Abstract
Objective
Atrial fibrillation (AF) is a condition that occurs in the presence of comorbidities. With the accumulation of comorbidities (multimorbidity), some combinations may more often occur together than others. Information on the impact of clustering of these on incident AF is sparse. We aimed to investigate clustering of cardiovascular and renal comorbidities and study the association between comorbidity clusters and incident AF.
Methods
We used the community-based Prevention of Renal and Vascular ENd-stage Disease (PREVEND) cohort in which 8592 individuals participated. Latent class analysis was performed to assess clustering of 10 cardiovascular and renal comorbidities.
Results
We excluded individuals with prior AF or missing ECG data, leaving 8265 individuals for analysis (mean age 48.9±12.6 years, 50.2% women). During 9.2±2.1 years of follow-up, 251 individuals (3.0%) developed AF. A model with three clusters was the optimal model, with one cluster being young (44.5±10.8 years) and healthy, carrying a low (1.0%) risk of incident AF; one cluster being older (63.0±8.4 years) and multimorbid, carrying a high (16.2%) risk of incident AF and a third middle-aged (57.0±11.3 years), obese and hypertensive cluster carrying an intermediate risk (5.9%) of incident AF. While the prevalence of the comorbidities differed between classes, no clear combination(s) of comorbidities was observed within the classes.
Conclusions
We identified three clusters of comorbidities in individuals in the community-based PREVEND cohort. The three clusters contained different amount of comorbidities carrying different risks of incident AF. However, there were no differences between the clusters regarding specific combination(s) of comorbidities.
Keywords: Atrial Fibrillation, EPIDEMIOLOGY, RISK FACTORS
WHAT IS ALREADY KNOWN ON THIS TOPIC
Individual cardiovascular risk factors and comorbidities associated with incident atrial fibrillation (AF) are well known.
Multimorbidity is common in individuals and increases risk of incident cardiovascular diseases.
Information on the impact of clustering of cardiovascular and renal comorbidities on incident AF is sparse.
WHAT THIS STUDY ADDS
In the community-based study of >8500 individuals, we found that multimorbidity increased the risk of incident AF.
In this general population cohort, three clusters of comorbidities were identified.
The three clusters contained different amount of comorbidities carrying different risks of incident AF.
There were no differences between the clusters regarding specific combination(s) of comorbidities.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This knowledge may aid clinicians in identifying individuals at an increased risk of developing AF.
This study implicates that management of multiple comorbidities at early stages may be need to alter risk of incident AF
We did not find clear combinations of comorbidities, and further studies are needed to explore comorbidity clusters, also including non-cardiovascular comorbidities and subsequent risk of incident AF.
Introduction
Atrial fibrillation (AF) occurs in the presence of comorbidities. Comorbidities that are frequently seen in patients with AF are, among others, hypertension, obesity, coronary heart disease and diabetes.1 Presence of specific cardiovascular risk factors and/or comorbidities increase the risk of incident AF.2–6 Both the prevalence of AF and of comorbidities increase with age, causing the prevalence of multimorbidity (the presence of two or more comorbidities in an individual) to increase as well.7–11
With the accumulation of comorbidities over time, certain comorbidities may more often occur together than others, that is, clustering of comorbidities. In AF, acknowledging clustering of certain comorbidities may have implications for early diagnosing previously unrecognised comorbidities, and clusters of comorbidities may carry differential risks of AF. Although that the individual cardiovascular comorbidities and/or risk factors for it are well known, information on the impact of clustering on incident AF is sparse.
In this study, we aim to investigate the presence of clusters of cardiovascular and renal comorbidities and study the association between cardiovascular and renal comorbidity clusters and incident AF in individuals of the community-based Prevention of Renal and Vascular ENd-stage Disease (PREVEND) cohort.
Methods
Study design, setting, population
For the current study, we used data from the PREVEND cohort. The study protocol has been described elsewhere in detail.12 In short, PREVEND is a community-based cohort. The original aim of the PREVEND study was to investigate the relation of urinary albumin excretion with renal and cardiovascular disease. This prospective study was initiated in 1997 and included 8592 individuals. By design, the cohort was enriched for elevated urinary albumin excretion with approximately 70% of individuals having a urinary albumin concentration >10 mg/L.
For the present analysis, we excluded 248 individuals without ECG data, 37 individuals diagnosed with AF prior to inclusion and 42 individuals with AF on baseline ECG, leaving 8265 individuals for analysis.
Ascertainment of AF
AF was diagnosed if either AF or atrial flutter was present on a 12-lead ECG during one of the study visits, or at an outpatient visit or hospitalisation. All ECGs were stored digitally, electronically screened, and validated by two independent observers. The assessment has been described in detail elsewhere.13 For the date of incident AF, the date of the first ECG with a definite diagnosis of AF or atrial flutter was used.
Definitions of comorbidities
Hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg (based on the mean of two measurements), or use of antihypertensive medication. Obesity was defined as body mass index >30.0 kg/m2, where body mass index has been calculated as weight divided by height squared (kg/m2). Type 2 diabetes was defined as a fasting plasma glucose level >7.0 mmol/L, a non-fasting plasma glucose level of >11.1 mmol/L or use of antidiabetic medication. All individuals with heart failure at inclusion were adjudicated by a committee of heart failure experts according to previously published criteria.14 Hypercholesterolaemia was defined as total serum cholesterol >6.5 mmol/L or serum cholesterol ≥5.0 mmol/L with use of lipid-lowering medication or if a history of myocardial infarction was present. Previous myocardial infarction or stroke was established as self-reported hospitalisation for at least 3 days for one or both conditions. Peripheral artery disease was defined as an ankle-brachial index <0.9. Renal impairment was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2, in which estimated glomerular filtration rate was estimated with the simplified modification of diet in renal disease formula. Urinary albumin excretion was calculated as an average of two consecutive 24-hour urine collections and microalbuminuria was defined as urinary albumin excretion ≥10 mg/L. Smoking was defined as current smoker or quit smoking within the last 5 years.
Follow-up
The follow-up duration was calculated as the time between the screening visit and incident AF, death or the last contact date to a maximum of 10 years.
Statistical analyses
Individual characteristics were described for the total population and the latent classes. Continuous variables were presented as mean±SD or median (IQR) and categorical variables as number (percentage). Latent class analysis was performed using 10 comorbidities: hypertension, heart failure, type 2 diabetes, hypercholesterolaemia, obesity, previous myocardial infarction, previous stroke, peripheral artery disease, renal impairment, microalbuminuria. We included the study outcome incident AF as covariate for the cluster analysis, according to the method by Lanza et al.15 As secondary analysis, latent class analysis was repeated with additionally including age, sex and ethnicity. A priori, the optimum number of the latent classes was unknown. To estimate the optimum number of classes, we increased the number of classes while comparing the fit of the models. The optimum number of classes was determined by the number of classes for which the Bayesian information criterion reached a minimum value.
Additional model fit statistics were calculated, including the normalised χ2 (Pearson χ2 for model fit divided by the number of residual df), root mean square error, log-likelihood and Madansky’s measure for local independence. For internal validation of the optimum number of latent classes, we performed parametric bootstrapping to check if the observed statistics were within the Efron’s 95% CI, which would imply a good model fit.
The risk of incident AF for each class was calculated by using latent class analysis. In addition, Kaplan-Meier analysis was performed to calculate the cumulative probability of incident AF, stratified by the latent classes. The log-rank test was used to compare the latent classes. We assessed missing data and found that this was <5% for all covariates, and no missing data for the primary outcome incident AF. No imputation strategies to account for missing data were applied. All analyses were performed using R package (V.4.0.5). The significance level was set at p<0.05.
Patient and public involvement
No patients were involved in the development of the research question and outcome measures.
Results
Individual characteristics
The characteristics of the total population are shown in table 1. Mean age was 48.9±12.6 years and 50.2% were women. Of the total population of 8265 individuals, 1424 individuals (18.2%) had no comorbidity, 6030 individuals (77.1%) had 1–3 comorbidities and 363 individuals (4.6%) had ≥4 comorbidities. The median number of comorbidities was 1.0 (1.0–2.0). The distribution of the number of comorbidities is shown in the upper panel of figure 1. During a mean follow-up duration of 9.2±2.1 years, 251 individuals (3.0%) developed AF.
Table 1.
Baseline characteristics
| Characteristic | Total population (n=8265) |
| Age (years) | 48.9±12.6 |
| Women | 4145 (50.2%) |
| Caucasian ethnicity | |
| Follow-up duration (years) | 9.2±2.1 |
| No of cardiovascular and renal comorbidities | 1.0 (1.0–2.0) |
| 0 | 1424 (18.2%) |
| 1–3 | 6030 (77.1%) |
| ≥4 | 363 (4.6%) |
| Hypertension | 2542 (31.6%) |
| Heart failure | 18 (0.2%) |
| Type 2 diabetes | 310 (3.8%) |
| Hypercholesterolemia | 361 (4.6%) |
| Obesity | 1287 (15.7%) |
| Previous myocardial infarction | 251 (3.1%) |
| Previous stroke | 81 (1.0%) |
| Peripheral artery disease | 291 (3.7%) |
| Renal impairment | 466 (5.7%) |
| Microalbuminuria | 5759 (69.7%) |
Cardiovascular and renal comorbidities include hypertension, heart failure, type 2 diabetes, hypercholesterolaemia, obesity, previous myocardial infarction, previous stroke, peripheral artery disease, renal impairment and microalbuminuria.
Figure 1.
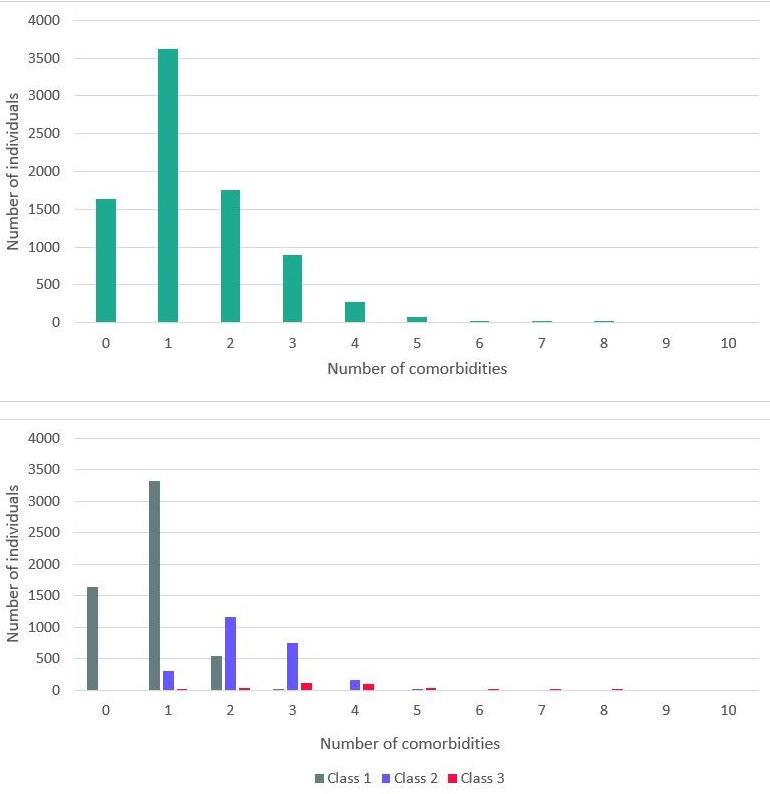
Distribution of the number of comorbidities: upper panel: total population; lower panel: divided by latent classes. AF, atrial fibrillation.
Latent class model of cardiovascular and renal comorbidities
Individuals were clustered based on 10 cardiovascular and renal comorbidities: hypertension, heart failure, type 2 diabetes, hypercholesterolaemia, obesity, previous myocardial infarction, previous stroke, peripheral artery disease, renal impairment, microalbuminuria. A model with three latent classes was the optimal model, with a minimum Bayesian information criterion value compared with other models with different numbers of classes. Additional fit model statistics all resulted in observed statistic values within the Efron’s 95% CI, implying good model fit (table 2).
Table 2.
Additional fit model statistics
| Observed statistic | Efron’s 95% CI | |
| Normalised χ2 | 0.596 | (0.385 to 1.966) |
| Root mean square error | 0.052 | (0.029 to 0.114) |
| Log-likelihood | −18320.840 | (−34638.885 to −18371.516) |
| Madansky | 1 845 806.951 | (1 657 859.509 to 7 513 280.270) |
Characteristics of the three latent classes
Characteristics of the population by latent classes are shown in table 3. Except for ethnicity, the characteristics were significantly different between the classes. Class 1 (n=5527) consisted of younger (44.5±10.8 years), relatively healthy individuals with low prevalence of cardiovascular and renal comorbidities (median number of comorbidities 1.0 (0.0–1.0)). Of these individuals, 55.1% were women. In contrast, individuals in class 3 (n=327) were older (63.0±8.4 years), less likely to be women (26.6%) and had a higher prevalence of all cardiovascular and renal comorbidities (median number of comorbidities 4.0 (3.0–4.0)), compared with class 1. Class 2 (n=2411) is characterised as an intermediate group, with a mean age of 57.0±11.3 years, 42.0% women and a median number of comorbidities of 2.0 (2.0–3.0)). However, both hypertension and obesity were more prevalent in this class compared with class 1 and 3 (hypertension: 98.3% in class 2 vs 0.0% vs 64.9% in class 1 and 3, respectively; obesity: 29.0% in class 2 vs 9.7% vs 19.1% in class 1 and 3, respectively). The characteristics of each class are shown in figure 2. While the prevalence of the comorbidities differed between classes, no clear combination(s) of comorbidities could be observed within the classes. The distribution of the number of comorbidities for each class is shown in the lower panel of figure 1. When adding age, sex and ethnicity to the latent class analysis, similar results were observed (online supplemental table 1 and online supplemental figure 1).
Table 3.
Characteristics of the population by latent classes
| Characteristic | Class 1 (n=5527) |
Class 2 (n=2411) |
Class 3 (n=327) |
P value |
| Age (years) | 44.5±10.8 | 57.0±11.3 | 63.0±8.4 | <0.001 |
| Women | 3046 (55.1%) | 1012 (42.0%) | 87 (26.6%) | <0.001 |
| Caucasian ethnicity | 5239 (95.4%) | 2288 (96.1%) | 317 (97.8%) | 0.062 |
| Follow-up duration (years) | 9.3±2.0 | 9.2±2.1 | 8.5±2.6 | <0.001 |
| No of comorbidities | 1.0 (0.0–1.0) | 2.0 (2.0–3.0) | 4.0 (3.0–4.0) | <0.001 |
| 0 | 1424 (29.0%) | 0 (0.0%) | 0 (0.0%) | |
| 1–3 | 3863 (73.1%) | 2039 (91.5%) | 128 (42.5%) | |
| ≥4 | 0 (0.0%) | 190 (8.5%) | 173 (57.5%) | |
| Hypertension | 0 (0.0%) | 2337 (98.3%) | 205 (64.9%) | <0.001 |
| Heart failure | 0 (0.0%) | 0 (0.0%) | 18 (5.5%) | <0.001 |
| Type 2 diabetes | 53 (1.0%) | 201 (8.5%) | 56 (17.6%) | <0.001 |
| Hypercholesterolaemia | 69 (1.3%) | 103 (4.6%) | 189 (59.6%) | <0.001 |
| Obesity | 531 (9.7%) | 694 (29.0%) | 62 (19.1%) | <0.001 |
| Previous myocardial infarction | 5 (0.1%) | 0 (0.0%) | 246 (75.9%) | <0.001 |
| Previous stroke | 28 (0.5%) | 36 (1.5%) | 17 (5.3%) | <0.001 |
| Peripheral artery disease | 82 (1.5%) | 152 (6.7%) | 57 (18.8%) | <0.001 |
| Renal impairment | 120 (2.2%) | 257 (10.7%) | 99 (27.5%) | <0.001 |
| Microalbuminuria | 3597 (65.1%) | 1895 (78.6%) | 267 (81.7%) | <0.001 |
Latent class analysis based on hypertension, heart failure, type 2 diabetes, hypercholesterolaemia, obesity, previous myocardial infarction, previous stroke, peripheral artery disease, renal impairment, microalbuminuria and incident atrial fibrillation.
Figure 2.
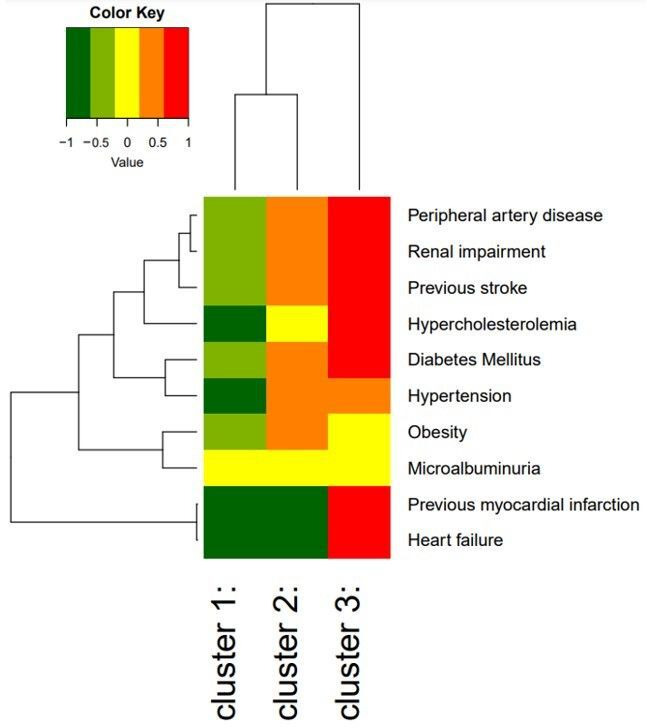
Heat map of individual cardiovascular and renal comorbidities within each cluster the colours represent the log relative risk of the presence of the comorbidity compared with the average individual in this cohort. The branching diagrams represent the hierarchy of categories based on degree of similarity between the comorbidities (rows) or clusters (columns).
openhrt-2023-002315supp001.pdf (149.4KB, pdf)
Latent classes and association with incident AF
The incidence of AF varied between the latent classes and was 1.0% for class 1, 5.9% for class 2 and 16.2% for class 3 (p<0.001). The incidence rates of AF were 0.11 (0.08–0.14), 0.64 (0.54–0.75) and 1.91 (1.43–2.50), respectively. The cumulative probability of incident AF for each class is shown in figure 3. Similar results were observed when adding age, sex and ethnicity to the latent class analysis (online supplemental figure 2).
Figure 3.
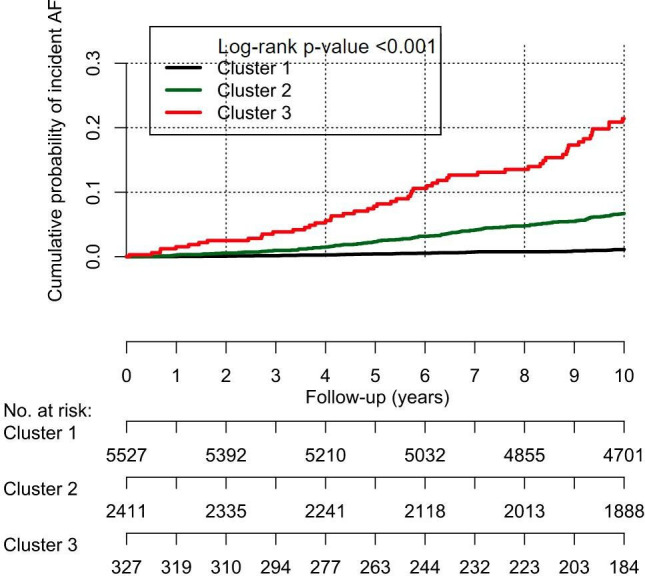
Cumulative probability of incident AF by comorbidity cluster. AF, atrial fibrillation.
Discussion
In the community-based PREVEND cohort, we found three distinct clusters of individuals with similar comorbidity burden, based on 10 cardiovascular and renal comorbidities. We found no specific combination(s) of comorbidities carrying different risks of incident AF, rather we found three clusters with different number of comorbidities. Our results indicate that multimorbidity itself carries an increased risk of incident AF. One young and healthy cluster with only one comorbidity, carrying a low (1.0%) risk of incident AF. One older and multimorbid cluster, with on average four comorbidities, carrying a high (16.2%) risk of incident AF. The last cluster is situated in between the other two, this intermediate middle-aged, obese and hypertensive cluster, with on average two comorbidities, carries an intermediate risk (5.9%) of incident AF.
In clinical practice, it is common to approach different risk factors and comorbidities individually. This is also reflected in research; studies would look at the association of individual risk factors and comorbidities with incident AF.2–6 However, individuals with AF commonly have more than one comorbidity and thus multimorbidity.1 16–18 This study has shown that latent class analysis can be used to define clusters of comorbidities, but that the clusters seem to be based on the amount of comorbidities, rather than specific combinations of comorbidities.
The association between the number of comorbidities and incident AF has been shown before. Andersson et al identified 272 186 individuals with incident AF diagnosed in a hospital setting and found that all predefined comorbidities were more common in individuals with incident AF than in controls, with 69.5% of individuals with AF having ‘any’ comorbidity compared with 27.2% of controls.19 Also Chamberlain et al found a significant difference in the number of comorbidities between individuals with incident AF and without incident AF, with a mean of 5.6 comorbidities in those with incident AF vs 4.5 in those without incident AF. 75% of individuals with incident AF had four or more comorbidities vs 62% in those without incident AF.20 Although we did not compare individuals with and without incident AF as done by the previous studies, we compared the clusters and found differences in the risk of incident AF with different number of comorbidities.
Individuals in cluster 3 had a significantly higher risk of incident AF compared with cluster 1 (16.2% vs 1.0%, respectively), and also had more comorbidities compared with cluster 1 (median of 4.0 (3.0–4.0) comorbidities with 57.5% of the individuals having four or more comorbidities vs median of 1.0 (0.0–1.0) comorbidities, no individuals with four or more comorbidities, respectively). Thus, multimorbidity is an important determinant of development of AF in our community-based cohort.
For this study, we used data from individuals with no prior AF in the PREVEND cohort. Data on comorbidity clusters and the risk of incident AF in the general population is sparse. A previous study by Rienstra et al used latent class analysis to determine the risk of incident AF in the PREVEND cohort and found that clustering by latent class analysis can be used to unravel distinct pathophysiological mechanisms underlying individuals with shared risk factors.21 However, this study focused on different risk factors and not specifically comorbidity clusters and incident AF as we did in the present analysis. While our clusters seem to be based on the number of comorbidities, we did not find clear combinations of comorbidities giving direction towards distinct pathophysiological mechanisms. Rather, our results indicate that multimorbidity, and with increasing number of comorbidities clustered in an individual the risk of incident AF increases.
Multimorbidity in individuals with AF is of importance, as the amount of multimorbidity has also been associated with impaired outcomes. In the UK Biobank cohort, individuals with AF and multimorbidity had a higher risk of mortality compared with individuals with AF without multimorbidity.1 Others have studied AF patient populations and the risk of impaired cardiovascular outcomes using latent cluster analysis. In the ORBIT-AF registry, Inohara et al performed a cluster analysis based on 60 patient characteristics and found 4 distinct clusters: (1) AF patients with low prevalence of risk factors and comorbidities; (2) AF patients at younger ages and/or behavioural disorders as comorbidity; (3) AF patients and similarities to patients with brady-tachy-syndrome with device implantation and (4) AF patients and atherosclerotic comorbidities.22 Compared with the cluster with low prevalence of risk factors and comorbidities, the other clusters of AF patients had a higher risk of major adverse cardiovascular or neurological evens and major bleeding. Similarly, Ogawa et al performed latent cluster analysis in AF patients included in the Fushimi AF Registry, using 42 patient characteristics.23 They found six comorbidity clusters: (1) AF patients with younger age and low prevalence of risk factors and comorbidities; (2) old AF patients with low prevalence of risk factors and comorbidities; (3) AF patients with high prevalence of atherosclerotic risk factors, but without atherosclerotic disease; (4) AF patients with atherosclerotic comorbidities; (5) AF patients with history of stroke and (6) very old AF patient. The authors found again different risk of major adverse cardiovascular or neurological events in the AF patient clusters. Both studies concluded that AF patients can have different phenotypic presentations that were associated with different clinical outcomes.
In this study, we found that multimorbidity increased the risk of incident AF in the general population. This knowledge is relevant as this may aid clinicians in identifying individuals at an increased risk of developing AF. We did not find clear combinations of comorbidities, and further studies are needed to explore comorbidity clusters with a larger number of comorbidities, also including non-cardiovascular comorbidities, and subsequent risk of incident AF. This information may not only be relevant to identify individuals at risk of incident AF or other comorbidities, but also may identify underlying pathways and consequently influence treatment strategies to improve outcomes.
This study also has some limitations. While we have studied a well-phenotyped community-based cohort of >8500 individuals with an average age below 50 years in the Netherlands, with follow-up of approximately 10 years, our population was virtually complete Caucasian. Also, by design, individuals with microalbuminuria were overrepresented. Both limit the generalisability of our results to other populations. Second, the advantages of latent class clustering methodology are clear; it is a model-based clustering approach which makes the choice of a cluster criterion less arbitrary, it can include variables with different measurement levels, no decisions have to make about the scaling and the number of classes can be based on more formal criteria than in other methods. However, continuous variables must be recoded into categories which may have led to loss of information, reification may be an issue when interpreting the results, and the selection of variables is of importance. The modest number of comorbidities may have limited our ability to find a larger number of classes and may have limited the possibility of finding specific clustering of certain comorbidities. Third, we did not validate our results externally in an independent cohort. Fourth, no distinction was made between AF and atrial flutter, or between different temporal patterns of AF, nor was information on treatment of comorbidities during follow-up available. Fifth, information on the presence of comorbidities, and interaction with advancing age, during follow-up was not available for this analysis.
Conclusion
Based on 10 cardiovascular and renal comorbidities, there were three clusters of comorbidities in individuals in the community-based PREVEND cohort. These clusters contained different amount of comorbidities carrying different risks of incident AF, indicating that multimorbidity and increasing number of comorbidities determine risk of incident AF. No specific combination(s) of comorbidities were identified.
Footnotes
Contributors: All authors contributed to the conception, design and acquisition of the work. BG, CVD and MR contributed to the data analysis and interpretation. CVD and MR drafted the manuscript and are responsible for the overall content of the work as guarantors. All authors contributed to the critical revision of the manuscript, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Funding: The PREVEND study is supported by the Dutch Kidney Foundation (grant E0.13). CVD, ICVG and MR are supported by a grant from the European Union’s Horizon 2020 research and innovation programme (grant agreement No 945260).
Competing interests: For the current manuscript, the authors declare no potential conflicts of interest. Outside of the submitted work, the authors disclosed the following financial support: MR reports grants from the Dutch Heart Foundation (CVON RACE V, grant 2014-09; CVON RED-CVD, grant 2017-11; CVON-AI, grant 2018B017; DECISION, grant 2018B024). The UMCG, which employs MR, has received grants from SJM/Abbott (VIP-HF study) and Medtronic (Cryoballoon AF registry/biobank study). ICVG reports grants from the Dutch Heart Foundation (CVON RACE V, grant 2014-09).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. For more information, please visit: https://groningendatacatalogus.nl/menu/main/dataexplorer/details/umcg_collections/17https://groningendatacatalogus.nl/menu/main/dataexplorer/details/umcg_collections/17
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Medical Ethical Committee Academic Hospital Groningen (MEC 96/01/022). Participants gave informed consent to participate in the study before taking part.
References
- 1.Jani BD, Nicholl BI, McQueenie R, et al. Multimorbidity and Co-morbidity in atrial fibrillation and effects on survival: findings from UK Biobank cohort. Europace 2018;20(FI_3):f329–36. 10.1093/europace/eux322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham heart study. JAMA 1994;271:840–4. [PubMed] [Google Scholar]
- 3.Lee S-R, Park CS, Choi E-K, et al. Hypertension burden and the risk of new-onset atrial fibrillation: A nationwide population-based study. Hypertension 2021;77:919–28. 10.1161/HYPERTENSIONAHA.120.16659 [DOI] [PubMed] [Google Scholar]
- 4.Ninni S, Lemesle G, Meurice T, et al. Real-life incident atrial fibrillation in outpatients with coronary artery disease. J Clin Med 2020;9. 10.3390/jcm9082367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharashova E, Wilsgaard T, Ball J, et al. Long-term blood pressure Trajectories and incident atrial fibrillation in women and men: the Tromsø study. Eur Heart J 2020;41:1554–62. 10.1093/eurheartj/ehz234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D, Feng T, Schlesinger S, et al. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J Diabetes Complications 2018;32:501–11. 10.1016/j.jdiacomp.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 7.van den Akker M, Buntinx F, Metsemakers JF, et al. Multimorbidity in general practice: prevalence, incidence, and determinants of Co-occurring chronic and recurrent diseases. J Clin Epidemiol 1998;51:367–75. 10.1016/s0895-4356(97)00306-5 [DOI] [PubMed] [Google Scholar]
- 8.Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of Multimorbidity. BMC Public Health 2015;15:415. 10.1186/s12889-015-1733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract 2008;14 Suppl 1:28–32. 10.1080/13814780802436093 [DOI] [PubMed] [Google Scholar]
- 10.van den Akker M, Vaes B, Goderis G, et al. Trends in Multimorbidity and Polypharmacy in the Flemish-Belgian population between 2000 and 2015. PLoS One 2019;14. 10.1371/journal.pone.0212046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Keshavjee S, Atun R. Trends, patterns and health consequences of Multimorbidity among South Korea adults: analysis of nationally representative survey data 2007-2016. J Glob Health 2020;10. 10.7189/jogh.10.020426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic, Nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 2001;249:519–26. 10.1046/j.1365-2796.2001.00833.x [DOI] [PubMed] [Google Scholar]
- 13.Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: A community-based study from the Netherlands. J Am Coll Cardiol 2015;66:1000–7. 10.1016/j.jacc.2015.06.1314 [DOI] [PubMed] [Google Scholar]
- 14.Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34:1424–31. 10.1093/eurheartj/eht066 [DOI] [PubMed] [Google Scholar]
- 15.Lanza ST, Tan X, Bray BC. Latent class analysis with distal outcomes: A flexible model-based approach. Struct Equ Modeling 2013;20:1–26. 10.1080/10705511.2013.742377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu HO, Saczynski J, Mehawej J, et al. Multimorbidity, physical Frailty, and self-rated health in older patients with atrial fibrillation. BMC Geriatr 2020;20:343. 10.1186/s12877-020-01755-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozieł M, Simovic S, Pavlovic N, et al. Impact of Multimorbidity and Polypharmacy on the management of patients with atrial fibrillation: insights from the BALKAN-AF survey. Ann Med 2021;53:17–25. 10.1080/07853890.2020.1799241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruzieh M, Mandrola J, Dyer A-M, et al. A Multimorbidity-based, risk-stratified Reanalysis of the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) trial. Drugs Aging 2020;37:839–44. 10.1007/s40266-020-00797-4 [DOI] [PubMed] [Google Scholar]
- 19.Andersson T, Magnuson A, Bryngelsson I-L, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061–7. 10.1093/eurheartj/ehs469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlain AM, Alonso A, Gersh BJ, et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am Heart J 2017;185:74–84. 10.1016/j.ahj.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rienstra M, Geelhoed B, Yin X, et al. Cluster individuals based on phenotype and determine the risk for atrial fibrillation in the PREVEND and Framingham heart study populations. PLoS One 2016;11:e0165828. 10.1371/journal.pone.0165828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inohara T, Shrader P, Pieper K, et al. Association of of atrial fibrillation clinical phenotypes with treatment patterns and outcomes: A multicenter Registry study. JAMA Cardiol 2018;3:54–63. 10.1001/jamacardio.2017.4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa H, An Y, Nishi H, et al. Characteristics and clinical outcomes in atrial fibrillation patients classified using cluster analysis: the Fushimi AF Registry. Europace 2021;23:1369–79. 10.1093/europace/euab079 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002315supp001.pdf (149.4KB, pdf)
Data Availability Statement
Data are available on reasonable request. For more information, please visit: https://groningendatacatalogus.nl/menu/main/dataexplorer/details/umcg_collections/17https://groningendatacatalogus.nl/menu/main/dataexplorer/details/umcg_collections/17


