Abstract
Introduction
The study aimed to evaluate the objective response level to neoadjuvant platinum-based chemotherapy and tumour complexity reduction in patients with invasive upper tract urothelial cancer (UTUC), and to estimate the functional and oncological outcomes of the combined organ-sparing approach compared to radical nephroureterectomy.
Material and methods
This prospective, non-randomised cohort study was conducted by the National Cancer Institute of Ukraine. Patients with invasive UTUC were enrolled between October 2016 and January 2021. Patients were allocated to one of two cohorts depending on the estimated glomerular filtration rate (eGFR) of the affected kidney. In cases where eGFR was preserved, neoadjuvant chemotherapy with an organ-sparing approach was used; all other cases proceeded directly to radical nephroureterectomy.
Results
A total of 64 patients (32 in each cohort) with invasive UTUC were enrolled. Both groups were comparable in terms of age, sex, T stage, maximal tumour size, eGFR, Eastern Cooperation Oncology Group (ECOG) performance status, body mass Index (BMI), and haemoglobin level. After four cycles of chemotherapy, there were no cases of progressive disease, stable disease [16 (50%), partial response; 12 (38%); and complete response, 4 (12%)]. The average maximal tumour size decreased by 2.3 cm. Prior to surgical treatment, total GFR according to scintigraphy did not statistically differ in both groups (р = 0.13). However, 3 months after surgery patients who underwent the organ-sparing approach had a better total eGFR (р = 0.0039), which was probably owing to the preserved kidney function (18.9 +5.1 mL/min). Better 2-year recurrence-free survival was also observed in the organ-sparing management group (85% vs 72%, log-rank test; p = 0.03).
Conclusions
Neoadjuvant systemic therapy reduces the surgical complexity of invasive UTUC without influencing the safety profile. The gemcitabine/cisplatin regimen leads to high regression rates among invasive UTUC, which could result in an organ-sparing approach in selected cases. Kidney function preservation remains a key parameter that can increase the possibility of effective systemic treatment.
Keywords: kidney function, nephrectomy, upper urinary tract cancer
INTRODUCTION
Upper tract urothelial cancer (UTUC) is a rare but highly aggressive oncologic disease. The 5-year cancer-specific survival of patients with pT2/pT3 non-metastatic disease is approximately 50%, with a rapid decline to 10% in patients with pT4 stage disease [1]. Considering these numbers, it is understandable that most of these patients will require a combined treatment approach of surgical tumour removal and systemic therapy [2].
Current data suggests that the best treatment option for patients with invasive UTUC is radical nephroureterectomy (RNU) with bladder cuff removal accompanied by systemic cisplatin-based chemotherapy, which could be done in neoadjuvant/adjuvant settings [3]. Although immune checkpoint inhibitors could be seen as prospective systemic agents, clinical data are still lacking and the level of objective response is not high [4]. Second-line taxane-based chemotherapy and radiation therapy also have low efficacy in patients with invasive UTUC [1, 4, 5].
Another crucial point is that nearly 50% of patients with UTUC present with chronic kidney disease at diagnosis, with an approximate 20% decline in glomerular filtration rate (GFR) postoperatively. This means that these patients are probably not candidates for platinum-based chemotherapy after kidney removal [6, 7]. In view of this information, we chose to use neoadjuvant chemotherapy, which potentially decreases the risk of systemic side effects caused by kidney insufficiency and is better tolerated by patients [8, 9]. However, a question that remains is, “why should we remove the kidney if there is a good treatment response to systemic agents and we can technically remove only the affected upper tract segment?”
Although currently available information suggests that RNU is the best treatment option for high-risk/invasive UTUC, the role of organ-sparing management remains unclear. Endoscopic treatment is feasible for patients with low-risk non-invasive UTUC, but this can be used only within imperative settings in high-risk cases [10, 11]. Partial ureterectomy can be successfully used for invasive low-ureter cancer when kidney function is preserved [12]. Surgeons often consider a higher tumour location as clinically complex and not feasible for the organ-sparing approach. Another potential diagnostic feature that could be used to switch between surgical options is tumour size, which could be a potential predictor of muscle-invasion [13]. Nevertheless, some retrospective studies suggest that the response to such treatment often depends on tumour aggressiveness and the efficacy of systemic therapy, but not the surgery type [14, 15]. Another potential benefit of such a curative option is preservation of kidney function.
The primary goal of this study was to evaluate the objective response level to neoadjuvant platinum-based chemotherapy and tumour complexity reduction in patients with invasive UTUC. The secondary goal of the study was to estimate the functional and oncological outcomes of the combined organ-sparing approach compared to RNU alone in patients with invasive UTUC.
MATERIAL AND METHODS
Study design
A prospective, non-randomised cohort study was conducted by the Research Department of Plastic and Reconstructive Oncology at the National Cancer Institute of Ukraine, and aimed to evaluate UTUC complexity reduction using neoadjuvant chemotherapy, and to compare the combined organ-sparing approach to RNU alone. The study was approved by the Institutional Review Board and local ethics committee, and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. A special informed consent form was developed by the study team and signed by the participants along with the National Health Care System form.
Patients were allocated to one of the two cohorts depending on GFR of the affected kidney. In cases where GFR was preserved, neoadjuvant chemotherapy with an organ-sparing approach was used; all other cases proceeded directly to RNU.
Patients
We included patients with muscular invasive or locally advanced UTUC (T2–T3) and no radiographic evidence of regional lymph node involvement or distant metastases. Urothelial cancer histology was confirmed endoscopically prior to patient inclusion in the study. The investigation was performed in only those patients with urothelial cancer belonging to the high-grade prognostic group. The presence of muscle/local tissue invasion was estimated using contrast-enhanced computed tomography (CT) and multiparametric magnetic resonance imaging (MRI). Clinical staging between T2 and T3 was done using imaging results.
Patients with chronic kidney disease, an expected eGFR of <45 mL/min after surgery, coexisting malignant disease, concomitant bladder cancer, history of bladder cancer, multifocal ureteral lesions or prior use of any other systemic therapies were excluded. Kidney function was measured using renal scintigraphy to determine the filtration rate of each unit. The main selection criteria used to include patients in the combined organ-sparing approach group were affected kidney GFR >15 mL/min. These cases were thought to have functional benefits from preoperative treatment and further kidney preservation.
Treatment
After informed consent form (ICF) assignment, patients were divided into two cohorts depending on the affected kidney GFR. The RNU cohort underwent surgical treatment within 3 weeks after ICF assignment. The surgical approach could have been performed using either the open method or laparoscopically, depending on the patient’s and surgeon’s preferences. All RNU procedures included bladder cuff excision according to guidelines. Patients in this group received only a single intravesical dose of doxorubicin 50 mg instillation within 72 hours postoperatively. Subsequently, no chemotherapy was administered, and the patient was moved to the follow-up phase.
The combined organ-sparing management group primarily received four cycles of neoadjuvant gemcitabine-cisplatin chemotherapy regimen (cisplatin 70 mg/m2 on the 2nd day and gemcitabine 1000 mg/m2 on the 1st and 8th day) with an interval of 2 weeks between the cycles. Two weeks after the completion of chemotherapy, the patient underwent surgery. It was intended to remove the affected upper urinary tract segment with further reconstruction. In cases of ureteral lesions and tumours of the pelvis, where there was a possibility of restoring the urinary tract with self-tissues - neocystostomy, end-to-end anastomosis or Andersen-Hynes plasty was used. Intestinal plasty of the ureter was performed when self-tissue upper urinary tract reconstruction was impossible. When a calyx tumour was present, partial nephrectomy was performed to remove the lesion and the kidney parenchyma above it. Patients in this group also received a single intravesical doxorubicin 50 mg instillation within 72 hours after surgery. Subsequently, the patient was moved to the follow-up phase.
Surgical procedures were performed by five surgeons that perform no less than 20 nephrouretectomies per year.
Follow-up
A follow-up examination was performed every 3 months until disease progression or death. After 2 years of follow-up, frequency of CT examination could be reduced depending on surgeon’s choice. Follow-up included regular laboratory assessments, contrast-enhanced computed tomography, white-light cystoscopy, and urinary cytology. Ureteroscopy was used in cases where upper tract recurrence was suspected on CT or in cases of positive upper tract cytology. Any type of urinary tract relapse or regional or distant metastases were assessed as progressive disease. When upper tract recurrence was observed, radical nephrectomy was performed. Bladder recurrence was treated according to bladder cancer guidelines. Patients with regional and distant metastases were discussed on a multidisciplinary board, targeting further treatment strategies.
Outcomes
Tumour complexity reduction was evaluated using CT according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. and T-stage decrease. Primary local tissue invasion was assessed using CT and MRI. The main criteria were T-stage, size decrease, and differences in positive cytology rates. Complications were estimated using the Clavien-Dindo scoring system, intraoperative blood loss, surgery duration, and duration of postoperative stay. Performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) grading. Kidney function was assessed using dynamic nephroscintigraphy.
Statistical analysis
Statistical significance was determined using the SPSS software version 24.0 (IBM United States Software Announcement 216-071 March 15, 2016). Demographic and clinical parameters of both groups were compared using the t-test (within the normal distribution), Mann–Whitney U test (for non-parametric values), and Chi-squared test (with non-verification correction). Survival was assessed using the Kaplan-Meier estimator with the log-rank test. We estimated recurrence-free survival, as long-term overall and cancer-specific survival were not achieved during the study.
RESULTS
A total of 64 patients (32 in each cohort) with invasive UTUC were included in the study between October 2016 and January 2021. Clinical and demographic parameters are shown in Table 1. Both groups were comparable in terms of age, sex, T stage, maximal tumour size, total eGFR, ECOG performance status, BMI, and haemoglobin level. Positive high-grade cytology was present in 25 (78%) patients in the combined organ-sparing management group and in 23 (72%) patients in the RNU group. Lesion located in renal pelvis in 8 (25%) patients in the combined treatment group and in 12 (38%) of the RNU alone group. Ureteral location was found in 24 (75%) and 20 (62%) cases respectively. Multifocality was an exclusion criteria, patients which had suspicion for multiple lesion were not included in the study.
Table 1.
Clinical and demographic parameters of the study groups
| Indicator | Statistical parameters | Combined organ-sparing management, n = 32 | Radical nephroureterectomy, n = 32 | p-value |
|---|---|---|---|---|
| Age, years | Me, IQR [25%; 75%] |
69 [58–77] |
70 [57–76] |
Mann-Whitney U test; р = 0.088 |
| Sex Male Female |
n (%) n (%) |
19 (59) 13 (41) |
22 (68) 10 (32) |
χ2 = 2.49 р = 0.57 |
| T-stage 2 3 |
n (%) n (%) |
22 (68) 10 (32) |
20 (63) 12 (37) |
χ2 = 1.89; р = 0.51 |
| Pelvicalyceal location | n (%) | 8 (25) | 12 (38) | χ2 = 1.16; р = 0.28 |
| Ureteral location | n (%) | 24 (75) | 20 (62) | |
| Maximal tumor size, mm | Me, IQR [25%; 75%] |
41.7 [25.2–56.4] |
49.4 [30.8–59.2] |
Mann-Whitney U test; р = 0.39 |
| Total eGFR, mL/min | M ±SD | 66.1 ±9.5 | 62.3 ±8.9 | Mann-Whitney U test; р = 0.72 |
| ECOG | Me, IQR [25%; 75%] |
0 [0–1] | 0 [0–1] | Mann-Whitney U test; р = 0.14 |
| Body mass index | Me, IQR [25%; 75%] |
29.4 [25.0–33.7] |
28.9 [26.6–31.6] |
Mann-Whitney U test; р = 0.45 |
| Hemoglobin, g/L | Me, IQR [25%; 75%] |
122 [106–132] |
114 [101–133] |
Mann-Whitney U test; р = 0.09 |
Me – median; M – mean; IQR – interquartile range; SD – standard deviation; CI – confidence interval, ECOG – Eastern Cooperative Oncology Group score; eGFR – estimated glomerular filtration rate; n – number of patients
All 32 patients in the combined treatment group underwent four cycles of neoadjuvant chemotherapy. Dose reduction to 70% owing to toxicity was performed in only five cases. Chemotherapy was well tolerated by the patients, as only six (15%) patients experienced grade 3 adverse events at the end of the cycle, and these patients fully recovered before the next cycle.
After four cycles of chemotherapy, there were no cases of progressive disease; however, there were 16 (50%) cases of stable disease, 12 (38%) cases of partial response, and 4 (12%) cases of complete response. The average maximal tumour size decreased by 2.3 cm. The T-stage decreased from T3 to T2 according to MRI in 8 cases (25%). Negative cytology results after chemotherapy were observed in 17 patients (53%).
Chemotherapy did not affect the surgical complication rate according to the Clavien-Dindo classification. Intraoperative and postoperative complication data are shown in Tables 2 and 3, respectively. The safety profile of the groups did not differ.
Table 2.
Intraoperative complications in the investigation groups
| Complication | Combined organ-sparing management group | Radical nephroureterectomy group | p-value |
|---|---|---|---|
| Blood loss over 1000 mL, n (%) | 1 (3) | 1 (7) | χ2 = 0.53; р = 0.47 |
| Blood loss over 500 mL, n (%) | 4 (29) | 2 (12) | χ2 = 1.26; р = 0.28 |
| Near lying organ trauma, n (%) | 0 | 1 | χ2 = 0.19; р = 0.61 |
| Average blood loss [mL], M ±SD (95% CI) |
250 ±96 (161–339) |
278 ±160 (196–352) |
Mann-Whitney U-test; р = 0.70 |
| Surgical duration [min], M ±SD (95% CI) |
132 ±18 (86–119) |
141 ±31 (87–135) |
Mann-Whitney U-test; р = 0.78 |
| Postoperative stay [days], M ±SD (95% CI) |
6.5 ±1.7 (4.6–7.7) |
5.9 ±2.0 (3.7–6.7) |
Mann-Whitney U-test; р = 0,69 |
n – number of patients; M – mean; SD – standard deviation; CI – confidence interval
Table 3.
Postoperative complication rates in the investigational groups
| Postoperative complications | Combined organ-sparing management | Radical nephroureterectomy | Statistical evaluation |
|---|---|---|---|
| Urine fistula, n (%) | 2 (2.6) | 1 (1.3) | χ2 = 0.34 р = 0.099 |
| Inflammation, n (%) | 4 (2.6) | 2 (2.6) | |
| Postoperative wound suppuration, n (%) | 1 (1.3) | 2 (1.3) | |
| Total, n (%) | 7 (21) | 5 (16) |
n – number of patients
In the combined treatment group, 4 (12%) patients underwent partial nephrectomy, 8 (25%) – pelvis or upper ureter resection with Andersen-Hynes plasty, 12 (38%) – low ureter resection with neocystostomy and 8 (25%) – ureteral resection with intestinal plasty of the ureter.
Pathological reports confirmed pT2 in 19 (59%) and pT3 in 13 (41%) patients in the RNU group. In the combined treatment group, 25% (8/32) of patients had pathological response with <pT2 disease on the final pathology among which4 (12%) patients experienced complete pathological response (pT0). Among the remaining patients, pathological reports confirmed pT2 in 17 (53%) and pT3 in 7 (22%) cases.
The kidney function data of the study groups are shown in Table 4. Prior to surgical treatment, the total GFR according to scintigraphy did not statistically differ between both groups (p = 0.13); however, 3 months after surgery patients who underwent the organ-sparing approach had a better total GFR (p = 0.0039), which was probably due to the preserved kidney function (18.9 ±5.1 mL/min).
Table 4.
Kidney function in the groups of comparison
| Indicator | Combined organ-sparing approach | Radical nephroureterectomy |
|---|---|---|
| Prior to surgery | ||
| Total GFR, 3/min | 66.1 ±9.5 | 62.3 ±8.9 |
| GFR of the affected kidney, mL/min | 16.4 +5.1 | 4.9 +2.2 |
| 3 months after surgery | ||
| Total GFR, mL/min | 69 +5.1 | 52 +9.8 |
| GFR of the affected kidney, mL/min | 18.9 +5.1 | – |
GFR – glomerular filtration rate
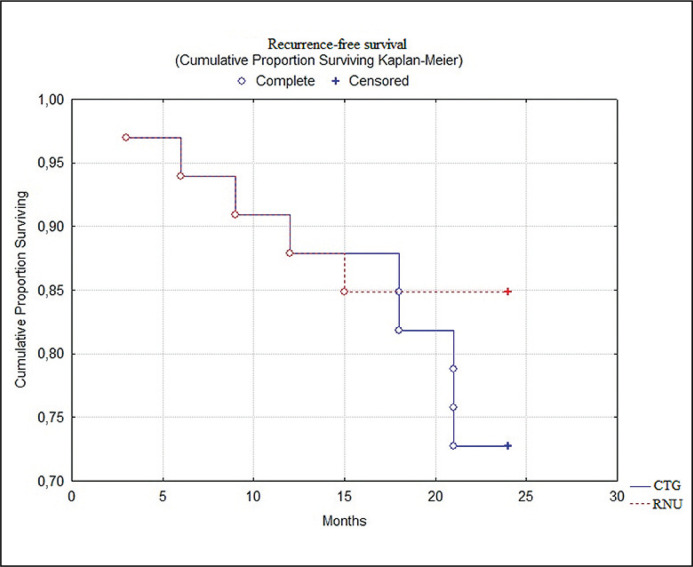
Median follow-up on the data-cut-off was 32.2 months. Furthermore, we analysed the 2-year recurrence-free cumulative survival as the main marker of effective local control. As shown in Figure 1, better recurrence-free survival was observed in the organ-sparing management group (84% vs 72%, log-rank test; p = 0.03). Bladder recurrence was seen in 3 (9%) and 5 (16%) patients of the combined and RNU alone groups. Data about progression and urinary tract reccurences is shown in Table 5.
Figure 1.
Comparison of recurrence-free survival between groups.
CTG – combined treatment group; RNU – radical nephroureterectomy
Table 5.
Progression data at 2-years of follow-up in the study groups
| Site of reccurence / progression | Combined organ-sparing management | Radical nephroureterectomy | Statistical evaluation |
|---|---|---|---|
| Upper tract reccurence | 3 (9%) | – | χ2 = 1.65; р = 0.79 |
| Bladder reccurence | 3 (9%) | 5 (15%) | |
| Nodal metastases | 4 (12%) | 5 (15%) | |
| Distant metastases | 3 (9%) | 4 (12%) | |
| UTUC-associated death | 3 (9%) | 4 (12%) |
UTUC – upper tract urothelial cancer
DISCUSSION
Urothelial cancer is a complicated oncological pathology with a high progression rate and low incidence [1]. The treatment strategy for localised disease according to current guidelines is limited to endoscopic surgery for low-grade tumours and RNU for high-grade tumours [1]. Systemic treatment is mainly used before surgery for invasive upper tract urinary cancer to reduce tumour burden and influence micrometastases. Postsurgical chemotherapy is often limited because of the low general kidney filtration rate [4]. In these cases, checkpoint inhibitors could be used, although the response rate to such systemic treatment remains low compared to chemotherapy. Therefore, neoadjuvant systemic therapy with radical nephroureterectomy is the best treatment option for high-risk upper tract urinary cancer. Patients who experience disease progression are more likely to receive checkpoint inhibitors, which is mainly due to the limited efficacy of chemotherapy regimens and low kidney function.
One of the key problems in invasive UTUC treatment is poor kidney function [6, 7]. According to the data, up to 50% of patients have a reduced general filtration rate prior to surgery. This means that by performing kidney removal, we proceeded with further renal function deterioration. According to the guidelines, endoscopic treatment can only be applied to low-risk cases. Patients with high-risk UTUC often experience recurrence and require further nephroureterectomy [10]. Partial ureterectomy has been suggested as an effective alternative for surgical management of patients with invasive ureteral tumours. The survival of these patients was equal to that of those who underwent radical nephroureterectomy, although the general kidney filtration rate was statistically better in the organ-sparing management group. This study’s findings suggest that prognosis of such patients depends mainly on tumour biology rather than the treatment type. The main limitations of such treatment are higher surgical complexity and longer recovery periods [16].
Currently, partial nephrectomy is rarely used for the treatment of UTUC. Investigations have analysed the efficacy of organ-sparing surgery for pelvic and caliceal kidney tumours. These groups were mainly limited to single centres and imperative indications. Although the recurrence rates were high, the function of the affected kidney was preserved in all patients.
In our study, we aimed to evaluate a combined organ-sparing approach for invasive upper tract tumours. The idea of using neoadjuvant chemotherapy is mainly driven by the following:
application of a nephrotoxic agent when the patient has two functional kidney units to decrease toxicity
susceptibility to chemotherapy may indicate cancer aggressiveness and further surgical strategy
decreased tumour surgical complexity can influence the possibility of conducting organ-sparing surgery
The data generated in this study show promising results. Under the influence of chemotherapy, 50% of patients responded to treatment and experienced regression. The other important parameter was a 25% decrease in positive cytology rates. The benefit of such treatment is significant as it permits organ-sparing surgery. As chemotherapy was well tolerated, all patients in the experimental group received all four planned cycles of therapy. This means that by applying preoperative chemotherapy, our intervention did not only affect micrometastatic disease but also reduced local surgical complexity, which could be achieved in a relatively high number of cases.
Systemic treatment administered preoperatively did not affect the complication rates, as the rates were equal in both groups. Chemotherapy in these cases decreased the primary tumour size and did not negatively influence the occurrence of complications. The safety profile of this approach did not differ significantly from that of RNU. Nevertheless, preservation of the affected kidney unit positively influences the general kidney filtration rate. The survival curves, which were better in the organ-sparing management group, could have been biased by the use of chemotherapy in one of the groups.
The combined organ-sparing strategy proved to be effective and not inferior to the standard treatment. We believe that patients with impaired kidney function will not benefit from systemic treatment. In cases where the function of the kidney affected is preserved, it is important to commence systemic treatment, which could provide further information for optimal surgical strategy treatment. In responders, organ-sparing management could be advised in selected patients, where the affected segment could be safely removed.
The main limitations of the study were small number of patients in cohorts, short observation period and presence of locally advanced cases in both groups that could strongly impact prognosis. Nevertheless, the study primarily evaluated chemotherapy efficacy, possibility to conduct organ-sparing surgery and short-term functional and oncological results. Further investigation is needed to determine long-term oncological outcomes.
CONCLUSIONS
Neoadjuvant systemic therapy reduces the complexity of surgical interventions for invasive UTUC without influencing its safety profile. The gemcitabine/cisplatin regimen leads to high regression rates in patients with invasive UTUC, and this could lead to the use of the organ-sparing approach in selected cases. Preservation of kidney function remains a key parameter that could increase the effectiveness of systemic treatment.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Investigation was funded by National Health Care System of Ukraine as a research project of National Cancer Institute of Ukraine.
CONTRIBUTION
M Pikul: protocol/project development, manuscript writing/editing, data analysis, data collection or management; E Stakhovskiy: protocol/project development.
FUNDING
This work was supported by the National Cancer Institute of Ukraine as part of the National Health Care System Scientific Program.
References
- 1.Rouprêt M, Babjuk M, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol. 2021; 79: 62-79. [DOI] [PubMed] [Google Scholar]
- 2.Ruvolo CC, Nocera L, Stolzenbach LF, et al. Incidence and Survival Rates of Contemporary Patients with Invasive Upper Tract Urothelial Carcinoma. Eur Urol Oncol. 2020; 4: 792-801. [DOI] [PubMed] [Google Scholar]
- 3.Peyronnet B, Seisen T, Dominguez-Escrig JL, et al. Oncological Outcomes of Laparoscopic Nephroureterectomy Versus Open Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An European Association of Urology Guidelines Systematic Review. Eur Urol Focus. 2019; 5: 205-223. [DOI] [PubMed] [Google Scholar]
- 4.Gust KM, Resch I, D'Andrea D, Shariat SF. Update on systemic treatment of upper tract urothelial carcinoma: a narrative review of the literature. Transl Androl Urol. 2021; 10: 4051-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YC, Chang YH, Chiu KH, Shindel AW, Lai CH. Adjuvant radiotherapy for locally advanced upper tract urothelial carcinoma. Sci Rep. 2016; 6: 38175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006; 7: 735-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellenthal NJ, Shariat SF, Margulis V, et al. Adjuvant chemotherapy for high-risk upper tract urothelial carcinoma: results from the upper tract urothelial carcinoma collaboration. J Urol. 2009; 182: 900-906. [DOI] [PubMed] [Google Scholar]
- 8.Porten S, Siefker-Radtke AO, Xiao L, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014; 120: 1794-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosogoe S, Hatakeyama S, Kusaka A, et al. Platinum-based Neoadjuvant Chemotherapy Improves Oncological Outcomes in Patients with Locally Advanced Upper Tract Urothelial Carcinoma. Eur Urol Focus. 2018; 4: 946-953. [DOI] [PubMed] [Google Scholar]
- 10.Vemana G, Kim EH, Bhayani SB, Vetter JM, Strope SA. Survival Comparison Between Endoscopic and Surgical Management for Patients With Upper Tract Urothelial Cancer: A Matched Propensity Score Analysis Using Surveillance, Epidemiology and End Results-Medicare Data. Urology. 2016; 95: 115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seisen T, Peyronnet B, Dominguez-Escrig J, et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur Urol. 2016; 70: 1052-1068. [DOI] [PubMed] [Google Scholar]
- 12.Colin P, Ouzzane A, Pignot G, et al. Comparison of oncological outcomes after segmental ureterectomy or radical nephroureterectomy in urothelial carcinomas of the upper urinary tract: results from a large French multicentre study. BJU Int. 2012; 110: 1134-1141. [DOI] [PubMed] [Google Scholar]
- 13.Ruvolo CC, Nocera L, Stolzenbach LF, et al. Tumor Size Predicts Muscle-invasive and Non-organ-confined Disease in Upper Tract Urothelial Carcinoma at Radical Nephroureterectomy. Eur Urol Focus. 2022; 8: 498-505. [DOI] [PubMed] [Google Scholar]
- 14.Ou YC, Hu CY, Cheng HL, Yang WH. Long-term outcomes of total ureterectomy with ileal-ureteral substitution treatment for ureteral cancer: a single-center experience. BMC Urol. 2018; 18: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lughezzani G, Jeldres C, Isbarn H, et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: A population-based study of 2299 patients. Euro J Cancer. 2009; 45: 3291-3297. [DOI] [PubMed] [Google Scholar]
- 16.Kim T H, Lee CU, Kang M, et al. Comparison of oncologic and functional outcomes between radical nephroureterectomy and segmental ureterectomy for upper urinary tract urothelial carcinoma. Sci Rep. 2021; 11: 7828. [DOI] [PMC free article] [PubMed] [Google Scholar]