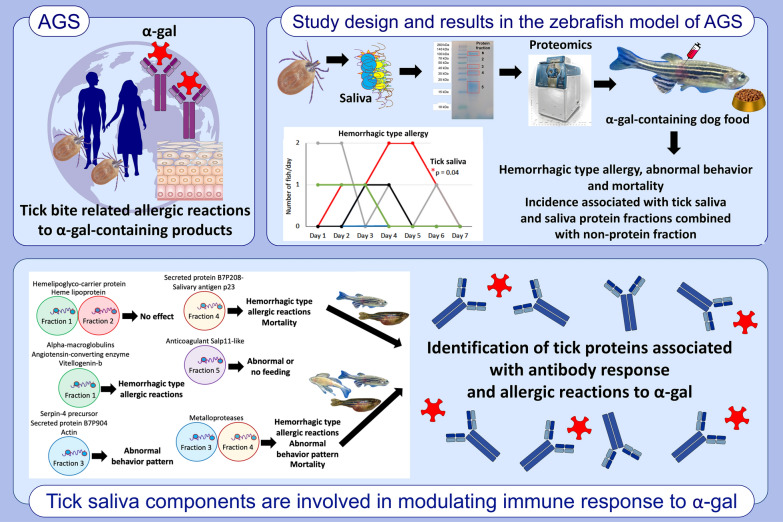
Abstract
Background
Alpha-Gal syndrome (AGS) is a tick-borne food allergy caused by IgE antibodies against the glycan galactose-alpha-1,3-galactose (α-Gal) present in glycoproteins and glycolipids from mammalian meat. To advance in the diagnosis and treatment of AGS, further research is needed to unravel the molecular and immune mechanisms underlying this syndrome. The objective of this study is the characterization of tick salivary components and proteins with and without α-Gal modifications involved in modulating human immune response against this carbohydrate.
Methods
Protein and α-Gal content were determined in tick saliva components, and proteins were identified by proteomics analysis of tick saliva fractions. Pathophysiological changes were recorded in the zebrafish (Danio rerio) model after exposure to distinct Ixodes ricinus tick salivary components. Serum samples were collected from zebrafish at day 8 of exposure to determine anti-α-Gal, anti-glycan, and anti-tick saliva protein IgM antibody titers by enzyme-linked immunosorbent assay (ELISA).
Results
Zebrafish treated with tick saliva and saliva protein fractions combined with non-protein fractions demonstrated significantly higher incidence of hemorrhagic type allergic reactions, abnormal behavioral patterns, or mortality when compared to the phosphate-buffered saline (PBS)-treated control group. The main tick salivary proteins identified in these fractions with possible functional implication in AGS were the secreted protein B7P208-salivary antigen p23 and metalloproteases. Anti-α-Gal and anti-tick salivary gland IgM antibody titers were significantly higher in distinct saliva protein fractions and deglycosylated saliva group when compared with PBS-treated controls. Anti-glycan antibodies showed group-related profiles.
Conclusions
Results support the hypothesis that tick salivary biomolecules with and without α-Gal modifications are involved in modulating immune response against this carbohydrate.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-05874-2.
Keywords: Allergy, Alpha-gal syndrome, Glycan, Tick, Zebrafish
Background
Alpha-Gal syndrome (AGS), also known as mammalian meat allergy, is a tick-borne allergy caused by immunoglobulin E (IgE) response to glycan galactose-alpha-1,3-galactose (α-Gal) modification of protein and lipid glycoconjugates [1–6]. The initial IgE sensitization is linked to bites from hard-bodied ticks such as the castor bean tick Ixodes ricinus in Europe and the lone star tick Amblyomma americanum in North America [7, 8]. Clinical presentation comprises delayed hypersensitivity to the consumption of non-catarrhine mammalian meat and its derivatives and immediate-onset anaphylaxis to α-Gal-containing drugs (mammalian-based substances), likely because drugs are parenterally administered and not consumed [9, 10].
The tick saliva immunogenic agents and triggering pathway of AGS have not yet been totally revealed. AGS patients typically show a Th2-skewed profile with increased anti-α-Gal IgE and IgG levels and allergen-specific B cells and basophil stimulation [8, 11–14]. Recently, the enzyme α-D-galactosidase has been identified as a regulator of α-Gal production in tick salivary glands [15, 16]. Tick saliva contains various biogenic substances with main components such as water, ions, non-peptide molecules such as glycans, tick and host proteins, and exosomes [17–21].
To advance in the diagnosis, treatment, and prevention of AGS, it is important to address the question of why only some individuals exposed to tick bites develop AGS [22]. In addition to differences in tick α-Gal content [23], there is variability in who will and will not become sensitized, and among individuals who have become sensitized, some become allergic to mammalian meat but others can continue tolerating it. To address this question, we hypothesize that tick salivary components with and without α-Gal modifications are involved in modulating the human immune response against this carbohydrate.
To help address this hypothesis, herein we used the proposed α-Gal-negative zebrafish (Danio rerio) animal model for AGS [24]. In a previous study [24], zebrafish treated with tick saliva and fed mammalian meat showed AGS-associated responses such as differential granulocyte profiles with basophils/eosinophils and upregulation of allergic disease biomarkers such as interleukin-1 beta and interleukin-4 [9, 25] also associated with responses to allergens in zebrafish [26] and mice [27]. Furthermore, as recently reviewed [28], zebrafish have been previously used as an animal model for food-associated allergy and immunity [29, 30]. Additionally, when compared to phosphate-buffered saline (PBS)-treated controls, zebrafish treated with tick saliva following mammalian meat consumption but not with any of these components alone presented a higher incidence of wide inter- and intrapersonal variability signs associated with AGS [31] such as hemorrhagic type allergic reactions (urticaria and angioedema in AGS), abnormal behavior (respiratory distress in AGS) and feeding (gastrointestinal symptoms, diarrhea, abdominal pain, reflux, emesis in AGS), and mortality [24]. These findings support the use of a zebrafish animal model to characterize AGS-associated signs in response to tick saliva and mammalian meat consumption.
In this study, zebrafish were exposed to I. ricinus tick saliva protein and non-protein components to evaluate the incidence of local hemorrhagic type allergic reactions, altered behavior patterns and feeding, and mortality. The results identified tick saliva proteins as candidate immunoregulatory in combination with non-protein salivary components involved in AGS.
Methods
Ethics statement
Experiments in zebrafish were conducted in strict accordance with the recommendations of the European Guide for the Care and Use of Laboratory Animals. Fish were housed and experiments were conducted at an experimental facility (Catalonia Institute for Energy Research [IREC], Ciudad Real, Spain) with the approval and supervision of the Ethics Committee on Animal Experimentation of the University of Castilla La Mancha (PR-2021-09-14) and the Department of Agriculture, Environment and Rural Development of Castilla La Mancha (REGA code ES130340000218).
Experimental design
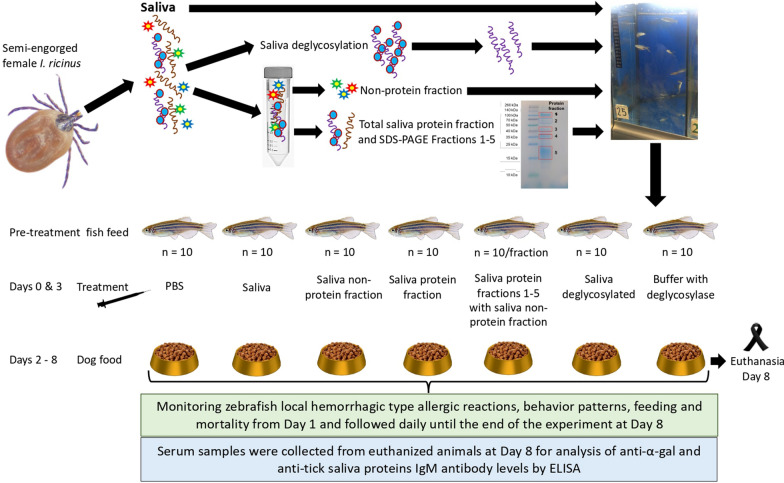
The experiment was designed to characterize tick saliva components associated with allergic reactions to mammalian meat consumption in the zebrafish model of AGS (Fig. 1, Ref. [24]) Saliva from semi-engorged I. ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated fractions. The α-Gal content was quantified in tick saliva in comparison with pig kidney (positive control) and human Caucasian promyelocytic leukemia HL60 cells (negative control) as described previously [24]. Protein content was quantified in tick saliva and its fractions used for treatment of zebrafish (Fig. 2A). The amount of protein administered by fish is shown in Fig. 2A. PBS and buffer with deglycosylase were used as negative controls. Wild-type adult [6–8-month-old) AB strain zebrafish (10 animals per group; 1:1 female-to-male ratio; 330 ± 70 mg weight) were kept on fish feed during pretreatment and until day 2. At days 0 and 3, zebrafish were intramuscularly injected with each treatment, and from day 2 until the end of the experiment at day 8 fish were fed dog food containing mammalian meat. Zebrafish hemorrhagic type allergic reactions (skin redness), behavior (abnormal behavior patterns and abnormal or no feeding), and cumulative mortality were examined throughout the experiment and compared between groups to assess the effect of treatments and dog food after feed change and treatment between days 1 and 7 as reported previously [24]. After fish euthanasia, serum was collected from each animal to determine anti-α-Gal and anti-tick saliva protein IgM antibody titers equivalent to human IgE/IgG antibodies [32]. Kidney and intestine samples were collected from euthanized animals at day 8 and stored at −80 °C for further analysis.
Fig. 1.
Experimental design to characterize tick saliva components associated with allergic reactions to mammalian meat consumption in the zebrafish model of alpha-Gal syndrome (AGS). Saliva from semi-engorged Ixodes ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated saliva fractions. Tick saliva fractions with quantified protein content were used for treatment of zebrafish. PBS and buffer with deglycosylase were used as negative controls. Wild-type adult AB strain zebrafish (10 animals per group; 1:1 female to male ratio) were kept on fish feed during pretreatment and until day 2. Zebrafish were injected with each treatment at days 0 and 3, and from day 2 until the end of the experiment at day 8 fish were fed dog food containing mammalian meat. Zebrafish hemorrhagic type allergic reactions, abnormal behavior patterns and abnormal or no feeding, and cumulative mortality were examined after feed change and treatment at day 3 and followed daily until the end of the experiment at day 8. After fish euthanasia, serum was collected from each animal to determine anti-α-Gal and anti-tick saliva protein IgM antibody titers
Fig. 2.
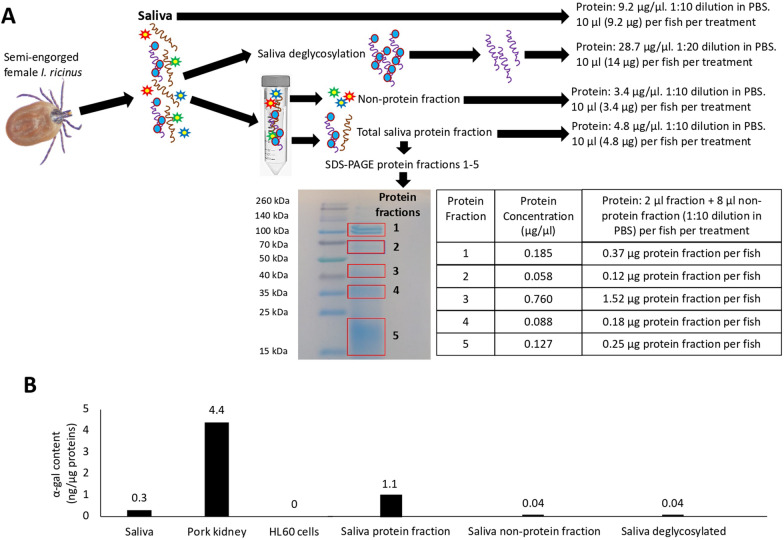
Protein and α-Gal content in tick saliva components and fractions. A Saliva from semi-engorged Ixodes ricinus female ticks was collected and used to prepare protein, non-protein, and deglycosylated saliva fractions. Protein content was quantified in tick saliva fractions used for treatment of zebrafish. B The α-Gal content was quantified by ELISA in tick saliva and tick saliva protein, non-protein, and deglycosylated components in comparison with pig kidney (positive control) and human Caucasian promyelocytic leukemia HL60 cells (negative control). The quantitation of α-Gal content was performed twice, with similar results
Ixodes ricinus tick saliva
Pathogen-free I. ricinus ticks were obtained from the laboratory colony maintained at the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences of the Czech Republic (IPBCAS), in České Budějovice. All animal experiments were performed in accordance with the Animal Protection Law of the Czech Republic no. 246/1992 Sb (ethics approval no. 34/2018). Semi-engorged female ticks fed for 6–7 days on guinea pigs were inoculated into the hemocoel with 5 μl of a 2% (w/v) solution of pilocarpine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) in PBS, and approximately 0.6 μl of saliva was collected per tick as described previously [24, 33]. Saliva was then transported and stored at −80 °C until use.
Tick saliva protein and non-protein fractions
Tick saliva (135 µl) was diluted 1:1 in PBS, and 255 µl was filtered twice through an Amicon 3 kDa unit (Merck & Co., Inc., Kenilworth, NJ, USA). Of this, 200 µl passed through the Amicon membrane and was considered the non-protein fraction. The 50 µl that did not pass through the Amicon membrane was considered the protein fraction.
Glycosidase treatment of tick saliva
For protein deglycosylation, 20 µl of tick saliva was incubated under denaturing conditions with a cocktail of α-Gal-free glycosidases (PNGase F, 36 kDa; α-(2-3,6,8,9)-neuraminidase, 69 kDa; O-glycosidase, 180 kDa; β(1-4)-galactosidase, 350 kDa; β-N-acetylglucosaminidase, 140 kDa) that removes both asparagine-linked (N-linked) and serine/threonine-linked (O-linked) oligosaccharides using the EDEGLY enzymatic protein deglycosylation kit (Merck & Co., Inc.) and following the manufacturer’s recommendations [34]. After deglycosylation, the tick saliva sample was diluted 1:20 in PBS for filtration first through an Amicon 50 kDa unit (Merck & Co., Inc.) to remove deglycosylases except PNGase F, and then the flow-through with tick salivary proteins (most proteins had less than 50 kDa; Fig. 2A) was filtered through the Amicon 3 kDa (Merck & Co., Inc.) to remove buffer and retain proteins.
Tick saliva protein fractionation by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and characterization by mass spectrometry analysis
To obtain the different tick saliva protein fractions, 70 µg from the saliva protein fraction was mixed in 1:1 proportion with Laemmli sample buffer and applied onto two 1.2-cm-wide wells on two 10% SDS-PAGE gels. In one gel, protein bands were visualized by staining with GelCode Blue Stain Reagent (Thermo Fisher Scientific, Waltham, MA, USA), excised, cut into 2 × 2 mm cubes, and digested overnight at 37 °C with 12.5 ng/μl sequencing grade trypsin (Promega, Madison, WI, USA) at a ratio of 5:1 protein/trypsin (w/w) in 50 mM ammonium bicarbonate, pH 8.8, containing 10% (v/v) acetonitrile [35]. The resulting tryptic peptides from each band were extracted by incubation for 30 min in 12 mM ammonium bicarbonate, pH 8.8. Trifluoroacetic acid was added to a final concentration of 0.1%, and the peptides were finally desalted onto OMIX C18 pipette tips (Agilent Technologies, Santa Clara, CA, USA), dried down, and stored at −20 °C until use for mass spectrometry analysis. The desalted protein digests were resuspended in 0.1% formic acid and analyzed by reversed-phase liquid chromatography coupled to mass spectrometry (RP-LC–MS/MS) using an EASY-nLC II system coupled online to an LTQ Linear Ion Trap mass spectrometer (Thermo Fisher Scientific). The peptides were concentrated using a 0.1× 20 mm C18 reversed-phase (RP) precolumn (Thermo Fisher Scientific) and separated using a 0.075× 100 mm C18 RP column (Thermo Fisher Scientific) operating at 0.3 μl/min. Peptides were eluted using a 60-min gradient from 5 to 40% solvent B in solvent A (solvent A: 0.1% formic acid in water, solvent B: 0.1% formic acid, 80% acetonitrile in water). Electrospray ionization (ESI) was carried out using a nano-bore emitter stainless steel ID 30 μm (Thermo Fisher Scientific) interface. Peptides were detected in survey scans from 400 to 1600 atomic mass units (amu, 1 μscan), followed by 15 data-dependent MS/MS scans (Top 15), using an isolation width of two mass-to-charge ratio units, normalized collision energy of 35%, and dynamic exclusion applied for periods of 30 s. Peptide identification from the MS/MS raw data was carried out using the SEQUEST algorithm (Proteome Discoverer 1.4; Thermo Fisher Scientific). A search was performed against the Ixodidae UniProt protein database (184,796 entries in July 2020). The following constraints were used for the searches: tryptic cleavage after Arg and Lys, up to two missed cleavage sites, and tolerance of 1 Da for precursor ions and 0.8 Da for MS/MS fragment ions, and the search was performed allowing optional methionine oxidation and cysteine carbamidomethylation. A search was performed against a decoy database in an integrated decoy approach. A false discovery rate (FDR) < 0.01 was considered as a condition for successful peptide assignments, and at least two peptides per protein was the condition for successful protein identification. Protein bands from the second gel were excised and cut into small cubes, covered with PBS with 0.1% SDS, and incubated in a rotator overnight at 4 °C. The supernatants containing the protein fractions were methanol/chloroform-precipitated, resuspended in PBS for quantification by bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Hercules, CA, USA), and stored at −20 °C until fish treatment.
Tick protein annotations
Tick proteins identified after proteomics analysis were annotated for Gene Ontology in UniProt (https://www.uniprot.org) and VectorBase (https://vectorbase.org/vectorbase/app/). Biological processes include annotations in Drosophila or human proteins that may be related to AGS when information is not available in tick species. Sequences from all identified proteins were used for Basic Local Alignment Search Tool (BLAST) analysis (UniProtKB reference proteomes plus Swiss-Prot; E-threshold = 10) in UniProt (Table 1, Additional file 1: Dataset S1). Additionally, for the secreted protein B7P208—salivary antigen p23 A0A0K8RKR7 (Table 1, Additional file 1: Dataset S1), match to 3UV1_A Chain(A) PDB structure of allergen from dust mite (https://www.rcsb.org/structure/3UV1) was predicted using PredictProtein (https://predictprotein.org) tool (identity = 0.20, expected value = 1e−28, matched length = 205 of 222 to A0A0K8RKR7) (Table 2, Additional file 1: Dataset S1).
Table 1.
Protein identification by mass spectrometry in tick saliva fractions. Full data are provided in Additional file 1: Dataset S1
| UniProt Protein ID | Protein description | Mass spectrometry data | ||
|---|---|---|---|---|
| Score | Coverage (%) No. proteins unique peptides PSMs |
No. amino acids MW (kDa) Calculated pI |
||
| Fraction 1: Hemorrhagic type allergic reactions | ||||
| B7Q407 | Heme lipoprotein | 272.12 | 21.75, 1, 23, 94 | 1329, 152.5, 6.73 |
| B7Q406 | Hemelipoglyco-carrier protein | 145.37 | 14.78, 2, 18, 56 | 1556, 177.5, 6.76 |
| B7QGE3 | 91.39 | 5.36, 2, 1, 33 | 1325, 151.6, 6.77 | |
| B7PJC0 | 35.37 | 23.93, 1, 2, 12 | 117, 13.2, 8.29 | |
| B7PU24 | Alpha-2-macroglobulin | 20.16 | 13.80, 1, 6, 7 | 413, 44.7, 5.24 |
| B7QMC8 | 10.01 | 3.11, 1, 4, 4 | 1092, 121.1, 5.73 | |
| B7P1G7 | Angiotensin-converting enzyme | 7.28 | 5.94, 2, 2, 3 | 320, 37.4, 6.13 |
| B7PH04/A0A4D5RL95 | Vitellogenin-b | 5.78 | 7.97, 1, 2, 2 | 276, 31.7, 7.24 |
| Fraction 2: No effect | ||||
| B7Q407 | Heme lipoprotein | 11.25 | 2.86, 3, 4, 5 | 1329, 152.5, 6.73 |
| B7Q406 | Hemelipoglyco-carrier protein | 4.76 | 1.09, 1, 2, 2 | 1556, 177.5, 6.76 |
| Fraction 3: Abnormal behavior pattern | ||||
| B7QM90/ A0A0K8RB81 | Salivary gland metalloprotease | 61.10 | 10.30, 2, 4, 26 | 437, 50.2, 7.49 |
| B7QM92 | Peptidase M12B domain-containing protein | 50.88 | 7.58, 1, 3, 22 | 488, 55.7, 7.25 |
| B7QM91 | Secreted metalloprotease | 15.82 | 7.94, 1, 4, 7 | 403, 45.9, 8.90 |
| B7PST4 | Serpin-4 precursor | 12.66 | 7.51, 1, 3, 5 | 213, 23.6, 6.55 |
| B7P904 | Secreted protein | 10.94 | 10.13, 1, 4, 5 | 306, 34.1, 6.02 |
| B7PBG2 | Actin | 5.17 | 6.27, 1, 2, 2 | 335, 37.6, 5.55 |
| Fraction 4: Hemorrhagic type allergic reactions and mortality | ||||
| B7QM92/A0A0K8RCY8 | Peptidase M12B domain-containing protein. Putative metalloprotease | 41.91 | 6.35, 2, 3, 16 | 488, 55.7, 7.25 |
| B7P208/A0A0K8RKR7 | Secreted protein-salivary antigen p23 | 12.00 | 11.59, 1, 2, 4 | 164, 18.3, 9.58 |
| B7Q2B8 | Metalloprotease | 10.93 | 3.42, 2, 2, 4 | 468, 53.7, 6.58 |
| Fraction 5: Abnormal or no feeding | ||||
| B7QKC1/ Q4PMH7 | Anticoagulant Salp11-like | 16.50 | 35.59, 1, 2, 5 | 59, 6.8, 4.22 |
Table 2.
Gene Ontology annotations of tick saliva proteins associated with AGS
| Tick proteins | Cellular component | Molecular function | Biological process |
|---|---|---|---|
| Alpha-macroglobulin | Extracellular region or secreted | Endopeptidase inhibitor activity | Complement activation |
|
Angiotensin-converting enzyme Cofactor: Zn2+ |
Membrane |
Carboxypeptidase activity Metal ion binding Metallopeptidase activity Peptidyl-dipeptidase activity |
Regulation of inflammatory response, cytokine production, transmembrane transporter activity |
| Vitellogenin | Membrane |
Glycosyltransferase activity Lipid transporter activity |
Cellular response to heat, estradiol, insulin, polycyclic arene |
| Metalloprotease | Membrane | Metallopeptidase activity | Membrane protein ectodomain proteolysis |
| Actin |
Cytoskeleton Nucleus |
ATP binding Constituent of cytoskeleton |
Mitotic cytokinesis Substantia nigra development Regulation of transmembrane transporter activity |
| Anticoagulant | Extracellular region and/or exosome | Unknown | Blood coagulation |
| Serpin | Extracellular region or secreted |
Protein serine kinase activity Serine-type endopeptidase inhibitor activity |
Negative regulation of endopeptidase activity, protein processing |
| Secreted protein B7P904 | Secreted | Protein serine/threonine phosphatase activity | |
| Secreted protein B7P208-Salivary antigen p23 | Secreted |
Match to 3UV1_A Chain(A) PDB structure. Allergen from dust mite, Dermatophagoides farinae (DOI:10.2210/pdb3UV1/pdb) Bactericidal permeability-increasing protein |
|
Quantitation of tick saliva proteins and α-Gal content
Protein and α-Gal content in tick saliva were determined in whole saliva and protein, non-protein, and deglycosylated fractions (Fig. 2A). The α-Gal levels were determined by an in-house enzyme-linked immunosorbent assay (ELISA) using tick saliva fractions in comparison with pig kidney (α-Gal-positive control) and human promyelocytic leukemia HL60 cells ATCC CCL-240 (α-Gal-negative control) (Fig. 2B) as described previously [22]. Tick saliva was diluted 1:1 in PBS and used to quantify α-Gal and protein content. Tick saliva, pig kidney, and HL60 protein concentrations were determined using a BCA Protein Assay Kit (Thermo Fisher Scientific) following the manufacturer’s recommendations. Briefly, ELISA plates were coated with 100 ng proteins per well from different samples in carbonate/bicarbonate buffer (Sigma-Aldrich), incubated overnight at 4 °C following five washes with PBS containing 0.05% Tween 20 (PBST), and unspecific unions blocked with 1% human serum albumin (HSA; Sigma-Aldrich). Anti-α-Gal epitope monoclonal antibodies (M86; Enzo Life Sciences Inc., Farmingdale, NY, USA) were added at 1:100 dilution in PBS and incubated for 1 h at 37 °C followed by four washes with PBST, and anti-mouse IgM (μ-chain-specific)-peroxidase antibodies produced in goat (Sigma-Aldrich) were added at 1:2000 dilution in PBS. The average value of the blanks (wells without sample proteins; n = 5) was subtracted from all reads, and the analysis was conducted using a calibration curve with 0.0 to 10.0 ng α-Gal (Galα1-3Gal-BSA, 3 atom spacer, product code NGP0203; Dextra, Shinfield, UK) and optical density (OD) values at 450 nm using Microsoft Excel for Mac (v. 16.26) to convert ELISA reader values to α-Gal content per sample (R2 = 0.96). Values for α-Gal content on each sample were represented as nanograms of α-Gal per microgram of proteins. As a control, wells coated with tick saliva protein fraction (n = 3) were incubated with secondary anti-mouse IgM-peroxidase antibodies alone, and α-Gal content values were below 0.0005 ng/µg proteins, thus ruling out non-specific reactions.
Zebrafish
Wild-type adult (6–8-month-old) AB male and female zebrafish were provided by Dr. Juan Galcerán Sáez from the Instituto de Neurociencias (IN-CSIC-UMH, Sant Joan d'Alacant, Alicante, Spain) and certified by Biosait Europe S.L. (Barcelona, Spain; https://biosait.com) as free of major fish pathogens [24]. Zebrafish were maintained in a flow-through water system at 27 °C with a light/dark cycle of 14 h/10 h and were fed twice daily at 9:30 and 13:30 with dry fish feed (Premium food tropical fish, DAPC, Valladolid, Spain; 50–70 μg/fish). On day 2 and until the end of the experiment at day 8, fish were fed dog food (Classic Red, ACANA, Champion Petfoods LP, Edmonton, Canada; 150–200 μg/fish). The composition of fish feed (cereals, fish and fish byproducts, soya, yeast, crustaceans, and algae) and dog food (23% lamb meat meal, 22% steel-cut oats, 5% fresh ranch-raised beef, 5% fresh Yorkshire pork, 5% lamb fat, 4% raw grass-fed lamb, 2% whole oats, 2% fresh beef liver, 2% pork meat meal, 2% herring oil, 2% fresh pork liver, 1% fresh beef tripe, 0.1% freeze-dried beef liver, whole red lentils, whole green peas, whole green lentils, whole garbanzo beans, whole yellow peas, sun-cured alfalfa, lentil fiber, dried brown kelp, fresh pumpkin, fresh butternut squash, fresh parsnips, fresh green kale, fresh spinach, fresh carrots, fresh Red Delicious apples, fresh Bartlett pears, fresh cranberries, fresh blueberries, chicory root, turmeric root, milk thistle, burdock root, lavender, marshmallow root, and rosehips) were as used in previous zebrafish studies [24]. Zebrafish were euthanized by overdose of tricaine methane sulfonate (MS222, 200–300 mg/l) by prolonged immersion (https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=32ed904477ecfcc4b0ac4f7ece7483d888149694) [36].
Characterization of anti-tick protein IgM antibody titers in zebrafish
Tick salivary gland protein extracts were prepared from salivary glands of I. ricinus eight female ticks-pool feeding for 5 days. Salivary glands were resuspended in 100 µl 1% Triton X-100-PBS solution and vortexed three times for 30 s. Then, the suspension was digested through a pellet pestle (DWK Life Sciences Kontes™ Pellet Pestle) and sonicated three times for 3 min. The BCA protein assay (Bio-Rad) was used for total protein quantification. For ELISA IgM titers quantification, high absorption capacity polystyrene microtiter plates were coated with 50 ng per well of tick saliva proteins in carbonate/bicarbonate buffer (Sigma-Aldrich). After overnight incubation at 4 °C, coated plates were washed once with 200 µl PBST (Sigma-Aldrich) and then blocked with 100 µl per well of 5% skim milk (Condalab, Madrid, Spain) in PBST (blocking solution) at room temperature (RT) with gentle shaking. Zebrafish serum samples from different groups of treatment were added at 1:100 dilution in blocking solution and incubated at 37 °C for 1 h. Plates were washed three times with PBST and 100 µl per well of specific rabbit anti-zebrafish IgM antibody diluted at 1:1000 in blocking solution. Plates were then incubated for 1 h at RT with gentle shaking. Plates were washed three times with PBST. A goat anti-rabbit IgG-peroxidase conjugate (Sigma-Aldrich) was added at 1:1000 and incubated for 1 h at RT with agitation. After three washes with 100 µl per well of PBST, 100 µl/well of TMB One Solution (Promega) was added and incubated for 15 min at RT in the dark. Finally, the reaction was stopped with 50 µl/well of 2 N H2SO4 and the OD at 450 nm was measured in a spectrophotometer (Multiskan, Thermo Fisher Scientific).
Characterization of anti-α-Gal IgM antibody titers in zebrafish
The ELISA was conducted as for tick proteins, but plates were coated with 100 ng α-Gal (Galα1-3Gal-BSA, 3 atom spacer, approximately 1.82 × 1020 Gal epitopes/g [37]; product code NGP0203; Dextra, Shinfield, UK) per well in carbonate/bicarbonate buffer (Sigma-Aldrich), incubated overnight at 4 °C following five washes with PBST. Unspecific unions were blocked with 1% HSA (Sigma-Aldrich) for 1 h at RT. Serum peritoneal fluid samples were diluted (1:100, v/v) in blocking solution, followed by the addition of 100 μl/well and incubation for 1.5 h at 37 °C. Plates were washed three times with PBST, and 100 μl/well of rabbit anti-zebrafish IgM antibodies diluted (1:1,000, v/v) in blocking solution was added and incubated for 1 h at RT. Plates were washed with PBST, and goat anti-rabbit IgG-peroxidase conjugate (Sigma-Aldrich) diluted 1:3000 in blocking solution was added and incubated for 1 h at RT. After washes with PBST, 100 μl/well of TMB (Promega) was added and incubated for 15 min at RT. Reactions were stopped with 50 μl/well of 2N H2SO4, and the OD at 450 nm was measured in a spectrophotometer (Multiskan, Thermo Fisher Scientific). Only hemorrhagic type allergic reactions were associated with individual fishes treated with tick saliva, and thus a correlation analysis was conducted between anti-α-Gal IgM antibody titers and these signs in this group (P < 0.05; n = 6).
Characterization of anti-glycan IgM antibody response in zebrafish
The glycochip array containing 378 glycans (20 µM) and 225 bacterial polysaccharides (2 µg/ml) was prepared as previously described (Semiotik LLC, Russia) [38]. Pooled sera obtained from a previous experiment [39] of 10 zebrafish for each group immunized by immersion with bovine serum albumin (BSA) coated with α-Gal (α-Gal; Dextra, Shinfield, UK) and PBS-treated control were diluted 1:10 in PBST (Sigma-Aldrich) and incubated with glycochip arrays overnight at 4 °C in a humidified chamber. After thorough washing with PBST to remove the proteins, glycochips were incubated with IgGs from rabbits immunized with zebrafish IgM diluted 1:1000 in PBST for 45 min at 20 °C. Then, glycochips were washed with PBST and incubated with goat anti-rabbit IgG (H + L)-Alexa Fluor 532 nm (Thermo Fisher Scientific) diluted 1:1000 in PBST at 20 °C for 1 h. Fluorescence signal intensity corresponding to the antibodies bound to printed glycans was measured with a GenePix 4100A fluorescence scanner (Molecular Devices, San Jose, CA, USA) at 500 PMT and a resolution of 10 µm. The images were processed using ScanArray Express 4.0 (fixed circle method) and then by Microsoft Excel software. Six spots represent each oligosaccharide or polysaccharide on the array, and data are reported as median relative fluorescence units (RFU) of replicates, given as a percentage ratio of maximum RFU on the chip (normRFU). The normRFU above 10% was considered significant (Additional file 2: Dataset S2).
Statistical analyses
The incidence of allergic reactions, abnormal behavior and feeding patterns, and mortality in zebrafish were compared between treatments by one-way analysis of variance (ANOVA) test with post hoc Tukey honestly significant difference (HSD) test (P < 0.05; https://astatsa.com/OneWay_Anova_with_TukeyHSD/). Anti-tick proteins and anti-α-Gal IgM antibody titers (OD at 450 nm) in zebrafish were compared between treatments by one-way ANOVA test with Bonferroni–Holm multiple comparisons with only pairs relative to PBS simultaneously compared (P < 0.05; n = 6–10 biological replicates; https://astatsa.com/OneWay_Anova_with_TukeyHSD/).
Results
Characterization of protein and α-Gal content in tick saliva fractions and antibody response in zebrafish
Protein content varied between different tick saliva fractions with the lowest concentration in SDS-PAGE protein fractions 1–5 (Fig. 2A). Protein and α-Gal content was higher in whole tick saliva and protein fractions when compared with non-protein and deglycosylated components (Fig. 2B).
The anti-α-Gal IgM antibody titers were significantly higher only in zebrafish treated with tick saliva when compared with PBS-treated controls (P < 0.05; Fig. 3A). The results for tick saliva were in accordance with α-Gal content in this fraction (Fig. 2B). However, other unknown factors affected the anti-α-Gal IgM antibody titers in zebrafish treated with saliva protein fraction as the α-Gal content was relatively high in this fraction (Fig. 2B). Regarding the anti-tick salivary gland IgM antibodies, zebrafish treated with protein fraction 5, deglycosylated saliva and deglycosylase with buffer showed significantly higher antibody titers than PBS-treated control (P < 0.05; Fig. 3B). These results suggest that multiple factors affect antibody response to tick salivary gland biomolecules and agree with the polynomial correlation that was obtained between the amount of protein injected per fish and average anti-tick salivary gland IgM titers (R2 = 0.9; Fig. 3C). Furthermore, two protein components in the EDEGLY enzymatic protein deglycosylation kit (Merck & Co., Inc.), peptidylglycine monooxygenase (PNGase F) and glycogen debranching enzyme (β(1-4)-galactosidase) are highly conserved and present in I. ricinus ticks (A0A0K8R5I7 and A0A147BME2, respectively), which probably explains the high antibody titers in fish treated with deglycosylated saliva containing PNGase F and deglycosylases in buffer fractions (Fig. 3B). A positive correlation was obtained between hemorrhagic type allergic reactions and anti-α-Gal IgM antibody titers in zebrafish treated with tick saliva (P < 0.001; Fig. 3D).
Fig. 3.
Antibody response in zebrafish treated with tick saliva components. After fish euthanasia, serum was collected from each animal to determine IgM antibody titers against A α-Gal and B tick proteins. Results were compared between treatments by one-way ANOVA test with Bonferroni–Holm multiple comparisons with only pairs relative to PBS simultaneously compared (P < 0.05; n = 6–10 biological replicates). C Polynomial correlation analysis between the amount of protein injected per fish and average anti-tick salivary gland IgM titers (R2 = 0.9). D Correlation analysis between hemorrhagic type allergic reactions and anti-α-Gal IgM antibody titers in fishes treated with tick saliva (R2 = 0.9, P < 0.001; n = 6). E Results of the anti-glycan IgM antibody response in zebrafish. Most of the reactive antibodies with significant differences were lower in response to α-Gal immunization, and the only glycan with higher RFU in α-Gal-immunized zebrafish was P antigen, Gb4 (https://www.omim.org/entry/615021). The glycochip array data and analysis are provided in full in Additional file 2: Dataset S2. Group numbers in panels A, B, and C correspond to treatments with PBS (1), saliva (2), saliva non-protein fraction (3), saliva protein fraction (4), protein fraction 1 (5), protein fraction 2 (6), protein fraction 3 (7), protein fraction 4 (8), protein fraction 5 (9), deglycosylated saliva (10), and deglycosylase plus buffer (11)
The results of the anti-glycan IgM antibody response in zebrafish showed that most of the reactive antibodies with significant differences were lower in response to α-Gal immunization (Fig. 3E; Additional file 2: Dataset S2). The only glycan with higher RFU in α-Gal-immunized zebrafish was GalNAcβ1-3Galα1-4Galβ1-4Glcβ (also known as globoside-4, Gb4 or P antigen), an antigen of the human GLOB blood group system (Fig. 3D; Additional file 2: Dataset S2).
Characterization of tick saliva components associated with allergic reactions to mammalian meat consumption in the zebrafish model of AGS
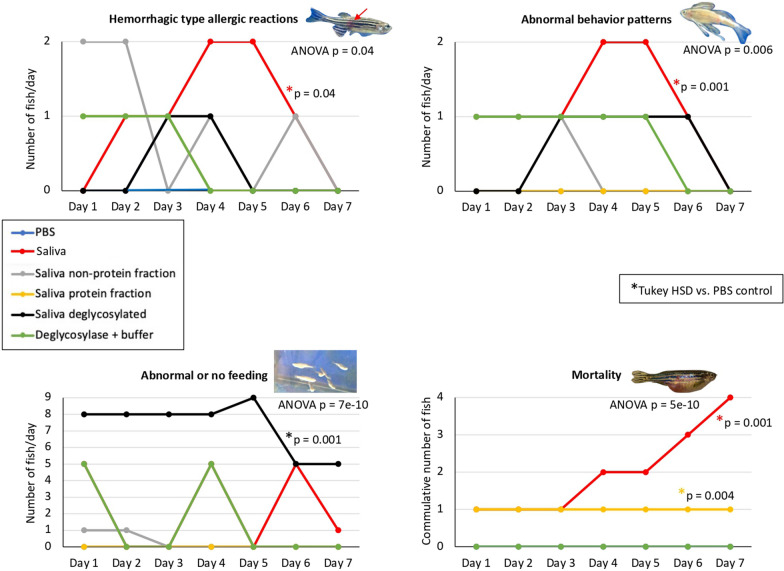
Treatment with tick saliva after feeding on dog food resulted in a significantly higher incidence of hemorrhagic type allergic reactions, abnormal behavior patterns, and mortality when compared with PBS-treated control (P ≤ 0.04; Fig. 4) and was associated with higher anti-α-Gal IgM antibody titers at least for hemorrhagic type allergic reactions (Fig. 3D). The signs seen on fish treated with different tick salivary components on day 1 before dog food challenge may be due to injection and/or direct toxic effect of tick saliva on the fish model and not saliva–mammalian meat-associated reactions (Figs. 4 and 5). Nevertheless, result analyses were based on statistically significant differences when compared with PBS-treated control (Figs. 4 and 5).
Fig. 4.
Allergic reaction of zebrafish to whole tick saliva and components. After treatment with whole tick saliva and saliva non-protein, protein, and deglycosylated components at days 0 and 3, with PBS and buffer with deglycosylase as controls, zebrafish were examined and the incidence of hemorrhagic type allergic reactions, abnormal behavior and feeding patterns, and mortality were compared between treatments by one-way ANOVA test with post hoc Tukey HSD test (P ≤ 0.04; n = 6–10 biological replicates). Significant differences between treatments and PBS control are shown with post hoc Tukey HSD P-values
Fig. 5.
Allergic reaction of zebrafish to whole tick saliva and protein fractions. After treatment at days 0 and 3 with tick whole saliva and different protein fractions (1–5) combined with non-protein fraction using PBS as control, zebrafish were examined and the incidence of hemorrhagic type allergic reactions, abnormal behavior and feeding patterns, and mortality were compared between treatments by one-way ANOVA test with post hoc Tukey HSD test (P ≤ 0.02; n = 6–10 biological replicates). Significant differences between treatments and control are shown with post hoc Tukey HSD P-values
Only treatment with deglycosylated saliva, but not deglycosylases with buffer, significantly altered feeding when compared with control (P = 0.001; Fig. 4). Then, treatment with five saliva protein fractions separated by SDS-PAGE in combination with the non-protein fraction to mimic saliva were compared (Fig. 4). The incidence of hemorrhagic type allergic reactions was significantly higher in saliva protein fractions 1 and 4 treated groups when compared with PBS control (P = 0.02). Abnormal behavior and feeding were associated with treatment using saliva protein fractions 3 (P = 0.003) and 5 (P = 0.001), respectively. Significant mortality was caused by treatment with whole saliva and protein fraction 4 (P = 0.001). However, it should be considered that the fractionation of the salivary proteins by SDS-PAGE may affect in different degrees the structure and activity of enzymes, which may translate into differences in signs produced in zebrafish after treatment with tick saliva protein fractions.
The identification of proteins in the five saliva fractions separated by SDS-PAGE was then approached using mass spectrometry analysis (Fig. 6, Table 1, Additional file 1: Dataset S1). For functional implication in AGS, identified proteins were then associated with their effect on zebrafish allergic reaction to treatment with different protein fractions (Fig. 5) to identify those associated with major effects on zebrafish related to AGS (Fig. 6, Table 1, Additional file 1: Dataset S1). Major effects were related to secreted protein B7P208-salivary antigen p23 (predicted allergen; Additional file 1: Dataset S1) in protein fraction 4 associated with hemorrhagic type allergic reactions and mortality, and metalloproteases identified in fractions 3 and 4 and associated with hemorrhagic type allergic reactions, abnormal behavior patterns, and mortality (Fig. 6, Table 1). Other tick salivary proteins were identified as associated with hemorrhagic type allergic reactions (fraction 1: alpha-macroglobulins, angiotensin-converting enzyme, vitellogenin-b), abnormal behavior pattern (fraction 3: serpin-4 precursor, secreted protein B7P904, actin), and abnormal or no feeding (fraction 5: anticoagulant salp11-like) (Fig. 6, Table 1).
Fig. 6.
Identification and functional association with the alpha-Gal syndrome (AGS) of proteins in tick saliva. Tick saliva proteins were fractionated by SDS-PAGE, and five fractions were extracted and proteins identified by mass spectrometry analysis. The results (Additional file 1: Dataset S1) were then associated with their effect on zebrafish allergic reaction to treatment with different protein fractions to identify those associated with major effects on zebrafish related to AGS
Gene Ontology annotation of identified tick saliva proteins associated with AGS in zebrafish showed as expected that most proteins were extracellular or secreted and associated with multiple metabolic processes (Table 2). Some of the identified tick proteins associated with AGS in the zebrafish model were previously reported as recognized by patients IgE but not by healthy individuals or using an allergenomics approach and reactive or not with α-Gal (Table 3). Other proteins were associated with allergenic compounds or acquired resistance to I. scapularis (Table 3).
Table 3.
Tick salivary proteins associated with allergic reactions, AGS, or acquired resistance to tick infestations
| Tick proteins | Signs associated with AGS in zebrafish model | Association with allergy, AGS in humans | Association with acquired resistance to tick infestations | α-Gal-positive | References |
|---|---|---|---|---|---|
| Alpha-macroglobulin | Hemorrhagic type allergic reactions | Yes | Yes | Yes | [13, 34, 41, 54, 55] |
| Vitellogenin | Hemorrhagic type allergic reactions | Yes | No | Yes | [13, 55] |
| Metalloprotease | Hemorrhagic type allergic reactions, abnormal behavior pattern, and mortality | Yes | No | No | [55] |
| Actin | Abnormal behavior pattern | Yes | No | No | [13, 55] |
| Anticoagulant | Abnormal or no feeding | No | Yes | Unknown | [34, 54] |
| Serpin | Abnormal behavior pattern | No | Yes | Yes | [34, 54, 55] |
| Secreted protein B7P208-Salivary antigen p23 (allergen) | Hemorrhagic type allergic reactions and mortality | Yes | No | Unknown | [55, 56] |
Discussion
As previously reported in the proposed zebrafish model of AGS [24], treatment with tick saliva resulted in a significantly higher incidence of hemorrhagic type allergic reactions, abnormal behavior patterns, and mortality, with a positive correlation between hemorrhagic type allergic reactions and anti-α-Gal IgM antibody titers. These results provided additional support for the use of zebrafish as an AGS animal model.
The results obtained here support the contention that exposure to tick saliva is associated with these signs, which have been described in cases with AGS [1, 2, 8, 22, 31, 40–45]. However, it is important to consider the potential effect of pilocarpine used for tick saliva extraction and which may be mixed in tick salivary components. Pilocarpine treatment in zebrafish has been shown to cause behavioral and biochemical alterations in chronic seizure-like conditions after repeated treatments [46]. Some of the abnormal behavior patterns observed in zebrafish treated with tick saliva and saliva protein fraction 3 may be associated with pilocarpine residues in these treatments. Considering this possibility, we compared the dose of pilocarpine causing seizure-like conditions in zebrafish (> 200 mg/kg) [46] with that used in our experiment. Considering that we treated ticks with 5 μl of a 2% (w/v) solution, equivalent to 2 g/100 ml or 20 mg/ml, and pilocarpine recovered in I. scapularis saliva is 11.5 μg/μl when treated with 2 μl of 50 mg/ml solution [47], the estimate in our experiment is also of 11.5 μg/μl pilocarpine in I. ricinus saliva. In the experiment reported here, 1 μl saliva was injected per treatment in zebrafish, equivalent to 33 mg/kg of pilocarpine, which is one sixth the amount causing seizure-like conditions in zebrafish [46]. Therefore, it is unlikely that the signs observed here in zebrafish in response to tick saliva components are associated with pilocarpine residues. Furthermore, toll-like receptor 4 (tlr4) messenger RNA (mRNA) levels were reported to increase in response to high pilocarpine doses in zebrafish with seizure-like conditions [46], but in zebrafish treated with tick saliva, tlr4 upregulation was observed only in the intestine of animals fed α-Gal-positive dog food and not those with α-Gal-negative fish feed [24].
Regarding tick saliva components, the results suggested a role for saliva non-protein biomolecules in the production of hemorrhagic type allergic reactions. However, glycosylation of salivary proteins appears to confer protection from abnormal or no feeding behavior, which correlates with higher anti-α-Gal IgM antibody titers in some zebrafish treated with deglycosylated saliva (Fig. 3A). In accordance with these results, Park et al. [48] proposed that glycan α-Gal serves as a molecular mimic of bioactive proteins during tick feeding on mammalian hosts but contributes as a sensitizer to allergic reactions associated with AGS in an atypical human host and in the zebrafish model.
The allergic response to the combination of tick saliva proteins with the non-protein fraction supports that both protein and non-protein components are involved in the modulation of the host immune response that may be associated with AGS. As reported in this study, some of the tick proteins associated with allergic reactions (i.e., metalloproteases) do not contain α-Gal modifications but may be associated with allergy/AGS in humans through activation of immune-related mechanisms (Table 3).
As previously reported in chickens [49], and observed here in zebrafish, immunization with α-Gal-containing biomolecules from tick saliva may reduce the natural humoral immune response that may affect reactions to tick bites. Furthermore, IgM and IgG antibody levels in response to the P antigen, Gb4, a prominent glycosphingolipid on human erythrocytes [50, 51], increased in α-Gal-immunized zebrafish, which may be associated with hemolytic transfusion reactions and paroxysmal cold hemoglobinuria [52, 53].
Conclusions
The limitations of the study to be considered include (1) the need to provide immune- and allergic-related biomarkers to support the response to tick saliva biomolecules in the zebrafish model, (2) the need to correlate at the molecular level the response in zebrafish treated with tick saliva components and fed mammalian meat with those reported in patients with AGS, (3) the low incidence of allergic reactions in zebrafish and the possibility that the presence of α-Gal in tick saliva might be important for α-Gal sensitization and allergic symptoms in the zebrafish model of AGS, (4) the wide inter- and intrapersonal variability in AGS symptomatology that may also be represented in the zebrafish model, (5) the fact that proteins > 50 kDa were removed from the deglycosylated fraction, (f) considering in future experiments saliva protein fractionation alternatives to SDS-PAGE approaches to reduce the effect on protein structure and activity, and (g) including control treatments with tick saliva unrelated proteins.
Despite these limitations, the results provide new insights to support the hypothesis that tick saliva biomolecules with and without α-Gal modifications are involved in modulating human immune response against this carbohydrate. The next step to advance in the diagnosis, treatment, and prevention of AGS is deciphering the immune-related mechanisms activated in response to these tick saliva components. These studies will be conducted by analysis of human sera for IgE to these proteins and using omics approaches in intestine samples collected from zebrafish in this study.
Supplementary Information
Additional file 1: Dataset S1. Identification and characterization of tick saliva proteins in SDS-PAGE fractions 1–5 by mass spectrometry and association with allergic reactions in zebrafish.
Additional file 2: Dataset S2. Glycochip data analysis.
Acknowledgements
We thank David Fernández (SaBio, IREC, Spain) for his support with the zebrafish facility and Polyakova Svetlana (Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russia) for glycochip data processing.
Abbreviations
- AGS
Alpha-Gal syndrome
- α-Gal
Galactose-alpha-1,3-galactose
- amu
Atomic mass units
- BCA
Bicinchoninic acid
- ELISA
Enzyme-linked immunosorbent assay
- ESI
Electrospray ionization
- FDR
False discovery rate
- HSD
Honestly significant difference
- Ig
Immunoglobulin
- OD
Optical density
- PBS
Phosphate-buffered saline
- PBST
PBS/0.05% Tween 20
- RFU
Relative fluorescence units
- RP-LC–MS/MS
Reversed-phase liquid chromatography coupled to mass spectrometry
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- RT
Room temperature
Author contributions
MC and MV contributed to study design and undertook longitudinal data collection and manuscript preparation. RV-R, SD-S, SA-J, AG-G, LM, EF-C, NMS, NVB, and IP contributed to data collection and analysis and manuscript preparation. JC, PK, ACC, and CG contributed to study implementation and manuscript preparation. JF contributed to study design, drafted the initial manuscript, and acts as guarantor for the paper. All authors approved the final version of the manuscript as submitted.
Funding
This study was supported by Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación MCIN/AEI/10.13039/501100011033, Spain and EU-FEDER (Grant BIOGAL PID2020-116761GB-I00). M. Contreras was funded by the Ministerio de Ciencia, Innovación y Universidades, Spain, grant IJC2020-042710-I. R. Vaz-Rodrigues was supported by a doctoral contract (2022/20675), from Universidad de Castilla-La Mancha (UCLM), Spain, co-financed by the European Social Fund (ESF). L. Mazuecos was supported by UCLM co-financed by ESF, post-doctoral grant 2021/32002). E. Ferreras-Colino was funded by UCLM co-financed by ESF, doctoral grant 2020/3836. The funding organizations were not involved in the collection, management, or analysis of the data; preparation or approval of the manuscript; or decision to submit the manuscript for publication.
Availability of data and materials
The data presented in this study are disclosed in the paper and Supplementary information.
Declarations
Ethics approval and consent to participate
Experiments in zebrafish were conducted in strict accordance with the recommendations of the European Guide for the Care and Use of Laboratory Animals. Fish were housed and experiments conducted at an experimental facility (IREC, Ciudad Real, Spain) with the approval and supervision of the Ethics Committee on Animal Experimentation of the University of Castilla La Mancha (PR-2021-09-14) and the Counseling of Agriculture, Environment and Rural Development of Castilla La Mancha (REGA code ES130340000218).
Competing interests
The authors declare that the research was conducted in the absence of financial relationships that could be construed as a potential conflict of interest.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marinela Contreras and Rita Vaz-Rodrigues contributed equally to this work.
Change history
10/27/2023
The Funding declaration has been corrected since original publication of this article.
References
- 1.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–511. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 2.Mullins RJ, James H, Platts-Mills TAE, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012;129:1334–42.e1. doi: 10.1016/j.jaci.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Çelebioğlu E, Akarsu A, Şahiner ÜM. IgE-mediated food allergy throughout life. Turk J Med Sci. 2021;51:49–60. doi: 10.3906/sag-2006-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy R. Mosquito vector proteins homologous to α1-3 galactosyl transferases of tick vectors in the context of protective immunity against malaria and hypersensitivity to vector bites. Parasit Vectors. 2021;14:303. doi: 10.1186/s13071-021-04801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galili U. Evolution in primates by “Catastrophic-selection” interplay between enveloped virus epidemics, mutated genes of enzymes synthesizing carbohydrate antigens, and natural anti-carbohydrate antibodies. Am J Phys Anthropol. 2019;168:352–363. doi: 10.1002/ajpa.23745. [DOI] [PubMed] [Google Scholar]
- 6.Kersh GJ, Salzer J, Jones ES, et al. Tick bite as a risk factor for alpha-gal-specific immunoglobulin E antibodies and development of alpha-gal syndrome. Ann Allergy Asthma Immunol. 2023;130:472–478. doi: 10.1016/j.anai.2022.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma SR, Karim S. Tick saliva and the alpha-gal syndrome: finding a needle in a haystack. Front Cell Infect Microbiol. 2021;11:680264. doi: 10.3389/fcimb.2021.680264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson AS, Gardner A, Iweala OI. Where’s the beef? Understanding allergic responses to red meat in Alpha-Gal Syndrome. J Immunol. 2022;208:267–277. doi: 10.4049/jimmunol.2100712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabezas-Cruz A, Hodžić A, Román-Carrasco P, Mateos-Hernández L, Duscher GG, Sinha DK, et al. Environmental and molecular drivers of the α-Gal syndrome. Front Immunol. 2019;10:1210. doi: 10.3389/fimmu.2019.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saretta F, Giovannini M, Mori F, Arasi S, Liotti L, Pecoraro L, et al. Alpha-Gal Syndrome in children: peculiarities of a “tick-borne” allergic disease. Front Pediatr. 2021;9:801753. doi: 10.3389/fped.2021.801753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrasekhar JL, Cox KM, Erickson LD. B Cell responses in the development of mammalian meat allergy. Front Immunol. 2020;11:1532. doi: 10.3389/fimmu.2020.01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of α-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy. 2018;73:1525–1531. doi: 10.1111/all.13400. [DOI] [PubMed] [Google Scholar]
- 13.Apostolovic D, Mihailovic J, Commins SP, Wijnveld M, Kazimirova M, Starkhammar M, et al. Allergenomics of the tick Ixodes ricinus reveals important α-Gal-carrying IgE-binding proteins in red meat allergy. Allergy. 2020;75:217–220. doi: 10.1111/all.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung CH, Chan E, Morse M, Hosen J, Zhou Q, Hicklin DJ. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. New England J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabezas-Cruz A, Espinosa PJ, Alberdi P, Šimo L, Valdés JJ, Mateos-Hernánde L, et al. Tick galactosyltransferases are involved in α-Gal synthesis and play a role during Anaplasma phagocytophilum infection and Ixodes scapularis tick vector development. Sci Rep. 2018;8:14224. doi: 10.1038/s41598-018-32664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma SR, Crispell G, Mohamed A, Cox C, Lange J, Choudhary S, et al. Alpha-Gal Syndrome: Involvement of Amblyomma americanum α-D-Galactosidase and β-1,4 Galactosyltransferase enzymes in α-Gal metabolism. Front Cell Infect Microbiol. 2021;11:775371. doi: 10.3389/fcimb.2021.775371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vora A, Taank V, Dutta SM, Anderson JF, Fish D, Sonenshine DE, et al. Ticks elicit variable fibrinogenolytic activities upon feeding on hosts with different immune backgrounds. Sci Rep. 2017;7:44593. doi: 10.1038/srep44593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuttall PA. Wonders of tick saliva. Ticks Tick-borne Dis. 2019;10:470–481. doi: 10.1016/j.ttbdis.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Villar M, Pacheco I, Merino O, Contreras M, Mateos-Hernández L, Padro E, et al. Tick and host derived compounds detected in the cement complex substance. Biomolecules. 2020;10:E555. doi: 10.3390/biom10040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neelakanta G, Sultana H. Tick saliva and salivary glands: what do we know so far on their role in arthropod blood feeding and pathogen transmission. Front Cell Infect Microbiol. 2022;11:816547. doi: 10.3389/fcimb.2021.816547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Gong H, Zhou Y, Xuan X, Fujisaki K. Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol Res. 2006;100:77–84. doi: 10.1007/s00436-006-0243-7. [DOI] [PubMed] [Google Scholar]
- 22.Vaz-Rodrigues R, Mazuecos L, de la Fuente J. current and future strategies for the diagnosis and treatment of the Alpha-Gal Syndrome (AGS) J Asthma Allergy. 2022;15:957–970. doi: 10.2147/JAA.S265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villar M, Pacheco I, Mateos-Hernández L, et al. Characterization of tick salivary gland and saliva alphagalactome reveals candidate alpha-gal syndrome disease biomarkers. Expert Rev Proteomics. 2021;18:1099–1116. doi: 10.1080/14789450.2021.2018305. [DOI] [PubMed] [Google Scholar]
- 24.Contreras M, Pacheco I, Alberdi P, Díaz-Sánchez S, Artigas-Jerónimo S, Mateos-Hernández L, et al. Allergic reactions and immunity in response to tick salivary biogenic substances and red meat consumption in the zebrafish model. Front Cell Infect Microbiol. 2020;10:78. doi: 10.3389/fcimb.2020.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kageyama R, Fujiyama T, Satoh T, Keneko Y, Kitano S, Tokura Y, et al. The contribution made by skin-infiltrating basophils to the development of alpha gal syndrome. Allergy. 2019;74:1805–1807. doi: 10.1111/all.13794. [DOI] [PubMed] [Google Scholar]
- 26.Balla KM, Lugo-Villarino G, Spitsbergen JM, et al. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood. 2010;116:3944–3954. doi: 10.1182/blood-2010-03-267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li CL, Liu N. Expression of interleukin-1β, interleukin-4, interferon-γ and tumour necrosis factor α in different tissue in a dinitrochlorobenzene-induced ear swelling test in mice. Skin Res Technol. 2023;29:e13255. doi: 10.1111/srt.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KrstićRistivojević M, Apostolović D, Smiljanić K. Enterocytes in food hypersensitivity reactions. Animals. 2021;11:2713. doi: 10.3390/ani11092713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailone RL, de Aguiar LK, de Oliveira Roca R, Borra RC, Corrêa T, Janke H, Fukushima HCS. Zebrafish as an animal model for food safety research: Trends in the animal research. Food Biotechnol. 2019;33:283–302. doi: 10.1080/08905436.2019.1673173. [DOI] [Google Scholar]
- 30.Fuentes-Appelgren P, Opazo R, Barros L, Feijoó CG, Urzúa V, Romero J. Effect of the dietary inclusion of soybean components on the innate immune system in zebrafish. Zebrafish. 2014;11:41–49. doi: 10.1089/zeb.2013.0934. [DOI] [PubMed] [Google Scholar]
- 31.Macdougall JD, Thomas KO, Iweala OI. The meat of the matter: understanding and managing alpha-gal syndrome. Immunotargets Ther. 2022;11:37–54. doi: 10.2147/ITT.S276872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mashoof S, Criscitiello M. Fish immunoglobulins. Biology. 2016;5:45. doi: 10.3390/biology5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kýčková K, Kopecký J. Effect of tick saliva on mechanisms of innate immune response against Borrelia afzelii. J Med Entomol. 2006;43:1208–1214. doi: 10.1093/jmedent/43.6.1208. [DOI] [PubMed] [Google Scholar]
- 34.Narasimhan S, Kurokawa C, Diktas H, Strank NO, Černý J, Murfin K, et al. Ixodes scapularis saliva components that elicit responses associated with acquired tick-resistance. Ticks Tick Borne Dis. 2020;11:101369. doi: 10.1016/j.ttbdis.2019.101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shevchenko A, Tomas H, Havli J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 36.Matthews M, Varga ZM. Anesthesia and euthanasia in zebrafish. ILAR J. 2012;53:192–204. doi: 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Shao A, Shan Y, Zhao H, Leiguo M, Zhang Y, Tang Y, Zhang W, Jin Y, Xu L. A standardized quantitative method for detecting remnant alpha-Gal antigen in animal tissues or animal tissue-derived biomaterials and its application. Sci Rep. 2018;8:15424. doi: 10.1038/s41598-018-32959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obukhova P, Tsygankova S, Chinarev A, et al. Are there specific antibodies against Neu5Gc epitopes in the blood of healthy individuals? [published correction appears in Glycobiology. 2020 May 19;30(6):415]. Glycobiology. 2020;30:395-406. 10.1093/glycob/cwz107 [DOI] [PubMed]
- 39.Pacheco I, Díaz-Sánchez S, Contreras M, et al. Probiotic bacteria with high Alpha-Gal content protect zebrafish against mycobacteriosis. Pharmaceuticals. 2021;14:635. doi: 10.3390/ph14070635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mateos-Hernández L, Villar M, Moral A, Rodríguez CG, Arias TA, de la Osa V, et al. Tick-host conflict: immunoglobulin E antibodies to tick proteins in patients with anaphylaxis to tick bite. Oncotarget. 2017;8:20630–20644. doi: 10.18632/oncotarget.15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Fuente J, Urra JM, Contreras M, Pacheco I, Ferreras-Colina E, Doncel-Pérez E, et al. A dataset for the analysis of antibody response to glycan alpha-Gal in individuals with immune-mediated disorders. F1000Res. 2021;9:1366. 10.12688/f1000research.27495.2 [DOI] [PMC free article] [PubMed]
- 43.Pacheco I, Fernández de Mera IG, Feo Brito F, Gómez Torrijos E, Villar M, Contreras M, et al. Characterization of the anti-α-Gal antibody profile in association with Guillain-Barré syndrome, implications for tick-related allergic reactions. Ticks Tick Borne Dis. 2021;12:101651. 10.1016/j.ttbdis.2021.101651 [DOI] [PubMed]
- 44.Young I, Prematunge C, Pussegoda K, Corrin T, Waddell L. Tick exposures and alpha-gal syndrome: a systematic review of the evidence. Ticks Tick Borne Dis. 2021;12:101674. doi: 10.1016/j.ttbdis.2021.101674. [DOI] [PubMed] [Google Scholar]
- 45.CDC. Alpha-gal syndrome | CDC. Centers for Disease Control and Prevention. Published March 28, 2022. https://www.cdc.gov/ticks/alpha-gal/index.html. Accessed 12 Sep 2022.
- 46.Paudel YN, Kumari Y, Abidin SAZ, Othman I, Shaikh MF. Pilocarpine induced behavioral and biochemical alterations in chronic seizure-like condition in adult zebrafish. Int J Mol Sci. 2020;21:2492. doi: 10.3390/ijms21072492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribeiro JM, Zeidner NS, Ledin K, Dolan MC, Mather TN. How much pilocarpine contaminates pilocarpine-induced tick saliva? Med Vet Entomol. 2004;18:20–24. doi: 10.1111/j.0269-283x.2003.0469.x. [DOI] [PubMed] [Google Scholar]
- 48.Park Y, Kim D, Boorgula GD, De Schutter K, Smagghe G, Šimo L, Archer-Hartmann SA, Azadi P. Alpha-Gal and cross-reactive carbohydrate determinants in the N-glycans of salivary glands in the lone star tick. Amblyomma americanum Vaccines. 2020;8:18. doi: 10.3390/vaccines8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shilova NV, Navakouski MJ, Huflejt M, et al. Changes in the repertoire of natural antibodies caused by immunization with bacterial antigens. Biochemistry (Mosc) 2011;76:862–866. doi: 10.1134/S0006297911070170. [DOI] [PubMed] [Google Scholar]
- 50.Ricci Hagman J, Westman JS, Hellberg Å, Olsson ML. An update on the GLOB blood group system (and former GLOB collection) Immunohematology. 2018;34:161–163. doi: 10.21307/immunohematology-2018-026. [DOI] [PubMed] [Google Scholar]
- 51.Okajima T, Nakamura Y, Uchikawa M, et al. Expression cloning of human globoside synthase cDNAs. Identification of beta 3Gal-T3 as UDP-N-acetylgalactosamine:globotriaosylceramide beta 1,3-N-acetylgalactosaminyltransferase. J Biol Chem. 2000;275:40498–40503. doi: 10.1074/jbc.M006902200. [DOI] [PubMed] [Google Scholar]
- 52.Thuresson B, Westman JS, Olsson ML. Identification of a novel A4GALT exon reveals the genetic basis of the P1/P2 histo-blood groups. Blood. 2011;117:678–687. doi: 10.1182/blood-2010-08-301333. [DOI] [PubMed] [Google Scholar]
- 53.Spitalnik PF, Spitalnik SL. The P blood group system: biochemical, serological, and clinical aspects. Transfus Med Rev. 1995;9:110–122. doi: 10.1016/s0887-7963(05)80050-1. [DOI] [PubMed] [Google Scholar]
- 54.Černý J, Lynn G, DePonte K, Ledizet M, Narasimhan S, Fikrig E. Fractionation of tick saliva reveals proteins associated with the development of acquired resistance to Ixodes scapularis. Vaccine. 2020;38:8121–8129. doi: 10.1016/j.vaccine.2020.10.087. [DOI] [PubMed] [Google Scholar]
- 55.Villar M, Urra JM, Rodríguez-Del-Río FJ, Artigas-Jerónimo S, Jiménez-Collados N, Ferreras-Colino E, et al. Characterization by quantitative serum proteomics of immune-related prognostic biomarkers for COVID-19 symptomatology. Front Immunol. 2021;12:730710. doi: 10.3389/fimmu.2021.730710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan KW, Jobichen C, Ong TC, Gao YF, Tiong YS, Wong KN, et al. Crystal structure of Der f 7, a dust mite allergen from Dermatophagoides farinae. PLoS ONE. 2012;7:e44850. doi: 10.1371/journal.pone.0044850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Dataset S1. Identification and characterization of tick saliva proteins in SDS-PAGE fractions 1–5 by mass spectrometry and association with allergic reactions in zebrafish.
Additional file 2: Dataset S2. Glycochip data analysis.
Data Availability Statement
The data presented in this study are disclosed in the paper and Supplementary information.