Abstract
Background
Traditionally inhaled treatment for asthma has used separate preventer and reliever therapies. The combination of formoterol and budesonide in one inhaler has made possible a single inhaler for both prevention and relief of symptoms (single inhaler therapy or SiT).
Objectives
To assess the efficacy and safety of budesonide and formoterol in a single inhaler for maintenance and reliever therapy in asthma compared with maintenance with inhaled corticosteroids (ICS) (alone or as part of current best practice) and any reliever therapy.
Search methods
We searched the Cochrane Airways Group trials register in February 2013.
Selection criteria
Parallel, randomised controlled trials of 12 weeks or longer in adults and children with chronic asthma. Studies had to assess the combination of formoterol and budesonide as SiT, against a control group that received inhaled steroids and a separate reliever inhaler.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 13 trials involving 13,152 adults and one of the trials also involved 224 children (which have been separately reported). All studies were sponsored by the manufacturer of the SiT inhaler. We considered the nine studies assessing SiT against best practice to be at a low risk of selection bias, but a high risk of detection bias as they were unblinded.
In adults whose asthma was not well‐controlled on ICS, the reduction in hospital admission with SiT did not reach statistical significance (Peto odds ratio (OR) 0.81; 95% confidence interval (CI) 0.45 to 1.44, eight trials, N = 8841, low quality evidence due to risk of detection bias in open studies and imprecision). The rates of hospital admission were low; for every 1000 people treated with current best practice six would experience a hospital admission over six months compared with between three and eight treated with SiT. The odds of experiencing exacerbations needing treatment with oral steroids were lower with SiT compared with control (OR 0.83; 95% CI 0.70 to 0.98, eight trials, N = 8841, moderate quality evidence due to risk of detection bias). For every 100 adults treated with current best practice over six months, seven required a course of oral steroids, whilst for SiT there would be six (95% CI 5 to 7). The small reduction in time to first severe exacerbation needing medical intervention was not statistically significant (hazard ratio (HR) 0.94; 95% CI 0.85 to 1.04, five trials, N = 7355). Most trials demonstrated a reduction in the mean total daily dose of ICS with SiT (mean reduction was based on self‐reported data from patient diaries and ranged from 107 to 385 µg/day). Withdrawals due to adverse events were more common in people treated with SiT in comparison to current best practice (OR 2.85; 95% CI 1.89 to 4.30, moderate quality evidence due to risk of detection bias).
Three studies including 4209 adults compared SiT with higher dose budesonide maintenance and terbutaline for symptom relief. The studies were considered as low risk of bias. The run‐in for these studies involved withdrawal of LABA, and patients were recruited who were symptomatic during run‐in. The reduction in the odds of hospitalisation with SiT compared with higher dose ICS did not reach statistical significance (Peto OR; 0.56; 95% CI 0.28 to 1.09, moderate quality evidence due to imprecision). Fewer patients on SiT needed a course of oral corticosteroids (OR 0.54; 95% CI 0.45 to 0.64, high quality evidence). For every 100 adults treated with ICS over 11 months, 18 required a course of oral steroids, whilst for SiT there would be 11 (95% CI 9 to 12). Withdrawals due to adverse events were less common in people treated with SiT in comparison to higher dose budesonide maintenance (OR 0.57; 95% CI 0.35 to 0.93, high quality evidence).
One study included children (N = 224), in which SiT was compared with higher dose budesonide. There was a significant reduction in participants who needed an increase in their inhaled steroids with SiT, but there were only two hospitalisations for asthma and no separate data on courses of oral corticosteroids. Less inhaled and oral corticosteroids were used in the SiT group and the annual height gain was also 1 cm greater in the SiT group, (95% CI 0.3 cm to 1.7 cm).
The results for fatal serious adverse events were too rare to rule out either treatment being harmful. There was no significant difference found in non‐fatal serious adverse events for any of the comparisons.
Authors' conclusions
Single inhaler therapy has now been demonstrated to reduce exacerbations requiring oral corticosteroids against current best practice strategies and against a fixed higher dose of inhaled steroids. The strength of evidence that SiT reduces hospitalisation against these same treatments is weak. There were more discontinuations due to adverse events on SiT compared to current best practice, but no significant differences in serious adverse events. Our confidence in these conclusions is limited by the open‐label design of the trials, and by the unknown adherence to treatment in the current best practice arms of the trials.
Single inhaler therapy can reduce the risk of asthma exacerbations needing oral corticosteroids in comparison with fixed dose maintenance ICS and separate relief medication. The reduced odds of exacerbations with SiT compared with higher dose ICS should be viewed in the context of the possible impact of LABA withdrawal during study run‐in. This may have made the study populations more likely to respond to SiT.
Single inhaler therapy is not currently licensed for children under 18 years of age in the United Kingdom and there is currently very little research evidence for this approach in children or adolescents.
Plain language summary
In people with asthma are single inhalers that contain both formoterol and budesonide better than current best practice?
Background to the review ‘Single inhaler therapy’ means that a single inhaler containing two drugs is used. One of these drugs acts quickly and is called the "reliever". The other works much more slowly and is called the "preventer". The reliever is a beta‐agonist bronchodilator, which help to open the airways and help people breathe more easily. The preventer is a steroid that controls the underlying inflammation in the lungs, which is caused by the asthma. People on 'single inhaler therapy' (SiT) have one inhaler for use every day to control their underlying inflammation and also for symptom relief. The idea behind SiT is that when people take their inhalers to reduce their shortness of breath or wheezing they will also be getting an increased dose of the steroid preventer.
We wanted to discover whether using the SiT was better or worse than alternatives, such as receiving two separate inhalers for regular treatment and relief of symptoms. What did we do? We reviewed the clinical trials that looked at SiT against inhaled steroids and reliever medication given as two separate inhalers (sometimes called current best practice). What did we find out? We found 13 trials on 13,152 adults and one trial also included 224 children, up to February 2013. The trials were all sponsored by the manufacturer of the single inhaler. When compared with current best practice or higher doses of inhaled steroid, we found that SiT probably reduces the number of flare‐ups that will need treatment with an oral steroid in adults but we are uncertain whether the number of adults admitted to hospital would be reduced. When compared with high doses of inhaled steroids, we found that fewer people experienced a flare‐up that needed treatment with an oral steroid. The results for death (1 per 1000 people given either treatment), or life threatening problems (just under 50 per 1000 people given either treatment), were too imprecise to enable us to rule out either treatment being more harmful than the other. More adults left the trial early because they experienced adverse effects in the group taking single inhalers. There was only one small trial in children, so we are unable to make any firm conclusions in children. The studies were generally well‐designed, although in the studies which compared SiT against current best practice people knew which treatment they were getting, and this could have affected the reliability of the results. The studies comparing SiT against inhaled steroids were designed differently and were more reliable. Overall, we think that more evidence from future trials might change the strength of the conclusions for the question of whether SiT is better than current best practice. We believe that there is good quality evidence that SiT is more effective than high dose inhaled steroids, although the studies recruited people who were likely to respond.
Summary of findings
Summary of findings for the main comparison. 160/4.5 mcg BDF single inhaler therapy compared to current best practice for adult asthma that is not controlled on ICS.
| 160/4.5 µgBDF single inhaler therapy compared to current best practice for adults with asthma that is not controlled on ICS | ||||||
| Patient or population: adults with asthma that is not controlled on ICS Settings: community Intervention: 160/4.5 µg BDF single inhaler therapy Comparison: current best practice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Current best practice | 160/4.5 µgBDF single inhaler therapy | |||||
| Patients with exacerbations causing hospitalisation Follow‐up: mean 6 months | 6 per 1000 | 5 per 1000 (3 to 8) | OR 0.81 (0.45 to 1.44) | 8841 (8 studies) | ⊕⊕⊝⊝ low1,2 | |
| Patients with exacerbations treated with oral steroids Follow‐up: mean 6 months | 70 per 1000 | 59 per 1000 (50 to 69) | OR 0.83 (0.70 to 0.98) | 8841 (8 studies) | ⊕⊕⊕⊝ moderate1 | |
| Fatal serious adverse events Follow‐up: mean 6 months | 1 per 1000 | 1 per 1000 (0 to 5) | OR 1.95 (0.53 to 7.21) | 8841 (8 studies) | ⊕⊕⊝⊝ low1,2 | |
| Serious adverse events (non‐fatal) Follow‐up: mean 6 months | 20 per 1000 | 24 per 1000 (18 to 32) | OR 1.20 (0.90 to 1.60) | 8841 (8 studies) | ⊕⊕⊝⊝ low1,2 | |
| Discontinuation due to adverse events Follow‐up: mean 6 months | 7 per 1000 | 21 per 1000 (14 to 31) | OR 2.85 (1.89 to 4.3) | 8411 (7 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unblinded trials 2 Confidence interval cannot rule out important differences in either direction
BDF: budesonide plus formoterol; ICS: inhaled corticosteroids
Summary of findings 2. Single inhaler therapy compared to fixed dose ICS for asthma in adults not controlled on regular ICS.
| Single inhaler therapy compared to fixed dose ICS for asthma in adults not controlled on regular ICS | ||||||
| Patient or population: patients with asthma in adults not controlled on regular ICS Settings: community Intervention: Single inhaler therapy Comparison: fixed dose ICS | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Fixed dose ICS | Single inhaler therapy | |||||
| Patients with exacerbations causing hospitalisation Follow‐up: mean 11 months | 10 per 1000 | 6 per 1000 (3 to 11) | OR 0.56 (0.28 to 1.09) | 4209 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Patients with exacerbations treated with oral steroids Follow‐up: mean 11 months | 181 per 1000 | 107 per 1000 (90 to 124) | OR 0.54 (0.45 to 0.64) | 4280 (4 studies) | ⊕⊕⊕⊕ high | |
| Fatal serious adverse events Follow‐up: mean 11 months | 1 per 1000 | 1 per 1000 (0 to 4) | OR 0.37 (0.05 to 2.62) | 4209 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Serious adverse events (non‐fatal) Follow‐up: mean 11 months | 48 per 1000 | 47 per 1000 (36 to 62) | OR 0.97 (0.73 to 1.29) | 4209 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Discontinuation due to adverse events Follow‐up: mean 11 months | 36 per 1000 | 21 per 1000 (13 to 33) | OR 0.57 (0.35 to 0.93) | 2586 (2 studies) | ⊕⊕⊕⊕ high | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Confidence interval cannot rule out important differences in either direction ICS: inhaled corticosteroids
Background
Description of the condition
There is currently no universally accepted definition of the term 'asthma'. This is in part due to an overlap of symptoms with other diseases such as chronic bronchitis, but is also due to the probable existence of more than one underlying pathophysiological process. There are, for example, wide variations in the age of onset, symptoms, triggers, association with allergic disease and the type of inflammatory cell infiltrate seen in patients diagnosed with asthma. Patients will typically all have intermittent symptoms of cough, wheeze, breathlessness or both. Underlying these symptoms there is a process of variable, at least partially reversible airway obstruction, airway hyper responsiveness and (with the possible exception of solely exercise‐induced asthma) chronic inflammation.
Description of the intervention
People with persistent asthma can use preventer therapy (usually low dose inhaled corticosteroid (ICS)) to maintain symptom control, improve lung function and reduce emergency care requirement. However, when symptoms deteriorate reliever medication in the form of short‐acting beta2‐agonists such as salbutamol or terbutaline, or formoterol (a fast‐acting but longer lasting formulation) can be used on an 'as‐needed' basis (SIGN/BTS 2012). Since most exacerbations have an onset over several days (Tattersfield 1999), there is potential for the person with asthma to increase both budesonide and formoterol at an early stage in response to increased symptoms of asthma. The pharmacological properties of another long‐acting beta2‐agonist (LABA), salmeterol, result in slower onset of bronchodilation (Palmqvist 2001), and it is not licensed for use on an 'as‐needed' basis. The inclusion of ICS in a reliever inhaler for use during episodes of loss of control requires monitoring and assessment of overall ICS dose (SIGN/BTS 2012).
How the intervention might work
The combination of ICS and LABA in one inhaler is an effective way of delivering maintenance anti‐inflammatory and bronchodilator therapy in chronic asthma (Ducharme 2010; Ducharme 2011; Ni Chroinin 2005; Ni Chroinin 2009). The anti‐inflammatory properties of the ICS and the bronchodilatory effect of the LABA play complementary roles in reducing inflammation in the airways and improving lung function with relief of symptoms related to bronchospasm (Adams 2008; Walters 2007). Both are recommended when low dose ICS alone is not sufficient to control asthma, which is at step three in British asthma guidelines (SIGN/BTS 2012). Concerns have been raised about the use of single inhaler LABA in chronic asthma, in particular where it is used without a regular ICS, in relation to the possible increased risk of severe adverse events and asthma‐related death (Cates 2008; Cates 2010a; Cates 2012a; Cates 2012b; Walters 2007). The concomitant delivery of ICS and LABA avoids the inadvertent use of LABA without prescribed ICS treatment (Cates 2009a; Cates 2009b).
Why it is important to do this review
It is recognised that many patients who are prescribed ICS do not take their inhaler every day, and combination inhalers can increase ICS use both as maintenance (Delea 2008) and single inhaler therapy (SiT) (Sovani 2008). Whilst the trials that have investigated doubling the dose of ICS early in exacerbations have been disappointing (FitzGerald 2004; Harrison 2004), there is the potential with SiT for the patient to automatically increase both LABA and ICS when their asthma is worse and cut down again as their symptoms improve. This holds out the prospect of maintaining control of asthma and preventing exacerbations with lower overall exposure to ICS.
This review has identified and summarised clinical trials that compare SiT for maintenance and relief with budesonide/formoterol against maintenance with ICS and a separate reliever therapy. The 2013 update to this review now includes data from an additional 4556 adults from trials comparing SiT with current best practice. The comparison of budesonide/formoterol for maintenance and relief against a higher dose maintenance ICS/LABA combination inhaler and a separate reliever therapy will be covered in another review (Cates 2011).
Objectives
To assess the efficacy and safety of budesonide and formoterol in a single inhaler for maintenance and reliever therapy in asthma compared with maintenance with inhaled corticosteroids (alone or as part of current best practice) and any reliever therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials of parallel group design.
Types of participants
Adults and children with a diagnosis of chronic asthma. We accepted trialist‐defined asthma, recording the definition of asthma used in the studies, and the entry criteria. We did not include studies conducted in an emergency department setting.
Types of interventions
Eligible treatment group intervention
Combined inhaled steroid and long‐acting beta2‐agonist (LABA) delivered through a single inhaler device for regular maintenance and the relief of asthma symptoms.
Eligible control group treatment
Inhaled corticosteroid given as regular maintenance treatment with a separate reliever inhaler. This included "current best practice" according to local or international guidelines (which included an inhaled corticosteroid, GINA 2006).
We included studies lasting at least 12 weeks.
We did not consider studies that compared different combination therapy inhalers (regular fluticasone/salmeterol versus regular budesonide/formoterol has been reviewed elsewhere Lasserson 2008), or titration of maintenance dosing of combination therapy based on clinical signs and symptoms. Trials randomising participants to SiT versus a fixed dose combination inhaler are included in another review (Cates 2011).
Types of outcome measures
Primary outcomes
Patients with exacerbations requiring hospitalisation
Patients with exacerbations requiring oral corticosteroids
Serious adverse events (including mortality and life‐threatening events)
Growth (in children)
Secondary outcomes
Severe exacerbations (composite outcome of hospitalisation/emergency room (ER) visit/oral steroid course)
Diary card morning and evening peak expiratory flow (PEF)
Clinic spirometry (FEV1)
Number of rescue medication puffs required per day
Symptoms/symptom‐free days
Nocturnal awakenings
Quality of life
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
("single inhaler therapy" or SiT or SMART or relie* or "as need*" or as‐need* or prn or flexible or titrat*) and ((combin* or symbicort or viani) or ((steroid* or corticosteroid* or ICS or budesonide or BUD or Pulmicort or beclomethasone or BDP or becotide) and ("beta*agonist" or "beta*adrenergic agonist" or formoterol or eformoterol or oxis or foradil)))
Date of last search was February 2013.
Searching other resources
We contacted trialists and manufacturers to confirm data and establish whether other unpublished or ongoing studies were available for assessment. We handsearched clinical trials web sites (www.clinicaltrials.gov and www.fda.gov) and the clinical trial web sites of combination inhaler manufacturers (www.ctr.gsk.co.uk; www.astrazenecaclinicaltrials.com).
Data collection and analysis
Selection of studies
Following electronic literature searches, two review authors (CC & TL or CK) independently selected articles on the basis of title and/or abstract for full text scrutiny. The authors agreed a list of articles which were retrieved, and subsequently assessed each study to determine whether it was a secondary publication of a primary study publication, and to determine whether the study met the entry criteria of the review.
Data extraction and management
We extracted information from each study for the following characteristics.
Design (description of randomisation, blinding, number of study centres and location, number of study withdrawals)
Participants (N, mean age, age range of the study, gender ratio, baseline lung function, % on maintenance ICS or ICS/LABA combination & average daily dose of steroid (BDP (beclomethasone dipropionate) equivalent), entry criteria)
Intervention (type and dose of component ICS and LABA, control limb dosing schedule, intervention limb dose adjustment schedule, inhaler device, study duration and run‐in)
Outcomes (type of outcome analysis, outcomes analysed)
We summarised baseline severity of lung function and persistence of symptoms, and collected data on pre‐study maintenance therapies.
Assessment of risk of bias in included studies
We assessed trial bias protection in the following domains study quality according to whether studies meet the following pre‐specified quality criteria (as met, unmet or unclear, Higgins 2008).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Incomplete reporting of results (selective reporting bias)
Measures of treatment effect
We extracted data for each of the outcomes considered by the review from the trial publication(s) or from correspondence with the trialist or manufacturer. Exacerbations were the primary outcome for this review and were reported by subtype (hospitalisation and courses of oral steroids), rather than just as a composite outcome. Serious adverse events were considered separately as fatal and non‐fatal events.
Unit of analysis issues
We sought to obtain data from the trial sponsors that was reported with patients (rather than events) as the unit of analysis for the primary outcomes. Some patients may have suffered more than one exacerbation over the course of the studies and these events would not have been independent.
Data synthesis
Data were combined with RevMan 5.0, using a fixed‐effect mean difference (calculated as a weighted mean difference) for continuous data variables, and a fixed‐effect Odds Ratio for dichotomous variables. When zero cells were present for an outcome in any of the included studies the Peto Odds Ratio was used to combine the results as it does not require a continuity correction to be used. For the primary outcomes of exacerbations and serious adverse events, when a significant Odds Ratio was found, we calculated an number needed to treat (NNT) (benefit or harm) for the different levels of risk as represented by control group event rates over a specified time period using the pooled Odds Ratio and its confidence interval (Visual Rx).
Subgroup analysis and investigation of heterogeneity
We pooled data from adults and children in separate subgroups. Adult studies were considered as those that recruited participants from 18 years of age upwards. Adult and adolescent studies were considered as those that recruit participants from 12 years of age upwards. We considered participants in studies where the upper age limit was 12 years as children, and in studies where the upper age limit was 18 years as children and adolescents. Subgroup analyses were not possible in relation to asthma severity and degree of control of symptoms at baseline.
We measured statistical variation between combined studies by the I2 statistic (Higgins 2003). Where this exceeded 20%, we investigated the heterogeneity found, before deciding whether to combine the study results for the outcome.
Sensitivity analysis
Sensitivity analyses were planned on the basis of risk of bias in studies and methods of data analysis (fixed‐effect and random‐effects models).
'Summary of findings' tables
We applied methods recommended by the GRADE working group to rate the quality of evidence of SiT in adults. We present separate Summary of Findings tables for these two comparisons which also include estimates of the absolute effects based on the results of our analyses. We rated the quality of evidence for five main outcomes:
Patients with exacerbations causing hospitalisation
Patients with exacerbations treated with oral steroids
Fatal serious adverse events
Serious adverse events (non‐fatal)
Discontinuation due to adverse events
Results
Description of studies
Results of the search
An updated search was carried out in February 2013 and identified 72 new abstracts (since the search in September 2008). These were independently assessed for inclusion by CJC and CK. Additionally, a new citation and two web‐reports were identified by handsearching the identifiers for the included studies (Riemersma (NCT00235911) and DE‐SOLO). One of the previously identified ongoing studies was excluded as it compared two different SiT regimens (EUROSMART or NCT00463866a). New reports were found on the AstraZenca web site for the five other studies comparing single inhaler therapy (SiT) with current best practice (PASSION; Riemersma (NCT00235911); SPAIN; STYLE; SYMPHONIE) and paper publications were also identified for SPAIN (Quirce 2011) and Riemersma (NCT00235911) (Riemersma 2012).
The search that was previously carried out in September 2008 included 198 citations. From these, 51 were retrieved as full text articles, representing 24 unique studies. There were originally 10 studies (21 citations) included in the review and 14 studies (31 citations) that were excluded. Full details are given in the lists of Included studies and Excluded studies.
Included studies
Five adult studies contributed new outcome data on 4560 adults and adolescents comparing SiT with current best practice for the 2013 update (DE‐SOLO; PASSION; SPAIN; STYLE; SYMPHONIE). Furthermore, new data on the characteristics of Riemersma (NCT00235911) are now available, indicating that this study recruited participants with well‐controlled asthma (FEV1 was nearly 100% predicted), and used a lower dose of budesonide/formoterol than the other studies comparing SiT with current best practice. This trial has therefore been considered in a separate comparison for the 2013 update. All of the included studies were sponsored or supported by AstraZeneca, the manufacturers of Symbicort.
Thirteen studies involved 13,152 adults and adolescents, and one study also recruited children (STAY ‐ Children). The results from the 224 children included in the STAY study were reported in a separate paper by Bisgaard 2006. This has therefore been regarded separately from STAY ‐ Adults, which reported the adult results from the STAY study.
Intervention
The active treatment in most studies was budesonide/formoterol 160/4.5 µg one inhalation twice daily plus as‐needed; this is the delivered dose and is the same as 200/6 µg actuator dose described in some of the studies. In STEAM and Riemersma (NCT00235911), the maintenance treatment inhaler was 80/4.5 µg given as two inhalations in the evening, and in STAY ‐ Children. The maintenance dose was budesonide/formoterol 80/4.5 µg one inhalation in the evening.
Of the 13 adult studies, nine studies compared SiT with current best practice; these were DE‐SOLO; MONO; Riemersma (NCT00235911); PASSION; SALTO; SOLO; SPAIN; STYLE and SYMPHONIE. In these studies long‐acting beta2‐agonists (LABA) were allowed in the control arm, and many of the participants were already using LABA when recruited. The only restriction on the current best practice group was that they must continue on inhaled corticosteroids (ICS).
The other four studies compared SiT with ICS as maintenance and terbutaline as reliever (Scicchitano 2004; STAY ‐ Adults; STEAM; Sovani 2008) and of these, the first three compared SiT with a higher dose of maintenance ICS. In these studies LABA were not allowed in the control arm, and were withdrawn from those patients previously taking them. Sovani 2008 compared to the same dose of ICS (as the main aim of this study was to assess compliance with ICS).
The reported mean daily dose of ICS previously used by participants was reported as: MONO (1035 µg), Riemersma (NCT00235911) (538 µg); SALTO (579 µg), Scicchitano 2004 (746 µg), SOLO (569 µg), Sovani 2008 (590 µg, but poor compliance was an inclusion criterion), SPAIN (1034 µg, BDP equivalent), STAY ‐ Adults (660 µg), STAY ‐ Children (315 µg), STYLE (506 µg) and SYMPHONIE (792 µg). The mean daily dose was lower in STEAM (340 µg) and was not available from PASSION or DE‐SOLO.
Inclusion criteria for DE‐SOLO, MONO, SALTO, SOLO, STYLE and SYMPHONIE were that the participants had to be stable on a combination of LABA and ICS or symptomatic on ICS maintenance, and 82% of the participants used LABA at study entry with an average ICS dose of 979 µg/day (BDP equivalent). PASSION, and SPAIN required adults to be symptomatic on ICS maintenance (with or without LABA). Scicchitano 2004, STAY ‐ Adults, and STAY ‐ Children included participants who had suffered a clinically important exacerbation in the previous year. In SALTO, 27% of patients were reported to have mild persistent asthma, 37% moderate persistent and 36% severe persistent.
Some studies included a run‐in of about two weeks in which LABA was withdrawn (Scicchitano 2004; STAY ‐ Adults; STAY ‐ Children; STEAM) and in the case of STEAM, the maintenance dose of ICS was reduced from an average of 350 µg/day to 200 µg/day as well, and in order to secure "a symptomatic population that could respond differently to different treatments, patients were required to have at least 7 inhalations of as‐needed medication during the last 10 days of the run‐in period". SALTO and SOLO continued usual therapy over the two‐week run‐in period, but details of any run‐in period are not currently available for the other studies.
Outcomes
The primary outcomes for the studies are shown in the Characteristics of included studies. For the majority of studies this was time to first severe asthma exacerbation, which usually included hospitalisation, visits to an emergency department (ED), a course of oral steroids for the studies comparing with current best practice. Sometimes a 30% drop in peak expiratory flow (PEF) was also counted as an exacerbation, such as in Scicchitano 2004; STEAM; STAY ‐ Adults; STAY ‐ Children. Sovani 2008 had a different design from the other studies and the primary outcome was the dose of ICS.
All of the studies (apart from Sovani 2008) are multicentre studies, and no information has been found in relation to differences between centres or countries in any of these trials.
Excluded studies
The reasons for the exclusion of 15 studies are documented in the Characteristics of excluded studies.
Risk of bias in included studies
An overview of risk of bias judgements is shown in Figure 1
1.
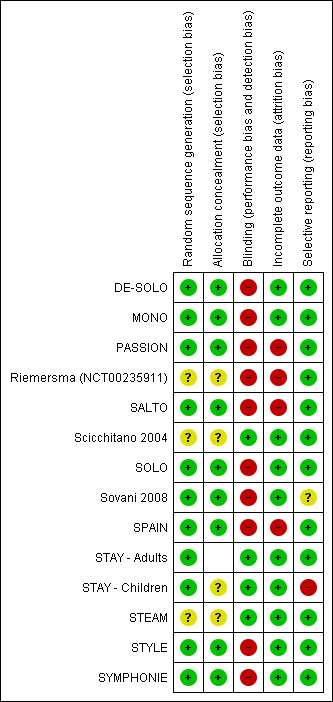
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Demoly 2009 has reported details for six of the included studies indicating low risk of allocation bias. As the rest of the trials were also sponsored by the same manufacturer, the risk of selection bias was assessed as low.
Blinding
All the studies comparing SiT with current best practice were unblinded, and therefore were judged to be at high risk of performance and detection bias, whilst those comparing with higher doses of ICS were double‐blind and at low risk. Sovani 2008 was also unblinded as adherence was the primary outcome for this study.
Incomplete outcome data
There were imbalanced discontinuations in Riemersma (NCT00235911), SALTO, Sovani 2008 and SPAIN, and around 20% discontinuations in each group in PASSION. Othewise, the included studies were judged to be at low risk of attrition bias.
Selective reporting
Missing data from five large trials on 4556 adults (DE‐SOLO; PASSION; SPAIN; STYLE; SYMPHONIE) comparing SiT with current best practice have been included in the 2013 update of this review. We are therefore now less concerned about missing trial reports in relation to the primary outcomes of this review. However, we have still not been able to obtain data from STAY ‐ Children for the exacerbations requiring a course of oral corticosteroids.
Effects of interventions
The primary outcomes for this review were exacerbations leading to hospitalisation, exacerbations treated with a course of oral corticosteroids and serious adverse events. Initially the trials reported composite outcomes that include the above types of exacerbation combined with emergency room (ER) visits and sometimes a 30% drop in peak flow. We have obtained data on our primary outcomes from the trial sponsor and recent web reports (for the 2013 update). Most of the studies are also multi‐site randomised controlled trials and have not reported data from any of the individual sites.
Adults and adolescents treated with 160/4.5 µg single inhaler therapy (SiT) (twice daily and as‐needed) versus current best practice
Eight trials on 8841 adults and adolescents now contribute to this comparison for the 2013 update (DE‐SOLO; MONO; PASSION; SALTO; SOLO; SPAIN; STYLE; SYMPHONIE). All of these studies ran for six months and recruited participants whose asthma was not controlled in spite of regular inhaled corticosteroids, or who were on treatment with LABA and ICS at recruitment (around 80% of those recruited). We have presented the quality of evidence for relevant outcomes in Table 1.
Primary outcomes
Exacerbations of asthma causing hospital admissions
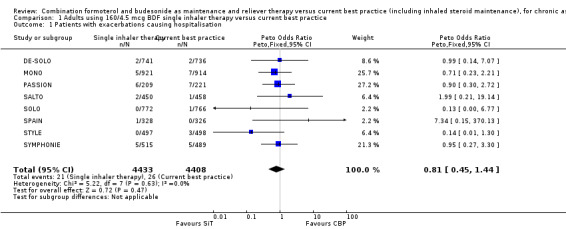
There were 47 people who suffered one or more hospitalisations from a total of 8841 participants in the eight trials providing data for this outcome (21 in the SiT arms and 26 in the current best practice arms). SALTO reported two asthma exacerbations that required hospitalisation in the SiT arm of the study, and we have obtained confirmation from the sponsors that the two events were in separate participants. There was no significant difference in the pooled outcome: (Peto odds ratio (OR) 0.81; 95% confidence interval (CI) 0.45 to 1.44; low quality evidence due to risk of bias and imprecision), see Figure 2.
2.
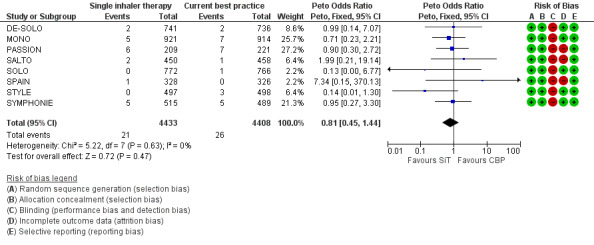
Forest plot of comparison: 1 Adults and Adolescents treated with Single Inhaler Therapy versus Conventional Best Practice, outcome: 1.1 Patients with exacerbations causing hospitalisation.
Exacerbations of asthma treated with oral corticosteroids
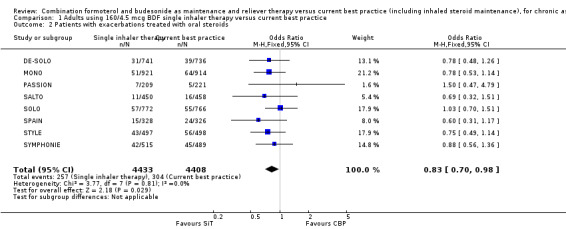
We originally obtained data from four studies (MONO, Riemersma (NCT00235911), SOLO and STYLE, 4470 participants) on patients with one or more courses of oral corticosteroids and the reduction with SiT was not significant at that time (OR 0.83; 95% CI 0.66 to 1.03; moderate quality evidence due to risk of bias).
However, with the update in 2013 there are now eight trials on 8841 participants contributing to the meta‐analysis and with the new data the reduction with SiT is now statistically significant (OR 0.83; 95% CI 0.70 to 0.98) see Figure 3. Three hundred and four out of 4408 participants (7%) were treated with oral corticosteroids using current best practice over six months, and this translates into a number needed to treat to benefit (NNTB) of 90 (95% CI 51 to 767), to prevent one patient needing oral corticosteroids over an 11‐month period, see Figure 4
3.
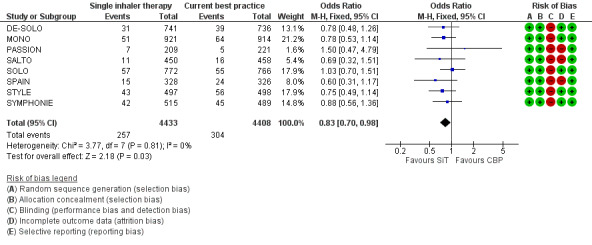
Forest plot of comparison: 1 adults and adolescents treated with Single Inhaler Therapy versus Conventional Best Practice, outcome: 1.2 Patients with exacerbations treated with oral steroids.
4.
In the current best practice group 7 people out of 100 had exacerbations treated with oral steroids over 6 months, compared to 6 (95% CI 5 to 7) out of 100 for the single inhaler therapy group. NNT(B) = 90, (95% CI: 51 to 767).
Serious adverse events
No significant difference was seen in people who suffered a fatal or non‐fatal serious adverse event from the combined results of the eight trials on 8841 participants; for fatal events (Peto OR 1.95; 95% CI 0.53 to 7.21; low quality evidence due to risk of bias and imprecision) Analysis 1.3, and for non‐fatal events (OR 1.20; 95% CI 0.90 to 1.60; low quality evidence due to risk of bias and imprecision) Analysis 1.4. However, the overall number of events was still too small to rule out the possibility of a clinically important increase or decrease in serious adverse events (as reflected in the wide confidence intervals). Peto OR was used for the fatal serious adverse events analysis in view of the presence of trials with no deaths in some of the treatment arms.
1.3. Analysis.
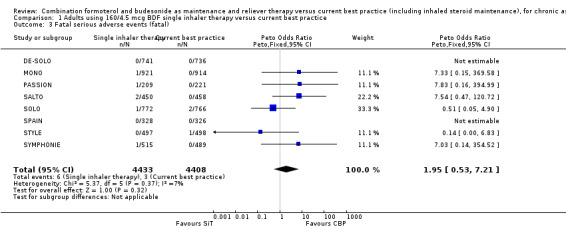
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 3 Fatal serious adverse events (fatal).
1.4. Analysis.
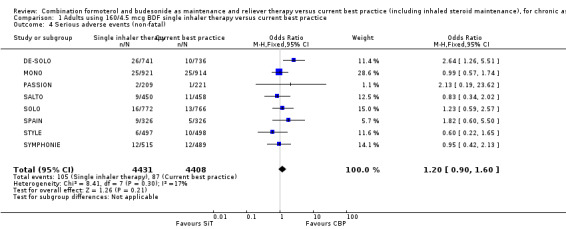
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 4 Serious adverse events (non‐fatal).
A post‐hoc observation was that there was a higher number of discontinuations due to adverse events with SiT (OR 2.85; 95% CI 1.89 to 4.30; moderate quality evidence due to risk of bias), see Analysis 1.5. This finding was attributed by the investigators to patients in the SiT arm who changed from metered‐dose inhaler (MDI) to dry‐powder inhaler devices, and who were not allowed to change their maintenance treatment during the course of the study.
1.5. Analysis.
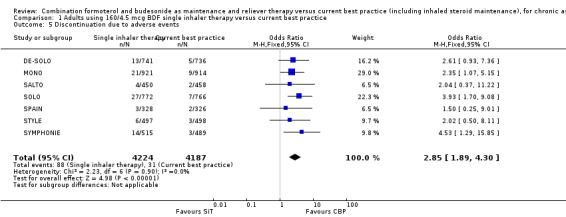
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 5 Discontinuation due to adverse events.
Secondary outcomes
Severe exacerbations requiring medical intervention
There was no overall significant reduction in the time to a severe exacerbation, as defined by the investigators, which was the primary outcome measure for these trials (Hazard Ratio (HR) 0.94; 95% CI 0.85 to 1.04, seven studies, N = 7355), Analysis 1.6. The HR was not reported for DE‐SOLO.
1.6. Analysis.
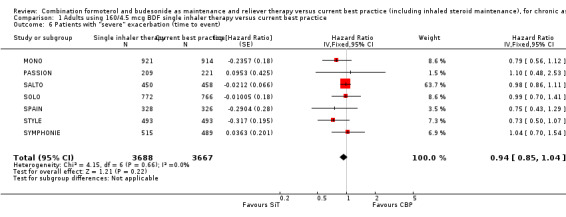
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 6 Patients with "severe" exacerbation (time to event).
Change in morning peak expiratory flow (PEF)
The change in morning PEF (% predicted) in SOLO was 1.00% (95% CI ‐0.96 to 2.96) which was in favour of SiT, but neither clinically nor statistically significant (Analysis 1.7). Data were not available for this outcome from MONO and SALTO.
1.7. Analysis.
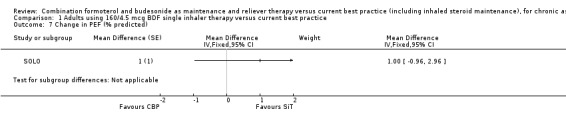
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 7 Change in PEF (% predicted).
Rescue medication use
There was a difference of ‐0.16 (95% CI ‐0.27 to ‐0.05) puffs per day of rescue medication use in the SiT arm of SOLO compared to current best practice, Analysis 1.8.
1.8. Analysis.
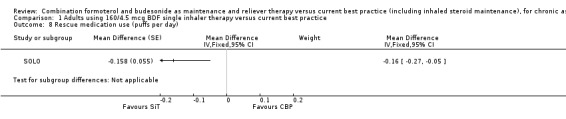
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 8 Rescue medication use (puffs per day).
Quality of life (change in ACQ score)
The five studies reporting this outcome had heterogeneous findings (Analysis 1.9). SALTO, SPAIN and SYMPHONIE demonstrated an improvement in the ACQ score in favour of SiT, whilst in DE‐SOLO and SOLO the direction of effect favoured current best practice. The results have not been combined in view of the heterogeneity (I2 = 68%) .
1.9. Analysis.
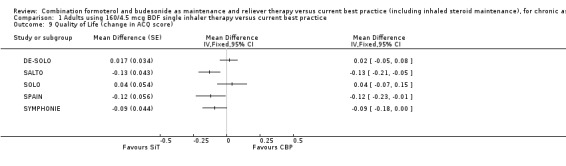
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 9 Quality of Life (change in ACQ score).
Steroid load
Five studies demonstrated a significantly lower intake of inhaled steroids in the SiT arms in comparison with current best practice but again, heterogeneity was high (I2 = 91%) and the results were therefore not combined. It is expected that the size of the reduction in ICS dose will reflect the trial design for each study, giving rise to the heterogeneity, and in the mean differences that ranged from 107 µg/day in SALTO to 267 µg/day in SOLO (Analysis 1.10). Data from SYMPHONIE indicates a mean difference of 549 µg/day, but this trial has not been reported in full and it is not clear whether this difference includes the inhaled steroid used "as required" in each arm. STYLE also indicated a lower mean daily dose of ICS on SiT (472 versus 516 µg) but provides no variance data in the web report. Furthermore, some trials reported the differences as BDP equivalent doses (e.g. SPAIN); this was not combined with the other trial results.
1.10. Analysis.
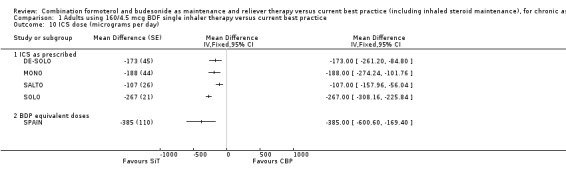
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 10 ICS dose (micrograms per day).
Adults treated with 80/4.5 µg single inhaler therapy (SiT) (two doses in the evening and as‐needed) versus current best practice
One trial on 102 adults was included for this comparison (Riemersma (NCT00235911). The primary aim of the study was to assess bronchial hyper responsiveness in primary care in adults with mild to moderate persistent asthma who were well controlled on ICS (Analysis 2.6).
2.6. Analysis.
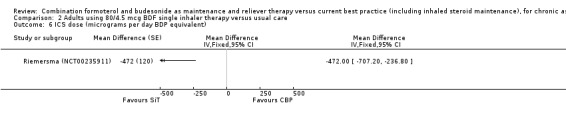
Comparison 2 Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care, Outcome 6 ICS dose (micrograms per day BDP equivalent).
The daily average dose of ICS was lower on the SiT arm (326 µg/day in comparison to 798 µg/day on usual care), but the number of participants with exacerbations and adverse events was too small to assess whether the treatments were equivalent or different for the primary outcomes of this review (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5).
2.1. Analysis.
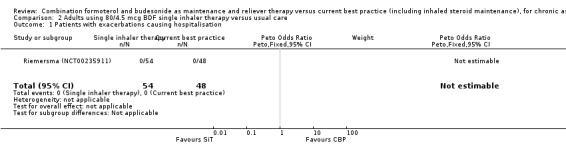
Comparison 2 Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care, Outcome 1 Patients with exacerbations causing hospitalisation.
2.2. Analysis.
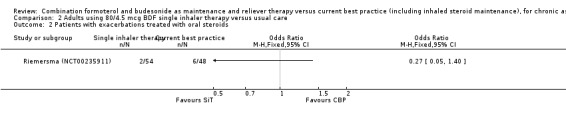
Comparison 2 Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care, Outcome 2 Patients with exacerbations treated with oral steroids.
2.3. Analysis.
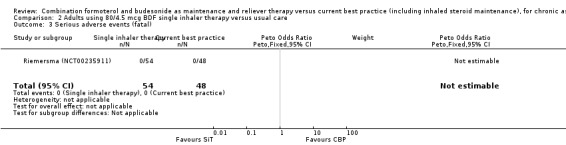
Comparison 2 Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care, Outcome 3 Serious adverse events (fatal).
2.4. Analysis.
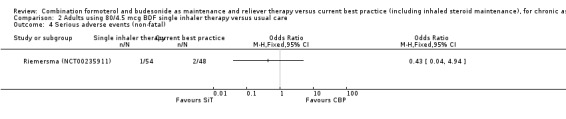
Comparison 2 Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care, Outcome 4 Serious adverse events (non‐fatal).
2.5. Analysis.
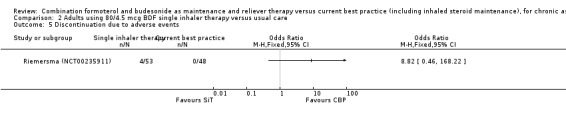
Comparison 2 Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care, Outcome 5 Discontinuation due to adverse events.
Adults and adolescents treated with single inhaler therapy (SiT) versus maintenance inhaled corticosteroids (ICS)with separate reliever inhaler
We have presented the quality of evidence for relevant outcomes in Table 2.
Primary outcomes
Exacerbations of asthma causing hospital admissions
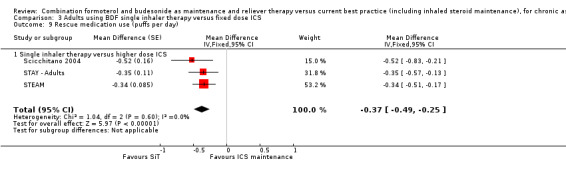
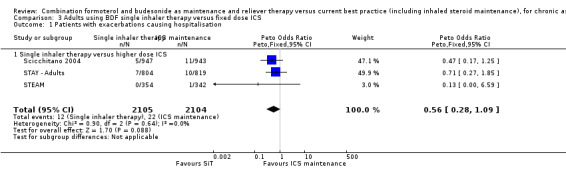
Scicchitano 2004, STAY ‐ Adults and STEAM contributed 4209 participants to this outcome, and overall there were fewer admissions on SiT in comparison to ICS, but this was not statistically significant. The number of admitted patients was small (12 in total on SiT and 22 on ICS) and the pooled result is (Peto OR 0.56; 95% CI 0.28 to 1.09; moderate quality evidence due to imprecision), as shown in Figure 5.
5.
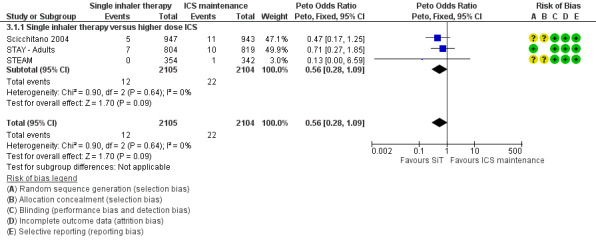
Forest plot of comparison: 2 Adults and Adolescents treated with Single Inhaler Therapy versus higher fixed dose ICS, outcome: 2.1 Patients with exacerbations causing hospitalisation.
The data used for Scicchitano 2004 and STEAM were on file from AstraZeneca, and were reported as patients with at least one asthma‐aggravated serious adverse event that required hospitalisation. The composite outcome of hospitalisation or ED visits was dominated by participants attending ED and was therefore not used for this outcome. In total,17 patients were admitted to hospital in comparison to 38 seen in ED from these two studies. Peto OR was chosen for this meta‐analysis as this method does not require a continuity correction for zero cells. Sensitivity analysis using Mantel‐Haenszel OR gave very similar results (OR 0.56; 95% CI 0.28 to 1.11).
Exacerbations of asthma treated with oral corticosteroids
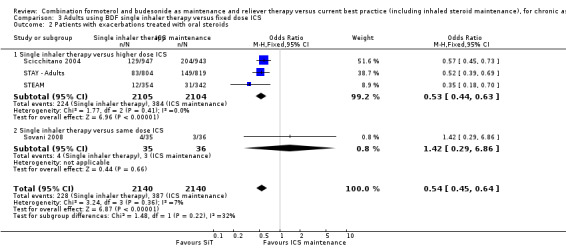
Scicchitano 2004, Sovani 2008, STAY ‐ Adults and STEAM contributed data on this outcome from 4280 participants; unpublished data on file from AstraZeneca has been obtained from Scicchitano 2004 and STAY ‐ Adults. The STAY ‐ Adults paper reported descriptive statistics only of courses of oral steroids per year, and in adults this was 0.19 on SiT and 0.38 on budesonide. The pooled result showed a significant reduction in the number of patients requiring a course of steroids (OR 0.54; 95% CI 0.45 to 0.64; high quality evidence) and with a total of 228 patients with an event on SiT and 387 on budesonide, Figure 6. For every 100 adults treated with ICS over 11 months, 18 required a course of oral steroids, whilst for SiT there would be 11 (95% CI: 9 to 12), Figure 7. This translates into a number needed to treat to prevent one patient needing oral corticosteroids over an 11‐month period of 14 (95% CI 12 to 18).
6.
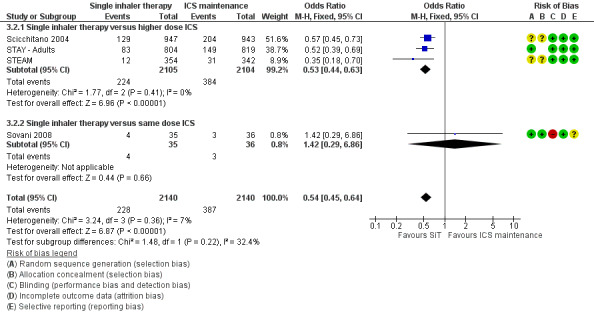
Forest plot of comparison: 2 adults and adolescents treated with Single Inhaler Therapy versus higher fixed dose ICS, outcome: 2.2 Patients with exacerbations treated with oral steroids.
7.
In the fixed dose ICS group 18 people out of 100 had exacerbation treated with oral steroids over 11 months, compared to 11 (95% CI 9 to 12) out of 100 treated with single inhaler therapy. NNT(B) = 14, (95% CI 12 to 18).
Sensitivity analysis using random‐effects Mantel‐Haenszel OR gave a marginally wider confidence interval (OR 0.54; 95% CI 0.44 to 0.65). The design of Sovani 2008 was different from the other studies in that adherence with ICSwas the primary concern of the study.
Serious adverse events
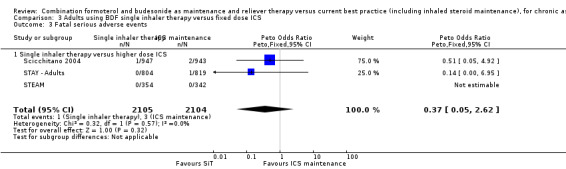
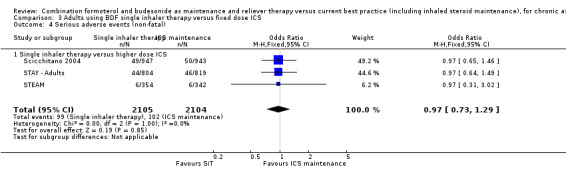
No significant difference was seen in either fatal or non‐fatal serious adverse events from the combined results of Scicchitano 2004, STAY ‐ Adults and STEAM; for fatal events (Peto OR 0.37; 95% CI 0.05 to 2.62; moderate quality evidence due to imprecision), and for non‐fatal events (OR 0.97; 95% CI 0.73 to 1.29; moderate quality evidence due to imprecision). Again, the number of events was small (four fatal and 201 non‐fatal) so the confidence interval includes the possibility of important increase or decrease with SiT.
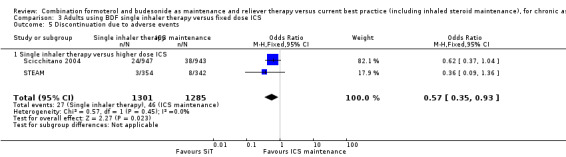
In contrast to the studies comparing SiT to current best practice, a post hoc inspection of discontinuations due to adverse events in Scicchitano 2004 and STEAM found a significant decrease in favour of SiT (OR 0.57; 95% CI 0.35 to 0.93; high quality evidence).
Secondary outcomes
Severe exacerbations requiring medical intervention
There were 1301 on SiT compared with 1285 on twice the dose of budesonide in Scicchitano 2004 and STEAM. There was a significant reduction in the time to a serious exacerbation, as defined by the investigators, (HR 0.59; 95% CI 0.49 to 0.70), Analysis 3.6.
3.6. Analysis.
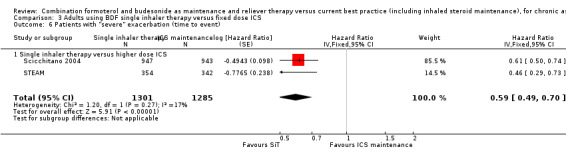
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 6 Patients with "severe" exacerbation (time to event).
Change in morning peak expiratory flow (PEF) and clinic FEV1
There was a significant increase in PEF in the SiT arms of Scicchitano 2004, STAY ‐ Adults and STEAM compared to higher doses of budesonide (mean difference (MD) 22.29 L/min; 95% CI 17.62 to 26.95), Analysis 3.7. Similarly, an increase of FEV1 in favour of SiT was found (MD 0.10 L ; 95% CI 0.07 to 0.13), Analysis 3.8.
3.7. Analysis.
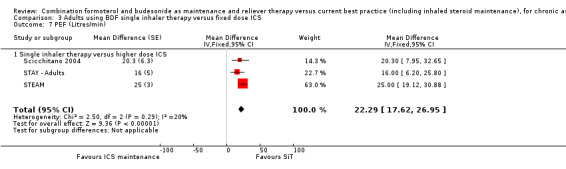
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 7 PEF (Litres/min).
3.8. Analysis.
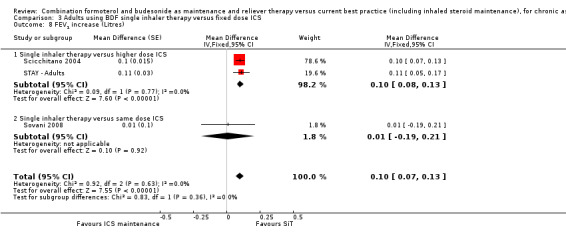
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 8 FEV1 increase (Litres).
Rescue medication use
There was a reduction in rescue medication use in favour of SiT (MD ‐0.37 puffs per day; 95% CI ‐0.49 to ‐0.25), Analysis 3.9.
3.9. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 9 Rescue medication use (puffs per day).
Quality of life (change in ACQ score)
The only study reporting ACQ scores was Sovani 2008 and no significant difference was found, Analysis 3.10.
3.10. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 10 Quality of Life (fall in ACQ score).
Steroid load
Scicchitano 2004 reported a mean daily budesonide dose of 466 µg/day in the SiT arm in comparison with 640 µg/day in the ICS arm, and 1776 days on oral corticosteroids in comparison with 3177 days. STAY ‐ Adults did not report the mean daily doses of budesonide, but reported 0.19 courses of oral corticosteroids per year for SiT compared to 0.38 per year for higher dose budesonide. STEAM reported a mean daily budesonide dose of 240 µg/day with SiT and 320 µg/day in the higher dose ICS arm. However, the paper also reported five patients on SiT who had a mean daily dose of > 640 µg/day on SiT. Again, STEAM reported a total of 114 days of oral corticosteroids with SiT and 498 days with higher dose budesonide.
Sovani 2008 was designed to investigate whether SiT could overcome poor adherence with ICS, and found an increase in mean daily use of budesonide (448 versus 252 µg/day, MD 196 µg/day, 95% CI 113 to 279 µg/day).
Children treated with single inhaler therapy (SiT) versus higher doses of inhaled corticosteroids (ICS)
Primary outcomes
Exacerbations of asthma causing hospital admissions
We obtained clarification from the sponsors in relation to the hospitalisations for children in each treatment arm of STAY ‐ Children. Hospitalisations related to asthma were reported as none on SiT and one with ICS (this does not quite match the asthma serious adverse event data, as one child was already in hospital with laryngitis, and the stay was prolonged due to an asthma exacerbation) Analysis 4.1. These events were too few to draw any conclusions.
4.1. Analysis.
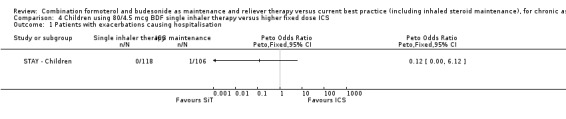
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 1 Patients with exacerbations causing hospitalisation.
Exacerbations of asthma treated with oral corticosteroids
The only information in the report of STAY ‐ Children in relation to oral corticosteroid use relates to the total number of treatment days in each group (32 days for SiT and 141 days for ICS). This is not suitable for use in meta‐analysis as there is no report of how many children were treated with oral corticosteroids in each group. The sponsors have not been able to provide these data.
Serious adverse events
There were no fatal serious adverse events in STAY ‐ Children and non‐fatal events occurred in two out of 118 children on SiT compared with five out of 106 on ICS (a non‐significant reduction) Analysis 4.3.
4.3. Analysis.
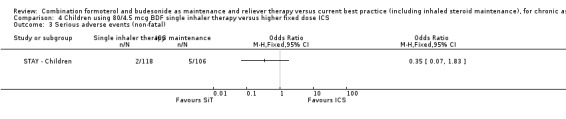
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 3 Serious adverse events (non‐fatal).
Annual height gain
The mean increase in height over one year in the SiT group was 5.3 cm (range 1 to 14 cm) and in the ICS group the mean increase was 4.3 cm (range ‐2 to 15 cm). The fact that some children appear to have become shorter raises concerns about the accuracy of the measurements carried out in some of the 246 centres (as the paper reports that local procedures were used to measure height), but the average advantage of 1 cm for SiT was statistically significant (95% CI 0.3 to 1.7 cms) Analysis 4.4.
4.4. Analysis.

Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 4 Annual height gain (cms).
Secondary outcomes
Severe exacerbations requiring medical intervention
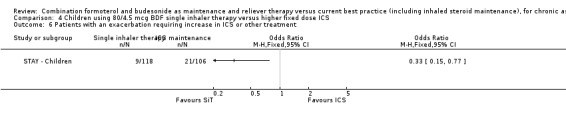
There were nine patients on SiT with exacerbations requiring medical intervention (hospitalisation or ER visit or course of oral steroids) which was significantly less than the 21 patients given ICS, (OR 0.33; 95% CI 0.15 to 0.77).
Change in morning peak expiratory flow (PEF)
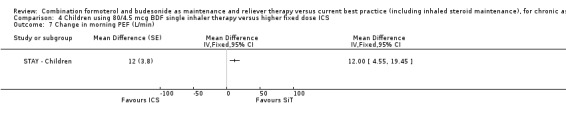
The children given SiT therapy in STAY ‐ Children had an average increase in morning PEF of 12 L/min (95% CI 4.55 to 19.45) in comparison with those given ICS.
Clinic spirometry (FEV1)
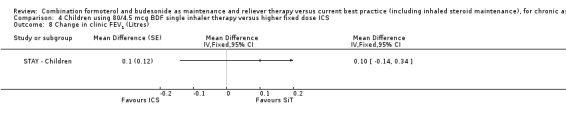
There was no significant difference in FEV1 between the SiT and ICS groups in STAY ‐ Children (0.10 L; 95% CI ‐0.14 to 0.34).
Nocturnal awakenings
There were, on average, two less nocturnal awakenings per night for children on SiT than those on ICS in STAY ‐ Children (‐2.00 [95% CI ‐3.33 to ‐0.67]).
Steroid load
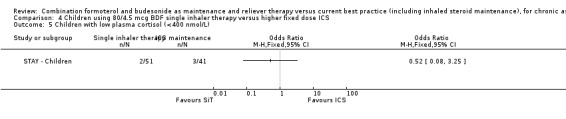
The mean daily dose of budesonide in children given SiT in STAY ‐ Children was 126 µg/day in comparison to 320 µg/day in the group randomised to fixed dose budesonide. There were also less days spent on oral corticosteroids in the SiT group (32 versus 141 days). Two of 51 children given SiT had abnormally low cortisol levels in comparison with three of 41 on ICS, a non‐significant reduction (OR 0.52; 95% CI 0.08 to 3.25).
Discussion
Summary of main results
In comparison to current best practice, which allowed the use of long‐acting beta2‐agonists (LABA) in the control arms, the updated evidence from eight trials on 841 adults and adolescents has not demonstrated significant advantages for SiT in exacerbations needing hospital admission (Peto OR 0.81; 95% CI 0.45 to 1.44), but has shown a significant reduction in exacerbations treated with a course of oral steroids (OR 0.83; 95% CI 0.70 to 0.98). For every 100 adults treated with current best practice over six months, seven required a course of oral steroids, whilst for SiT there would be six (95% CI 5 to 7), Figure 4. There was no significant difference found in fatal or non‐fatal serious adverse events, nor in the hazard ratio (HR) of time to first exacerbation (HR 0.94; 95% CI 0.85 to 1.04). All the studies found a reduction in ICS dose when using SiT.
In comparison to higher maintenance doses of budesonide (with no LABA in the control arms), four studies (Scicchitano 2004, STAY ‐ Adults, STEAM and Sovani 2008) involving 4280 patients demonstrated significant reductions in patients with an exacerbation needing oral steroids (OR 0.54; 95% CI 0.45 to 0.64). For every 100 adults treated with inhaled corticosteroids (ICS) over 11 months, 18 required a course of oral steroids, whilst for SiT there would be 11 (95% CI 9 to 12), Figure 7. There was no significant reduction in exacerbations leading to hospitalisation (Peto OR 0.56; 95% CI 0.28 to 1.09). There was no significant difference found in fatal or non‐fatal serious adverse events. The studies also found a reduction in ICS dose when using SiT.
Sovani 2008 demonstrated increased adherence with ICS using SiT in comparison to maintenance budesonide at the same dose.
Only one study included 224 children (STAY ‐ Children) and compared SiT to four times the dose of regular budesonide. There was a significant reduction in exacerbations needing increase in inhaled steroid treatment, or additional treatment or both in this study, but there were only two hospitalised patients and no separate data on courses of oral corticosteroids. There was no significant difference found in fatal or non‐fatal serious adverse events. Less inhaled and oral corticosteroids were used in the SiT group and the annual height gain was also greater in the SiT group.
Overall completeness and applicability of evidence
We found very little evidence in relation to the safety or efficacy of SiT in children, in whom the use of LABA is more contentious (Bisgaard 2003). There is also no separate reporting of results from adolescents in any of the trials of adults and adolescents.
It is possible that the difference between the results in trials comparing SiT with current best practice as opposed to higher doses of ICS alone as a comparison arm could relate to the design of the trials. Around 80% of the patients in the control arm of the current best practice trials were taking LABA, whereas none of the control arm patients in the higher dose maintenance ICS were taking LABA (as they were withdrawn from those patients previously using them). Further information will also be available in the review comparing SiT with combination therapy in double‐blind randomised trials (Cates 2011)
The inclusion of patients who were symptomatic during run‐in periods in which LABA was withdrawn may favour SiT, as formoterol would be expected to control symptoms more quickly than ICS in Scicchitano 2004, STEAM, STAY ‐ Adults and STAY ‐ Children. The plots of individual patient exacerbations show steeper gradients initially for the budesonide arms of these trials but similar gradients in the two groups towards the end of the study period.
This implies that the current evidence comparing SiT with fixed doses of ICS may only be directly applicable to patients who become symptomatic when maintenance treatment with ICS (with or without LABA) is reduced. How these results should be extrapolated to other groups of patients remains a matter for debate.
Interpretation of the results of the studies comparing SiT with current best practice is not straightforward, as the compliance with ICS in the current best practice groups and the SiT groups was based on self‐reporting in a patient note‐book. The lower dose of ICS prescribed in the SiT arms may, therefore, not reflect the true difference in the ICS doses that were actually taken by the participants.
Quality of the evidence
For key outcomes relating to exacerbations, we regarded the risk of detection bias in studies comparing SiT against best practice to be sufficient to downgrade the quality of evidence. Taken with varying degrees of statistical imprecision, we have downgraded to either low or moderate quality evidence for hospital admissions and steroid‐treated exacerbations. In contrast the blinded design of the studies comparing SiT against higher doses of ICS protected the studies against detection bias in our view. The quality of evidence for a reduction in the exacerbations was therefore higher for this comparison.
Agreements and disagreements with other studies or reviews
A meta‐analysis of six of the trials (DE‐SOLO; MONO; SALTO; SOLO; STYLE; SYMPHONIE) comparing SiT with current best practice was published before the 2013 update of this review and the findings for hospitalisation due to exacerbations, courses of oral steroids and time to first exacerbation were very similar to the findings of the updated review (Demoly 2009).
We agree with the reservations voiced by Lipworth 2008 in his response in the BMJ to the review of single inhaler by Barnes 2007. Lipworth comments that the run‐in for the trials comparing SiT with fixed dose ICS was designed to select patients who became symptomatic when their maintenance treatment was reduced or when LABA were withdrawn. This feature of the trial design may contribute to the improvement in time to first asthma exacerbation on SiT, because the onset of action of higher dose ICS would be expected to be slower, and more patients may therefore have suffered an early exacerbation in the higher dose ICS arm of the trials.
Authors' conclusions
Implications for practice.
Guidelines suggest the addition of regular LABA or increasing the dose of ICS for asthma that is not controlled on regular low dose ICS. SiT did not significantly reduce exacerbations leading to hospitalisation in comparison with current best practice. However, SiT can reduce the risk of asthma exacerbations needing oral corticosteroids in comparison with fixed dose maintenance ICS, and to a lesser degree in comparison with current best practice in adults who were not well controlled on regular corticosteroids. There were more discontinuations due to adverse events on SiT compared with current best practice, but no significant differences in fatal or non‐fatal serious adverse events.
Our confidence in these conclusions is limited by the open‐label design of the trials that compared SiT with current best practice, and by the reliability of the self‐reporting of adherence to treatment in the trials. The main limitation of the results from studies comparing SiT with higher dose inhaled steroids was the possible selection of participants with diminished asthma control following the withdrawal of LABA during run‐in.
SiT is not currently licensed for children under 18 years of age in the United Kingdom and there is currently very little research evidence for this approach in children or adolescents.
Implications for research.
More research is required on the efficacy and safety of SiT in children and adolescents, and on adults whose asthma is well‐controlled on low or moderate doses of ICS without additional LABA.
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2016 | Amended | We have corrected an error in the abstract in reporting the withdrawals due to adverse events (which were higher in comparison to current best practice but lower in comparison to inhaled steroid maintenance). |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 11 April 2013 | Amended | NIHR acknowledgement inserted |
| 11 February 2013 | New search has been performed | New literature search run and new trials incorporated. |
| 11 February 2013 | New citation required and conclusions have changed | Review updated following new search in February 2013. Five outstanding study reports (for DE‐SOLO; PASSION; SPAIN; STYLE; SYMPHONIE) have contributed new data to this review on 4560 adults comparing single inhaler therapy with current best practice. The additional data shows that single inhaler therapy also significantly reduced exacerbations requiring oral steroids in comparison to current best practice, although the absolute reduction was smaller in comparison to current best practice than in comparison to inhaled steroid maintenance. The title has been expanded to contain both comparator groups. We added 'Summary of findings' tables and incorporated the GRADE judgements within the review text. We rewrote a significant proportion of the review. |
| 11 November 2009 | Amended | Typological errors corrected (doses corrected to mcg not mg). The Sovani study was designed to assess compliance with inhaled (not oral) corticosteroids. |
Acknowledgements
We thank Susan Hansen and Elizabeth Stovold of the Cochrane Airways Group for their assistance in searching for trials and obtaining the abstracts and full reports, and John White for editorial help. We also thank Robyn Von Maltzahn, Joe Gray and Roberta Karlstrom from AstraZeneca for help in obtaining data from MONO, PASSION, SALTO, SPAIN, Scicchitano 2004, STEAM, STAY ‐ Adults and STYLE. Thanks also to Helen Reddel for pointing out the typological errors that now have been corrected.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Data and analyses
Comparison 1. Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations causing hospitalisation | 8 | 8841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.45, 1.44] |
| 2 Patients with exacerbations treated with oral steroids | 8 | 8841 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 3 Fatal serious adverse events (fatal) | 8 | 8841 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.53, 7.21] |
| 4 Serious adverse events (non‐fatal) | 8 | 8839 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.90, 1.60] |
| 5 Discontinuation due to adverse events | 7 | 8411 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.89, 4.30] |
| 6 Patients with "severe" exacerbation (time to event) | 7 | 7355 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.85, 1.04] |
| 7 Change in PEF (% predicted) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 8 Rescue medication use (puffs per day) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9 Quality of Life (change in ACQ score) | 5 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10 ICS dose (micrograms per day) | 5 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10.1 ICS as prescribed | 4 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 BDP equivalent doses | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 1 Patients with exacerbations causing hospitalisation.
1.2. Analysis.
Comparison 1 Adults using 160/4.5 mcg BDF single inhaler therapy versus current best practice, Outcome 2 Patients with exacerbations treated with oral steroids.
Comparison 2. Adults using 80/4.5 mcg BDF single inhaler therapy versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations causing hospitalisation | 1 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Patients with exacerbations treated with oral steroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Serious adverse events (fatal) | 1 | 102 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious adverse events (non‐fatal) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Discontinuation due to adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 ICS dose (micrograms per day BDP equivalent) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 3. Adults using BDF single inhaler therapy versus fixed dose ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations causing hospitalisation | 3 | 4209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.28, 1.09] |
| 1.1 Single inhaler therapy versus higher dose ICS | 3 | 4209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.28, 1.09] |
| 2 Patients with exacerbations treated with oral steroids | 4 | 4280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.45, 0.64] |
| 2.1 Single inhaler therapy versus higher dose ICS | 3 | 4209 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.44, 0.63] |
| 2.2 Single inhaler therapy versus same dose ICS | 1 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.29, 6.86] |
| 3 Fatal serious adverse events | 3 | 4209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.05, 2.62] |
| 3.1 Single inhaler therapy versus higher dose ICS | 3 | 4209 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.05, 2.62] |
| 4 Serious adverse events (non‐fatal) | 3 | 4209 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 4.1 Single inhaler therapy versus higher dose ICS | 3 | 4209 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.29] |
| 5 Discontinuation due to adverse events | 2 | 2586 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.93] |
| 5.1 Single inhaler therapy versus higher dose ICS | 2 | 2586 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.35, 0.93] |
| 6 Patients with "severe" exacerbation (time to event) | 2 | 2586 | Hazard Ratio (Fixed, 95% CI) | 0.59 [0.49, 0.70] |
| 6.1 Single inhaler therapy versus higher dose ICS | 2 | 2586 | Hazard Ratio (Fixed, 95% CI) | 0.59 [0.49, 0.70] |
| 7 PEF (Litres/min) | 3 | Mean Difference (Fixed, 95% CI) | 22.29 [17.62, 26.95] | |
| 7.1 Single inhaler therapy versus higher dose ICS | 3 | Mean Difference (Fixed, 95% CI) | 22.29 [17.62, 26.95] | |
| 8 FEV1 increase (Litres) | 3 | Mean Difference (Fixed, 95% CI) | 0.10 [0.07, 0.13] | |
| 8.1 Single inhaler therapy versus higher dose ICS | 2 | Mean Difference (Fixed, 95% CI) | 0.10 [0.08, 0.13] | |
| 8.2 Single inhaler therapy versus same dose ICS | 1 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.19, 0.21] | |
| 9 Rescue medication use (puffs per day) | 3 | Mean Difference (Fixed, 95% CI) | ‐0.37 [‐0.49, ‐0.25] | |
| 9.1 Single inhaler therapy versus higher dose ICS | 3 | Mean Difference (Fixed, 95% CI) | ‐0.37 [‐0.49, ‐0.25] | |
| 10 Quality of Life (fall in ACQ score) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10.1 Single inhaler therapy versus same dose ICS | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 1 Patients with exacerbations causing hospitalisation.
3.2. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 2 Patients with exacerbations treated with oral steroids.
3.3. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 3 Fatal serious adverse events.
3.4. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 4 Serious adverse events (non‐fatal).
3.5. Analysis.
Comparison 3 Adults using BDF single inhaler therapy versus fixed dose ICS, Outcome 5 Discontinuation due to adverse events.
Comparison 4. Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations causing hospitalisation | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Fatal serious adverse events | 1 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious adverse events (non‐fatal) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Annual height gain (cms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Children with low plasma cortisol (<400 nmol/L) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Patients with an exacerbation requiring increase in ICS or other treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Change in morning PEF (L/min) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 Change in clinic FEV1 (Litres) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 As‐needed medication use over 24 hours | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10 Nocturnal awakenings | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
4.2. Analysis.
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 2 Fatal serious adverse events.
4.5. Analysis.
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 5 Children with low plasma cortisol (<400 nmol/L).
4.6. Analysis.
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 6 Patients with an exacerbation requiring increase in ICS or other treatment.
4.7. Analysis.
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 7 Change in morning PEF (L/min).
4.8. Analysis.
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 8 Change in clinic FEV1 (Litres).
4.9. Analysis.
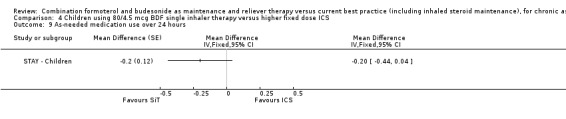
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 9 As‐needed medication use over 24 hours.
4.10. Analysis.
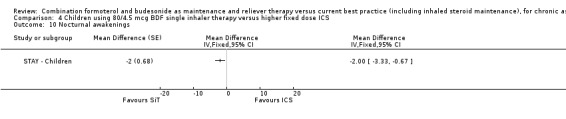
Comparison 4 Children using 80/4.5 mcg BDF single inhaler therapy versus higher fixed dose ICS, Outcome 10 Nocturnal awakenings.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DE‐SOLO.
| Methods | A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler 160/4.5 µg, 1 inhalation b.i.d. plus as‐needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised, open‐label, parallel‐group, multicentre study. Dec 2004 to October 2006. 169 centres in Germany. No report of run‐in. The purpose of this study is to determine whether Symbicort dosed according to the Symbicort Maintenance and Reliever Therapy (SMART) concept is superior to standard asthma treatment according to the local German treatment guidelines. |
|
| Participants | 1477 adults aged 18 years or older Inclusion Criteria:
Exclusion Criteria:
|
|
| Interventions |
|
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
Definition of severe exacerbation Treatment with oral corticosteroids (including one patient with IV corticosteroids), hospitalisation or ER treatment |
|
| Notes | Results obtained from a report on AstraZeneca web site. No results posted for NCT00252863 on ClinicalTrials.gov in December 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code assigned from a computer generated randomisation schedule" Demoly 2009 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised, strictly sequentially...using coded envelopes. When a patient had been randomised, the envelope was opened and the code was revealed." Demoly 2009 |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43/736 (5.8%) on SiT and 54/724 (7.5%) on current best practice discontinued treatment |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures (with the exception of hazard ratio of time to first exacerbation) |
MONO.
| Methods | This was a randomised, open‐label, parallel‐group, multicentre study in 1900 patients (planned number) with persistent asthma. Patients were treated with either Symbicort® SMART (i.e. Symbicort® Turbuhaler® (budesonide/formoterol) 160/4.5 μg (delivered dose), 1 inhalation b.i.d. plus as‐needed), or conventional best practice according to the investigator’s judgement, following GINA guidelines (Ref: Global Initiative for Asthma 2002). The treatment period lasted for 26 weeks, with no mention of any run‐in period. This study was conducted in Denmark (123 centres), Finland (69 centres) and Norway (83 centres) between September 2004 and October 2006. |
|
| Participants | 1854 patients were randomised, 1835 took at least one treatment and contributed to the analysis, and 1667 completed the study. 75% were taking LABA and daily ICS dose was 1035 µg/day (BDP equivalence). Male and female patients, > 12 years of age, with persistent asthma who were currently treated with inhaled glucocorticosteroids (IGCSs) and LABA. |
|
| Interventions |
Investigational product was Symbicort® Turbuhaler®, 160/4.5 μg/dose budesonide/formoterol (delivered dose), 1 inhalation b.i.d. as maintenance treatment plus as‐needed, in response to symptoms. Comparator products were any conventional best practice treatments, except Symbicort® SMART and/or maintenance with oral glucocorticosteroids prescribed at the discretion of the investigator according to GINA treatment guidelines Ref: Global Initiative for Asthma 2002). |
|
| Outcomes |
Primary variable
Secondary variables
Safety Only information regarding SAEs and discontinuations due to AE (DAEs) were collected in this study. Definition of severe exacerbation Not specified Additional Data Data on file from AstraZeneca indicated 51/64 patients with at least one course of oral steroids and 5/7 with at least one hospitalisation on single inhaler therapy/current best practice (921/914) |
|
| Notes | One (1) death was reported in the study in the SiT group in Denmark. The patient contacted the investigator 16 August due to asthma deterioration. The patient discontinued the study and study medication on 9 September 2005 due to “Subject not willing to continue study” and experienced asthma exacerbation on 30 September 2005. The event was considered serious due to hospitalisation, and the patient died the same day. The events pneumonia and in compensatio cordis led to death and not the event of asthma exacerbation. The investigator considered the event to be unrelated to the study therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code assigned from a computer generated randomisation schedule" Demoly 2009 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised, strictly sequentially...using coded envelopes. When a patient had been randomised, the envelope was opened and the code was revealed." Demoly 2009 |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90% of randomised patients completed the study and analyses was ITT |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
PASSION.
| Methods | Study design: 26‐week randomised, open‐label, active control, parallel group multicentre study conducted at 15 centres in Turkey between March 2006 and September 2008 | |
| Participants | 430 adults aged 18 years or older Inclusion Criteria:
Exclusion Criteria:
|
|
| Interventions | A comparison of Symbicort single inhaler therapy 200/6 (Symbicort Turbuhaler delivered dose 160/4.5 µg, 1 inhalation b.i.d. plus as‐needed) and conventional best practice (according to guidelines) | |
| Outcomes | The primary outcome variable was time to first severe asthma exacerbations (hospitalisation for at least one day or at least 3 days of oral steroids). A secondary objective was to collect safety data for treatment in the two treatment groups in adult patients with persistent asthma |
|
| Notes | Results posted this trial (NCT00628758) on clinicaltrials.gov in July 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list (confirmed by sponsors) |
| Allocation concealment (selection bias) | Low risk | "in order to not reveal the randomised treatment of the next patient and to ensure that patients received randomised treatment, coded envelopes were prepared which revealed randomised treatment when opened" (information from sponsors) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44/209 (21%) on SiT and 42/221 (19%) on conventional best practice discontinued from the trial |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
Riemersma (NCT00235911).
| Methods |
Study Design: Randomised, open‐label, active control, parallel assignment, efficacy study. August 2003 to September 2006 Effects of Symbicort single inhaler therapy on bronchial hyper responsiveness, asthma control and safety in mild to moderate asthmatics in general practice, compared to usual care therapy. The primary objective is to compare the effects of Symbicort SiT and treatment according to NHG‐guidelines on bronchial hyper responsiveness in asthmatic patients, as measured by PD20 histamine, and to validate the Bronchial Hyperresponsiveness Questionnaire (BHQ). Two research centres in the Netherlands. |
|
| Participants | 102 adults enrolled with mild to moderate persistent asthma Inclusion Criteria:
Exclusion Criteria:
|
|
| Interventions |
|
|
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures
Definition of severe exacerbation Not specified Additional Data Data on file from AstraZeneca indicate that no patients were hospitalised, and 2/54 compared to 6/48 patients had at least one course of oral steroids on SMART and current best practice respectively |
|
| Notes | Reported in full for the 2013 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/54 (15%) discontinued on SiT and 4/48 (8% discontinued on usual care. |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
SALTO.
| Methods | This was a randomised, open‐label, phase IIIB, multicentre study with a parallel group design. Patients were treated with either SMART i.e. Symbicort® Turbohaler® 160/4.5μg/inhalation (delivered dose), 1 inhalation b.i.d. plus as‐needed (in response to symptoms), or conventional best practice. The study consisted of the following periods: 2‐week run‐in period followed by a 26‐week randomised treatment period. Usual therapy used in run‐in period. A total of 194 centres in Belgium and Luxembourg participated in this study, between December 2004 and June 2006 |
|
| Participants |
Population: 908 adults and adolescents were randomised. All were analysed for efficacy and safety and 867 completed the study. 38% classified as moderate persistent asthma, 36% severe persistent asthma and 27% mild persistent asthma. Mean ICS daily dose during run‐in 579 µg/day (range 100 to 2000). Inclusion criteria: Male and female, adolescent (≥ 12 years of age) and adult patients with persistent asthma, currently treated with IGCS or IGCS and LABA. |
|
| Interventions |
|
|
| Outcomes |
Primary variable
Secondary variables
Patient‐reported outcomes (PROs)
Safety
Definition of severe exacerbation Not specified |
|
| Notes | Twenty patients reported a total of 20 SAEs during treatment (9 in the SMART group and 11 in the current best practice group). Six patients discontinued treatment due to an SAE/AE [4 in the SMART group (including two patients who died : one suicide and one myocardial infarction with no relation with the treatment) and 2 in the current best practice group]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code assigned from a computer generated randomisation schedule" Demoly 2009 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised, strictly sequentially...using coded envelopes. When a patient had been randomised, the envelope was opened and the code was revealed." Demoly 2009 |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27/450 (6%) on SiT and 14/458 (3%) discontinued treatment |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
Scicchitano 2004.
| Methods | This was a double‐blind, double‐dummy, randomised, active‐controlled, parallel‐group, multicentre study comparing the efficacy and safety of Symbicort 160/4.5 µg/inhalation, two inhalations once daily + Symbicort 160/4.5 µg/inhalation as‐needed (Symbicort SiT) with Pulmicort 160 µg/inhalation, two inhalations bid + Bricanyl 0.4 mg/inhalation as‐needed, in adults and adolescents (12‐80 years) for a period of 12 months in the treatment of asthma.(Carried out between May 2001 and January 2003). The run‐in period was on usual ICS dose but LABA was withdrawn. This was a multicentre study with 211 centres participating from the following 18 countries: Argentina (6 centres), Australia (10 centres), Canada (22 centres), Czech republic (5 centres), Finland (6 centres), France (29 centres), Germany (20 centres), Hungary (7 centres), Israel (17 centres), Italy (11 centres), Mexico (5 centres), the Netherlands (24 centres), New Zealand (4 centres), Norway (13 centres), Portugal (7 centres), Russia (6 centres), South Africa (11 centres) and Turkey (8 centres). |
|
| Participants |
Population: 1890 adults aged 12 years or older. Mean age: 43 years. FEV170% predicted. Mean ICS dose at enrolment 746 µg/day. Hospital admission for asthma in the past year: unknown%. Course of oral steroids for asthma in past year: unknown%. Previous clinically important exacerbation required for eligibility. 45% of enrolled patients were already on LABA as well as ICS. Inclusion criteria: Male and female participants, 12 to 80 years with asthma, previously treated with IGCS 400‐1600 µg per day, with a FEV1 of 50% to 90% of predicted normal (% P.N. ), a history of at least one clinical important asthma exacerbation 1‐12 months prior to inclusion, a reversibility in FEV1 from baseline of at least 12%, and who had an asthma symptom score ≥ 1 during 4 of the last 7 days of the run‐in period (in which usual dose of ICS was used but LABA was withdrawn from the 45% taking LABA previously). |
|
| Interventions |
Maximum of 10 as‐needed inhalations could be used per day before contacting the investigator. *200/6 µg actuator dose is described as 160/4.5 µg delivered dose in the paper. |
|
| Outcomes |
Primary efficacy variable
Additional secondary efficacy variables
FEV1, overall treatment evaluation, and asthma quality of life questionnaire, standardised version (AQLQ(S)) overall and domain scores. N.B. As‐needed‐free days and symptom‐free days were added as variables to the statistical analyses to conform with previous Symbicort studies. It was done after finalisation of the study protocol, but before un blinding of study data. Safety Safety assessments including physical examination, AEs, pulse and blood pressure, were obtained in all participants. Definition of severe exacerbation Included PEF less than 70% baseline on two consecutive days, severe exacerbations requiring medical intervention were also reported (hospitalisation, ED visit or course of oral steroids), but all severe exacerbations were to be treated with a 10‐day course of oral prednisolone. Additional Data Data on file from AstraZeneca indicated the number patients given at least one course of oral steroids was129/947 on SMART and 204/943 on Pulmicort. For hospitalisation/ER treatment there were 12/947 and 20/947 respectively. Asthma SAE was 5/947 and 11/943 which have been used as the hospital admission outcomes for this study. |
|
| Notes | There were three deaths reported in the study, two in the Pulmicort group and one in the Symbicort SiT group. None of the deaths were related to asthma or, as judged by the investigator causally related to investigational product. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1573/1890 completed (83%) |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
SOLO.
| Methods | Randomised, open‐label, parallel group study over a 6‐month period. In the two‐week run‐in patients used current asthma treatment (pre‐study ICS +/‐ LABA). Unknown number of centres in Canada. | |
| Participants |
Population: 1538 asthmatic adults aged 12 years and over with asthma on at least 400 µg/day ICS and symptomatic unless also on LABA (74% of those randomised were on LABA and ICS). Inclusion Criteria: Aged 12 years or more and asthma diagnosis for a minimum of three months. Previous treatment with ICS for at least 3 months (at least 400µg/day) with at least 3 inhalations of relief medication in the last 7 days of run‐in, or concurrent use of LABA. Patients with a smoking history of over 10 pack‐years or exacerbation requiring a change in asthma treatment in the past 14 days were not included; nor were patients already using SiT. Baseline Characteristics: Mean age: 40 years. FEV1 not measured but PEF 94% predicted. Mean ICS dose at enrolment 569 µg/day, and 74% were also using LABA. Hospital admission for asthma in the past year:unknown. Course of oral steroids for asthma in past year: unknown. |
|
| Interventions |
|
|
| Outcomes |
Primary outcome:
Secondary outcomes
Definition of severe exacerbation Hospitalisation, or ER visit or course of oral corticosteroids for at least 3 days due to asthma |
|
| Notes | Study D5890L00004 is now reported on the AstraZeneca trials web site (for 2013 update). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code assigned from a computer generated randomisation schedule" Demoly 2009 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised, strictly sequentially...using coded envelopes. When a patient had been randomised, the envelope was opened and the code was revealed." Demoly 2009 |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1400/1538 (91%) completed the study |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
Sovani 2008.
| Methods | Randomised, open‐label, parallel group study over a 6‐month period. | |
| Participants |
Population: 71 adults aged 18‐70 years with asthma, on at least 400‐1000 µg/day ICS who demonstrated poor compliance and were poorly controlled. Inclusion Criteria: Aged 18‐70 years. Previous treatment with ICS (400‐1000 µg/day beclomethasone equivalent) who demonstrated poor compliance by collecting less than 70% of expected ICS prescriptions in the previous year. Poor control demonstrated by at least two prescriptions of prednisolone or 10 canisters of reliever inhaler in previous year. At least four puffs of reliever for at least 4 days per week over past 4 weeks. Patients with a smoking history of over 20 pack‐years or exacerbation requiring oral steroids in the past 4 weeks were not included. Baseline characteristics: Mean age: 36 years. FEV1 85% predicted. Mean ICS dose at enrolment 590 µg/day, but only 278 µg/day was being taken! Hospital admission for asthma in the past year: unknown. Course of oral steroids for asthma in past year: mean of one course per year (SD 1). |
|
| Interventions |
|
|
| Outcomes |
Primary outcome:
Secondary outcomes
|
|
| Notes | Supported by an unconditional grant from AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | An independent pharmacist used computer‐generated random numbers to randomise each participant to one of two groups. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 55/71 (77%) completed the study and there were more withdrawals in the control arm: 13 compared to 3 in active arm (all 3 reported difficulty using the inhaler) |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome for this study was compliance and data were not presented on exacerbations |
SPAIN.
| Methods | Study design: 26‐week randomised, open‐label, active control, parallel group study conducted in France, Lituania, Spain and UK between September 2006 and December 2007 | |
| Participants | 654 adults aged 18 years or older. Inclusion Criteria:
Exclusion Criteria:
|
|
| Interventions | A comparison of Symbicort SiT 200/6 (Symbicort Turbuhaler delivered dose 160/4.5 µg, 1 inhalation b.i.d. plus as‐needed) and conventional best practice (according to GINA guidelines) | |
| Outcomes | The primary outcome variable was time to first severe asthma exacerbations. The definition of a severe asthma exacerbation was oral corticosteroids for at least three days, ER treatment or hospitalisation for asthma. A secondary objective is to collect safety data for treatment in the two treatment groups in adult patients with persistent asthma |
|
| Notes | Results posted this trial (NCT 00385593) on clinicaltrials.gov in November 2010 (accessed December 2012). The AstraZeneca web report is inconsistent in describing the participants with SAE but confirmation of correct figures has been obtained from the sponsors.. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list (confirmed by sponsors) |
| Allocation concealment (selection bias) | Low risk | "The investigator phoned to a free number of AstraZeneca randomisation centre. After checking that the patient met all selection and randomisation criteria, the investigator received the patient study number together with the assigned therapy. This was done consecutively from a centre specific randomisation listing, previously designed by AstraZeneca biometrical Unit All patients were rigorously assigned in a sequential manner, and always having previously checked that they met eligibility criteria." (Information provided by sponsors) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐study design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 58/328 (18%) on SiT did not complete the study, compared to 37/326 (11%) on conventional best practice |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
STAY ‐ Adults.
| Methods | Study Design: Randomised, double‐blind, parallel group study over a 12‐month period in 246 centres in 22 countries (between Jan 2001 and Jan 2003). In the 14‐18 day run‐in patients used pre‐study ICS with terbutaline for symptom relief (LABA had to be discontinued at least 3 days before run‐in). | |
| Participants |
Adult Population: 2419 asthmatic adults aged 12 years or more with asthma uncontrolled on ICS (400‐1000 µg/day) and a history of at least one "clinically important" exacerbation in the past year. The maintenance ICS dose was cut to a quarter with additional formoterol (SiT) or maintenance with terbutaline for relief. Adults Mean age: 40 years. FEV173% predicted pre bronchodilator. Mean ICS dose at enrolment 660 µg/day. Hospital admission for asthma in the past year: unknown proportion. Course of oral steroids for asthma in past year: unknown proportion. Inclusion Criteria: Aged 12‐80 years, with a constant dose of ICS (400‐1000 µg/day) at least 3 months. Prebronchodilator FEV1 of 60% to 90% predicted normal value and at least 12% reversibility following Terbutaline. To be included patients had to need at least 12 rescue inhalations in the last 10 days of run‐in. Adults using 10 or more rescue inhalations in a single day or with an exacerbation during run‐in were not randomised. |
|
| Interventions | 1. Budesonide/formoterol 100/6 µg twice daily [200 µgbudesonide/day] and as‐needed (one maintenance and one relief Turbuhaler) 2. Budesonide/formoterol 100/6 µg twice daily [200 µgbudesonide/day] and terbutaline as‐needed (one maintenance and one relief Turbuhaler) 3. Budesonide 400 µg twice daily [800 µgbudesonide/day] and terbutaline as‐needed (one maintenance and one relief Turbuhaler) Maximum of 10 as‐needed inhalations could be used per day before contacting the investigator. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Exacerbation Definition: Severe ‐ Deterioration in asthma requiring hospital or ER treatment, or oral steroids (or other additional treatment) or morning PEF 70% or less of baseline on two consecutive days. Severe exacerbations requiring medical intervention were analysed separately . Mild exacerbation day ‐ PEF 80% or less of baseline, relief medication 2 or more inhalations above baseline, or awakenings due to asthma. Mild exacerbation defined as 2 consecutive mild exacerbation days using the same criteria. Additional Data Data on file from AstraZeneca indicate that 7/804 adults and adolescents had at least one asthma related SAE on SMART and 10/819 on Pulmicort. Similarly 83/804 on SMART and 149/819 on Pulmicort had at least one course of oral corticosteroids. |
|
| Notes | SAE data (44,46,42) in the adult population; deaths given for whole trial (0,2,1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
STAY ‐ Children.
| Methods | Study Design: Randomised, double‐blind, parallel group study over a 12‐month period in 41 centres in 12 countries (between Jan 2001 and Jan 2003). In the 14‐18 day run‐in patients used pre‐study ICS with terbutaline for symptom relief (LABA had to be discontinued at least 3 days before run‐in). | |
| Participants |
Children in Study: 341 asthmatic children aged 4‐11 years with asthma uncontrolled on ICS (200‐500 µg/day) and a history of at least one "clinically important" exacerbation in the past year. 224 children were in arms considered in this review. Mean age: 8 years. Mean morning PEF: 220 L/min. FEV176% predicted pre bronchodilator. Mean ICS dose at enrolment 315 µg/day. Hospital admission for asthma in the past year: unknown proportion. Course of oral steroids for asthma in past year: unknown proportion. Inclusion Criteria: Aged 4‐11 years, with a constant dose of ICS (200‐500 µg/day) at least 3 months. Prebronchodilator FEV1 of 60‐100% predicted normal value and at least 12% reversibility following terbutaline. To be included patients had to need at least 8 rescue inhalations in the last 10 days of run‐in. Children using seven or more rescue inhalations in a single day or with an exacerbation during run‐in were not randomised. |
|
| Interventions | 1. Budesonide 100 µg (80 µg delivered dose) and Formoterol 4.5 µg in the evening and as‐needed (one maintenance and one relief Turbuhaler) 2. Budesonide 100 µg (80 µg delivered dose) and Formoterol 4.5 µg in the evening and terbutaline as‐needed (one maintenance and one relief Turbuhaler) 3. Budesonide 400 µg (320 µg delivered dose) in the evening and terbutaline as‐needed (one maintenance and one relief Turbuhaler) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Exacerbation Definition: Severe ‐ Deterioration in asthma requiring hospital or emergency room treatment, or oral steroids (or an increase in ICS or other additional treatment) or morning PEF 70% or less of baseline on two consecutive days. Severe exacerbations requiring medical intervention were analysed separately . Mild exacerbation day ‐ PEF 80% or less of baseline, relief medication 2 or more inhalations above baseline, or awakenings due to asthma. Mild exacerbation defined as 2 consecutive mild exacerbation days using the same criteria. Additional Data Data on file from AstraZeneca indicate that 0/118 children under the age of 12 years had at least one asthma related SAE on SMART and 2/106 on Pulmicort. Data on children given a course of oral corticosteroids have not been obtained. |
|
| Notes | Adverse Events: SAE data given (2,16,5) of these (0,7,2) were related to asthma. Change from baseline nights with awakenings were the same in both groups, P value in the paper not used as it related to post treatment levels not changes. No SD data published in the paper with respect to growth comparing budesonide/formoterol to terbutaline as reliever, so SD calculated from the other comparisons presented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See STAY ‐ Adults |
| Allocation concealment (selection bias) | Unclear risk | See STAY ‐ Adults |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See STAY ‐ Adults |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See STAY ‐ Adults |
| Selective reporting (reporting bias) | High risk | Data on children given a course of oral corticosteroids have not been reported. |
STEAM.
| Methods | The study was double‐blind, randomised, active‐controlled, multicentre and multinational with a parallel group design comparing the efficacy and safety of Symbicort 80/4.5 µg/inhalation, 2 inhalations once daily plus Symbicort 80/4.5 µg/inhalation as‐needed (Symbicort SiT) with that of Pulmicort 160 µg/inhalation, 2 inhalations once daily plus Bricanyl 0.4 mg/dose as‐needed when given to adults and adolescents (12‐80 years) for a period of 6 months in the treatment of asthma. This was a multicentre study with 77 centres participating from the following countries: Argentina (5 centres), Brazil (7 centres), China (4 centres), Denmark (15 centres), Indonesia (6 centres), Norway (10 centres), The Philippines (10 centres), Spain (9 centres), and Sweden (11 centres). |
|
| Participants |
Population: Male and female participants (n = 696), 12 to 80 years with asthma, previously treated with 200‐500 µg per day of IGCS. They had to have a FEV1 of 60% to 100% of predicted normal at Visit 1 and a reversibility in FEV1 from baseline of at least 12% at Visit 1 or 2, or a PEF variability of at least 12% on at least 3 out of the last 10 days of the run‐in. During the last 10 days of the run‐in period the participants also had to have used at least 7 inhalations of the as‐needed medication. Run‐in was on Budesonide 100µg bd with terbutaline prn (this represents around half the previous dose of ICS and LABA was withdrawn from the 20% participants who were taking LABA previously). Baseline Characteristics: Mean age: 38 years. FEV175% predicted. Mean ICS dose at enrolment 348 µg/day and 20% were also on LABA. Hospital admission for asthma in the past year: unknown. Course of oral steroids for asthma in past year: unknown. Previous exacerbation not required for eligibility. |
|
| Interventions | 1. Budesonide/formoterol 100/6 µgtwo inhalation in the evening [200 µgbudesonide/day], with additional doses as‐needed as reliever 2. Budesonide 200 µg two inhalations once daily [400 µgbudesonide/day], with terbutaline reliever *200 µg actuator dose is described as 160 µg delivered dose in the paper. |
|
| Outcomes |
Primary outcome:
Secondary outcomes
Exacerbation Definition: Severe ‐ Deterioration in asthma requiring hospital or emergency room treatment, or oral steroids , or at least 30% fall in PEF from baseline on two consecutive days. If prednisone was needed beyond 10 days this was counted as a second exacerbation. Mild exacerbation day ‐ defined in other studies as PEF 80% or less of baseline(average of last 10 days of run‐in), relief medication 2 or more inhalations above baseline, or a night with awakenings due to asthma. No report of definition in trial report. Additional Data Data on file from AstraZeneca indicate 12/354 patients with at least one course of oral steroids on SMART and 31/342 on Pulmicort. For asthma‐aggravated SAE the figures were 0/1 and for hospitalisation or ER visits 1/9 (which suggests that most of these were ER visits as hospitalisation for asthma is a mandatory category for asthma SAE). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 639/697 (92%) completed the study |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
STYLE.
| Methods | A comparison of the efficacy of Symbicort SiT (Symbicort Turbuhaler® 160/4.5 µg 1 inhalation b.i.d. plus as‐needed) and conventional best practice for the treatment of persistent asthma in adolescents and adults ‐ a 26‐week, randomised, open‐label, parallel‐group, multicentre study. July 2005 to December 2006 53 study locations in Chile, Croatia, Czech Republic, Greece, Iceland, Latvia, Lithuania, Portugal, Slovakia and Slovenia |
|
| Participants | Age at least 12 years. 1008 participants enrolled (986 analysed for safety). Inclusion Criteria: ‐ ‐ Diagnosis of asthma at least 3 months ‐ Prescribed daily use of glucocorticosteroids at a dose > 320 µg/ day for at least 3 months prior to Visit 1 Exclusion Criteria: ‐ Smoking history > 10 pack‐years ‐ Asthma exacerbation requiring change in asthma treatment during the last 14 days prior to inclusion ‐ Any significant disease or disorder that may jeopardize the safety of the patient. |
|
| Interventions | The purpose of the study is to compare the efficacy of a flexible dose of Symbicort with conventional stepwise treatment according to asthma treatment guidelines in patients with persistent asthma
|
|
| Outcomes |
Primary outcome:
Secondary outcome:
Safety: SAEs and discontinuations due to AEs. All variables assessed over the 6‐month treatment period Definition of severe exacerbation Not specified Additional Data Data on file from AstraZeneca indicates no patients with admission for asthma and 43 with at least one course of oral steroids (N = 497) on SMART and 3/498 and 56/498 respectively on current best practice. |
|
| Notes | This study was completed in December 2006 but no results have yet been published in medical journals. A trial report (published in 2008 on the AstraZeneca web site) has been used for the 2013 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code assigned from a computer generated randomisation schedule" Demoly 2009 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised, strictly sequentially...using coded envelopes. When a patient had been randomised, the envelope was opened and the code was revealed." Demoly 2009 |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36/493 (7.1%) on SiT and 35/493 (7.0%) discontinued treatment |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
SYMPHONIE.
| Methods | Study design: 26‐week randomised, open‐label, active control, parallel‐group study conducted in 82 centres in France between September 2004 and January 2006 | |
| Participants | Inclusion Criteria:
|
|
| Interventions | A comparison of Symbicort SiT 200/6 (Symbicort Turbuhaler delivered dose 160/4.5 µg, 1 inhalation b.i.d. plus as‐needed) and conventional best practice (according to GINA and ANAES guidelines) | |
| Outcomes | The primary outcome variable was time to first severe asthma exacerbations. The definition of a severe asthma exacerbation was oral corticosteroids for at least three days, ER treatment or hospitalisation for asthma. | |
| Notes | No results posted this trial (NCT00259792) on clinicaltrials.gov by December 12th 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization code assigned from a computer generated randomisation schedule" Demoly 2009 |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised, strictly sequentially...using coded envelopes. When a patient had been randomised, the envelope was opened and the code was revealed." Demoly 2009 |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 53/517 (10.3%) on SiT and 40/491 (8.2%) on conventional best practice discontinued prematurely |
| Selective reporting (reporting bias) | Low risk | Data have been obtained for all primary outcome measures |
ACQ: Asthma Control Questionnaire; AE: adverse events; BDP: budesonide plus formoterol; BHQ: Bronchial Hyperresponsiveness Questionnaire; b.i.d: twice daily; DAE: discontinuations due to AE; ED/ER: emergency department/room; FEV1: forced expiratory volume in 1 second; GINA: Global Initiative for Asthma; ICS: inhaled corticosteroids; IGCS: inhaled glucocorticosteroids; ITT: intention‐to‐treat; IV: intravenous; LABA: long‐acting β2‐agonist; PEF: peak expiratory flow; PRO: patient‐reported outcomes; QOL: quality of life; SAE: serious adverse event; SATQ: Satisfaction with Asthma Treatment Questionnaire; SD: standard deviation; SiT: single inhaler therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balanzat 2004 | Overview of three existing trials |
| Bousquet 2007 | Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high‐dose salmeterol/fluticasone |
| COMPASS | Different doses of Symbicort used for maintenance |
| COSMOS | Comparison with maintenance on fluticasone/salmeterol |
| D5890C00003 | SiT compared to higher dose maintenance regimen on BDF and budesonide |
| Ind 2002 | Formoterol vs. terbutaline as reliever |
| Jenkins 2007 | Budesonide/formoterol dose adjustment with FeNO (not used as reliever) |
| Jonkers 2006 | Single‐dose study |
| Loukides 2005 | SiT compared to separate inhalers for maintenance treatment and formoterol relief |
| Lundborg 2006 | Higher dose combination maintenance therapy but no ICS maintenance arm |
| NCT00463866 | Comparison of two different SiT regimens |
| Richter 2007 | Formoterol not combination therapy as reliever |
| SMILE | No comparison with maintenance ICS arm |
| SOMA | No maintenance ICS arm |
| Tattersfield 2001 | Formoterol v terbutaline as reliever |
BDF: budesonide plus formoterol; FeNO: fractional exhaled nitric oxide; ICS: inhaled corticosteroids; SiT: single inhaler therapy
Differences between protocol and review
This review has been focused on formoterol and budesonide as maintenance and reliever therapy, rather than formoterol with any inhaled corticosteroid. Steroid load and discontinuation due to adverse events were post hoc outcomes, added after the protocol. When zero cells were present for an outcome in any of the included studies the Peto Odds Ratio was used to combine the results, as it does not require a continuity correction to be used.
For the 2013 update Riemersma (NCT00235911) has been considered in a separate comparison group, as the SiT regimen was different from the other trials compared to current best practice, and the level of asthma control was better (FEV1 nearly 100% predicted in the patients at recruitment).
Contributions of authors
Chris Cates and Toby Lasserson conceived of the idea for this review, wrote the protocol, assessed the studies for inclusion, extracted the data from the papers and web reports and co‐wrote the review. Charlotta Karner and Chris Cates assessed studies for inclusion and extracted data for the 2013 update, and co‐wrote the updated version. Toby Lasserson has taken on editorial responsibility for the update, and has therefore stood down as an author of the review.
Sources of support
Internal sources
St George's University of London, UK.
External sources
-
NIHR, UK.
Programme grant funding
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
DE‐SOLO {published and unpublished data}
- AstraZeneca (D5890L00011). A comparison of Symbicort® single inhaler therapy (Symbicort® Turbuhaler® 160/4.5 mcg, 1 inhalation b.i.d. plus as‐needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised, open‐label, parallel‐group, multicentre study. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐trials/resources/pdf/D5890L00011 Accessed December 12th 2012. [NCT00252863]
MONO {published data only}
- AstraZeneca (D5890L00008). MONO: Symbicort single inhaler therapy and conventional best standard treatment for the treatment of persistent asthma in adolescents and adults. clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00242411 [Accessed 12/04/2006].
PASSION {published data only}
- AstraZeneca. A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler160/4.5 mg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised, open‐label,parallel‐group, multicentre study – PASSION Study [D5890L00016]. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐trials/resources/pdf/8610707 Accessed July 2012. [NCT00628758]
Riemersma (NCT00235911) {published and unpublished data}
- AstraZeneca (BN‐00S‐0011). Symbicort single inhaler therapy for asthma in a general practice setting. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐trials/resources/pdf/8610351 Accessed Jluy 2012. [NCT00235911]
- Riemersma RA, Postma D, Molen T. Budesonide/formoterol maintenance and reliever therapy in primary care asthma management: effects on bronchial hyperresponsiveness and asthma control. Primary Care Respiratory Journal 2012;21:50‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
SALTO {published data only}
- AstraZeneca (D5890L00009). SALTO ‐ symbicort single inhaler therapy use in adolescent adults and adults with persistent asthma. www.clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00290264 [Accessed 22/02/2008].
Scicchitano 2004 {published data only}
- Scicchitano R, Aalbers R, Ukena D, Manjra A, Fouquert L, Centann S, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Current Medical Research and Opinion 2004;20(9):1403‐18. [DOI] [PubMed] [Google Scholar]
SOLO {published data only}
- Sears MR, Boulet L‐P, Laviolette M, FitzGerald JM, Bai R, SmiljanicGeorijev N, et al. Budesonide/formoterol maintenance and reliever therapy for asthma compared to conventional best practice a randomised real life study. European Respiratory Journal 2006;28(Suppl 50):613s. [Google Scholar]
- Sears MR, Boulet L‐P, Laviolette M, FitzGerald JM, Bai TR, Kaplan A, et al. Budesonide/formoterol maintenance and reliever therapy: impact on airway inflammation in asthma. European Respiratory Journal 2008:Epub: doi: 10.1183/09031936.00104007. [DOI] [PubMed]
Sovani 2008 {published data only}
- Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekström T, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?. British Journal of General Practice 2008;58(546):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
SPAIN {published and unpublished data}
- AstraZeneca. A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler160/4.5 mcg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adults ‐ a 26‐week, randomised,open‐label, parallel‐group, multicentre study. Study SPAIN [D5890L00010]. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐trials/resources/pdf/8610692 Accessed July 2012.
- AstraZeneca. Symbicort single inhaler therapy vs conventional best practice for the treatment of persistent asthma in adults. http://clinicaltrials.gov/ct2/show/results/NCT00385593?sect=X30125#evnt Accessed December 12th 2012. [NCT00385593]
- Quirce S, Barcina C, Plaza V, Calvo E, Munoz M, Ampudia R, et al. A comparison of budesonide/formoterol maintenance and reliever therapy versus conventional best practice in asthma management in Spain. Journal of Asthma 2011;48:839‐47. [DOI] [PubMed] [Google Scholar]
STAY ‐ Adults {published and unpublished data}
- Bateman ED, Palmqvist M, Juniper EF, Zhu Y, Ekstrom T. Single inhaler therapy with budesonide/formoterol has superior efficacy to fixed‐dose budesonide/formoterol or a higher dose of budesonide alone. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004.
- Bruce SA, Scherer YK. Maintenance and symptom relief with budesonide plus formoterol reduced severe asthma exacerbations. Evidence‐Based Nursing 2005;8(3):78. [DOI] [PubMed] [Google Scholar]
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Budesonide and formoterol in mild persistent asthma compared with doubling the dose of budesonide ‐ a cost‐effectiveness analysis. European Respiratory Journal. 2001; Vol. 18, issue Suppl 33:517s.
- Jönsson BG, Berggren FE, Svensson K, O'Byrne PM. Economic results of adding formoterol to budesonide in mild persistent asthma. European Respiratory Journal 2001; Vol. 18, issue Suppl 33:331s.
- O'Byrne PM. Acute asthma intervention: Insights from the STAY study. Journal of Allergy and Clinical Immunology 2007;119(6):1332‐6. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(2):129‐36. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Godard P, Pistolesi M, Ekstrom T. Single inhaler therapy with budesonide/formoterol improves asthma control compared with fixed dosing with budesonide/formoterol or a higher dose of budesonide alone [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:Poster J93.
- SD‐039‐673. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler® as single therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). http://www.astrazenecaclinicaltrials.com 2006.
STAY ‐ Children {published and unpublished data}
- Bisgaard H, Hultquist C. Budesonide/formoterol for maintenance and as needed ‐ a new approach to asthma management in children [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1060. [Google Scholar]
- Bisgaard H, Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy ‐ a new strategy in pediatric asthma. Chest 2006;130(6):1733‐43. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM. Acute asthma intervention: Insights from the STAY study. Journal of Allergy and Clinical Immunology 2007;119(6):1332‐6. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(2):129‐36. [DOI] [PubMed] [Google Scholar]
- SD‐039‐673. Efficacy and safety of budesonide/formoterol (Symbicort) Turbuhaler® as single therapy in patients with mild‐moderate asthma. Comparison with Symbicort Turbuhaler and Pulmicort® Turbuhaler as maintenance therapy, both complemented with Bricanyl® Turbuhaler (STAY). http://www.astrazenecaclinicaltrials.com 2006.
STEAM {published data only}
- Astrazenca (SD‐039‐0667). Efficacy and safety of Symbicort® Turbuhaler® as single therapy in patients with mild to moderate asthma ‐ STEAM (SD‐039‐0667). Astrazeneca Clinical Trials Register 2005:http://www.astrazenecaclinicaltrials.com (accessed 20th February 2008).
- Rabe KF, Pizzichini E, Stallberg B, Romero S, Balanzat AM, Atienza T, et al. Budisonide/formoterol in a single inhaler for maintenance and relief in mild‐to‐moderate asthma: A randomized, double‐blind trial. Chest 2006;129:245‐56. [DOI] [PubMed] [Google Scholar]
STYLE {published data only}
- AstraZeneca. STYLE ‐ A Comparison of Symbicort SMART (Symbicort Turbuhaler160/4,5 mcg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adolescents and adults – a 26‐week, open‐labelled, parallel‐group, multicentre study. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐trials/resources/pdf/8610702 Accessed July 2012. [NCT00252824]
- AstraZeneca (D5890L00014). STYLE ‐ symbicort single inhaler therapy vs. conventional therapy in treatment of persistent asthma. www.clinicaltrials.gov 2005:http://www.clinicaltrials.gov/ct/show/ NCT00252824 [Accessed 22/02/2008].
SYMPHONIE {published data only}
- AstraZeneca. A comparison of Symbicort single inhaler therapy (Symbicort Turbuhaler 200/6 μg, 1 inhalation b.i.d. plus as needed) and conventional best practice for the treatment of persistent asthma in adolescents and adults – a 26‐week, randomised, open, parallel group multicentre study. [D5890L00005]. http://www.astrazenecaclinicaltrials.com/_mshost800325/content/clinical‐trials/resources/pdf/8610675 Accessed July 2012. [NCT00259792]
References to studies excluded from this review
Balanzat 2004 {published data only}
- Balanzat A, Centanni S, Palmqvist M, Rabe K. Budesonide/formoterol single inhaler therapy reduces over reliance on rapid acting reliever medication [Abstract]. European Respiratory Journal 2004;24(Suppl 48):344s. [Google Scholar]
Bousquet 2007 {unpublished data only}
- Astrazeneca (D5890C00002). Efficacy and safety of Symbicort®Turbuhaler®160/4.5 mcg/inhalation, two inhalations twice daily plus as‐needed compared with Seretide™ Diskus™ 50/500 mcg/inhalation, one inhalation twice daily plus terbutaline Turbuhaler 0.4 mg/inhalation as‐needed ‐ a 6‐month, randomised, double‐blind, parallel‐group, active controlled, multinational phase IIIB study in adult and adolescent patients with persistent asthma. AstraZeneca Clinical Trials Register 2006, issue www.astrazenecaclinicaltrials.com (accessed 21/02/2008).
- Bousquet J, Boulet L‐P, Peters MJ, Magnussen H, Quiralte J, Martinez‐Aguilar NE, et al. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high‐dose salmeterol/fluticasone. Respiratory Medicine 2007;101(12):2437‐46. [DOI] [PubMed] [Google Scholar]
COMPASS {published and unpublished data}
- AstraZeneca. SYM/050/DEC2007. Data on File.
- Bleecker ER, Postma DS, Lawrance R, Meyers DA, Ambrose H, Goldman M. Effect of polymorphisms in the beta2‐adrenergic receptor gene (ADRB2) on response to long‐acting beta2‐agonist (LABA) therapy. Journal of Allergy and Clinical Immunology 2007;119(2):523. [Google Scholar]
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Current Medical Research and Opinion 2007;23(8):1867‐78. [DOI] [PubMed] [Google Scholar]
- Kuna P, Peters MJ, Buhl R. Budesonide/formoterol as maintenance and reliever therapy reduces asthma exacerbations a higher maintenance dose of budesonide/versus formoterol or salmeterol/fluticasone. European Respiratory Journal 2006;28(Suppl 50):205s. [Google Scholar]
- Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martinez‐Jimenez NE, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. International Journal of Clinical Practice 2007;61(5):725‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D, Wiren A, Kuna P. Budesonide/formoterol (B/F) as maintenance and relief for asthma improves efficacy and is cost saving versus higher maintenance dose of B/F or salmeterol/fluticasone (S/F) [Abstract]. European Respiratory Journal 2006;28(Suupl 50):214s. [Google Scholar]
- Price D, Wiren A, Kuna P. Cost‐effectiveness of budesonide/formoterol for maintenance and reliever asthma therapy. Allergy 2007;62(10):1189‐98. [DOI] [PubMed] [Google Scholar]
COSMOS {published data only}
- Buhl R, Vogelmeier C. Budesonide/formoterol maintenance and reliever therapy: a new treatment approach for adult patients with asthma. Current Medical Research and Opinion 2007;23(8):1867‐78. [DOI] [PubMed] [Google Scholar]
- D'Urzo A, Vogeimeier C, Jaspal M, Merino JM, Boulet S. Symbicort (budesonide/formoterol) for both maintenance and relief reduces the exacerbation burden compared with titration of seretide (salmeterol/fluticasone) in patients with asthma, a real life study. American Thoracic Society International Conference; May 20‐25; San Diego, California. 2005:Poster G24.
- Johansson G, Andreasson EB, Larsson PE, Vogelmeier CF. Cost effectiveness of budesonide/formoterol for maintenance and reliever therapy versus salmeterol/fluticasone plus salbutamol in the treatment of asthma. Pharmacoeconomics 2006;24(7):695‐708. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, D'Urzo A. Maintenance plus as‐needed budesonide/formoterol vs salmeterol/fluticasone in a real‐life setting. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 2770. [Google Scholar]
- Vogelmeier C, D'Urzo A, Jaspal M, Merino JM, Johansson G, Boulet S. Symbicort for both maintenance and relief reduces exacerbations compared with a titration of Seretide (Advair) in patients with asthma: a real life study. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:Poster F67.
- Vogelmeier C, D'Urzo A, Pauwels R, Merino JM, Jaspal M, Boutet S, et al. Budesonide/formoterol maintenance and reliever therapy: An effective asthma treatment option?. European Respiratory Journal 2005;26(5):819‐28. [DOI] [PubMed] [Google Scholar]
D5890C00003 {published data only}
- AstraZeneca (D5890C00003). A comparison of the control of asthma inflammation provided by Symbicort Turbuhaler 160/4.5 mcg/inhalation bid plus as‐needed versus symbicort turbuhaler 320/9 mcg/inhalation bid plus Pulmicort Turbuhaler 400mcg/dose bid plus terbutaline turbuhaler 0.4mg/inhalation as‐needed. www.clinicaltrials.gov 2006:http://www.clinicaltrials.gov/ct/show/ NCT00244608 [Accessed22/02/2008].
Ind 2002 {published data only}
- Ind PW, Villasante C, Shiner RJ, Pietinalho A, Boszormenyi NG, Soliman S, et al. Safety of formoterol by Turbuhaler as reliever medication compared with terbutaline in moderate asthma. European Respiratory Journal 2002;20(4):859‐66. [DOI] [PubMed] [Google Scholar]
Jenkins 2007 {published data only}
- Jenkins CR, Marks GB, Gibson PG, Wark PAB, Thien FC, Belousova EG, et al. A randomised controlled trial of two algorithms for maintaining asthma control on long‐acting bronchodilators (LABA) and inhaled corticosteroids (ICS). Thoracic Society of Australia and New Zealand Annual Scientific Meeting, 25‐28 March 2007, Auckland. 2007:Abstract TP044.
Jonkers 2006 {published data only}
- Jonkers RE, Bantje TA, Aalbers R. Onset of relief of dyspnoea with budesonide/formoterol or salbutamol following methacholine‐induced severe bronchoconstriction in adults with asthma: a double‐blind, placebo‐controlled study. Respiratory Research 2006;7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loukides 2005 {published data only}
- Loukides S, Papageorgiou M, Karokis A, Zervas E, Christodoulopoulou A, Papageorgiou N, et al. Single inhaler therapy (SiT) with budesonide/formoterol (BUD/FUM) is effective in asthma control. European Respiratory Journal. 2005; Vol. 26(Suppl 49):124S.
Lundborg 2006 {published data only}
- Astrazeneca (LD‐039‐0003). An open, randomized, parallel‐group, multicentre, phase IIIB study to evaluate the efficacy of Symbicort® Turbuhaler® single inhaler therapy (SiT), given as a low maintenance dose once or twice daily plus as needed, compared to a higher maintenance dose of Symbicort Turbuhaler given twice daily plus Oxis® Turbuhaler® as needed during 24 weeks in asthmatic patients (LD‐039‐0003). AstraZeneca Clinical Trials Register 2006, issue http://www.astrazenecaclinicaltrials.com/ncmprint.aspx?type=article¶m=516829 (accessed 21st February 2008).
- Lundborg M, Wille S, Bjermer L, Tilling B, Lundgren M, Telg G, et al. Maintenance plus reliever budesonide/formoterol compared with a higher maintenance dose of budesonide/formoterol plus formoterol as reliever in asthma: An efficacy and cost‐effectiveness study. Current Medical Research and Opinion 2006;22(5):809‐21. [DOI] [PubMed] [Google Scholar]
NCT00463866 {published data only}
Richter 2007 {published data only}
- Richter K, Hartmann U, Metzenauer P, Magnussen H. Randomised trial comparing as‐needed versus regular treatment with formoterol in patients with persistent asthma. Respiratory Medicine 2007;101(3):467‐75. [DOI] [PubMed] [Google Scholar]
SMILE {published and unpublished data}
- Rabe F, Atienza T, Magyar P, Larsoon P, Jorup C, Lalloo G. A new combination therapeutic approach challenging the current dogma of using inhaled corticosteroids as maintenance only to control asthma. European Respiratory Journal 2006;28(Suppl 50):666s. [Google Scholar]
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double‐blind study. Lancet 2006;938(9537):744‐53. [DOI] [PubMed] [Google Scholar]
SOMA {published and unpublished data}
- Haahtela T, Tamminen K, Malmberg LP, Zetterstrom O, Karjalainen J, Yla‐Outinen H, et al. Formoterol as needed with or without budesonide in patients with intermittent asthma and raised NO levels in exhaled air: A SOMA study. European Respiratory Journal 2006;28(4):748‐55. [DOI] [PubMed] [Google Scholar]
- Haahtela T, Tamminen K, Malmberg P, Karjalainen J, Yia‐Outinen H, Zetterstrom O, et al. As‐needed treatment with a b2‐agonist/ corticosteroid combination in mild intermittent asthma (SOMA). European Respiratory Journal 2005;26(Suppl 45):Abstract No. 1722. [Google Scholar]
Tattersfield 2001 {published data only}
- Berggren F, Ekström T. A cost‐effectiveness study comparing the as‐needed use of formoterol (Oxis) and terbutaline (Bricanyl) in patients with moderate to severe asthma. Respiratory Medicine 2001;98(9):753‐8. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Löfdahl CG, Postma DS, Eivindson A, Schreurs AGM, Rasidakis A, et al. Comparison of formoterol and terbutaline for as‐needed treatment of asthma: a randomised trial. Lancet 2001;357(9252):257‐61. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2008
- Adams NP, Bestall JC, Lasserson TJ, Jones PW, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [PubMed] [Google Scholar]
Barnes 2007
- Barnes PJ. Using a combination inhaler (budesonide plus formoterol) as rescue therapy improves asthma control. BMJ 2007; Vol. 335, issue 7618:513. [DOI] [PMC free article] [PubMed]
Bisgaard 2003
Cates 2008
- Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2009a
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006922.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2009b
- Cates CJ, Lasserson TJ, Jaeschke R. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006924.pub2] [DOI] [PubMed] [Google Scholar]
Cates 2010a
- Cates CJ, Lasserson TJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007694.pub2; CD007694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2011
- Cates CJ, Lasserson TJ. Combination formoterol and inhaled steroid as maintenance and reliever therapy versus higher dose combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009019] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2012a
- Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006923.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2012b
- Cates CJ, Lasserson TJ. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD007695.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Delea 2008
Demoly 2009
- Demoly P, Louis R, Soes‐Petersen U, Naya I, Carlsheimer A, Worth H, et al. Budesonide/formoterol maintenance and reliever therapy versus conventional best practice. Respiratory Medicine 2009;103(11):1623‐32. [DOI] [PubMed] [Google Scholar]
Ducharme 2010
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005533.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducharme 2011
- Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long‐acting beta2‐agonists versus anti‐leukotrienes for chronic asthma. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD003137.pub4] [DOI] [PubMed] [Google Scholar]
FitzGerald 2004
- FitzGerald JM, Becker A, Sears MR, Mink S, Chung K, Lee J. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax 2004; Vol. 59, issue 7:550‐6. [DOI] [PMC free article] [PubMed]
GINA 2006
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2006. http://www.ginasthma.org (accessed 2006).
Harrison 2004
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] Available from: www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Lasserson 2008
- Lasserson TJ, Cates CJ, Ferrara G, Casali L. Combination fluticasone and salmeterol versus fixed dose combination budesonide and formoterol for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004106.pub3] [DOI] [PubMed] [Google Scholar]
Lipworth 2008
- Lipworth BJ, Jackson C. A SMART choice for primary care asthma therapy?. http://www.bmj.com/cgi/eletters/335/7618/513#178078 13 October 2007.
Ni Chroinin 2005
- Ni Chroinin M, Greenstone IR, Danish A, Magdolinos H, Masse V, Zhang X, et al. Long‐acting beta2‐agonists versus placebo in addition to inhaled corticosteroids in children and adults with chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005535] [DOI] [PubMed] [Google Scholar]
Ni Chroinin 2009
- Ni Chroinin M, Lasserson TJ, Greenstone I, Ducharme FM. Addition of long‐acting beta‐agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2009, issue 3. [DOI: 10.1002/14651858.CD007949] [DOI] [PMC free article] [PubMed]
Palmqvist 2001
- Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lotvall J. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulmonary Pharmacology and Therapeutics 2001;14(1):29‐34. [DOI] [PubMed] [Google Scholar]
SIGN/BTS 2012
- Scottish Intercollegiate Guidelines Network/British Thoracic Society. British guideline on the management of asthma: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2008 (revised 2012). [Google Scholar]
Tattersfield 1999
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM, et al. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. American Journal of Respiratory and Critical Care Medicine 1999;160(2):594‐9. [DOI] [PubMed] [Google Scholar]
Visual Rx
- Visual Rx. www.nntonline.net/visualrx/ Accessed June 2012.
Walters 2007
- Walters EH, Gibson PG, Lasserson TJ, Walters JAE. Long‐acting beta2‐agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001385.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cates 2009
- Cates CJ, Lasserson TJ. Combination formoterol and budesonide as maintenance and reliever therapy versus inhaled steroid maintenance for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007313.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


