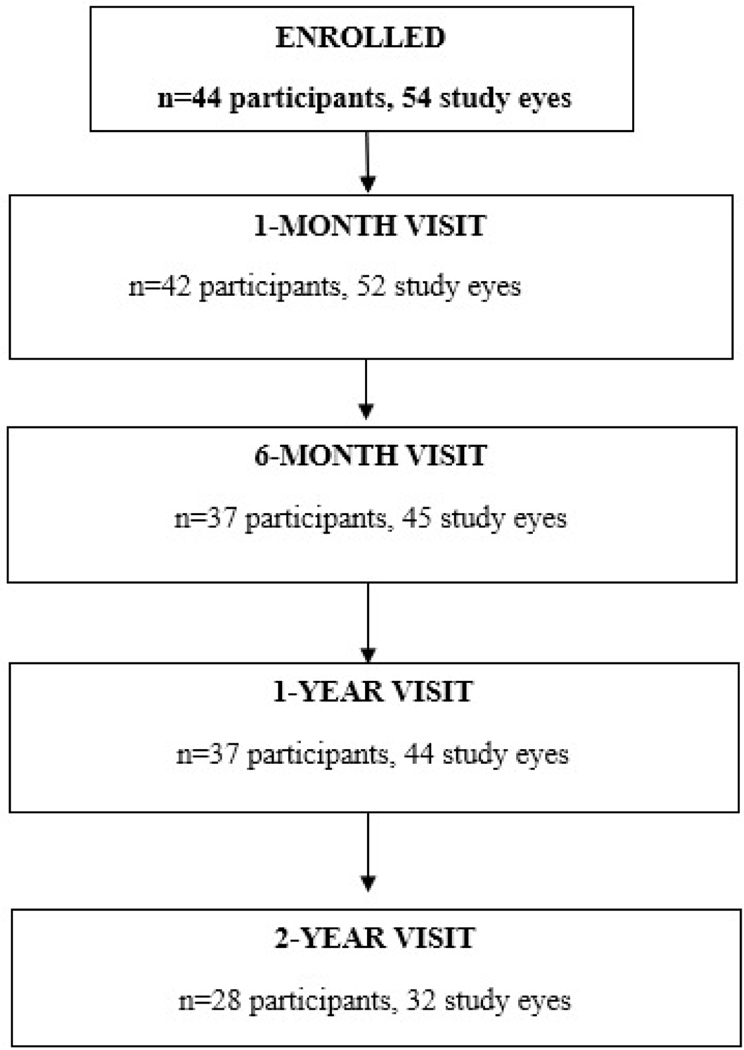
We enrolled 44 participants (54 eyes) between June 2016 and July 2018 at 23 sites (range 1–15 participants/site); 28 participants (32 eyes) completed the 2-year follow-up visit with HCVA (Figure 1, Table 1). The median age at enrollment of the 28 participants was 10.3 years (range 5–15); 46% were female; and 68% had unilateral ON at presentation. From the original study, 16 participants were not examined at 2 years. Of these, 15 were lost to follow-up after multiple attempts to contact them failed to result in their return for a final visit. One parent withdrew from the study for an unknown reason. 37 participants completed the 6-month and 1-year visits.
Figure 1.
Visit Completion with HCVA
Table 1.
Baseline Characteristics of Participants by 2-Year Visit Completion Status
| Non-Completer (N=16) | Completer (N=28) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Sex: Female | 5 | 31% | 13 | 46% |
| Age (Years) | ||||
| 3 to <7 | 3 | 19% | 7 | 25% |
| 7 to <10 | 2 | 13% | 6 | 21% |
| 10 to <13 | 7 | 44% | 9 | 32% |
| >=13 | 4 | 25% | 6 | 21% |
| Mean (SD) | 10.3 (3.5) | 10.2 (3.5) | ||
| Race/Ethnicity | ||||
| White | 7 | 44% | 16 | 57% |
| Black/African American | 2 | 13% | 4 | 14% |
| Hispanic | 4 | 25% | 6 | 21% |
| Asian | 3 | 19% | 1 | 4% |
| Unknown/not reported | 0 | 1 | 4% | |
| Pre-Puberty Status by Tanner Questionnaire | ||||
| Yesa | 3 | 19% | 6 | 21% |
| No | 11 | 69% | 17 | 61% |
| Not Done/Unknown | 2 | 13% | 5 | 18% |
| Systemic Symptoms Reported: | ||||
| None | 10 | 63% | 11 | 39% |
| Any | 6 | 38% | 17 | 61% |
| Family History of Medical Conditions (listed below): Yes | ||||
| None | 11 | 69% | 17 | 61% |
| Optic neuritis | 2 | 13% | 1 | 4% |
| Autoimmune conditions | 4 | 25% | 6 | 21% |
| Cardiovascular disease | 1 | 6% | 6 | 21% |
| Laterality at PON presentation | ||||
| Bilateralb | 7 | 44% | 9 | 32% |
| Bilateral sequential | 0 | 1 | 4% | |
| Bilateral simultaneous | 7 | 44% | 8 | 29% |
| Unilateral | 9 | 56% | 19 | 68% |
| Corticosteroid Treatment at Time of Enrollment c | ||||
| No | 3 | 19% | 1 | 4% |
| Yes | 13 | 81% | 27 | 96% |
| History of Demyelination | ||||
| None | 15 | 94% | 22 | 79% |
| Optic neuritis | 0 | 1 | 4% | |
| Multiple sclerosis | 0 | 1 | 4% | |
| Acute disseminated encephalomyelitis (ADEM) | 1 | 6% | 2 | 7% |
| Transverse myelitis | 0 | 1 | 4% | |
| Not reported | 0 | 1 | 4% | |
| Brain Lesion(s) on Magnetic Resonance Imaging (MRI) d | ||||
| Site Assessment | ||||
| No | 8 | 50% | 13 | 46% |
| Yes | 8 | 50% | 15 | 54% |
| Independent Masked Assessment | ||||
| No | 9 | 56% | 11 | 39% |
| Yes | 6 | 38% | 17 | 61% |
| Unknown/Unable to determine | 1 | 6% | 0 | 0% |
| Diagnosis based on initial site impression e | ||||
| Bilateral isolated optic neuritis | 6 | 38% | 3 | 11% |
| Unilateral isolated optic neuritis only | 4 | 25% | 9 | 32% |
| Acute disseminated encephalomyelitis (ADEM) | 2 | 13% | 6 | 21% |
| Multiple sclerosis | 2 | 13% | 3 | 11% |
| Neuromyelitis optica spectrum disorder (NMO) | 1 | 6% | 4 | 14% |
| Myelin oligodendrocyte glycoprotein positive demyelinating disorder (MOG) | 0 | 0% | 1 | 4% |
| MS vs seronegative NMO | 1 | 6% | 1 | 4% |
| Unknown/ Not Reported f | 0 | 0% | 1 | 4% |
Pre-puberty defined as Tanner Questionnaire Level 1 response = scrotum and penis size are the same as when young (if male) or breasts are flat (if female).
Of those with bilateral optic neuritis at presentation, laterality was defined as simultaneous bilateral if both eyes developed an initial episode of acute optic neuritis within 1 month of each other; otherwise, participants were classified as having sequential bilateral optic neuritis.
Participants were classified as having corticosteroid treatment (oral or intravenous) at enrollment if corticosteroid use was current and/or reported prior to enrollment or if corticosteroid treatment was prescribed at enrollment.
Based on MRI scan performed at enrollment (or within 2 weeks after enrollment).
Site-reported diagnosis was recorded at the 1-month visit. Cases where the investigator indicated that the diagnosis was clinically isolated syndrome were reclassified as either unilateral optic neuritis only or bilateral optic neuritis only according to laterality at optic neuritis presentation.
Site did not report a diagnosis because the participant missed the 1-month visit.
All (100%) 28 participants completed the 2-year exam were treated with corticosteroids at some time during the study period, typically within the first month (27 at enrollment and 1 by one month). MRI findings at enrollment revealed cerebral WML (aside from optic nerve enhancement) in 17 of 28 (61%, 95% CI = 0.41–0.78) based upon masked reading of the MRI. Of the 8 (29%) 2-year visit completers who participated in the optional study for AQP4 testing at enrollment, 1 (13%, 95% CI= 0–53%) tested positive for AQP4 antibodies. Consent for optional MOG testing at enrollment was obtained for 8 (29%) of the 2-year visit completers, of whom 3 (38%, 95% CI = 9–76%) tested positive for MOG antibodies. Based upon the combination of clinical findings, MRI review, and serological testing (including serologic testing as part of clinical care), the final 2-year diagnosis was isolated ON in 39% (n=11) of participants. Other final 2-year diagnoses were: MOG-associated demyelination (n=8, 29%), MS (n=4, 14%), ADEM (n=2, 7%), and NMOSD (n=3, 11%) (Table 2). The adjudicated diagnosis at enrollment was consistent with the 2-year diagnosis for 16/28 (57%) (Table 3).
Table 2:
Initial and Final Neurological Diagnosis and Proportion of Participants with Age-Normal Visual Acuity (VA) at 2 Years
| Initial Neurological Diagnosis | Age-Normal VA at 2 Years | ||||
|---|---|---|---|---|---|
| Diagnosis | N (%) | 95% CI | # Eyes | # Eyes total | Exact Proportion (95% CI) |
| ADEM | 6 (21%) | (0.08, 0.41) | 5 | 7 | .71 (0.29, 0.96) |
| Bilateral optic neuritis only | 3 (11%) | (0.2, 0.28) | 4 | 6 | 0.67 (0.22, 0.96) |
| MOG + associated | 1 (4%) | (0.0, 0.18) | 1 | 1 | 1.00 (0.03, 1.00) |
| Multiple sclerosis vs Seronegative NMO | 1 (4%) | (0.0, 0.18) | 1 | 1 | 1.00 (0.03, 1.00) |
| Multiple sclerosis | 3 (11%) | (0.02, 0.28) | 1 | 3 | 0.33 (0.01, 0.91) |
| NMOSD | 4 (14%) | (0.04, 0.33) | 3 | 4 | 0.75 (0.19, 0.99) |
| Unilateral optic neuritis only | 9 (32%) | (0.16, 0.52) | 8 | 9 | 0.89 (0.52, 1.00) |
| Final Neurological Diagnosis at 2 Years | Age-Normal VA at 2 Years | ||||
| Diagnosis | N (%) | 95% CI | # Eyes | # Eyes total | Exact Proportion (95% CI) |
| ADEM | 2 (7%) | (0.01, 0.24) | 2 | 2 | 1.00 (0.16, 1.00) |
| Bilateral optic neuritis only | 3 (11%) | (0.2, 0.28) | 4 | 6 | 0.67 (0.22, 0.96) |
| MOG + demyelinating disorder | 8 (29%) | (0.13, 0.49) | 8 | 8 | 1.00 (0.63, 1.00) |
| Multiple sclerosis | 4 (14%) | (0.04, 0.33) | 2 | 4 | 0.5 (0.07, 0.93) |
| NMOSD | 3 (11%) | (0.2, 0.28) | 1 | 4 | 0.25 (0.01, 0.81) |
| Unilateral optic neuritis only | 8 (29%) | (0.13, 0.49) | 7 | 8 | 0.88 (0.47, 1.00) |
ADEM=Acute disseminated encephalomyelitis; CI= Confidence Interval; MOG=Myelin oligodendrocyte glycoprotein; NMOSD: Neuromyelitis optica spectrum disorder
Table 3:
Enrollment and Final Diagnoses at 2-years
| Enrollment diagnosis | Final 2 year diagnosis | Count |
|---|---|---|
| Missing | Multiple sclerosis | 1 |
| ADEM | ADEM | 2 |
| ADEM | MOG+ demyelinating disorder | 3 |
| ADEM | NMOSD | 1 |
| Bilateral optic neuritis only | Bilateral optic neuritis only | 3 |
| MOG associated | MOG+ demyelinating disorder | 1 |
| MS vs Seronegative NMO | MOG+ demyelinating disorder | 1 |
| Multiple sclerosis | Multiple sclerosis | 2 |
| Multiple sclerosis | Unilateral optic neuritis only | 1 |
| NMOSD | MOG+ demyelinating disorder | 2 |
| NMOSD | NMOSD | 2 |
| Unilateral optic neuritis only | MOG+ demyelinating disorder | 1 |
| Unilateral optic neuritis only | Multiple sclerosis | 1 |
| Unilateral optic neuritis only | Unilateral optic neuritis only | 7 |
Distance Visual Acuity
Non-completers had worse point estimates of VA at enrollment than those who completed the study (estimate (95% CI) for the logMAR difference for a two-year completer was −.35 (−.76, 0.06) logMAR lower than non-completers, p=0.09). Table 4, available at https://www.aaojournal.org, shows the distribution of visual acuities for the non-completers at each visit. The adjusted mean distance HCVA at enrollment was 1.16 logMAR (~20/250 , 95% CI = 0.94–1.38). Three of 22 eyes (9%, 95% CI = 2–32%) had distance HCVA at enrollment that was age-normal. By 1 year, the mean distance HCVA improved in the 12 remaining eyes (9 participants) by 10.7 lines (95% CI= 6.89–14.51) to 0.18 logMAR (~20/32, 95% CI=−0.11–0.47); 6 of 12 eyes (44%, 95% CI = 18–75%) had age-normal HCVA at 1 year.
In the 2-year completers, the adjusted mean distance HCVA at enrollment was 0.81 logMAR (~20/125 , 95% CI = 0.62–1.0). Of 32 eyes, 8 (25%, 95% CI = 14–42%) had distance HCVA at enrollment that was age-normal. Seven eyes (22%) had distance HCVA between 20/250 to 20/800, and 7 eyes (22%) had HCVA worse than 20/800 (Table 5). By 2 years, mean distance HCVA improved by 7.09 lines (95% CI= 4.32–9.85) to 0.11 logMAR (~20/25, 95% CI=−0.08–0.30); 24 of 32 eyes (79%, 95% CI = 60–90%) had age-normal HCVA at 2 years and 21 had 20/20 vision or better at 2 years. Only 2 (6%) participants had distance HCVA worse than 20/200, with only 1 of those 2 (3%) having worse VA than 20/800. One participant each with MS and NMOSD had unilateral VA worsen by 3 and 6 lines respectively from enrollment to 2 years. Of the four participants with worse than 20/40 at the two year visit, two were improvements from baseline. One unilateral participant improved from >20/800 to 20/50. A bilateral participant improved from >20/800 in both eyes to 20/63 and 20/40. The other two participants were unilateral cases worsening form 20/100 to 20/400 and 20/500 to >20/800.
Table 5.
Distribution of Distance Visual Acuity Scores for 2-year Completer Eyes at Enrollment to Two Yearsa
| High Contrast Visual Acuity | Low Contrast Visual Acuity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Enrollment (N eyes = 32) | 6 Months (N eyes = 31) | 1 Year (N eyes = 32) | 2 Years (N eyes = 32) | Enrollment (N eyes = 32) | 6 Months (N eyes = 30) | 1 Year (N eyes = 31) | 2 Years (N eyes = 30) |
|||||||||
|
| ||||||||||||||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||||||
| Visual acuity in affected eye(s), Snellen Equivalent (Number of Letters) | ||||||||||||||||
| Worse than 20/800 (<5) | 7 | (22%) | 1 | (3%) | 1 | (3%) | 1 | (3%) | 18 | (56%) | 5 | (17%) | 5 | (16%) | 4 | (13%) |
| 20/250 to 20/800 (5–30) | 7 | (22%) | 1 | (3%) | 1 | (3%) | 1 | (3%) | 8 | (25%) | 2 | (7%) | 2 | (6%) | 1 | (3%) |
| 20/50 to 20/200 (35–65) | 6 | (19%) | 3 | (10%) | 3 | (9%) | 2 | (6%) | 4 | (13%) | 18 | (60%) | 14 | (45%) | 16 | (53%) |
| 20/20 to 20/40 (70–85) | 9 | (28%) | 15 | (48%) | 13 | (41%) | 16 | (50%) | 2 | (6%) | 5 | (17%) | 10 | (32%) | 9 | (30%) |
| Better than 20/20 (>85) | 3 | (9%) | 11 | (35%) | 14 | (44%) | 12 | (38%) | 0 | (0%) | 0 | (0%) | 0 | (0%) | 0 | (0%) |
|
| ||||||||||||||||
| Mean (95% CI) logMAR VA (95% CI)b | 0.81 (0.62 to 1.0) |
0.14 (−0.05 to 0.33) |
0.12 (−0.07 to 0.31) |
0.11 (−0.08 to 0.30) |
1.45 (1.25 to 1.64) |
0.78 (0.58 to 0.97) |
0.69 (0.50 to 0.89) |
0.68 (0.48 to 0.88) |
||||||||
VA=visual acuity, SD=standard deviation, CI = confidence interval
logMAR converted to Snellen as follows: −0.2 = 20/12; −0.1 = 20/16; 0 = 20/20; 0.1 = 20/25; 0.2 = 20/32; 0.3 = 20/40; 0.4 = 20/50; 0.5 = 20/63; 0.6 = 20/80; 0.7 = 20/100; 0.8 = 20/125; 0.9 = 20/160; 1 = 20/200; 1.1 = 20/250; 1.2 = 20/320; 1.3 = 20/400; 1.4 = 20/500; 1.5 = 20/640; 1.6 = 20/800; 1.7 = <20/800
Adjusted for inter-eye correlation between eyes.
Of the patients who did not complete the 2-year follow up, 3 of them did not follow-up for the six-month visit. Of those, there were two unilateral cases (ADEM and isolated ON with enrollment VA of 1.7 and 0.6 logMAR, respectively). The other patient had bilateral ON and presenting VA of 1.3 and 0.8 logMAR. Four additional patients did not complete any visits after their 6-month follow-up. Of these four patients, 3 had bilateral ON and 1 had unilateral ON. Enrollment VA for the 3 bilateral cases was 1.4 and 1.6 logMAR improving to 0.2 and 0.0 logMAR at 6 months, and 1.5 logMAR improving to −0.3 at 6 months, and 1.7 and 0.2 logMAR improving to −0.1 and 0.1 logMAR at 6 months. The fourth patient who only completed 6 months of follow-up with unilateral ON presented with 0.5 logMAR and improved to 0.1 logMAR.
Low Contrast Visual Acuity
In non-completers, the adjusted mean distance LCVA at enrollment was 1.59 logMAR (~20/800, 95% CI = 1.4–1.78); 20 of 22 eyes (91%) had distance LCVA worse than 20/200, and 15 eyes (68%) had LCVA worse than 20/800 (Table 4, available at https://www.aaojournal.org). By 1 year, the LCVA for the 9 remaining eyes (7 participants) improved by 7.57 lines (95% CI = 3.66–11.47) to 0.81 logMAR (~20/125, 95% CI = 0.53 to 1.09); 1/9 (11%) eyes had distance LCVA worse than 20/800 (Table 4, available at https://www.aaojournal.org).
In the 2-year completers, the adjusted mean distance LCVA at enrollment was 1.45 logMAR (~20/640, 95% CI = 1.25–1.64); 26 of 32 eyes (81%) had distance LCVA worse than 20/200, and 18 eyes (56%) had LCVA worse than 20/800 (Table 5). By 2 years, the LCVA improved by 7.24 lines (95% CI = 5.06–9.41) to 0.68 logMAR (~20/100, 95% CI = 0.48 to 0.88); 4/32 (13%) eyes had distance LCVA worse than 20/800 (Table 5).
The results of subgroup analyses evaluating the proportion of completers with age-normal HCVA by final diagnosis are included in Table 2.
Discussion
This 2-year observational study provides the longest prospective follow-up of VA outcomes for children and teenagers with ON and provide useful insights for clinicians and patients. The data suggest that a clinical diagnosis of isolated ON at the time of onset may reduce the risk of recurrence and the development of an associated neurologic condition; In this study, only 2 of the 12 participants with a clinical diagnosis of isolated ON with 2 years of follow-up developed an associated condition, with one having a recurrence. This finding is consistent with previous studies that have reported that MRI brain abnormalities at presentation are one of the strongest predictors of the development of MS.14 In addition, despite poor VA at presentation, most children had marked improvement in VA at 2 years, which was present by 6 months from onset.
Associated neurologic autoimmune conditions were common, occurring in 61% of the participants who completed their 2-year visit. MOG-associated demyelination was the most common associated disease in 29% of participants, although these children were commonly labelled with ADEM at the enrollment exam (Table 3). At enrollment, the majority of participants who were mis-labelled were initially diagnosed with ADEM or NMOSD and eventually diagnosed with MOG+ disease. Many of these initial mis-attribution may have been related to the rapid evolution of the understanding of the role of MOG testing15–17 in pediatric ON resulting in increased standard-of-care MOG testing that occurred during the time span of this study. Only one patient who was eventually determined to have NMOSD after later serologic testing was mis-labelled at the enrollment visit (Table 3). Correctly identifying NMOSD is important because NMOSD requires a different treatment regimen such as an anti-B cell agent, and the disease course may be aggravated by disease-modifying drugs for MS.18
Poor outcomes (defined as HCVA worse than 20/50 and LCVA worse than 20/250), persisted at 2 years in 4 (14%) of 28 participants who had HCVA 20/50 or worse at enrollment [isolated unilateral ON (n=1), NMO (n=2), MS (n=1)] and 5(18%) participants who had LCVA 20/250 or worse at enrollment [isolated unilateral ON (n=1), isolated bilateral ON (n=1), NMO (n=1), MOG+ ON (n=1) and MS (n=1)]. Prior studies have identified NMO-associated ON as a risk factor for more severe, persistent vision loss in children.19 Interestingly, no participant who relapsed or had poor recovery of HCVA had a diagnosis of MOG-associated ON [although only 8/28 (29%) underwent MOG testing at enrollment]. This finding is consistent with other studies which identify MOG-related disease as having a low risk of severe, permanent vision loss although our small number of participants cannot rule out the possibility of poor outcomes in all cases of MOG-related disease.15–17 Of note, we may have missed some MOG-associated diagnoses due the low frequency of MOG testing.
Although this study is the first prospective study of visual outcomes in pediatric ON, the study has limitations, including a large number of participants who did not complete their 2-year follow-up visit and a lower enrollment than was initially planned due to poor recruitment. This resulted in some results that are difficult to interpret, with wide confidence intervals. The participants in the non-completer group had a higher proportion of isolated ON although it is possible that with longer follow-up, an associated neurologic diagnosis may have been made. In addition, there was a non-statistically significant difference in the enrollment VA between the completer and non-completer groups, with a better point estimate of enrollment VA in those who completed the two-year visit. Therefore, it is possible that our two-year results may be biased towards a better presenting VA compared to the population at large. Despite these limitations, our study provides data on the long-term visual and neurologic prognosis of children with ON, and we found that the majority had marked improvement in VA by 6 months. These data also provide insights on the risk of ON recurrence and development of associated neurologic autoimmune disorders. Future studies of pediatric ON should consider the recruitment and retention difficulties experienced in PON1 and adjust their enrollment goals accordingly. Furthermore, our results suggest that the presence of certain associated neurologic syndrome may affect visual prognosis. Future studies should evaluate treatment regimens to minimize systemic and visual comorbidities.
Supplementary Material
Acknowledgements
Funding Statement:
Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health, under Award Numbers EY011751, EY018810, and EY023198. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Meeting Presentations:
This manuscript has been presented in part at the North American Neuro Ophthalmology Society Annual Meeting (February 22, 2021), and the American Association for Pediatric Ophthalmology and Strabismus Annual Meeting (April 9, 2021).
Funding/Support:
Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751, EY023198, and EY018810. The funding organization had no role in the design or conduct of this research.
Footnotes
Access to Data: Robert Henderson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical Sites
Sites are listed in order by number of participants enrolled (in parenthesis).
Personnel are listed as (I) for Investigator, (C) for Coordinator or (E) for Examiner.
Los Angeles, CA - Children’s Hospital Los Angeles (5)
Mark S. Borchert (I); Melinda Y. Chang (I); Dilshad Contractor (C); Emily J. Zolfaghari (C); Aarti Vyas (E); Tiffany Yuen (E)
Houston, TX - Texas Children’s Hospital – Department of Ophthalmology (4)
Veeral S. Shah (I); Evelyn A. Paysse (I); Gihan Romany (C)
Atlanta, GA - The Emory Eye Center (3)
Jason H. Peragallo (I); Judy L. Brower (C)
Boston, MA - Boston Children`s Hospital (3)
Aparna Raghuram (I); Gena Heidary (I); Bilal AlWattar (C); Ryan Chinn (C); Srishti Kothari (C)
Oklahoma City, OK - Dean A. McGee Eye Institute, University of Oklahoma (3)
R.Michael Siatkowski (I); Janine E. Collinge (I); Maria E. Lim (I); Alisha N. Brewer (C);
Annette M. Doughty (C); Sonny W. Icks (C); Shannon
Almeida (E)
San Francisco, CA – University of California San Francisco Department of Ophthalmology (3)
Alejandra de Alba Campomanes (I); Premilla Banwait (I); Jennifer S. Graves (I); Leila
Hajkazemshirazi (I); Yizhuo Bastea-Forte (C); Jennifer K.
Arjona (C); Jeremy Chen (C); Karen Cooper (E)
St. Louis, MO - Saint Louis University Institute (3)
Rafif Ghadban (I); Sophia M. Chung (I); Oscar A. Cruz (I); Sangeeta Khanna (I); Traci A.
Christenson (C); Lisa L. Breeding (C); Dawn M.
Govreau (C); Beth A. Wallis (C)
Grand Rapids, MI - Helen DeVos Children’s Hospital Pediatric Ophthalmology (2)
Brooke E. Geddie (I); Julie A. Conley (I); Elisabeth T. Wolinski (C)
Lisle, IL - Progressive Eye Care (2)
Patricia L. Davis (I); Indre M. Rudaitis (I); Jacqueline Twite (C); Carrie S. Bloomquist (E);
Sarah R. Laboy (E); Jackie M. Twite (E)
Los Angeles, CA - Jules Stein Eye Institute at the University of California, Los Angeles (2)
Stacy L. Pineles (I); Melinda Y. Chang (I); Michelle V. Doan (C); Marianne J. Bernardo (C)
Rochester, MN - Mayo Clinic (2)
Michael C. Brodsky (I); John J. Chen (I); Jonathan M. Holmes (I); Suzanne M. Wernimont (C);
Lindsay L. Czaplewski (E); Stacy L. Eastman
(E); Moriah A. Keehn (E); Debbie M. Priebe (E)
Columbus, OH - Pediatric Ophthalmology Associates, Inc. (1)
Don L. Bremer (I); Richard P. Golden (I); Catherine O. Jordan (I); Mary Lou McGregor (I);
Rachel E. Reem (I); David L. Rogers (I); Amanda N.
Schreckengost (C); Sara A. Maletic (C)
Durham, NC - Duke University Eye Center (1)
Mays A. Dairi (I); Laura B. Enyedi (I); Sarah K. Jones (C); Navajyoti R. Barman (C); Robert J.
House (C); David A. Nasrazadani (C)
Kansas City, MO - Children’s Mercy Hospitals and Clinics (1)
Sean M. Gratton (I); Justin D. Marsh (I); Rebecca J. Dent (C); Lezlie L. Bond (C); Lori L. Soske (C)
Lexington, KY - University of Kentucky Department of Neurology (1)
Padmaja Sudhakar (I); Christi M. Willen (I); Deborah Taylor (C); Nathaniel Q. Moliterno (C); Michael Nsoesie (C); Shaista Vally (E)
Little Rock, AR - Arkansas Childrens Hospital/ University of Arkansas Medical Sciences (1)
Paul H. Phillips (I); Robert S. Lowery (I); Beth Colon (C); Nancy L. Stotts (C); Kelly D. To (C)
Minneapolis, MN - University of Minnesota-Minnesota Lions Children’s Eye Clinic (1)
Collin M. McClelland (I); Raymond G. Areaux (I); Ann M. Holleschau (C); Kim S. Merrill (E)
Montreal, Quebec, Canada - Centre Hospitalier Universitaire - Sainte-Justine (1)
Luis H. Ospina (I); Rosanne Superstein (I); Maryse Thibeault (C); Helene Gagnon (C)
Nashville, TN - Vanderbilt University Medical Center (1)
Sean P. Donahue (I); Scott T. Ruark (C); Lisa A. Fraine (C); Petrice A. Sprouse (C); Ronald J. Biernacki (E)
Philadelphia, PA - Children’s Hospital of Philadelphia (1)
Grant T. Liu (I); Robert A. Avery (I); Brian J. Forbes (I); Imran Jivraj (I); Anita A. Kohli (I); Meg M. Richter (C); Agnieshka Baumritter (C)
Pittsburgh, PA - University of Pittsburgh Medical Center- Children`s Eye Center of
Children`s Hospital of Pittsburgh (1)
Ellen B. Mitchell (I); Ken K. Nischal (I); Lauren M. Runkel (C); Bianca Blaha (E); Whitney
Churchfield (E); Christina Fulwylie (E)
Syracuse, NY – State University of New York Upstate Medical University (1)
Melissa W. Ko (I); Luis J. Mejico (I); Muhammad Iqbal (C); Catherine E. Attanasio (C); Lena F.
Deb (C); Courtney B. Goodrich (C); Alisha M.
Hartwell (C); Jennifer A. Moore (C)
West Bloomfield, MI - Children`s Eye Care PC (1)
Lisa Bohra (I); Alexandra O. Apkarian (I); Elena M. Gianfermi (I); John D. Roarty (I); Leemor
B. Rotberg (I); Susan N. Perzyk (C)
PEDIG Coordinating Center - Tampa, FL
Raymond T. Kraker, Roy W. Beck, Darrell S. Austin, Nicole M. Boyle, Danielle L. Chandler, Patricia L. Connelly, Courtney L. Conner, Trevano W. Dean, Quayleen Donahue, Brooke P. Fimbel, Robert J. Henderson, Amra Hercinovic, James E. Hoepner, Joseph D. Kaplon, Zhuokai Li, Gillaine Ortiz, Julianne L. Robinson, Kathleen M. Stutz, David O. Toro, Victoria C. Woodard, Rui Wu.
Pediatric Optic Neuritis Planning Committee
Stacy L. Pineles (lead), Michael X. Repka (lead), Laura Balcer, Roy W. Beck, Mark S. Borchert, Sean P. Donahue, Gena Heidary, Raymond T. Kraker, Mark Kupersmith, Elizabeth L. Lazar, Grant T. Liu, Paul H. Phillips, Amy Waldman, David K. Wallace
PEDIG Executive Committee
Susan A. Cotter (Co-chair), Jonathan M. Holmes (Co-chair), Roy W. Beck, Eileen E. Birch, Angela M. Chen (2017–2018), Stephen P. Christiansen, Laura B. Enyedi (2014–2016), S. Ayse Erzurum (2016–2017), Donald F. Everett, Sharon F. Freedman (2016–2018), William V. Good (2017-present), Raymond T. Kraker, Katherine A. Lee (2014–2016), Richard London, Vivian M. Manh (2016–2018), Ruth E. Manny, David G. Morrison (2018-present), Michael X. Repka, Scott T. Ruark, Bonita R. Schweinler (2016–2018), Jayne L. Silver (2014–2016), Lisa C. Verderber (2015–2017), David K. Wallace, Katherine K. Weise.
Pediatric Optic Neuritis Steering Committee
Stacy L. Pineles (lead), Michael X. Repka (lead), Laura Balcer, Mark S. Borchert, Sean P. Donahue, Ari Green, Gena Heidary, Raymond T. Kraker, Mark Kupersmith, Elizabeth L. Lazar, Grant T. Liu, Paul H. Phillips, Amy Waldman, David K. Wallace
National Eye Institute - Bethesda, MD
Donald F. Everett
Data and Safety Monitoring Committee
Marie Diener-West (chair), John D. Baker, Barry Davis, Dale L. Phelps, Stephen W. Poff,
Richard A. Saunders, Lawrence Tychsen
A list of participating sites appears as an Appendix
An address for reprints will not be provided.
Conflict of Interest: No conflicting relationship exists for any author
Conflict of Interest: The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Liu GT, Volpe NJ, Galetta SL. Liu, Volpe and Galetta’s Neuro-ophthalmology: diagnosis and management. London: Elsevier Saunders; 2019. [Google Scholar]
- 2.O’Mahony J, Marrie RA, Laporte A, et al. Recovery from central nervous system acute demyelination in children. Pediatrics. 2015;136(1):e115–123. [DOI] [PubMed] [Google Scholar]
- 3.Pineles SL, Liu GT, Waldman AT, Lazar E, Kupersmith MJ, Repka MX. Pediatric Optic Neuritis Prospective Outcomes Study. J Neuroophthalmol. 2016;36(2):115–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Committee for the Pediatric Eye Disease Investigator Group. Assessment of Pediatric Optic Neuritis Visual Acuity Outcomes at 6 Months. JAMA Ophthalmology. 2020;138(12):1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan Y, Tarczy-Hornoch K, Cotter SA, et al. Visual acuity norms in pre-school children: the Multi-Ethnic Pediatric Eye Disease Study. Optom Vis Sci. 2009;86(6):607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drover JR, Felius J, Cheng CS, Morale SE, Wyatt L, Birch EE. Normative pediatric visual acuity using single surrounded HOTV optotypes on the Electronic Visual Acuity Tester following the Amblyopia Treatment Study protocol. J AAPOS. 2008;12(2):145149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes JM, Beck RW, Repka MX, et al. The Amblyopia Treatment Study visual acuity testing protocol. Arch Ophthalmol. 2001;119(9):1345–1353. [DOI] [PubMed] [Google Scholar]
- 8.Cotter SA, Chu RH, Chandler DL, et al. Reliability of the Electronic Early Treatment Diabetic Retinopathy Study testing protocol in children 7 to <13 years old. Am J Ophthalmol. 2003;136(4):655–661. [DOI] [PubMed] [Google Scholar]
- 9.Chen JJ, Pineles SL, Repka MX, et al. MOG-IgG Among Participants in the Pediatric Optic Neuritis Prospective Outcomes Study. JAMA Ophthalmol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. Journal of youth and adolescence. 1980;9(3):271–280. [DOI] [PubMed] [Google Scholar]
- 11.Waldman AT, Yeshokumar AK, Lavery A, et al. Validation of a symptom-based questionnaire for pediatric CNS demyelinating diseases. J AAPOS. 2019;23(3):157.e151157.e157. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada T, Hatt SR, Leske DA, et al. A new computer-based pediatric vision-screening test. J AAPOS. 2015;19(2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldman AT, Stull LB, Galetta SL, Balcer LJ, Liu GT. Pediatric optic neuritis and risk of multiple sclerosis: meta-analysis of observational studies. J AAPOS. 2011;15(5):441446. [DOI] [PubMed] [Google Scholar]
- 15.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry. 2015;86(3):265272. [DOI] [PubMed] [Google Scholar]
- 16.Chen JJ, Flanagan EP, Jitprapaikulsan J, et al. Myelin Oligodendrocyte Glycoprotein Antibody-Positive Optic Neuritis: Clinical Characteristics, Radiologic Clues, and Outcome. Am J Ophthalmol. 2018;195:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Zhao G, Huang Y, et al. Clinical Characteristics of Pediatric Optic Neuritis With Myelin Oligodendrocyte Glycoprotein Seropositive: A Cohort Study. Pediatr Neurol. 2018;83:42–49. [DOI] [PubMed] [Google Scholar]
- 18.Fujihara K, Palace J. Neuroimmunology: towards more-accurate diagnosis in neuromyelitis optica. Nature reviews Neurology. 2014;10(12):679–681. [DOI] [PubMed] [Google Scholar]
- 19.Absoud M, Lim MJ, Appleton R, et al. Paediatric neuromyelitis optica: clinical, MRI of the brain and prognostic features. J Neurol Neurosurg Psychiatry. 2015;86(4):470–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.