Abstract
Introduction
Inkjet-dispensing printing is a promising additive manufacturing method for pharmaceutical applications such as drug discovery. The unique advantages of this technology, including low cost, programmability, high resolution, high throughput, high speed, and biocompatibility, may reduce the financial resources needed to discover new drug candidates. Sophisticated and miniaturized assays have been developed to accomplish drug discovery and drug screening using modern inkjet dispensing printers.
Areas covered
This paper reviews recent advancements in the field of inkjet printer technology for drug discovery. Various types of inkjet printers and their recent use for the drug discovery are summarized; physical and biological limitations of this technology are also examined. Furthermore, typical inks used in the inkjet printing technology are introduced.
Expert opinion
Inkjet bioprinting technology is a promising tool for many biological and pharmaceutical applications. Several bottlenecks associated with this technology need to be addressed before commercialization. For example, sophisticated inks need to be synthesized to meet both biological and engineering restrictions. Further progress of parallel technologies will enhance the performance and functionality of the printers. It is also worth emphasizing that inkjet printing technologies must meet the requirement of regulatory agencies (e.g., the US Food & Drug Administration) for commercialization by the pharmaceutical industry.
Keywords: inkjet dispensing technology, inkjet printer, drug discovery, drug screening, ink
1. Introduction
Inkjet printing refers to a class of additive manufacturing technologies for reproducing images or characters on a material or substrate from digital data by precisely ejecting and steering the ink as droplets to predefined positions [1]. The droplets are usually transferred to the receiving substrates from the printhead in a non-contact mode using gravity, pressure, and fluidic mechanisms. Inkjet-based bioprinting technologies offer many advantages such as low cost, programmability, high resolution, high throughput, high speed, and compatibility with many biological materials. Furthermore, concentration gradients containing cells, materials and/or growth factors may be introduced throughout a three-dimensional structure by altering drop densities or sizes [2, 3]. This technology is traditionally used in office printers and microengineering to print electronic boards. The first study on biomedical applications of the inkjet printer was reported by Klebe in 1988 [4]. He used HP inkjet printers to produce two- and three-dimensional synthetic tissues by depositing collagen and fibronectin. Since then, studies have examined various biomedical applications of inkjet printers in fields such as regenerative medicine [5, 6, 7], toxicology [8], disease modeling [8], and pharmaceutical science [9, 10, 11].
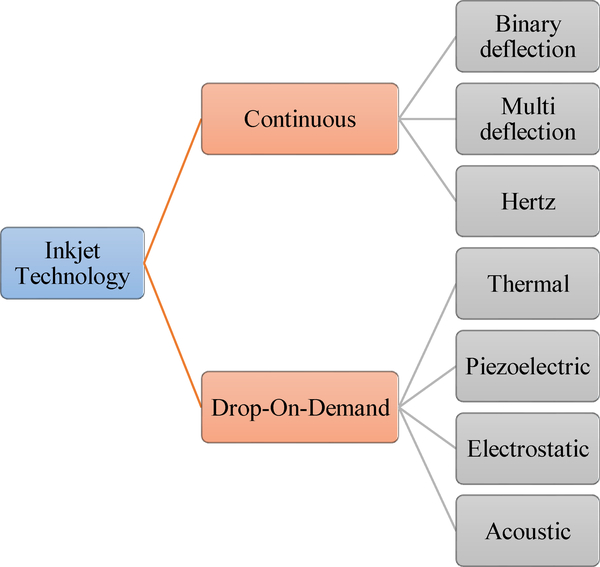
Inkjet printing technology is usually classified based on the physical process of generating droplets in two main categories: continuous inkjet printing (CIJ) and drop-on-demand (DOD) printing. Figure 1 illustrates different types of this technology. In the CIJ bioprinters, a stream of liquid is continuously ejected out the printhead by applying continuous hydrostatic pressure. It was shown in the nineteenth century by Raleigh that a stream of fluid may naturally tend to break into a stream of drops because of surface tension forces [1, 12]. The CIJ bioprinters exploit this effect to produce droplets from the ejected stream of liquid. The DOD bioprinters directly eject the inks in the form of droplets onto the substrates by various mechanisms such as thermal, electrical and piezoelectric actuators. For more information, see references [1, 7, 12] for additional information on the classifications and working principles of DOD bioprinters.
Figure 1.
Classification of inkjet printing technologies. Reproduced from [16] with permission of IS&T: The Society for Imaging Science and Technology; sole copyright owners of The Journal of Imaging Science and Technology..
A review of recent published research studies and available commercial inkjet printing technologies shows that the DOD inkjet technology dominates the CIJ inkjet technology for biomedical and pharmaceutical applications. The current review mainly examines the recent development of DOD inkjet technology for printing functional materials that are used in drug discovery and other pharmaceutical applications. Other pharmaceutical applications of this cutting-edge technology such as drug screening and drug delivery can be found elsewhere [11, 12, 13, 14]. The current article mainly examines published studies between the years 2011and 2018. Readers may consult the other review paper published by the same journal in 2012 to become more familiar with the older studies on this topic [15].
A process of discovering new candidate medications is known as drug discovery. Multiple tests should be conducted in the drug discovery process to examine new drug and verify various factors such as the efficacy, safety, and dose of the drug. Inkjet printing technologies can rapidly develop more reliable and cost-effective in vitro models, which will enhance the prediction of the efficacy, toxicity, and pharmacokinetics of the drug compounds in humans. In the following section, we will consider the working principles of the DOD inkjet bioprinters and will review recent studies of the various types of printing technologies. The critical parameters and current limitations of the inkjet printing technology will be examined in Section 3. Popular inks will be reviewed in Section 4. Section 5 will briefly explain regulatory affairs associated with inkjet printing technology. Concluding remarks and the future direction of this technology will be considered at the end of this article.
2. Inkjet printers
2.1. Drop on demand inkjet printers
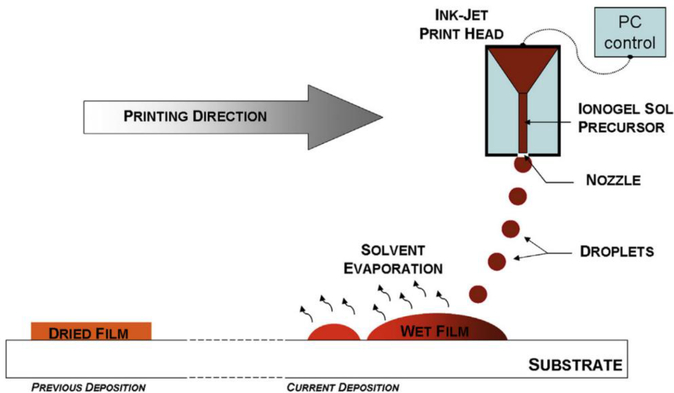
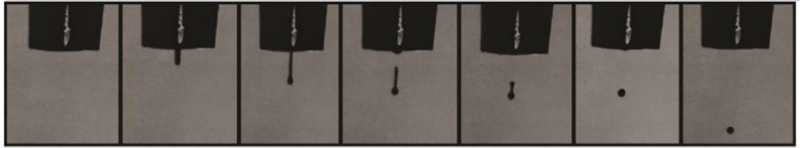
The DOD bioprinters are dominant in pharmaceutical industries compared with their counterparts. The characteristics of high throughput, low cost, and ease of fabrication have attracted many scientists to inkjet printing technology since its initial development [17, 18, 19, 20, 21]. Based on the mechanisms of generating droplets, the DOD inkjet bioprinters are classified into four main groups: thermal inkjet printers, piezoelectric inkjet printers, electrostatic inkjet printers, and electrohydrodynamic inkjet printers. Figure 2 shows a schematic of the inkjet printing process. Figure 3 illustrates the droplet formation sequence. In the following sections, the working principles of these bioprinters will be briefly introduced. In addition, recent studies involving the use of inkjet printing technologies for drug discovery will be reviewed.
Figure 2.
Schematic of various inkjet printing process (reprinted from [22] with permission of Elsevier).
Figure 3.
Droplet formation sequence for PEGDA ink (reprinted from [23] with permission of Elsevier).
2.1.1. Thermal inkjet printers
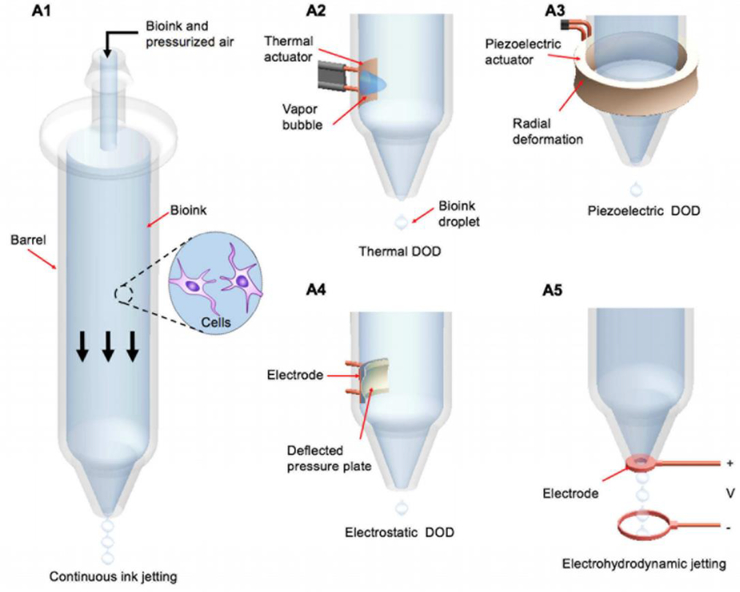
The fabrication of tablets by inkjet bioprinters is different from traditional methods. The differences between traditional/compression methods and bioprinting for fabricating tablets were considered by Khaled et al. The results show that the 3D inkjet printing produces more porous and friable tablets. [24]. Incomplete interaction with the printed binder solution results in higher porosity [25]. Therefore, these properties are beneficial for the rapid dissolution of the drug with a small amount of water. Furthermore, bioprinters address the problem of incorporating a complex drug dosage in a single tablet and controlled drug release by using different types of layers. The thermal inkjet printer uses thermal actuators to generate droplets. Figure 4(A2) schemes the working principle of these printers. They usually have a micro-resistor, which is in direct contact with the ink. The temperature of this heat source is usually in the range of 200–300 °C. The direct contact of the ink and the heating source for about 2 μs leads to an approximately 4–10 °C increase in the overall ink temperature, which is not harmful for biological molecules such as DNA [26, 27, 28, 29, 30]. This amount of heating will be enough to produce and expand bubbles in the inks and eject them out of the nozzle. For thermal printing technologies, ink viscosity, boiling point, and surface tension are usually around 2.5 mPas, 90–95 °C, and 33 mN/m, respectively [30, 31, 32]. The main drawbacks of the thermal inkjet (TIJ) printers are clogging at the nozzle tip and cell encapsulation [30]. These shortcomings must be addresses carefully in the design of this type of inkjet printers.
Figure 4.
The working principles of various types of bioprinters, including continuous (A1), thermal (A2), piezoelectric (A3), acoustic (A4), and electrohydrodynamic(A5) inkjet printing technologies (reprinted from [33] with permission of Elsevier).
Recent studies used TIJ printers for bioprinting of oral drugs with well-controlled drug dosage. As an example, Buanz et al. used a TIJ printer to coat salbutamol sulfate on top of a potato starch oral film with a proper drug dosage for pediatric studies [34]. Previous studies used a fiberglass film for preparation of prednisolone and paclitaxel for oral drug screening [35, 36].
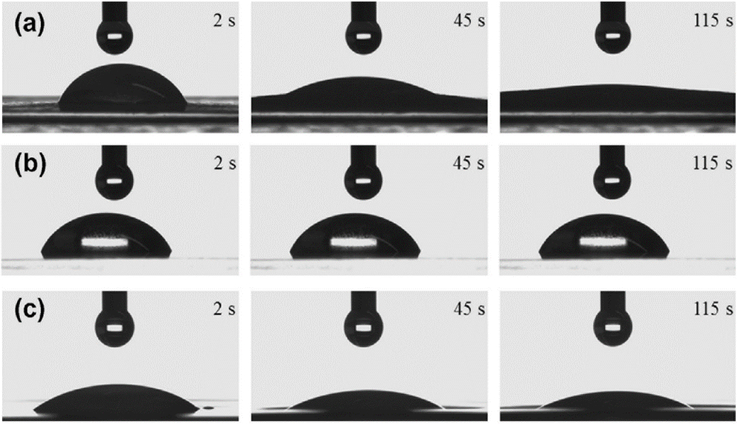
Genina et al. used the TIJ printer for printing low-dosage rasagiline mesylate, the Parkinson’s disease drug, and examining the wettability of this drug on three different substrate types. Propylene glycol was selected as the ink, and different amounts of API (active pharmaceutical ingredient) were distributed in different layers. Figure 5 shows images of these experiments. The amount of printed drug per dosage unit and the applied printing layer numbers were suitable for printing on copy paper (Figure 5a). They concluded that edible films with absorption properties similar to copy paper would be an appropriate substrate for the TIJ printers due to mechanical strength and absorptivity. The fabricated drug form was analyzed with light microscopy. Because the ink was absorbed into the substrate matrix, no crystals were observed on the copying paper [37]. In another study, they combined inkjet printing technology with flexographic printing to obtain a precise drug dosage and a controlled release form of the drug. Riboflavin sodium phosphate and propranolol hydrochloride are water-soluble drugs. They were printed onto a porous substrate by inkjet printing and coated with a water-insoluble polymeric film with flexographic printing. Their results show that different numbers of coating layers as well as the water-soluble and water-insoluble polymer ratio were able to control the drug release profile [38].
Figure 5.
Drop shape of ink on various substrates: (a) copy paper, (b) transparency film, and (c) orodispersible film (reprinted from [37] with permission).
2.1.2. Piezoelectric inkjet printers
Figure 4(A3) shows the mechanism utilized in piezoelectric inkjet printers. The piezoelectric crystals convert an electric voltage to mechanical stress. These bioprinters use piezoelectric crystals in their printheads to generate an acoustic wave by applying an electric field to produce and eject droplets [39, 40]. Various characteristics of the ejected droplets, such as volume and shape, can be controlled by the applied electric field and wave parameters. These bioprinters do not use heat; this parameter may be beneficial for many biomedical applications. However, the generated high frequencies (15–25 kHz) may lead to cell membrane disruption or cell death [41].
Piezoelectric-based bioprinters can be used to facilitate many types of drug discovery studies. The efficacy, the safety, and the dose are crucial characteristics of drugs and need to be verified in the process of discovering new therapies [42]. Park et al. used piezoelectric inkjet printers to fabricate a cancer microtissue array in a multi-well format that exhibits a heterogeneous structure by continuous deposition of collagen-suspended HeLa cells on a fibroblast-layered nanofibrous membrane via inkjet printing. They treated the printed microtissue with an anticancer drug and showed that high drug resistance to doxorubicin occurred in cancer microtissues but not in fibroblast-free microtissues. Their results show the potential of piezoelectric inkjet bioprinting technology for the drug discovery applications and 3D cancer studies [43]. Lorber et al. used a piezoelectric bioprinter to process retinal glial cells and disassociated retinal cells with 57% and 33% cell death for glial and retinal cell types, respectively [44].
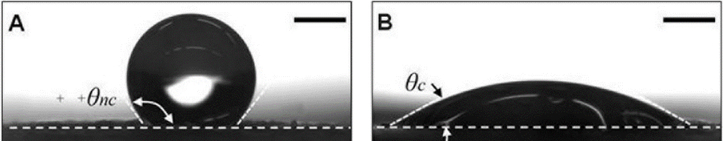
Inks should be low viscosity fluids [34]; therefore, high viscous inks may need to be heated in inkjet printing systems [38]. Planchette et al. used both piezoelectric- and solenoid valve-based inkjet printing technologies for depositing pharmaceutical materials onto orodispersible films. Simultaneous use of these two inkjet technologies may be helpful for dispensing aqueous drug solutions in a viscous polymer coating material. Their results show that the printed films had a consistent surface texture. The printed films were not uniform on hydrophobic and non-porous substrates due to a lack of spreading and absorption by the hydrophobic surface. Their results show that pre-coating of the substrates with a hydrophilic PEG-based solution resulted in a more homogenous printed pattern of APIs on the substrate [45]. Figure 6 shows the effect of the coating solution on the droplet contact angle.
Figure 6.
Schematic showing the difference of contact angles on uncoated (A) and coated (B) hydrophobic nonporous films (reprinted from [45] with permission of Elsevier).
2.1.3. Electrostatic inkjet printers
In electrostatic inkjet technology, droplets are produced and steered by electrostatic fields. Mechanical impulses are directly applied to the fluid chamber in order to cause a displacement (Figure 4(A4)) [40]. This technology is considered to be compatible with cell-containing inks since it avoids the use of heat for generating droplets [7]. To print different layers of a hydrogel structure, the height of the nozzle needs to be adjusted for each layer; this will alter the electric field around the nozzle tip and cause a reduction in printing resolution. This shortcoming can be addressed by installing an electrode ring between the nozzle tip and the target [46]. Nakamura et al. investigated the feasibility of using electrostatic inkjet printers for printing bovine vascular endothelial cells onto culture disks [39]. This type of printers was used to print encapsulated B50 neuroblastoma rat cells in an alginate hydrogel for determining the role of depth in nutrient transport [47]; it was also used to print protein on polyacrylamide substrates for mechanobiology applications [48].
2.1.4. Electrohydrodynamic inkjet printers
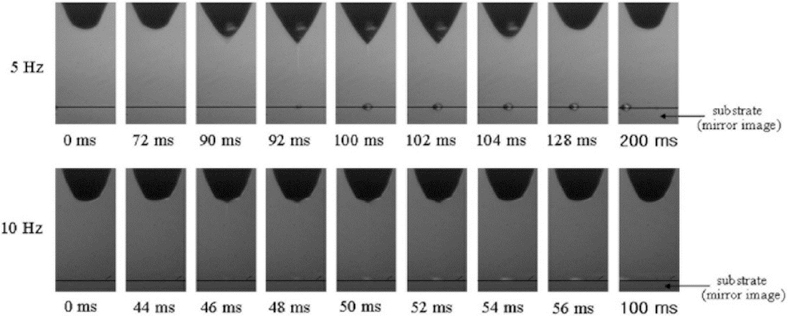
Electrohydrodynamic inkjet (EHIJ) printers use electric force to push ink through orifice [49]. Figure 4(A5) shows the schematic of this type of DOD printer. A high voltage (0.5–20 kV) between the nozzle and the substrate acts as a source of the electric force, which overcomes the surface tension of the orifice and ejects the droplet from the nozzle. The high voltage and flow rate of the ink are the drawbacks of this method [50]. A continuous ink stream in this technique creates a continuous cone, which is known as cone-jet-mode. Figure 7 shows the formed cone-jet-mode pulse. A low voltage in this technique results in a dripping mode and distinct droplet formation [33].
Figure 7.
Sequence images of the formed droplet at the nozzle tip with frequencies of 5 and 10 Hz. (Reprinted from [51] with permission of Elsevier).
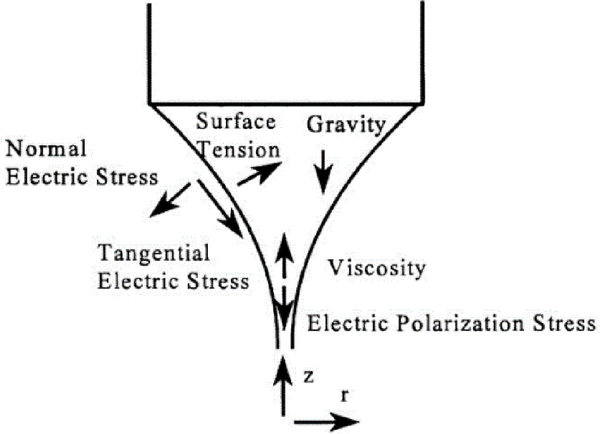
Figure 8 shows all the forces applied to the liquid at the nozzle of this type of printer.
Figure 8.
Schematic showing the forces associated with the liquid cone (Reprinted from [52] with permission).
2.2. Continuous inkjet printer
In the continuous inkjet printer, a pressure wave is applied to the ink stream, which vibrates the nozzle and breaks up the ink into droplets of uniform size (Figure 4(A1)) [53]. These bioprinters continuously eject a stream of fluids droplet even when the droplets are not required. This feature of continuous inkjet technology may result in wastage of the ink. One of the main advantages of continuous inkjet printers is high speed; the nozzle does not clog easily due to continuous droplet generation. However, the resolution associated with this approach is low. Furthermore, maintenance of these printers is relatively expensive [54].
Table 1 summarizes recent inkjet printing studies for various drug carriers and the materials for adjusting drug release profiles.
Table 1.
Summary of the recent studies that used 3D bioprinting technology for drug discovery.
| Ink | Drug | Disease | Printing technology | Release adjuster | Advantages | Ref |
|---|---|---|---|---|---|---|
| hyaluronic acid | ropinirole | Parkinson | PIJ printer | 60% (15 min) | [55] | |
| PEGDA, eosin Y, methoxide-poly(ethylene glycol)-amine | naproxen | anti-inflammatory drug | inkjet printer | PEG | [23] | |
| propylene glycol | paracetamol, theophylline, | fever, asthma | inkjet printer | control drug deposition and crystallization by porous substrate | [56] | |
| propylene glycol | rasagiline mesylate, | Parkinson’s disease | TIJ printer | no crystals were found on the substrate paper | [37] | |
| Kollidon SR and hydroxypropyl-methyl cellulose (HPMC) | water-soluble drugs | Kollidon SR-HPMC | water- soluble drug printing |
[57] | ||
| Sodium picosulfate | aqueous drug solutions | piezoelectric- and solenoid valve-based inkjet printer | PEG | PEG for making homogenous AIPs | [45] | |
| PLGA | paclitaxel | cancer | PIJ printer | surface area | higher surface area was associated with faster drug release | [36] |
| PLGA | hydrochloro-thiazide (HCT) | high blood pressure | inkjet printer | produced solid dispersion of poorly soluble drugs | [58] | |
| PVP | felodipine | high blood pressure | inkjet printer | uniform distribution of the active ingredient | [58] |
3. Critical process parameters
Physical restrictions along with biological and environmental criteria must be met to have consistent and functional printed patterns. The physical parameters affecting the dynamics and functionality of the printed patterns are orifice size, mechanical vibrations, matrix composition, ink viscosity, and temperature. These parameters have considerable impacts on the droplet size, the resolution, and the precision of the printed pattern.
The printer resolution and the quality of the printed pattern are the most essential characteristics in the development of a novel printing technology. In inkjet bioprinting technologies, a series of droplets are ejected and placed on the substrates; they merge to form printed patterns and lines. This drop-by-drop deposition of the inks affects the resolution of the printed patterns. Lowering the droplet size and precise control of the printing speed serve to enhance the printing quality. However, several physical factors control these two parameters [59]. The size of the droplet depends on parameters such as nozzle diameter, printing mechanism, material viscosity, and wettability of the substrate [60, 61]. In general, the smaller the print head, the higher the resolution of the printed pattern. However, the size of the print head does not always directly correlate with the resolution of the bioprinter. The droplet may spread on the substrates and rebound from the substrate, lowering the resolution. To overcome these shortcomings, the surface properties and wettability of the substrates should be modified; new inks may be developed to control the spreading and dynamics of the droplets on the substrates [62]. Reducing the surface energy and endowing the substrate with improved chemical/physical properties are effective approaches to decrease the wetting of the printed droplets and increase the printing resolution [63, 64, 65, 66]. The propensity of rebounding and splashing by the droplets on the substrates increase in more dried substrates; however, the droplets tend to spread more on wet surfaces, which results in the formation of a flat disc shape dot [36, 45]. The surface roughness of the substrate may also cause splashing the droplets, reducing the resolution of the pattern [67, 68].
Other important parameters affecting the printing resolution include (a) the distance between the printhead and the target [69], (b) the vibration of the printing set-up [70], and (c) air conditioning in the room [69]. An increase of the distance between the printhead and the target will intensify the destructive effects of the sources of error on the printing resolution. These kinds of errors can be diminished by holding the printhead on a stationary platform and utilizing a movable stage beneath the printhead that is used create the biological structure [69].
The viscosity of the ink also affects the resolution. Low viscosity inks spread more thoroughly on the substrate, resulting in lower resolution features. On the other hand, increasing the viscosity intensifies the ink surface tension, enhancing the possibility of nozzle clogging [71]. The addition of cells to the ink will result in a slight increase in viscosity [72]. The chemical compositions of the inks can also be modified to reduce viscosity and surface tension, thereby enhancing printing efficiency [73]. As an example, Inzana et al. modified a phosphoric acid-based binder solution concentration to maximize cytocompatibility and mechanical strength; supplementation with Tween 80 served to improve printing efficiency. They also dissolved collagen into the binder solution to fabricate collagen-calcium phosphate composites. Physiologic heat treatment and Tween 80 reduced the viscosity and the surface tension, respectively, enabling reliable thermal inkjet printing of the collagen solutions [74].
Using cross-linkers is an alternative for increasing printing resolution with low viscosity inks [73, 75]. The ink materials can be crosslinked after inkjet deposition using chemical, pH or ultraviolet mechanisms [76, 77]. However, the incorporation of a crosslinking step often requires a reduction in the rate of the bioprinting process. It also involves chemical modification of the chemical and material properties of ECM materials. Furthermore, some crosslinking mechanisms generate toxic products or harmful conditions to the cells; the reduced viability and functionality of the bio-printed patterns may be associated with the crosslinking process [26, 78].
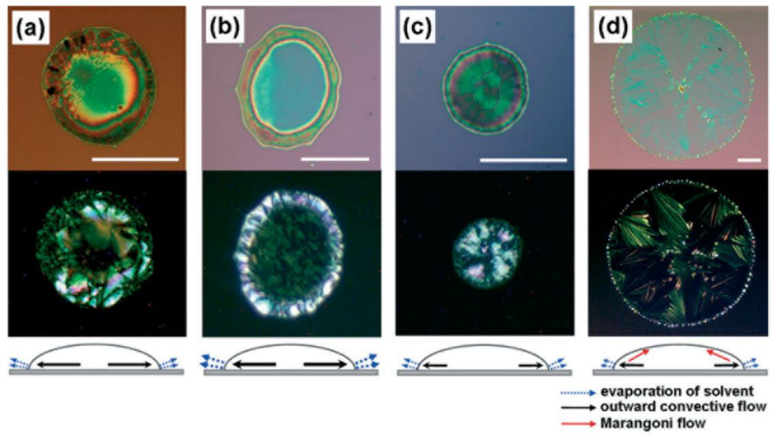
The homogeneity of the printed pattern is one of the main challenges of bio-printing technologies. It is mainly affected by the drying process of the printed droplets. The coffee ring effect and Marangoni flow are known as the two main deficiencies of an inhomogeneous drying process (Figure 9). One of the main effects of inhomogeneous evaporation of the solvents in the bio-printed patterns is an accumulation of solute material at the rim of the drying droplet, which is known as coffee ring effect. Several mechanisms have been reported to minimize this effect, which mostly involve controlled evaporation of the solvent [79, 80, 81, 82, 83, 84, 85].
Figure 9.
Optical microscope and polarized images of (triisopropylsilylethynyl) pentacene droplets in various solvents: a) chlorobenzene b) hexane, c) o-dichlorobenzene, and d) dodecane (reprinted from [88] with permission of John Wiley and Sons).
The cell density during inkjet-bioprinting should be kept low (fewer than 10 million cells/ml) to facilitate droplet formation, avoid nozzle clogging, and reduce shear stresses [86]. Some of the hydrogel crosslinking mechanisms may also be prevented by the use of higher cell concentrations [87].
4. Inks
Proper bioinks for inkjet printing technologies not only must satisfy biocompatibility and biodegradability criteria, but also must satisfy the physical restrictions of bioprinters such as resolution, orifice size, and viscosity. These limitations will restrict the choice of inks. It should be noted that the bioinks must remain in liquid form or contain a considerable amount of water for many bioprinting applications [89]. In this section, we introduce commonly used bioinks that satisfy these restrictions and review the currently published studies involving the use of these bioinks for drug discovery applications. These inks include polyethylene glycol (PEG), gelatin, collagen, fibrin, alginate, and methacrylated gelatin (GelMA) [1, 39]. Table 2 summarizes bioinks, cell types, and results from recent studies. Inks have been classified into four categories based on their applications: A) screening platform, B) drug formulation (pharmaceutical), C) bioinks (inks that contain cells), and D) druginks (inks that contain drugs).
Table 2.
Shows the previous studies materials and results for 3D bioprinting of cells
| Ink | Cell | Printing technology | Cell viability | Advantages | Ref |
|---|---|---|---|---|---|
| Retinal glial cells (curing blindness) | PIJ printer | 43% glial, 67% retinal | [44] | ||
| collagen | cancer microtissue array | PIJ printer | 100 % Hela cells | [43] | |
| sodium alginate (calcium chloride as crosslinker) | hepatocytes pluripotent stem cells, embryonic stem cells | microvalve bioprinter | >84% | using short nozzle for achieving greater cell viability | [95] |
| sodium alginate- collagen solution | amniotic fluid-derived stem cells, bovine aortic endothelial cells, and canine smooth muscle | TIJ printer | vascularization on bone functional tissue was sufficient | [96] | |
| type II collagen and glycosamino-glycans | chondrocytes (cartilage) | electro--spinning and inkjet printer | >80% (after one week) | improved biological and mechanical properties | [97] |
| fibrin collagen gel | amniotic fluid-derived stem and bone marrow-derived mesenchymal stem cells | cells were not permanent in scaffold, but delivered secreted trophic factors | re-epithelialization and closure of skin wounds became greater | [98] | |
| PEGDMA | chondrocytes, osteochondral (cartilage) cells | TIJ printer | ~ 26% | compressive modulus was near to the native tissue | [99] |
| acrylated peptides + PEG | mesenchymal stem cells | inkjet printer | 87.9 ±5.3% | minimize nozzle clogging; mechanical strength is increased | [100] |
| alginate hydrogel | hydroxyapatite (HA) | TIJ printer | satisfactory against control group | formation of a proteoglycan- and type II collagen was promoted by HA | [101] |
| calcium phosphate + Tween 80 | collagen (bone regeneration) | TIJ printer | cytocompatibility and mechanical strength were maximized | [74] |
A). Screening platforms (biological)
One of the main applications of inks in pharmaceutical science is drug screening. As an example, RodrƖguez-Devora et al. used alginate ink to develop a miniature drug-screening platform for realistic, inexpensive, and rapid evaluation of biochemical reactions in a picoliter-scale volume [90]. To show the effectiveness of the design, they used their inkjet bioprinter to process kidney cells and showed that the cell viability was over 98% and the DNA damage was negligible. They proposed the effectiveness of this mechanism to improve the drug discovery process at the target evaluation stage.
B). Drug formulation
Another important application of inks in pharmaceutical science is to formulate drugs. As an example, Copmaan et al. examined cell proliferation and spreading in hydrogels. Slow or harsh gelation condition in silk fibroin decreased the printability of the ink; therefore, they used a two-step gelation process for silk fibroin printing, which utilized alginate as a sacrificial hydrogel. The first step was near-instantaneous ionic gelation with calcium chloride, and the second step was covalent gelation by enzymatic crosslinking with silk fibroin protein [91]. Shu et al. improved the mechanical strength of tripolyphosphate (TPP)/chitosan beads at 4 °C in the presence of gelatin. These beads can be used in drug delivery applications. They loaded the beads with a model drug to show their drug releasing efficiency. Their results show that modifying surface properties of the TPP/chitosan beads by using sodium alginate will form polyelectrolyte complex film, which will provide continuous and sustained drug release for a long period of time (around two months) [92]. Wickström et al. illustrated the possibility of formulating and printing of poorly water-soluble (BCS class II, IV), poorly permeable (BCS class III, IV), and unstable drugs with mesoporous silica nanoparticles (MSN) and PEI by the inkjet printing technology in a digital and non-contact method [93]. In this study, polyester transparency and hydroxypropylmethyl cellulose (HPMC) used as a substrate; combinations of mesoporous silica nanoparticles and polyethyleneimine, as well as the drug furosemide, were printed on the substrate. Propylene glycol was added to the ink to increase the viscosity and reduce the surface tension to reach the recommended viscosity value [93]. In order to fabricate independent functional material droplets, Choi et al. designed several ink reservoirs with poly-L-lysine, hyaluronic acid, and immunosuppressive drugs (RAPA, Cyc A, and MPA) and controlled them by software to fabricate a patterned multilayer nanofilm. Hyaluronic acid and poly-L-lysine were used to modulate the stability and release profile [94]. Sandler et al. printed the model drug substances paracetamol, theophylline, and caffeine on a paper substrate and penetrated them into porous substances. They demonstrated the ability to control both drug deposition and crystallization [56]. Acosta-Vélez engineered inks containing PEGDA (poly ethylene glycol diacrylate), eosin Y, and methoxide-poly (ethylene glycol)-amine as the cross-linkable monomer, photoinitiator, and co-initiator, respectively; these inks were used to package a hydrophobic API, naproxen. The drug release profile was also controlled by both the poly(ethylene glycol) (200 Da) plasticizer and the light exposure time of ink curing [23]. Paclitaxel has been loaded in PLGA using this approach; the physical properties of the ink were optimized for the PIJ printer. Various geometries of the drug carriers were prepared using this approach [36].
C). Bioinks
Inks may also contain cells in order to regenerate functional and live organs. This type of inks usually refers as bioink. Faulkner-Jones et al. printed human embryonic stem cells in a 3D alginate matrix to mimic hepatocyte-like cells; they examined the cell markers and concluded that they were hepatic in nature. Since a long nozzle led to more cell death, they used a short nozzle [95]. Xu et al. mixed human amniotic fluid-derived stem cells, bovine aortic endothelial cells, and canine smooth muscle cells with calcium chloride separately to prepare three bioinks for TIJ printing [96]. These three cell types were successfully transferred into a sodium- alginate collagen. Finally, artificial constructs formed functional bone constructs with vascularization. In another study, Xu et al. fabricated cartilage tissue with type II collagen and glycosaminoglycans; they illustrated that this scaffold has greater mechanical strength than fibrin-collagen gels. They also showed that the viability of chondrocytes was more than 80% one week after the printing process[97]. In another study, amniotic fluid-derived stem cells (AFS) and bone marrow-derived mesenchymal stem cells (MSCs) were suspended in a fibrin-collagen gel. This printed tissue was used at wound sites. Wound closure was satisfactory; re-epithelialization and wound closure with AFS and MSCs were significantly improved as compared with treatment using fibrin collagen [98]. Cui et al. printed polyethylene glycol dimethacrylate (PEGDMA) with human chondrocytes in a layer-by-layer manner to repair defects in osteochondral plugs (3D biopaper)[99]. The compressive modulus of printed PEGDMA was 395.73 ±80.40 kPa, which was close to the natural value for this type of tissue. The other achievement of this method was increasing the viability of printed human chondrocytes by up to 26%. Another hydrogel that satisfies many of the requirements of bioprinters is polyethylene glycol (PEG). Gao et al. developed acrylated peptides, which were co-printed with PEG using a photopolymerization process. Bone marrow-derived human mesenchymal stem cells were also printed. All of the scaffold synthesis and cell coating steps were combined into one step by bioprinting; cell viability was satisfactory (87.9 ±5.3%) using this approach. The low viscosity of PEG helped to maintain the low viscosity of the ink and minimize nozzle clogging. Mechanical strength was increased; mineral and cartilage matrix formation were excellent because of osteogenic and chondrogenic differentiation [100]. Khanarian et al. created an osteochondral interface using hydroxyapatite (HA) and alginate (Alg) hydrogel. The results of this study showed that the scaffold containing alginate and hydroxyapatite improved the deep-zone chondrocyte-mediated formation of an artificial cartilage matrix; this material exhibited greater strength than ceramic-free alginate scaffolds. The effects of HA on the response of chondrocytes were evaluated, focusing on changes in matrix production, mineralization, as well as mechanical properties of the scaffold over time. The results of this study showed that hydrogel-ceramic scaffold is appropriate for osteochondral interface tissue engineering [101]. Peng et al. printed human ovarian cancer (OVCAR-5) cells and MRC-5 fibroblasts using inkjet printers with dual nozzles (ejected droplets in a one spot at the same time)[102].
D). Drugink
Druginks are another category of inks in which pharmaceutical agents serve as ingredients. Direct-writing, drop-on-demand, and piezoelectric inkjet printing system were used for the fabrication of drug carriers with various geometries [36]. It should be noted that a high concentration of cells in ink or bioprinted objects will change the printability characteristics. For example, Billiet et al. compared the properties of methacrylamide-modified gelatin with and without the presence of hepatocarcinoma cells. Their results show that cells changed the rheological properties such as viscosity which will alter the printability by itself. The viscosity will decrease by the cell density [103]. In another study, Skardal et al. evaluated the effect of human intestinal epithelial cells on hyaluronan-based hydrogels. Their results show that there is a threshold for cell density; a greater density than this threshold value will not allow hydrogels to be formed [104]. In another study, felodipine and hydrochlorothiazide were printed with PVP (polyvinyl pyrrolidone) and PLGA polymers. Both felodipine and hydrochlorothiazide are considered poorly soluble drugs [58]. This shortcoming was addressed by the stability of the solid dispersion technique. The PVP produced a uniform API/excipient(felodipine) distribution; however, the HCT distribution in PLGA was heterogeneous [58]. Ropinirole, a non-ergoline dopamine agonist, is an example of a highly hydrophilic drug that is used for the treatment of Parkinson’s disease and restless legs syndrome. It was printed in a tablet shape using a synthesized hyaluronic acid photocurable ink. Over 80% of the drug was released in 30 minutes [55].
E). Other application of inkjet bioprinter in medical sciences
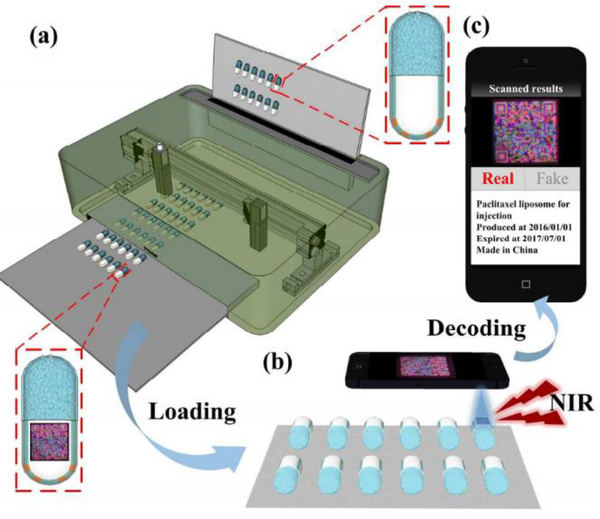
Counterfeit drugs lead to significant human morbidity and mortality. You et al. reported a low-cost upconversion fluorescent 3D QR code for tracking drugs and finding counterfeit ones using the inkjet printer. This process involves two steps. First, a 3D QR code is printed on capsule using the inkjet printer. Second, the printed code on the capsules is identified by smartphones. They claimed that this technology could provide high throughput, high information capacity, and reliability for anti-counterfeiting applications[17]. Figure 10 shows 3D QR inkjet printing on capsules and drug identification by smart phone.
Figure 10.
3D QR inkjet printing schematic on capsules and identifying by smartphones. a) Printing 3D QR code on capsule by an inkjet printer. b) Identifying the printed code on capsuled by smart phones. c) The capsule before printing QR code ([17]-Reproduced by permission of The Royal Society of Chemistry)
5. Regulatory Considerations
Similar to other medical and pharmaceutical technologies, inkjet bioprinters must satisfy regulatory requirements. Several regulatory considerations have been described by the US Food & Drug Administration (FDA) for additive manufacturing processes, including the inkjet printing process [105]. Various phases of development, including the production process, process validation, semi-finished device testing, and finished device testing, must be considered [105]. Readers may consult the published document for additional details [105]. The overall process of developing the inkjet bioprinting technologies, including the use of inkjet bioprinting for drug discovery applications, can be categorized in the following steps:
-
Design: The inkjet bioprinting process provides the capability to create structures for drug discovery applications that cannot be readily made using other manufacturing processes. However, the FDA suggests comparing the required resolution and feature size of the final finished device to the minimum possible feature size of the available inkjet printing process and the associated manufacturing tolerances to understand the suitability of the inkjet bioprinting process for the desired application.
The inkjet bioprinting process usually consists of several steps to build a device. The FDA recommends preparing a production flow diagram to summarize the steps from the initial device design to the final device. It will be helpful in all phases of product development. Furthermore, root-cause-analysis of the possible failures of the device will be made more straightforward by using this diagram.
Software workflow: This inkjet bioprinting processes usually involves the utilization of several interacting software packages. The files need to be compatible with all the software that is utilized in the process. Furthermore, the process of converting the digital device design needs to be well defined and regulated to assure the accuracy of the inkjet bioprinting process.
- Material Control: The raw materials used in the inkjet bioprinting process play an important role in the accuracy of the bioprinting process and the functionality of the bioprinted structure. This process by may also significantly alter physical and chemical properties of the feedstock materials. The FDA recommends that manufacturers document the identity of the feedstock materials, the material suppliers, the incoming material specifications, and the material certificates of analysis to ensure the consistency of the starting (raw) materials and the final product. For materials are reused in the bioprinting process, the FDA recommends describing the material reuse process. The inkjet bioprinters usually print inks in the form of liquid, polymer, or monomer mixtures. The documented specifications include the following parameters:
- For fluids: viscosity, viscoelasticity, and pot life (duration over which the material is curing) for fluids
- For polymer/monomer: composition, purity, water content, molecular formula, chemical structure, molecular weight, molecular weight distribution, glass transition temperature, melting point temperature, and crystallization point temperature
Process validation: The quality of the printed feature is affected by the inkjet bioprinting process parameters, process steps, and feedstock materials. Based on FDA regulations, analysis of each of these parameters on the final printed feature is critical to ensure the quality of the printed features. Furthermore, revalidation of the process will be essential in the case of any changes in the manufacturing process and/or the feedstock materials.
Postprocessing: The accuracy and resolution of the printed features can be affected by the postprocessing technique. The FDA recommends documenting all of the postprocessing steps and the possible effects of these steps on the final structure.
6. Conclusions
Inkjet printing technology has several advantages compared with other patterning technologies, such as high precision, high throughput, and low cost. Many biological and physical restrictions limit functionality and design of inkjet bioprinters. The ink viscosity, surface tension, and resolution are considered as non-biological constraints of bioprinter design. Furthermore, biocompatibility, biodegradability, mechanical strength, release profile, and cell viability are biological restrictions that should be considered when designing bioprinters. Bioprinting technologies may require approval by the regulatory agencies such as US FDA for commercial use in the medical and the pharmaceutical industries. Many studies have been conducted to advance this technology and to address the above-mentioned limitations and restrictions. More studies will also be required to surmount the current technological challenges as well as to develop more functional and reliable inkjet printing technologies.
7. Expert Opinion
This opinion covers recent developments in the use of inkjet bioprinters for drug discovery and drug screening applications. The ultimate goal is to commercialize inkjet bioprinting technology for use by the medical and pharmaceutical industries. Several limitations to conventional inkjet dispensing technology need to be addressed prior to commercialization of this technology. Formation of the proper inks is one of the main bottlenecks associated with conventional inkjet dispensing technology. Simultaneously satisfying both engineering and biological requirements may be difficult. As an example, the low-viscosity inks spread more on the substrate and are associated with lower resolution features. On the other hand, the nozzle clogging is one of the main consequences of increasing the viscosity. Adding cells to the inks will also increase the viscosity. Higher temperatures are associated with lower ink viscosity; however, higher temperatures may preclude the introduction of cells in the ink. Fabricating successful scaffolds or drug carriers is a multidisciplinary effort. As an example, chemistry and material science are required for synthesizing inks and modifying the surface properties of substrates. Pharmacists must be involved in the development and performance of drug and cell studies. Engineering is essential to develop novel types of inkjet printers. More sophisticated inkjet printing procedures are required for fragile drugs and cells. The development of new inks should satisfy these restrictions as much as possible. The surface properties of the substrates should also be modified to increase the resolution and functionality of the printed patterns. Furthermore, it should be noted that almost all the current considerations of this cutting-edge technology assume inks act as a Newtonian fluid. However, many inks of practical importance are non-Newtonian in nature. The coffee drop effect and the Marangoni flow are some of the effects that can decrease the resolution and quality of the printed features. The chemical and mechanical properties of the inks, the surface properties of the substrates, and the ink/substrate interactions should be modified to diminish the effects of these two phenomena during the inkjet printing process. Each type of inkjet printer has unique advantages and disadvantages; therefore, inkjet printer design should be customized for each application. One of the future trends of inkjet printer technology is the development of polymer inkjet printers. Systematic studies of printability and the characteristics of polymers as inks are necessary. There are many parallel technologies that need to be developed to advance inkjet printing biotechnology. For example, further development of automation and machinery of microscale devices can increase the resolutions of bio-printed patterns by reducing the vibration of printheads and increasing the accuracy of droplet ejection. Also, developments in cell patterning using inkjet printers are required. This technology is beneficial to examine diseases that cannot be replicated in animal models. Inks in this field must be optimized to facilitate commercial translation and wider use. Ink jet printing is useful for depositing an accurate drug concentration, adjusting the drug releasing profile, and developing a homogenous API of drug carriers. Some of the drugs should be released promptly while the others need continuous and/or slower release over the time. Ink printers can be used to fabricate complicated in vitro models to analyze drug delivery schemes and dosing strategies for newly developed medications. Inkjet printers need to satisfy regulatory requirements (such as US FDA regulations) prior to commercialization by the pharmaceutical and medical industries. Despite these restrictions, the utilization of inkjet dispensing technology may be seen as one of the main breakthroughs in the fields of drug discovery and drug delivery.
Article highlights.
Inkjet printers open many opportunities in the fields of drug discovery, drug delivery, and tissue engineering.
Inkjet printing technology is one of the common forms of bioprinting due to its high throughput, precision, and low cost.
Several inkjet printing parameters need to be satisfied to obtain optimized results for drug discovery and other pharmaceutical applications.
Each type of inkjet printer has unique characteristics and functionalities that need to be customized for various drugs, cells, and applications.
There are many biological and physical constraints for inks used in inkjet printing technologies.
Recent studies seek to develop inkjet printing technology for additional drug discovery applications.
Acknowledgments
Funding:
R Narayan is the recipient of fellowship funding from the NCSU Community Engaged Faculty Fellow program, the Visiting Advanced Joint Research (VAJRA) Faculty Award at the Indian Institute of Technology-BHU, the UNC Rajkumar Faculty Fellowship and the Burroughs Welcome Fund Travel Grant with the Technical University of Vienna. They have also received grant funding from: The National Science Foundation, the National Institute of Innovation in Manufacturing Biopharmaceuticals, the National Institutes of Health, the NCSU Adelaide Grant Program. He has also the recipient of a NC Space Grant. S Movahed has further received grant funding via the National Science Foundation.
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Chameettachal S, Pati F. Inkjet-based 3D bioprinting. 3D Bioprinting in Regenerative Engineering:: Principles and Applications. 2018. [Google Scholar]
- 2.Campbell PG, Miller ED, Fisher GW, et al. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials. 2005;26(33):6762–6770. [DOI] [PubMed] [Google Scholar]
- 3.Phillippi JA, Miller E, Weiss L, et al. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle‐and bone‐like subpopulations. Stem cells. 2008;26(1):127–134. [DOI] [PubMed] [Google Scholar]
- 4.Klebe RJ. Cytoscribing: a method for micropositioning cells and the construction of two-and three-dimensional synthetic tissues. Experimental cell research. 1988;179(2):362–373. [DOI] [PubMed] [Google Scholar]
- 5.Lee VK, Dai G. Printing of three-dimensional tissue analogs for regenerative medicine. Annals of biomedical engineering. 2017;45(1):115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hikita A, Chung U-i, Hoshi K, et al. Bone regenerative medicine in oral and maxillofacial region using a three-dimensional printer. Tissue Engineering Part A. 2017;23(11–12):515–521. [DOI] [PubMed] [Google Scholar]
- 7.Zohora FT, Azim AYMA. Inkjet printing: an emerging technology for 3D tissue or organ printing. European Scientific Journal, ESJ. 2014;10(30). [Google Scholar]
- 8.Nguyen DG, Pentoney SL Jr. Bioprinted three dimensional human tissues for toxicology and disease modeling. Drug Discovery Today: Technologies. 2017;23:37–44. [DOI] [PubMed] [Google Scholar]
- 9.Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. International journal of pharmaceutics. 2016;499(1–2):376–394. [DOI] [PubMed] [Google Scholar]
- 10.Peng W, Datta P, Ayan B, et al. 3D bioprinting for drug discovery and development in pharmaceutics. Acta biomaterialia. 2017;57:26–46. [DOI] [PubMed] [Google Scholar]
- 11.Prasad LK, Smyth H. 3D Printing technologies for drug delivery: a review. Drug development and industrial pharmacy. 2016;42(7):1019–1031. [DOI] [PubMed] [Google Scholar]
- 12.Daly R, Harrington TS, Martin GD, et al. Inkjet printing for pharmaceutics–a review of research and manufacturing. International journal of pharmaceutics. 2015;494(2):554–567. [DOI] [PubMed] [Google Scholar]
- 13.Matsusaki M, Sakaue K, Kadowaki K, et al. Three‐dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Advanced healthcare materials. 2013;2(4):534–539. [DOI] [PubMed] [Google Scholar]
- 14.Inamura M, Kawabata K, Takayama K, et al. Efficient generation of hepatoblasts from human ES cells and iPS cells by transient overexpression of homeobox gene HEX. Molecular Therapy. 2011;19(2):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Zheng Q, Yang H, et al. Recent advances in inkjet dispensing technologies: applications in drug discovery. Expert opinion on drug discovery. 2012;7(9):761–770. [DOI] [PubMed] [Google Scholar]
- 16.Le HP. Progress and trends in ink-jet printing technology. Journal of Imaging Science and Technology. 1998;42(1):49–62. [Google Scholar]
- 17.You M, Lin M, Wang S, et al. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale. 2016;8(19):10096–10104. [DOI] [PubMed] [Google Scholar]
- 18.You M, Zhong J, Hong Y, et al. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale. 2015;7(10):4423–4431. [DOI] [PubMed] [Google Scholar]
- 19.Park DH, Jeong W, Seo M, et al. Inkjet‐printable amphiphilic polydiacetylene precursor for hydrochromic imaging on paper. Advanced Functional Materials. 2016;26(4):498–506. [Google Scholar]
- 20.Khan Y, Pavinatto FJ, Lin MC, et al. Inkjet‐printed flexible gold electrode arrays for bioelectronic interfaces. Advanced Functional Materials. 2016;26(7):1004–1013. [Google Scholar]
- 21.Yakovlev AV, Milichko VA, Vinogradov VV, et al. Sol–Gel Assisted Inkjet Hologram Patterning. Advanced Functional Materials. 2015;25(47):7375–7380. [Google Scholar]
- 22.Delannoy P-E, Riou B, Lestriez B, et al. Toward fast and cost-effective ink-jet printing of solid electrolyte for lithium microbatteries. Journal of Power Sources. 2015;274:1085–1090. [Google Scholar]
- 23.Acosta-Vélez GF, Zhu TZ, Linsley CS, et al. Photocurable poly (ethylene glycol) as a bioink for the inkjet 3D pharming of hydrophobic drugs. International journal of pharmaceutics. 2018;546(1–2):145–153. [DOI] [PubMed] [Google Scholar]
- 24.Khaled SA, Burley JC, Alexander MR, et al. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. International journal of pharmaceutics. 2014;461(1–2):105–111. [DOI] [PubMed] [Google Scholar]
- 25.Gross BC, Erkal JL, Lockwood SY, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. ACS Publications; 2014. [DOI] [PubMed] [Google Scholar]
- 26.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nature biotechnology. 2014;32(8):773. [DOI] [PubMed] [Google Scholar]
- 27.Gomes T, Constantino C, Lopes E, et al. Thermal inkjet printing of polyaniline on paper. Thin Solid Films. 2012;520(24):7200–7204. [Google Scholar]
- 28.Okamoto T, Suzuki T, Yamamoto N. Microarray fabrication with covalent attachment of DNA using bubble jet technology. Nature biotechnology. 2000;18(4):438. [DOI] [PubMed] [Google Scholar]
- 29.Goldmann T, Gonzalez JS. DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. Journal of Biochemical and Biophysical Methods. 2000;42(3):105–110. [DOI] [PubMed] [Google Scholar]
- 30.Calvert P Inkjet printing for materials and devices. Chemistry of materials. 2001;13(10):3299–3305. [Google Scholar]
- 31.De Gans BJ, Duineveld PC, Schubert US. Inkjet printing of polymers: state of the art and future developments. Advanced materials. 2004;16(3):203–213. [Google Scholar]
- 32.Ballarin B, Fraleoni-Morgera A, Frascaro D, et al. Thermal inkjet microdeposition of PEDOT: PSS on ITO-coated glass and characterization of the obtained film. Synthetic Metals. 2004;146(2):201–205. [Google Scholar]
- 33.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. [DOI] [PubMed] [Google Scholar]
- 34.Buanz AB, Saunders MH, Basit AW, et al. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharmaceutical research. 2011;28(10):2386. [DOI] [PubMed] [Google Scholar]
- 35.Meléndez PA, Kane KM, Ashvar CS, et al. Thermal inkjet application in the preparation of oral dosage forms: dispensing of prednisolone solutions and polymorphic characterization by solid-state spectroscopic techniques. Journal of pharmaceutical sciences. 2008;97(7):2619–2636. [DOI] [PubMed] [Google Scholar]
- 36.Lee BK, Yun YH, Choi JS, et al. Fabrication of drug-loaded polymer microparticles with arbitrary geometries using a piezoelectric inkjet printing system. International journal of pharmaceutics. 2012;427(2):305–310. [DOI] [PubMed] [Google Scholar]
- 37.Genina N, Janßen EM, Breitenbach A, et al. Evaluation of different substrates for inkjet printing of rasagiline mesylate. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85(3):1075–1083. [DOI] [PubMed] [Google Scholar]
- 38.Genina N, Fors D, Vakili H, et al. Tailoring controlled-release oral dosage forms by combining inkjet and flexographic printing techniques. European Journal of Pharmaceutical Sciences. 2012;47(3):615–623. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura M, Kobayashi A, Takagi F, et al. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue engineering. 2005;11(11–12):1658–1666. [DOI] [PubMed] [Google Scholar]
- 40.Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008;29(2):193–203. [DOI] [PubMed] [Google Scholar]
- 41.Cui X, Boland T, DD’Lima D, et al. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent patents on drug delivery & formulation. 2012;6(2):149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunz-Schughart LA, Freyer JP, Hofstaedter F, et al. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. Journal of biomolecular screening. 2004;9(4):273–285. [DOI] [PubMed] [Google Scholar]
- 43.Park T-M, Kang D, Jang I, et al. Fabrication of In Vitro Cancer Microtissue Array on Fibroblast-Layered Nanofibrous Membrane by Inkjet Printing. International journal of molecular sciences. 2017;18(11):2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorber B, Hsiao W-K, Hutchings IM, et al. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2013;6(1):015001. [DOI] [PubMed] [Google Scholar]
- 45.Planchette C, Pichler H, Wimmer-Teubenbacher M, et al. Printing medicines as orodispersible dosage forms: Effect of substrate on the printed micro-structure. International journal of pharmaceutics. 2016;509(1–2):518–527. [DOI] [PubMed] [Google Scholar]
- 46.Umezu S Precision printing of gelatin utilizing electrostatic inkjet. Japanese journal of applied physics. 2014;53(5S3):05HC01. [Google Scholar]
- 47.Gasperini L, Maniglio D, Migliaresi C. Microencapsulation of cells in alginate through an electrohydrodynamic process. Journal of Bioactive and Compatible Polymers. 2013;28(5):413–425. [Google Scholar]
- 48.Poellmann MJ, Barton KL, Mishra S, et al. Patterned hydrogel substrates for cell culture with electrohydrodynamic jet printing. Macromolecular Bioscience. 2011;11(9):1164–1168. [DOI] [PubMed] [Google Scholar]
- 49.Onses MS, Sutanto E, Ferreira PM, et al. Mechanisms, capabilities, and applications of high‐resolution electrohydrodynamic jet printing. Small. 2015;11(34):4237–4266. [DOI] [PubMed] [Google Scholar]
- 50.Workman VL, Tezera LB, Elkington PT, et al. Controlled Generation of Microspheres Incorporating Extracellular Matrix Fibrils for Three‐Dimensional Cell Culture. Advanced functional materials. 2014;24(18):2648–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Oh H, Kim SS. Electrohydrodynamic drop-on-demand patterning in pulsed cone-jet mode at various frequencies. Journal of Aerosol Science. 2008;39(9):819–825. [Google Scholar]
- 52.Hartman R, Brunner D, Camelot D, et al. Electrohydrodynamic atomization in the cone–jet mode physical modeling of the liquid cone and jet. Journal of Aerosol Science. 1999;30(7):823–849. [Google Scholar]
- 53.Sweet RG. Fluid droplet recorder. Google Patents; 1971. [Google Scholar]
- 54.Li J, Rossignol F, Macdonald J. Inkjet printing for biosensor fabrication: combining chemistry and technology for advanced manufacturing. Lab on a Chip. 2015;15(12):2538–2558. [DOI] [PubMed] [Google Scholar]
- 55.Acosta-Vélez GF, Linsley CS, Craig MC, et al. Photocurable bioink for the inkjet 3D pharming of hydrophilic drugs. Bioengineering. 2017;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandler N, Määttänen A, Ihalainen P, et al. Inkjet printing of drug substances and use of porous substrates‐towards individualized dosing. Journal of pharmaceutical sciences. 2011;100(8):3386–3395. [DOI] [PubMed] [Google Scholar]
- 57.Wang C-C, Tejwani MR, Roach WJ, et al. Development of near zero-order release dosage forms using three-dimensional printing (3-DP™) technology. Drug development and industrial pharmacy. 2006;32(3):367–376. [DOI] [PubMed] [Google Scholar]
- 58.Scoutaris N, Hook AL, Gellert PR, et al. ToF-SIMS analysis of chemical heterogeneities in inkjet micro-array printed drug/polymer formulations. Journal of Materials Science: Materials in Medicine. 2012;23(2):385–391. [DOI] [PubMed] [Google Scholar]
- 59.Lee VK, Dias A, Ozturk MS, et al. 3D bioprinting and 3D imaging for stem cell engineering. Bioprinting in Regenerative Medicine: Springer; 2015. p. 33–66. [Google Scholar]
- 60.Caviezel D, Narayanan C, Lakehal D. Adherence and bouncing of liquid droplets impacting on dry surfaces. Microfluidics and Nanofluidics. 2008;5(4):469–478. [Google Scholar]
- 61.Rioboo R, Tropea C, Marengo M. Outcomes from a drop impact on solid surfaces. Atomization and Sprays. 2001;11(2). [Google Scholar]
- 62.Kuang M, Wang L, Song Y. Controllable printing droplets for high‐resolution patterns. Advanced materials. 2014;26(40):6950–6958. [DOI] [PubMed] [Google Scholar]
- 63.Ko H-Y, Park J, Shin H, et al. Rapid self-assembly of monodisperse colloidal spheres in an ink-jet printed droplet. Chemistry of materials. 2004;16(22):4212–4215. [Google Scholar]
- 64.Wang J, Zheng Z, Li H, et al. Dewetting of conducting polymer inkjet droplets on patterned surfaces. Nature materials. 2004;3(3):171. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Wang J, Zhang Y, et al. Closed-air induced composite wetting on hydrophilic ordered nanoporous anodic alumina. Applied Physics Letters. 2010;97(23):233107. [Google Scholar]
- 66.Hendriks CE, Smith PJ, Perelaer J, et al. “Invisible” silver tracks produced by combining hot‐embossing and inkjet printing. Advanced Functional Materials. 2008;18(7):1031–1038. [Google Scholar]
- 67.Latka A, Strandburg-Peshkin A, Driscoll MM, et al. Creation of prompt and thin-sheet splashing by varying surface roughness or increasing air pressure. Physical review letters. 2012;109(5):054501. [DOI] [PubMed] [Google Scholar]
- 68.Bolleddula D, Berchielli A, Aliseda A. Impact of a heterogeneous liquid droplet on a dry surface: Application to the pharmaceutical industry. Advances in colloid and interface science. 2010;159(2):144–159. [DOI] [PubMed] [Google Scholar]
- 69.Binder KW, Allen AJ, Yoo JJ, et al. Drop-on-demand inkjet bioprinting: a primer. Gene therapy and regulation. 2011;6(01):33–49. [Google Scholar]
- 70.Merrin J, Leibler S, Chuang JS. Printing multistrain bacterial patterns with a piezoelectric inkjet printer. PLoS One. 2007;2(7):e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoch E, Hirth T, Tovar GE, et al. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. Journal of Materials Chemistry B. 2013;1(41):5675–5685. [DOI] [PubMed] [Google Scholar]
- 72.Munaz A, Vadivelu RK, John JS, et al. Three-dimensional printing of biological matters. Journal of Science: Advanced Materials and Devices. 2016;1(1):1–17. [Google Scholar]
- 73.Pereira RF, Bártolo PJ. 3D bioprinting of photocrosslinkable hydrogel constructs. Journal of Applied Polymer Science. 2015;132(48). [Google Scholar]
- 74.Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selimović Š, Oh J, Bae H, et al. Microscale strategies for generating cell-encapsulating hydrogels. Polymers. 2012;4(3):1554–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio‐printing applications. Journal of Biomedical Materials Research Part A. 2013;101(1):272–284. [DOI] [PubMed] [Google Scholar]
- 77.Khalil S, Sun W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Materials Science and Engineering: C. 2007;27(3):469–478. [Google Scholar]
- 78.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Advanced drug delivery reviews. 2012;64:223–236. [DOI] [PubMed] [Google Scholar]
- 79.Teichler A, Perelaer J, Schubert US. Inkjet printing of organic electronics–comparison of deposition techniques and state-of-the-art developments. Journal of Materials Chemistry C. 2013;1(10):1910–1925. [Google Scholar]
- 80.Oh Y, Kim J, Yoon YJ, et al. Inkjet printing of Al2O3 dots, lines, and films: From uniform dots to uniform films. Current Applied Physics. 2011;11(3):S359–S363. [Google Scholar]
- 81.Kajiya T, Kobayashi W, Okuzono T, et al. Controlling profiles of polymer dots by switching between evaporation and condensation. Langmuir. 2010;26(13):10429–10432. [DOI] [PubMed] [Google Scholar]
- 82.Park JS, Kim JP, Song C, et al. Control of inkjet printed profiles by solvent-vapor annealing. Displays. 2010;31(3):164–167. [Google Scholar]
- 83.Tekin E, de Gans B-J, Schubert US. Ink-jet printing of polymers–from single dots to thin film libraries. Journal of Materials Chemistry. 2004;14(17):2627–2632. [Google Scholar]
- 84.Hu H, Larson RG. Marangoni effect reverses coffee-ring depositions. The Journal of Physical Chemistry B. 2006;110(14):7090–7094. [DOI] [PubMed] [Google Scholar]
- 85.Zhou JX, Fuh JY, Loh HT, et al. Characterization of drop-on-demand microdroplet printing. The International Journal of Advanced Manufacturing Technology. 2010;48(1–4):243–250. [Google Scholar]
- 86.Xu T, Jin J, Gregory C, et al. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26(1):93–99. [DOI] [PubMed] [Google Scholar]
- 87.Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31(24):6173–6181. [DOI] [PubMed] [Google Scholar]
- 88.Lim JA, Lee WH, Lee HS, et al. Self‐organization of ink‐jet‐printed triisopropylsilylethynyl pentacene via evaporation‐induced flows in a drying droplet. Advanced functional materials. 2008;18(2):229–234. [Google Scholar]
- 89.Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering: bioprinting. International Materials Reviews. 2014;59(8):430–448. [Google Scholar]
- 90.Rodríguez-Dévora JI, Zhang B, Reyna D, et al. High throughput miniature drug-screening platform using bioprinting technology. Biofabrication. 2012;4(3):035001. [DOI] [PubMed] [Google Scholar]
- 91.Compaan AM, Christensen K, Huang Y. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomaterials Science & Engineering. 2016;3(8):1519–1526. [DOI] [PubMed] [Google Scholar]
- 92.Shu X, Zhu K. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled release drug delivery. International Journal of Pharmaceutics. 2000;201(1):51–58. [DOI] [PubMed] [Google Scholar]
- 93.Wickström H, Hilgert E, Nyman JO, et al. Inkjet Printing of Drug-Loaded Mesoporous Silica Nanoparticles—A Platform for Drug Development. Molecules. 2017;22(11):2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi M, Hwang J, Choi J, et al. Multicomponent High-throughput Drug Screening via Inkjet Printing to Verify the Effect of Immunosuppressive Drugs on Immune T Lymphocytes. Scientific reports. 2017;7(1):6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faulkner-Jones A, Fyfe C, Cornelissen D-J, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7(4):044102. [DOI] [PubMed] [Google Scholar]
- 96.Xu T, Zhao W, Zhu J-M, et al. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34(1):130–139. [DOI] [PubMed] [Google Scholar]
- 97.Xu T, Binder KW, Albanna MZ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2012;5(1):015001. [DOI] [PubMed] [Google Scholar]
- 98.Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid‐derived stem cells accelerate healing of large skin wounds. Stem cells translational medicine. 2012;1(11):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui X, Breitenkamp K, Finn M, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Engineering Part A. 2012;18(11–12):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao G, Yonezawa T, Hubbell K, et al. Inkjet‐bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnology Journal. 2015;10(10):1568–1577. [DOI] [PubMed] [Google Scholar]
- 101.Khanarian NT, Jiang J, Wan LQ, et al. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Engineering Part A. 2011;18(5–6):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peng W, Unutmaz D, Ozbolat IT. Bioprinting towards physiologically relevant tissue models for pharmaceutics. Trends in biotechnology. 2016;34(9):722–732. [DOI] [PubMed] [Google Scholar]
- 103.Billiet T, Gevaert E, De Schryver T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. [DOI] [PubMed] [Google Scholar]
- 104.Skardal A, Atala A. Biomaterials for integration with 3-D bioprinting. Annals of biomedical engineering. 2015;43(3):730–746. [DOI] [PubMed] [Google Scholar]
- 105.Di Prima M Technical Considerations for Additive Manufactured Medical Devices. Federal Register Volume 82, Issue 232 (December 5, 2017), Silver Spring, MD. [Google Scholar]