Abstract
Cerebral small vessel disease (CSVD) has emerged as a common factor driving age-dependent diseases, including stroke and dementia. CSVD-related dementia will affect a growing fraction of the aging population, requiring improved recognition, understanding, and treatments. This review describes evolving criteria and imaging biomarkers for the diagnosis of CSVD-related dementia. We describe diagnostic challenges, particularly in the context of mixed pathologies and the absence of highly effective biomarkers for CSVD-related dementia. We review evidence regarding CSVD as a risk factor for developing neurodegenerative disease and potential mechanisms by which CSVD leads to progressive brain injury. Finally, we summarize recent studies on the effects of major classes of cardiovascular medicines relevant to CSVD-related cognitive impairment. While many key questions remain, the increased attention to CSVD has resulted in a sharper vision for what will be needed to meet the upcoming challenges imposed by this disease.
Introduction
The broad term cerebral small vessel disease (CSVD) describes heterogeneous conditions affecting blood vessels of 50 to 500 micrometers in diameter. Multiple distinct pathologies can cause CSVD, most commonly hypertensive vasculopathy and cerebral amyloid angiopathy (CAA). The clinical challenge is that rarely are there indications for obtaining tissue for a definitive diagnosis. Neuroimaging, however, is readily available, and standards exist for using imaging to diagnose CVSD 1 (Fig 1). The STRIVE group proposed standardized terminology and suggested minimum standards for imaging suspected CSVD, though imaging technology continues to evolve. Current and future standards for CSVD diagnosis will certainly depend on feasible, reliable, and quantitative neuroimaging.
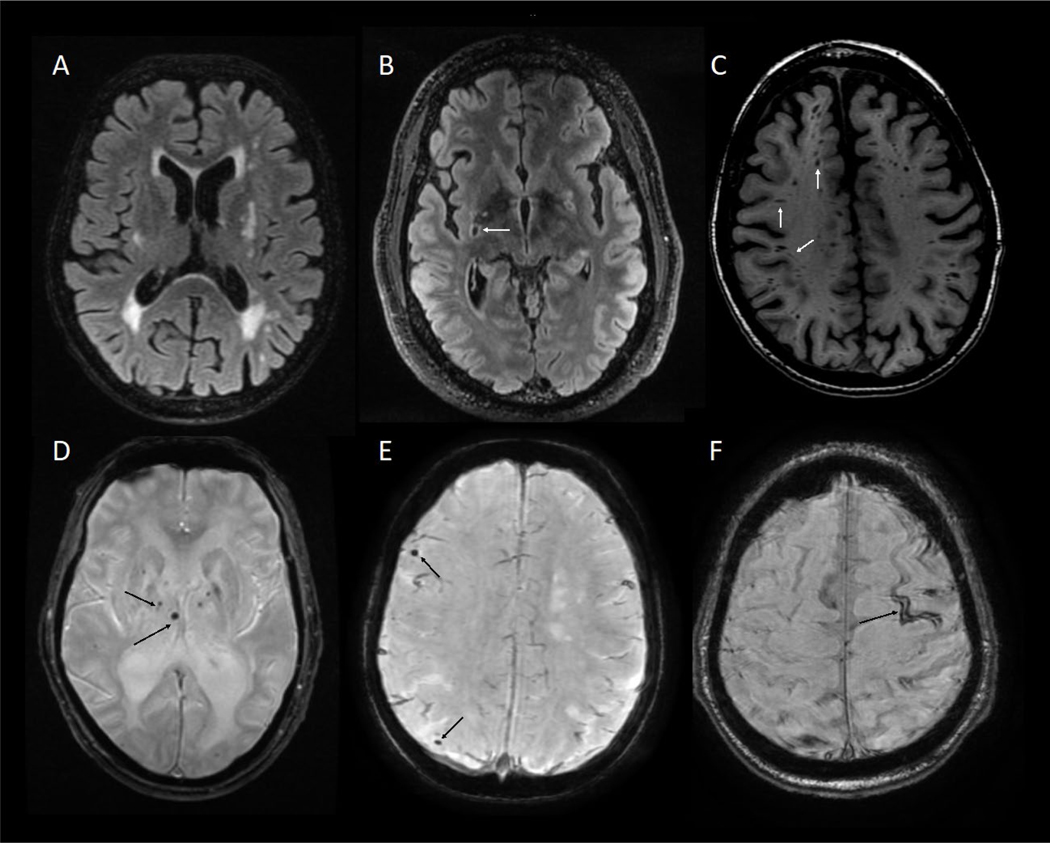
Figure 1.
Neuroimaging in CSVD-VCID. (A) subcortical and periventricular white matter hyperintensities (WMH) on T2/FLAIR (fluid-attenuated inversion recovery) sequences. (B) Lacunar stroke a cavity with a rim of hyperintensity on FLAIR. (C) Enlarged perivascular spaces (ePVS) on T1 sequence, can appear both as streak-like spaces as well as punctate. (D) Subcortical microhemorrhages on T2* SWI (susceptibility weighted imaging) sequence. (E) Cortical microhemorrhages on T2* SWI sequence. (F) Superficial siderosis on T2* SWI sequence.
CSVD can manifest as symptomatic cerebral hemorrhages, small ischemic strokes, and cognitive impairment. Symptomatic hemorrhages are easily recognized and diagnosed. Small ischemic strokes are also easy to recognize, but small strokes from CSVD must be distinguished from strokes due to small emboli. Thus, even when pre-test probability is high, neuroimaging is essential for diagnosing CSVD.
Diverse CSVD markers seen on MRI (Fig 1) correlate in aggregate with cognitive and functional decline 2. Findings such as white matter hyperintensity raise suspicion for CSVD. Additional imaging findings with greater specificity but unknown sensitivity for CSVD include enlarged perivascular spaces 3, abnormal diffusion measures 4, and blood-brain barrier (BBB) leakage seen with dynamic contrast enhanced MRI (DCE-MRI) 5. Other lesions, such as recent small subcortical infarcts, lacunae of vascular origin, and microbleeds are the most specific, clinically available CSVD biomarkers.
CSVD-related dementia is a major subgroup within vascular cognitive impairment and dementia (VCID). VCID is a challenging disorder to ascertain, prompting numerous groups to evaluate diagnostic criteria to make the diagnosis more reliable and enable cross-study comparisons. A comparison of 5 major diagnostic criteria for vascular dementia applied to consecutive ischemic stroke patients followed for 3 months with MRI and cognitive testing found wide variability in the prevalence of vascular dementia depending on criteria used from 32.7% (ADDTC criteria) to 91.6% (ICD-10 criteria)6. The same study found that only 37.4% of patients had focal neurological deficits at 3 months, highlighting the importance of imaging to detect cerebrovascular disease.
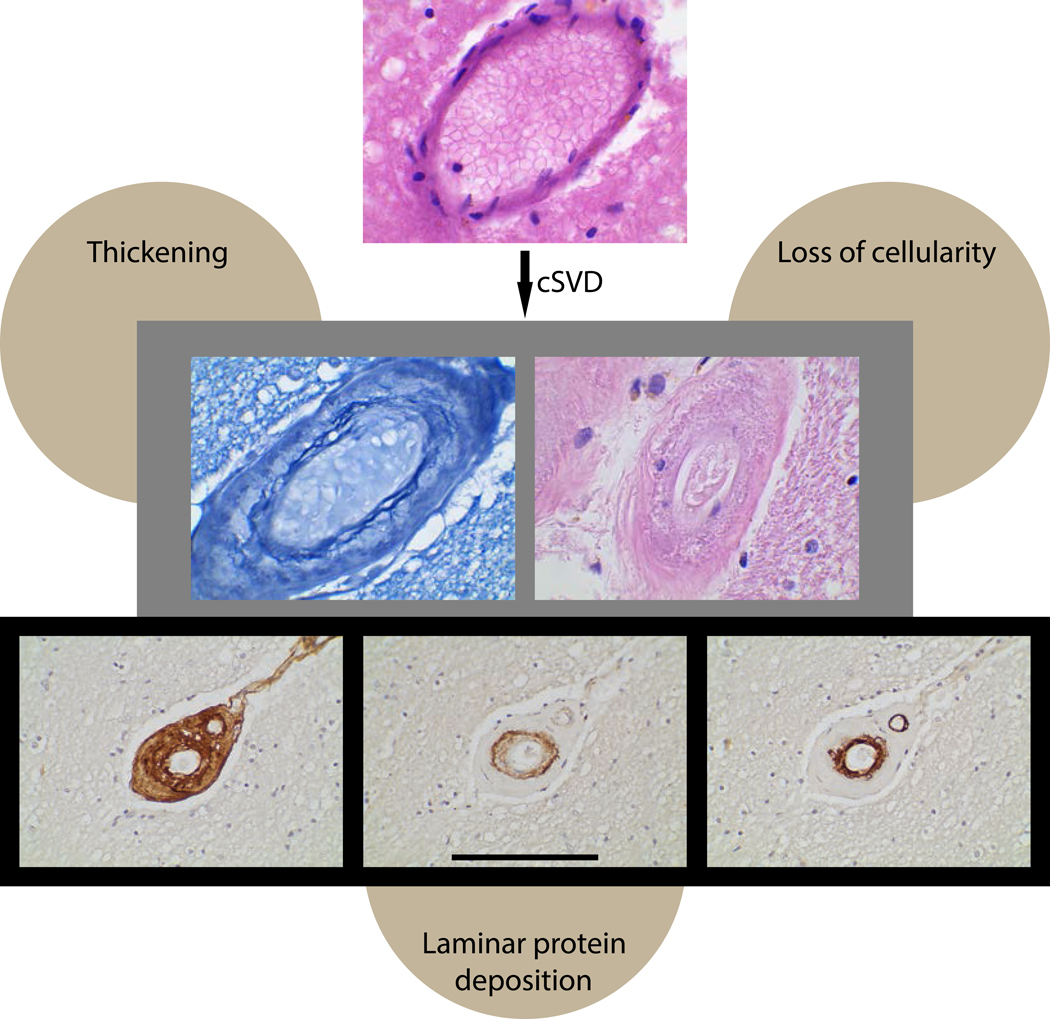
The limited clinicopathological studies of VCID highlight the challenges of making a premortem diagnosis that correlates well with pathology. An autopsy study of 89 patients with dementia found no statistically significant relationship between a neuropathological diagnosis of vascular dementia and any of three clinical criteria (ICD-10, ADDTC, and NINDS-AIREN) 7. Another challenge is that there is no agreed upon histopathological diagnosis for VCID 8, though commonly cited features of CSVD include wall thickening, vascular cell loss, and laminar protein accumulation (Fig 2). Ongoing studies correlating imaging with pathology should help in making reliable premortem diagnosis of clinically significant CSVD9.
Figure 2.
Histopathological features of CSVD. Common histopathological features include arterial wall thickening, vascular cell loss, and protein accumulation in a laminar pattern. The top photo shows a normal appearing white matter artery stained with hematoxylin and eosin (H&E). The middle row shows markedly thickened small arteries in a patient with CADASIL, an inherited CSVD. On the left is a vessel after Miller’s staining, which shows massive vessel thickening and fraying elastin fibers of the intimal/medial function. Cell loss is demonstrated in the H&E stained CADASIL artery on the right. The lower panel illustrates layered protein accumulation in a patient with CADASIL. The left photo shows staining for all collagens using a collagen binding peptide probe (B-CHP) that stains all three layers of the vessel (adventitia, media, and intima). The middle photo shows staining with a NOTCH3 neo-epitope antibody that principally highlights the media; in CAA, the media accumulates Aβ. The right photo shows staining for COL4A that localizes predominantly to the intima. Scale bar span 40 microns for rows 1–2 and 100 microns for row 3.
CSVD-related dementia is thus in a state of evolution: though well-recognized as an important entity, many improvements in understanding await. Since many excellent discussions have already been presented, this review focuses on five common questions.
My patient has CSVD. Will it cause dementia?
CSVD does not always lead to dementia or even cognitive impairment. In routine practice, MRI obtained for a variety of concerns in cognitively normal middle aged or older individuals will frequently demonstrate findings associated with CSVD. Although the presence of CSVD does not ensure dementia, there appears to be a strong correlation between CSVD and development of dementia in diverse populations. The best evidence of this is provided by longitudinal studies. At least 20 studies10–21 have investigated white matter hyperintensities and incident dementia that, in large part, include European and North American populations; two studies of these investigations were performed in East Asia.
A meta-analysis of studies over the last two decades by Debette et al 22 places the hazard ratio imposed by CSVD as 1.84 (p<0.001; over 9,338 study subjects); the total incidence of dementia among these subjects was 12%, though the length of observation for each of the studies varied significantly. Two separate overlapping meta-analyses by Rensma 23 and Bos 24 concluded that white matter hyperintensities presented a significant risk of incident dementia. As such, the pragmatic counseling for patients found to have CSVD is that this condition does not always cause dementia, but that it substantially increases the risk.
Although in practice these principles are applied broadly, current studies do not adequately cover all ethnicities and races. It is also not known whether there are other clinical characteristics, combinations of risk factors, or genetic determinants that render CSVD deterministic in some individuals.
Since CSVD is associated with several abnormalities on MRI, there could be subtypes of sporadic age-related CSVD 25. One question that has emerged is whether specific MRI markers of CSVD could be useful in prognostication of dementia. Debette et al 22 analyzed several CSVD features for as predictors of dementia. Although white matter hyperintensities were clearly associated with increased risk of incident dementia, subcortical brain infarcts or cerebral microbleeds did not reach significance, despite the size of the aggregate study population (8,736 individuals). Rensma 23 and Bos 24 found that dementia was increased or borderline significant in studies of patients with lacunar infarcts but not microbleeds. The ability to compare these MRI findings is limited because most studies lacked direct comparison of MRI features. Studies on perivascular space3 , have reported increased incident dementia risk in a small number of studies 26 27 .
My patient has dementia. Is this CSVD-related dementia?
In dementia, especially late-life sporadic disease, definite diagnoses are based on neuropathological examination only available at autopsy 28,29. Marching back from that, select in vivo biomarkers, such as Alzheimer’s disease Amyloid/Tau/Neurodegeneration (A/T/N) biomarkers, aid in pre-mortem diagnoses with high confidence. In contrast, outside of monogenic causes of VCID, cognitive impairment due to CSVD faces diagnostic obstacles. The first problem is lack of clear neuropathological consensus criteria. The second is the scarcity of sensitive and specific in vivo biomarkers for CSVD-related VCID. The third is lack of distinct clinical syndromes for VCID. The fourth is the variable lag between diagnosis of vascular risk factors or vascular disease and onset of cognitive and neurobehavioral symptoms, diminishing the power of predictive models with limited longitudinal follow-up.
In post-mortem analyses of individuals with dementia, vascular disease, especially CSVD lesions and tissue abnormalities, such as microinfarcts, microbleeds, and arteriolosclerosis, have been noted as common pathologies and co-pathologies, increasing the odds of dementia in the aging brain30. The challenge, however, has been in attributing the cause of pre-mortem dementia to CSVD, especially in the setting of quantifiable non-vascular neurodegenerative diseases 31.
The likelihood of causal assignment of cognitive impairment to vascular disease is further decreased in the setting of co-existing non-vascular neurodegenerative disease syndromes that have syndromic presentations and/or biomarkers (e.g., AD)29. This in part is due to lack of clear clinical syndromes associated with CSVD and, critically, a lack of highly accurate CSVD in vivo biomarkers. For example, in AD, post-mortem analyses have demonstrated that 80–100% of individuals with parenchymal amyloid plaques have CAA. When CAA is significant, especially in APOE4 carriers, cortical microbleeds can be seen on brain imaging. These microbleeds can be seen prior to significant cognitive and functional impairment, and in some instances are the first imaging abnormality noted for a patient who may also demonstrate white matter hyperintensities, arteriolosclerosis, and lipohyalinosis 32. Even so, attribution of brain dysfunction to AD in patients with mixed AD and CSVD has traditionally underestimated vascular contributions.
AD has several clinical syndromes such as the typical amnestic-predominant or limbic AD, behavioral-executive AD (bvAD), posterior cortical atrophy (PCA), and logopenic variant primary progressive aphasia (lvPPA). The syndromic presentations of these variants are assumed to be non-vascular, but the prevalence of CAA is thought to be similar across AD syndromes; thus, in such syndromes, the vascular contribution to dementia deserves more investigation. Outside of AD, additional clinical syndromes, such as frontotemporal dementias and Lewy body disorders are considered non-vascular neurodegenerative diseases. The prevalence of CSVD in these syndromes remains understudied. Yet, CSVD affects white matter and brain connectivity, causing cognitive speed and executive function decline which are common to non-vascular disorders and CVSD. Symmetric parkinsonism is another clinical symptom that could be due to subcortical CSVD. These motor manifestations are also not sufficiently specific enough to point the diagnostic compass toward VCID.
Teasing apart diverse CSVD contributions to clinical symptoms would require in vivo molecular biomarkers that are not currently available. MRI-apparent CSVD lesions, detailed above, are unlikely to be the earliest manifestations of disease. Moreover, none have been demonstrated to be both sensitive (as an early abnormality) and specific (capturing disease of vasculature rather than neuro-glial degeneration). Importantly, dysfunction of the BBB (discussed below), measured by DCE-MRI as well as with CSF/serum albumin quotient, is an uncommonly assessed parameter in practice and research, though recent studies suggesting a potentially early causal association with brain dysfunction and degeneration.
Notwithstanding, according to the 2020 Lancet Commission’s Report on Dementia Prevention, Intervention, and Care, up to 40% of dementias worldwide are preventable 33. Many of these modifiable risk factors either directly or indirectly fall under the rubric of vascular disease. Therefore, CSVD is an important contributor to brain dysfunction and an ideal therapeutic target for cognitive impairment and dementia. Brain blood vessels have numerous roles, such as gating communication between peripheral organs, blood, and brain, interacting with immune cells and controlling entry and exit of molecules, and cells to and from the brain. In addition, a molecular cross-talk between blood vessel cells and neurons control steady state perfusion as well as rapid on-demand increases in cerebral blood flow to active neuronal networks34. Therefore, CSVD is a reasonable contributor to disorders of cognition.
In summary, in routine clinical practice, whether CSVD causes dementia in a particular patient is not easily answerable. Brain dysfunction frequently occurs in the setting of multiple pathologies. Current barriers to assignment of roles of CSVD in neuropsychiatric symptoms, motor dysfunction, and cognitive impairment include the need for better in vivo molecular and imaging biomarkers, methods to disentangle mixed vascular and non-vascular pathologies, and better clinical recognition of mechanisms connecting CSVD to brain dysfunction.
My patient has CSVD-related dementia. What is the differential diagnosis of CSVD dementia?
Vascular risk factors and qualitative evaluations of small and large vessel diseases are considered treatable, potentially modifiable risk factors for neurodegenerative disorders. But the extent to which CSVD and other vasculopathies contribute to brain degeneration in each patient generally remains a matter of opinion and escapes a specific diagnosis. Monogenic vasculopathies are the exception to the diagnostic ambiguity regarding VCID. Clinical syndrome, family history, and imaging biomarkers raise suspicion for a genetic cause of SVD-related dementia (Table 1), which genetic testing can confirm. Several are described below.
Table 1.
Monogenic CSVD disorders
| Syndrome | Gene | Mutation Prevalence | Disease Incidence | Clinical Syndrome | Imaging | Pathological Findings |
|---|---|---|---|---|---|---|
| Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) |
NOTCH3 Autosomal dominant |
>230 mutations in 34 EGFR regions, >95% missense, mainly cysteine altering; cysteine-sparing mutations are less common Mutations lead to aggregation of NOTCH3 Extra-cellular domain (ECD) |
Classical syndrome: 2–5 in 100,000 Mutations discovered 2.2 in 1,000 |
Transient ischemic attacks and strokes, neuropsychiatric symptoms, cognitive impairment, apathy, mood disturbance including depression, rarely psychosis; migraine with or without aura; seizures (5–10%) | Confluent WMH by fifth decade of life in anterior temporal lobes, external capsule, periventricular areas, centrum semiovale, superior frontal gyrus Lacunar infarcts, enlarged perivascular spaces, cerebral microhemorrhages Brain atrophy (late life) |
• Deposition of granular osmiophilic material (GOM) adjacent to VSMCs caused by mutated NOTCH3 ECD, containing other proteins • Arteriopathy, most severe in small penetrating cerebral and leptomeningeal arteries • Widespread cortical neuronal apoptosis • Arterial wall thickening and fibrosis, stenosis • Degeneration of VSMCs and pericytes • Myelin degeneration • Blood-brain barrier dysfunction |
| Cerebral Autosomal Recessive Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CARASIL) |
HTRA1 Autosomal recessive (mainly) |
Missense and nonsense mutations, and a few compound heterozygous individuals affecting protease activity of this serine protease | ~5100 cases reported | Brain: ischemic strokes, cognitive decline and dementia by 30–40yo, gait disturbance, Spine: lumbago (low back pain), Hair: alopecia (hair loss) Heterozygous HTRA1 CSVD has milder presentation without extra-CNS symptoms, and some HTRA1 mutation carriers can be asymptomatic |
Symmetrical WMH in periventricular and deep WM, occasionally in anterior temporal lobes and external capsules Notable hyperintense arc from pons to middle cerebellar peduncle in late disease stages. Lacunar infarcts in basal ganglia and thalamus, Cerebral microhemorrhages Herniated lumbar and cervical disks with degeneration Brain atrophy |
• Loss of VSMCs • Hyalinosis, fibrosis and thickening of blood vessel walls • Thinning of cerebral arterioles ECM leading to enlargement, loss of vascular elasticity and collapse |
| Gould syndrome |
COL4A1/2 (Collagen IV A1 and A2) Autosomal dominant |
Missense mutations mainly in highly conserved glycine residues in the triple helical domain of the COL4A1 gene. Other mutations impairing translation; insertions also reported. Mutations in the 3’ untranslated region of COL4A1 cause Pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL). |
Highly variable multi-system disease BBB dysfunction and recurrent subcortical hemorrhages. Disease affects brain, spinal cord, eye, muscles, and kidneys. Brain: cerebral SVD, cerebral aneurysms, stroke (hemorrhagic and ischemic). Eye: retinal arterial tortuosity, cataract, developmental microphthalmia, and Axenfeld–Rieger syndrome Other affected organs: kidney, anemia, muscular cramps, cardiac arrhythmias, and Raynaud’s PADMAL: dysarthria, ataxia, paresis, mood disturbance, gait abnormality, stroke and dementia. |
White matter disease, lacunar infarcts, intracranial aneurysms of carotid siphon even in asymptomatic PADMAL: lacunar infarcts of BG, brain stem, pons, and periventricularly, pyramidal tract degeneration |
• Intra and extracellular accumulation of defective collagen in vessel walls, small vessel fragility and barrier dysfunction • Basement membrane instability • PADMAL: proliferation of intima, increased elastic fibers, atrophy of tunica media of arterioles |
|
| Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic manifestations (RVCL-S) |
TREX1 (Three prime Repair EXonuclease 1) Autosomal dominant |
C-terminal frameshift mutations, mis-localization, immune dysfunction | Exceedingly rare (<100 families known) | Brain capillary rarefaction, strokes, tumor-like lesions, proteinuria, hematuria, macular edema with perifoveal microangiopathic telangiectasias, migraines, psychiatric disturbances, possibly early death | Focal T2 hyperintense lesions (tumor like in appearance) in periventricular and deep WM Contrast-enhanced pseudotumors focal calcifications visible before symptoms Frontal lobes heavily affected; corpus callosum and infratentorial tissue spared |
• Thicker and multilayered vascular basement membrane • Vessels with fibrinoid vascular necrosis or thickened hyalinized walls |
| Fabry’s disease | GLA (α galactosidase A) X-linked recessive |
Insufficient activity of αGAL (early and late onset depend on x-inactivation and other factors) | 1 in 3,100–117,000 | Neuropathy, angiokeratomas, hypohidrosis, corneal opacity, and hearing loss. Internal organs, such as the kidney, heart or brain, may also be affected, leading to progressive kidney damage, heart attacks, and strokes (type 1 or early onset); diffuse white matter lesion with severe intracerebral hemorrhage and epilepsy can occur. Type 2 or late onset spears kidney and other organs | Deep WM lesions, T1 hyperintensities in pulvinar (thalamus) are pathognomic infarcts in posterior circulation and vertebrobasilar dolichoectasia microbleeds, lacunar infarcts |
• Accumulation of glycosphingolipids in ECs/VSMCs |
| Hereditary Cerebral Hemorrhage with Amyloidosis (HCHWA) | Dutch, Italian, Flemish, Iowa and Piedmont types: Aβ precursor protein (APP) Icelandic type: cystatin C (CST3) |
CAA associated disease | Rare | Misfolded Aβ 42 amyloid deposition in arteries, arterioles, capillaries, and veins, and parenchyma, degeneration of VSMCs Icelandic: amyloid fibril deposition in cerebral arteries, lymphoid organs, skin, salivary glands, testes |
CADASIL, caused by NOTCH3 mutations, is a uniquely pure form of CSVD that features insidious onset, gradually progressive neurobehavioral decline, chronic white matter disease (with involvement of anterior temporal lobes), lacunes, microbleeds, and migraines 35. Less frequently, parkinsonism or seizures have also been reported 36. Separate from CADASIL, NOTCH3 mutation disease can present with either syndromic findings or lead to other neurological presentations37; NOTCH3 mutations are also associated with AD 38 and Parkinson’s disease syndromes 39. Recent studies indicate that up to 1:300 individuals carry mutations in NOTCH3 40,41.
CARASIL, an autosomal recessive disease related to mutations in HTRA1, presents with CSVD and non-neurological symptoms of alopecia, spondyloarthropathies and changes in vision. Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic manifestations (RVCL-S), affects mainly brain and retina and is caused by mutations in TREX1. A unique feature of RVCL is that lesions may appear and behave like tumors.
Gould syndrome caused by mutations in COL4A1 and COL4A2 leads to defects in extracellular matrix, notably vascular basement membranes with multi-organ involvement including a characteristic CSVD, cerebral cortical developmental abnormalities, myopathy, renal and lung dysfunction, and marked ophthalmic abnormalities with developmental defects (ocular dysgenesis). Mutations in the 3’ untranslated region of COL4A1 mRNA can cause PADMAL (Pontine Autosomal Dominant MicroAngiopathy with Leukoencephalopathy), characterized by early adult-onset pontine strokes.
Although these genetic CSVD syndromes can be confirmed easily using genetic testing, they are uncommon. Sporadic, age dependent CSVD is by far the most common cause of this condition in the general population. But discoveries in rare genetic VCID syndromes could inform the mechanisms and therapy of sporadic disease.
What is the biological basis of CSVD dementia?
Advanced morphological and molecular techniques have enabled a more nuanced understanding of the structural changes that result from CSVD. Further, advanced imaging has enabled investigations in cross-sectional and longitudinal cohorts. Physiological studies of animal models of CSVD, modeling human genetic disorders, permit investigation of how CSVD leads to functional alterations of the brain that are presumed to cause dementia. What has emerged from these multidisciplinary studies are three core potential mechanisms by which CSVD leads to imaging changes – an imperfect proxy for dementia.
Decreased cerebral blood flow.
Lowering of overall blood flow in CSVD has been suggested as a mechanism driving dementia. It is reasoned that CSVD results in chronic hypoperfusion, particularly to watershed regions such as the deep white matter, which demonstrates the most severe MRI abnormalities. If true, one would expect lower cerebral blood flow (CBF) in patients with CSVD; moreover, lower blood flow should predict development of MRI changes and dementia.
The drop in global cerebral blood flow in patients with CSVD has been observed in numerous populations in cross-sectional analyses 42–48, though not universally 49–51 . Meta-analysis describes an association between low cerebral blood flow and CSVD inferred by MRI 49 . In many studies in which blood flow dropped, the fall in flow was associated with atrophy, suggesting to some that flow changes were a result of tissue injury and not the cause of brain parenchymal damage. In longitudinal studies, there have been mixed results: some studies show CBF deficiency predicting MRI hyperintensities while others find falling CBF after development of lesions 52 53 54 55 . Further complicating the issue, regulation of flow may change at different stages of disease 56; in early CSVD, resting CBF may increase, raising the possibility of shunting that may injure tissue.
Protein markers of hypoxia within white matter lesions have been presented 57 in a histopathological study of postmortem brain. But whether these markers presage the development of structural changes is not clear.
Alteration of the blood brain barrier.
Other investigations have been presented that support CSVD causes brain parenchymal damage via changes in two vascular functions: 1) blood brain barrier integrity and 2) vasoreactivity determined by activity.
Structural abnormalities observed in CSVD can decrease BBB integrity. Pathological studies performed on autopsy-derived samples from patients with sporadic CSVD have shown extravasation of blood proteins 58–63. The ultrastructural components of the BBB are affected in mice expressing NOTCH3 mutant protein 64 . In CSVD patients 65–70, BBB breakdown has been described in multiple cohorts. Longitudinal studies suggest that areas of BBB breakdown predict evolution of WMH 71,72 . The relative importance of specific substances that leak through the BBB to damage brain parenchyma remains an unknown, but serum proteins have been suspected to be harmful. For example, fibrinogen can cross a leaky BBB 58,60 and has multiple cellular targets, including activation of microglia 73,74 and blockade of oligodendrocyte replacement 75.
Not all investigations support a role for BBB breakdown in CSVD. There was little evidence of BBB breakdown in neuropathological investigations of general CSVD 76 or in CADASIL pathological samples 77 and pre-clinical models 78 . Nonetheless, a causal role of BBB impairment deserves further investigation.
Attenuation of regional cerebral vasoreactivity.
The brain vasculature features an autoregulatory system that couples flow to demand at a microscopic level. Impaired activity-related changes in flow may selectively stress regions of metabolic demand, resulting in chronic and intermittent hypoperfusion that may not be appreciated in bulk CBF investigations.
Sam et al 79 reported decreased cerebrovascular reactivity in regional patterns that correlated with abnormal diffusion and perfusion patterns. Importantly, in longitudinal analysis, areas of loss of cerebrovascular reactivity developed white matter hyperintensities at one year follow-up 80,81 . Further, Blair and colleagues 82 described decreased vascular reactivity in CSVD as assessed by MRI BOLD during a hypercapnic challenge that was related to the burden of white matter hyperintensities.
In CADASIL, Chabriat et al 55 did not observe decreases in vasoreactivity in normal appearing white matter, although blood flow and reactivity were decreased in areas of white matter hyperintensity. But Liem et al described a cohort of CADASIL subjects with decrease reactivity to acetazolamide 83 that correlated with worsening of white matter hyperintensities 7 years later. Although total cerebral blood flow was decreased in this CADASIL cohort, unlike reactivity, it did not correspond with radiological worsening. Separately, ASL demonstrated impaired vascular reactivity in CADASIL in response to visual and motor stimulation 84 , with unimpaired evoked potentials.
In CADASIL animal models of CSVD, arteriolar vasoreactivity to chemical stimulation of downstream endothelial cells or to whisker stimulation is impaired via attenuation of an endothelial inward rectifying potassium channel Kir2.1. Features upstream of Kir2.1 impairment, including TIMP3-mediated suppression of EGFR signaling, implicates a potential comprehensive pathological pathway to CSVD85.
Other pathways to dementia.
A role for pathology of oligodendrocytes has also been investigated, though these sites of injury are likely downstream from the initiating vascular problem 86,87. In line with this, oligodendrocyte precursor cell progression, which is thought to aid in repair of white matter injury, has been shown to be inhibited by endothelial damage in cathepsin A-related arteriopathy88.
What are the core recommendations for the management of CSVD dementia?
Antihypertensives.
Treating hypertension prevents first and recurrent stroke, and, as the SPRINT trial demonstrated, intensive blood pressure targets reduced fatal and non-fatal cardiovascular events and all-cause mortality 889. Meta-analyses of randomized trials of antihypertensive treatments found that antihypertensive treatment reduces white matter hyperintensities, and more intensive therapy is more effective 90,91.
However, evidence for intensive blood pressure control having a beneficial effect on cognitive outcomes (cognitive decline, MCI, or dementia) is not certain. SPRINT found that intensive blood pressure control reduced the incidence of MCI but did not significantly reduce risk of dementia 92. A SPRINT substudy did not show benefit from intensive therapy on memory or speed of mental processing 93. This lack of clear benefit on dementia, memory, or speed of mental processing is particularly disappointing because SPRINT found favorable effects of intensive blood pressure lowering on white matter disease, whole brain volume, and cerebral blood flow (Table 2). A meta-analysis involving over seventeen thousand patients found no association of lower versus standard blood pressure targets with incidence of cognitive decline, MCI, and dementia 98. It is possible that the neutral results from intensive blood pressure trials are the product of intervening later in life and not following patients long enough to detect treatment group curve separation.
Table 2.
Lessons from SPRINT trial and SPRINT MIND substudy. Target for intensive systolic BP control was <120 mm Hg and for standard systolic BP control <140 mm Hg.
| Clinical outcomes | |
|---|---|
| Wright et al 89 | Intensive BP therapy did not reduce risk of stroke but did reduce all-cause mortality by 27%. |
| Williamson et al92 | Intensive BP group had a significantly reduced risk of MCI and composite endpoint of MCI and dementia compared to standard group. |
| Rapp et al94 | No clinically significant difference was observed between intensive and standard treatment groups for memory or processing speed. |
| Radiographic and physiological outcomes | |
| Nasrallah et al95 | Intensive BP group had 0.54cm3 less increase in white matter lesion volume than the standard group over a median follow-up of 3.40 years. |
| Goldstein et al96 | SPRINT-MIND post hoc analysis showed that use of ACE inhibitors was most consistently associated with decreased white matter progression. |
| Nasrallah et al95 | Intensive BP group had 3.7 cm3 less total brain volume loss than standard BP group. |
| Dolui et al97 | Intensive BP group had 2.30 ml/100g/min higher whole brain perfusion change than standard BP group. |
BP denotes blood pressure; MCI, mild cognitive impairment.
While blood pressure lowering has been shown to improve radiographic and physiological parameters, on balance one cannot currently conclude that intensive pharmacological blood pressure lowering definitively protects patients from dementia. However, there does not appear to be cognitive harm from lowering blood pressure. Elderly patients are at greatest risk of incident cognitive impairment and dementia, and older patients show the same relative risk reduction of cardiovascular events as younger patients from pharmacological lowering of blood pressure 99.
Antiplatelet therapy.
Antiplatelet therapy has been studied extensively in patients with ischemic strokes due to CSVD. A meta-analysis of seventeen trials found that single antiplatelet therapy (SAPT) effectively reduces risk of recurrent stroke in patients with recent small subcortical infarct 100. However, the SPS3 trial found that dual antiplatelet therapy (DAPT) with aspirin and clopidogrel doubled risk of bleeding without lowering the risk of recurrent stroke 101. Much less is known about the effects of antiplatelets on dementia associated with CSVD.
Table 3 proposes how to manage antiplatelet therapies in patients with cognitive impairment based on clinical and imaging characteristics. In patients without recent small subcortical infarct who are found to have signs of CSVD either incidentally (e.g., for evaluation of headache) or during evaluation of MCI or dementia probably ought not be prescribed aspirin unless they have manifest atherosclerotic disease. A series of recent randomized trials do not support aspirin for primary prevention in several adult populations without manifest atherosclerosis 102. Also, when the timing of occurrence of a radiographically detected lacune is unknown (asymptomatic and not DWI+), aspirin may well be of no benefit in reducing recurrence. A meta-analysis of secondary prevention trials showed that benefits in reducing recurrent stroke seen before 12 weeks of stroke onset were no longer evident beyond 12 weeks 103. To add further concern about broad use of antiplatelets for CSVD, microbleeds are a marker of risk of hemorrhagic stroke in patients taking aspirin, and meta-analysis of 37 observational studies showed that antiplatelet therapy increases the risk of lobar CMBs and intracranial hemorrhage 104.
Table 3.
Use of antiplatelet therapy in patients with MCI or dementia and CSVD.
| Recent small subcortical infarct | Lacune of presumed vascular origin with symptomatic atherosclerosis | Lacune of presumed vascular origin without symptomatic atherosclerosis | Lacune of presumed vascular origin with >5–10 CMBs and no symptomatic atherosclerosis | CSVD without lacunes, with symptomatic atherosclerosis | CSVD without lacunes, without symptomatic atherosclerosis | |
| Indicated | SAPT & DAPT | SAPT | SAPT | |||
| Possibly indicated | SAPT | SAPT | ||||
| Contraindicated | DAPT | DAPT | SAPT & DAPT | DAPT | DAPT |
CMB denotes cerebral microbleeds; DAPT, dual antiplatelet therapy; and SAPT, single antiplatelet therapy.
Statins.
A meta-analysis of studies through 2017, found that use of statins reduces the risk of all-type dementia, mild cognitive impairment, and AD 105. Interestingly, statin use did not significantly lower the risk of vascular dementia 105. Ott et al analyzed the literature up to 2015 and suggested that fears that statins impair cognition appear unfounded 106.
Though trial data are limited, statins appear to reduce new silent infarcts and reduce white matter hyperintensities 107. Statins have no appreciable effect on microbleeds overall but may increase risk of lobar bleeds 108. Interestingly, a 2×2 factorial trial of telmisartan and low dose rosuvastatin in elderly hypertensive individuals, found that rosuvastatin lowered the incidence of Fazekas ≥2 white matter changes and that there was a favorable interaction with telmisartan 109. At this point, it is appropriate to use statins in accordance with primary and secondary guidelines for prevention of cardiovascular and cerebrovascular disease 110.
Diabetes control.
Randomized trials have shown that intensive glycemic control in patients with diabetes reduces risk of microvascular complications (neuropathy, nephropathy, and retinopathy), but has not been shown to reduce risk of impaired memory or cognitive function111. There is limited evidence for intensive glycemic control preserving small vessel function. However, diabetes is not a contraindication to tight blood pressure control. Secondary analysis of the ACCORD MIND trial showed that in patients with type 2 diabetes mellitus tight blood pressure control reduced progression in white matter hyperintensities 112.
Cholinesterase inhibitors.
Many patients with vascular cognitive impairment will have CSVD. Cholinesterase inhibitors have demonstrated modest significant benefit in cognition in patients with vascular dementia 113. However, this modest benefit comes at a cost of side-effects that may include dizziness, nausea, vomiting, and diarrhea. If the patient is willing to accept the risks, a therapeutic trial is reasonable, but if clinically significant benefits are not observed by 3 months, the medication should be discontinued.
Conclusions and future directions
CSVD has attracted significant attention due to its high prevalence, impact on neurological health, and clear role in dementia. Several features of CSVD-related dementia have been highlighted: 1) CSVD-related dementia is a group of heterogenous pathologies that currently depend on MRI imaging for diagnosis; 2) CSVD is not deterministic of cognitive impairment but is an important risk factor for dementia; 3) CSVD-related dementia very often co-exists with other neurodegenerative conditions that are obscure its recognition; 4) CSVD may cause brain injury via physiological mechanisms which extend beyond simple blood flow reduction; 5) monogenic forms of CSVD dementia have been described and promise to provide important footholds to understand mechanisms of disease; and 6) emerging evidence indicates that wisely selected cardiovascular medications could modify disease. Many of these features have been supported by recent work, but none are definitive; what is more certain is that rigorous investigations are still needed, particularly with respect to establishing robust, standardized disease markers that enable refinement of natural history, mechanistic, and therapeutic studies.
Supplementary Material
Acknowledgements
We thank Soo Jung Lee for histology and preparation of images.
Funding sources
FME receives support from the Department of Veterans Affairs and the National Institute of Aging (IK2CX002180), Larry L. Hillblom Foundation (2019A012SUP), and New Vision Research.
MMW is supported by grants from the Department of Veterans Affairs (BX003824) and the NIH (NS099160). He has also received support from CADASIL Together We Have Hope.
JFM is supported by grants from the National Institute of Neurological Disorders and Stroke for the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2; U01NS080168); CREST-2-Hemodynamics (CREST-H; R01NS097876); the Long-term Observational Extension of Participants in the CREST-2 Randomized Clinical Trial (C2LOE; U01 NS119169); and the DISCOVERY Recruitment and Retention Core (DISCOVERY RRC; U19NS115388).
Non-standard Abbreviations and Acronyms
- ACCORD MIND
Action to Control Cardiovasular Risk in Diabetes - Memory in Diabetes
- AD
Alzheimer’s Disease
- ADDTC
Alzheimer’s Disease Diagnostic and Treatment Centers
- ARIEN
Association Internationale pour la Recherche et l’Enseignement en Neurosciences
- BBB
blood brain barrier
- CAA
cerebral amyloid angiopathy
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CBF
cerebral blood flow
- CMB
cerebral microbleeds
- CSF
cerebral spinal fluid
- CVSD
cerebral small vessel disease
- DAPT
dual antiplatelet therapy
- DCE-MRI
dynamic contrast enhanced magnetic resonance imaging
- DWI
diffusion weighted imaging
- ICD-10
International Statistical Classification of Diseases and Related Health Problems Version 10
- MRI
magnetic resonance imaging
- NINDS
National Institute of Neurological Disorders and Stroke
- SAPT
single antiplatelet therapy
- SPRINT
Systolic Blood Pressure Intervention Trial
- STRIVE
STandards for Reporting Vascular changes on Euroimaging
- VCID
vascular cognitive impairment and dementia
- WMH
white matter hyperintensity
Footnotes
Disclosure statement
FME, MMW have no competing interests. JFM reports compensation from NIH for data and safety monitoring services.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jokinen H, Koikkalainen J, Laakso HM, Melkas S, Nieminen T, Brander A, Korvenoja A, Rueckert D, Barkhof F, Scheltens P, et al. Global Burden of Small Vessel Disease-Related Brain Changes on MRI Predicts Cognitive and Functional Decline. Stroke. 2020;51:170–178. doi: 10.1161/STROKEAHA.119.026170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bown CW, Carare RO, Schrag MS, Jefferson AL. Physiology and Clinical Relevance of Enlarged Perivascular Spaces in the Aging Brain. Neurology. 2022;98:107–117. doi: 10.1212/WNL.0000000000013077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maillard P, Fletcher E, Singh B, Martinez O, Johnson DK, Olichney JM, Farias ST, DeCarli C. Cerebral white matter free water: A sensitive biomarker of cognition and function. Neurology. 2019;92:e2221–e2231. doi: 10.1212/WNL.0000000000007449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja R, Rosenberg GA, Caprihan A. MRI measurements of Blood-Brain Barrier function in dementia: A review of recent studies. Neuropharmacology. 2018;134:259–271. doi: 10.1016/j.neuropharm.2017.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohjasvaara T, Mäntylä R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Stroke. 2000;31:2952–2957. doi: 10.1161/01.str.31.12.2952 [DOI] [PubMed] [Google Scholar]
- 7.Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, Mayor PA, Michel JP, Giannakopoulos P. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002;159:82–87. doi: 10.1176/appi.ajp.159.1.82 [DOI] [PubMed] [Google Scholar]
- 8.McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, Hortobagyi T, Ince P, Jellinger K, Gao J, et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 2016;14:129. doi: 10.1186/s12916-016-0676-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys CA, Jansen MA, Muñoz Maniega S, González-Castro V, Pernet C, Deary IJ, Al-Shahi Salman R, Wardlaw JM, Smith C. A protocol for precise comparisons of small vessel disease lesions between ex vivo magnetic resonance imaging and histopathology. Int J Stroke. 2019;14:310–320. doi: 10.1177/1747493018799962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaffashian S, Soumaré A, Zhu YC, Mazoyer B, Debette S, Tzourio C. Long-Term Clinical Impact of Vascular Brain Lesions on Magnetic Resonance Imaging in Older Adults in the Population. Stroke. 2016;47:2865–2869. doi: 10.1161/STROKEAHA.116.014695 [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, Romero JR, Kase CS, Wolf PA, Seshadri S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109 [DOI] [PubMed] [Google Scholar]
- 13.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531 [DOI] [PubMed] [Google Scholar]
- 14.Bombois S, Debette S, Bruandet A, Delbeuck X, Delmaire C, Leys D, Pasquier F. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke. 2008;39:2046–2051. doi: 10.1161/STROKEAHA.107.505206 [DOI] [PubMed] [Google Scholar]
- 15.Eckerström C, Olsson E, Klasson N, Berge J, Nordlund A, Bjerke M, Wallin A. Multimodal prediction of dementia with up to 10 years follow up: the Gothenburg MCI study. J Alzheimers Dis. 2015;44:205–214. doi: 10.3233/JAD-141053 [DOI] [PubMed] [Google Scholar]
- 16.Firbank MJ, Burton EJ, Barber R, Stephens S, Kenny RA, Ballard C, Kalaria RN, O’Brien JT. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging. 2007;28:1664–1669. doi: 10.1016/j.neurobiolaging.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Geroldi C, Rossi R, Calvagna C, Testa C, Bresciani L, Binetti G, Zanetti O, Frisoni GB. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:1219–1222. doi: 10.1136/jnnp.2005.082651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, van der Flier WM, Scheltens P, Barkhof F, Visser MC, Fazekas F, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc Dis. 2009;27:384–391. doi: 10.1159/000207442 [DOI] [PubMed] [Google Scholar]
- 19.Kantarci K, Weigand SD, Przybelski SA, Shiung MM, Whitwell JL, Negash S, Knopman DS, Boeve BF, O’Brien PC, Petersen RC, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72:1519–1525. doi: 10.1212/WNL.0b013e3181a2e864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Choi SH, Lee YM, Kim MJ, Kim YD, Kim JY, Park JH, Myung W, Na HR, Han HJ, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatr. 2015;27:2069–2077. doi: 10.1017/S1041610215001076 [DOI] [PubMed] [Google Scholar]
- 21.Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, Tanzi RE, Albert MS, Greenberg SM, Guttmann CR. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23 [DOI] [PubMed] [Google Scholar]
- 22.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76:81–94. doi: 10.1001/jamaneurol.2018.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–173. doi: 10.1016/j.neubiorev.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos D, Wolters FJ, Darweesh SKL, Vernooij MW, de Wolf F, Ikram MA, Hofman A. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimers Dement. 2018;14:1482–1492. doi: 10.1016/j.jalz.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685 [DOI] [PubMed] [Google Scholar]
- 26.Zhu YC, Dufouil C, Soumaré A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimers Dis. 2010;22:663–672. doi: 10.3233/JAD-2010-100378 [DOI] [PubMed] [Google Scholar]
- 27.Ding J, Sigurðsson S, Jónsson PV, Eiriksdottir G, Charidimou A, Lopez OL, van Buchem MA, Guðnason V, Launer LJ. Large Perivascular Spaces Visible on Magnetic Resonance Imaging, Cerebral Small Vessel Disease Progression, and Risk of Dementia: The Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol. 2017;74:1105–1112. doi: 10.1001/jamaneurol.2017.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elahi FM, Miller BL. A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol. 2017;13:457–476. doi: 10.1038/nrneurol.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017;74:1246–1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003;62:1287–1301. doi: 10.1093/jnen/62.12.1287 [DOI] [PubMed] [Google Scholar]
- 33.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iadecola C The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9 [DOI] [PubMed] [Google Scholar]
- 36.Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Shungu DC, Naini AB, Mohr JP. CADASIL in a North American family: clinical, pathologic, and radiologic findings. Neurology. 1998;51:844–849. doi: 10.1212/wnl.51.3.844 [DOI] [PubMed] [Google Scholar]
- 37.Rutten JW, Hack RJ, Duering M, Gravesteijn G, Dauwerse JG, Overzier M, van den Akker EB, Slagboom E, Holstege H, Nho K, et al. Broad phenotype of cysteine-altering. Neurology. 2020;95:e1835–e1843. doi: 10.1212/WNL.0000000000010525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassi C, Nalls MA, Ridge PG, Gibbs JR, Lupton MK, Troakes C, Lunnon K, Al-Sarraj S, Brown KS, Medway C, et al. Mendelian adult-onset leukodystrophy genes in Alzheimer’s disease: critical influence of CSF1R and NOTCH3. Neurobiol Aging. 2018;66:179.e117–179.e129. doi: 10.1016/j.neurobiolaging.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramirez J, Dilliott AA, Binns MA, Breen DP, Evans EC, Beaton D, McLaughlin PM, Kwan D, Holmes MF, Ozzoude M, et al. Parkinson’s Disease, NOTCH3 Genetic Variants, and White Matter Hyperintensities. Mov Disord. 2020;35:2090–2095. doi: 10.1002/mds.28171 [DOI] [PubMed] [Google Scholar]
- 40.Rutten JW, Dauwerse HG, Gravesteijn G, van Belzen MJ, van der Grond J, Polke JM, Bernal-Quiros M, Lesnik Oberstein SA. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol. 2016;3:844–853. doi: 10.1002/acn3.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho BPH, Nannoni S, Harshfield EL, Tozer D, Gräf S, Bell S, Markus HS. variants are more common than expected in the general population and associated with stroke and vascular dementia: an analysis of 200 000 participants. J Neurol Neurosurg Psychiatry. 2021;92:694–701. doi: 10.1136/jnnp-2020-325838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisschops RH, van der Graaf Y, Mali WP, van der Grond J, group Ss. High total cerebral blood flow is associated with a decrease of white matter lesions. J Neurol. 2004;251:1481–1485. doi: 10.1007/s00415-004-0569-y [DOI] [PubMed] [Google Scholar]
- 43.van Es AC, van der Grond J, ten Dam VH, de Craen AJ, Blauw GJ, Westendorp RG, Admiraal-Behloul F, van Buchem MA, Group PS. Associations between total cerebral blood flow and age related changes of the brain. PLoS One. 2010;5:e9825. doi: 10.1371/journal.pone.0009825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Vrooman HA, Hofman A, Krestin GP, Breteler MM. Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008;28:412–419. doi: 10.1038/sj.jcbfm.9600526 [DOI] [PubMed] [Google Scholar]
- 45.Crane DE, Black SE, Ganda A, Mikulis DJ, Nestor SM, Donahue MJ, MacIntosh BJ. Gray matter blood flow and volume are reduced in association with white matter hyperintensity lesion burden: a cross-sectional MRI study. Front Aging Neurosci. 2015;7:131. doi: 10.3389/fnagi.2015.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heliopoulos I, Artemis D, Vadikolias K, Tripsianis G, Piperidou C, Tsivgoulis G. Association of ultrasonographic parameters with subclinical white-matter hyperintensities in hypertensive patients. Cardiovasc Psychiatry Neurol. 2012;2012:616572. doi: 10.1155/2012/616572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tzourio C, Lévy C, Dufouil C, Touboul PJ, Ducimetière P, Alpérovitch A. Low cerebral blood flow velocity and risk of white matter hyperintensities. Ann Neurol. 2001;49:411–414. [PubMed] [Google Scholar]
- 48.Alosco ML, Brickman AM, Spitznagel MB, Garcia SL, Narkhede A, Griffith EY, Raz N, Cohen R, Sweet LH, Colbert LH, et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest Heart Fail. 2013;19:E29–34. doi: 10.1111/chf.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi Y, Thrippleton MJ, Makin SD, Marshall I, Geerlings MI, de Craen AJM, van Buchem MA, Wardlaw JM. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2016;36:1653–1667. doi: 10.1177/0271678X16662891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ott BR, Faberman RS, Noto RB, Rogg JM, Hough TJ, Tung GA, Spencer PK. A SPECT imaging study of MRI white matter hyperintensity in patients with degenerative dementia. Dement Geriatr Cogn Disord. 1997;8:348–354. doi: 10.1159/000106654 [DOI] [PubMed] [Google Scholar]
- 51.Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, van Swieten JC, van Harskamp F, Hofman A. Vascular risk factors, atherosclerosis, cerebral white matter lesions and cerebral perfusion in a population-based study. Eur J Nucl Med. 1996;23:675–682. doi: 10.1007/BF00834530 [DOI] [PubMed] [Google Scholar]
- 52.Nylander R, Fahlström M, Rostrup E, Kullberg J, Damangir S, Ahlström H, Lind L, Larsson EM. Quantitative and qualitative MRI evaluation of cerebral small vessel disease in an elderly population: a longitudinal study. Acta Radiol. 2018;59:612–618. doi: 10.1177/0284185117727567 [DOI] [PubMed] [Google Scholar]
- 53.Promjunyakul NO, Dodge HH, Lahna D, Boespflug EL, Kaye JA, Rooney WD, Silbert LC. Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology. 2018;90:e2119–e2126. doi: 10.1212/WNL.0000000000005684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Veen PH, Muller M, Vincken KL, Hendrikse J, Mali WP, van der Graaf Y, Geerlings MI, Group SS. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke. 2015;46:1233–1238. doi: 10.1161/STROKEAHA.114.008030 [DOI] [PubMed] [Google Scholar]
- 55.Chabriat H, Pappata S, Ostergaard L, Clark CA, Pachot-Clouard M, Vahedi K, Jobert A, Le Bihan D, Bousser MG. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. 2000;31:1904–1912. doi: 10.1161/01.str.31.8.1904 [DOI] [PubMed] [Google Scholar]
- 56.Østergaard L, Engedal TS, Moreton F, Hansen MB, Wardlaw JM, Dalkara T, Markus HS, Muir KW. Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab. 2016;36:302–325. doi: 10.1177/0271678X15606723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14 [DOI] [PubMed] [Google Scholar]
- 58.Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, Kimura J. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer’s disease patients. Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069 [DOI] [PubMed] [Google Scholar]
- 59.Utter S, Tamboli IY, Walter J, Upadhaya AR, Birkenmeier G, Pietrzik CU, Ghebremedhin E, Thal DR. Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol. 2008;67:842–856. doi: 10.1097/NEN.0b013e3181836a71 [DOI] [PubMed] [Google Scholar]
- 60.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in Binswanger’s disease; an immunohistochemical study. Acta Neuropathol. 1998;95:78–84. doi: 10.1007/s004010050768 [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Meng H, Blaivas M, Rushing EJ, Moore BE, Schwartz J, Lopes MB, Worrall BB, Wang MM. Von Willebrand Factor permeates small vessels in CADASIL and inhibits smooth muscle gene expression. Transl Stroke Res. 2012;3:138–145. doi: 10.1007/s12975-011-0112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpson JE, Wharton SB, Cooper J, Gelsthorpe C, Baxter L, Forster G, Shaw PJ, Savva G, Matthews FE, Brayne C, et al. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci Lett. 2010;486:246–251. doi: 10.1016/j.neulet.2010.09.063 [DOI] [PubMed] [Google Scholar]
- 63.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54 [DOI] [PubMed] [Google Scholar]
- 64.Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol. 2015;78:887–900. doi: 10.1002/ana.24512 [DOI] [PubMed] [Google Scholar]
- 65.Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072 [DOI] [PubMed] [Google Scholar]
- 67.Wardlaw JM, Doubal FN, Valdes-Hernandez M, Wang X, Chappell FM, Shuler K, Armitage PA, Carpenter TC, Dennis MS. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke. 2013;44:525–527. doi: 10.1161/STROKEAHA.112.669994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Li M, Zuo L, Shi Q, Qin W, Yang L, Jiang T, Hu W. Compromised Blood-Brain Barrier Integrity Is Associated With Total Magnetic Resonance Imaging Burden of Cerebral Small Vessel Disease. Front Neurol. 2018;9:221. doi: 10.3389/fneur.2018.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang CE, Wong SM, Uiterwijk R, Backes WH, Jansen JFA, Jeukens CRLP, van Oostenbrugge RJ, Staals J. Blood-brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging. Brain Imaging Behav. 2019;13:389–395. doi: 10.1007/s11682-018-9855-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muñoz Maniega S, Chappell FM, Valdés Hernández MC, Armitage PA, Makin SD, Heye AK, Thrippleton MJ, Sakka E, Shuler K, Dennis MS, et al. Integrity of normal-appearing white matter: Influence of age, visible lesion burden and hypertension in patients with small-vessel disease. J Cereb Blood Flow Metab. 2017;37:644–656. doi: 10.1177/0271678X16635657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-Term Blood-Brain Barrier Permeability Changes in Binswanger Disease. Stroke. 2015;46:2413–2418. doi: 10.1161/STROKEAHA.115.009589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wardlaw JM, Makin SJ, Valdés Hernández MC, Armitage PA, Heye AK, Chappell FM, Muñoz-Maniega S, Sakka E, Shuler K, Dennis MS, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer’s & Dementia. 2017;13:634–643. doi: 10.1016/j.jalz.2016.09.006 [DOI] [Google Scholar]
- 73.Merlini M, Rafalski VA, Rios Coronado PE, Gill TM, Ellisman M, Muthukumar G, Subramanian KS, Ryu JK, Syme CA, Davalos D, et al. Fibrinogen Induces Microglia-Mediated Spine Elimination and Cognitive Impairment in an Alzheimer’s Disease Model. Neuron. 2019;101:1099–1108.e1096. doi: 10.1016/j.neuron.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ryu JK, McLarnon JG. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J Cell Mol Med. 2009;13:2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen MA, Ryu JK, Chang KJ, Etxeberria A, Bardehle S, Mendiola AS, Kamau-Devers W, Fancy SPJ, Thor A, Bushong EA, et al. Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron. 2017;96:1003–1012.e1007. doi: 10.1016/j.neuron.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015;25:44–50. doi: 10.1111/bpa.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajani RM, Ratelade J, Domenga-Denier V, Hase Y, Kalimo H, Kalaria RN, Joutel A. Blood brain barrier leakage is not a consistent feature of white matter lesions in CADASIL. Acta Neuropathol Commun. 2019;7:187. doi: 10.1186/s40478-019-0844-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joutel A, Monet-Leprêtre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, Lemaire-Carrette B, Domenga V, Schedl A, Lacombe P, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–445. doi: 10.1172/JCI39733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sam K, Peltenburg B, Conklin J, Sobczyk O, Poublanc J, Crawley AP, Mandell DM, Venkatraghavan L, Duffin J, Fisher JA, et al. Cerebrovascular reactivity and white matter integrity. Neurology. 2016;87:2333–2339. doi: 10.1212/WNL.0000000000003373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sam K, Crawley AP, Conklin J, Poublanc J, Sobczyk O, Mandell DM, Venkatraghavan L, Duffin J, Fisher JA, Black SE, et al. Development of White Matter Hyperintensity Is Preceded by Reduced Cerebrovascular Reactivity. Ann Neurol. 2016;80:277–285. doi: 10.1002/ana.24712 [DOI] [PubMed] [Google Scholar]
- 81.Sam K, Conklin J, Holmes KR, Sobczyk O, Poublanc J, Crawley AP, Mandell DM, Venkatraghavan L, Duffin J, Fisher JA, et al. Impaired dynamic cerebrovascular response to hypercapnia predicts development of white matter hyperintensities. Neuroimage Clin. 2016;11:796–801. doi: 10.1016/j.nicl.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blair GW, Thrippleton MJ, Shi Y, Hamilton I, Stringer M, Chappell F, Dickie DA, Andrews P, Marshall I, Doubal FN, et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology. 2020;94:e2258–e2269. doi: 10.1212/WNL.0000000000009483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liem MK, Lesnik Oberstein SA, Haan J, Boom R, Ferrari MD, Buchem MA, Grond J. Cerebrovascular reactivity is a main determinant of white matter hyperintensity progression in CADASIL. AJNR Am J Neuroradiol. 2009;30:1244–1247. doi: 10.3174/ajnr.A1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huneau C, Houot M, Joutel A, Béranger B, Giroux C, Benali H, Chabriat H. Altered dynamics of neurovascular coupling in CADASIL. Ann Clin Transl Neurol. 2018;5:788–802. doi: 10.1002/acn3.574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dabertrand F, Harraz OF, Koide M, Longden TA, Rosehart AC, Hill-Eubanks DC, Joutel A, Nelson MT. PIP. Proc Natl Acad Sci U S A. 2021;118. doi: 10.1073/pnas.2025998118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajani RM, Williams A. Endothelial cell-oligodendrocyte interactions in small vessel disease and aging. Clin Sci (Lond). 2017;131:369–379. doi: 10.1042/CS20160618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joutel A, Chabriat H. Pathogenesis of white matter changes in cerebral small vessel diseases: beyond vessel-intrinsic mechanisms. Clin Sci (Lond). 2017;131:635–651. doi: 10.1042/CS20160380 [DOI] [PubMed] [Google Scholar]
- 88.Bugiani M, Kevelam SH, Bakels HS, Waisfisz Q, Ceuterick-de Groote C, Niessen HW, Abbink TE, Lesnik Oberstein SA, van der Knaap MS. Cathepsin A-related arteriopathy with strokes and leukoencephalopathy (CARASAL). Neurology. 2016;87:1777–1786. doi: 10.1212/WNL.0000000000003251 [DOI] [PubMed] [Google Scholar]
- 89.Wright JT, Whelton PK, Johnson KC, Snyder JK, Reboussin DM, Cushman WC, Williamson JD, Pajewski NM, Cheung AK, Lewis CE, et al. SPRINT Revisited: Updated Results and Implications. Hypertension. 2021;78:1701–1710. doi: 10.1161/HYPERTENSIONAHA.121.17682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su C, Wu H, Yang X, Zhao B, Zhao R. The relation between antihypertensive treatment and progression of cerebral small vessel disease: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100:e26749. doi: 10.1097/MD.0000000000026749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai Y, Jiang C, Du X, Sang C, Guo X, Bai R, Tang R, Dong J, Ma C. Effect of intensive blood pressure control on the prevention of white matter hyperintensity: Systematic review and meta-analysis of randomized trials. J Clin Hypertens (Greenwich). 2020;22:1968–1973. doi: 10.1111/jch.14030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pearce LA, McClure LA, Anderson DC, Jacova C, Sharma M, Hart RG, Benavente OR, Investigators S. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol. 2014;13:1177–1185. doi: 10.1016/S1474-4422(14)70224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rapp SR, Gaussoin SA, Sachs BC, Chelune G, Supiano MA, Lerner AJ, Wadley VG, Wilson VM, Fine LJ, Whittle JC, et al. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol. 2020;19:899–907. doi: 10.1016/S1474-4422(20)30319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral White Matter Lesions. JAMA. 2019;322:524–534. doi: 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldstein ED, Wolcott Z, Garg G, Navarro K, Delic A, Yaghi S, Sederholm B, Prabhakaran S, Wong KH, McLean K, et al. Effect of Antihypertensives by Class on Cerebral Small Vessel Disease: A Post Hoc Analysis of SPRINT-MIND. Stroke. 2022;53:2435–2440. doi: 10.1161/STROKEAHA.121.037997 [DOI] [PubMed] [Google Scholar]
- 97.Dolui S, Detre JA, Gaussoin SA, Herrick JS, Wang DJJ, Tamura MK, Cho ME, Haley WE, Launer LJ, Punzi HA, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral Blood Flow: Secondary Analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Neurol. 2022;79:380–389. doi: 10.1001/jamaneurol.2022.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dallaire-Théroux C, Quesnel-Olivo MH, Brochu K, Bergeron F, O’Connor S, Turgeon AF, Laforce RJ, Verreault S, Camden MC, Duchesne S. Evaluation of Intensive vs Standard Blood Pressure Reduction and Association With Cognitive Decline and Dementia: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e2134553. doi: 10.1001/jamanetworkopen.2021.34553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collaboration BPLTT. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398:1053–1064. doi: 10.1016/S0140-6736(21)01921-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwok CS, Shoamanesh A, Copley HC, Myint PK, Loke YK, Benavente OR. Efficacy of antiplatelet therapy in secondary prevention following lacunar stroke: pooled analysis of randomized trials. Stroke. 2015;46:1014–1023. doi: 10.1161/STROKEAHA.114.008422 [DOI] [PubMed] [Google Scholar]
- 101.Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM, Group SS. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marquis-Gravel G, Roe MT, Harrington RA, Muñoz D, Hernandez AF, Jones WS. Revisiting the Role of Aspirin for the Primary Prevention of Cardiovascular Disease. Circulation. 2019;140:1115–1124. doi: 10.1161/CIRCULATIONAHA.119.040205 [DOI] [PubMed] [Google Scholar]
- 103.Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365–375. doi: 10.1016/S0140-6736(16)30468-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qiu J, Ye H, Wang J, Yan J, Wang Y. Antiplatelet Therapy, Cerebral Microbleeds, and Intracerebral Hemorrhage: A Meta-Analysis. Stroke. 2018;49:1751–1754. doi: 10.1161/STROKEAHA.118.021789 [DOI] [PubMed] [Google Scholar]
- 105.Chu CS, Tseng PT, Stubbs B, Chen TY, Tang CH, Li DJ, Yang WC, Chen YW, Wu CK, Veronese N, et al. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci Rep. 2018;8:5804. doi: 10.1038/s41598-018-24248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–358. doi: 10.1007/s11606-014-3115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Katsanos AH, Lioutas VA, Charidimou A, Catanese L, Ng KKH, Perera K, de Sa Boasquevisque D, Tsivgoulis G, Smith EE, Sharma M, et al. Statin treatment and accrual of covert cerebral ischaemia on neuroimaging: a systematic review and meta-analysis of randomized trials. Eur J Neurol. 2020;27:1023–1027. doi: 10.1111/ene.14196 [DOI] [PubMed] [Google Scholar]
- 108.Katsanos AH, Lioutas VA, Charidimou A, Catanese L, Ng KKH, Perera K, de Sa Boasquevisque D, Falcone GJ, Sheth KN, Romero JR, et al. Statin treatment and cerebral microbleeds: A systematic review and meta-analysis. J Neurol Sci. 2021;420:117224. doi: 10.1016/j.jns.2020.117224 [DOI] [PubMed] [Google Scholar]
- 109.Zhang H, Cui Y, Zhao Y, Dong Y, Duan D, Wang J, Sheng L, Ji T, Zhou T, Hu W, et al. Effects of sartans and low-dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: a randomized, double-blind and placebo-controlled clinical trial. Hypertens Res. 2019;42:717–729. doi: 10.1038/s41440-018-0165-7 [DOI] [PubMed] [Google Scholar]
- 110.Appleton JP, Scutt P, Sprigg N, Bath PM. Hypercholesterolaemia and vascular dementia. Clin Sci (Lond). 2017;131:1561–1578. doi: 10.1042/CS20160382 [DOI] [PubMed] [Google Scholar]
- 111.Crabtree T, Ogendo JJ, Vinogradova Y, Gordon J, Idris I. Intensive glycemic control and macrovascular, microvascular, hypoglycemia complications and mortality in older (age ≥60years) or frail adults with type 2 diabetes: a systematic review and meta-analysis from randomized controlled trial and observation studies. Expert Rev Endocrinol Metab. 2022;17:255–267. doi: 10.1080/17446651.2022.2079495 [DOI] [PubMed] [Google Scholar]
- 112.de Havenon A, Majersik JJ, Tirschwell DL, McNally JS, Stoddard G, Rost NS. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology. 2019;92:e1168–e1175. doi: 10.1212/WNL.0000000000007093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst Rev. 2021;2:CD013306. doi: 10.1002/14651858.CD013306.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.