Abstract
Introduction
Metrics for posttransplant immune monitoring to prevent over or under immunosuppression in renal transplant recipients (RTRs) are lacking.
Methods
We surveyed 132 RTRs, 38 in the first year posttransplant and 94 >1-year posttransplant, to study the clinical expression of immunosuppressive therapy. A questionnaire administered to these RTRs was divided into physical (Q physical) and mental (Q mental) symptoms.
Results
In multivariable models for the association between the calculated Q physical and Q mental scores and different clinical and biochemical variables in the 38 RTRs who filled out the questionnaire 130 times during the first year posttransplant, it was found that mycophenolic acid (MPA) and prednisone use increased the mean Q physical score by 0.59 (95% CI: 0.21–0.98, p = 0.002) and 0.53 (95% CI: 0.26–0.81, p = 0.00), respectively, while MPA use increased the mean Q mental score by 0.72 (95% CI: 0.31–1.12, p = 0.001). Among the 94 RTRs who each completed the questionnaire only once, the odds for the mean Q mental score to be above the median value were more than 3 times higher for RTRs treated versus non-treated with MPA (OR 3.38, 95% CI: 1.1–10.3, p = 0.03). MPA-treated RTRs had higher mean scores for questions related to sleep disorders (1.83 ± 1.06 vs. 1.32 ± 0.67 for not treated, p = 0.037), to difficulty falling asleep (1.72 ± 1.11 vs. 1.16 ± 0.5, p = 0.02), and to depression and anxiety.
Conclusion
We concluded that prednisone and MPA use are associated with an increased Q physical and Q mental scores in RTRs. Routine monitoring of physical and mental status of RTRs should be implemented to improve the diagnosis of overimmunosuppression. Dose reduction or discontinuation of MPA should be considered in RTRs who report sleep disorders, depression, and anxiety.
Keywords: Renal transplant recipients, Overimmunosuppression, Immunosuppressive therapy
Introduction
Renal transplant recipients (RTRs) receive combination immunosuppressive therapy throughout the life of the renal allograft to prevent rejection. Inhibition of the immune system is, however, not specific to the renal allograft, resulting in many adverse effects secondary to intense immunosuppression. Among these side effects, the long-term use of steroids can lead to hypertension, hyperlipidemia, the appearance of new diabetes after transplant, the loss of bone mineral density, and mood swings [1–3]. In parallel, long-term use of other drugs in immunosuppression regimes exacerbate the RTR’s susceptibility to malignancies and infections, including opportunistic bacterial, fungal, and viral infections. For example, calcineurin inhibitors (CNIs), mycophenolic acid (MPA), and lymphodepleting agents were found to increase the incidence of cytomegalovirus and BK virus infections [4–8]. Immunosuppression has also been shown to lead to the development of malignancies, such as skin cancer [9].
Determining the type and intensity of immunosuppressive therapy is based on an assessment of the patient’s immunological risk, i.e., the risk of rejection versus infection and malignancy. The goal is therefore to personalize immunosuppressive therapy, thereby preventing excess or deficient immunosuppression [10, 11]. However, there is currently no universally accepted approach to assessing individual patient risk profiles, although some previous studies have indeed investigated risk factors for rejection [12, 13].
As mentioned above, posttransplant immune monitoring is necessary to prevent a state of over or inadequate immunosuppression, but an integrated immune-monitoring approach, including relevant side effects of immunosuppressive therapy, is currently not available. The follow-up tools currently available in clinical practice for monitoring the intensity of immunosuppression are based mainly on blood and urine tests. These tests include monitoring blood for CNI 12-h trough levels and the appearance of antihuman leukocyte antigen antibodies [14] and BK viremia [15], assessing renal allograft function and monitoring urine for albumin and protein. Other tests include blood testing for donor-derived cell-free DNA [16] and more invasive tests, such as renal allograft biopsy.
We believe that a better understanding of the clinical manifestations of the immunosuppressive therapy administered to RTRs can complement existing laboratory immune-monitoring tools so as to improve the diagnosis of excess or deficient immunosuppression. Our working hypothesis in this study was that the level of immune suppression is linked to the severity of patients’ symptoms, which may include sleep disorders, restlessness, general weakness, anxiety, depression, and joint pain, among others. This study seeks to elucidate the association between different clinical symptoms and the type and intensity of immunosuppressive therapy aiming to improve immune-monitoring in RTRs.
Methods
Study Population and Design
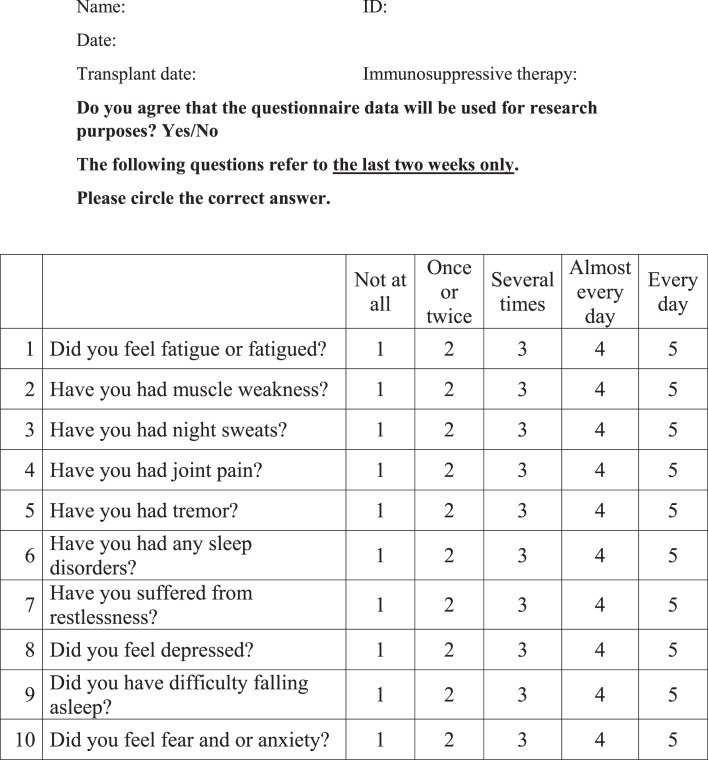
On a routine follow-up visit to the out-patient RTR clinic at the Sheba Medical Center, RTRs were asked to complete a questionnaire regarding their symptoms, after giving consent to participate in the study (Fig. 1). The questionnaire consists of 10 questions for which the answers reflect the level of severity of symptoms in the range of 1–5 (low to high) in the 2 weeks prior to completing the questionnaire. Questions 1–5 refer to the physical status of the transplant recipient (fatigue, muscle weakness, night sweats, joint pain, and tremor), while questions 6–10 refer to the mental state of the transplant recipient (sleep disorders, difficulty falling asleep, restlessness, depression, fear, and anxiety). For each questionnaire, mean Q physical and mean Q mental scores were calculated based on the means of answers to questions 1–5 and 6–10, respectively. On the same day that the questionnaire was completed, patients were weighed (physical index) and blood was drawn for laboratory tests. The reliability of the questionnaire was tested by Cronbach’s-alpha index for internal consistency between questions reflecting similar characteristics.
Fig. 1.
Questionnaire.
One hundred and ninety-two RTRs completed the questionnaire. Excluded from the analysis were transplant recipients with active rejection, infection, and/or malignancy, recipients who received maintenance immunosuppressive therapy other than a regimen comprising tacrolimus, MPA, and prednisone (e.g., cyclosporine, mammalian target of rapamycin inhibitors, or azathioprine) or any treatment for anxiety and/or depression, and recipients with an estimated glomerular filtration rate (eGFR) < 30 mL/min or with C-reactive protein > 30 mg/L. To ensure the questions were well understood, only Hebrew-speaking transplant recipients were asked to fill in the questionnaire.
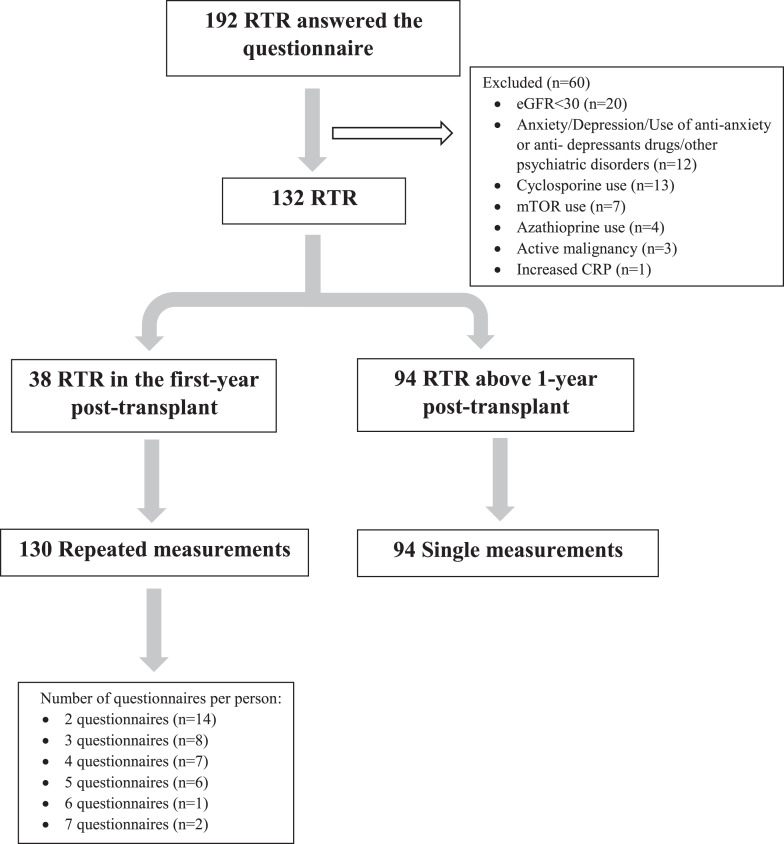
The final study cohort included 132 RTRs, of whom 38 were in the first year posttransplant and 94 were >1-year posttransplant. Given the frequent changes in immunosuppressive therapy early posttransplant and the possible effect of these changes on the physical and mental status of RTRs, the study design allowed patients in the first year posttransplant to fill out the questionnaire several times as long as at least 2 weeks had passed since the last time they did so. The 38 RTRs in the first year posttransplant completed the questionnaire between 2 and 7 times, with a total of 130 repeated measurements. The 94 RTR who were >1-year posttransplant each filled out the questionnaire only once (Fig. 2). The study was approved by our Institutional Review Board (SMC-7053-20).
Fig. 2.
Consort diagram.
Immunosuppression
At our medical center, the standard maintenance immunosuppression regimen for RTRs comprises a CNI (usually tacrolimus), an antimetabolite (usually a mycophenolate-based drug, mainly MPA), and prednisone, as described previously [17]. For RTRs with a low-immunological risk of rejection, early steroid withdrawal is implemented 5–8 days after transplant, and the maintenance regimen thus consists of tacrolimus and MPA. Conversion to an mammalian target of rapamycin inhibitor (sirolimus or everolimus) is instituted according to the patient’s risk of malignancy and lack of tolerance to CNIs.
Primary Outcome
The primary outcome was defined as mean Q physical and Q mental scores below or above the median score of RTR population tested.
Data Extraction and Study Assessments
Patient information on the day of questionnaire completion was obtained from electronic patient records and included: age, gender, weight, time from kidney transplantation, etiology of end-stage renal disease (ESRD), dialysis pretransplant (yes/no), transplant number, donor type, and relevant medical history, specifically a history of hypertension, congestive heart failure, ischemic heart disease, diabetes, and smoking (yes/no). In addition, information on the types and daily doses of all immunosuppressive medications the patient was taking on the day of questionnaire completion was collected. For patients treated with mycophenolate, the total daily mycophenolate dose was converted to the equivalent MPA dose by dividing the mycophenolate dose by 1.388. Laboratory results obtained on the day of clinic visit were tacrolimus trough blood level, hemoglobin level, white blood cell count, total lymphocytes, total neutrophils, globulins, albumin, C-reactive protein, and creatinine. eGFR was calculated based on the CKD-EPI equation.
Statistical Analysis
Categorical variables were described as frequencies and percentages. Continuous variables were evaluated for normal distribution using histograms and reported as means and standard deviations, or medians and interquartile ranges. Since the physical and mental scores were not normally distributed, they were categorized into two categories using the median as the cut-off value. Differences in baseline characteristics between the groups were tested using the χ2 or Fisher’s exact test for the categorical variables, and the independent sample t test or the Mann-Whitney tests were applied to compare continuous variables.
In the repeated-measurements study, a logistic mixed model was used for the univariate and multivariable analysis. In further analysis, the scores were analyzed as continuous variables using interaction terms between MPA level and other treatments.
In the single-measurements study, χ2 and Fisher’s exact tests were used to compare categorical variables, while the independent sample t test or the Mann-Whitney test was applied to compare continuous and ordinal variables. All statistical tests were two-sided and a p value <0.05 was considered statistically significant. SPSS software was used for all statistical analyses (IBM SPSS Statistics for Windows, Version 25.0 IBM Corp. Released 2017. Armonk, NY: IBM Corp.).
Results
First Year Posttransplant RTRs – Repeated Measurements
Clinical and Biochemical Characteristics
For the 38 first year posttransplant RTRs, mean age at completion of the questionnaire was 52.1 (±12.7) years; 31 (81.6%) of the patients were men; 32 (84.2%) had a living donor; and 29 (76.3%) were treated with dialysis pretransplant. The cause of ESRD was nephrosclerosis in 12 (31.6%), polycystic kidney disease in 12 (26.3%), and diabetic nephropathy in 10 (26.3%) of the RTRs. A medical history of hypertension, diabetes, and ischemic heart disease was recorded for 34 (89.5%), 13 (34.2%), and 10 (26.3%) of the 38 RTRs, respectively. The number of questionnaire completions per patient ranged from 2 to 7, with 14 (36.8%) RTRs filling out the questionnaire twice and 2 (5.3%) filling it out 7 times. Other clinical characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the cohort of 38 RTRs in the first year posttransplant – repeated measurements
| Patient characteristics | |
|---|---|
| Gender, n (%) | |
| Male | 31 (81.6) |
| Female | 7 (18.4) |
| Weight, mean (SD), kg | 81.7 (16.35) |
| Age at questionnaire, mean (SD), years | 52.11 (12.67) |
| Time posttransplantation, median (IQR), months | 4.8 (1.9–6.3) |
| Dialysis pretransplantation – yes, n (%) | 29 (76.3) |
| Transplant number, n (%) | |
| 1 | 35 (92.1) |
| 2 | 1 (2.6) |
| 3 | 2 (5.3) |
| Donor type, n (%) | |
| Living | 32 (84.2) |
| Deceased | 6 (15.8) |
| ESRD cause, n (%) | |
| Diabetic nephropathy | 10 (26.3) |
| Nephrosclerosis | 12 (31.6) |
| PCKD | 10 (26.3) |
| Glomerulonephritis | 3 (7.9) |
| Other | 3 (7.9) |
| Medical history, n (%) | |
| HTN | 34 (89.5) |
| Diabetes | 13 (34.2) |
| Smoking | 2 (5.3) |
| IHD | 10 (26.3) |
| CHF | 1 (2.6) |
| Number of questionnaires per person, n (%) | |
| 2 | 14 (36.8) |
| 3 | 8 (21.1) |
| 4 | 7 (18.4) |
| 5 | 6 (15.8) |
| 6 | 1 (2.6) |
| 7 | 2 (5.3) |
CHF, congestive heart failure; ESRD, end-stage renal disease; HTN, hypertension; IHD, ischemic heart disease; PCKD, polycystic kidney disease; RTR, renal transplant recipient; SD, standard deviation.
Questionnaire Results
Table 1 in the online supplementary file presents the median score for the whole questionnaire (Q general; 1.4 [IQR, 1.175–1.825]). The table also gives the median scores for Q physical and Q mental (1.4 [IQR, 1–1.8] and 1.2 [IQR, 1–1.8], respectively) (for all online suppl. material, see https://doi.org/10.1159/000530855).
Univariate Logistic Regression for the Association between Q Physical Score above the Median Score and Different Predictors, Adjusted for Time Posttransplant
The odds for the Q physical score to be above the median score were almost 4 times higher in female RTRs (odds ratio [OR] 3.96, 95% confidence interval [CI]: 1.09–14.44, p = 0.037). In addition, for every increase of 1 mg in the prednisone daily dose, the odds for the Q physical score to be above the median score were 8% higher (OR 1.08, 95% CI: 1.01–1.15, p = 0.017) (Table 2A).
Table 2.
Association between Q physical score or Q mental score (above the median score) and different predictors adjusted for time posttransplantation (months) – repeated measurements (N = 130)
| A. Q physical score |
| OR (95% CI) | p value | |
|---|---|---|
| Patient characteristics | ||
| Gender – female | 3.96 (1.09–14.44) | 0.037* |
| Weight (kg) | 1.00 (0.97–1.04) | 0.872 |
| Age at questionnaire (years) | 0.99 (0.95–1.03) | 0.491 |
| Time posttransplantation (months) | 1.03 (0.86–1.23) | 0.712 |
| Dialysis pretransplantation | 0.94 (0.27–3.24) | 0.926 |
| Medical history | ||
| HTN | 0.77 (0.19–3.05) | 0.711 |
| Diabetes | 0.67 (0.21–2.15) | 0.506 |
| IHD | 0.50 (0.14–1.71) | 0.266 |
| Medication use | ||
| MPA (yes) | 2.96 (0.48–18.18) | 0.242 |
| MPA dose (mg/day) | ||
| Off MPA | 1 | 0.111 |
| 360–720 mg | 4.53 (0.73–28.30) | |
| ≥1,080 mg | 2.01 (0.30–13.51) | |
| Prednisone (yes) | 2.82 (0.78–10.13) | 0.112 |
| Prednisone dose (mg/day) | ||
| Off prednisone | 1 | 0.117 |
| 1–10 mg | 2.26 (0.53–9.61) | |
| ≥11 mg | 4.25 (1.08–16.69) | |
| Medication doses | ||
| Tacrolimus dose (for every increase of 1 mg/day) | 0.99 (0.88–1.11) | 0.843 |
| MPA dose (for every increase of 1 mg/day) | 1.00 (0.10–1.00) | 0.825 |
| Prednisone dose (for every increase of 1 mg/day) | 1.08 (1.01–1.15) | 0.017* |
| Laboratory results | ||
| WBC (K/μL) | 0.87 (0.71–1.06) | 0.173 |
| Absolute neutrophils (K/μL) | 0.87 (0.68–1.12) | 0.274 |
| Absolute lymphocytes (K/μL) | 0.72 (0.33–1.58) | 0.416 |
| Hb (g/dL) | 1.09 (0.72–1.64) | 0.677 |
| Scr (mg/dL) | 0.12 (0.01–1.18) | 0.069 |
| eGFR (mL/min) | 1.05 (1.01–1.09) | 0.016 |
| Tacrolimus blood level (ng/mL) | 0.90 (0.79–1.04) | 0.151 |
| CRP (mg/L) | 0.95 (0.86–1.04) | 0.263 |
| Albumin (g/dL) | 4.76 (0.94–24.02) | 0.059 |
| Globulins (g/dL) | 1.21 (0.38–3.87) | 0.75 |
| B. Q mental score |
| OR (95% CI) | p value | |
|---|---|---|
| Patient characteristics | ||
| Gender – female | 0.78 (0.14–4.37) | 0.777 |
| Weight (kg) | 1.03 (0.99–1.07) | 0.17 |
| Age at questionnaire (years) | 1.01 (0.97–1.06) | 0.654 |
| Time posttransplantation (months) | 1.02 (0.85–1.23) | 0.802 |
| Dialysis pretransplantation | 0.63 (0.16–2.44) | 0.507 |
| Medical history | ||
| HTN | 0.99 (0.20–4.94) | 0.996 |
| Diabetes | 1.20 (0.36–4.00) | 0.768 |
| IHD | 1.50 (0.36–6.21) | 0.572 |
| Medication use | ||
| MPA (yes) | 3.79 (0.90–15.88) | 0.068 |
| MPA (mg/day) | ||
| Off MPA | 1 | 0.143 |
| 360–720 mg | 2.86 (0.61–13.32) | |
| ≥1,080 mg | 4.92 (1–24.18) | |
| Prednisone (yes) | 0.89 (0.20–3.85) | 0.876 |
| Prednisone dose (mg/day) | ||
| Off prednisone | 1 | 0.98 |
| 1–10 mg | 0.91 (0.18–4.59) | |
| ≥11 mg | 0.86 (0.19–3.84) | |
| Medication dose | ||
| Tacrolimus dose (for every increase of 1 mg/day) | 0.92 (0.81–1.06) | 0.25 |
| MPA dose (for every increase of 1 mg/day) | 2.63 (0.99–6.99) | 0.053 |
| Prednisone dose (for every increase of 1 mg/day) | 1.01 (0.94–1.09) | 0.768 |
| Laboratory results | ||
| WBC (K/μL) | 0.93 (0.75–1.14) | 0.469 |
| Absolute neutrophils (K/μL) | 0.93 (0.72–1.20) | 0.584 |
| Absolute lymphocytes (K/μL) | 0.99 (0.41–2.44) | 0.994 |
| Hb (g/dL) | 1.01 (0.63–1.63) | 0.958 |
| Scr (mg/dL) | 0.97 (0.12–7.82) | 0.98 |
| eGFR (mL/min) | 1.01 (0.97–1.04) | 0.681 |
| Tacrolimus blood level (ng/mL) | 0.73 (0.61–0.87) | 0.001** |
| CRP (mg/L) | 0.98 (0.89–1.08) | 0.679 |
| Albumin (g/dL) | 0.53 (0.10–2.96) | 0.472 |
| Globulins (g/dL) | 1.12 (0.26–4.83) | 0.877 |
CI, confidence interval; CRP, C-reactive protein; Hb, hemoglobin; HTN, hypertension; IHD, ischemic heart disease; MPA, mycophenolic acid; OR, odds ratio; Scr, serum creatinine; WBC, white blood cells.
Numbers in bold indicate significant at *p < 0.05.
Numbers in bold indicate significant at **p < 0.01.
Univariate Logistic Regression for the Association between Q Mental Score above the Median Score and Different Predictors, Adjusted for Time Posttransplant
For MPA use and an increase in MPA dose, the odds for the mean Q mental to be above the median score increased (OR 3.79, 95% CI: 0.9–15.9, p = 0.068 and OR 2.63, 95% CI: 0.99–6.99, p = 0.053 respectively), but the increases were not significant (as CI crosses 1). For every increase in tacrolimus blood level of 1 mg/mL, the odds for Q mental to be above the median score were 27% lower (OR 0.73, 95% CI: 0.61–0.87, p = 0.001) (Table 2B).
Multivariable Logistic Regression Models for the Association between Mean Q Physical and Mental Scores above the Median Score and Different Predictors, Adjusted for Time Posttransplant
In a multivariable model adjusted for age, gender, time posttransplant, dialysis pretransplant, diabetes, treatment with MPA and prednisone, tacrolimus blood level and absolute lymphocyte count, the odds for mean Q physical to be above the median score were more than 5 times higher in female RTRs (OR 5.47, 95% CI: 1.13–26.5, p = 0.035) and 7 times higher in RTRs treated with prednisone versus non-prednisone treated (OR 7, 95% CI: 2.38–20.61, p = 0.00). In a multivariable model adjusted for the same variables, the odds for mean Q mental to be above the median score were more than 4 times higher in RTRs treated with MPA compared to non-treated (OR 4.38, 95% CI: 1.21–15.8, p = 0.024). Each 1 ng/mL increase in tacrolimus blood level reduced the odds for mean Q mental to be above the median score by 29% (OR 0.71, 95% CI: 0.59–0.86, p = 0.00) (data not shown).
Multivariable Linear Regression Model for the Association between Mean Q Physical Score and the Different Variables Tested
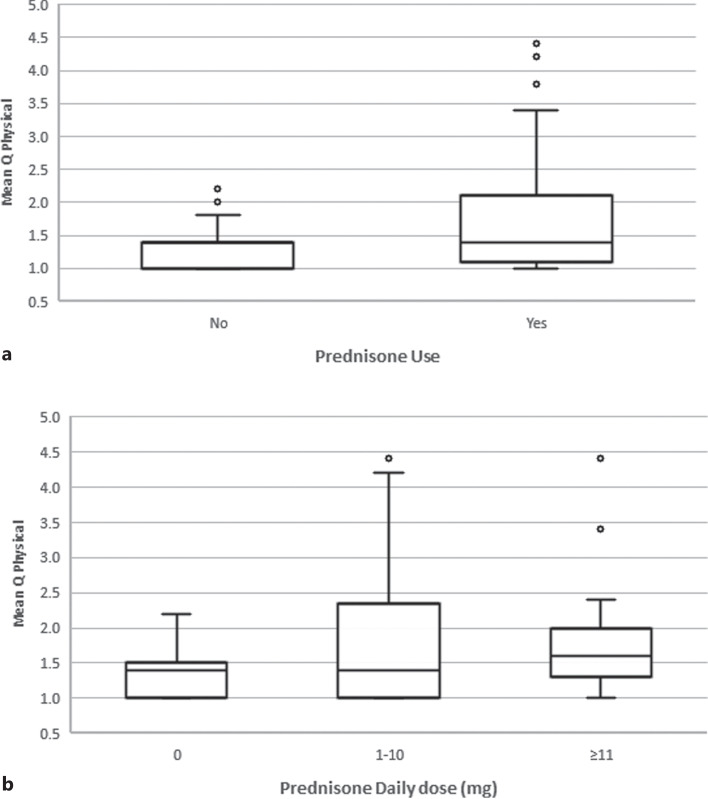
In a multivariable model adjusted for age, gender, time posttransplant, dialysis pretransplant, diabetes, treatment with MPA and prednisone, tacrolimus blood level and absolute lymphocyte count, independent predictors for the Q physical score were found to be female gender, MPA and prednisone use and tacrolimus blood level. The mean Q physical score was 0.47 higher for women than for men (95% CI: 0.03–0.9, p = 0.036). In RTRs receiving MPA or prednisone, compared with patients not taking those drugs, the mean score increased by 0.59 (95% CI: 0.21–0.98, p = 0.002) and 0.53 (95% CI: 0.26–0.81, p = 0.00), respectively (Fig. 3). Each 1 ng/mL increase in tacrolimus blood level lowered the mean score by 0.038 (95% CI: [−] 0.07 – [−] 0.01, p = 0.013) (Table 3A).
Fig. 3.
Box plot diagrams for mean Q physical score for 38 RTRs in the first year posttransplant on and off prednisone treatment (a), and divided by prednisone daily dose (b).
Table 3.
Multivariable model for the association between mean Q physical score or mean Q mental score and the different variables tested.
| A. Mean Q physical score |
| Effect | Mean (95% CI) | p value |
|---|---|---|
| Gender (female vs. male) | 0.47 (0.03–0.90) | 0.036* |
| Age at questionnaire (for every increase of 1 year) | (−0.001) [(−0.01)–0.01] | 0.926 |
| Time posttransplantation (for every increase of 1 month) | 0.01 (−0.06–0.08) | 0.704 |
| Dialysis pretransplantation (yes/no) | (−0.1) [(−0.49)–0.29] | 0.616 |
| Diabetes (yes/no) | 0.08 (−0.35–0.51) | 0.714 |
| Medication use (yes/no) | ||
| MPA | 0.59 (0.21–0.98) | 0.002** |
| Prednisone | 0.53 (0.26–0.81) | 0.000** |
| Laboratory results | ||
| Absolute lymphocytes (per 1 K/μL increase) | (−0.015) [(−0.24)–0.21) | 0.899 |
| Tacrolimus blood level (per 1 ng/mL increase) | (−0.038) [(−0.07)–(−0.01)] | 0.013* |
| B. Mean Q mental score |
| Effect | Mean (95% CI) | p value |
|---|---|---|
| Gender (female vs. male) | 0.22 [(−0.43)–0.86)] | 0.510 |
| Age at questionnaire (for every increase of 1 year) | 0.01 [(−0.01)–0.03] | 0.149 |
| Time posttransplantation (for every increase of 1 month) | 0.01 [(−0.07)–0.09] | 0.823 |
| Dialysis pretransplantation (yes/no) | 0.01 [(−0.5)–0.52] | 0.957 |
| Diabetes (yes/no) | 0.1 [(−0.37)–0.57] | 0.672 |
| Medication use (yes/no) | ||
| MPA | 0.72 (0.31–1.12) | 0.001** |
| Prednisone | 0.25 [(−0.27)–0.77] | 0.346 |
| Laboratory results | ||
| Absolute lymphocytes (per 1 K/μL increase) | (−0.04) [(−0.29)–0.2)] | 0.722 |
| Tacrolimus blood level (per 1 ng/mL increase) | (−0.07) [(−0.11)–(−0.03)] | 0.001** |
CI, confidence interval; MPA, mycophenolic acid.
Numbers in bold indicate significant at *p < 0.05; **p < 0.01.
Multivariable Linear Regression Model for the Association between Mean Q Mental Score and the Different Variables Tested
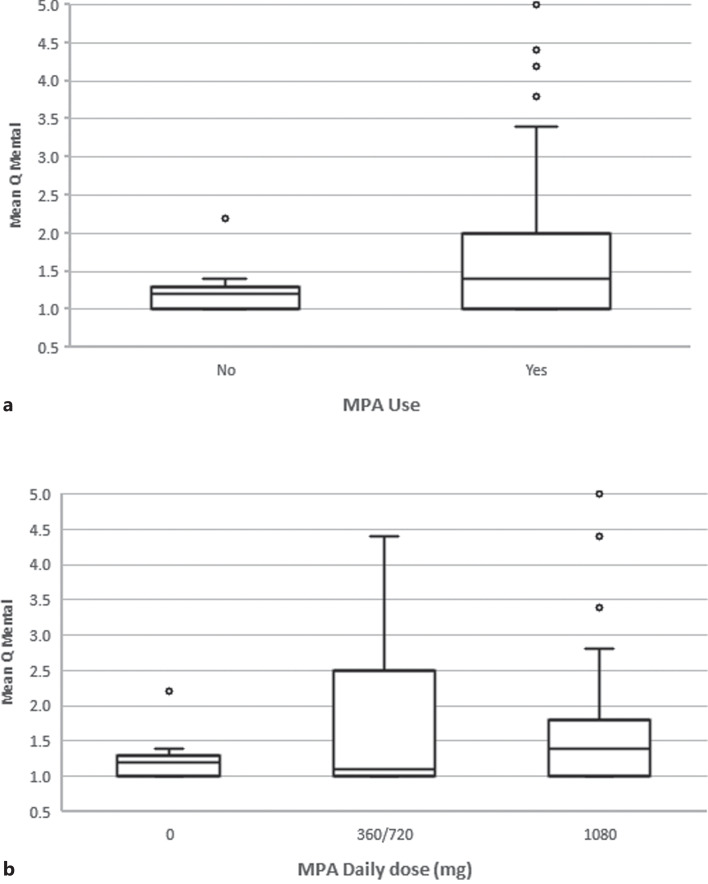
In a multivariable model adjusted for age, gender, time posttransplant, dialysis pretransplant, diabetes, treatment with MPA and prednisone, tacrolimus blood level and absolute lymphocyte count, independent predictors for mean Q mental score were found to be use of MPA and tacrolimus blood level. In RTRs receiving MPA compared to patients not taking MPA, the mean score increased by 0.72 (95% CI: 0.31–1.12, p = 0.001) (Fig. 4). For every increase in tacrolimus blood level by 1 ng/mL, the mean score decreased by 0.07 (95% CI: [−]0.11 – [−]0.03, p = 0.001) (Table 3B).
Fig. 4.
Box plot diagram for mean Q mental score for 38 RTRs in the first year posttransplant (a) on and off MPA treatment, and divided by MPA daily dose (b).
Multivariable Linear Regression Model for the Association between Mean Q Mental Score and the Different Variables Tested, Including the Interaction between MPA and Prednisone Use and Tacrolimus Blood Level
In the same multivariable model presented in Table 3B with the addition of interaction terms between MPA or prednisone use and tacrolimus blood level, only MPA use was found to be an independent predictor for the Q mental score, with the score increasing by 1.04 (95% CI: 0.27–1.8, p = 0.008) (online suppl. Table 2).
RTRs >1-Year Posttransplant – Single Measurements
Clinical and Biochemical Characteristics
Mean age at completion of the questionnaire was 60.2 (±12.4) years; 75 (79.8%) of the patients were men; 67 (71.3%) had a living donor; and 61 (64.9%) were treated with dialysis pretransplant. The cause of ESRD was glomerulonephritis in 27 (28.7%), polycystic kidney disease in 19 (20.2%), and diabetic nephropathy in 18 (19.1%) of the RTRs. A medical history of hypertension, diabetes, and smoking was recorded for 81 (86.2%), 32 (34%), and 16 (17%) RTRs, respectively. Posttransplant, 75 (79.8%) of the patients were treated with MPA and 73 (77.7%) with prednisone. Other clinical characteristics are shown in Table 4. Median eGFR was 51.1 (IQR, 42.4–65.3). Other biochemical characteristics are shown in online supplementary Table 3.
Table 4.
Demographic and clinical characteristics of the cohort of 94 RTRs >1-year posttransplant
| Patient characteristics | |
|---|---|
| Gender, n (%) | |
| Male | 75 (79.8) |
| Female | 19 (20.2) |
| Weight [mean (SD)], kg | 81.32 (15.3) |
| Age at questionnaire [mean (SD)], years | 60.2 (12.4) |
| Time posttransplantation [median (IQR)], years | 4.54 (2.25–9.8) |
| Dialysis pretransplantation, n (%) | 61 (64.9) |
| Transplantation number, n (%) | |
| 1 | 86 (91.5) |
| 2 | 7 (7.4) |
| 3 | 1 (1.1) |
| Donor type, n (%) | |
| Living | 67 (71.3) |
| Deceased | 26 (27.7) |
| Unknown | 1 (1.1) |
| ESRD cause, n (%) | |
| Diabetic nephropathy | 18 (19.1) |
| Nephrosclerosis | 13 (13.8) |
| PCKD | 19 (20.2) |
| Glomerulonephritis | 27 (28.7) |
| Other | 11 (11.7) |
| Unknown | 6 (6.4) |
| Medical history, n (%) | |
| HTN | 81 (86.2) |
| Diabetes | 32 (34) |
| Smoker | 16 (17.02) |
| IHD | 14 (14.9) |
| CHF | 4 (4.3) |
| Medication use, n (%) | |
| MPA | 75 (79.8) |
| Prednisone | 73 (77.7) |
| Medications doses [median (IQR)] | |
| Tacrolimus dose, mg/day | 3 (2–4) |
| MPA dose, mg/day | 720 (720–720) |
| Prednisone dose, mg/day | 5 (4.37–5) |
CHF, congestive heart failure; ESRD, end-stage renal disease; HTN, hypertension; IHD, ischemic heart disease; MPA, mycophenolic acid; PCKD, polycystic kidney disease; RTR, renal transplant recipient; SD, standard deviation.
Questionnaire Results
Table 4 in the online supplementary file presents the mean score for each question separately and the median score for the whole questionnaire (Q general). In addition, we divided the questionnaire into questions that focus on physical (Q physical – questions 1–5) and mental (Q mental – questions 6–10) symptoms. The median scores for Q physical and Q mental were 1.2 (IQR, 1–1.8) and 1.2 (IQR, 1–1.6), respectively.
Univariate Analysis Based on the Division of Patients according to the Mean Score for Q Physical above and below the Median Score for All 94 RTRs Tested
A mean Q physical score less than or equal to the median score was obtained for 54 RTRs, while 40 had a mean Q physical score above the median score. No statistically significant differences were found between the two groups (data not shown).
Univariate Analysis Based on the Division of Patients according to the Mean Score for Q Mental above and below the Median Score for all 94 RTRs Tested
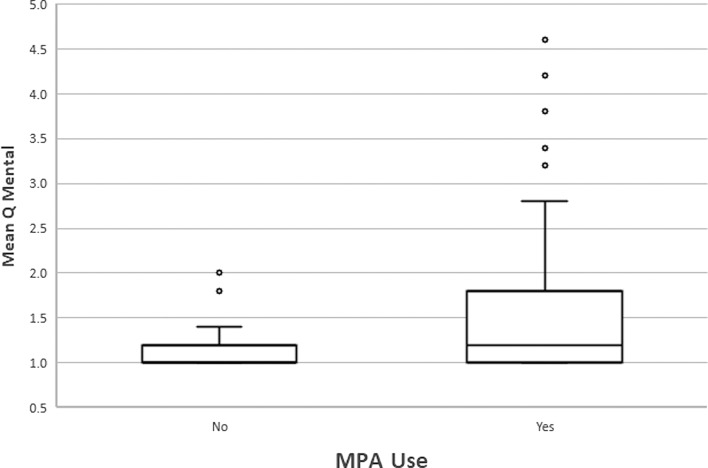
A mean Q mental score less than or equal to the median score was obtained for 57 RTRs, while 37 patients had a mean score above the median value. Of the RTRs with a mean score below the median, 41 (71.9%) were treated with MPA as opposed to 31 (91.9%) RTRs with a mean score above the median (p = 0.019). The response to taking MPA and to the dose itself also varied among the RTRs: 16 (28.1%) RTRs with a mean score below the median had been taken off MPA compared to only 3 (8.1%) RTRs with a mean score above the median score, and for the latter group, 81.1% of RTRs with a score above the median received a daily dose of 360–720 mg compared to 61.4% of RTRs with a score below the median score (p = 0.058). The median daily dose was also higher in RTRs with a mean score above (720 mg, IQR, 720-720) compared to below (720 mg, IQR, 0–720) the median score, with a p value approaching significance (0.065). All other variables tested were not significantly different between the groups, as shown in Table 5A. The odds for the mean Q mental score to be above the median were found to be more than 3 times higher for RTRs who were treated with MPA compared to those who did not receive the medication (OR 3.38, 95% CI: 1.1–10.3, p = 0.03) (Fig. 5).
Table 5.
RTRs >1-year posttransplant
| A. Demographic, clinical, and biochemical characteristics of 94 RTRs >1-year posttransplant stratified by Q mental score |
| Score ≤1.2 (n = 57) | Score ≥1.3 (n = 37) | p value | |
|---|---|---|---|
| Gender – female, n (%) | 8 (14%) | 11 (29.7) | 0.064 |
| Weight [mean (SD)], kg | 81.43 (15.66) | 81.15 (14.95) | 0.932 |
| Age at questionnaire [mean (SD)], years | 61.23 (12.45) | 58.57 (12.33) | 0.312 |
| Transplant number, n (%) | |||
| 1 | 53 (93) | 33 (89.2) | 0.708 |
| 2 and above | 4 (7) | 4 (10.8) | |
| Living donor, n (%) | 38 (67.9) | 29 (78.4) | 0.268 |
| Time posttransplantation [median (IQR)], years | 4.62 (2.43–10.91) | 4.32 (1.84–9.34) | 0.403 |
| Dialysis pretransplantation – yes, n (%) | 37 (64.9) | 24 (64.9) | 0.996 |
| ESRD cause, n (%) | |||
| Diabetic nephropathy | 8 (15.1) | 10 (28.6) | 0.265 |
| Nephrosclerosis | 6 (11.3) | 7 (20) | |
| PCKD | 12 (22.6) | 7 (20) | |
| Glomerulonephritis | 20 (37.7) | 7 (20) | |
| Other | 7 (13.2) | 4 (11.4) | |
| Medical history, n (%) | |||
| HTN | 52 (91.2) | 29 (78.4) | 0.078 |
| Diabetes | 20 (35.1) | 12 (32.4) | 0.791 |
| Smoker | 2 (3.6) | 3 (8.1) | 0.636 |
| IHD | 6 (10.5) | 8 (21.6) | 0.14 |
| CHF | 2 (3.5) | 2 (5.4) | 0.645 |
| Medications use, n (%) | |||
| MPA | 41 (71.9) | 34 (91.9) | 0.019** |
| Prednisone | 46 (80.7) | 27 (73) | 0.379 |
| MPA dose (mg/day), n (%) | |||
| Off MPA | 16 (28.1) | 3 (8.1) | 0.058 |
| 360–720 mg | 35 (61.4) | 30 (81.1) | |
| ≥1,080 mg | 6 (10.5) | 4 (10.8) | |
| Medications doses [median (IQR)] | |||
| Tacrolimus dose, mg/day | 2.5 (2–3) | 3 (2–4) | 0.098 |
| MPA dose, mg/day | 720 (0–720) | 720 (720–720) | 0.065 |
| Prednisone dose, mg/day | 5 (5–5) | 5 (0–5) | 0.261 |
| Laboratory results [median (IQR)] | |||
| WBC, K/μL | 7.9 (6.33–9.58) | 7.74 (5.91–8.28) | 0.301 |
| Absolute neutrophils, K/μL | 4.94 (3.77–5.98) | 4.25 (3.73–5.49) | 0.592 |
| Absolute lymphocytes, K/μL | 2.01 (1.69–2.46) | 1.87 (1.41–2.4) | 0.201 |
| NLR | 2.29 (1.75–2.95) | 2.15 (1.64–3.33) | 0.816 |
| Hb, g/dL | 13.7 (12.69–14.41) | 13.22 (12.18–14.6) | 0.623 |
| Scr, mg/dL | 1.16 (0.99–1.32) | 1.11 (0.86–1.35) | 0.314 |
| eGFR, mL/min | 49.62 (42.34–63.17) | 57.33 (42.98–69.2) | 0.2 |
| Tacrolimus blood level, ng/mL | 7.3 (6.1–8.3) | 6.5 (5.55–8.2) | 0.161 |
| CRP, mg/L | 3 (1.35–5.43) | 2.45 (1.32–5.51) | 0.864 |
| Albumin, g/dL | 4.1 (3.9–4.3) | 4.1 (3.85–4.25) | 0.671 |
| Globulins, g/dL | 2.5 (2.2–2.8) | 2.6 (2.4–3) | 0.395 |
| B. Clinical expression of MPA use in 94 RTRs >1 year posttransplant |
| Questionnaire results | |||
|---|---|---|---|
| RTRs on MPA (N = 75), mean (SD) | RTRs off MPA (N = 19), mean (SD) | p value | |
| Question | |||
| Fatigue | 1.88 (1.13) | 1.47 (0.7) | 0.183 |
| Muscle weakness | 1.51 (0.83) | 1.47 (0.96) | 0.623 |
| Night sweats | 1.24 (0.57) | 1.21 (0.92) | 0.226 |
| Joint pain | 1.45 (0.83) | 1.37 (0.83) | 0.481 |
| Tremor | 1.49 (1.01) | 1.16 (0.37) | 0.198 |
| Sleep disorder | 1.83 (1.06) | 1.32 (0.67) | 0.037* |
| Restlessness | 1.56 (1.07) | 1.21 (0.42) | 0.372 |
| Depression | 1.59 (1.15) | 1.11 (0.31) | 0.09 |
| Difficulty falling asleep | 1.72 (1.11) | 1.16 (0.5) | 0.021* |
| Anxiety | 1.45 (0.98) | 1.05 (0.23) | 0.064 |
SD, standard deviation; CHF, congestive heart failure; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; Hb, hemoglobin; HTN, hypertension; IHD, ischemic heart disease; MPA, mycophenolic acid; NLR, neutrophil-to-lymphocyte ratio; PCKD, polycystic kidney disease; RTR, renal transplant recipient; Scr, serum creatinine; WBC, white blood cells.
Numbers in bold indicate significant at *p < 0.05; **p < 0.01.
Fig. 5.
Box plot diagram for mean Q mental score for 94 RTRs >1-year posttransplant on and off MPA treatment.
Univariate Analysis Comparing the Mean Score for Each Question Separately between RTRs on and off MPA Treatment
RTRs taking MPA had higher mean scores in several questions related to sleep disorders (1.83 ± 1.06 as opposed to 1.32 ± 0.67, p = 0.037), to difficulty falling asleep (1.72 ± 1.11 compared to 1.16 ± 0.5, p = 0.02), and to depression and anxiety (p values approaching significance), as shown in Table 5B. There was no significant difference in the Q physical score for questions 1–5 and the question related to a feeling of restlessness.
Discussion
To date, tools available for the diagnosis of overimmunosuppression are limited, and there are no metrics for identifying transplant recipients with overimmunosuppression. In this study, we aimed to better characterize the adverse effects of immunosuppressive medications administered to RTRs. In multivariable models for a total of 130 measurements in 38 RTRs during the first year posttransplant, MPA use increased Q physical and Q mental scores, while prednisone use increased the Q physical score alone. These findings were in line with those for 94 RTRs >1-year posttransplant, for whom a higher rate of MPA use and an increased daily dose were associated with an increase in the Q mental score. Moreover, the odds for a mean Q mental score above the median score were more than 3 times higher in RTRs treated with MPA as opposed to non-treated. An examination of the results for the specific questions revealed that RTRs treated with MPA experienced more sleep disorders and feelings of depression and anxiety versus non-treated.
The link between MPA use for various conditions and sleep disorders has been described previously [18]. For example, insomnia and diarrhea were the main side effects experienced by 10 patients with refractory inflammatory eye disease who received 2–3 g of mycophenolate mofetil daily [19]. Furthermore, a number of studies have reported MPA use to be associated with depression [20, 21]. Depression and migraine necessitated drug withdrawal in 2 out of 24 patients with chronic active inflammatory bowel disease who received mycophenolate mofetil, 2 g daily [22]. To the best of our knowledge, this is the first study to describe an increased rate of MPA-induced sleep disorders, depression, and anxiety in RTRs, both early and late posttransplant. MPA treatment was also associated with an increased Q physical score in RTRs in the first year posttransplant but not in RTRs >1-year posttransplant. This finding is most probably related to the higher doses of MPAs given to RTRs early posttransplant.
Prednisone treatment has been associated with a variety of side effects. The literature is rife with examples, so we present but a few. Sarcoidosis patients receiving prednisone therapy exhibited higher fatigue scores compared to those not treated with prednisone [23, 24]. Similarly, women with sarcoidosis treated with oral steroids awarded lower scores in a quality-of-life questionnaire in the facets of Energy and Fatigue [25]. Corticosteroid-induced myopathy associated with muscle weakness is highly prevalent [26], and glucocorticoid-induced osteoporosis may cause joint pain [27]. In this study, the association between prednisone therapy and the Q physical score for RTRs during the first year posttransplant is probably related to the higher doses of prednisone prescribed early posttransplant to prevent rejection. This association was not found in RTRs >1-year posttransplant, who are almost always on a low maintenance dose of prednisone of up to 5 mg daily.
The effect of tacrolimus blood level on Q physical and Q mental scores in the first year posttransplant was negated when a model including the interaction terms tacrolimus blood level × prednisone use and tacrolimus blood level × MPA use was applied. This finding indicates that tacrolimus blood level is not an independent predictor for the questionnaire results but rather is affected by drug-drug interactions with MPA and prednisone. In keeping with this finding, a 20% increase in tacrolimus concentrations and a 40% increase in tacrolimus area under the curve after corticosteroid withdrawal were reported in randomized controlled studies [28, 29]. In a different study, tacrolimus dose-adjusted trough concentrations were 30% lower for patients who had received prednisone for 3 months than for patients who had received daclizumab without corticosteroid therapy [30]. The mechanism for this interaction is not clear, but corticosteroids may induce the CYP3A4 or CYP3A5 enzymes that are responsible for tacrolimus metabolism [31]. On the other hand, mycophenolate may increase the serum concentration of tacrolimus [32, 33]. It is thus possible that the net effect of simultaneous treatment with prednisone and MPA leads to a reduction in the tacrolimus blood level, which, in turn, is manifested as increased Q physical and Q mental scores early posttransplant. This effect was not shown later posttransplant, most probably due to the lower doses of MPA and prednisone prescribed and fewer changes in the immunosuppressive regimen resulting in drug-drug interactions. In the current study, we did not show an effect of tacrolimus blood level on the questionnaire results early posttransplant due to its interaction with MPA and prednisone, but we assume that the overall immunosuppression regimen, including tacrolimus treatment, led to increases in the Q physical and mental scores during the first year posttransplant.
Several limitations should be taken into consideration in the interpretation of our results. To obtain maximum cooperation from the patients, we used a short and easy-to-fill-out questionnaire that was not validated. The selection of the questions was based on a previous investigation of the various symptoms experienced by RTRs early and late posttransplant. The reliability of the questionnaire was tested by Cronbach’s-alpha index for internal consistency between questions expressing similar characteristics and was found to be 0.893, 0.774, and 0.905 for Q general, physical and mental, respectively. Although there is no way to differentiate between the side effects of the immunosuppressive drugs and the effects caused by the immunosuppression itself, it is assumed that the more powerful the immunosuppressive treatment, the more significant the side effects and/or the effects caused by the immunosuppression itself. The strengths of this study include the use of two RTR cohorts that differed in the time posttransplant, and power derived from examining multiple confounders, including potential clinical and biochemical confounders obtained on the day of completion of the questionnaire. We cannot exclude potential residual confounding.
We believe that RTR should be proactively followed with regard to sleep difficulties and feelings of depression and anxiety. For patients who report these symptoms, reducing the dose – or stopping MPA in those who do not respond to dose reduction – should be considered with the aim to prevent excess immunosuppression, with its short- and long-term complications. RTRs should also be asked about feelings of fatigue, muscle weakness, night sweats, joint pain, and tremor. In patients who suffer from these symptoms, a reduction of overall immunosuppression should be considered.
In conclusion, by using a simple tool of asking RTRs about the clinical symptoms they experience as part of the routine follow-up, it is possible to improve the diagnosis of excess immunosuppression. In particular, by focusing on questions related to sleep disorders, depression, and anxiety, overimmunosuppression related to MPA treatment can be better diagnosed.
Statement of Ethics
The study was approved by our Institutional Review Board (7053-20-SMC). Written informed consent was not required.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
There were no funding sources.
Author Contributions
Noa Scott: data collection and interpretation and writing; Aharon Ben-David: data acquisition; Yana Davidov and Renana Yemini: data interpretation; Keren Cohen-hagai: conception and design; Ronen Ghinea: data collection; Eytan Mor: revising; Tammy Hod: conception and design, data acquisition, data interpretation, writing, and revising.
Funding Statement
There were no funding sources.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Rizzari MD, Suszynski TM, Gillingham KJ, Dunn TB, Ibrahim HN, Payne WD, et al. Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol. 2012 Mar;7(3):494–503. 10.2215/CJN.08630811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Opelz G, Dohler B. Association between steroid dosage and death with a functioning graft after kidney transplantation. Am J Transpl. 2013 Aug;13(8):2096–105. 10.1111/ajt.12313. [DOI] [PubMed] [Google Scholar]
- 3. Laurent MR, Goemaere S, Verroken C, Bergmann P, Body JJ, Bruyere O, et al. Prevention and treatment of glucocorticoid-induced osteoporosis in adults: consensus recommendations from the Belgian bone club. Front Endocrinol. 2022;13:908727. 10.3389/fendo.2022.908727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lebranchu Y, Bridoux F, Buchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transpl. 2002 Jan;2(1):48–56. 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 5. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Thymoglobulin Induction Study G. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006 Nov 9;355(19):1967–77. 10.1056/nejmoa060068. [DOI] [PubMed] [Google Scholar]
- 6. Dadhania D, Snopkowski C, Ding R, Muthukumar T, Chang C, Aull M, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008 Aug 27;86(4):521–8. 10.1097/TP.0b013e31817c6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prince O, Savic S, Dickenmann M, Steiger J, Bubendorf L, Mihatsch MJ. Risk factors for polyoma virus nephropathy. Nephrol Dial Transpl. 2009 Mar;24(3):1024–33. 10.1093/ndt/gfn671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bataille S, Moal V, Gaudart J, Indreies M, Purgus R, Dussol B, et al. Cytomegalovirus risk factors in renal transplantation with modern immunosuppression. Transpl Infect Dis. 2010 Dec;12(6):480–8. 10.1111/j.1399-3062.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- 9. Luppi M, Barozzi P, Torelli G. Skin cancers after organ transplantation. N Engl J Med. 2003 Aug 7;349(6):612–4; author reply 12-4. [PubMed] [Google Scholar]
- 10. Cippa PE, Schiesser M, Ekberg H, van Gelder T, Mueller NJ, Cao CA, et al. Risk stratification for rejection and infection after kidney transplantation. Clin J Am Soc Nephrol. 2015 Dec 7;10(12):2213–20. 10.2215/CJN.01790215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi J, Chandraker A. Immunologic risk assessment and approach to immunosuppression regimen in kidney transplantation. Clin Lab Med. 2019 Dec;39(4):643–56. 10.1016/j.cll.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 12. Almond PS, Matas A, Gillingham K, Dunn DL, Payne WD, Gores P, et al. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993 Apr;55(4):752–6; discussion 56-7. 10.1097/00007890-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 13. Massy ZA, Guijarro C, Wiederkehr MR, Ma JZ, Kasiske BL. Chronic renal allograft rejection: immunologic and nonimmunologic risk factors. Kidney Int. 1996 Feb;49(2):518–24. 10.1038/ki.1996.74. [DOI] [PubMed] [Google Scholar]
- 14. Wan SS, Chadban SJ, Watson N, Wyburn K. Development and outcomes of de novo donor-specific antibodies in low, moderate, and high immunological risk kidney transplant recipients. Am J Transpl. 2020 May;20(5):1351–64. 10.1111/ajt.15754. [DOI] [PubMed] [Google Scholar]
- 15. Sawinski D, Trofe-Clark J. BK virus nephropathy. Clin J Am Soc Nephrol. 2018 Dec 7;13(12):1893–6. 10.2215/cjn.04080318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halloran PF, Reeve J, Madill-Thomsen KS, Kaur N, Ahmed E, Cantos C, et al. Combining donor-derived cell-free DNA fraction and quantity to detect kidney transplant rejection using molecular diagnoses and histology as confirmation. Transplantation. 2022 Jun 29;106(12):2435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hod T, Ben-David A, Olmer L, Scott N, Ghinea R, Mor E, et al. BNT162b2 third booster dose significantly increases the humoral response assessed by both RBD IgG and neutralizing antibodies in renal transplant recipients. Transpl Int. 2022;35:10239. 10.3389/ti.2022.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Namgoong JM, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A pilot study on the safety and efficacy of generic mycophenolate agent as conversion maintenance therapy in stable liver transplant recipients. Transpl Proc. 2013 Oct;45(8):3035–7. 10.1016/j.transproceed.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 19. Choudhary A, Harding SP, Bucknall RC, Pearce IA. Mycophenolate mofetil as an immunosuppressive agent in refractory inflammatory eye disease. J Ocul Pharmacol Ther. 2006 Jun;22(3):168–75. 10.1089/jop.2006.22.168. [DOI] [PubMed] [Google Scholar]
- 20. Draper HM. Depressive disorder associated with mycophenolate mofetil. Pharmacotherapy. 2008 Jan;28(1):136–9. 10.1592/phco.28.1.136. [DOI] [PubMed] [Google Scholar]
- 21. Goebel A, Jacob A, Frank B, Sacco P, Alexander G, Philips C, et al. Mycophenolate for persistent complex regional pain syndrome, a parallel, open, randomised, proof of concept trial. Scand J Pain. 2018 Jan 26;18(1):29–37. 10.1515/sjpain-2017-0154. [DOI] [PubMed] [Google Scholar]
- 22. Fellermann K, Steffen M, Stein J, Raedler A, Hamling J, Ludwig D, et al. Mycophenolate mofetil: lack of efficacy in chronic active inflammatory bowel disease. Aliment Pharmacol Ther. 2000 Feb;14(2):171–6. 10.1046/j.1365-2036.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- 23. De Vries J, Michielsen H, Van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS). Br J Health Psychol. 2004 Sep;9(Pt 3):279–91. 10.1348/1359107041557048. [DOI] [PubMed] [Google Scholar]
- 24. de Kleijn WP, De Vries J, Lower EE, Elfferich MD, Baughman RP, Drent M. Fatigue in sarcoidosis: a systematic review. Curr Opin Pulm Med. 2009 Sep;15(5):499–506. 10.1097/MCP.0b013e32832d0403. [DOI] [PubMed] [Google Scholar]
- 25. De Vries J, Van Heck GL, Drent M. Gender differences in sarcoidosis: symptoms, quality of life, and medical consumption. Women Health. 1999;30(2):99–114. 10.1300/j013v30n02_07. [DOI] [PubMed] [Google Scholar]
- 26. Surmachevska N, Tiwari V. Corticosteroid induced myopathy. StatPearls. Treasure Island (FL); 2022. [PubMed] [Google Scholar]
- 27. Ilias I, Milionis C, Zoumakis E. An overview of glucocorticoid-induced osteoporosis. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext. South Dartmouth (MA); 2000. [Google Scholar]
- 28. Park SH, Kim HJ, Cho WH, Kim JH, Oh MH, Kim SH, et al. Tacrolimus pharmacokinetic drug interactions: effect of prednisone, mycophenolic acid or sirolimus. FEMS Microbiol Lett. 2009 Feb;301(1):137–46. 10.1111/j.1574-6968.2009.01809.x. [DOI] [PubMed] [Google Scholar]
- 29. Shihab FS, Lee ST, Smith LD, Woodle ES, Pirsch JD, Gaber AO, et al. Effect of corticosteroid withdrawal on tacrolimus and mycophenolate mofetil exposure in a randomized multicenter study. Am J Transpl. 2013 Feb;13(2):474–84. 10.1111/j.1600-6143.2012.04327.x. [DOI] [PubMed] [Google Scholar]
- 30. Hesselink DA, Ngyuen H, Wabbijn M, Gregoor PJ, Steyerberg EW, van Riemsdijk IC, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br J Clin Pharmacol. 2003 Sep;56(3):327–30. 10.1046/j.0306-5251.2003.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosohata K, Uesugi M, Hashi S, Hosokawa M, Inui K, Matsubara K, et al. Association between CYP3A5 genotypes in graft liver and increase in tacrolimus biotransformation from steroid treatment in living-donor liver transplant patients. Drug Metab Pharmacokinet. 2014;29(1):83–9. 10.2133/dmpk.dmpk-13-rg-060. [DOI] [PubMed] [Google Scholar]
- 32. Pirsch J, Bekersky I, Vincenti F, Boswell G, Woodle ES, Alak A, et al. Coadministration of tacrolimus and mycophenolate mofetil in stable kidney transplant patients: pharmacokinetics and tolerability. J Clin Pharmacol. 2000 May;40(5):527–32. 10.1177/00912700022009143. [DOI] [PubMed] [Google Scholar]
- 33. Kim JH, Han N, Kim MG, Yun HY, Lee S, Bae E, et al. Increased exposure of tacrolimus by Co-administered mycophenolate mofetil: population pharmacokinetic analysis in healthy volunteers. Sci Rep. 2018 Jan 26;8(1):1687. 10.1038/s41598-018-20071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.