Abstract
The study was designed to examine microglia morphology in early and late forms of multiple sclerosis (MS). Archival paraffin embedded tissue samples from 25 cases were examined immunohistochemically. Pío del Río Hortega reported that phagocytes in acute focal destructive CNS lesions develop from microglia with no early contribution from infiltrating monocytes. In this study, we were unable to identify the changes cited by del Río Hortega in support of his theory. Instead, myelin phagocytes in MS appear to originate chiefly from infiltrating monocytes. In 4 cases, walls composed of MHC class II antigen-positive “wall microglia” were observed at plaque margins separating demyelinated and bordering myelinated tissue. Wall microglia in 2 plaques were accompanied by AQP4-positive fiber-forming astrocytes. In chronic but not early disease MS cases, microglia were seen to interact with infiltrating monocytes to form microglial nodules of several types. Also, MHC II-positive “activated” microglia in bordering intact tissue were exceptionally prominent where there was little evidence of ongoing myelin loss. It is concluded that myelin phagocytes in MS derive entirely from infiltrating MRP14-positive monocytes and not from resident microglia and that Río Hortega’s microglia play an anti-inflammatory role in MS and not the destructive role favored by the current literature.
Keywords: Microglia nodules, Monocyte encephalopathy, Phagocytes, Río Hortega, Secondary progressive multiple sclerosis, Wall microglia
INTRODUCTION
Microglia are small cells with unusually shaped thin branching processes that are present in large numbers throughout gray and white matter of the healthy brain and spinal cord. They are hard to see in sections stained using routine stains but are stained selectively using silver impregnation techniques (1). They were discovered and named by the Spanish neuropathologist Pío del Río Hortega (2).
In a series of studies published between 1919 and 1932, del Río Hortega reported evidence that phagocytes swollen with cell debris appearing within days in and around acute focal destructive lesions are transformed microglia. The changes in size and shape and overall appearances accompanying this transformation were the same in the various natural and experimental traumatic, inflammatory ischemic and other conditions that he examined. He concluded, based on these observations, that the chief function of these phagocytes was to scavenge cell debris, an activity he considered beneficial to the host.
In the current literature, while there are differing opinions as to their role in disease pathogenesis, it is generally accepted that these phagocytes originate and transform as described by Río Hortega and that the morphological changes accompanying their transformation are similar in different diseases. The major change from Río Hortega’s original conception of microglia functioning solely as scavengers is the now dominant view that microglia-derived phagocytes, alone or together with hematogenous leucocytes, contribute to tissue breakdown as well as removal of damaged tissues (3–12).
There is an alternative view, not often mentioned in the current literature, that refers to phagocytes including foamy macrophages as originating chiefly or solely from infiltrating blood-borne monocytes (3, 13–15).
In multiple sclerosis (MS), the prevailing view based on traditional histopathological and immunopathological investigations as well as advanced techniques of single cell proteomic and transcriptomic profiling is that microglia act in this disease as resident immune effector cells, that myelin phagocytes originate in part from microglia, and that these phagocytes together with lymphocytes exhibit, in addition to scavenging, targeting, and destruction of glia and myelin (16–20).
We reported in a recent study of myeloid cells in developing MS lesions that the morphological subtypes described by Río Hortega accompanying the transformation of ramified microglia into foamy macrophages in focal inflammatory and other diseases of central nervous tissue were not readily identifiable (21). The same lesions, however, showed monocyte infiltration of a type discussed but rejected by Río Hortega as a source of lipid macrophages in most diseases of nervous tissue. We concluded that monocytes are an important source of myelin phagocytes and foamy macrophages in actively demyelinating MS lesions but we were uncertain as to the contribution of microglia to this population. The present study of the same group of patients examines in greater detail the role of microglia in disease progression and resolution in early and late MS.
MATERIALS AND METHODS
This is the third of a series of studies of the same group of patients designed to investigate the pathogenesis of myelin destruction in early and late forms of MS. The first reported evidence that astrocytes as well as myelin are destroyed in early lesions (22). The second showed that monocytes are an important source of phagocytes in MS (21).
Clinical material
The age, sex, and duration of the illness and the source of tissue in each of 25 cases are listed in Table 1. Identifying case numbers are the same as those used in the 2 previous studies. The study complies with the requirements of the Human Ethics Committee of the University of Sydney.
Table 1.
Acute, subacute, and chronic multiple sclerosis cases
| Case | Sex/age | Duration of illness/terminal illness | Neuropathologist | Institution |
|---|---|---|---|---|
| 1 | F 31 | 3 months/7 days | CWM Adams | Guy’s Hospital Medical School, London, UK |
| 2 | F 20 | 2 weeks | CWM Adams | Guy’s Hospital Medical School, London, UK |
| 3 | M 25 | 29 days | S Pogacar | Brown University Medical School, Providence, RI, USA |
| 4 | F 42 (40) | 2.5 months/2 weeks | RD Terry | Albert Einstein College of Medicine, The Bronx, NY, USA |
| 5 | M 14 | 18 days | J McLaughlin | Royal Free Hospital, Hampstead, London, UK |
| 6 | F 70 | 21 days | S Love | The National Hospital, Queen Square, London, UK |
| 7 | M 32 | 10 months/8 months | R Doshi | The Maudsley Institute of Psychiatry, London, UK |
| 8 | F 48 | 18 days | CG Harper | Royal Perth Hospital, Perth, WA, Australia |
| 9 | F 14 | 9 months/17 hours | GN Budzilovich | New York University Medical Center, NY, USA |
| 10 | M 36 | 3 years/not known | DM Boehme | VA Hospital, East Orange, NJ, USA |
| 11 | F 23 | 5 weeks | CJ Bruton | Runwell Hospital, Wickford, UK |
| 12 | F 23 | 30 days/60 hours | BA Kakulas | Royal Perth Hospital, Perth, WA, Australia |
| 13 | F 27 | 32 months | RO Barnard | Maida Vale Hospital For Nervous Diseases, London, UK |
| 14 | F 29 | 12 months | W Evans | Oliver Latham Laboratory, Macquarie Hospital North Ryde, NSW, Australia |
| 15 | F 27 | 49 months | TH Moss | Frenchay Hospital, Bristol, UK |
| 16 | M 34 | 3 years 11 months | DM Boehme | VA Hospital, East Orange, NJ, USA |
| 17 | M 43 | 13 years | JW Prineas | Dover General Hospital, NJ, USA |
| 18 | M 55 | “Long standing” | CJ Bruton | Runwell Hospital, Wickford, UK |
| 19 | F 39 | 14 years | JW Prineas | Concord Hospital, Sydney, Australia |
| 20 | F 30 | 11 years | CS Raine | Albert Einstein College of Medicine, The Bronx, NY, USA |
| 21 | M 60 | 20 years | Eun-Sook Cho | University Hospital, UMDNJ—New Jersey Medical School, Newark, NJ, USA |
| 22 | M 55 | 20 years | Dr Krumerman | Jersey Shore Medical Center, Neptune, NJ, USA |
| 23 | M47 | 7 months | TH Moss | Frenchay Hospital, Bristol, UK |
| 24 | M 30 | 14 years/10 months | G Palffy/L Leel-Ossy/WW Tourtellotte | University Medical School of Pecs, Hungary |
| 25 | F 37 | 3 weeks/6 days | H Heng Teoh | Auckland Hospital, Auckland, New Zealand |
Remyelinating lesions were present in the same section or elsewhere in Cases 1, 3, 4, 9, 10, 14, 18, and 24. Prominent concentric bands were present in Case 8. Neurogenic pulmonary edema occurred in Cases 9 and 10. Herniation occurred in Cases 3 and 8. Treatment: Cases 3, 7, 9, and 10 received corticosteroids; Case 3 received azathioprine: Cases 16 and 17 received cyclophosphamide. Case 24 received a subcutaneous injection of pig brain 8 months before death; treatments unknown for other 15 cases.
Immunohistochemistry
Paraffin sections 4- to 6-µm-thick were stained using hematoxylin and eosin (H&E), Luxol fast blue-periodic acid-Schiff (LFB-PAS) for myelin, and Bodian silver stain for axons. Frozen sections were prepared from mirror blocks of selected lesions and stained for neutral lipids using Oil red O and hematoxylin. Immunohistochemical staining was performed as described previously using primary antibodies listed here and in Table 2, biotinylated or polymer-bound horseradish peroxidase-labelled second antibodies (Vector ABC Elite Kit, Vector Laboratories, Burlingame, CA; EnVision+ and LSAB+ Kits, Dako Cytomation, Inc., Carpinteria, CA), and diaminobenzidine as chromogen.
Table 2.
Antibodies
| Antigen | Clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| CD45 | PD7-26,2B11 | 1:200 | Microwave/citrate | Dako, Carpinteria, CA |
| CD68 | PG/M1 | 1:50 | Pronase | Dako |
| MAC-1 | HAM56 | 1:1000 | Proteinase K | Dako |
| MHC-class ll | CR3/43 | 1:50 | Heat/high pH Dako | Dako |
| MRP14 | BMA-S36,48 | 1:25 | Microwave/citrate | Accurate Chemical, Westbury, NY |
| RCA-1 lectin | 1:4000 | Vector, Burlingame, CA | ||
| CD209 DC-SIGN | 1:100 | Heat/high pH Dako | R&D Systems, Minneapolis, MN | |
| IgG | 1:8000 | Proteinase K | Dako | |
| C9neo (MAC) | B7 | 1:20 | Proteinase K | BP Morgan, Cardiff University, Cardiff, UK |
| CD3 | 1:50 | Microwave/citrate | Dako | |
| CD4 | 1:10 | Microwave/citrate | Novocastra, Newcastle Upon Tyne, UK | |
| CD8 | C8/144B | 1:100 | Heat/high pH Dako | Dako |
| CD45RO | UCHL1 | 1:150 | Microwave/citrate | Novocastra |
| PCNA | PC10 | 1:80 | Dako | |
| CD16 | 2H7 | 1; 30 | Microwave/citrate | Novocastra |
| AQP4 | 1:500–1000 | Microwave/citrate | Millipore Corp, Billerica, MA |
Antigens and lectins examined included: Riccinus communis agglutinin 1 lectin ([RCA1], endothelial cells, microglia, macrophages), MRP-14 (activated monocyte marker), HAM56 (human alveolar macrophages, pan-macrophages), CD45 (myeloid cells including microglia), CD68 (macrophage lineage marker, lysosomes), CD209 (dendritic cells), CD16 (FcRγIII), MHC class II antigens, UCHL1 (CD45RO, activated and memory T cells), lymphocytes (CD3, CD4, CD8), IgG, complement proteins (C3d, monoclonal B7 anti-MAC 5b-9), and PCNA and Ki67 (proliferating cells).
Also examined were sections prepared during previous immunohistochemical studies of the same cases using antibodies recognizing MBP, MOG, CNPase (myelin, oligodendrocytes), HNK-1 (immature oligodendrocytes and type 2 astrocytes), activated caspase-3, and GFAP. The sources and other details of the antibodies are cited where relevant.
RESULTS
Río Hortega classified microglia as ramified, nonramified pseudopodic and amoeboid, and phagocytic (foamy macrophages). In the present study, microglial subtypes and phagocytes identified in developing MS lesions stained using the myeloid cell marker CD45 are divided into 4 groups—ramified, nonramified, phagocytic, and nonphagocytic (foamy macrophages). The immunohistochemical profiles of the various subtypes are summarized in Table 3.
Table 3.
Microglia and macrophages
| CD45 | RCA1 | MHC II | CD16 | IgG | CD68 | MRP14 | HAM56 | |
|---|---|---|---|---|---|---|---|---|
| Resident microglia | + | ± to + | o to ± | o | o | ± to + | o | o |
| Reactive microglia | + | ± to + | o to ± | ± | o | ± to + | o | o |
| Demyelinated tissue | ||||||||
| Corralling | ||||||||
| Reticulate | ||||||||
| Mitotic | ||||||||
| Perivascular microglia | ||||||||
| Activated microglia | + | * | + | ± | o | ± | o | o |
| Transitioning | ||||||||
| Phagosomes | ||||||||
| MHCll | ||||||||
| Perivascular microglia | ||||||||
| Wall microglia | + | ± | + | * | o to ± | * | o | ± |
| Blood-brain barrier | ||||||||
| Nodules of microglia | + | * | o to + | o to ± | o | o to + | o to + | * |
| C3d | ||||||||
| Microglia/monocyte | ||||||||
| Compacted | ||||||||
| Ensheathed monocytes | ||||||||
| Residual microglia | * | * | * | * | * | * | * | * |
| Phagocytic macrophages | + | o | + | * | + | + | + | + |
| Round and amoeboid | ||||||||
| Phagosomes | ||||||||
| Proliferation | ||||||||
| Spindle shaped | ||||||||
| Perivascular demyelination | ||||||||
| Foamy macrophages | + | + | + | o to + | o to ± | + | o to + | ± to + |
| Remyelination |
Resident and reactive: microglia with branching processes. Activated: nonramified MHC ll-positive microglia. Wall microglia: wall-forming microglia. Nodules: aggregates of microglia. Residual: parenchymal phagocytes with distinctive secondary lysosomes observed in chronic cases of multiple sclerosis. Probably microglia in origin. Currently no reliable immunohistochemical data available. Phagocytic: cells with Luxol Fast Blue (LFB)-positive particles of myelin. Foamy macrophages: LFB-negative cells distended with lipids.
+, positive; ±, variable; o, negative; *, not known.
Ramified microglia
Resident microglia
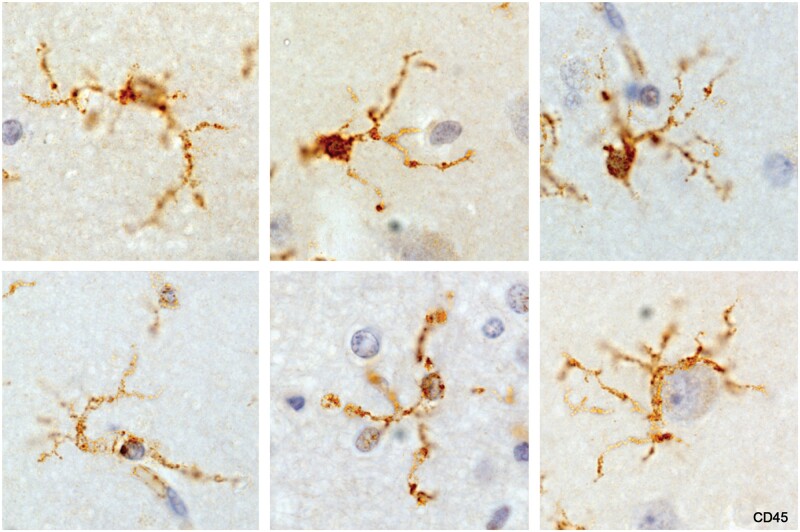
Microglia, referred to as resting or resident microglia, located in intact tissue remote from lesions, appeared as small cells with extremely thin branching processes as described by Río Hortega in normal animal and human tissues (Fig. 1). Also, characteristic was the presence of small granular irregularities along and at the tips of processes. The granules stained strongly for CD68, a lysosome marker.
Figure 1.
Resting microglia, normal cerebral cortex. Processes are extremely thin and branching (ramified). The small swellings were positive for the lysosomal marker CD68. Bottom right: A microglial cell corralling a neuron (Case 2, CD45, ×630).
Reactive microglia
Increased numbers of ramified microglia, some with enlarged cell bodies and thickened smooth branching processes, were observed in some newly forming plaques and in intact tissue distant from chronic plaques (Figs. 2–4). Increased numbers of ramified microglia around blood vessels were not seen, including around vessels with early upregulation of expression of GFAP.
Figure 2.
Reactive microglia, molecular layer of the cerebellum (Case 17, CD45, ×720).
Figure 3.
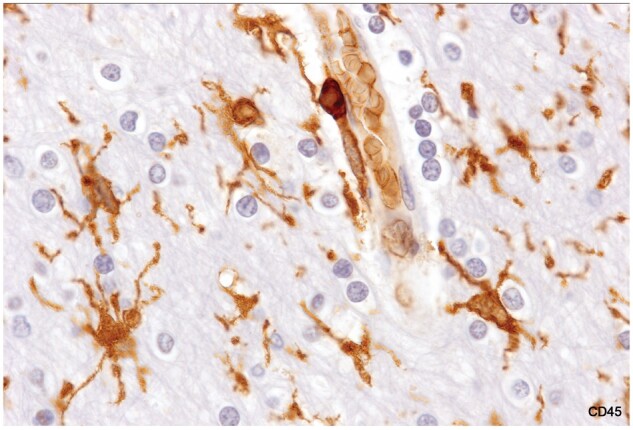
Reactive microglia, intact white matter. Cell bodies are enlarged, and processes are thickened. The myelinated tissue appears otherwise normal with normal-appearing oligodendrocytes. There is a monocyte in the wall of the blood vessel (Case 17, CD45, ×1020).
Figure 4.
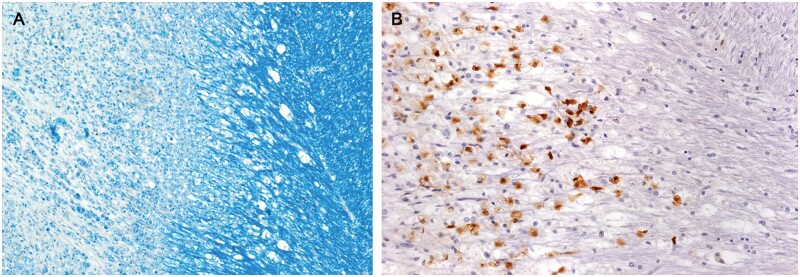
A still myelinated early plaque. (A, B) Myelin sheaths are intact but pale; oligodendrocytes may be reduced in number; ramified microglia are numerous, and there is a vessel with a cuff of what appear to be mostly monocytes (higher magnification in C) (Case 12, CD45, A, ×25, B, ×50, C, ×200).
Demyelinated tissue
Most chronic plaques showed varying usually small numbers of small, ramified microglia (Fig. 5). In demyelinated tissue with numerous lipid macrophages, it was often difficult to detect any ramified microglia located in spaces separating foamy macrophages.
Figure 5.
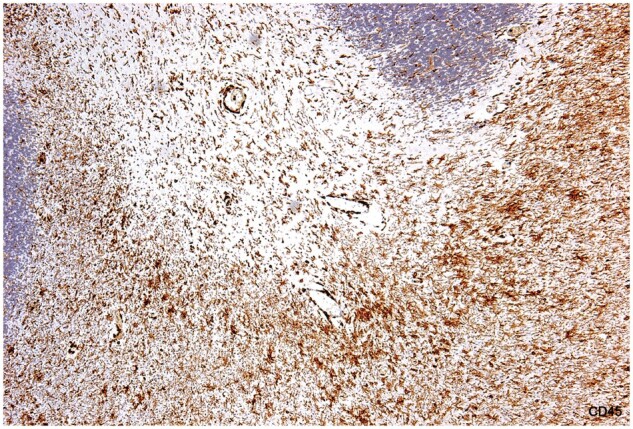
Chronic MS. An old, demyelinated plaque in the cerebellum (no lipid macrophages, no LFB-positive macrophages). Ramified microglia are present in demyelinated tissue, and are somewhat more numerous in bordering myelinated tissue where there are also microglial nodules. Monocytes were relatively common in the plaque and in bordering tissue (Case 17, CD45, ×80).
Corralling microglia
While it was common to see ramified microglia in normal cerebral cortex tissue contacting neurons, it was unusual to see corralling activity in normal white matter. An exception was Case 4 in which corralling by ramified microglia of unidentified cells was seen in tissue bordering an expanding basal ganglia plaque (21).
Reticulate microglia
In Case 16, elongated sometimes branching MHC II-positive microglia with spongy looking cytoplasm were seen ensheathing monocytes and other cells in cerebral cortex tissue (Fig. 6, Supplementary Data Fig. S1). In our previous study, the MHC II-positive walls of the cavities in which the monocytes were located were thought to be MHC II-positive capillary walls. Examination of further examples indicated that these profiles were in fact formed by these odd looking spongy ramified microglia ensheathing infiltrating monocytes.
Figure 6.
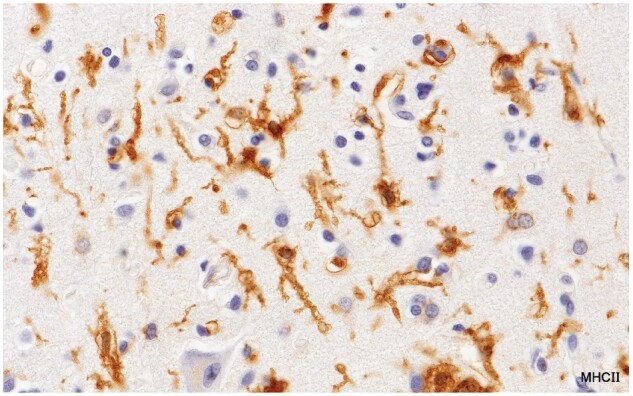
MHC II-positive reticulate ramified microglia located in an area in the cerebral cortex infiltrated by small numbers of monocytes (Case 16, MHC II, ×650).
Mitotic microglia
Microglia in mitosis, a relatively common finding in developing lesions in Case 7 (a patient thought to have MS but on review a variant of neuromyelitis optica (NMO) [21]), were not observed in any of the 24 MS cases. Macrophages with mitotic figures were observed in biopsy tissue from a patient with a symptomatic lesion of 6 days duration. Mitotic figures were also observed in rare gemistocytic astrocytes.
Nonramified microglia
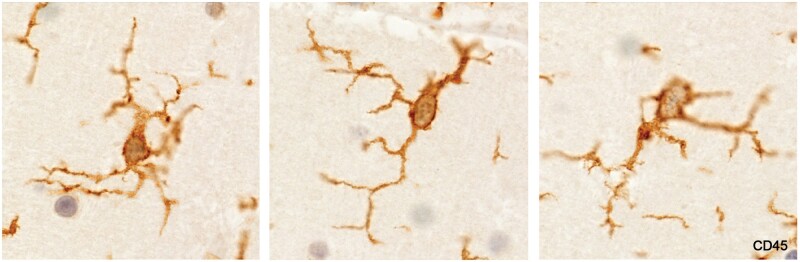
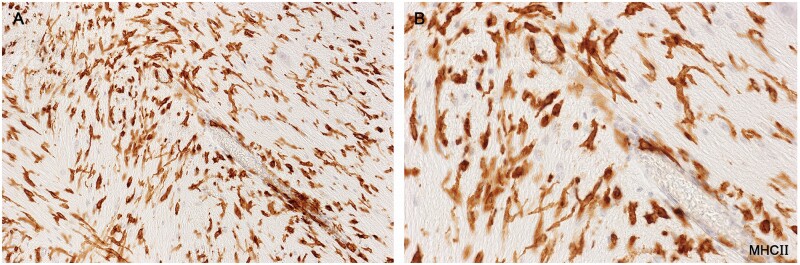
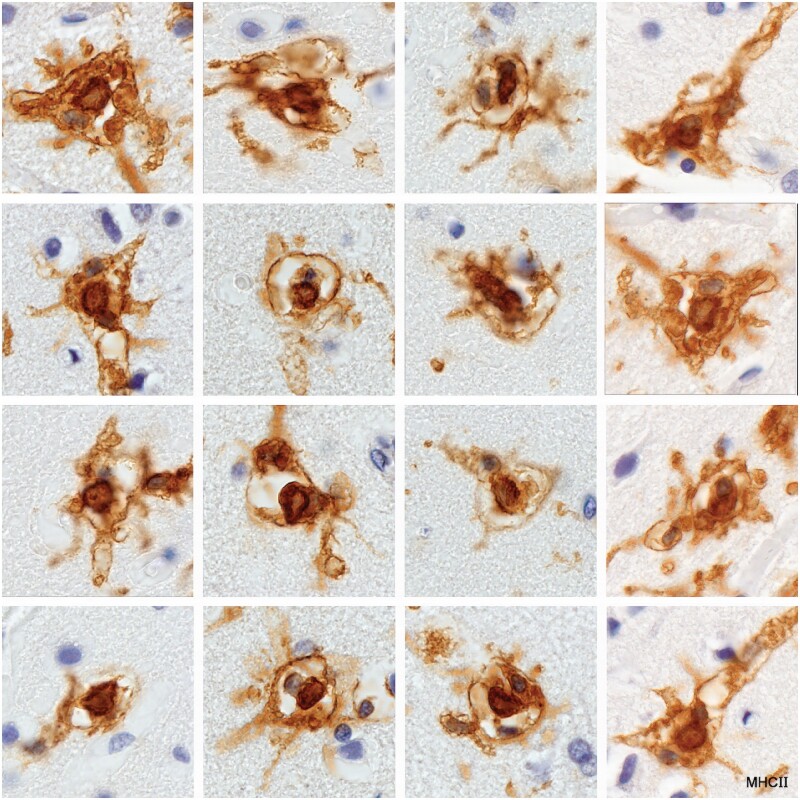
Activated microglia
Described variously as spiky or furry in appearance (Fig. 7; Supplementary Data Figs. S2 and S3), and with characteristic kidney shaped nuclei without prominent nucleoli, this microglia subtype, (commonly referred to as “activated”) stained positively for MHC class II antigens (Fig. 8). The cell stained positively for CD16, although lightly (Fig. 9), and negatively for the monocyte/macrophage marker MRP14. Importantly, they were seen only in intact white matter immediately bordering demyelinated plaques, never in demyelinated tissue or in remote unaffected tissue. This subtype was not observed in tissue bordering newly forming lesions in most cases of acute MS, or generally in cases of subacute MS.
Figure 7.
A largely prephagocytic early plaque capped by an area of myelin pallor (A, B), largely preserved oligodendrocytes, and activated microglia (C). Immediately below this zone is a band of vacuolated myelin sheaths and oligodendrocyte loss. It was difficult to identify any LFB-positive phagocytes in the lesion and there were no MRP14-positive cells except in blood vessels (Case 2, A, CD45, ×4.5, B, Luxol fast blue, ×30, C, CD45, ×400).
Figure 8.
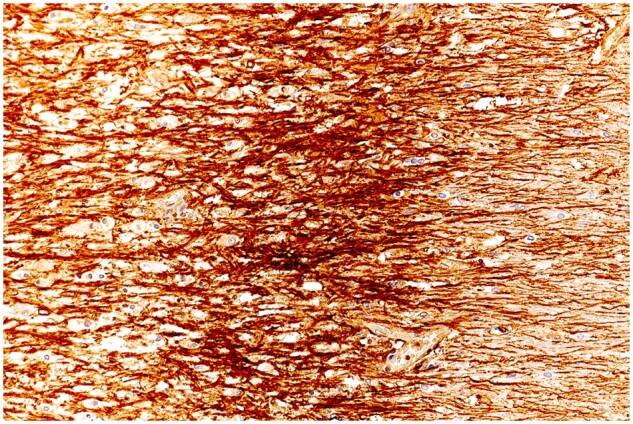
Activated microglia in tissue bordering a spinal cord plaque in a patient with clinical disease of 12 months duration (Case 14, MHC II, ×320).
Figure 9.
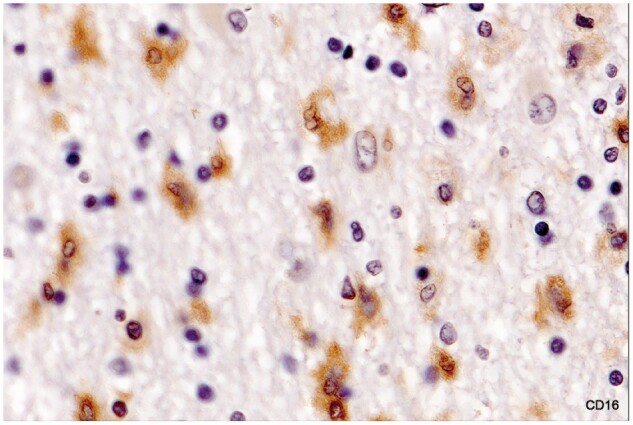
Activated microglia in intact white matter immediately bordering an inactive demyelinated plaque (no LFB-positive phagocytes). Numerous foamy microphages were present in demyelinated tissue at the lesion margin. Microglia stain positively for FcγRIII (CD16). The cytoplasm is so lightly stained, however, that the kidney-shaped cell nuclei remain visible (Case 16, CD16 digitally enhanced, ×650).
The origin of MHC II-positive activated microglia remained uncertain as no clear intermediate forms were identified at the point where they were replaced by normal-appearing ramified microglia some distance from the plaque margins.
Activated microglia with the characteristics just described appeared not to be actively phagocytic of myelin as lipid-containing phagosomes and phagosomes containing LFB-positive particles of myelin (both easily identifiable by light microscopy in perivascular space macrophages and phagocytes in other locations) were absent. CD68 immunoreactivity remained uncertain. MHC II immunoreactivity was not restricted to this microglia subtype. Rare, ramified microglia sometimes stained positively for MHC class II antigens as did otherwise unidentified flattened cells in the walls of some capillaries and small blood vessels in tissue bordering expanding early lesions.
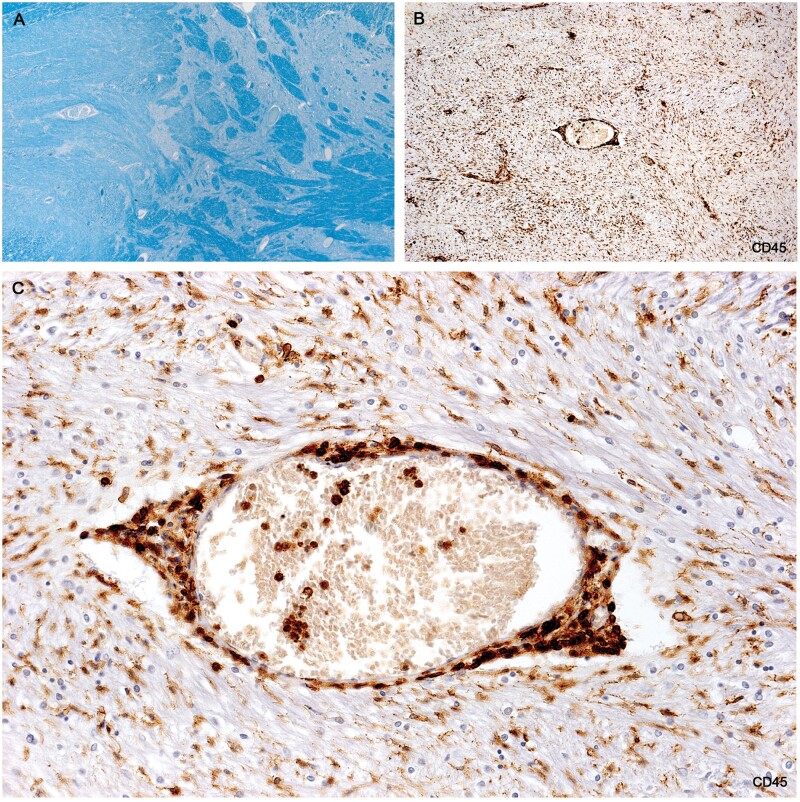
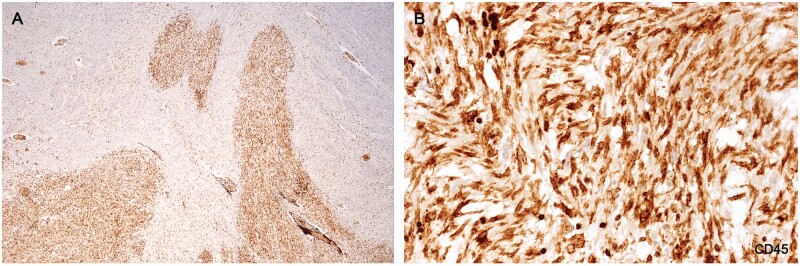
Wall microglia
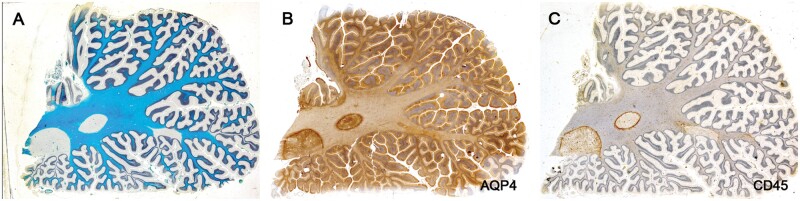
Plaques in Cases 2, 18, 23, and 24 showed at their margins, narrow bands of elongated nonramified CD45-positive, MHC class II-positive, weakly RCA1-positive cells separating demyelinated tissue and intact bordering myelinated tissue (Figs. 10–16; Supplementary Data Figs. S4 and S5). In Case 18, sections stained for AQP4 used in previous studies of this case, showed the walls in 2 such plaques to be packed with fiber-forming AQP4-positive astrocyte processes. Monocytes were identified in the vicinity of the walls, chiefly on the plaque side of the wall. The fact that AQP4-positive astrocytes were present in the walls was especially interesting as earlier studies of blood-brain barrier integrity in Case 18 showed leakage of serum proteins to be sharply restricted at the boundary between demyelinated tissue and intact bordering myelinated tissue (23).
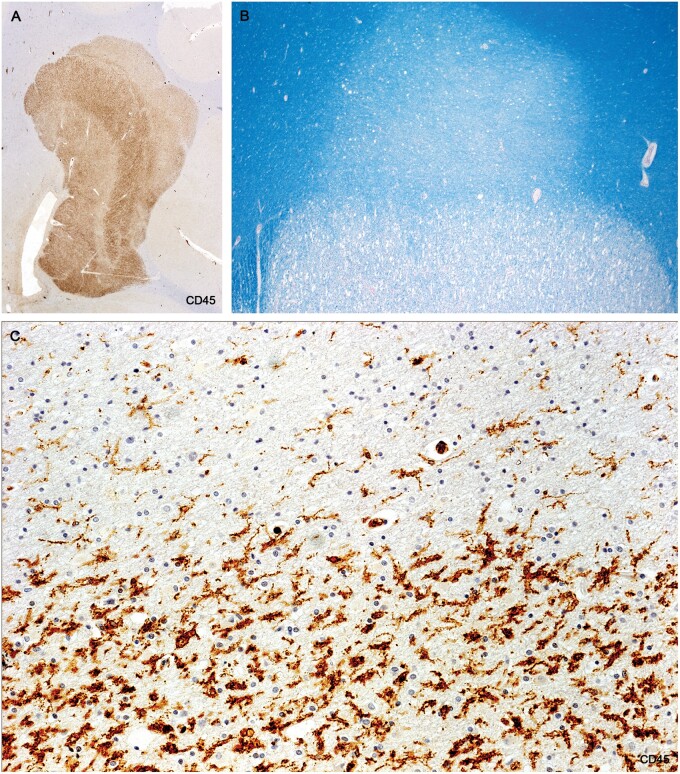
Figure 10.
Sections of the cerebellum in a patient with longstanding disease showing that 2 of the 4 plaques in the white matter have CD45-positive walls. Higher magnifications of the walled plaque at the left in (B) and (C) stained for CD45 and AQP4 are shown in Figures 11 and 12 (Case 18, A, LFB, B, AQP4, C, CD45. A–C, ×1.2).
Figure 11.
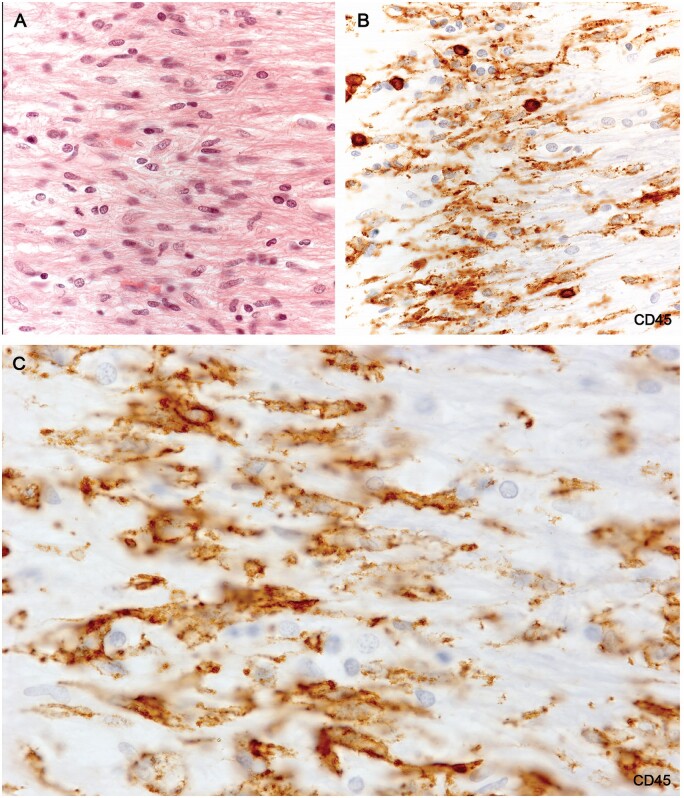
(A) H&E-stained section of the microglial wall of the large round plaque bottom left Figure 10A shows tightly packed cells with elongated nuclei. (B) The same myelinated margin reacted for CD45 showing aligned elongated wall microglia together with 6 cells with round shapes thought to be monocytes. (C) Higher magnification showing typical nonramified wall microglia with enhanced CD45 edge immunoreactivity (Case 18, A, H&E, ×400; B, CD45, ×400; C, CD45, ×1020).
Figure 12.
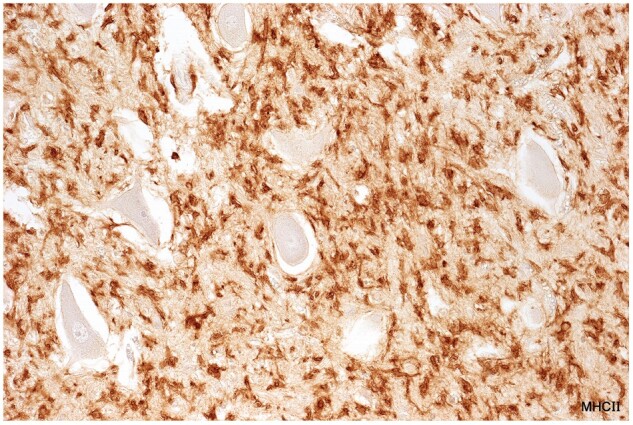
A microglial wall immunostained for AQP4 shows the wall packed with AQP4-positive process-bearing astrocytes (Case 18, AQP4, ×200).
Figure 13.
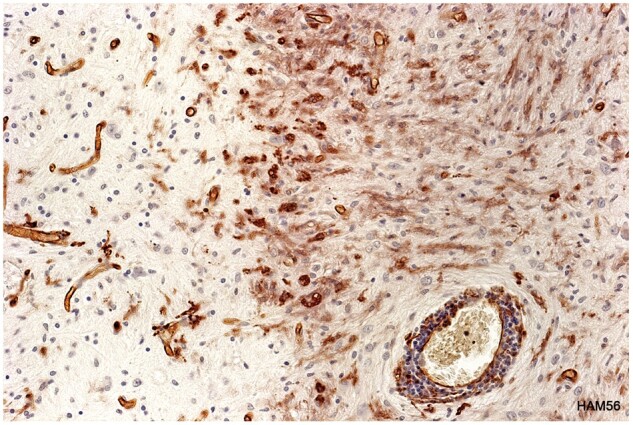
HAM56-positive macrophages, capillaries, and elongated HAM56-positive microglia combine to form a wall separating demyelinated and remyelinated tissue in a developing plaque. (Case 24, plaque 3, HAM56, ×320).
Figure 14.
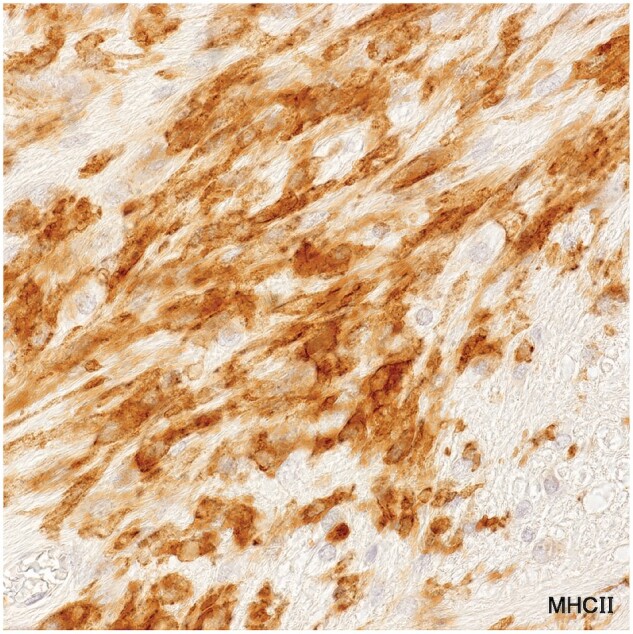
MHC II-positive wall microglia (Case 24, plaque 3, MHC II, ×650).
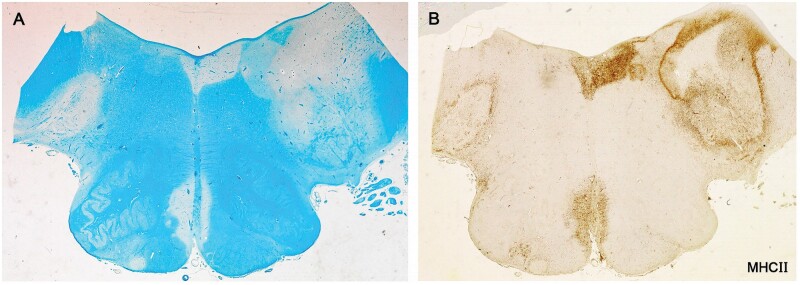
Figure 15.
Microglia walls. Of 7 plaques analyzed in the medulla. There were two with MHC II-positive microglial walls, one seen on the left (plaque 1) and the other the complex lesion on the right (plaque 3) (Case 24, A, LFB, B, MHC II, A, B, ×3.5).
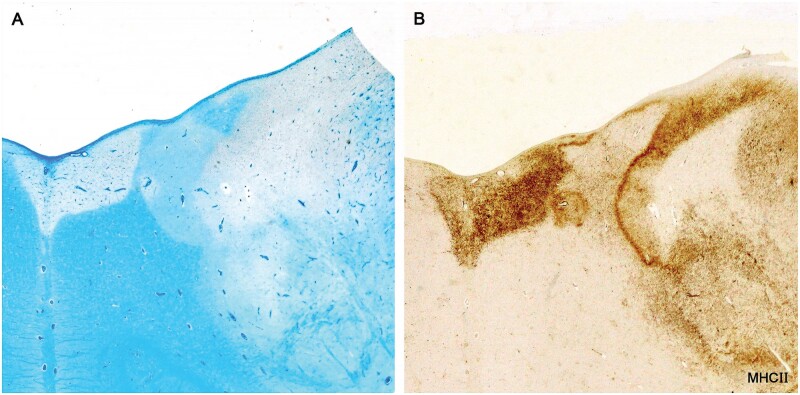
Figure 16.
Microglia walls and remyelinated shadow plaques. This example shows a wall of microglia and macrophages at the edge of a fresh incursion of myelin phagocytes into contiguous remyelinated tissue (Case 24, MHC II, A, B, ×7).
Regarding the origin of wall cells, in 2 of the cases, Cases 2 and 24, there were areas in the plaques packed with large numbers of thickened and elongated cells with a somewhat similar appearance (Figs. 17 and 18). It is likely that these correspond to cells referred to by Río Hortega as, “hordes or clusters of migrating globular, cuboidal, or flattened microglioblasts seen close to fields of branched microgliocytes in the brains of rabbits and kittens 1 to 5 days old” (3). In the present material, there was some evidence suggesting that such hordes may originate from blood-borne mononuclear cells (Fig. 19).
Figure 17.
(A) An early prephagocytic stage in the formation of a new plaque in a patient with exceptionally early multiple sclerosis. (B) The whole plaque consists of a mass of elongated cells resembling wall microglia. Monocytes and myelin phagocytes were present in small numbers mainly at the at the edge of the lesion (Case 2, plaque 1, CD45, A, ×25, B, ×200).
Figure 18.
MHC II-positive wall microglia (Case 24, plaque 3, MHC II, A ×200, B ×400).
Figure 19.
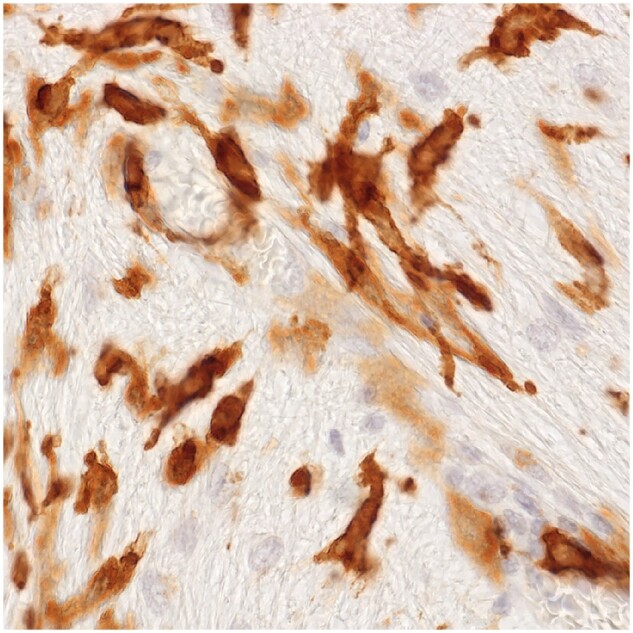
Wall microglia progenitors. A patch of tissue in a developing lesion in Case 24 crowded with elongated MHC II-positive cells resembling those seen in a newly forming lesion in a patient with a clinical disease duration of 2 weeks (Case 24, MHC II, ×860).
Microglial nodules
Small aggregates of microglia, sometimes together with monocytes, were observed in various locations in all cases of chronic MS and in some subacute cases but not in early cases of the disease.
Microglial nodules composed only of microglia
Nodules of elongated nonramified microglia clustered along short lengths of myelin sheaths that stained positively for activated complement (C3d) were especially common in otherwise intact myelinated tissue close to and at a distance from the edge of established plaques in Cases 16 and 17 (24). Importantly C3d-positive microglial nodules were observed only in myelinated tissue, never in demyelinated plaque tissue, a fact that argues against reports that C3d-positive microglial clusters in MS target axons rather than altered myelin.
The microglia stained negatively for IgG and complement membrane attack complex (C9neo) and there was no evidence of uptake (phagocytosis) of C3d-positive myelin. The fact that only short lengths of affected nerve fibers attracted such microglia activity suggests a possible relationship to the nodal pathology reported in MS (25).
Nodules of microglia together with monocytes
Nodules of microglia together with monocytes were observed, in small numbers in intact white matter bordering and remote from lesions in Cases 16–18 and 22.
Nodules of compacted microglia and unidentified cells and gray matter nodules
Dense knots of cells forming a distinctive type of MHC II-positive nodule located in demyelinated plaque tissue and in bordering intact myelinated tissue were common in Cases 16 and 17 (Supplementary Data Fig. S6).
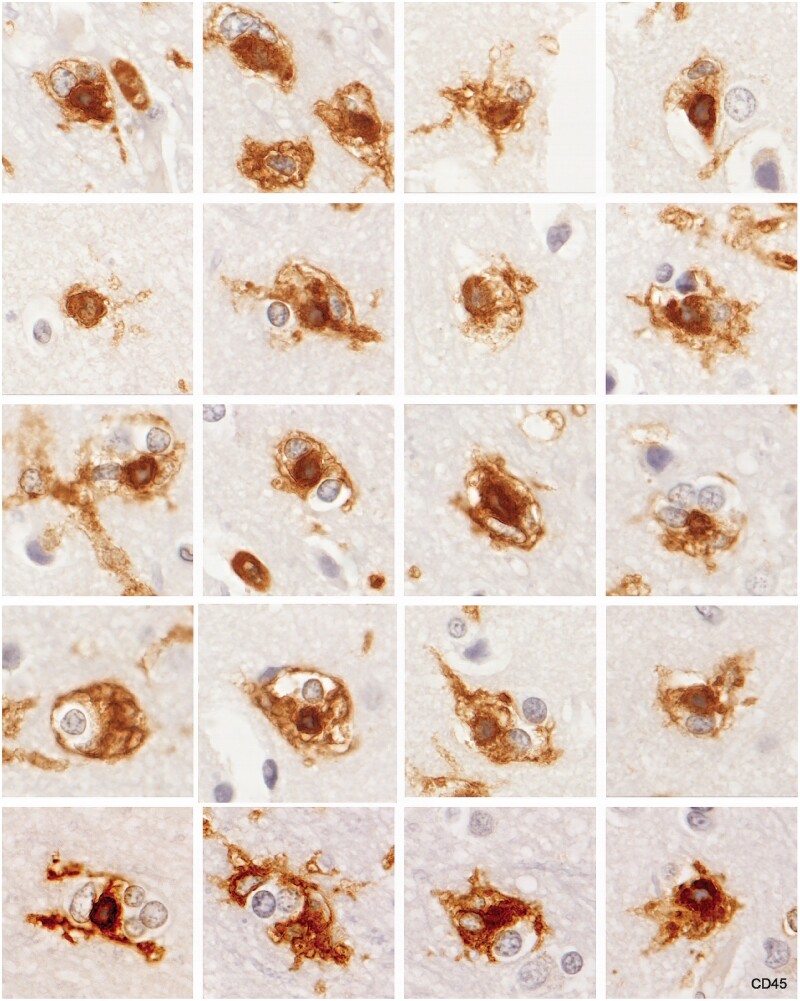
Grey matter nodules of monocytes ensheathed by reticulate microglia were observed only in one patient, Case 16, in the cerebral cortex (Figs. 20 and 21). These occurred in groups, sometimes in the vicinity of plaques located at the corticomedullary junction. The tissue looked otherwise normal except for the presence of monocyte capillary plugs (CD16-positive, MRP14-positive elongated monocytes located in narrow capillaries in intact grey matter seen in most of the early MS cases). In a few instances reactive/degenerative changes were observed in the cell bodies of neurons. We have termed this gray matter lesion “monocyte encephalopathy” (21). Whether it is peculiar to MS or occurs in other conditions is unknown.
Figure 20.
MHC II-positive reticulate microglia ensheathing MHC II-positive cells thought to be monocytes in the cerebral cortex (Case 16, MHC II, each image ×400).
Figure 21.
Grey matter microglia nodules in the cerebral cortex. Most nodules are composed of an encircling reticulate microglial cell, a CD45-positive cell that may be a monocyte, and a CD45-negative (nonmyeloid) cell (Case 16, CD45, each image ×400).
Spindle-shaped myelin phagocytes
Newly formed plaques in the cerebral cortex showed small spindle-shaped cells as well as slightly larger irregularly shaped cells that stained positively for neutral lipids. These may have been microglia in origin as monocytes were rare or absent in such lesions (Supplementary Data Fig. S7).
Residual microglia
Motile cells with distinctive secondary lysosomes (26) were seen entering perivascular spaces in old plaques where they were phagocytosed by perivascular space macrophages (Figs. 22 and 23). These have been described by many authors. Whether these cells derive from microglia or monocytes is unknown.
Figure 22.
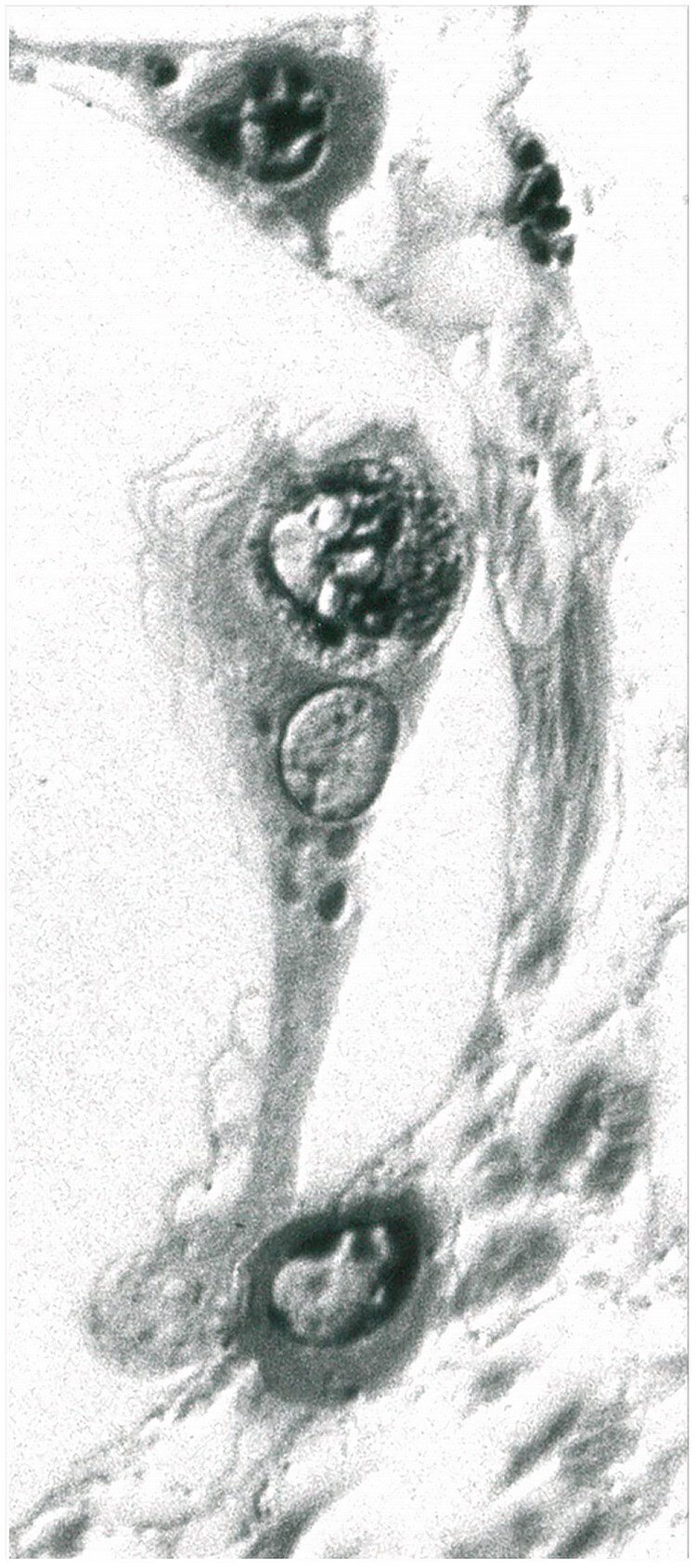
A perivascular space macrophage englobing cell detritus. Also present are 2 plasma cells located in other channels in the perivascular space (Case 19, 1-µm-thick epoxy section, Toluidine blue, ×2, 200).
Figure 23.
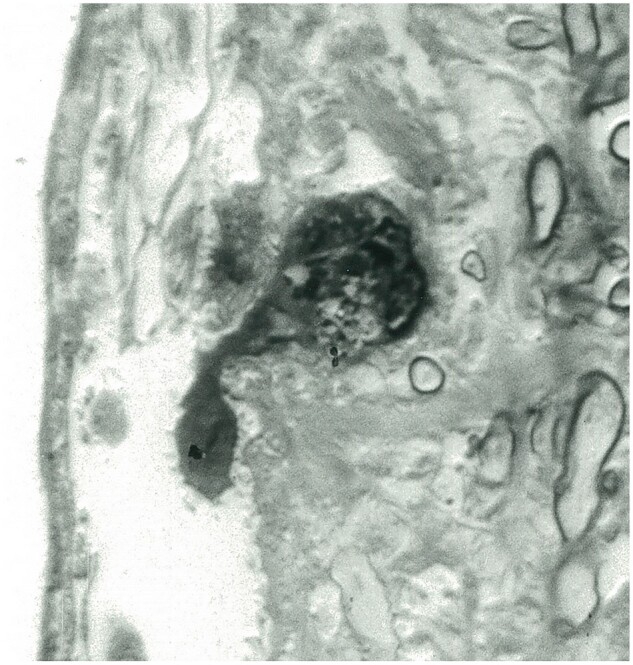
Residual microglia. The cell with a contained phagosome is seen entering a perivascular space at the edge of an old plaque (Case 19, 1-µm-thick epoxy section, Toluidine blue, ×1900).
Phagocytic macrophages
Amoeboid, pseudopodic, and phagocytic early phagocytes
Commencing destruction of myelin at plaque margins occurred in vacuolated myelinated tissue infiltrated by cells with a rounded, pseudopodic, or amoeboid outline (Fig. 24) (Supplementary Data Figs. S8–S10). Myelin sheaths appeared vacuolated due to the presence of intramyelinic edema, and they reacted positively for activated complement (C9neo). Early phagocytes with commencing accumulation of myelin particles were identified but very rarely in lesions in Cases 1, 3, 5, 9, 20, 24, and 25.
Figure 24.
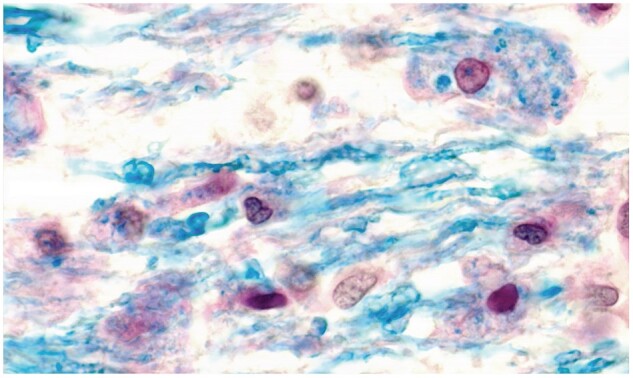
Round cells and phagocytes in vacuolated myelinated tissue bordering an expanding plaque. Six early phagocytes are seen together with one filled with undigested myelin. No oligodendrocytes are seen in the field (Case 3, LFB PAS, ×650).
Phagosomes containing myelin particles recognizable light microscopically in LFB-stained sections were not detected in resident or ramified microglia or MHC-II-positive activated microglia, reticulate microglia or wall microglia. Macrophages with LFB-positive myelin fragments were observed in newly forming lesions in Cases 1–12, 14, 23, 24, and 25. LFB-positive macrophages were absent or rare in chronic cases (Cases 15–19, 21, 22).
Destruction by phagocytes of large AQP4-positive astrocytes was not uncommon in Cases 2 (plaque 4) and 4 (Supplementary Data Fig. S11). Case 4 was the first case reported in the literature with evidence of astrocyte injury in early MS (destruction of glial limiting membrane perivascular foot processes [27, 28]). The same picture of macrophage-mediated destruction of AQP4-positive astrocytes in the presence of normal looking gemistocytic astrocytes was also seen in Case 14 as reported previously (22).
MRP14
Staining for MRP14 showed these rarely seen early phagocyte to be MRP14-positive. Importantly MRP14-positivity in the present MS cases was restricted to monocytes, polymorphs, and early phagocytes. In Case 3, some large hypercellular plaques filled with foamy macrophages stained completely negatively for MRP14 while in intact tissue bordering the same lesion MRP14-positive amoeboid early phagocytes could be seen emerging from vessels in developing small perivascular lesions (21). In Case 24, ongoing active demyelination at the edge of the lesion was associated with the presence of LFB-positive MRP14-positive, RAC1-negative, amoeboid, and early phagocytes, while deeper in the lesion there were only LFB-negative, MRP14-negative, RAC1-positive foamy macrophages (Figs. 25 and 26). Case 2, a patient with a clinical disease duration of 2 weeks, was unusual in that 4 developing plaques (plaques 1–3 and 4) stained negatively for MRP14, except for cells located intravascularly. LFB-positive macrophages and foamy macrophages were detected in 2 of the lesions but only in very small numbers.
Figure 25.
(A, B) An expanding (actively demyelinating) plaque. Vacuolated myelin at the advancing edge of the lesion is infiltrated by monocyte-derived phagocytes. Those at the extreme edge could be described as amoeboid (Case 24, MRP14, A, B, ×50).
Figure 26.
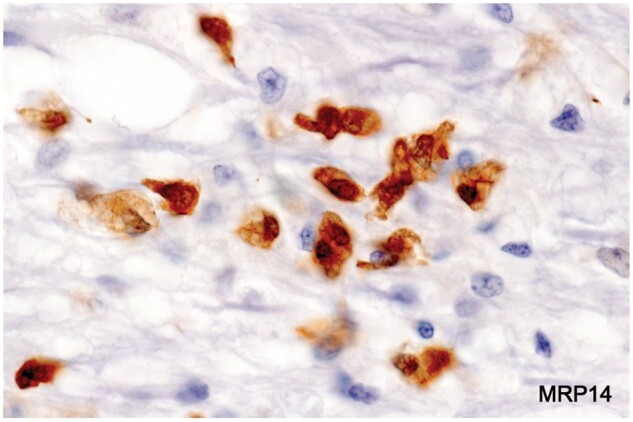
Monocyte-derived early myelin phagocytes (Case 24, MRP14, ×200).
Proliferation
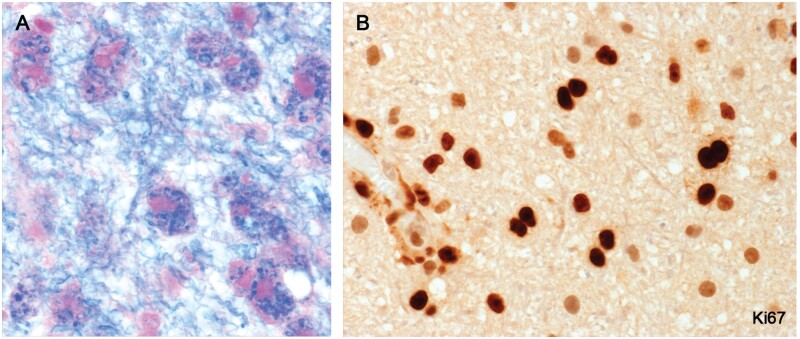
New lesions in 3 cases with particularly early symptomatic lesions (Cases 9 and 25 and a third nonindex biopsy case described previously [21]) immunostained for the proliferation cell markers Ki67 and PCNA, showed locally proliferating parenchymal perivascular space monocytes and early macrophages as they emerged from blood vessels (Fig. 27; Supplementary Data Fig. S12).
Figure 27.
Locally proliferating monocytes and early macrophages in still-myelinated tissue bordering a newly forming plaque (Case 25, A, LFB PAS, B, Ki67, A, B, ×400).
Perivascular demyelination
Small isolated perivascular foci of myelin loss or commencing myelin loss in the presence of MRP14-positive monocytes and histiocytic macrophages were observed in Cases 2, 3, 4, 8, 15, and 24 and other early and subacute cases. They occurred as satellites near plaques, or in 1 instance (Case 2), in a field of MHC II-positive activated microglia bordering a partially remyelinated shadow plaque. No concentration of ramified microglia or MHC II-positive activated microglia was observed in perivascular tissue in or near developing lesions.
Foamy macrophages
Frozen sections stained for neutral lipids showed 2 staining patterns, one the well-known image of foamy macrophages distended with large Oil Red O-positive globules located throughout the plaque or concentrated in demyelinated tissue toward the edge of the lesion (Fig. 28), and a second showing macrophages with small lipid vesicles located in the myelinated margin of the plaque (Fig. 29). In sections reacted for CD16 (FcRγIII) and IgG, staining was often restricted to the cell margin, or in the case of IgG, to surface caps corresponding to the location of Galc-positive MBP-positive particles adherent to the cell surface (29). The source of the IgG, whether from serum or locally synthesized, is uncertain as an important feature of these postphagocytic plaques was the presence of plasma cells. In sections stained for CD68 (or using biotinylated normal or MS CSF IgG [21]), staining was restricted to discrete particles in the cell cytoplasm. In sections stained for MHC class II antigens, staining was concentrated near the Golgi complex.
Figure 28.
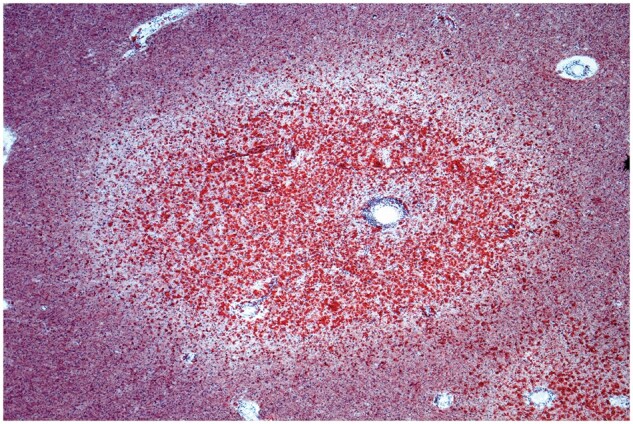
A typical new plaque filled with foamy macrophages. The halo effect is due in part to myelin loss and the presence of numerous gemistocytic astrocytes. Commencing clearing around the central vessel is due to beginning migration of foamy macrophages to the perivascular space of the vessel (Case 14, frozen section, Oil Red O, ×80).
Figure 29.
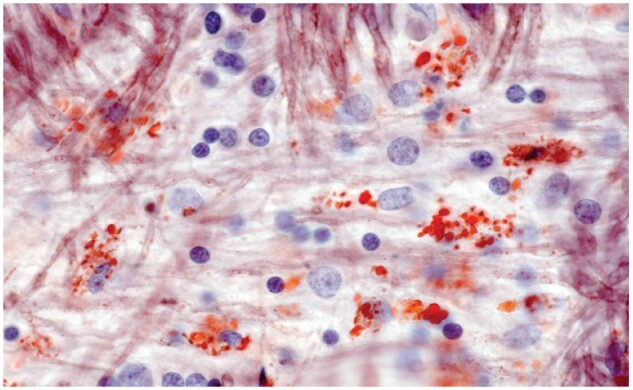
Myelinated tissue bordering a typical old plaque (moderate numbers of large foamy macrophages at the edge of the lesion but no identifiable LFB-positive phagocytes) is infiltrated by scattered small foamy macrophages. Some normal-appearing oligodendrocytes are present (Case 17, frozen section, Oil Red O, ×650).
Remyelination
A common finding in remyelinated shadow plaques and older remyelinated lesions was the presence of lipid macrophages located close to newly formed myelin internodes (Figs. 30 and 31). Atypical partially remyelinated shadow plaques (myelin sheaths and oligodendrocytes immunoreactive for activated complement C9neo together with few or no MRP14-positive phagocytes) were observed in Case 2 (Supplementary Data Fig. S13A, B).
Figure 30.
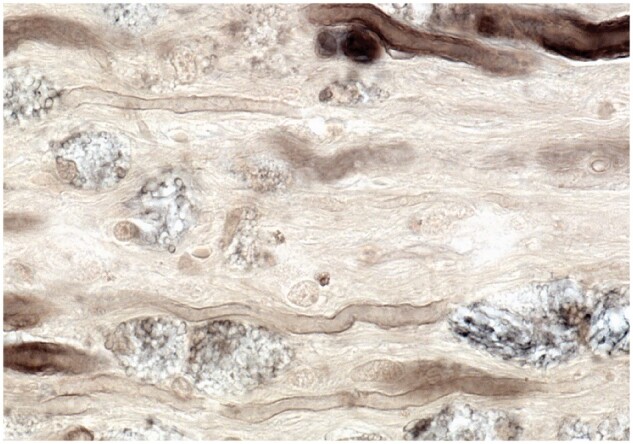
Remyelination of recently demyelinated axons in the presence of foamy macrophages in recently demyelinated tissue (Case 14, 2-µm-thick epoxy section, ×650).
Figure 31.
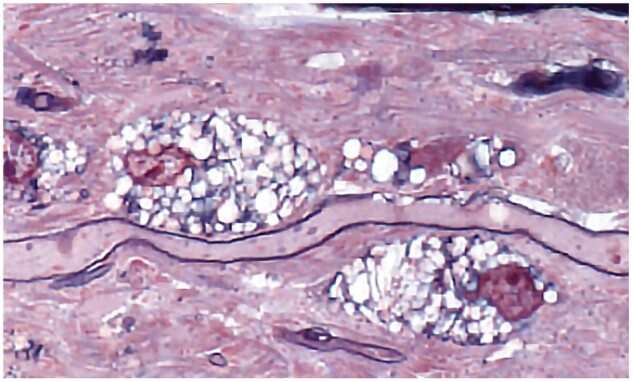
A 1-µm-thick epoxy section of an old spinal cord plaque. Foamy macrophages are aligned along a remyelinated myelin internode (Case 14, Toluidine blue and Safranin, ×650).
There is a famous drawing in Babinski’s doctoral thesis published in Paris in 1885 of macrophages attached to nerve fibers with progressive thinning of myelin sheaths (Fig. 32) (30). His suggestion that debris-laden cells remove myelin directly from the sheath and that this involves transfer of myelin into the cells cytoplasm by some unknown process was written at about the time Elie Metchnikoff, the discoverer of phagocytosis, moved his laboratory to Paris from Odessa (31).
Figure 32.
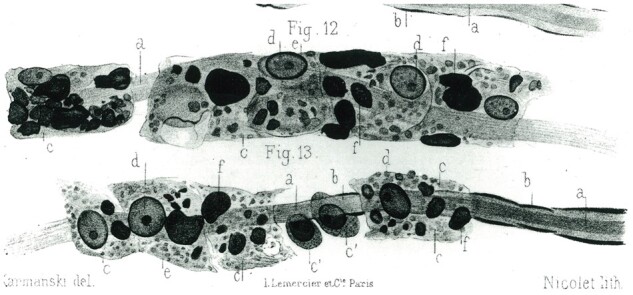
Macrophage-mediated demyelination circa 1885. A drawing made by Babinski of “corpuscles in some way absorbing into their cytoplasm myelin directly off the sheath”, an early description of what at about that time came to be known as phagocytosis.
Monocytes and lymphocytes
CD45-positive mononuclear leukocytes together with lymphocytes were detected in plaque parenchyma and perivascular spaces, and in the walls of small blood vessels in all early cases and in most of the chronic cases (Supplementary Data Figs. S14, S15). Monocytes were also common in the meninges, and in grey matter capillaries (cerebral cortex, brain stem). MHC II-positive and MHC II-negative lymphocytes were identified in large perivascular spaces in one case (Case 4) (Supplementary Data Fig. S15A). Plasma cells were seen in the parenchyma and perivascular spaces in and near subacute and chronic lesions, never in the earliest lesions. CD209-positive dendritic cells were particularly numerous in the walls of small blood vessels bordering an expanding lesion in Case 4 (Supplementary Data Fig. S15B).
Disease duration
Plaques of different histological ages were observed in most of the cases examined (Supplementary Data Fig. S17). Early plaques in cases of early MS were essentially destructive in nature, with few or no reactive changes in microglia in immediately bordering tissues. By contrast in cases of severe subacute disease, plaques presented a mixed picture of active macrophage-mediated destruction of astrocytes, myelin, and oligodendrocytes with at the same time evidence (sometimes in the same lesion) of increased numbers of regenerating astrocytes, oligodendrocytes, and nonramified activated microglia. Plaques in chronic cases showed a markedly reduced rate of ongoing myelin and oligodendrocyte loss, together with increased numbers of nonramified activated microglia in bordering tissue.
Acute MS
In patients with the most acute disease as judged by histology, some plaques were identified of a type not seen in patients with more protracted disease. These were seen most notably in Cases 1–6, 10, and 12. Features characterizing these early lesions as observed in Case 1 (1 plaque), Case 2 (6 plaques), Case 3 (1 plaque), Case 5 (1 plaque), Case 9 (1 plaque), and Case 10 (1 plaque). Case 12 (2 plaques) were as follows.
In sections stained for myelin, new plaques appeared as large sharply circumscribed patches of myelin pallor. From the beginning, quite large volumes of tissue were affected, something that occurred probably over the course of some hours or days (Supplementary Data Fig. S18).
The fate of oligodendrocytes in most of these still myelinated lesions could not be determined. There were several lesions, however, with evidence of oligodendrocyte degeneration, notably nuclear pyknosis or loss (Cases 1, 3, 9, 10).
Phagocytes
Myelin sheaths stained positively for activated complement (membrane attack complex C9neo) in most typical lesions. Although no macrophages containing LFB-positive myelin remnants were readily discernible, there were lesions with small to moderate numbers of easily identified HAM56-positive phagocytes scattered throughout the lesion or located around blood vessels (Case 2 plaque 6, Case 5, Case 10). Although LFB-positive phagocytes were difficult to detect in sections stained for myelin, close inspection of plaque 6 in Case 2 revealed barely visible minute “myelin balls” located in areas where myelin sheaths appeared thin and irregular, appearances consistent with other features suggesting that at least some of these still myelinated lesions would, over the course of a few days or weeks, acquire the usual appearance of an actively demyelinating lesion. The important question of what changes if any precede (and possibly determine) the appearance in the tissue of phagocytes remains uncertain (19, 32–35).
Monocytes were detected usually in small numbers in the walls of small blood vessels within or close to lesions. The inflammatory profile appeared to be the same as that seen in lesions in less acute cases, namely the presence of lymphocytes and monocytes around blood vessels, with monocytes also prominent in the meninges. Importantly no plasma cells were seen in these lesions.
Microglia
A further important characteristic was an absence of any sign of microglia reaction immediately bordering the lesion (no increase in number or size of ramified microglia, no MHC II-positive microglia). Within these still-myelinated developing plaques and plaque extensions, CD45-positive, RCA1-positive, MRP14-negative ramified microglia were present in increased numbers. One unusual early lesion in Case 2 (plaque 1) consisted largely of massed wall microglia with commencing myelin breakdown restricted to a small area infiltrated by monocytes at the lesion edge.
Subacute MS
Plaques especially characteristic of severe progressive early MS sampled some months after clinical onset were lesions with ongoing myelin destruction coexisting with lesions showing evidence of repair and regeneration, in some instance with tissue breakdown and repair occurring in different areas within the same plaque.
Microglia
Wall microglia and what we judge to be precursors were present in some developing plaques in Cases 2, 18, 23, and 24.
Phagocytes and myelin, oligodendrocyte, and astrocyte loss
Areas of ongoing breakdown of tissue in the form of broad areas of active or recent demyelination (presence of LFB-positive macrophages) involved both normally myelinated tissue and remyelinated tissue. Intact remyelinated shadow plaques, exceptionally common in this type of MS, appeared as evenly remyelinated tissue affecting most of the plaque or as extensive areas of new myelin formation located at plaque margins. Interestingly new myelin sheaths in these early newly formed shadow plaques merged imperceptibly with surrounding intact myelin, indicating perhaps an initial reduction in myelin sheath thickness rather than complete myelin loss. This would also explain the fact that these newly formed shadow plaques showed no perivascular accumulation of foamy macrophages of the sort of normally evident following recent myelin breakdown.
Oligodendrocyte pyknosis and/or apoptosis (fragmenting nuclei, nuclear bodies) were observed in still myelinated tissue bordering, or in some instances, within lesions in Cases 1–3, 9, 10, and 24. The numbers of typical small oligodendrocytes present in recently demyelinated tissue (partially demyelinated tissue with infiltrating macrophages immunoreactive for MBP, MAG, and CNPase) were markedly reduced compared to normal white matter in Cases 4 and 8, as reported previously. Swollen oligodendrocytes immunoreactive for activated complement (membrane attack complex C9neo) located in partially myelinated tissue were observed in plaque 4, Case 2 (plaque 4).
Large numbers of HNK1-positive oligodendrocyte precursors in recently demyelinated tissue and in older postphagocytic plaques were noted in Cases 2, 4, 8, 13, 14, and 16 (36). Oligodendrocytes located within gemistocytic astrocytes were a common finding in several cases, as noted also in an EM study of similar lesions (37).
Loss of astrocytes and or astrocyte foot processes was seen in Cases 4, 5, 6, and 15. Macrophage-associated destruction of AQP4-positive astrocytes was noted in cases 2, 7–9, and 15. Proliferating (mitotic) GFAPδ-positive AQP4-negative astrocyte precursors in bordering tissue was seen in Cases 2, 3, 4, and 15.
Chronic MS
Plaques in the 5 cases with clinical disease duration 4 years or longer (Cases 15–18, 22) had features which were absent or rare in patients with more acute disease.
Phagocytes
The most outstanding characteristic of these plaques was the absence of evidence of significant ongoing loss of myelin shown by the rarity of plaques with LFB-positive macrophages. This included plaques with variable sometimes numerous foamy macrophages. Tobin et al reported a similar experience in a study of a much larger number of chronic MS cases (38).
Microglia
Typical of this group of cases was a marked increase in number and types of ramified and nonramified microglial subtypes in intact white and grey matter tissue immediately bordering and at a distance from plaque margins. In Case 18, for example, this affected all intact white matter in sections of the whole cerebellum.
Activated microglia
MHC II-positive nonramified activated microglia, a population not seen in acute and subacute forms of MS, were common in tissue immediately bordering many chronic plaques. Uncertain immunoreactivity for CD68 and an absence of other evidence of phagocytosis argue against this population as a source of brain phagocytes including foamy macrophages.
Microglial nodules
Nodules of several types were observed especially in Cases 16–18, but also in lesser numbers in Case 22. They were located typically in myelinated tissue bordering plaques and in 1 case (Case 16) also in the cerebral cortex. Nodules of any type were rare or not observed in any case of early or subacute disease. Ramified microglia of a particular subtype interacting with infiltrating monocytes in the cerebral cortex as in Case 16 can be added to the list of gray matter lesions yet to be explained in MS (39).
Astrocytes
There was no evidence of ongoing destruction of astrocytes in patients with chronic disease. Small bipolar AQP4-negative GFAPδ-positive astrocyte precursors similar to those that populate early non-necrotic NMO lesions were observed at the edges of plaques in Case 15.
Repair of astrocyte foot processes at perivascular glial limiting membranes was observed in Cases 16 and 22. That such restorative activity sometimes results in the formation of abnormal foot processes is illustrated in Supplementary Data Figure S16.
Blood-brain barrier integrity was examined in Cases 16 and 22 together with 33 other cases of longstanding MS (23). Extravascular serum proteins, signifying defective blood-brain barrier function was detected in one active (LFB-positive macrophages) and 26 inactive plaques (no LFB-positive macrophages); intact blood-brain barrier function, interpreted as evidence of repair of blood-brain barrier function, was observed in 8 of 34 inactive plaques.
DISCUSSION
Myelin phagocytes
The original objective of this study was to describe the morphological subtypes of microglia involved in disease progression in early and late forms of MS, and how such subtypes relate to Río Hortega’s theory that brain phagocytes in acute destructive focal lesions are transformed ramified resident microglia.
While the results of the study do not rule out the possibility that a proportion of myelin phagocytes in MS derive from microglia, the findings indicate that a significant proportion of phagocytes responsible for the destruction of myelin in MS are MHC II-positive, MRP14-positive, IgG-positive monocyte-derived macrophages. Brück et al also report that hematogenous MRP14-positive phagocytes contribute significantly to macrophage populations in early MS lesions (40, 41). The first report suggesting that all myelin phagocytes in MS are macrophages of hematogenous origin is a study by Adams et al (15). The authors studied 20 autopsy cases of exceptionally early MS at Guy’s Hospital Medical School. The clinical duration was <12 weeks in most cases. All macrophages initially stained positively for markers characteristic of hematogenous macrophages (muramidase, anti-alpha1-antitrypsin, MAC, and HAM56). Except for HAM56, staining intensity of each marker subsequently declined. Such fading also occurs with respect to foamy macrophage MRP14, IgG, and MHC II immunoreactivity (40, 42).
Polak et al (Polak was a close associate and biographer of Río Hortega) describe a number of early reports that directly support this view that monocytes and not microglia are the true source of brain phagocytes (3). Kitamura, for example, in an investigation of the effects of stab wounds of the cerebral cortex of mice given tritiated thymidine (3H-T) and killed 6–12 hours postoperatively, concluded that almost all brain macrophages were derived from circulating leucocytes, particularly mononuclear leucocytes (monocytes), and that “the resting microglia of Hortega play no significant role in the production of brain macrophages” (43). In a separate review of the role of microglia and phagocytes in disease, Federof noted that while monocyte-derived macrophages are present in the CNS in large numbers throughout embryonic development, and that their presence there is related to neural cell death, the density and distribution of mature microglia are not related to sites of neural cell death (44).
Microglia phenotypes
An important recent review (135 first authors) concerning the nomenclature of microglia phenotypes in disease recommends discarding certain terms because “they are of no practical value”. Terms mentioned include pro- and anti-inflammatory microglia, MI and M2 microglia, and activated microglia among others (45). The paper also advises against classifying microglia based on morphological features without including proteomic, transcriptomic, and other core determining characteristics. These and other classifications of microglia subtypes based on “shape to contents” studies appear, at this time, to be of little practical value when it comes to identifying microglia subtypes as they occur in actual diseased tissue (9–11, 45–49).
Contemporary accounts of microglia subtypes in MS and other CNS diseases describe 4 morphologically distinct forms, ie, resting, reactive, activated, and phagocytic, the last to include foamy macrophages (4, 5). The present study has added a number of new morphologic and functional subtypes to this list as they occur in MS, as well as details of MRP14 and MHC11 expression by microglia and phagocytes during the course of the disease.
MRP14 is a calcium binding protein expressed by early activated monocytes and monocyte derived macrophages in acutely inflamed tissue. In the present study and in our previous study (21) the only MRP14-positive cells identified in MS tissue were monocytes, polymorphs, and early phagocytes. Microglia of all types stained negatively for the marker including resident, reactive, activated, aggregated (nodules), reticulate and wall microglia. Foamy macrophages usually stained negatively for the marker. In other CNS diseases, exceptionally, MRP14-positive microglia nodules and ramified reactive microglia have been observed. Akiyama et al, for example, describe such MRP14 microglia in a patient with Alzheimer Disease and bacterial encephalitis (50).
Disease duration
The present study shows that as the disease progresses, distinct and discrete populations of microglia appear at particular times and in different locations in developing and maturing MS plaques. Three of these populations exhibit features suggesting an anti-inflammatory role in disease progression: First, the population of MHC II-positive microglia termed wall microglia that together with AQP4-positive reactive astrocytes form what could be described as levees or walls bordering some early plaques; second, reticulate and other microglia that interact directly with infiltrating monocytes resulting in the formation of different types of nodules; third, the population of MHC II-positive microglia, referred to as activated microglia that become increasingly prominent in tissue immediately bordering increasingly quiescent chronic plaques.
Limitations of the study
The main problems with this report are the relatively small number of cases studied and the lack of knowledge in most of the cases of treatment received during the course of the disease, particularly the duration and doses received of corticosteroids, anti-B-cell antibodies, cyclophosphamide, and other disease modifying therapies. The absence of comparable publications of microglial morphology in other diseases seriously limits interpretation of our findings and it remains uncertain as to which if any of what is described here relate specifically to the pathogenesis of MS.
CONCLUSIONS
Phagocytes including foamy macrophages associated with the destruction of myelin, oligodendrocytes, and astrocytes in MS are hematogenous and locally proliferating monocytes and monocyte-derived macrophages. It remains uncertain as to whether microglia ever develop into effective phagocytes or contribute directly to the pool of phagocytes in MS.
Early in the disease MS lesions are destructive and largely unrelated to the activity of microglia. With increasing disease duration, the course of the disease moderates to a state dominated by repair and regeneration which is accompanied by increased microglia activity.
Microglia in MS and perhaps in other diseases exist chiefly to mitigate and or prevent inflammation and its effects in the CNS, effects which are far more dangerous for survival than those caused by similar lesions in other tissues.
Supplementary Material
ACKNOWLEDGMENTS
Control tissue was received from the Australian Brain Donor Programs NSW Tissue Resource Centre which is supported by The University of Sydney and the National Health and Medical Research Council of Australia. Also received was tissue and cerebrospinal fluid from The National Neurological Research Specimen Bank, VAMC Wadsworth Division, Los Angeles, CA 90073, which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society, and the Veterans Health Services and Research Administration, Department of Veterans Affairs. Technical assistance was provided by E. Kwon, J. Baverstock, S. Kum-jew, and Toan Nguyen.
Contributor Information
John W Prineas, Department of Medicine, The University of Sydney, Camperdown, NSW, Australia.
Sandra Lee, Department of Medicine, The University of Sydney, Camperdown, NSW, Australia.
FUNDING
The study was supported by grants from Multiple Sclerosis Research Australia, The Nerve Research Foundation, University of Sydney, The National Multiple Sclerosis Society (JWP RG 2731-A-B), and the NSW Ministry for Science and Medical Research.
DISCLOSURE/CONFLICT OF INTEREST
The authors have no duality or conflicts of interest to declare.
SUPPLEMENTARY DATA
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Lee AB. The Microtomist’s Vade-Mecum. A Handbook of the Methods of Microscopic Anatomy, 8th edn. Gatenby JB, ed. London: J. and A. Churchill; 1924:492–3 [Google Scholar]
- 2. del Río Hortega P. Microglia. In: Penfield W, ed. Cytology and Cellular Pathology of the Nervous System, Vol. 2. New York: Paul B. Hoeber; 1932:483–534 [Google Scholar]
- 3. Polak M, D’Amelio F, Johnson JE, et al. Microglial cells origins and reactions. In: Haymaker W, Adams RD, eds. Histology and Histopathology of the Nervous System. Springfield, IL: Charles C. Thomas; 1982:481–559 [Google Scholar]
- 4. Gehrmann J, Kreutzberg GW, Microglia in experimental neuropathology. In: Kettenmann H, Ransom BR, eds. Neuroglia. New York: Oxford University Press; 1995:883–904 [Google Scholar]
- 5. Streit WJ, Microglial cells. In: Kettenmann H, Ransom BR, eds. Neuroglia. New York: Oxford University Press; 1995:85–96 [Google Scholar]
- 6. Berry M, Butt AM, Wilkin G, et al. Structure and function of glia in the central nervous system. In: Graham DI, Lantos PL, eds. Greenfield’s Neuropathology, Vol. 1, 7th edn. London: Arnold; 2002:75–121 [Google Scholar]
- 7. Graeber MB, Blakemore WF, Kreutzberg GW, Cellular pathology of the central nervous system. In: Graham DI, Lantos PL, eds. Greenfield’s Neuropathology, Vol. 1, 7th edn. London: Arnold; 2002:123–93 [Google Scholar]
- 8. Vinters HV, Kleinschmidt-DeMasters BK. General pathology of the central nervous system. In: Love S, Louis DN, Ellison DW, eds. Greenfield’s Neuropathology, Vol. 1, 8th edn. London: Hodder Arnold; 2008:1–56 [Google Scholar]
- 9. Spiteri AG, Wishart CL, Pamphlett R, et al. Microglia and monocytes in inflammatory CNS disease: Integrating phenotype and function. Acta Neuropathol 2022;143:179–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwabenland M, Brück W, Priller J, et al. Analyzing microglial phenotypes across neuropathologies: A practical guide. Acta Neuropathol 2021;142:923–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prinz M, Masuda T, Wheeler MA, et al. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol 2021;39:251–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma X, Ma R, Zhang M, et al. Recent progress in multiple sclerosis treatment using immune cells as targets. Pharmaceutics 2023;15:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams RD, Sidman RL. Introduction to Neuropathology. New York: McGraw-Hill; 1968:36–7 [Google Scholar]
- 14. Dolman CL. Microglia. In: Davis RL, Robertson DM, eds. Textbook of Neuropathology. Baltimore, MD: Williams and Wilkins; 1991:141–63 [Google Scholar]
- 15. Adams CW, Poston RN, Buk SJ. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci 1989;92:291–306 [DOI] [PubMed] [Google Scholar]
- 16. Compston A. Neurobiology of multiple sclerosis. In: Compston A, Ebers G, Lassmann H, et al. , eds. McAlpine’s Multiple Sclerosis, 3rd edn. London: Churchill Livingstone; 1998:283–321 [Google Scholar]
- 17. Zrzavy T, Hametner S, Wimmer I, et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 2017;140:1900–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Attfield KE, Jensen LT, Kaufmann M, et al. The immunology of multiple sclerosis. Nat Rev Immunol 2022;22:734–50 [DOI] [PubMed] [Google Scholar]
- 19. Pachner AR. The neuroimmunology of multiple sclerosis: fictions and facts. Front Neurol 2021;12:796378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain 2019;142:133–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prineas JW, Parratt JDE. Multiple sclerosis: microglia, monocytes, and macrophage-mediated demyelination. J Neuropathol Exp Neurol 2021;80:975–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prineas JW, Lee S. Multiple sclerosis: destruction and regeneration of astrocytes in acute lesions. J Neuropathol Exp Neurol 2019;78:140–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon EE, Prineas JW. Blood-brain barrier abnormalities in longstanding multiple sclerosis lesions. An immunohistochemical study. J Neuropathol Exp Neurol 1994;53:625–36 [DOI] [PubMed] [Google Scholar]
- 24. Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol 2001;50:646–57 [DOI] [PubMed] [Google Scholar]
- 25. Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain 2003;126:1638–49 [DOI] [PubMed] [Google Scholar]
- 26. Prineas JW, Raine CS. Electron microscopy and immunoperoxidase studies of early multiple sclerosis lesions. Neurology 1976;26:29–32 [DOI] [PubMed] [Google Scholar]
- 27. Parratt JD, Prineas JW. The astrocyte lesion in neuromyelitis optica. Mult Scler 2009;15:S17 [Google Scholar]
- 28. Parratt JD, Prineas JW. Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler 2010;16:1156–72 [DOI] [PubMed] [Google Scholar]
- 29. Prineas JW, Kwon EE, Sternberger NH, et al. The distribution of myelin-associated glycoprotein and myelin basic protein in actively demyelinating multiple sclerosis lesions. J Neuroimmunol 1984;6:251–64 [DOI] [PubMed] [Google Scholar]
- 30. Babinski J. Étude anatomies et clinique sur la sclérose en plaques. Paris: A. Parent, imprimeur de la Faculte de Medicine; 1885
- 31. Metchnikoff E. Lectures on the comparative pathology of inflammation. In: Starling FA, Starling EH, eds. Delivered at the Pasteur Institute in 1891. New York: Dover Publications; 1968:180–97 [Google Scholar]
- 32. Raine CS, Cannella B, Hauser SL, et al. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann Neurol 1999;46:144–60 [DOI] [PubMed] [Google Scholar]
- 33. Molina-Gonzalez I, Miron VE, Antel JP. Chronic oligodendrocyte injury in central nervous system pathologies. Commun Biol 2022;5:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol 1996;55:300–9 [DOI] [PubMed] [Google Scholar]
- 35. Sobel RA, Albertelli M, Hinojoza JR, et al. Azetidine-2-carboxylic acid-induced oligodendrogliopathy: relevance to the pathogenesis of multiple sclerosis. J Neuropathol Exp Neurol 2022;81:414–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prineas JW, Kwon EE, Goldenberg PZ, et al. Multiple sclerosis. Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest 1989;61:489–503 [PubMed] [Google Scholar]
- 37. Ghatak NR. Occurrence of oligodendrocytes within astrocytes in demyelinating lesions. J Neuropathol Exp Neurol 1992;51:40–6 [DOI] [PubMed] [Google Scholar]
- 38. Tobin WO, Kalinowska-Lyszczarz A, Weigand SD, et al. Clinical correlation of multiple sclerosis immunopathologic subtypes. Neurology 2021;97:e1906–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zuroff LR, Benjamins JA, Bar-Or A, et al. Inflammatory mechanisms underlying cortical injury in progressive multiple sclerosis. Neuroimmunol Neuroinflammation 2021;8:111–33 [Google Scholar]
- 40. Brück W, Porada P, Poser S, et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995;38:788–96 [DOI] [PubMed] [Google Scholar]
- 41. Brück W, Sommermeier N, Bergmann M, et al. Macrophages in multiple sclerosis. Immunobiology 1996;195:588–600 [DOI] [PubMed] [Google Scholar]
- 42. Prineas JW, Parratt JD. Oligodendrocytes and the early multiple sclerosis lesion. Ann Neurol 2012;72:18–31 [DOI] [PubMed] [Google Scholar]
- 43. Kitamura T. The origin of brain macrophages. Some considerations on the microglia theory of del Rio Hortega. Acta Pathol Jpn 1973;23:11–26 [DOI] [PubMed] [Google Scholar]
- 44. Fedorof S, Development of microglia. In: Kettenmann H, Ransom BR, eds. Neuroglia. New York: Oxford University Press; 1995:162–81 [Google Scholar]
- 45. Paolicelli RC, Sierra A, Stevens B, et al. Microglia states and nomenclature: a field at its crossroads. Neuron 2022;110:3458–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amor S, Peferoen LA, Vogel DY, et al. Inflammation in neurodegenerative diseases-an update. Immunology 2014;142:151–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peferoen LA, Vogel DY, Ummenthum K, et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol 2015;74:48–63 [DOI] [PubMed] [Google Scholar]
- 48. Voet S, Prinz M, van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med 2019;25:112–23 [DOI] [PubMed] [Google Scholar]
- 49. Amor S, McNamara NB, Gerrits E, et al. White matter microglia heterogeneity in the CNS. Acta Neuropathol 2022;143:125–41 [DOI] [PubMed] [Google Scholar]
- 50. Akiyama H, Ikeda K, Katoh M, et al. Expression of MRP14, 27E10, interferon-alpha and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J Neuroimmunol 1994;50:195–201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.