Abstract
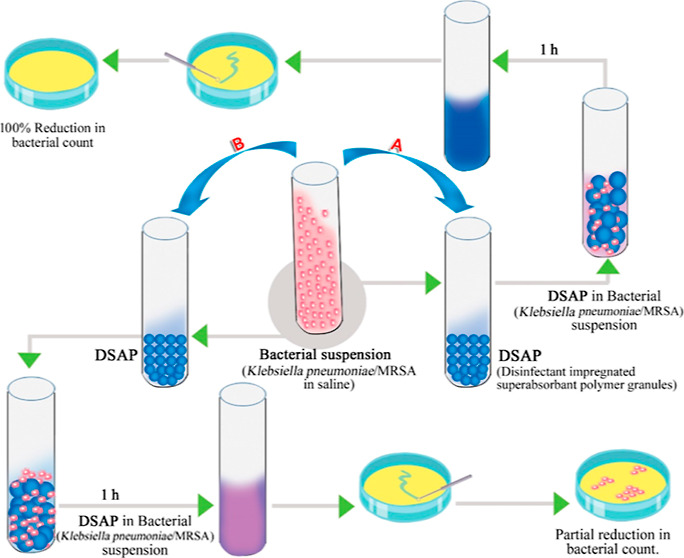
Disposal of respiratory secretions from patients having contagious diseases (e.g., COVID-19 and tuberculosis) poses a high risk of infection for healthcare workers. AcryloSorb canister liner bags are highly efficient for the safe handling of contagious respiratory secretions via solidification and disinfection processes. The canister liner bags are lined with disinfectant-impregnated superabsorbent polymer (DSAP) granules. The liner structure in the bag has a patented design that has upward progressive absorbent availability (Indian Patent application # 202041019872). AcryloSorb canister liner bags can decontaminate the fluid secretions absorbed in the bag and solidify within 10 min. The present study focused on the bactericidal effect of DSAP using Gram-negative bacteria, Klebsiella pneumoniae, and Gram-positive bacteria, methicillin-resistantStaphylococcus aureus (MRSA). Disinfectants such as peracetic acid (ethaneperoxic acid), sodium dichloroisocyanurate (sodium 3,5-dichloro-2,4,6-trioxo-1,3,5-triazinan-1-ide), rose bengal (disodium; 2,3,4,5-tetrachloro-6-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl) benzoate), and N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride at different weight ratios were impregnated in superabsorbent polymer (SAP) granules. The bactericidal activities of DSAP were studied along with its solidification capacity. Disinfectants showed different bactericidal activities when impregnated with SAP granules. For example, peracetic acid-impregnated SAP granules (DSAP-P) showed 100% bactericidal activity for both Klebsiella pneumoniae and MRSA at 0.5 wt % peracetic acid. Sodium dichloroisocyanurate-impregnated SAP granules showed 100% bactericidal activity only at 5 wt % sodium dichloroisocyanurate (DSAP-S5). Even though peracetic acid was highly effective, SAP granules collapsed when impregnated with peracetic acid. The ease of handling, disinfection efficacy, and preserving the morphology of SAP granules make DSAP-S5, a suitable candidate for AcryloSorb canister liner bags.
Introduction
Disposal of infected secretion from patients poses a great challenge to every hospital. This is particularly in the case of secretions of patients with highly contagious diseases such as COVID-19, tuberculosis, and so forth.1−3 Collection and disposal of such wastes expose the nursing and cleaning staff to high risk. Generally, in the intensive care unit, secretions are sucked by a suction machine into bottles or canisters which have to be emptied when full, then subjected to a decontamination process in a sluice room, and discarded through the waste fluid disposal system. Apart from recontamination risk during handling involved in these processes, there is a need for a well-equipped sluice room with disinfection facilities, which can be an issue in less-equipped hospitals.
AcryloSorb canister liner bags are highly efficient for the safe handling of contagious respiratory secretions via solidification and disinfection processes. These canister bags are lined with DSAP granules. The liner structure has a patented design which allows the progressive absorbent availability upward (Indian Patent application # 202041019872). The AcryloSorb canister liner bags can decontaminate the fluid secretions absorbed in the bag and solidify them immediately. Solidification and immediate disinfection that occur inside these bags eliminate the risk of secondary infections by preventing spillage and aerosol formation, thereby protecting healthcare workers and promoting safe workplace management. AcryloSorb canister liner bags are enclosed in a customizable sealer bag which can pack the used canister bag as spill-proof decontaminated biomedical waste that can be disposed of through incineration.
Superabsorbent polymers (SAPs) are three-dimensional cross-linked polymer structures that can absorb and retain a large amount of aqueous fluid compared to their dry weight.4,5 High swelling capacity and good water retention ability of SAPs lead to potential applications in various fields including hygiene products (e.g., baby diapers, adult diapers, and sanitary napkins)6−9 and biomedical healthcare products (e.g., drug delivery systems and wound dressing).10−13 Several fluid solidification products are available in the international market including Isolyser from Ecolab, Medi-Solid Plus from Cardinal Health, SaniSorb from Multisorb filtration group, SafeSorb from DiSorb Systems, and so forth. However, there is not much information regarding the antimicrobial effect of these fluid solidification products. As part of the development of AcryloSorb canister liner bags, disinfectant-impregnated SAP (DSAP) granules were developed, and antimicrobial effects were evaluated. The present study aims to evaluate the antimicrobial activity of various disinfectant-impregnated SAP granules. Bactericidal activities of disinfectants such as peracetic acid (ethaneperoxic acid), sodium dichloroisocyanurate (sodium 3,5-dichloro-2,4,6-trioxo-1,3,5-triazinan-1-ide), rose bengal (disodium; 2,3,4,5-tetrachloro-6-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl)benzoate), and N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride at different weight ratios in DSAP (say 0.5, 1, 5 wt %, etc.) were evaluated using Gram-negative bacteria, Klebsiella pneumoniae (K. Pneumoniae), and Gram-positive bacteria, methicillin-resistantStaphylococcus aureus (MRSA).
Materials and Methods
Materials
Acrylic acid (prop-2-enoic acid), sodium hydroxide, N,N’methylenebisacrylamide (N-[(prop-2-enoylamino)methyl]prop-2-enamide), ammonium persulfate (diazonium; sulfonatooxy sulfate), sodium dichloroisocyanurate (sodium 3,5-dichloro-2,4,6-trioxo-1,3,5-triazinan-1-ide), rose bengal (disodium; 2,3,4,5-tetrachloro-6-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl)benzoate), and N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride were purchased from Sigma-Aldrich, Bangalore, India. Peracetic acid (ethaneperoxoic acid) was purchased from GK Health Care, Bengaluru. Bacterial strains, K. pneumoniae(ATCC 4532) and methicillin-resistantS. aureus (ATCC 700698), were purchased from an American-type culture collection, PO Box 1549, Manassas, VA. Bacterial culture media, tryptone soya agar and tryptone soya broth, were purchased from HiMedia, Mumbai. All other reagents were of analytical grade and used as such without any further purification.
Methods
Preparation of SAP Granules
SAP granules were prepared using partially neutralized acrylic acid via the solution-based free radical polymerization technique followed by oven drying at 80–85 °C. In brief, 10 g of acrylic acid (139 mmol) was stirred using a magnetic stirrer at 200 rpm in a 500 mL round-bottom flask. A sodium hydroxide solution (5 N) was added slowly to the above solution until the pH turned 4.5. Throughout the neutralization reaction, the temperature of the acrylic acid solution was maintained at 15–20 °C using an ice bath. 0.0427 g of N,N’methylene bisacrylamide (0.276 mmol), 0.05 g of ammonium persulfate (2.19 mmol), and 6 mL of distilled water were added to the above solution and stirred at 300 rpm and 85 °C. A hydrogel was formed within 4 h of the reaction. The hydrogel formed was transferred to a glass tray, cut into small pieces, and dried in an oven at 105 °C for 48 h. The dried pieces were ground, and polymer granules of size ≤710 μm were collected. These granules were mixed well with 0.405 mmol N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride and 8.32 mmol quartz, incubated at 25 °C for 2 h. Complete consumption of the monomer (acrylic acid) during the reaction was confirmed using high-performance liquid chromatography (HPLC) and Fourier transform infrared (FTIR) spectroscopy. SAP granules obtained were stored in a desiccator for further use.
Preparation of Disinfectant-Impregnated SAP Granules
SAP granules were mixed with different weight ratios of the disinfectant (say, 5, 2, 1, and 0.5 wt %), incubated for 15 min at 25 °C, and dried in an oven at 60 °C for 24 h. DSAP granules that contained peracetic acid, rose bengal, N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride, and sodium dichloroisocyanurate were abbreviated as DSAP-P, DSAP-R, DSAP-QA, and DSAP-S, respectively.
Determination of Particle Size Distribution of SAP Granules by Sieve Fractionation
10 g of SAP granules was taken in a beaker (150 L) and transferred to the top sieve on the sieve tester (size of sieves used from top to bottom: 710, 500, 250, and 45 μm, respectively). The lid was placed and sieved at an intensity of 40% for 30 min. Granules in each sieve were carefully removed and weighed (m2). The mass percentage of each fraction (w) was calculated from the equation
| 1 |
m2 = mass of the retained fraction of SAP granules along with the sieve.ms = mass of the empty sieve.m1 = total mass of the sample.
Evaluation of Residual Monomer Content
Residual acrylic acid was extracted from 10 g of SAP granules using 200 mL of 0.9% saline. The solution was stirred at 500 rpm for 1 h and allowed to settle for 5 min. The supernatant was filtered through a 0.45 μm filter (AXIVA nylon syringe filer) and analyzed using HPLC from Waters, USA (Waters 600 E, pump, 7725 Rheodyne injectors). The stationary phase was the Merck RP-C18 column (5 μm, 4.6 × 250 mm) at 30 °C. An acetonitrile/0.1% phosphoric acid solution (90:10) was used as the mobile phase. The flow rate was fixed at 0.8 mL/min. A Waters 2484 dual-wavelength ultraviolet (UV) detector at 210 nm was used as the detector. In brief, 0.020 mL of the extract was injected into the column and the retention time was monitored. Calibration curves were constructed using standard solutions of acrylic acid (1, 2, 3, and 4 mg/L) by plotting the peak area against the concentration. Residual monomer content was calculated using the equation.
| 2 |
W = mass fraction of residual acrylic acid.C = concentration of the extract expressed in mg/L.Msap = mass of SAP granules expressed in grams.
Fourier Transform Infrared Spectroscopy Studies
The presence of acrylic acid in SAP granules was analyzed using a Nicolet 5700 Fourier transform infrared spectrometer in the attenuated total reflection mode. Transmission spectra were collected in the range of 4000–400 cm–1 by placing a thin uniform layer of acrylic acid or SAP granules perpendicular to the infrared radiation path.
Scanning Electron Microscopy
SAP granules were sputter-coated with gold using a Hitachi E–1010 ion sputter, Japan. SEM images were recorded on a Hitachi S-2400-SEM, Japan, at an accelerated voltage of 15 kV.
Thermogravimetric Analysis
The thermogravimetric analysis (TGA) of SAP was carried out on SDT Q600 (simultaneous TGA-DTA, TA Instruments) in the temperature range of 25–850 °C at a scan rate of 0.333 °C s–1 under a nitrogen atmosphere.
Free Swelling Capacity
0.2 g of SAP granules was weighed and transferred to a porous heat-sealable bag. The powder was distributed horizontally throughout the bag and laid on the surface of 0.9% saline along with blank bags. Each bag was allowed to wet for 60 s and pushed under the liquid surface. After the desired time (e.g., 15, 30, 60, and 120 min), the bags were removed from the saline solution and hung diagonally on a line from one of the double-sealed corners for 10 min. The same experiment was repeated by changing the medium to distilled water and artificial saliva.
Each bag was weighed, and mass (mw) was recorded. Experiments were done in quadruplicate. Free swell capacity was expressed in mass fraction (w)
| 3 |
ms = mass of dry SAP granules.mb = average mass of the wet blank bag.mw = mass of the wet bag with SAP granules.
Antimicrobial Studies
Studies were performed based on ASTM E2149-13a (American Society for Testing and Materials), a test method designed to evaluate the antimicrobial activities of nonleaching materials under dynamic conditions. Being nonleaching, the antimicrobial activity was surface-bound and was assayed against both Gram-negative and Gram-positive bacteria by the dynamic contact method. Gram-negative bacteria (Klebsiellapneumonia ATCC 4532) and Gram-positive bacteria (MRSA, MRSA ATCC 700698) were used to study the disinfection ability of DSAP. In brief, 50 ± 0.001 mL of the working inoculum containing approximately 9.3 × 108 cfu/L of bacterial load in a 0.3 mM KH2(PO4) (potassium dihydrogen phosphate) buffer was incubated with 0.2 g of DSAP at 200 rpm for an hour. After 1 h, the viable bacterial load in the suspension was determined. The viable bacterial load in the suspension was estimated after serially diluting the suspension in physiological saline followed by plating on tryptone soya agar. The plates were incubated at 37 ± 1 °C overnight (∼18 h) and examined for microbial growth. The number of colonies in the plate was counted using a microbial colony counter (Schuett Biotec GmbH, Germany). The number of colonies grown was multiplied by the dilution factor to estimate the total microbial load in the suspension. Percentage reduction (R) was calculated based on the viable count of control (D) and viable count of treated samples (C)
| 4 |
Statistical Analysis
All the quantitative data were expressed as mean ± standard deviation. Statistical significance was determined using GraphPad Prism 9 by performing a suited one-way/two-way analysis of variance followed by Tukey’s multiple comparison test. Significance was accepted at p-values ≤0.0005.
Results and Discussion
SAP granules were prepared by crosslinking partially neutralized acrylic acid using N,N’methylenebisacrylamide via the free radical solution polymerization technique. After the reaction, the hydrogel pieces were dried in an oven at 105 °C, ground, and sieved to obtain particles of size ≤710 μm. SAP granules were surface-treated using 2 wt % N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride) (DMTPA) and 5 wt % quartz to evade the adverse effect of gel blocking. Hydrophobic groups were imparted on the surface as the hydrocarbon chain of DMTPA protruded out, while the quaternary ammonium group interacted with carboxylate ions in SAP granules via an ionic interaction. Quartz was bound to the surface of SAP granules via physical (e.g., hydrogen bonding) and chemical adsorption. The hydroxyl groups of quartz were chemically bound with the siloxane groups of DMTPA, and stable SAP granules were formed.
The extent of monomer consumption during SAP synthesis was confirmed using FTIR spectroscopy analysis (Figure 1A). The wide absorption bands around 3200–3600 cm–1 were due to O–H stretching, and a sharp, intense peak at ∼1695 cm–1 was assigned to the C=O stretching vibration of carboxyl groups in acrylic acid and SAP granules. As indicated in the figure, the absorption band of acrylic acid due to =C–H bending at 815 cm–1 was diminished in the spectrum of SAP. The absence of a peak at 815 cm–1 in SAP was attributed to the absence of a significant amount of free acrylic groups in SAP granules. The high monomer conversion as evident from the absence of an acrylic peak in the spectra of SAP enabled the use of SAPs without purification. The residual monomer content was further analyzed using HPLC. Calibration curves were constructed by plotting the peak area against the known concentration of acrylic acid. Acrylic acid elutes at 4.2 min. The saline extract of SAP granules was calculated using the calibration curve. The data showed the presence of 6.4 ± 0.0001 g of acrylic acid/kg of SAP granules. As the concentration of residual monomer content was within the acceptable limit, no purification process was introduced.
Figure 1.
(A) FTIR spectra of acrylic acid (black line) and SAP granules (red line). There is an intense peak of =C–H at 815 cm–1 in the acrylic acid spectrum. No significant peak of =C–H bending was present for SAP granules, indicating the absence of a significant amount of acrylic acid in SAP granules (B) TGA curve of SAP granules indicating the percentage weight loss in the temperature range of 25–850 °C.
TGA conducted under a N2 atmosphere was shown in Figure 1B. A mass loss of 10% was observed at ∼250 °C as a result of the evaporation of water along with unreacted monomers and impurities. An additional mass loss of 40 wt % was observed around 380 °C which was attributed to the pyrolysis of cross-linked polyacrylic acid networks in SAP granules. Thermal decomposition of SAP granules continued, and a weight loss of ∼90% was observed at 835 °C.
SEM images (Figure 2) showed the presence of a wide size range of particles of irregular morphology in SAP granules. No macro- or micropores were observed in these particles in their dry state. Solid particles in the size range of ∼1–500 μm were observed in SEM images. The wide size range of particles in SAP granules was also confirmed by measuring the particle size distribution by sieve fractionation. Mass fractions of 30.427, 34.779, 17.036, and 17.758% were measured in the size range of <710 < 500; <500 < 250; <250 < 45; and <45 μm, respectively. Different size ranges of particles are important in a liquid solidification system as this helps to progressively absorb liquid. Small particles with high surface area absorb liquid at a fast rate. Larger particles absorb liquid slowly compared to smaller particles.
Figure 2.
SEM images of SAP granules at different magnifications, (A) 50× and (B) 5000×, indicating the presence of a wide range of particles in the size range of 700–1 μm or less.
The free swelling capacity of SAP granules was studied using different liquids including distilled water, saline, and artificial saliva. The free swelling capacity of the SAP granules depends on many parameters including crosslinking density of the polymer network, degree of neutralization of the carboxylic group of the polymer network, ionic strength/chemical potential of the liquid, and so forth. As we optimize disinfectant-impregnated SAP granules, the chemical potential of the liquid is the only variant used to study the free swelling capacities of SAP granules.
It was observed that distilled water with the highest chemical potential among the test liquids had the greatest ability to flow through the polymer network (Figure 3A). Each gram of SAP granules absorbed 129.4 ± 7.35 g/g in 15 min. No significant increase in free swelling capacity was observed with time as shown in Figure 3. The fast swelling nature of SAP granules is highly useful while using them in AcryloSorb canister liner bags. Salt resistance of SAP granules was a major challenge. The end use of the AcryloSorb canister liner bag is to disinfect and solidify respiratory secretions that contain a variety of electrolytes or salts intended to interact with SAP granules. Therefore, the resistance of SAP granules to swell in saline and artificial saliva was studied along with free swelling capacity in distilled water. The free swelling capacity of SAPs in saline and artificial saliva was shown in Figure 3B,C. The free swelling capacity of SAP granules in 15 min was reduced by ∼65 and ∼58% in saline and artificial saliva, respectively, compared to that of distilled water. Each gram of SAP granules absorbed 43.74 ± 1.18 g/g of saline and 40.65 ± 2.58 g/g of artificial saliva in 15 min. As in the case of free swelling capacity in distilled water, no significant change in the free swelling capacity of SAP granules was observed in saline with an increase in time (Figure 3). However, the free swelling capacity of SAP granules increased to 40.87% in artificial saliva at 120 min compared to that at 15 min. The integrity of the swollen SAP granules was retained for more than 72 h in all these media. The free swelling capacity of SAP granules in artificial saliva at different time points is statistically significant with P ≤ 0.0001 compared to the free swelling capacity at 15 min. The high ionic concentration of both saline and artificial saliva reduces the osmotic pressure difference between the internal and external environment of the gel system which leads to reduced swelling. Artificial saliva having higher viscosity and salt concentration hinders the free swelling capacity of SAP granules to a greater extent compared to that of distilled water and saline.
Figure 3.
Free swelling capacity of SAP granules in (A) distilled water, (B) 0.9% saline, and (C) artificial saliva at different time points.
DSAP at different weight ratios of the disinfectant was analyzed for antimicrobial activities. The percentage reduction in the microbial count, when exposed to different types of DSAP is shown in Figures 4 and 5. All the disinfectants used in the study had a wide spectrum of antimicrobial activities.14 However, an entirely different disinfection behavior was observed while impregnated with SAP granules. To optimize the minimum concentration of the disinfectant in SAP granules to provide 100% bactericidal activities, different weight ratios of the disinfectant in DSAP were evaluated. Based on the chemical nature and interactions of the disinfectant with SAP granules, different antimicrobial activities were observed at different weight ratios. During the study, 2 wt % disinfectant-impregnated SAP granules were evaluated initially irrespective of their chemical nature. Based on the results, higher/lower concentrations of disinfectant-impregnated SAP granules were assessed to obtain the minimum bactericidal concentration of the disinfectant in DSAP. For example, two weight ratios of peracetic acid (2 and 0.5 wt %) in DSAP-P were evaluated, whereas three weight ratios of N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride (2, 5, and 10 wt %) in DSAP-QA were studied.
Figure 4.
Percentage reduction in the bacterial count (A), Gram-negative K. pneumoniae incubated with DSAP granules (B) Gram-positive MRSA incubated with DSAP granules.
Figure 5.
Percentage reduction in the bacterial count. (A) DSAP-S granules using different weight ratios of sodium dichloroisocyanurate. (B) DSAP-QA granules using different weight ratios of N,N-dimethyl-N-[3-(triethoxysilyl)propyl]ctadecane-1-aminium chloride.
The bactericidal activities of peracetic acid (PAA)-impregnated SAP granules (DSAP-P) were evaluated at different weight ratios of PAA such as 2 and 0.5 wt % represented as DSAP-P 2 and DSAP-P 0.5, respectively. As shown in Figure 4, a 100 % reduction in the microbial count was observed when exposed to both DSAP-P2 and DSAP-P0.5. This data was in accordance with earlier studies that showed disinfection efficacy of PAA even at low concentrations.15−18
The bactericidal effect of rose bengal-impregnated SAP granules (DSAP-R) was evaluated without additional illumination or ultrasonic treatment. Rose bengal is a commonly used photosensitizer for photodynamic antimicrobial therapies.19−21 However, the current data showed no significant bactericidal effect for DSAP-R. Very low microbial reductions of 18 ± 22 and 9 ± 11% were observed for DSAP-R2 and DSAP-R0.5, respectively, toward K. pneumoniae. It may be attributed to the reduced effect of rose bengal without specific light exposure during the experiment. As evident from Figure 4, the bactericidal activity of DSAP-R was more toward MRSA. DSAP-R2 and DSAP-R0.5 showed a bactericidal activity of 84 ± 9 and 61 ± 12%, respectively. As discussed earlier, these reductions could not provide a significant disinfection effect as the remaining microbes can multiply within zero time.
The bactericidal effect of various quaternary ammonium compounds including N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride (DMTPA) is well studied.22−24 As discussed earlier, DMTPA has been used as one of the surface cross-linkers during the synthesis of SAP granules. However, bare SAP granules did not show bactericidal activities. It may be attributed to the absence of free quaternary ammonium groups due to ionic interactions between the surface of SAP granules and quaternary ammonium groups in DMTPA. To check whether an additional amount of DMTPA could impart an antimicrobial effect, DSAP-QA was prepared using three different weight ratios of DMTPA (e.g., 2, 5, and 10 wt %). However, all these formulations show only a partial reduction in microbial load. For example, DSAP-QA2, DSAP-QA5, and DSAP-QA10 showed a reduction of 3.87 ± 2.28, 24 ± 4, and 66 ± 1%, respectively, for K. pneumoniae. However, an increased reduction in microbial growth was observed in the case of MRSA as shown in Figure 5. DSAP-QA10 showed a microbial reduction of 73.6 ± 5%. The reduced bactericidal effects of DSAP-QA could be attributed to the availability of a limited number of free quaternary ammonium groups due to strong ionic interactions between carboxylate ions and quaternary ammonium groups in DSAP-QA.
Sodium dichloroisocyanurate (NaDCC) is a synthetic chlorine donor containing high “free available chlorine”.25−27 NaDCC reacts with water to release chlorine in the form of hypochlorous acid. Hypochlorous acid (HOCl)/hypochlorite (ClO–) is an excellent oxidizing agent and disinfectant.28−31 The disinfection effect of NaDCC-impregnated SAP granules (DSAP-S) was evaluated at different weight ratios of NaDCC, say 5, 2, and 0.5 wt % represented as DSAP-S 5, DSAP-S 2, and DSAP-S 0.5, respectively. The results showed 100% bactericidal activity for DSAP-S5 against K. pneumoniae and MRSA (Figure 5A). As the weight percentage of NaDCC was reduced in DSAP-S, a corresponding reduction in bactericidal activities was also observed. For example, DSAP-S2 showed a microbial reduction of 98 ± 9 and 70 ± 4.5% toward K. pneumoniae and MRSA, respectively.
As discussed above, peracetic acid showed highly effective bactericidal activities at low concentrations in DSAP. However, the morphology of DSAP-P granules collapsed while interacting with peracetic acid. Collapsed DSAP-P granules aggregated and became a solid mass within 10 min. Even though excellent bactericidal activities were observed in DSAP-P, the adverse effect discovered in the morphology and surface texture constrained the use of peracetic acid as a disinfectant in DSAP. Similarly, rose bengal and N,N-dimethyl-N-[3-(triethoxysilyl)propyl]octadecan-1-aminium chloride were also excluded from the list of disinfectants to be used in AcryloSorb canister liner bags due to partial bactericidal activities of both DSAP-R and DSAP-QA. However, 100% bactericidal activities observed for DSAP-S5 render sodium dichloroisocyanurate a promising candidate for disinfectants in DSAP. It also preserves the morphology and surface texture of DSAP granules effectively. The ease of handling and preserving the morphology of DSAP along with 100% bactericidal activities promotes the use of DSAP-S5 in AcryloSorb canister liner bags used for the safe handling of contagious respiratory secretions. AcryloSorb canister liner bags were also evaluated using patient secretions.
Conclusions
DSAP granules were prepared, and antimicrobial effects were evaluated using K. pneumoniae and MRSA. Peracetic acid-impregnated SAP granules (DSAP-P) showed the most effective bactericidal activities compared to DSAP-S, DSAP-R, and DSAP-QA. A 100% reduction in bacterial counts was seen in the case of both K. pneumoniae and MRSA even at 0.5 wt % of peracetic acid. DSAP-S showed 100% bactericidal activity in the presence of 5 wt % sodium dichloroisocyanurate. However, both DSAP-R and DSAP-QA showed a partial reduction in bactericidal activities. Partial reduction in the microbial count was not effective in providing disinfection as the remaining microbes multiplied within no time and could increase the microbial load significantly. The study reports sodium dichloroisocyanurate as an effective disinfectant in DSAP that can be used in the AcryloSorb secretion solidification system for the safe management of contagious secretions of patients.
Acknowledgments
The authors gratefully acknowledge the Technology Research Center (TRC) grant (#8224), Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Trivandrum, India, for the financial support and Dr. Rekha M. R, Biosurface Technology, Biomedical Technology Wing, SCTIMST, for providing disodium 2,3,4,5-tetrachloro-6-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl) benzoate for the study.
The authors declare no competing financial interest.
References
- Xiangdong C.; You S.; Shanglong Y.; Renyu L.; Henry L.. Perioperative Care Provider’s Considerations in Managing Patients with the COVID-19 Infections. Transl. Perioper. Pain Med. 2020, 7(), 10.31480/2330-4871/116. [DOI] [Google Scholar]
- Wang D.; Hu B.; Hu C.; Zhu F.; Liu X.; Zhang J.; Wang B.; Xiang H.; Cheng Z.; Xiong Y.; Zhao Y.; Li Y.; Wang X.; Peng Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA, J. Am. Med. Assoc. 2020, 323, 1061. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.; Liu X. f.; Jia Z. 2019-nCoV transmission through the ocular surface must not be ignored. The Lancet 2020, 395, e39 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon A.; De Belie N.; Dubruel P.; Van Vlierberghe S. Superabsorbent Polymers: A Review on the Characteristics and Applications of Synthetic, Polysaccharide-Based, Semi-Synthetic and ‘Smart’ Derivatives. Eur. Polym. J. 2019, 117, 165–178. 10.1016/j.eurpolymj.2019.04.054. [DOI] [Google Scholar]
- Ma X.; Wen G. Development History and Synthesis of Super-Absorbent Polymers: A Review. J. Polym. Res. 2020, 27, 136. 10.1007/s10965-020-02097-2. [DOI] [Google Scholar]
- Behera S.; Mahanwar P. A. Superabsorbent Polymers in Agriculture and Other Applications: A Review. Polym.-Plast. Technol. Mater. 2020, 59, 341–356. 10.1080/25740881.2019.1647239. [DOI] [Google Scholar]
- Venkatachalam D.; Kaliappa S. Superabsorbent Polymers: A State-of-Art Review on Their Classification, Synthesis, Physicochemical Properties, and Applications. Rev. Chem. Eng 2021, 39, 127. 10.1515/revce-2020-0102. [DOI] [Google Scholar]
- Fang S.; Wang G.; Li P.; Xing R.; Liu S.; Qin Y.; Yu H.; Chen X.; Li K. Synthesis of Chitosan Derivative Graft Acrylic Acid Superabsorbent Polymers and Its Application as Water Retaining Agent. Int. J. Biol. Macromol. 2018, 115, 754–761. 10.1016/j.ijbiomac.2018.04.072. [DOI] [PubMed] [Google Scholar]
- Jafari M.; Najafi G. R.; Sharif M. A.; Elyasi Z. Superabsorbent Polymer Composites Derived from Polyacrylic Acid: Design and Synthesis, Characterization, and Swelling Capacities. Polym. Polym. Compos. 2021, 29, 733–739. 10.1177/0967391120933482. [DOI] [Google Scholar]
- Chang L.; Xu L.; Liu Y.; Qiu D. Superabsorbent Polymers Used for Agricultural Water Retention. Polym. Test. 2021, 94, 107021. 10.1016/j.polymertesting.2020.107021. [DOI] [Google Scholar]
- Borden M. A.; Leibfarth F. A. From Disposable Diapers to Adhesives. Nat. Chem. 2021, 13, 930–932. 10.1038/s41557-021-00793-0. [DOI] [PubMed] [Google Scholar]
- Zohuriaan-Mehr M. J.; Omidian H.; Doroudiani S.; Kabiri K. Advances in Non-Hygienic Applications of Superabsorbent Hydrogel Materials. J. Mater. Sci. 2010, 45, 5711–5735. 10.1007/s10853-010-4780-1. [DOI] [Google Scholar]
- Hallam C.; Denton A.; Thirkell G. COVID-19: Considerations for the Safe Management and Disposal of Human Excreta. Infection Prevention in Practice 2020, 2, 100085. 10.1016/j.infpip.2020.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse A.; Temmam S.; Deboosere N.; Guillier L.; Delobel A.; Maris P.; Vialette M.; Morin T.; Perelle S. Comparison of Chlorine and Peroxyacetic-Based Disinfectant to Inactivate Feline Calicivirus, Murine Norovirus and Hepatitis A Virus on Lettuce. Int. J. Food Microbiol. 2011, 151, 98–104. 10.1016/j.ijfoodmicro.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Ao X.; Eloranta J.; Huang C. H.; Santoro D.; Sun W. j.; Lu Z. d.; Li C. Peracetic Acid-Based Advanced Oxidation Processes for Decontamination and Disinfection of Water: A Review. Water Res. 2021, 188, 116479. 10.1016/j.watres.2020.116479. [DOI] [PubMed] [Google Scholar]
- Kitis M. Disinfection of Wastewater with Peracetic Acid: A Review. Environ. Int. 2004, 30, 47–55. 10.1016/S0160-4120(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Cutts T.; Kasloff S.; Safronetz D.; Krishnan J. Decontamination of Common Healthcare Facility Surfaces Contaminated with SARS-CoV-2 Using Peracetic Acid Dry Fogging. J. Hosp. Infect. 2021, 109, 82–87. 10.1016/j.jhin.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow S. Peracetic Acid Sterilization: A Timely Development for a Busy Healthcare Industry. Infect Control Hosp Epidemiol 1992, 13, 111–113. 10.1086/646483. [DOI] [PubMed] [Google Scholar]
- Amescua G.; Arboleda A.; Nikpoor N.; Durkee H.; Relhan N.; Aguilar M. C.; Flynn H. W.; Miller D.; Parel J. M. Rose Bengal Photodynamic Antimicrobial Therapy: A Novel Treatment for Resistant Fusarium Keratitis. Cornea 2017, 36, 1141–1144. 10.1097/ICO.0000000000001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M.; Yoshida Y.; Horii K.; Hasegawa Y.; Shibuya Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch. Oral Biol. 2021, 122, 105024. 10.1016/j.archoralbio.2020.105024. [DOI] [PubMed] [Google Scholar]
- Fischer B. B.; Krieger-Liszkay A.; Eggen R. I. L. Photosensitizers Neutral Red (Type I) and Rose Bengal (Type II) Cause Light-Dependent Toxicity in Chlamydomonas Reinhardtii and Induce the Gpxh Gene via Increased Singlet Oxygen Formation. Environ. Sci. Technol. 2004, 38, 6307–6313. 10.1021/es049673y. [DOI] [PubMed] [Google Scholar]
- Grigoras A. G. Natural and Synthetic Polymeric Antimicrobials with Quaternary Ammonium Moieties: A Review. Environ. Chem. Lett. 2021, 19, 3009–3022. 10.1007/s10311-021-01215-w. [DOI] [Google Scholar]
- Daood U.; Matinlinna J. P.; Pichika M. R.; Mak K. K.; Nagendrababu V.; Fawzy A. S. A Quaternary Ammonium Silane Antimicrobial Triggers Bacterial Membrane and Biofilm Destruction. Sci. Rep. 2020, 10, 10970–11014. 10.1038/s41598-020-67616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Bao H.; Bok K. X.; Lee C. Y.; Li B.; Zin M. T.; Kang L. High Durability and Low Toxicity Antimicrobial Coatings Fabricated by Quaternary Ammonium Silane Copolymers. Biomater. Sci. 2016, 4, 299–309. 10.1039/c5bm00353a. [DOI] [PubMed] [Google Scholar]
- Goda H.; Nakayama-Imaohji H.; Yamaoka H.; Tada A.; Nagao T.; Fujisawa T.; Koyama A. H.; Kuwahara T. Inactivation of Human Norovirus by Chlorous Acid Water, a Novel Chlorine-Based Disinfectant. J. Infect. Chemother. 2022, 28, 67–72. 10.1016/j.jiac.2021.10.001. [DOI] [PubMed] [Google Scholar]
- Byun K. H.; Han S. H.; Yoon J.; Park S. H.; Ha S. Do. Efficacy of Chlorine-Based Disinfectants (Sodium Hypochlorite and Chlorine Dioxide) on Salmonella Enteritidis Planktonic Cells, Biofilms on Food Contact Surfaces and Chicken Skin. Food Control 2021, 123, 107838. 10.1016/j.foodcont.2020.107838. [DOI] [Google Scholar]
- Gallandat K.; Kolus R. C.; Julian T. R.; Lantagne D. S. A Systematic Review of Chlorine-Based Surface Disinfection Efficacy to Inform Recommendations for Low-Resource Outbreak Settings. Am. J. Infect. Control 2021, 49, 90–103. 10.1016/j.ajic.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proto A.; Zarrella I.; Cucciniello R.; Pironti C.; De Caro F.; Motta O. Bactericidal and Fungicidal Activity in the Gas Phase of Sodium Dichloroisocyanurate (NaDCC). Curr. Microbiol. 2016, 73, 287–291. 10.1007/s00284-016-1040-x. [DOI] [PubMed] [Google Scholar]
- El Zawawy L. A.; El-Said D.; Ali S. M.; Fathy F. M. Disinfection Efficacy of Sodium Dichloroisocyanurate (NADCC) against Common Food-Borne Intestinal Protozoa. J. Egypt. Soc. Parasitol. 2010, 40, 165–185. [PubMed] [Google Scholar]
- Jin B.; Niu J.; Wang L.; Zhao J.; Li Y.; Pang L.; Zhang M. Effect of Sodium Dichloroisocyanurate Treatment on Enhancing the Biodegradability of Waste-Activated Sludge Anaerobic Fermentation. J. Environ. Manage. 2021, 287, 112353. 10.1016/j.jenvman.2021.112353. [DOI] [PubMed] [Google Scholar]
- Clasen T.; Edmondson P. Sodium Dichloroisocyanurate (NaDCC) Tablets as an Alternative to Sodium Hypochlorite for the Routine Treatment of Drinking Water at the Household Level. Water Res. 2006, 209, 173–181. 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]







