Abstract
The number of hypercholesterolemic people is increasing rapidly worldwide, with elevated lipid profiles representing a major risk factor of coronary heart diseases. Dietary intervention was shown to improve the lipid profile, thus enhancing the quality of life. Dietary fiber is a nondigestible form of carbohydrates, due to the lack of the digestive enzyme in humans required to digest fiber, and is classified according to its water solubility properties as either soluble (SDF) or insoluble dietary fiber (IDF). Consumption of SDF is associated with several health benefits such as reduced lipid levels, lower blood pressure, improved blood glucose control, improved immune function, and reduced inflammation. SDF has been shown to lower blood cholesterol by several action mechanisms including directly due to the gelling, mucilaginous, and viscous fiber nature, and indirectly due to its fermented products and modulation of the gut microbiome. This review aims to provide a holistic overview on how SDF impacts the lipid profile. We start by providing an overview of the chemical structure of the major SDFs including mucilage, gums (gum arabic and guar gum), pectin, and inulin.
Introduction
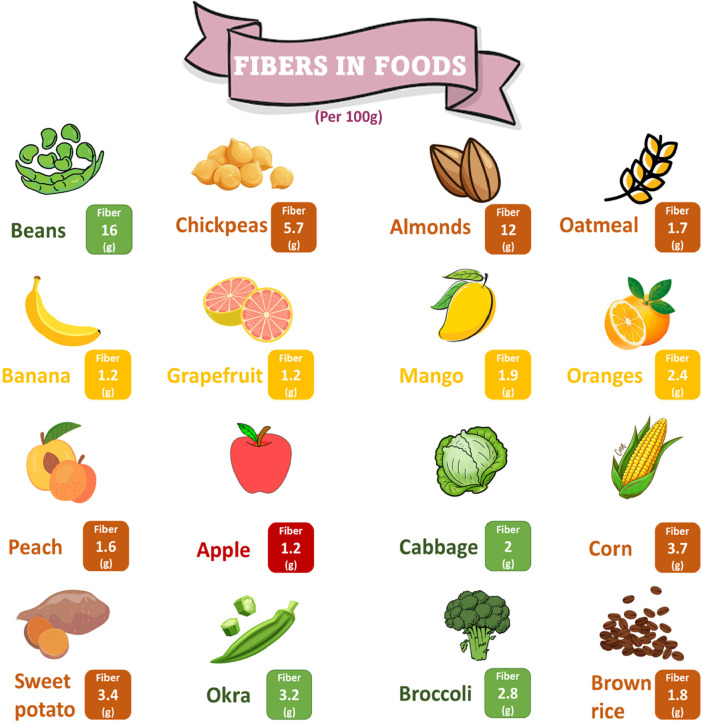
Dietary fiber (DF) is described in different ways all over the world. Several definitions are based on analytical approaches for separating fiber, whereas others define fiber according to a physiological basis. Conventionally, DF is defined as plant polysaccharides which cannot be digested by intestinal enzymes, but are adequately fermented entirely or partly by the intestinal microbiome.1 High fiber foods intake can improve the metabolic profile, decrease blood pressure, assist in weight management, and increase insulin sensitivity.2 The recommended daily allowances (RDAs) of fiber for men and women aged 19–50 years is 38 g/day for men and 25 g/day for women.3 RDA recommendations are for healthy people and not for individuals with chronic diseases. High fiber foods are presented in Figure 1.
Figure 1.
Quantity of fibers in different dietary sources per 100 g.
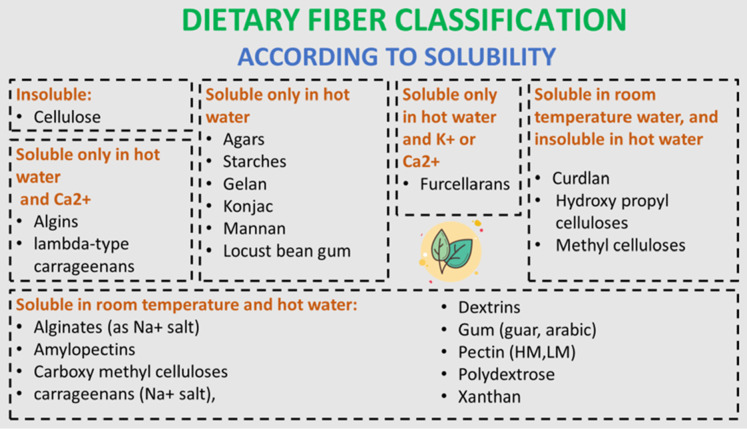
DF can be categorized according to the composition and solubility. Regarding structure, polysaccharides are classified into linear or branched molecules. On the basis of solubility, DF is classified into soluble fiber, which is made up of noncellulosic polysaccharides (e.g., pectin, gums, mucilage), or insoluble dietary fiber (IDF), which forms cell wall components (e.g., cellulose, lignin, hemicellulose).4 The solubility of fibers also can be totally changed depending on the temperature and pH value.5 For example, the chemical modification of insoluble fibers such as cellulose could produce a gelatinous methyl cellulose that is entirely fermented in water rapidly with the viscous structure vanishing.6 Moreover, the solubility of pectin is enhanced with the increase of the quantity of its terminal chains.7 The categorization of DF according to solubility is presented in Figure 2.
Figure 2.
Classification of dietary fibers according to their solubility.
Hyperlipidemia is defined as an increased level of total cholesterol with or without increased triglycerides. This could be attributed to genetic and/or environmental factors or as a comorbidity of another disease such as obesity, hypothyroidism, and diabetes.8 Cholesterol is an insoluble molecule transported in the blood via lipoproteins. There are two types of lipoproteins: high density lipoprotein (HDL), which absorbs cholesterol and transports it back to the liver where it is cleared out from the body, and low density lipoprotein (LDL), which is known as the bad cholesterol since it accumulates on the walls of blood vessels, forming plaques that result in narrowing or occlusion of blood vessels and eventually causing cardiovascular disease (CVD) and stroke.9 According to the World Health Organization (WHO), hypercholesterolemia accounts for 4.5% of total deaths or 2% of total disability-adjusted life years (DALYs).10
Despite the presence of lipid-lowering medications, such as statins,11 some studies showed beneficial effects from the intake of SDFs on the lipid profile. Recent studies state that a 10 g/day increase in intake of SDFs can lower the risk of coronary heart disease by 14% and the risk of coronary death by 27% with lower side effects compared to the use of statins, famous antihyperlipidemic drugs.12
Significantly, SDF supplies the gut microbiota with carbon and energy; besides, SDF improves the intestinal environment by augmenting the beneficial bacteria.13 Moreover, SDF increases mucus production by the intestinal epithelium that retains bacteria insulated from the intestinal lining epithelium.14 Insufficient consumption of SDFs will diminish the quantity of probiotics and affect the metabolism of the intestinal bacteria to consume amino acids which increases the possibility of intestinal injury by the accumulation of toxic metabolites, involving amines, ammonia, fatty acids, and phenolic compounds.15 Hence, a dietary pattern with low fiber and high fat, protein, and sugar may induce chronic diseases such as obesity and cardiovascular disease.16 Therefore, sufficient consumption of SDF is recommended to prevent the degradation of intestinal mucosa by intestinal microbiota and the incidence of these diseases.17
Besides increasing mucus production, SDF has other valuable physiological functions. SDF was found to decrease total cholesterol, LDL cholesterol, and serum triglyceride levels.18,19 The aim of this review is to summarize the clinical trials and in vivo research data interrelating the hypolipidemic properties of soluble dietary fibers involving mucilage, gum, pectin, and inulin.
Although SDF is beneficial for human health, the inappropriate consumption of SDF can result in several health hazards and limit its use.20 Consumption of large amounts of SDF suddenly may lead to several gastrointestinal tract (GIT) complaints including abdominal bloating, flatulence, constipation, and diarrhea.21 Recently, a study done by Singh et al. revealed that fortification of the diet with SDF such as refined inulin resulted in intestinal dysbiosis in mice.22 Moreover, the binding affinity of certain SDFs, such as pectin and guar gum, can reduce the bioavailability of certain bioactive compounds such as β-carotene, lycopene, and lutein as well as micronutrients.20 Hence, despite their physiological actions, a diet fortified with SDFs should be consumed with caution to avoid possible GIT disorders and disruption in gut microbiota.
Cholesterol-Lowering Mechanisms of SDF
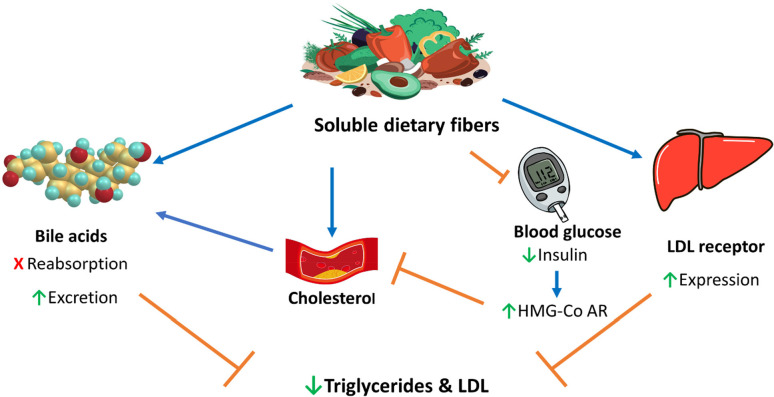
The water holding capacity of SDF increases the bulk weight and dilutes the nutrients inside the intestine due to the presence of water inside involving carbohydrates and lipids and their movement via the intestinal wall.19 These bulking and viscosity features of SDF prolong satiety and decrease food consumption, which was considered as one of the important mechanisms of lipid lowering.23 Another underlying principle behind the reduction of lipid by SDF is attributed to the ability of fibers to bind to bile acids/salts and prevent their reabsorption into the enterohepatic circulation as well as enhance their excretion into feces. Hence, formation of new bile salts from cholesterol occurs, so dropping blood cholesterol levels and having a lipid-lowering effect.24
Furthermore, hepatic LDL receptors become upregulated to restore hepatic cholesterol stores, which will lead to decreased serum LDL concentrations.25 Many studies indicate that SDF adsorbs and sequesters cholesterol; it increases the rate of bile acid excretion, leading to a reduction in triglycerides and LDL levels.26 A third mechanism involves reduction of the postprandial blood glucose which reduces insulin production. This is then reflected by a reduction of cholesterol biosynthesis, since insulin activates 3-hydroxy 3-methylglutaryl coenzyme A reductase (HMG-Co AR), a rate-limiting enzyme in the cholesterol biosynthesis pathway. Finally, through fermentation of SDF by the intestinal microbial flora into short-chain fatty acids (SCFAs), such as acetate, propionate, or butyrate, it may play a role in the inhibition of hepatic cholesterol synthesis.27 Butyrate is metabolized by colonic mucosal cells, while acetate and propionate are rapidly absorbed. The production of SCFAs and in particular changes in the propionate:acetate ratio may influence lipid metabolism, decreasing hepatic absorption and increasing excretion through bile and fecal lipids.28 Another hypothesis for the hypocholesterolemic effects of SDF is based on a lower overall energy intake. Since fiber-rich foods contain fewer calories and take a longer time to digest, SDF promotes increases of satiety.12 During adipogenesis, the MAPK signaling pathway controls the expression of both CCAAT-enhancer binding proteins α (C/EBPα) and peroxisome proliferator-activated receptors γ (PPARγ) mRNA. Guar gum (10% for 12 weeks) was found to regulate the metabolic changes caused by PPARγ suppression in mice fed a high fat diet. Afterward the expression of mitochondrial uncoupling protein 2 (UCP2) was upregulated, inducing the triggering of the AMPK pathway.29 The underlying hypolipidemic mechanism of SDF discussed in the text is illustrated in (Figure 3).
Figure 3.
Main lipid lowering action mechanisms of soluble dietary fiber. Brown lines point to blocking actions, and blue arrows point to stimulating actions.
Soluble Dietary Fibers
Mucilage
There were few reports on plant-derived mucilage before 1991; after that, curiosity in plant mucilage emerged.30 The progress of analytical instruments and better awareness of the chemical composition of mucilage has launched the way for novel applications of this biocompatible and nontoxic compound.
Mucilage (MC) is typically present as a jelly-like substance in different parts of plants including root, stem, or leaf besides being present in seeds following treatment with water. Characterization of MC varied according to their source as well as extraction method affect the ratio of individual constituents. The main sources of soluble mucilaginous dietary fiber are psyllium seed husk (outer coat),31Zizyphus mauritiana fruits,32 flaxseeds,33 chia seeds,33 and Aloe vera leaf.34
From a chemical perspective, MC is formed of uronic acids, glycoproteins, and highly branched polysaccharides as d-xylose, l-arabinose, d-galactose, l-rhamnose, d-mannose, d-glucose, or l-fucose linked by glycosidic bonds.35 The distribution and molecular weights of polysaccharides determine the features of mucilage. High-molecular-weight polysaccharides promote the ability of MC to be used as a thickening and gel-forming agent.36
On top of polysaccharides, the main constituents of mucilage are several proteins, lipids, minerals, and water. Moreover, small constituents are detected in mucilage such as tannins, flavonoids, sterols, and alkaloids. The protein concentration in mucilage influences its water-holding capacity.30 Greater protein concentrations in mucilage are recognized to enrich the quality of products having mucilage.37
Several studies revealed the capability of using mucilage in the pharmaceutical industry and in drug-delivery systems due to its binding features.38 Moreover, the thickening and binding capacities of mucilage also make it useful in the food industry.39 Since mucilage is principally an abundant source of dietary fiber, it exerts multiple therapeutic effects including a laxative effect, satiety regulation, and reduction of hyperlipidemia, hyperglycemia, and obesity.40 Mucilage was found to be poorly fermented inside the small intestine and has the ability to absorb water into the gut leading to the formation of gel.41 This feature provides a desired laxative effect, supplies a poorly fermented substance for microbial multiplication, and produces as a result a greater bacterial bulk and increases the content of colon.42 Conversely, mucilage has the capacity to hinder gastric and colon emptying time; this is helpful for the treatment of diarrhea,43 irritable bowel disease, ulcerative colitis, and hemorrhoids.44
As mentioned above, the mechanism of the hypocholesteremic action of gel-forming fiber is attributed to accordingly hindering intestinal motility and the gastric emptying rate.45 Moreover, mucilage could hinder the absorption of carbohydrates inside the small intestine and thus inhibit the cholesterol biosynthesis pathway as well as stimulate the secretion of insulin.46 MC was found to alter the gut microbiota in individuals with obesity. Administration of flaxseed mucilage daily for 6 weeks for obese patients reduced the quantity of Faecalibacterium prausnitzii species in intestine. Unexpectedly, decreasing the concentration of F. prausnitzii improves insulin sensitivity through producing SCFA butyrate in a large amount which has an anti-inflammatory effect and recovers the metabolic disorder related to obesity such as insulin resistance.47
Overall, several studies focused on mucilage over 20 years. Despite this, large numbers of studies presented only basic information regarding the chemical composition and properties of mucilage. Besides, few studies have relatively evaluated mucilage as a hypolipidemic agent in both human and animal models. The hypocholesterolemic effects of mucilage in animal models and in humans are listed in Table 1.
Table 1. Clinical Trials and In Vivo Studies on the Hypocholesterolemic Effect of Mucilage (MC)a.
| study | subject | dose of MC | results | ref |
|---|---|---|---|---|
| clinical trial | randomized, double blind, crossover study applied on 15 diabetic patients | pudding containing yellow mustard MC (15.5 g, 3.10 wt %), flaxseed MC (11.4 g, 2.28 wt %), fenugreek gum (5.9 g, 1.18 wt %) three times daily for 2 weeks | ↓ BG, insulin, and ↑ intestinal viscosity significantly | (48) |
| double blind, randomized, and placebo controlled study applied on 72 obesity patients | flaxseed MC (2560 mg and 1280 mg twice daily for 12 weeks) | ↓ BW (p < 0.001), TC (p = 0.038), TG (p = 0.040), compared to placebo group | (49) | |
| in vivo | hypercholesterolemic rabbits | fenugreek seeds MC (40 mg/kg for 3 months) | ↓ TC, TG, and LDL (p < 0.05) | (50) |
| hypercholesterolemic rats | diet containing 10% Lepidium sativum MC for 3 weeks | ↓ TC, TG, LDL, and VLDL | (51) | |
| hyperlipidemic rats | Cordia dichotoma MC (0.50 and 1.00 g/kg) for 4 weeks | ↓ TC and LDL | (52) | |
| diabetic rats | Aloe vera MC (1.2 g daily for 6 weeks) | ↑ genes involved in lipid metabolism | (53) | |
| hyperlipidemic rats | flaxseed or cress seed mucilage (40 mg/kg for 4 weeks) | ↓ TC, TG, LDL, and MDA | (54) | |
| diabetic rats | Aloe vera MC (1 mL daily for 3 weeks) | ↓ TC, TG, and BG | (34) | |
| rats | HFD plus Nopal MC 500 mg/kg daily for 30 days | ↓TC, TG, BG, and abdominal circumference | (55) | |
| hyperlipidemic rabbits | Trigonella foenum-graecum MC (75 mg/kg for 90 days) | ↓TC, TG, LDL, and ↑HDL | (56) | |
| diabetic mice | okra MC (150 mg/kg for 21 days) | ↓TC, TG, LDL, and BG | (57) |
BW, body weight; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; V-LDL, very low density lipoprotein; BG, blood glucose; HFD, high fructose diet. MDA, malondialdehyde.
Gums
Major gums used as sources of drugs include gum arabic and guar gum. Gum arabic (GA), African gum belt, is a natural edible gum from various species of the acacia seyal trees. GA is formed of polysaccharide sugars and glycoprotein, attached to carbohydrate (d-galactose and l-arabinose) with a covalent bond. GA includes amino acids and trace elements such as aluminum, phosphorus, magnesium, copper, zinc, and iron.58 GA exhibits antioxidant,59 renoprotective, and blood glucose reducing properties.60 GA is not degraded in the small intestine; thus it is digested with difficulty by animals and humans. It is fermented inside the colon, yielding short-chain fatty acids, mainly propionate, by the enzymatic action of intestinal bacteria.61
Several studies have reported on the lipid-lowering effects of GA in both animal and human models (Table 2). GA reduces lipid levels mostly through the downregulation of gene levels included in lipid metabolism such as11β-hydroxysteroid dehydrogenase type I, HMG-CoA reductase, and adipose triglyceride lipase.62 Likewise, GA was found to downregulate peroxisome proliferator-activated receptor γ and stearoyl-CoA desaturase mRNA expression in mice liver as well as steroid 17-α-monooxygenase (CYP17) in mice muscle.63 Further, GA hinders intestinal lipid absorption by increasing the viscosity of intestinal content.64
Table 2. List of Gum Arabic (GA) Effects as a Hypolipidemic Agent in Human and Animal Modelsa.
| study | subject | dose of GA | results | ref |
|---|---|---|---|---|
| clinical trial | controlled study applied on 55 hyperlipidemic patients | 30 mg of GA plus atorvastatin (20 mg) daily for 4 weeks | ↓ TC (7.8%), TG (2.9%), and LDL (8.1%) compared to atorvastatin treated group | (65) |
| controlled study applied on 40 diabetic patients | 10 g/day for 16 weeks | ↓ TC, TG, BG, and HbAc1 significantly | (66) | |
| randomized, placebo controlled, double blind trial applied on 100 diabetic patients | 30 g/day for 3 months | ↓ TC (8.28%), LDL (5.95%), TG (10.95%), and BG; ↑ HDL (19.89%) | (67) | |
| randomized, placebo controlled trial applied on 45 diabetic patients | 30 g/day for 3 months | ↓ body adiposity index (3.9%) and visceral adiposity index (23.7%) | (68) | |
| phase II, single-arm trial applied on 47 sickle cell anemia patients | 30 g/day for 12 weeks | ↓ TC, TG, and LDL significantly | (69) | |
| controlled, randomized, single blind, parallel-design study trial applied on 31 participants | 20 g/day for 12 weeks | ↓ (p = 0.008) and diastolic blood pressure (0.009), fat free mass (p = 0.03), mass, carbohydrate (p = 0.008), and calorie (p = 0.014) intake, and fasting plasma glucose (p = 0.046) | (70) | |
| in vivo | male albino rats | diet supplemented with 30% GA for 30 days | ↓ TC and LDL | (71) |
| male donkeys | 25 g/day for seven successive days | ↓ TC, TG, urea, and creatinine | (72) | |
| female CD-1 mice | GA (10%) in drinking water for 6 weeks | ↓ TC, HDL, and LDL but not TG | (63) | |
| diabetic rats | GA (10%) in drinking water for 10 weeks | ↓ TC, LDL, TG and ↑ HDL | (73) | |
| diabetic rats | GA in drinking water (10% w/v) for 12 weeks | ↓TG, TC, TG, LDL, urea, and creatinine | (74) | |
| metabolic syndrome (MS) rat model | diet containing GA (5%) mixed with Kishk Sa’eedi (KS, 10%) or pomegranate seed oil (1%) | ↓ plasma dyslipidemia, BG, AST, creatinine, and urea; ↑ HDL | (75) | |
| mice | high fat diet containing 10% w/w GA for 15 weeks | ↓ TC, LDL, BG, and ↑ HDL | (76) |
HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; V-LDL, very low density lipoprotein; BG, blood glucose.
The second example of gum is guar gum (GG). Guar gum, Cyamopsis tetragonoloba or cluster bean, is a viscous soluble fermentable fiber. Guar seeds were found to be formed of endosperm (42%) in which the main portion is gum.77 Roughly 80–85% of the gum is made up of a galactomannan polymer that develops a gel in water causing a thickening which makes it suitable for food processing.78
Moreover, GG has shown several beneficial therapeutic effects against diabetes, colon cancer, heart, and liver disease.79,80 Further, partially hydrolyzed guar gum (PHGG) was found to improve abdominal pain and bowel habits when consumed by irritable bowel syndrome patients at a dose of 5 g/day for 4 weeks.81 A meta-analysis of 34 trials indicated GG showed a nonsignificant difference in the body weight of patients compared to patients receiving placebo with the incidence of side effects such as diarrhea and cramps. Hence, GG cannot be recommended as a medicine for body weight reduction.82
Like gum arabic, GG exhibits its hypolipidemic effect by increasing the viscosity of the intestinal content besides altering levels of beneficial bacteria (e.g., bifidobacteria) and pathological microbiota (e.g., Clostridium species) resulting in limiting lipid absorption.83 Guar-containing bread readily incorporated into the diet with minimal alteration in the normal eating pattern and palatability is likely to increase compliance and be of considerable therapeutic use in the future owing to its added value in the management of hyperlipidemia.84 The use of large doses of high-molecular-weight guar galactomannan gum (∼2.4 × 106) can, however, lead to problems of palatability upon inclusion of the polymer into foods. Sensory studies have shown that improvements in the quality of guar wheat bread can be achieved by using low-molecular-weight guar galactomannan ((0.5–1.0) × 106), warranting production of different varieties of bread and other foods containing guar gum to provide a wide selection of products and improve long-term compliance for consumers.85 The hypolipidemic effects of GG in human and animal models are listed in Table 3.
Table 3. Guar Gum (GG) as a Hypolipidemic Agent in Human and Animal Modelsa.
| study | subject | dose of GG | results | ref |
|---|---|---|---|---|
| clinical trial | randomized trial applied on 141 hypertensive, overweight patients | 3.5 g twice daily before meal for 6 months | ↓ HbA1c, LDL, and ApoB without altering TG | (86) |
| controlled study applied on hyperlipidemic patients | guar gum bread for 4 weeks | ↓ TC (10%) | (84) | |
| randomized trial applied on 9 diabetic patients | 7 g three times daily for 3 months | ↓ TC and LDL | (87) | |
| controlled study applied on 60 diabetic patients | 10–20 g daily for 15–30 days | ↓ TC, TG, LDL, VLDL, and blood glucose; ↑ HDL | (88, 89) | |
| randomized trial applied on 17 diabetic patients | 21 g/day for 3 weeks | ↓ TC (14%) | (90) | |
| controlled trail applied on 20 nonalcoholic steatohepatitis (NASH) with obesity and osteoarthritis patients | GG (5 g) plus metadoxine (0.5 g) twice daily for 90 days | ↓ obesity, absorption of carbohydrates, and fermentation products in the intestine | (91) | |
| in vivo | guinea pigs (male, female, and ovariectomized) | diet mixed with GG (2.5 g/100) for 4 weeks | ↓ TC (64%), LDL (44%) cholesterol, and TG (22%)b | (92) |
| hyperlipidemic male rats | diet supplemented with 10% GG for 3 weeks | ↓ TC, TG, glucose, insulin, glucagon, and corticosterone levels | (93) | |
| pigs | diet supplemented with 3.5% GG for 4 weeks | ↓ TC, LDL, and TG | (94) | |
| hyperlipidemic male rats | flour mixed with GG (3 g/100 g) for 8 weeks | ↓ TC (17.2%), LDL (29.7%), and TG (28.4%) | (95) | |
| hyperlipidemic rats | diet containing 5% (w/w) GG for 28 days | ↓ TC | (96) | |
| hyperlipidemic rats | diet containing 5, 10, and 20% (w/w) GG for 28 days | ↓ TC, TG, and LDL | (97) | |
| diabetic rats | diet containing 5, 10, and 20% (w/w) GG for 28 days | ↓ TC, TG, LDL, and blood glucose | (79) | |
| hyperlipidemic guinea pigs | high fat diet mixed with partially hydrolyzed GG (12 g/100 g of diet) for 4 weeks | ↓ TC; ↑ excretion of fatty acids and neutral sterolsc | (98) |
HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; V-LDL, very low density lipoprotein.
Ovariectomized guinea pigs had higher TC and TG compared to males and females.
Excretion of fatty acids and neutral sterols did not alter the fecal fatty acid profile.
Pectin
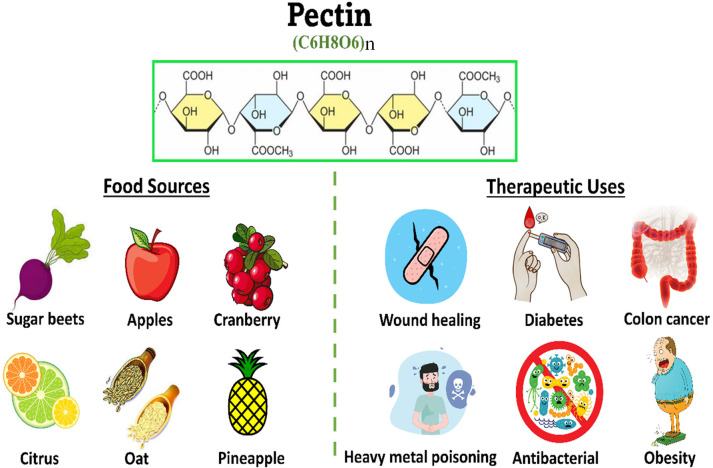
Pectin (PC) is the main structure of cell walls of several plants including in citrus peel, apple, and carrot.99 Pectin is extensively used in the food industry as a thickener and gelling agent.100 Moreover, pectin is considered a good source of SDF and has a several biological benefits for human health.100 Pectin is rich in galacturonic acid, where homogalacturonan (HG), formed by the α-1,4 linkage of d-galacturonic acids, represents the smooth region of the pectin backbone and is about 65% of pectin polysaccharides, and the hairy regions are comprised of rhamnogalacturonans I and II (RG-I and RG-II). The degree of methyl esterification (DE), whether it is high (DE > 50%) or low (DE < 50%), defines the solubility and gelling properties of pectin.101
Pectin was found to be indigestible by intestinal enzymes; conversely, it could be simply degraded by intestinal bacteria, resulting in the production of SCFAs.102 Besides, pectin enhances the intestinal integrity and supports the connection of intestinal epithelial cells with probiotic Lactobacillus strains to epithelial cells.103 Moreover, several studies have shown that PC stimulates the growth of bacterial populations such as lactobacilli, bifidobacteria, and F. prausnitzii.104 Pectin was found to be degraded by several bacteria species such as Bacteroides and Prevotella that secrete carbohydrate-active enzymes including lyases, methylesterases, and acetylesterases.105
Several in vitro and in vivo studies have been carried out to examine the lipid-lowering effects of pectin. A study by Brouns et al.106 explored the cholesterol-lowering effect of pectin from different sources, with different physicochemical properties. Pectin, extracted from citrus, apple, and orange pulp fiber, processed to achieve different DEs and molecular weights (MWs), were used in a crossover clinical study on individuals with mild hypercholesterolemia. Results showed that the pectin source, along with the DE and MW, accounted for the LDL-C lowering capacity, where high DE and MW pectin showed a better LDL-C lowering effect than low DE pectin. Whether such structural features are also linked to other effects like lowering the blood glucose level should be examined.
To assess the potential of pectin to serve as a prebiotic that confers health benefits, in vitro fermentation studies were done using human fecal samples to determine alterations of the microbiota profile and the produced SCFAs. Baobab fruit pulp powder (BP) was examined by Foltz et al.,163 owing to its high composition of HG pectin polysaccharide with a low DE. The study was done using a 48 h in vitro incubation with human microbiota from three different stool sample donors. Despite the observed interindividual differences, acetate and propionate SCFAs were constantly increased and butyrate was increased to some extent. It is worth noting that propionate is proposed to reduce the serum cholesterol level by inhibiting HMG-Co AR. Also, among the five taxonomic gut microbial groups quantified, Bacteroidetes was found enriched in the three samples, compared to the control. Another study by Min et al. investigated the effect of different pectin sources on gut microbiota and SCFA production. The bacterial fermentation of high methoxy pectin (HMP) and two low methoxy pectins extracted from sugar beet (SBP) and soy (SOY) was tested through anaerobic incubation with four human fecal samples. Although results showed different microbiota profiles, especially that three of the participants were overweight, the composition was altered after the 30 h post fermentation period. SCFA production was higher with citrus and apple pectin compared to SBP and oat. Moreover, propionate and butyrate production were found significantly higher in the case of soy fermentation compared to SBP, likely attributed to differences in structures leading to different fermentation levels acting as a better prebiotic.107 Moreover, pectin oligosaccharides derived from lemon peel waste (LPOS) and sugar beet pulp (SBPOS) provide better prebiotic potential than their pectin counterparts. Studying the fermentation profile by human fecal microbiota showed the enrichment of bifidobacteria and lactobacilli in all pectin preparations. However, a marked bifidogenic effect was evident by SBPOS fermentation, while LPOS showed a significant increase in lactobacilli count.104
Moreover, the hypocholesterolemic effect of pectin was verified in humans and animals. Food sources, the chemical structure, and therapeutic effects of pectin are concluded in Figure 4. The hypocholesterolemic effects of pectin in human and animal models are revealed in Table 4. The clinical trials are considered inadequate and poorly designed. Thus, more trials should be performed on humans in the future to evaluate the therapeutic effects of pectin as a hypolipidemic agent.
Figure 4.
Chemical composition, dietary sources, and therapeutic effects of pectin.
Table 4. Clinical Trials and In Vivo Studies Reported on the Hypolipidemic Effect of Pectin (PC)a.
| study | subject | dose of PC | results | ref |
|---|---|---|---|---|
| clinical trial | randomized trial applied on 53 healthy humans | diet contains 4.11 g of soluble fiber (PC, gums, and mucilage) and 25.08 g of insoluble fiber for 3 months | ↓ LDL (12.8%) and glucose (12.3%) | (108) |
| placebo controlled, randomized, parallel double blind study applied on 66 diabetic patients | sugar beet PC, 12 weeks | fasting plasma glucose concentration did not change; ↑ HbA1c and HDL significantly | (109) | |
| crossover studies applied on hypercholesterolemic patients | 15 g of apple low MW PC for 4 weeks; 6 g/day citrus and high MW PC for 3 weeks | apple PC reduced LDL by 7–10%, while citrus PC reduced LDL by 6–7% | (106) | |
| in vivo | male Sprague–Dawley rats | diet containing 15% fat and 6% PC | ↓ TC, V-LDL, and LDL; ↑ HDL and CoA reductase activity | (110, 111) |
| hamsters | high cholesterol (0.1% w/w) diets plus 3% lemon PC for 8 weeks | ↓ TC | (112) | |
| male Wistar rats | diet containing 5 g/100 g apple PC and 10 g/100 g high polyphenol freeze-dried apple for 21 days | ↓ TC, TG, and cholesterol absorption (13%) | (113) | |
| male rats | high cholesterol diet containing PC (60 g/kg) for 4 weeks | ↓ TC (from 2.08 to 1.67 μmol/mL) | (114) | |
| female Zucker fatty rats as a model of genetic obesity | diet containing 10% PC for 15 weeks | ↓ BW, TC, and TG | (115) | |
| laying hens | diet containing 0.5% PC | ↓ egg yolk cholesterol | (116) | |
| hypercholesterolemic swine | diet containing 30 g of PC daily for 4 weeks | ↓ LDL and TG | (117) | |
| diabetic rats | 0.5–25 mg/kg orally for 5 successive days | ↓ TG and blood glucose | (118) | |
| hypercholesterolemic rats | diets containing 5% of PC for 6 weeks | ↓ TC | (119) | |
| hypercholesterolemic New Zealand rabbits | combination of PC, niacin, and apple cider vinegar 3 mL/kg/day for 4 weeks | ↓ TC, LDL, and TG; ↑ HDL | (120) | |
| hypercholesterolemic rats | diet containing pea proteins (90%) and apple pectin (7.5%) | ↑ CYP7A1 and NTCP genes, which were involved in cholesterol turnover | (121) | |
| male Wistar rats | High fat diet plus GG with low, medium, or high viscosity degree (1%, w/v) and two different doses of PC (24%, LM or 70%, HM) for 3 weeks | both PC and GG ↓ TC, BG, and liver steatosis but to varying extent depending on degree of methoxylation and viscosity of fibers; only GG with medium and high viscosities ↑ levels of butyric acid in cecum and blood | (122) | |
| hypercholesterolemic rats | diet supplemented with PC pectin (5 wt %/wt) for 6 weeks | ↓ BW, TC, TG, and development of adipose tissue | (123) | |
| diabetic rats | diet containing citrus PC, 4 weeks | ↓ TC, LDL, TC, insulin resistance, and BG levels | (124) | |
| hypercholesterolemic mice | ε-polylysine and PC for 13 weeks | ↓ TC and TG | (125) | |
| hypercholesterolemic mice | high fat diet containing 4 or 8% of PC for 12 weeks | ↓ BW, TC, and LDL in a dose dependent manner | (126) | |
| male mice | high cholesterol diet containing 20 wt %/wt PC for 12 weeks | ↓ cholesterol absorption | (127) | |
| male C57BL/6 mice | high fat diet and 200 mg/kg/day PC orally for 17 weeks | ↓ TC, LDL, leptin, and adiponectin | (128) |
HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; V-LDL, very low density lipoprotein; BW, body weight; BG, blood glucose; LM, low methoxylation; HM, high methoxylation.
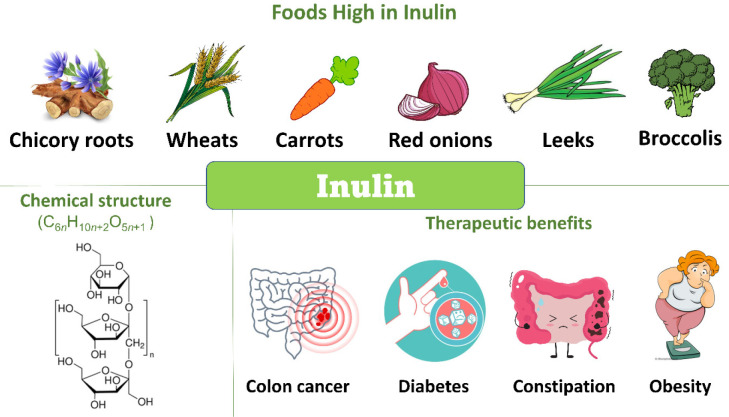
Inulin
Inulin is a nondigestible carbohydrate formed of d-fructose units joined by β-(2–1) bonds and an α-(1–2) terminal d-glucose.129 It is found in a variety of plants such as chicory, artichoke, leek, asparagus, and garlic. The ability of fructans to be selectively fermented by health promoting Bifidobacterium bacteria underscores their use as functional food and in nutraceuticals.130 A recent study has evaluated the effect of gut microbiota in mediating the antihyperlipidemic action of inulin. Briefly, inulin stimulated the production of SCFA-producing bacteria such as Ruminococcaceae and Lachnospiraceae as well as the expression of angiopoietin-like protein 4 that may be enhanced by the greater production of SCFAs. These findings pointed to the mediating effect of bacteria on the effect of inulin.131
A study by Van De Wiele et al.132 compared the prebiotic effects of fructans with a low degree of polymerization (DP), oligofructose (DP 2–20), and with a higher DP, chicory inulin (DP 3–60), under in vitro conditions using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) reactor inoculated with fecal bacteria of a healthy donor. Although inulin required a longer fermentation period, owing to its longer chain length, the prebiotic effectiveness was higher even in the distal region of the colon. Both fructans increased propionate and butyrate production; however, the propionate level was significantly higher with inulin fermentation in all colon vessels.133 Also, inulin was shown to exert a greater bifidogenic effect along with a significant reduction in the ammonium level than oligofructose; hence it was considered a safer option than oligofructose. Furthermore, increased abundance of Bifidobacterium and Anaerostipes bacteria together with decreased Bilophila bacteria were observed with the intake of 12 g of chicory inulin for a period of 4 weeks by healthy adults with mild constipation.133 Foods high in inulin and the chemical composition and therapeutic benefits of inulin are demonstrated in Figure 5. Besides, clinical trials and in vivo studies over 20 years are revealed in Table 5.
Figure 5.
Chemical composition, dietary sources, and therapeutic benefits of inulin.
Table 5. Clinical Trials and In Vivo Studies Evaluating the Hypocholesterolemic Effect of Inulin (IN)a.
| study | subject | dose of IN | results | ref |
|---|---|---|---|---|
| clinical trial | double blind, randomized, placebo trial applied on 12 obese patients | 7 g/day orally in morning for 4 weeks | ↓ TC (p = 0.028), LDL (p = 0.028), VLDL (p = 0.046), TG (p = 0.046) | (137) |
| randomized study applied on 15 obese patients | IN enriched cookies for 1 month | ↓ TC and LDL significantly | (138) | |
| randomized, controlled study applied on 30 obese Mexican women | 5 g/day for 3 months | ↓ TC, TC, LDL, and blood glucose level significantly | (139) | |
| randomized, placebo controlled trial applied on 24 diabetic women | 10 g/day for 8 weeks | ↓ FBS (8.5%), HbA1c (10.4%), TC (12.9%), TG (23.6%), LDL (35.3%), LDL/HDL ratio (16.25%), and TC/HDL ratio (25.2%), and ↑ HDL (19.9%) | (140) | |
| randomized, controlled trial applied on 25 diabetic women | 10 g/day for 4 weeks | ↓ TC, insulin, and blood glucose level | (141) | |
| randomized, controlled trial applied on 10 diabetic patients | 30 g/day for 9–18 weeks | ↓ BFI, FBG, and insulin | (142) | |
| randomized, controlled trial applied on 25 diabetic women | 10 g/day for 8 weeks | ↓ FBG (9.4%), HbA1c (8.4%), TC (14.1%), LDL (21.7%), TC/HDL ratio (20.7%), and LDL/HDL ratio (27.5%) | (143) | |
| randomized, controlled trial applied on 14 obese men | high fat milkshake containing 24 g of IN one time | ↑ fat oxidation, ↓ blood glucose, and insulin; no effects on TG free glycerol and satiety hormones GLP-1 | (132) | |
| randomized, controlled trial applied on 51 obese patients | 16 g/day for 3 months | ↓ FBG, insulin, HbA1c, TC, LDL, and TG | (144) | |
| randomized, placebo controlled, double blind applied on study 42 obese patients | 10 g of IN plus 10 g of resistant maltodextrin daily in 400 mL of milk for 12 weeks | ↓ TC, TG, and LDL | (145) | |
| randomized, controlled trial applied on 14 obese men | high fat milkshake with 24 g of fermentable IN | ↓ fat oxidation and ↑ FFA | .146 | |
| in vivo | Balb/c mice | diet containing 5% IN for 3 weeks | ↓ TC and TG | (147) |
| rats | FRD supplemented with IN (10 g/100 g) for 4 weeks | ↓ blood pressure and TG | (148) | |
| albino rats | FRD supplemented with 0.174 g/100 g body weight | ↓ TC, TG, LDL, serum glucose, and insulin level | (8) | |
| piglets | diet supplemented with 2% IN for 12 days | ↓ TC and ↑ IgA and IgG concentrations | (149) | |
| hyperlipidemic hamsters | combination of IN and Fibersol-2 orally at 864, 1727, or 2591 mg/kg/day for 9 weeks | ↓ TC, TG, LDL, LDL/HDL ratio; hepatic TC and TG levels in a dose dependent manner | (150) | |
| rats | diet containing 5% fat with 5% 5% IN for 28 days | ↓ TC and TG | (151) | |
| diabetic rats | 2.5, 5, or 10 g/kg daily for 8 weeks | ↓ blood glucose, insulin, TC, TG, and NEFA in a dose dependent manner | (152) | |
| C57BL/6J mice | HF/HS diet supplemented with IN (9%) for 4 weeks | ↓ TC and fecal cholesterol and bile acid excretion | (153) | |
| Syrian hamsters | HFD containing 20% IN for 3 weeks | ↓ TC (24%), TG (34%), and LDL (29%); ↑ fecal excretion of total fat, TG, and bile acids | (154) | |
| Sprague–Dawley rats | HFD containing IN (8% w/w) | ↓ TC, TG, and LDL | (155) | |
| C57BL/6 pregnant female mice | HF/HS diet plus 1.67 or 3.33 g/kg/day orally for 4 weeks | ↓ TC, TG, LDL, blood glucose level, and insulin level | (156) | |
| diabetic female rats | oral administration of 10 mg/kg daily for 2 weeks | ↓ TC, TG, LDL, serum glucose, and insulin level | (157) |
FRD, fructose-rich diet; HFD, high fat diet; HF/HS, high fat/high sucrose; NEFA, nonesterified fatty acid; HbA1c, glycosylated hemoglobin; TC, total cholesterol; TG, triglycerides; HDL, high density lipoprotein; LDL, low density lipoprotein; V-LDL, very low density lipoprotein; BFI, body fat index; FFA, free fatty acid.
Although inulin has been established to act as a prebiotic, studies regarding the lipid-lowering effect of inulin are somehow controversial. For example, intake of 20 g of chicory inulin per day for 3 weeks was found to significantly reduce serum TG, with a slight reduction in serum TC of 12 males with mild hypercholesterolemia.110,134 However, no significant effect on the lipid profile was shown in a randomized, double blind, placebo controlled study where healthy individuals consuming 10 g of fructan preparation (inulin and oligofructose) for a long term (6 months).135 These results are in line with those demonstrated by Mistry et al., where neither the lipid profile nor cholesterol metabolism changed in mice fed long-chain or short-chain inulin for 2 weeks, despite the significant increase in fecal SCFA level.136
Safety of SDF
Although SDF exhibits valuable health benefits, it cannot be disregarded that improper SDF consumption may induce specific health hazards that differ according to the nature and amount of SDF as well as the medical history of the subject.16 An unexpected increase of SDF ingestion could induce constipation, diarrhea, and other abdominal disorders.158 Besides, rapid fermentation of SDF (60 g/kg of fructooligosaccharides) by intestinal bacteria revealed a marked injury to intestinal mucosa as well as increased intestinal permeability in rats infected with Salmonella enterica.159 SDF also was found to increase the extent of several elements such as metal ions through its binding capacity. In spite of this, a previous study showed that the bioavailability of β-carotene, lycopene, and lutein were reduced significantly in six healthy young women by different types of SDF including pectin, guar gum, and alginate.160
Moreover, intake of a diet rich in purified inulin (7.5%), pectin (2.5%), and fructooligosaccharides (2.5%) for 6 months induced icteric hepatocellular carcinoma (HCC) to mice that were deficient in Toll-like receptor 5, through the promotion of secondary bile acids in the systemic circulation and cholestasis, followed by triggering of several inflammatory pathways that ended with necrosis of hepatocytes. These findings could not be induced by cellulose (7.5%), the insoluble and nonfermentable fiber.161
Similarly, it has been reported that daily administration of a high dose of psyllium (20.4 g) that was rich in mucilage for 3 months caused diarrhea in 30 hypocholesterolemic patients.162 However, the dose and time relationship was established in another study that revealed that administration of psyllium for 8 week at a dose of 3.0–20.4 g daily reduced cholesterol significantly in a dose and time dependent manner.88 This study was performed on 1039 mild and moderate hypercholesterolemic patients that were included in the meta-analysis studies including randomized placebo controlled trials, double blind or open label. These data suggested that, despite the prominent physiological benefits of a diet rich in SDF, its consumption should be made with caution.
Conclusions and Future Directions
Hypercholesterolemia is a risk factor for coronary heart disease, and nutrition management is the best therapeutic approach. Soluble dietary fibers have several protective effects against chronic diseases, including cardiovascular disease, diabetes, and metabolic syndrome. The attempt to manage these conditions through dietary intervention could improve the quality of life of many individuals, reducing morbidity and mortality rates while leading to suffering from fewer side effects compared to medical treatment strategies. Despite the differences of the gut microbiota composition due to either dietary habits or health condition, SDFs have shown to positively alter the microbiota profile with the production of beneficial SCFAs. SDFs also have multiple non-nutritive health effects that help improve lipid profiles via multiple action mechanisms. The inclusion of SDF in the diet appears to be the right approach to reduce the risk of hypercholesterolemia, atherosclerosis, and cardiovascular disease based on extensive reports in this review.
These studies will possibly drive scientists to establish novel antihyperlipidemic agents from natural resources and to validate their mechanistic approaches using pharmacological assessments. More clinical studies with a larger cohort group, a chemically well-characterized type of SDF by a specified clinical context, and suitable predictors of metabolic health and SDF pharmacokinetic/pharmacodynamic behaviors are still required to extrapolate the experimental data to human scenarios. The interaction of SDF in combination with specific antihypercholestorlemic drugs should also be considered in different cohort groups and populations.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
References
- Holscher H. D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut microbes 2017, 8 (2), 172–184. 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuwissen E.; Mensink R. P. Water-soluble dietary fibers and cardiovascular disease. Physiology & behavior 2008, 94 (2), 285–292. 10.1016/j.physbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Soliman G. A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11 (5), 1155. 10.3390/nu11051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert M. O.; Pfeiffer A. F. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. The Journal of nutrition 2018, 148 (1), 7–12. 10.1093/jn/nxx008. [DOI] [PubMed] [Google Scholar]
- Lovegrove A.; Edwards C.; De Noni I.; Patel H.; El S.; Grassby T.; Zielke C.; Ulmius M.; Nilsson L.; Butterworth P.; et al. Role of polysaccharides in food, digestion, and health. Critical Reviews in Food Science and Nutrition 2017, 57 (2), 237–253. 10.1080/10408398.2014.939263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasatto P. L.; Pignon F.; Silveira J. L.; Duarte M. E. R.; Noseda M. D.; Rinaudo M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7 (5), 777–803. 10.3390/polym7050777. [DOI] [Google Scholar]
- Ralet M.-C.; Dronnet V.; Buchholt H. C.; Thibault J.-F. Enzymatically and chemically de-esterified lime pectins: characterisation, polyelectrolyte behaviour and calcium binding properties. Carbohydrate research 2001, 336 (2), 117–125. 10.1016/S0008-6215(01)00248-8. [DOI] [PubMed] [Google Scholar]
- Nassar S. E.; Ismail G. M.; El-Damarawi M. A.; Alam El-Din A. A. Effect of inulin on metabolic changes produced by fructose rich diet. Life Sci. J 2013, 10, 1807–1814. [Google Scholar]
- Murli S.Identify the Risks of Cholesterol in Children. The Wellthy Magazine, January 17, 2020. https://www.wellthy.care/identify-the-risks-of-cholesterol-in-children/.
- Morales-Villegas E. C.; Yarleque C.; Almeida M. L. Management of hypertension and dyslipidemia in Mexico: Evidence, gaps, and approach. Archivos de Cardiologia de Mexico 2023, 93, 77–87. 10.24875/ACM.21000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahan K. Lipid-lowering drugs. Cellular and molecular life sciences CMLS 2006, 63 (10), 1165–1178. 10.1007/s00018-005-5406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surampudi P.; Enkhmaa B.; Anuurad E.; Berglund L. Lipid lowering with soluble dietary fiber. Current Atherosclerosis Reports 2016, 18 (12), 75. 10.1007/s11883-016-0624-z. [DOI] [PubMed] [Google Scholar]
- Tap J.; Furet J. P.; Bensaada M.; Philippe C.; Roth H.; Rabot S.; Lakhdari O.; Lombard V.; Henrissat B.; Corthier G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environmental Microbiology 2015, 17 (12), 4954–4964. 10.1111/1462-2920.13006. [DOI] [PubMed] [Google Scholar]
- Johansson M. E.; Phillipson M.; Petersson J.; Velcich A.; Holm L.; Hansson G. C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the national academy of sciences 2008, 105 (39), 15064–15069. 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wrzosek L.; Miquel S.; Noordine M.-L.; Bouet S.; Chevalier-Curt M. J.; Robert V.; Philippe C.; Bridonneau C.; Cherbuy C.; Robbe-Masselot C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biology 2013, 11 (1), 61. 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windey K.; De Preter V.; Verbeke K. Relevance of protein fermentation to gut health. Molecular nutrition & food research 2012, 56 (1), 184–196. 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- Guan Z.-W.; Yu E.-Z.; Feng Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26 (22), 6802. 10.3390/molecules26226802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B. O.; Birchenough G. M.; Ståhlman M.; Arike L.; Johansson M. E.; Hansson G. C.; Bäckhed F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host & Microbe 2018, 23 (1), 27–40. e27. 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Bowman R.; Welch A. A.; Luben R. N.; Wareham N.; Khaw K.-T.; Bingham S. A. Apolipoprotein E polymorphisms, dietary fat and fibre, and serum lipids: the EPIC Norfolk study. European heart journal 2007, 28 (23), 2930–2936. 10.1093/eurheartj/ehm482. [DOI] [PubMed] [Google Scholar]
- Dai F.-J.; Chau C.-F. Classification and regulatory perspectives of dietary fiber. Journal of food and drug analysis 2017, 25 (1), 37–42. 10.1016/j.jfda.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z.-W.; Yu E.-Z.; Feng Q. J. M. Soluble dietary fiber, one of the most important nutrients for the gut microbiota 2021, 26 (22), 6802. 10.3390/molecules26226802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Salhy M.; Gundersen D. J. N. j. Diet in irritable bowel syndrome. Nutr. J. 2015, 14 (1), 36. 10.1186/s12937-015-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.; Yeoh B. S.; Chassaing B.; Xiao X.; Saha P.; Aguilera Olvera R.; Lapek J. D. Jr.; Zhang L.; Wang W.-B.; Hao S.; et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 2018, 175 (3), 679–694. e622. 10.1016/j.cell.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y.; Luo F. Dietary fiber: an opportunity for a global control of hyperlipidemia. Oxidative Medicine and Cellular Longevity 2021, 2021, 5542342. 10.1155/2021/5542342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirouchi B.; Kawamura S.; Matsuoka R.; Baba S.; Nagata K.; Shiratake S.; Tomoyori H.; Imaizumi K.; Sato M. Dietary guar gum reduces lymph flow and diminishes lipid transport in thoracic duct-cannulated rats. Lipids 2011, 46 (8), 789–793. 10.1007/s11745-011-3570-0. [DOI] [PubMed] [Google Scholar]
- Tosh S. M.; Bordenave N. Emerging science on benefits of whole grain oat and barley and their soluble dietary fibers for heart health, glycemic response, and gut microbiota. Nutrition Reviews 2020, 78, 13–20. 10.1093/nutrit/nuz085. [DOI] [PubMed] [Google Scholar]
- Singh S.; Shetkar S. S.. Cholesterol Absorption Inhibitors. Handbook of Lipidology; Ghose T., Ed.; Jaypee Digital: 2016; p 152. 10.5005/jp/books/12757_16. [DOI] [Google Scholar]
- Gunness P.; Gidley M. J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food & function 2010, 1 (2), 149–155. 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- Wong J. M.; De Souza R.; Kendall C. W.; Emam A.; Jenkins D. J. Colonic health: fermentation and short chain fatty acids. Journal of clinical gastroenterology 2006, 40 (3), 235–243. 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- den Besten G.; Gerding A.; van Dijk T. H.; Ciapaite J.; Bleeker A.; van Eunen K.; Havinga R.; Groen A. K.; Reijngoud D.-J.; Bakker B. M. Protection against the metabolic syndrome by guar gum-derived short-chain fatty acids depends on peroxisome proliferator-activated receptor γ and glucagon-like peptide-1. PLoS One 2015, 10 (8), e0136364. 10.1371/journal.pone.0136364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosif M. M.; Najda A.; Bains A.; Kaushik R.; Dhull S. B.; Chawla P.; Walasek-Janusz M. A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13 (7), 1066. 10.3390/polym13071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A.; Mogra R. Psyllium (Plantago ovata) husk: a wonder food for good health. International Journal of Science and Research 2013, 4 (9), 1581–1585. [Google Scholar]
- Sangeethapriya M.; Siddhuraju P. Health related functional characteristics and antioxidant potential of mucilage (dietary fiber) from Zizyphus mauritiana fruits. Food Science and Human Wellness 2014, 3 (2), 79–88. 10.1016/j.fshw.2014.05.003. [DOI] [Google Scholar]
- HadiNezhad M.; Duc C.; Han N. F.; Hosseinian F. Flaxseed soluble dietary fibre enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. Journal of Food Research 2013, 2 (5), 152–152. 10.5539/jfr.v2n5p152. [DOI] [Google Scholar]
- Sefi M.; Chaâbane M.; Refrafi M.; Zeghal N. Hypoglycemic and hypolipidemic activities of aloe vera leaf mucilage in alloxan-induced diabetic rats. Pharmaceutical and Biomedical Research 2019, 5 (3), 29–34. 10.18502/pbr.v5i3.2116. [DOI] [Google Scholar]
- Dybka-Stępień K.; Otlewska A.; Góźdź P.; Piotrowska M. The renaissance of plant mucilage in health promotion and industrial applications: A review. Nutrients 2021, 13 (10), 3354. 10.3390/nu13103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes S. S.; de las Mercedes Salas-Mellado M. Addition of chia seed mucilage for reduction of fat content in bread and cakes. Food Chemistry 2017, 227, 237–244. 10.1016/j.foodchem.2017.01.075. [DOI] [PubMed] [Google Scholar]
- Kassem I. A.; Ashaolu T. J.; Kamel R.; Elkasabgy N. A.; Afifi S. M.; Farag M. A. Mucilage as a functional food hydrocolloid: Ongoing and potential applications in prebiotics and nutraceuticals. Food & Function 2021, 12 (11), 4738–4748. 10.1039/D1FO00438G. [DOI] [PubMed] [Google Scholar]
- Choudhary P. D.; Pawar H. A. Recently investigated natural gums and mucilages as pharmaceutical excipients: an overview. Journal of Pharmaceutics 2014, 2014, 204849. 10.1155/2014/204849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang J. H.; Ong D. S. M.; Ng F. S. K.; Hua X. Y.; Tay W. L. W.; Henry C. J. Application of chia (Salvia hispanica) mucilage as an ingredient replacer in foods. Trends in Food Science & Technology 2021, 115, 105–116. 10.1016/j.tifs.2021.06.039. [DOI] [Google Scholar]
- Fan Q.; Xu F.; Liang B.; Zou X. The anti-obesity effect of traditional Chinese medicine on lipid metabolism. Frontiers in Pharmacology 2021, 12, 696603. 10.3389/fphar.2021.696603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgulkar A. R.; Rao M. R.; Warrier D. Characterization of psyllium (Plantago ovata) polysaccharide and its uses. Polysaccharides 2015, 871–890. 10.1007/978-3-319-16298-0_49. [DOI] [Google Scholar]
- Chhikara N.; Panghal A.; Chaudhary G.. Functional Foods; John Wiley & Sons: 2022. [Google Scholar]
- Singh B. Psyllium as therapeutic and drug delivery agent. International journal of pharmaceutics 2007, 334 (1–2), 1–14. 10.1016/j.ijpharm.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Wärnberg J.; Marcos A.; Bueno G.; Moreno L. A. Functional benefits of psyllium fiber supplementation. Current Topics in Nutraceutical Research 2009, 7, 55–64. [Google Scholar]
- Peredo-Lovillo A.; Romero-Luna H.; Jiménez-Fernández M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Research International 2020, 136, 109473. 10.1016/j.foodres.2020.109473. [DOI] [PubMed] [Google Scholar]
- Willey J. Z.; Elkind M. S. 3-Hydroxy-3-methylglutaryl–coenzyme A reductase inhibitors in the treatment of central nervous system diseases. Archives of Neurology 2010, 67 (9), 1062–1067. 10.1001/archneurol.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hari A.; Neethu N.. Plant Mucilages and their Potential Applications—A Review. Advances in Bioscience and Biotechnology Research; A S., Senan V P., Eds.; Darshan Publishers: 2021; pp 57–70. [Google Scholar]
- Brahe L. K.; Le Chatelier E.; Prifti E.; Pons N.; Kennedy S.; Blædel T.; Håkansson J.; Dalsgaard T. K.; Hansen T.; Pedersen O.; et al. Dietary modulation of the gut microbiota–a randomised controlled trial in obese postmenopausal women. Br. J. Nutr. 2015, 114 (3), 406–417. 10.1017/S0007114515001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B. A.; Trigatti K.; MacNeil M. B.; Klingel S. L.; Repin N.; Goff H. D.; Wright A. J.; Duncan A. M. Pudding products enriched with yellow mustard mucilage, fenugreek gum or flaxseed mucilage and matched for simulated intestinal viscosity significantly reduce postprandial peak glucose and insulin in adults at risk for type 2 diabetes. Journal of Functional Foods 2017, 37, 603–611. 10.1016/j.jff.2017.08.017. [DOI] [Google Scholar]
- Bongartz U.; Hochmann U.; Grube B.; Uebelhack R.; Alt F.; Erlenbeck C.; Peng L. V.; Chong P. W.; De Costa P. Flaxseed Mucilage (IQP-LU-104) Reduces Body Weight in Overweight and Moderately Obese Individuals in a 12-week, Three-Arm, Double-Blind, Randomized, and Placebo-Controlled Clinical Study. Obesity Facts 2022, 15 (3), 395–404. 10.1159/000522082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boban P. T.; Nambisan B.; Sudhakaran P. R. Dietary mucilage promotes regression of atheromatous lesions in hypercholesterolemic rabbits. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 2009, 23 (5), 725–730. 10.1002/ptr.2728. [DOI] [PubMed] [Google Scholar]
- Al Hamedan W. Protective effect of Lepidium sativum L. seeds powder and extract on hypercholesterolemic rats. Journal of American Science 2010, 6 (11), 873–879. [Google Scholar]
- El-Newary S. A. Mucilage of Cordia dichotoma seeds pulp: Isolation, purification and a new hypolipidemic agent in normal and hyperlipidemic rats. Planta Med. 2015, 81 (16), PW_107. 10.1055/s-0035-1565731. [DOI] [Google Scholar]
- Zhou Z.; Ren X. Consumption of Aloe vera mucilage attenuates plasma oxidative stress and dyslipidemia in Type 2 diabetic rats. Global. Journal of Biotechnology and Biomaterial Science 2015, 1 (1), 001–003. 10.17352/gjbbs.000001. [DOI] [Google Scholar]
- Prajapati V. D.; Maheriya P. M.; Jani G. K.; Patil P. D.; Patel B. N. Lepidium sativum Linn.: a current addition to the family of mucilage and its applications. International journal of biological macromolecules 2014, 65, 72–80. 10.1016/j.ijbiomac.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Cárdenas Y.; Ríos-Silva M.; Huerta M.; López M.; Bricio-Barrios J.; Ortiz-Mesina M.; Urzúa Z.; Saavedra-Molina A.; Trujillo X. The comparative effect of nopal and mucilage in metabolic parameters in rats with a high-fructose diet. Journal of medicinal food 2019, 22 (5), 538–541. 10.1089/jmf.2018.0124. [DOI] [PubMed] [Google Scholar]
- G S.; Pushpan C. K.; S P.; Nambisan B.; A H. Trigonella foenum graecum derived mucilage supplementation in diet alleviates the progression of atherosclerosis in high cholesterol diet-fed rabbit model by regulating inflammation. Bioactive Carbohydrates and Dietary Fibre 2020, 24, 100246. 10.1016/j.bcdf.2020.100246. [DOI] [Google Scholar]
- Uddin Zim A. I.; Khatun J.; Khan M. F.; Hossain M. A.; Haque M. M. Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Science & Nutrition 2021, 9 (12), 6854–6865. 10.1002/fsn3.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadlelmoula A. A. In Gum Arabic: Structure, Properties, Application and Economics; Mariod A. A., Ed.; Academic Press: 2018; p 261. [Google Scholar]
- Gill S. S.; Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant physiology and biochemistry 2010, 48 (12), 909–930. 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Musa H.; Ahmed A.; Fedail J.; Musa T.; Sifaldin A. Gum Arabic attenuates the development of nephropathy in type 1 diabetes rat. Gums and Stabilisers for the Food Industry 2016, 18, 245–255. 10.1039/9781782623830-00245. [DOI] [Google Scholar]
- Kishimoto A.; Ushida K.; Phillips G. O.; Ogasawara T.; Sasaki Y. Identification of intestinal bacteria responsible for fermentation of gum arabic in pig model. Current microbiology 2006, 53 (3), 173–177. 10.1007/s00284-005-0219-3. [DOI] [PubMed] [Google Scholar]
- Ahmed A. A.; Fedail J. S.; Musa H. H.; Kamboh A. A.; Sifaldin A. Z.; Musa T. H. Gum Arabic extracts protect against hepatic oxidative stress in alloxan induced diabetes in rats. Pathophysiology 2015, 22 (4), 189–194. 10.1016/j.pathophys.2015.08.002. [DOI] [PubMed] [Google Scholar]; Park J. A.; Tirupathi Pichiah P.; Yu J. J.; Oh S. H.; Daily J. III; Cha Y. S. Anti-obesity effect of kimchi fermented with W eissella koreensis OK 1–6 as starter in high-fat diet-induced obese C57 BL/6J mice. J. Appl. Microbiol. 2012, 113 (6), 1507–1516. 10.1111/jam.12017. [DOI] [PubMed] [Google Scholar]
- Ahmed A. A.; Fedail J. S.; Musa H. H.; Sifaldin A. Z.; Musa T. H. Gum Arabic Down-Regulate Cholesterol Biosynthesis Enzyme Gene mRNA Expression in Mice Muscle. Mathews Journal of Diabetes & Obesity 2016, 1 (1), 003. [Google Scholar]
- Lattimer J. M.; Haub M. D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2 (12), 1266–1289. 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R. E.; Gadour M. O.; Adam I. The lowering effect of Gum Arabic on hyperlipidemia in Sudanese patients. Frontiers in physiology 2015, 6, 160. 10.3389/fphys.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir O.; Babiker S.; Salim A.-M. M. Protective effect of gum Arabic supplementation for type 2 diabetes mellitus and its complications. Int. J. Multidiscip. Curr. Res. 2016, 4, 288–294. [Google Scholar]
- Babiker R.; Elmusharaf K.; Keogh M. B.; Banaga A. S.; Saeed A. M. Metabolic effect of gum Arabic (Acacia Senegal) in patients with type 2 diabetes mellitus (T2DM): randomized, placebo controlled double blind trial. Functional Foods in Health and Disease 2017, 7 (3), 222–234. 10.31989/ffhd.v7i3.325. [DOI] [Google Scholar]
- Babiker R.; Elmusharaf K.; Keogh M. B.; Saeed A. M. Effect of Gum Arabic (Acacia Senegal) supplementation on visceral adiposity index (VAI) and blood pressure in patients with type 2 diabetes mellitus as indicators of cardiovascular disease (CVD): a randomized and placebo-controlled clinical trial. Lipids in health and disease 2018, 17 (1), 56. 10.1186/s12944-018-0711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddam L.; Fadl-Elmula I.; Eisawi O. A.; Abdelrazig H. A.; Saeed A. M. Acacia senegal (Gum Arabic) supplementation modulate lipid profile and ameliorated dyslipidemia among sickle cell anemia patients. Journal of Lipids 2019, 2019, 3129461. 10.1155/2019/3129461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrar A. H.; Stojanovska L.; Apostolopoulos V.; Feehan J.; Bataineh M. a. F.; Ismail L. C.; Al Dhaheri A. S. The effect of gum arabic (Acacia senegal) on cardiovascular risk factors and gastrointestinal symptoms in adults at risk of metabolic syndrome: A randomized clinical trial. Nutrients 2021, 13 (1), 194. 10.3390/nu13010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelwahed N.; Idris O. F.; Seri H. I. The effect of feeding Gum Arabic on serum total and lipoproteins cholesterol in hypercholesterolemic rats. Assiut Veterinary Medical Journal 2011, 57 (128), 1–12. 10.21608/avmj.2011.172234. [DOI] [Google Scholar]
- Elmekki S.; Seri H.; Bakhiet A.; Bulldan A. Evaluation of Anti-hyperlipidaemic potential of Gum Arabic in experimentally induced hyperlipidaemia in donkeys. J. Agric. Vet. Sci. 2014, 15, 27. [Google Scholar]
- Fedail J. S.; Ahmed A. A.; Musa H. H.; Ismail E.; Sifaldin A. Z.; Musa T. H. Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats. Asian Pacific Journal of Reproduction 2016, 5 (5), 434–441. 10.1016/j.apjr.2016.07.014. [DOI] [Google Scholar]
- Muataz-E M.; Rehab-M B.; Osama-M O.; Mohamed-D M.; Amr-M A.; Salah-Omer B.; Amal-M S. Preventive Role of Gum Arabic Administration on STZ Induced Diabetic Kidney Disease in Rats; Renal Antioxidant and Histopathological Evidence. International Journal of Morphology 2020, 38 (4), 1003. 10.4067/S0717-95022020000401003. [DOI] [Google Scholar]
- Al-Okbi S. Y.; Abd El Ghani S.; Elbakry H.; Mabrok H.; Nasr S.; Desouky H.; Mahmoud K. Kishk Sa′ eedi as a potential functional food for management of metabolic syndrome: A study of the possible interaction with pomegranate seed oil and/or gum Arabic. Journal of Herbmed Pharmacology 2021, 10 (3), 319–330. 10.34172/jhp.2021.37. [DOI] [Google Scholar]
- Ahmed A. A.; Essa M. E. A.; Mollica A.; Stefanucci A.; Zengin G.; Ahmed H. Gum Arabic modifies anti-inflammatory cytokine in mice fed with high fat diet induced obesity. Bioactive Carbohydrates and Dietary Fibre 2021, 25, 100258. 10.1016/j.bcdf.2020.100258. [DOI] [Google Scholar]
- Wang X.; Wang J.; Zhang J.; Zhao B.; Yao J.; Wang Y. Structure–antioxidant relationships of sulfated galactomannan from guar gum. International Journal of Biological Macromolecules 2010, 46 (1), 59–66. 10.1016/j.ijbiomac.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Kurakake M.; Sumida T.; Masuda D.; Oonishi S.; Komaki T. Production of galacto-manno-oligosaccharides from guar gum by β-mannanase from Penicillium oxalicum SO. Journal of agricultural and food chemistry 2006, 54 (20), 7885–7889. 10.1021/jf061502k. [DOI] [PubMed] [Google Scholar]
- Saeed S.; Mosa-Al-Reza H.; Fatemeh A. N.; Saeideh D. Antihyperglycemic and antihyperlipidemic effects of guar gum on streptozotocin-induced diabetes in male rats. Pharmacognosy magazine 2012, 8 (29), 65. 10.4103/0973-1296.93328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor M.; Singh J.; Bandral J. D.; Gani A.; Shams R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. International Journal of Biological Macromolecules 2020, 165, 554–567. 10.1016/j.ijbiomac.2020.09.182. [DOI] [PubMed] [Google Scholar]
- Parisi G.; Zilli M.; Miani M.; Carrara M.; Bottona E.; Verdianelli G.; Battaglia G.; Desideri S.; Faedo A.; Marzolino C.; et al. High-fiber diet supplementation in patients with irritable bowel syndrome (IBS): a multicenter, randomized, open trial comparison between wheat bran diet and partially hydrolyzed guar gum (PHGG). Digestive Diseases and Sciences 2002, 47 (8), 1697–1704. 10.1023/A:1016419906546. [DOI] [PubMed] [Google Scholar]
- Pittler M. H.; Ernst E. Guar gum for body weight reduction: meta-analysis of randomized trials. The American journal of medicine 2001, 110 (9), 724–730. 10.1016/S0002-9343(01)00702-1. [DOI] [PubMed] [Google Scholar]
- Kang J. W.The Role of Targeted Prebiotics for Improving Gut Microbiome Composition and Function in Individuals Consuming Diets Low in Fiber, and Individuals at Risk for Chronic Disease. Ph.D. Dissertation, University of California, Davis, 2022. [Google Scholar]
- Rideout T. C.; Harding S. V.; Jones P. J.; Fan M. Z. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Vascular Health and Risk Management 2008, 4 (5), 1023. 10.2147/VHRM.S3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeller D. A.; Westerterp M.. Advances in the Assessment of Dietary Intake; CRC Press: 2017. [Google Scholar]
- Cicero A. F.; Derosa G.; Manca M.; Bove M.; Borghi C.; Gaddi A. V. Different effect of psyllium and guar dietary supplementation on blood pressure control in hypertensive overweight patients: a six-month, randomized clinical trial. Clinical and experimental hypertension 2007, 29 (6), 383–394. 10.1080/10641960701578378. [DOI] [PubMed] [Google Scholar]
- Aronson J. K.Meyler’s Side Effects of Herbal Medicines; Elsevier: 2009. [Google Scholar]
- Wei Z.; Wang H.; Chen X.; Wang B.; Rong Z.; Su B.; Chen H.; Wang B. Time-and dose-dependent effect of psyllium on serum lipids in mild-to-moderate hypercholesterolemia: a meta-analysis of controlled clinical trials. European journal of clinical nutrition 2009, 63 (7), 821–827. 10.1038/ejcn.2008.49. [DOI] [PubMed] [Google Scholar]
- Soni Y.; Rajnee Effect of Cyamopsis Tetragonoloba (Guar) on Lipid Profile in Diabetic and Non-Diabetic Subjects. JPMI: Journal of Postgraduate Medical Institute 2011, 25 (3), 199–205. [Google Scholar]
- Wang N.; Pan D.; Guo Z.; Xiang X.; Wang S.; Zhu J.; Sun G. Effects of guar gum on blood lipid levels: A systematic review and meta-analysis on randomized clinical trials. Journal of Functional Foods 2021, 85, 104605. 10.1016/j.jff.2021.104605. [DOI] [Google Scholar]
- Khukhlina O.; Liakhovych O.; Shuper V.; Kanyovska L.; Hryniuk O. Y. Peculiar features of glucose homeostasis in patients suffering from non-alcoholic steatohepatitis with comorbid obesity and osteoarthritis on the background of metadoxine and guar gum administration. Bukovyna Medical Herald 2020, 24 (3 (95)), 143–150. 10.24061/2413-0737.XXIV.3.95.2020.85. [DOI] [Google Scholar]
- Fernandez M. L.; Volek J. S. Guinea pigs: a suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation. Nutrition & Metabolism 2006, 3, 17. 10.1186/1743-7075-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Roy S.; Vega-Lopez S.; Fernandez M. L. Gender and hormonal status affect the hypolipidemic mechanisms of dietary soluble fiber in guinea pigs. The Journal of nutrition 2000, 130 (3), 600–607. 10.1093/jn/130.3.600. [DOI] [PubMed] [Google Scholar]
- Grizard D.; Dalle M.; Barthomeuf C. Changes in insulin and corticosterone levels may partly mediate the hypolipidemic effect of guar gum and low-molecular weight pectin in rats. Nutr. Res. (N.Y.) 2001, 21 (8), 1185–1190. 10.1016/S0271-5317(01)00316-5. [DOI] [Google Scholar]
- Furgał-Dierżuk I. The effect of different dietary fibres on short-chain fatty acid concentrations in the caecum and lipid profile in pig serum. Journal of Animal and Feed Sciences 2004, 13 (2), 31–34. 10.22358/jafs/70288/2004. [DOI] [Google Scholar]
- Shahzadi N.; Butt M. S.; Sharif M. K.; Nasir M. Effect of guar gum on the serum lipid profile of Sprague Dawley rats. LWT-Food Science and Technology 2007, 40 (7), 1198–1205. 10.1016/j.lwt.2006.08.007. [DOI] [Google Scholar]
- Samarghandian S.; Hadjzadeh M.-A.-R.; Davari A. S.; Abachi M. Reduction of serum cholesterol in hypercholesterolemic rats by Guar gum. Avicenna Journal of Phytomedicine 2011, 1 (1), 36–42. 10.22038/AJP.2011.119. [DOI] [Google Scholar]
- Hajzadeh M.-A.-R.; Samarghandian S.; Davari A. S.; Abachi M. Comparison of the beneficial effects of guar gum on lipid profile in hyperlipidemic and normal rats. Journal of Medicinal Plants Research 2012, 6 (9), 1567–1575. 10.5897/JMPR11.887. [DOI] [Google Scholar]
- Santas J.; Espadaler J.; Cuñé J.; Rafecas M. Partially hydrolyzed guar gums reduce dietary fatty acid and sterol absorption in guinea pigs independent of viscosity. Lipids 2012, 47 (7), 697–705. 10.1007/s11745-012-3682-1. [DOI] [PubMed] [Google Scholar]
- Maxwell E. G.; Belshaw N. J.; Waldron K. W.; Morris V. J. Pectin–an emerging new bioactive food polysaccharide. Trends in Food Science & Technology 2012, 24 (2), 64–73. 10.1016/j.tifs.2011.11.002. [DOI] [Google Scholar]
- Xiang C.; Gao J.; Ye H.; Ren G.; Ma X.; Xie H.; Fang S.; Lei Q.; Fang W. Development of ovalbumin-pectin nanocomplexes for vitamin D3 encapsulation: Enhanced storage stability and sustained release in simulated gastrointestinal digestion. Food Hydrocolloids 2020, 106, 105926. 10.1016/j.foodhyd.2020.105926. [DOI] [Google Scholar]
- Moslemi M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. 10.1016/j.carbpol.2020.117324. [DOI] [PubMed] [Google Scholar]
- Shinohara K.; Ohashi Y.; Kawasumi K.; Terada A.; Fujisawa T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010, 16 (5), 510–515. 10.1016/j.anaerobe.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Larsen N.; Cahú T. B.; Saad S. M. I.; Blennow A.; Jespersen L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiology 2018, 74, 11–20. 10.1016/j.fm.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Gómez B.; Gullón B.; Yáñez R.; Schols H.; Alonso J. L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. Journal of Functional Foods 2016, 20, 108–121. 10.1016/j.jff.2015.10.029. [DOI] [Google Scholar]
- Larsen N.; Bussolo de Souza C.; Krych L.; Barbosa Cahú T.; Wiese M.; Kot W.; Hansen K. M.; Blennow A.; Venema K.; Jespersen L. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Frontiers in Microbiology 2019, 10, 223. 10.3389/fmicb.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns F.; Theuwissen E.; Adam A.; Bell M.; Berger A.; Mensink R. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. European journal of clinical nutrition 2012, 66 (5), 591–599. 10.1038/ejcn.2011.208. [DOI] [PubMed] [Google Scholar]
- Min B.; Koo O. K.; Park S. H.; Jarvis N.; Ricke S. C.; Crandall P. G.; Lee S.-O. Fermentation patterns of various pectin sources by human fecal microbiota. Food and Nutrition Sciences 2015, 6 (12), 1103. 10.4236/fns.2015.612115. [DOI] [Google Scholar]
- Aller R.; de Luis D. A.; Izaola O.; La Calle F.; del Olmo L.; Fernandez L.; Arranz T.; Hernandez J. G. Effect of soluble fiber intake in lipid and glucose leves in healthy subjects: a randomized clinical trial. Diabetes Research and Clinical Practice 2004, 65 (1), 7–11. 10.1016/j.diabres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Schwab U.; Louheranta A.; Törrönen A.; Uusitupa M. Impact of sugar beet pectin and Polydextrose on fasting and postprandial glycemia and fasting concentrations of serum total and lipoprotein lipids in middle-aged subjects with abnormal glucose metabolism. European journal of clinical nutrition 2006, 60 (9), 1073–1080. 10.1038/sj.ejcn.1602421. [DOI] [PubMed] [Google Scholar]
- Wong T. W.; Colombo G.; Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. Aaps PharmSciTech 2011, 12, 201–214. 10.1208/s12249-010-9564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.; Choi J.; Kim K. Docosahexaenoic acid-rich fish oil and pectin have a hypolipidemic effect, but pectin increases risk factor for colon cancer in rats. Nutr. Res. (N.Y.) 2000, 20 (12), 1783–1794. 10.1016/S0271-5317(00)00269-4. [DOI] [Google Scholar]
- Terpstra A.; Lapre J.; De Vries H.; Beynen A. Intact pectin and its polygalacturonic acid regions have similar hypocholesterolemic properties in hybrid F1B hamsters. Food/Nahrung 2002, 46 (2), 83–86. . [DOI] [PubMed] [Google Scholar]
- Aprikian O.; Duclos V.; Guyot S.; Besson C.; Manach C.; Bernalier A.; Morand C.; Remesy C.; Demigne C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. The Journal of nutrition 2003, 133 (6), 1860–1865. 10.1093/jn/133.6.1860. [DOI] [PubMed] [Google Scholar]
- Marounek M.; Volek Z.; Synytsya A.; Čopíková J. Effect of pectin and amidated pectin on cholesterol homeostasis and cecal metabolism in rats fed a high-cholesterol diet. Physiological Research 2007, 56 (4), 433. 10.33549/physiolres.930967. [DOI] [PubMed] [Google Scholar]
- Sanchez D.; Muguerza B.; Moulay L.; Hernández R.; Miguel M.; Aleixandre A. Highly methoxylated pectin improves insulin resistance and other cardiometabolic risk factors in Zucker fatty rats. Journal of agricultural and food chemistry 2008, 56 (10), 3574–3581. 10.1021/jf703598j. [DOI] [PubMed] [Google Scholar]
- Lien T. F.; Yeh H. S.; Su W. T. Effect of adding extracted hesperetin, naringenin and pectin on egg cholesterol, serum traits and antioxidant activity in laying hens. Archives of animal nutrition 2008, 62 (1), 33–43. 10.1080/17450390701780318. [DOI] [PubMed] [Google Scholar]
- Metzger B.; Barnes D.; Reed J. A comparison of pectin, polyphenols, and phytosterols, alone or in combination, to lovastatin for reduction of serum lipids in familial hypercholesterolemic swine. Journal of medicinal food 2009, 12 (4), 854–860. 10.1089/jmf.2008.0140. [DOI] [PubMed] [Google Scholar]
- Silva D. C.; Freitas A. L.; Pessoa C. D.; Paula R. C.; Mesquita J. X.; Leal L. K.; Brito G. A.; Gonçalves D. O.; Viana G. S. Pectin from Passiflora edulis shows anti-inflammatory action as well as hypoglycemic and hypotriglyceridemic properties in diabetic rats. Journal of medicinal food 2011, 14 (10), 1118–1126. 10.1089/jmf.2010.0220. [DOI] [PubMed] [Google Scholar]
- Krzysik M.; Grajeta H.; Prescha A.; Weber R. Effect of cellulose, pectin and chromium (III) on lipid and carbohydrate metabolism in rats. Journal of Trace Elements in Medicine and Biology 2011, 25 (2), 97–102. 10.1016/j.jtemb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Hameed B. J. Study the hypolipidemic effect of combination of pectin, niacin and apple cider vinegar supplied as Apple-cure effervescent tablets in rabbits. J. College Educ. Pure Sci. 2012, 2, 52–63. [Google Scholar]
- Parolini C.; Manzini S.; Busnelli M.; Rigamonti E.; Marchesi M.; Diani E.; Sirtori C. R.; Chiesa G. Effect of the combinations between pea proteins and soluble fibres on cholesterolaemia and cholesterol metabolism in rats. British journal of nutrition 2013, 110 (8), 1394–1401. 10.1017/S0007114513000639. [DOI] [PubMed] [Google Scholar]
- Fåk F.; Jakobsdottir G.; Kulcinskaja E.; Marungruang N.; Matziouridou C.; Nilsson U.; Stålbrand H.; Nyman M. The physico-chemical properties of dietary fibre determine metabolic responses, short-chain fatty acid profiles and gut microbiota composition in rats fed low-and high-fat diets. PloS one 2015, 10 (5), e0127252. 10.1371/journal.pone.0127252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T.; Gao X.; Wu C.; Tian F.; Lei Q.; Bi J.; Xie B.; Wang H. Y.; Chen S.; Wang X. Apple-derived pectin modulates gut microbiota, improves gut barrier function, and attenuates metabolic endotoxemia in rats with diet-induced obesity. Nutrients 2016, 8 (3), 126. 10.3390/nu8030126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Dong M.; Yang Z.; Pan S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. International Journal of Biological Macromolecules 2016, 89, 484–488. 10.1016/j.ijbiomac.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Song M.; Lopez-Pena C. L.; McClements D. J.; Decker E. A.; Xiao H. Safety evaluation and lipid-lowering effects of food-grade biopolymer complexes (ε-polylysine-pectin) in mice fed a high-fat diet. Food & function 2017, 8 (5), 1822–1829. 10.1039/C7FO00222J. [DOI] [PubMed] [Google Scholar]
- Li W.; Zhang K.; Yang H. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: possible role of short-chain fatty acids and gut microbiota regulated by pectin. J. Agric. Food Chem. 2018, 66 (30), 8015–8025. 10.1021/acs.jafc.8b02979. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Xu C.; Huang R.; Song J.; Li D.; Xia M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. The Journal of Nutritional Biochemistry 2018, 56, 175–182. 10.1016/j.jnutbio.2018.02.011. [DOI] [PubMed] [Google Scholar]
- Wu J.; Xu Y.; Su J.; Zhu B.; Wang S.; Liu K.; Wang H.; Shi S.; Zhang Q.; Qin L.; et al. Roles of gut microbiota and metabolites in a homogalacturonan-type pectic polysaccharide from Ficus pumila Linn. fruits mediated amelioration of obesity. Carbohydrate Polymers 2020, 248, 116780. 10.1016/j.carbpol.2020.116780. [DOI] [PubMed] [Google Scholar]
- Kolida S.; Gibson G. R. Prebiotic capacity of inulin-type fructans. The Journal of nutrition 2007, 137 (11), 2503S–2506S. 10.1093/jn/137.11.2503S. [DOI] [PubMed] [Google Scholar]
- Tawfick M. M.; Xie H.; Zhao C.; Shao P.; Farag M. A. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. International Journal of Biological Macromolecules 2022, 208, 948. 10.1016/j.ijbiomac.2022.03.218. [DOI] [PubMed] [Google Scholar]
- Guo J.; Zhang M.; Wang H.; Li N.; Lu Z.; Li L.; Hui S.; Xu H. Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice. Journal of Food Biochemistry 2022, 46 (5), e14063. 10.1111/jfbc.14063. [DOI] [PubMed] [Google Scholar]
- Van De Wiele T.; Boon N.; Possemiers S.; Jacobs H.; Verstraete W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. Journal of Applied Microbiology 2007, 102 (2), 452–460. 10.1111/j.1365-2672.2006.03084.x. [DOI] [PubMed] [Google Scholar]
- Vandeputte D.; Falony G.; Vieira-Silva S.; Wang J.; Sailer M.; Theis S.; Verbeke K.; Raes J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66 (11), 1968–1974. 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey J. L.; Feirtag J. M.; Gallaher D. D.; Tungland B. C.; Slavin J. L. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nutr. Res. (N.Y.) 2000, 20 (2), 191–201. 10.1016/S0271-5317(99)00152-9. [DOI] [Google Scholar]
- Forcheron F.; Beylot M. Long-term administration of inulin-type fructans has no significant lipid-lowering effect in normolipidemic humans. Metabolism 2007, 56 (8), 1093–1098. 10.1016/j.metabol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Mistry R. H.; Gu F.; Schols H. A.; Verkade H. J.; Tietge U. J. Effect of the prebiotic fiber inulin on cholesterol metabolism in wildtype mice. Scientific Reports 2018, 8 (1), 13238. 10.1038/s41598-018-31698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcázar-Muñoz B. R.; Martínez-Abundis E.; González-Ortiz M. Effect of oral inulin administration on lipid profile and insulin sensitivity in dyslipidemic obese subjects. Revista Médica de Chile 2003, 131 (6), 597–604. 10.4067/S0034-98872003000600002. [DOI] [PubMed] [Google Scholar]
- de Luis D.; de La Fuente B.; Izaola O.; Conde R.; Gutierrez S.; Morillo M. Randomized clinical trial with a inulin enriched cookie on risk cardiovascular factor in obese patients. Nutrición Hospitalaria 2010, 25 (1), 53–59. [PubMed] [Google Scholar]
- Tovar A. R.; Caamaño M. d. C.; Garcia-Padilla S.; García O. P.; Duarte M. A.; Rosado J. L. The inclusion of a partial meal replacement with or without inulin to a calorie restricted diet contributes to reach recommended intakes of micronutrients and decrease plasma triglycerides: A randomized clinical trial in obese Mexican women. Nutrition Journal 2012, 11 (1), 44. 10.1186/1475-2891-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan P.; Gargari B. P.; Asgharijafarabadi M. Effects of high performance inulin supplementation on glycemic status and lipid profile in women with type 2 diabetes: a randomized, placebo-controlled clinical trial. Health Promotion Perspectives 2013, 3 (1), 55. 10.5681/hpp.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan P.; Gargari B. P.; Jafar-Abadi M. A.; Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. International journal of food sciences and nutrition 2014, 65 (1), 117–123. 10.3109/09637486.2013.836738. [DOI] [PubMed] [Google Scholar]
- Guess N. D.; Dornhorst A.; Oliver N.; Bell J. D.; Thomas E. L.; Frost G. S. A randomized controlled trial: the effect of inulin on weight management and ectopic fat in subjects with prediabetes. Nutrition & Metabolism 2015, 12 (1), 36. 10.1186/s12986-015-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliasgharzadeh A.; Khalili M.; Mirtaheri E.; Gargari B. P.; Tavakoli F.; Farhangi M. A.; Babaei H.; Dehghan P. A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: A randomized controlled clinical trial. Advanced Pharmaceutical Bulletin 2015, 5 (4), 507. 10.15171/apb.2015.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M.; Gao C.; Xu L.; Jiang L.; Zhu J.; Chen G.; Law B. Y. K.; Xu Y. Effect of inulin-type carbohydrates on insulin resistance in patients with type 2 diabetes and obesity: a systematic review and meta-analysis. Journal of Diabetes Research 2019, 2019, 5101423. 10.1155/2019/5101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. L.; Benítez-Páez A.; Blædel T.; Larsen L. H.; Iglesias J. R.; Madera C.; Sanz Y.; Larsen T. M. The effect of inulin and resistant maltodextrin on weight loss during energy restriction: a randomised, placebo-controlled, double-blinded intervention. European journal of nutrition 2020, 59 (6), 2507–2524. 10.1007/s00394-019-02099-x. [DOI] [PubMed] [Google Scholar]
- van der Beek C. M.; Canfora E. E.; Kip A. M.; Gorissen S. H.; Olde Damink S. W.; van Eijk H. M.; Holst J. J.; Blaak E. E.; Dejong C. H.; Lenaerts K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. 10.1016/j.metabol.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Kolida S.; Tuohy K.; Gibson G. R. Prebiotic effects of inulin and oligofructose. Br. J. Nutr. 2002, 87 (S2), S193–S197. 10.1079/BJN/2002537. [DOI] [PubMed] [Google Scholar]; Chen H.-L.; Hsiang Y.-Y.; Wu W.-T.; Lin M.-S. Hypolipidemic effects of inulin and synthetic oligofructose in Balb/c mice. J. Chin. Nutr. Soc. 2005, 30 (2), 99–107. [Google Scholar]
- Rault-Nania M.-H.; Demougeot C.; Gueux E.; Berthelot A.; Dzimira S.; Rayssiguier Y.; Rock E.; Mazur A. Inulin supplementation prevents high fructose diet-induced hypertension in rats. Clinical Nutrition 2008, 27 (2), 276–282. 10.1016/j.clnu.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Grela E. R.; Sobolewska S.; Kowalczuk-Vasilev E.; Krasucki W. Effect of dietary inulin source on piglet performance, immunoglobulin concentration, and plasma lipid profile. Journal of Veterinary Research 2014, 58 (3), 453–458. 10.2478/bvip-2014-0069. [DOI] [Google Scholar]
- Huang W.-C.; Lin C.-L.; Hsu Y.-J.; Chiu Y.-S.; Chen Y.-M.; Wu M.-F.; Huang C.-C.; Wang M.-F. Inulin and fibersol-2 combined have hypolipidemic effects on high cholesterol diet-induced hyperlipidemia in hamsters. Molecules 2016, 21 (3), 313. 10.3390/molecules21030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K. H.; Yamamoto A.; Shimada K. i.; Kikuchi H.; Fukushima M. Dietary fat content modulates the hypolipidemic effect of dietary inulin in rats. Molecular nutrition & food research 2017, 61 (8), 1600635. 10.1002/mnfr.201600635. [DOI] [PubMed] [Google Scholar]
- Ning C.; Wang X.; Gao S.; Mu J.; Wang Y.; Liu S.; Zhu J.; Meng X. Chicory inulin ameliorates type 2 diabetes mellitus and suppresses JNK and MAPK pathways in vivo and in vitro. Molecular nutrition & food research 2017, 61 (8), 1600673. 10.1002/mnfr.201600673. [DOI] [PubMed] [Google Scholar]
- Yang J.; Zhang S.; Henning S. M.; Lee R.; Hsu M.; Grojean E.; Pisegna R.; Ly A.; Heber D.; Li Z. Cholesterol-lowering effects of dietary pomegranate extract and inulin in mice fed an obesogenic diet. The Journal of nutritional biochemistry 2018, 52, 62–69. 10.1016/j.jnutbio.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Villanueva-Suárez M. J.; Mateos-Aparicio I.; Pérez-Cózar M. L.; Yokoyama W.; Redondo-Cuenca A. Hypolipidemic effects of dietary fibre from an artichoke by-product in Syrian hamsters. Journal of Functional Foods 2019, 56, 156–162. 10.1016/j.jff.2019.03.013. [DOI] [Google Scholar]
- Hijova E.; Strojný L.; Bertková I.; Bomba A.; Štofilová J. Dietary Lactobacillus plantarum LS/07 and inulin in the management of chronic disease risk factors. Acta Biochimica Polonica 2020, 67 (4), 547–551. 10.18388/abp.2020_5404. [DOI] [PubMed] [Google Scholar]
- Miao M.; Dai Y.; Rui C.; Fan Y.; Wang X.; Fan C.; Mu J.; Hou W.; Dong Z.; Li P.; et al. Dietary supplementation of inulin alleviates metabolism disorders in gestational diabetes mellitus mice via RENT/AKT/IRS/GLUT4 pathway. Diabetology & Metabolic Syndrome 2021, 13 (1), 150. 10.1186/s13098-021-00768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. E.; Abouelnaga A. M.; Omran M. A.; Ghanem R. A. Modulatory Role of Inulin Extract against Metabolic and Structural Changes in the Ovaries and Uterus of Induced Diabetic albino Rats. Egyptian Journal of Histology 2021, 45 (1), 311–324. 10.21608/ejh.2021.65101.1437. [DOI] [Google Scholar]
- Eswaran S.; Muir J.; Chey W. D. Fiber and functional gastrointestinal disorders. American Journal of Gastroenterology 2013, 108 (5), 718–727. 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate S. J.; Bovee-Oudenhoven I. M.; Lettink-Wissink M. L.; Van der Meer R. Dietary fructooligosaccharides increase intestinal permeability in rats. The Journal of nutrition 2005, 135 (4), 837–842. 10.1093/jn/135.4.837. [DOI] [PubMed] [Google Scholar]
- Riedl J.; Linseisen J.; Hoffmann J. r.; Wolfram G. n. Some dietary fibers reduce the absorption of carotenoids in women. The Journal of nutrition 1999, 129 (12), 2170–2176. 10.1093/jn/129.12.2170. [DOI] [PubMed] [Google Scholar]
- Singh V.; San Yeoh B.; Chassaing B.; Xiao X.; Saha P.; Aguilera Olvera R. A.; Lapek J. D. Jr.; Zhang L.; Wang W.-B.; Hao S.; et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. Cell 2018, 175 (3), 679–694.e622. 10.1016/j.cell.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal G.; Balm T. Synergistic effects of psyllium in the dietary treatment of hypercholesterolemia. Southern medical journal 1990, 83 (10), 1131–1137. 10.1097/00007611-199010000-00005. [DOI] [PubMed] [Google Scholar]
- Foltz M.; Zahradnik A. C.; Van den Abbeele P.; Ghyselinck J.; Marzorati M. A pectin-rich, baobab fruit pulp powder exerts prebiotic potential on the human gut microbiome in vitro. Microorganisms 2021, 9, 1981. 10.3390/microorganisms9091981. [DOI] [PMC free article] [PubMed] [Google Scholar]