Abstract
The large pine weevil (Hylobius abietis) is a major regeneration pest in commercial forestry. Pesticide application has historically been the preferred control method, but pesticides are now being phased out in several countries for environmental reasons. There is, thus, a need for alternative plant protection strategies. We applied methyl jasmonate (MeJA), salicylic acid (SA) or oxalic acid (OxA) on the stem of 2-year-old Norway spruce (Picea abies) plants to determine effects on inducible defenses and plant growth. Anatomical examination of stem cross-sections 9 weeks after application of 100 mM MeJA revealed massive formation of traumatic resin ducts and greatly reduced sapwood growth. Application of high concentrations of SA or OxA (500 and 200 mM, respectively) induced much weaker physiological responses than 100 mM MeJA. All three treatments reduced plant height growth significantly, but the reduction was larger for MeJA (~55%) than for SA and OxA (34-35%). Lower MeJA concentrations (5-50 mM) induced comparable traumatic resin duct formation as the high MeJA concentration but caused moderate (and non-significant) reductions in plant growth. Two-year-old spruce plants treated with 100 mM MeJA showed reduced mortality after exposure to pine weevils in the field, and this enhanced resistance-effect was statistically significant for three years after treatment.
Keywords: methyl jasmonate (MeJA), oxalic acid, Picea abies (L) Karst., Hylobius abietis, salicylic acid, traumatic resin ducts
Introduction
Norway spruce (Picea abies) and Scots pine (Pinus sylvestris) are economically important and ecologically dominant tree species in Europe’s boreal and subalpine forests (Buras and Menzel, 2019). A major challenge to reforestation of these species after harvesting is attack by the large pine weevil (Hylobius abietis), a regeneration pest that may girdle and kill young plants (Nilsson et al., 2010). As Norway spruce forests usually are regenerated by planting nursery-grown plants after clear-cutting, pine weevil impacts are most severe in spruce forests. If no preventive measures are taken, pine weevil feeding may kill 60% or more of newly planted spruce trees (Örlander and Nilsson, 1999). Historically, insecticides such as DDT, lindane, and permethrin were used to protect young spruce plants from pine weevil attack (Nilsson et al., 2010). However, the use of insecticides is now being banned or phased out in many countries due to environmental concerns (Nilsson et al., 2010). In their place, integrated pest management incorporating soil scarification, physical stem protection, and other measures may be effective in reducing pine weevil damage (Dillon and Griffin, 2008; Galko et al., 2022). Yet, another and perhaps more cost-effective protection measure could be to stimulate the trees’ own defenses using defense elicitors.
Norway spruce has multiple constitutive and inducible defenses against pests and pathogens. Constitutive defenses are always present and provide the first line of protection (Franceschi et al., 2005). These defenses include physical barriers, such as cork bark and other tissues reinforced by lignin and suberin, as well as chemical defenses such as terpenes stored in preformed resin ducts and phenolic compounds stored in polyphenolic parenchyma (PP) cells (Krokene, 2015). Upon attack, injury or other stresses, Norway spruce activates inducible defenses for enhanced protection. Inducible defenses include increased resin production in preformed resin ducts, formation of novel traumatic resin ducts (Nagy et al., 2000), and activation of PP cells (Franceschi et al., 1998; Krokene et al., 2008a). Inducible defenses can be activated immediately upon attack or be delayed/primed, whereby a plant sensitized by previous stress responds quicker or more strongly to a subsequent attack (Conrath et al., 2015; Mageroy et al., 2020a).
Many studies have shown that exogenous application of the phytohormone methyl jasmonate (MeJA) triggers inducible resistance in Norway spruce against fungal infection and insect attack through a combination of direct activation and priming of tree defenses (Martin et al., 2002; Erbilgin et al., 2006; Krokene et al., 2008b; Mageroy et al., 2020b). However, using MeJA to boost tree resistance can also have negative effects such as reducing height growth (Heijari et al., 2005; Moreira et al., 2012; Zas et al., 2014), reducing sapwood growth, reducing tracheid cell lumen area, reducing net photosynthetic rate or causing stomatal closure (Heijari et al., 2005). The magnitude of these negative effects varies among studies and with the concentration of MeJA used, suggesting that optimal usage of MeJA for practical tree protection requires knowledge about the costs and benefits associated with different dosages. Additionally, there could be other chemical priming stimuli that increase tree resistance with less negative effects.
Salicylic acid (SA) and oxalic acid (OxA) are two candidate chemicals that are known to elicit inducible defenses in conifers. SA, a plant hormone with a key regulatory role in inducible defenses, has been shown to accumulate after pathogen infection in Norway spruce (Kozlowski and Metraux, 1998; Kozlowski et al., 1999; Likar and Regvar, 2008). Exogenous application of SA on Norway spruce can reduce colonization by the spruce bark beetle Ips typographus (Urbanek Krajnc et al., 2011; Felicijan et al., 2016). Treatment with SA also increases resin production in Pinus pinaster (Michavila Puente-Villegas et al., 2021) and Pinus elliotti (Rodrigues and Fett-Neto, 2009). OxA is a pathogenicity factor that enables several phytopathogenic fungi to infect their host (Ikotun, 1984; Kumari et al., 2014), but it can also directly trigger systemic resistance in plants (Doubrava et al., 1988; Singh et al., 2002; Zhang et al., 2015). Krokene et al. (2008b) found that exogenous application of OxA reduced symptoms of fungal infection in 13-year-old Norway spruce trees.
In this study, we assessed effects of exogenous application of OxA, SA, and MeJA on growth, defense responses, and resistance of 2-year-old Norway spruce plants. We also did a dose-response experiment with MeJA, the most commonly used chemical priming stimulus in Norway spruce, to test how different MeJA concentrations affects plant growth and defenses. Finally, we tested if MeJA treatment increased plant resistance to pine weevil attacks over three growing seasons in a field experiment.
Materials and methods
Treatment of plants with MeJA, SA or OxA
Two-year-old Norway spruce plants were treated with methyl jasmonate (MeJA), salicylic acid (SA) or oxalic acid (OxA) to study effects on growth and inducible defense responses. Containerized plants from two different full-sib families were obtained from a commercial nursery. Two different families were used because the nursery could not provide enough plants from a single family for two different experiments. Seedlings were potted in 8 cm pots on 5-6 June 2001 and placed in a greenhouse. When all plants had broken bud and started shoot elongation (21 June) they were treated with chemicals by carefully coating the first stem internode using a small, soft paintbrush. In experiment 1, 60 plants from one family were randomly allocated to treatment with 100 mM MeJA (in water with 0.1% Tween 20), 200 mM OxA (in water), 500 mM SA (in 60% ethanol), water with 0.1% Tween 20 or no treatment (“MeJA-OxA-SA experiment”; n = 12). Tween 20 was used to solubilize MeJA and to act as a surfactant to help spread the solution evenly on the hydrophobic bark surface (Franceschi et al., 2002). In experiment 2, 60 plants from the other family were randomly allocated to a dose-response experiment with MeJA (0, 5, 25, 50 or 100 mM MeJA in water with 0.1% Tween 20) (“MeJA dose-response experiment”; n = 12). For an overview of the experimental design, tree measurements and number of replicates, see Supplemental Figure 1.
Fungal inoculation of plants
About four weeks after chemical treatment (18 July), when the length of the apical shoot averaged 55 mm, plants were inoculated with a pathogenic fungus to test whether treatment with SA, OxA or different doses of MeJA affected plant resistance. Nine plants per treatment were inoculated with the pathogenic bluestain fungus Endoconidiophora polonica (isolate 93-208/115 from the culture collection of the Norwegian Institute of Bioeconomy Research) and three plants were left intact as uninoculated controls. This fungus is a necrotrophic pathogen vectored mainly by the spruce bark beetle Ips typographus (Kirisits, 2004). Normally, E. polonica infects the phloem and sapwood of mature Norway spruce trees that have been attacked by I. typographus but the fungus will also infect younger trees/plants if inoculated experimentally. It has therefore been used as a tool to induce tree defenses in several previous studies of resistance mechanisms in Norway spruce (e.g. Krokene et al. 2008b; Fossdal et al., 2012; Zhao et al., 2019). Plants were inoculated by incising a ~5 mm wide cut into the stem bark, extending around half the stem circumference midway up on the first internode. Inoculum, consisting of actively growing mycelium of fungus on malt agar (2% malt, 1.5% agar), was placed beneath the bark, and parafilm was wrapped around the inoculation wound to seal the bark back to the stem and to prevent contamination and excessive drying of phloem and sapwood. Five weeks after inoculation (22-23 August), stem cross-sections were cut from six inoculated plants per treatment and examined for symptoms of fungal infection under a Leitz stereomicroscope (Ernst Leitz GMBH, Wetzlar, Germany) (Supplemental Figure 1).
Stem anatomy and plant growth
Plants were harvested when they had completed shoot growth (22-23 August), ~5 weeks after fungal inoculation and ~9 weeks after chemical treatment. Stem tissues for anatomical studies were collected from three inoculated and three intact plants per treatment (Supplemental Figure 1). A stem section extending from the inoculation site and ~5 mm downwards was cut from each plant using a razor blade. The 5-mm stem sections were placed directly in fresh fixative (2% paraformaldehyde and 1.25% glutaraldehyde buffered in 50 mM L-piperazine-N-N’-bis (2-ethane sulfonic) acid, pH 7.2) and stored until further processing. Semi-thin sections (15 µm thick), including the whole cross-sectional stem area, were cut near the inoculation site using a cryotome (Cryo-Star HM 560, Microm International GmbH, Walldorf, Germany). Stem cross-sections were stained with Stevenel’s blue (del Cerro et al., 1980) and mounted with immersion oil for analysis of general anatomy, parenchyma cells, polyphenolic inclusions within these cells, and traumatic resin ducts (Nagy et al., 2000).
On each stem cross-section we measured total area of bark (i.e., all tissues outside the vascular cambium) and sapwood (both current-year sapwood and total sapwood) at 32× magnification using a Leitz stereomicroscope (Ernst Leitz GMBH, Wetzlar, Germany). We also measured the combined cross-sectional area of all resin ducts present in the sapwood and of cortical resin ducts in the phloem. On sapwood cross-sections, imaged at 5 or 10× using a Leitz Aristoplan light microscope (Ernst Leitz GMBH, Wetzlar, Germany) and bright-field optics, we counted the total number of tracheid cells in the current-year sapwood along three radial cell files per cross-section. If traumatic resin ducts were present, we also counted the number of tracheids laid down in the earlywood, before the traumatic resin ducts were formed. On phloem cross-sections imaged at 10× using the light microscope we measured the combined area of all polyphenolic inclusions inside parenchyma cells inside a randomly selected section of the secondary phloem. All cross-sections were imaged using a Leica DC200 or DC300 CCD camera (Leica Microscopy Systems Ltd., Heerbrugg) and anatomical structures were quantified as described in Nagy et al. (2004).
Height growth of the plants was determined by measuring the length of the apical shoot at the time of inoculation (18 July) and at harvesting, 5 weeks later (22-23 August) (Supplemental Figure 1). Height growth was calculated as the difference between the two measurements.
Plant resistance to the pine weevil Hylobius abietis
In a third experiment, two-year-old Norway spruce plants were obtained from a commercial nursery in October 2000 and cold-stored until spring 2001. On 14 June 2001, plants were planted out in a fresh clear-cut in Kjølstad, Ås, SE Norway. Before planting, groups of 30 plants were subjected to four different treatments: (1) treatment with 100 mM MeJA (in water with 0.1% Tween 20) on 11 May using the same procedure as for the greenhouse-grown plants, (2) spray application with the pesticide Gori 920 LX (active ingredient: permethrin, 235 gL-1) on 13 June as a positive control (this pesticide was the industry standard for chemical protection of young spruce plants in Norway at the time of the experiment), (3) mechanical wounding by puncturing the bark of the lower stem using a metal brush, or (4) no treatment (negative control). Spray application of the pesticide was done outdoors and away from the other plants, to prevent contamination of unsprayed plants. Plants were planted in a randomised complete block design, with 30 blocks containing one plant subjected to each of the four treatments (120 plants in total). On the clear-cut, plants were exposed to natural attacks of the pine weevil Hylobius abietis for three consecutive growing seasons. Each autumn, we recorded the number of plants that had been girdled and killed by the beetles.
Data treatment and statistical analysis
Statistical analyses were performed using RStudio Server (v2022.02.2 Build 485; R Core Team, 2022) and the packages emmeans (v1.7.3; Lenth et al., 2022), lme4 (Bates et al., 2015), mixlm (v1.2.6; Liland, 2022), rstatix (v0.7.0; Kassambara, 2021), and stats (v4.2.0; R Core Team, 2022). All anatomical parameters were fitted with a linear model (estimated using Ordinary Least Squares) to predict the effect of treatment on anatomical features (formula: feature ~ treatment + incoulation). We obtained linear models with a non-significant main effect of “inoculation” and a non-significant interaction term between “treatment” and “inoculation”. Therefore, we combined data from inoculated and uninoculated plants for analyses of anatomical parameters and height growth to not overfit the model. Statistical significance was determined using a one-way ANOVA (formula: feature ~ treatment) with Tukey’s HSD post-hoc testing. Because there were no statistical significant differences between the two control treatments used in the MeJA-OxA-SA experiment (water with 0.1% Tween or no treatment) we combined these two controls in the statistical analyses. Data on plant survival following weevil exposure was fitted with a logistic mixed model (estimated using Maximum Likelihood and Nelder-Mead optimizer) to predict the effect of treatment on survival status (dead or alive) (formula: survival status ~ treatment). The model included year and tree ID as random effects [formula: ~ treatment + (1|year)]. Analysis of deviance of survival data was performing using Type II Wald Chi-Square tests. Pairwise statistical significance was determined using Tukey’s HSD post-hoc testing. Figures were generated using ggfortify (Tang et al., 2016), ggplot2 (Wickham, 2016), ggpubr (v0.4.0; Kassambara, 2020), and RColorBrewer (v1.1-3, Neuwirth, 2022).
Results
Effects of MeJA, SA, and OxA on plant growth
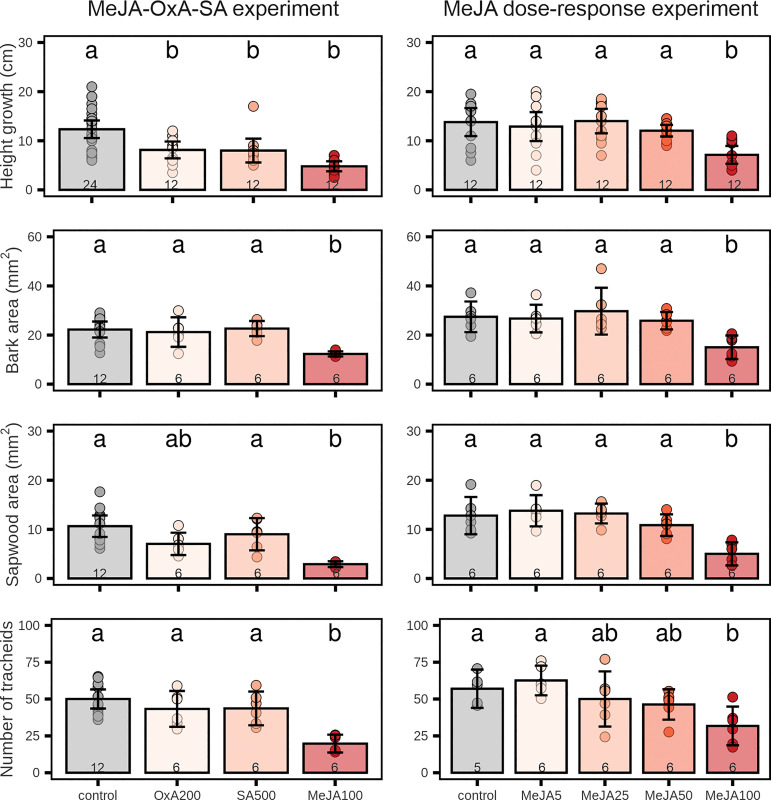
High concentrations of MeJA, SA or OxA reduced height growth significantly relative to the controls, with MeJA having the strongest effect (Figure 1). MeJA (but not OxA or SA) also inhibited xylem and phloem growth (Figures 1, 2). Growth reduction for the 100 mM MeJA treatment was greater in the MeJA-OxA-SA experiment (61% reduction relative to the control) than in the MeJA dose-response experiment (48% reduction). Trees treated with 100 mM MeJA had smaller phloem cross-sectional area than the controls (45% in both experiments), smaller current-year sapwood area (73% in MeJA-OxA-SA experiment, 61% in MeJA dose-response experiment), and laid down fewer tracheids in the current annual ring (60% in MeJA-OxA-SA experiment, 44% in MeJA dose-response experiment) (Figure 1). Lower concentrations of MeJA had small and non-significant effects on growth (Figure 1). OxA and SA reduced height growth significantly (by 34 and 35%, respectively) but had no significant effect on phloem area, sapwood area or tracheid numbers (Figure 1).
Figure 1.
Growth in 2-year-old Norway spruce plants after application of different chemicals on the stem bark. Left panels: treatment with high concentrations of salicylic acid (SA; 500 mM), oxalic acid (OxA; 200 mM) and methyl jasmonate (MeJA; 100 mM). Right panels: treatment with different MeJA concentrations (0, 5, 25, 50, 100 mM). Height growth was measured over a 9-week period after chemical treatment. Current-year sapwood area, total bark area, and tracheid numbers in the current-year sapwood were determined on stem cross-sections sampled 9 weeks after chemical treatment and visualized using a microscope. Bars represent treatment group means ± 95% confidence interval of the mean. Points represent individual replicates. Number inside the bars indicate the sample size (n). Treatment groups which do not share the same letter are significantly different (1-way ANOVA (~ treatment) followed by Tukey post-hoc test, p < 0.05).
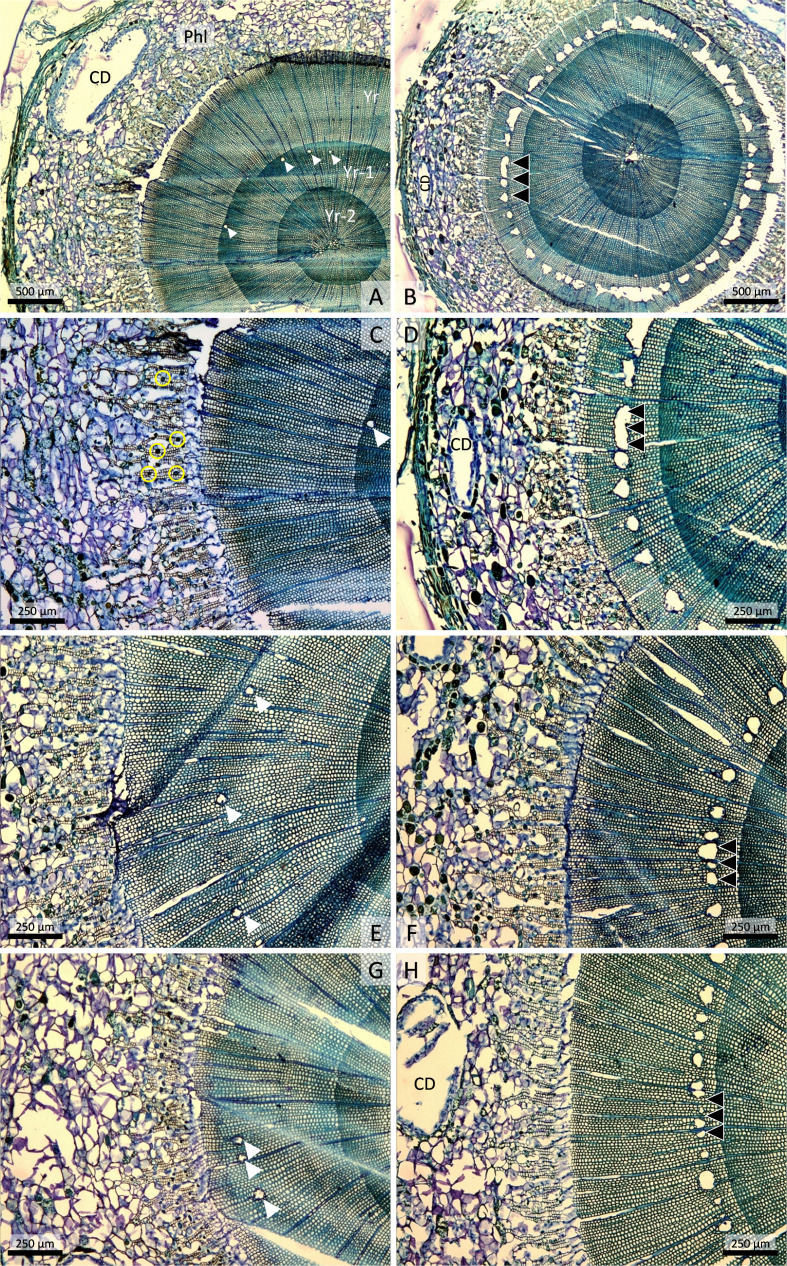
Figure 2.
Stem cross-sections of 2-year-old Norway spruce plants collected 9 weeks after the start of the experiment. (A) Untreated control plant with phloem (Phl), containing a large cortical resin duct (CD), and three annual rings of xylem growth (Yr, Yr-1, Yr-2). Scattered resin ducts (white arrowheads) are visible in the Yr-1 annual ring (Yr-1). (B) Plant treated with 100 mM methyl jasmonate (MeJA) 9 weeks earlier. An almost continuous ring of traumatic resin ducts (triple black arrowheads) is visible in the current annual xylem growth, which is much narrower than in the control. (C) Higher magnification of A showing sieve cells interspersed with rounded parenchyma cells with vacuolar polyphenolic inclusions in the phloem (small yellow circles). (D) Higher magnification of B showing traumatic resin ducts with very wide lumens and coalescence of adjoining ducts. (E) Untreated control plant that was inoculated with the fungus Endoconidiophora polonica 4 weeks after the start of the experiment. (F) Plant treated with 50 mM MeJA 9 weeks earlier has numerous traumatic resin ducts and normal annual xylem growth. (G) Plant treated with 200 mM oxalic acid 9 weeks earlier. (H) Plant treated with 5 mM MeJA 9 weeks earlier has extensive traumatic resin duct formation and normal annual xylem growth.
Plant resistance to fungal inoculation
To evaluate effects of SA, OxA and different doses of MeJA on induced resistance, plants were inoculated with the bluestain fungus E. polonica or left intact. Inoculated plants showed mild and inconsistent symptoms five weeks after inoculation. Symptomatic plants had some necrotic phloem near the inoculation site, but the necrotic area varied both within and between treatments and there were no consistent differences between control plants (not treated with chemicals) and plants treated with SA, OxA or different doses of MeJA.
Anatomical responses to MeJA, SA, and OxA
In stem cross-sections of untreated control plants, we observed phloem, mostly consisting of cortex, and three annual rings of xylem/sapwood growth (Figure 2A). The cortex, which represents primary growth, consisted of sieve cells, rounded parenchyma cells with polyphenolic vacuolar inclusions, and some large cortical resin ducts (Figures 2A, C). Only occasional axial resin ducts were observed in the last two annual layers of sapwood growth in the controls (Figures 2A, C, E). In plants treated with 100 mM MeJA, the surface areas of phloem and current-year xylem growth were smaller than in the control, and a nearly continuous ring of traumatic resin ducts was present (Figures 2B, D). Extensive traumatic resin ducts were also present in the current annual xylem growth in plants treated with lower concentrations of MeJA (Figures 2F, H). The traumatic resin ducts had wider lumens than regular axial resin ducts, and neighboring ducts had sometimes coalesced to form very wide resin ducts (Figures 2B, D). Plants treated with lower concentrations of MeJA had normal xylem growth similar to untreated controls (Figures 2F, H). Occasional axial resin ducts were also found in the xylem of control plants and plants treated with OxA, but these ducts always occurred further out in the annual ring (Figures 2E, G) and were probably induced by wounding associated with fungal inoculation (occurring 4 weeks after elicitor treatment).
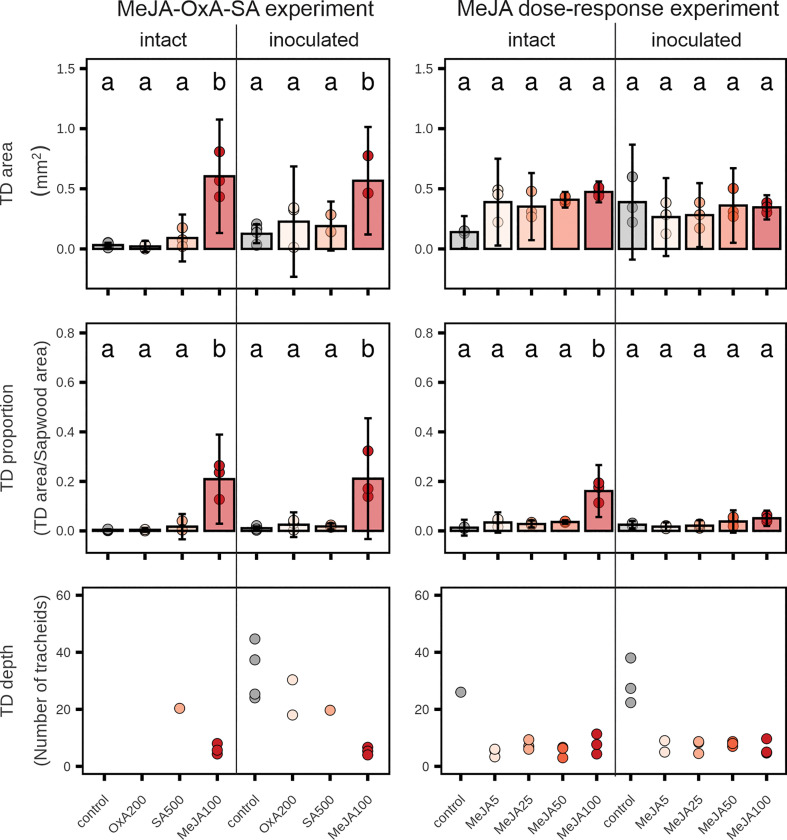
The total area of traumatic resin ducts was significantly larger in plants treated with 100 mM MeJA in the MeJA-OxA-SA experiment than in the other treatments (Figure 3). This was the case both in intact plants and in plants that had been inoculated with E. polonica (Figure 3). In the MeJA dose-response experiment, there were no significant differences in traumatic resin duct area between control and MeJA treatments. However, in uninoculated plants resin duct areas tended to be greater for all MeJA dosages compared to controls (Figure 3).
Figure 3.
Traumatic resin duct (TD) formation in 2-year-old Norway spruce plants 9 weeks after application of different chemicals on the stem bark. Four weeks after application, three plants per treatment were inoculated with the fungus Endoconidiophora polonica and three plants were left intact. Upper row: cross-sectional TD area after treatment with high concentrations of oxalic acid (OxA; 200 mM), salicylic acid (SA; 500 mM) or methyl jasmonate (MeJA; 100 mM) or different concentrations of MeJA (0, 5, 25, 50 or 100 mM with 0.1% Tween 20). Control plants in the MeJA-OxA-SA experiment were untreated or were treated with 0.1% Tween 20 (n = 3 + 3 = 6). Control plants in MeJA dose-response experiment were treated with 0.1% Tween 20 (n = 3). Middle row: cross-sectional TD area relative to the total sapwood area of the current annual ring. Lower row: the number of tracheids laid down in the xylem before the TDs were formed (only plants with resin duct formation are included; n = 1-4). Bars represent treatment group means ± 95% confidence interval of the mean. Points represent individual replicates. Treatment groups that do not share the same letter are significantly different (1-way ANOVA (~ treatment) followed by Tukey post-hoc, p < 0.05).
The proportion of total current-year sapwood area that was made up by traumatic resin ducts in the MeJA-OxA-SA experiment was significantly larger in plants treated with 100 mM MeJA than in the other treatments. The traumatic resin duct proportion was also significantly higher in plants treated with 100 mM MeJA than in other treatments in the MeJA dose-response experiment, but only in uninoculated plants (Figure 3).
The number of tracheids produced in the current-year sapwood before the traumatic resin ducts appeared was always lower in MeJA-treated plants than in control plants and plants treated with OxA or SA (Figure 3). The difference between MeJA-treated plants and non-MeJA-treated plants amounted to about 10-35 tracheids. No significant effects of OxA, SA or MeJA were observed on the size of cortical resin ducts or area of polyphenolic inclusions inside PP cells (Supplemental Figure S2).
Effect of methyl jasmonate on weevil damage
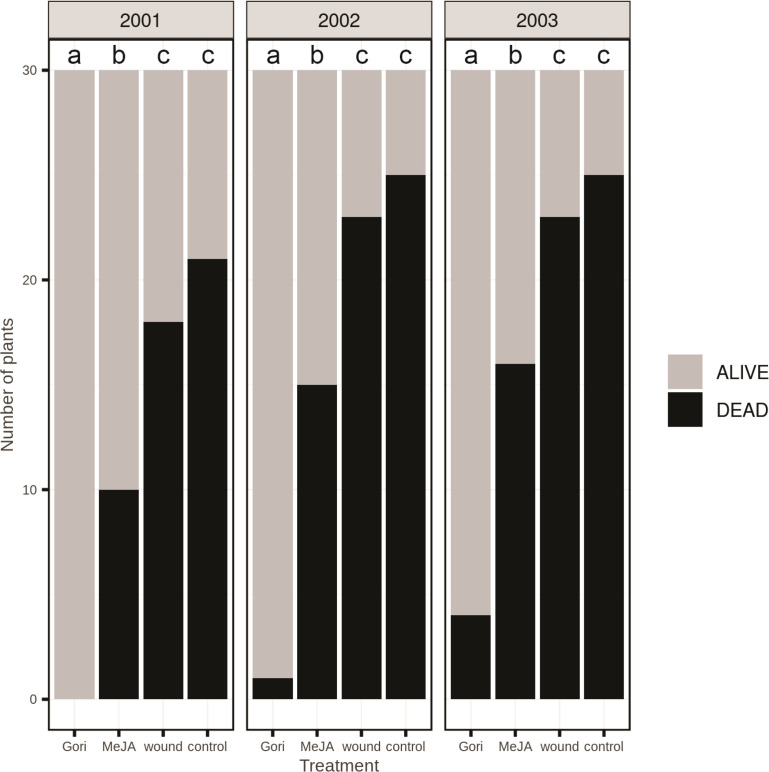
Treatment with MeJA reduced pine weevil damage considerably the first growing season, when plant mortality was only 33%, compared to 60% mortality in wounded plants and 70% in untreated control plants (Figure 4). Pesticide treatment gave complete protection the first season (no plant mortality). In the second year, mortality increased somewhat in MeJA-treated, wounded, and control plants, and accumulated mortality reached 50, 77, and 83%, respectively. Only one pesticide-treated plant died during the second year (3% accumulated mortality). In the third year, very few additional plants died in the different treatments, except for pesticide-treated plants where accumulated mortality reached 13%. Overall, pesticide treatment was most effective in reducing mortality due to pine weevil attack (χ2 (3, N = 120) = 39.9, p < 0.001). MeJA was significantly more effective than both control and wounding (p < 0.001), but less effective than the pesticide (p < 0.001). There was no significant difference between wounding and the untreated control (p = 0.62).
Figure 4.
Accumulated pine weevil-inflicted mortality of 2-year-old Norway spruce plants over three growing seasons following planting in the field. Shortly before planting, the stem of the plants was treated with the pyrethroid Gori 920 LX (Gori), 100 mM methyl jasmonate (MeJA), mechanical wounding (wound) or remained untreated as a control. Black bars = dead plants that had been completely girdled by the weevils; grey bars = plants with no or moderate weevil damage. n = 30 plants per treatment. Treatment groups that do not share the same letter are significantly different (Wald Chi square [~ treatment + (1|year)] followed by Tukey post-hoc, p < 0.05).
Discussion
The use of natural compounds to enhance plant resistance to pests and diseases is an attractive alternative crop protection concept to pesticide/fungicide application (Aranega-Bou et al., 2014; Cooper and Ton, 2022). In conifers, methyl jasmonate (MeJA) has been used for more than 20 years to study inducible defenses, including defense priming, mostly in spruce (Picea spp.) and pine (Pinus spp.) (Kozlowski and Metraux, 1998; Martin et al., 2002; Miller et al., 2005; Zeneli et al., 2006; Zulak and Bohlmann, 2010; Celedon et al., 2017; Mageroy et al., 2020a; Chen et al., 2021; Puentes et al., 2021). In this study, we quantified inducible defense responses in 2-year-old Norway spruce plants following stem application of oxalic acid (OxA), salicylic acid (SA), and MeJA. We found that MeJA induced much stronger defense responses than OxA and SA: plants treated with MeJA formed an almost continuous ring of traumatic resin ducts around the stem circumference, whereas traumatic resin ducts formed only sporadically in plants treated with OxA or SA (Figure 2). Previously, OxA has been shown to reduce disease symptoms in 13-year-old Norway spruce trees infected with a fungal pathogen, but to a much smaller extent than MeJA (Krokene et al., 2008b). SA has been found to reduce bark beetle colonization of mature Norway spruce trees when applied on the stem bark prior to beetle attack (Urbanek Krajnc et al., 2011; Felicijan et al., 2016). This plant hormone is an important activator of genes encoding pathogenesis-related proteins (Durner et al., 1997), i.e., proteins induced in response to pathogen infection (van Loon, 1997). SA accumulates in response to pathogen infection and MeJA treatment in spruce (Kozlowski and Metraux, 1998; Kozlowski et al., 1999) but there is still no evidence that SA induces conifer defense responses in planta and any mechanisms of SA-induced resistance in conifers remain unknown.
Unfortunately, we were unable to determine whether treatment with MeJA, SA or OxA increased plant resistance to fungal infection in this experiment, as the fungus failed to infect either chemically treated plants or control plants. The fungal isolate we used has been shown to be pathogenic to both 2-year-old and mature Norway spruce trees in previous studies (e.g., Krokene and Solheim, 1998; Krokene et al., 1999; Krokene et al., 2001). The restricted symptoms induced in the present study suggest that the inoculation load we used was too small relative to the resistance of the plants. In previous studies, MeJA treatment has been shown to increase resistance to fungal infection in Norway spruce. Stem treatment of mature Norway spruce trees with 5 to 100 mM MeJA reduced symptoms of fungal infection in a dose-dependent manner (Zeneli et al., 2006). Additionally, foliar treatment of 2-year-old plants with 10 mM MeJA reduced stem colonization of the bluestain fungus Grosmannia penicillata (Wilkinson et al., 2022). These previous studies demonstrate that even low MeJA concentrations can induce pathogen resistance in Norway spruce.
Application of high MeJA concentrations (100 mM) lead to massive formation of traumatic resin ducts and up to 72% reduced height or stem growth in Norway spruce plants (Figures 1, 3). Reduced growth following MeJA application has also been observed in previous studies and could be due to trade-offs between growth and defense (Heijari et al., 2005; Krokene et al., 2008b; Chen et al., 2021). We observed no toxic effects of MeJA, as the plants continued to grow for 9 weeks after MeJA application without browning, needle loss, or other visible signs of damage. Effects of MeJA on tree defenses and growth appeared to be genetically controlled, as treatment with 100 mM MeJA had more pronounced effects on growth reduction and traumatic resin duct formation in the family used in the MeJA-SA-OxA experiment than in the family used in the MeJA dose experiment (Figures 2, 4). Genotype-specific responses to MeJA-treatment have previously been observed by Zeneli et al. (2006) in mature, clonal Norway spruce trees: some clones had little or no response to MeJA, whereas others responded with extensive traumatic resin duct formation. Understanding the genetic and molecular mechanisms underlying this differential response would be of interest in future research.
Interestingly, low MeJA concentrations (5-50 mM) induced comparable traumatic resin duct formation as 100 mM MeJA but without any obvious negative effects on growth (Figures 1–3). In terms of absolute traumatic resin duct area, plants treated with low MeJA concentrations had 2-3 times more resin ducts than control plants but did not differ significantly from controls (probably due to the low number of replicates; n = 3). Stem cross-sectioning confirmed that even the lowest MeJA concentration we tested (5 mM) induced extensive traumatic resin duct formation (Figure 2H). Thus, low MeJA concentrations did induce defense responses (and, by extension, probably also resistance) in young Norway spruce plants. The fact that low MeJA concentrations elicited strong inducible defense responses with minimal negative effects on growth suggests that low concentrations may be optimal for practical tree protection, e.g., in tree nursery production. Chen et al. (2021) showed that low MeJA concentrations (10 mM) significantly reduced pine weevil feeding on 1- and 2-year-old Norway spruce plants when plants were exposed to weevil feeding for 48 hours in lab bioassays. In the field, treatment with 10 mM MeJA reduced weevil feeding by about 80% 3 and 12 months after planting but the effect was not statistically significant. The 10 mM treatment also reduced plant height growth significantly 3 and 12 months after planting (Chen et al., 2021). Thus, effects of low MeJA concentrations on growth and resistance of young Norway spruce plants appear to be variable and to depend on experimental conditions. More studies are therefore needed before optimal MeJA concentrations for robust operational plant protection in forest nurseries can be suggested.
Both MeJA treatment and inoculation/wounding induced traumatic resin duct formation in our study. The anatomical analyses suggested that in non-MeJA-treated plants traumatic resin ducts were mainly formed in response to wounding incurred by the inoculation process, as the fungus did not successfully colonize the stem. Formation of traumatic resin ducts in response to wounding has been observed in previous studies (Nagy et al., 2000; Byun-McKay et al., 2006). In our study, traumatic resin ducts in non-MeJA-treated plants occurred further out in the current-year sapwood growth compared to MeJA-treated plants, i.e., they were produced later in the season. The 10-35 additional tracheids that were produced prior to traumatic resin duct formation in non-MeJA-treated compared to MeJA-treated plants (Figure 3) correspond roughly to the number of tracheids Norway spruce trees produce in a 4-week period [the interval between chemical treatment (21 June) and fungal inoculation (18 July); Krekling et al., 2004]. Traumatic resin duct formation was always more extensive in MeJA-treated plants than in non-MeJA-treated plants (although not always significantly so). The ducts also occurred deeper in the current-year sapwood in MeJA-treated plants (Figure 3), indicating that they were induced earlier in the growing season. There were no clear differences in absolute traumatic resin duct area between MeJA concentrations, but the ducts made up a much larger proportion of the sapwood area in plants treated with the highest MeJA concentration (100 mM). This is simply because the highest MeJA concentration reduced sapwood growth several-fold compared to lower MeJA concentrations (Figures 2B, F, H).
In our field experiment we showed that treatment with 100 mM MeJA reduced pine weevil damage in 2-year-old Norway spruce plants compared to untreated plants and plants that had been mechanically wounded on the lower stem. MeJA was not as effective as pesticide application in protecting the plants but did reduce spruce mortality significantly. Furthermore, the effect lasted for three growing seasons after treatment. We did not test lower concentrations of MeJA in the field. However, Chen et al. (2021) showed that 1-year-old Norway spruce plants sprayed with 10 mM MeJA had 78% less weevil damage than untreated control plants, suggesting that low MeJA concentrations may provide good protection. However, it is probably not realistic to expect MeJA treatment to protect Norway spruce plants as effectively as pesticide application (Cooper and Ton, 2022). Rather, the best strategy for using chemical priming stimuli in crop protection is probably to integrate these stimuli with other environmentally sound control strategies, such as physical stem protection. Implementing effective integrated pest management strategies against the pine weevil is of great importance, as it will reduce the reliance on environmentally harmful pesticides.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PK: Conceptualization, Supervision, Methodology, Investigation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review and editing. KK: Methodology, Investigation, Writing – review and editing. NH: Formal analysis, Writing – original draft, Writing – review and editing. MM: Formal analysis, Visualization, Funding acquisition, Writing – original draft, Writing – review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Elin Ørmen (Norwegian University of Life Sciences) for assistance with sample processing and microscopy. Lars Dalen and Olaug Olsen (Norwegian Institute of Bioeconomy Research) helped with plant measurements and fungal inoculation.
Funding Statement
This work was supported by the Research Council of Norway (project numbers 324129 and 325319).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1155170/full#supplementary-material
Overview of experimental treatments, tree measurements, and number of replicates. Different chemicals were applied on the lower stem bark of 2-year-old Norway spruce plants: MeJA = methyl jasmonate, OxA = oxalic acid, SA = salicylic acid, Tween20 = water with 0.1% Tween20 (control). Different chemical concentrations were used in different sub-experiments (e.g., MeJA100 = 100 mM MeJA). Four weeks after treatment, plants were inoculated with the fungus Endoconidiophora polonica or left intact. Symptoms of fungal infection and different anatomical parameters were measured in the stem five weeks after fungal inoculation. Tree height growth was measured at the time of fungal inoculation and at harvest.
Quantification of anatomical defense structures in the stem bark of 2-year-old Norway spruce plants 9 weeks after application of different chemical priming stimuli. Four weeks after application, three plants per treatment were inoculated with the fungus Endoconidiophora polonica and three plants were left intact. Upper panels: cross-sectional area of polyphenolic inclusions inside phloem parenchyma cells after treatment with high concentrations of oxalic acid (OxA; 200 mM), salicylic acid (SA; 500 mM) or methyl jasmonate (MeJA; 100 mM) (left panels) or different concentrations of MeJA (0, 5, 25, 50 or 100 mM with 0.1% Tween 20) (right panels). Control plants in the left panels were untreated or were treated with 0.1% Tween 20 (n = 3 + 3 = 6). Control plants in the right panels were treated with 0.1% Tween 20 (n = 3). Lower panels: cross-sectional area of cortical resin ducts (CD). Bars represent treatment group means ± 95% confidence interval of the mean. Points represent individual replicates. No significant differences were found between treatments (1-way ANOVA (~ treatment) followed by Tukey post-hoc, p < 0.05).
References
- Aranega-Bou P., de la O Leyva M., Finiti I., García-Agustín P., González-Bosch C. (2014). Priming of plant resistance by natural compounds. hexanoic acid as a model. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 (1), 1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Buras A., Menzel A. (2019). Projecting tree species composition changes of European forests for 2061–2090 under RCP 4.5 and RCP 8.5 scenarios. Front. Plant Sci. 9, 1986. doi: 10.3389/fpls.2018.01986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun-McKay A., Godard K. A., Toudefallah M., Martin D. M., Alfaro R., King J., et al. (2006). Wound-induced terpene synthase gene expression in Sitka spruce that exhibit resistance or susceptibility to attack by the white pine weevil. Plant Physiol. 140 (3), 1009–1021. doi: 10.1104/pp.105.071803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celedon J. M., Yuen M. M. S., Chiang A., Henderson H., Reid K. E., Bohlmann J. (2017). Cell-type- and tissue-specific transcriptomes of the white spruce (Picea glauca) bark unmask fine-scale spatial patterns of constitutive and induced conifer defense. Plant J. 92 (4), 710–726. doi: 10.1111/tpj.13673 [DOI] [PubMed] [Google Scholar]
- Chen Y., Bylund H., Björkman C., Fedderwitz F., Puentes A. (2021). Seasonal timing and recurrence of methyl jasmonate treatment influence pine weevil damage to Norway spruce seedlings. New Forests 52 (3), 1–18. doi: 10.1007/s11056-020-09803-4 [DOI] [Google Scholar]
- Conrath U., Beckers G. J. M. M., Langenbach C. J. G. G., Jaskiewicz M. R. (2015). Priming for enhanced defense. Annu. Rev. Phytopathol. 53, 97–119. doi: 10.1146/annurev-phyto-080614-120132 [DOI] [PubMed] [Google Scholar]
- Cooper A., Ton J. (2022). Immune priming in plants: from the onset to transgenerational maintenance. Essays Biochem. 66 (5), 635–646. doi: 10.1042/EBC20210082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Cerro M., Standler N. S., del Cerro C. (1980). High resolution optical microscopy of animal tissues by the use of sub-micrometer thick sections and a new stain. Microscopica Acta 83 (3), 217–220. [PubMed] [Google Scholar]
- Dillon A. B., Griffin C. (2008). Controlling the large pine weevil, Hylobius abietis, using natural enemies. COFORD Connect 15, 1–8. [Google Scholar]
- Doubrava N. S., Dean R. A., Kuć J. (1988). Induction of systemic resistance to anthracnose caused by Colletotrichum lagenarium in cucumber by oxalate and extracts from spinach and rhubarb leaves. Physiol. Mol. Plant Pathol. 33 (1), 69–79. doi: 10.1016/0885-5765(88)90044-6 [DOI] [Google Scholar]
- Durner J., Shah J., Klessig D. F. (1997). Salicylic acid and disease resistance in plants. Trends Plant Sci. 2 (7), 266–274. doi: 10.1016/S1360-1385(97)86349-2 [DOI] [Google Scholar]
- Erbilgin N., Krokene P., Christiansen E., Zeneli G., Gershenzon J. (2006). Exogenous application of methyl jasmonate elicits defenses in Norway spruce (Picea abies) and reduces host colonization by the bark beetle Ips typographus . Oecologia 148 (3), 426–436. doi: 10.1007/s00442-006-0394-3 [DOI] [PubMed] [Google Scholar]
- Felicijan M., Kristl J., Krajnc A. U. (2016). Pre-treatment with salicylic acid induces phenolic responses of Norway spruce (Picea abies) bark to bark beetle (Ips typographus) attack. Trees 30 (6), 2117–2129. doi: 10.1007/s00468-016-1438-x [DOI] [Google Scholar]
- Fossdal C. G., Yaqoob N., Krokene P., Kvaalen H., Solheim H., Yakovlev I. A. (2012). Local and systemic changes in expression of resistance genes, nb-lrr genes and their putative microRNAs in Norway spruce after wounding and inoculation with the pathogen Ceratocystis polonica . BMC Plant Biol. 12, 105. doi: 10.1186/1471-2229-12-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Krekling T., Berryman A. A., Christiansen E. (1998). Specialized phloem parenchyma cells in Norway spruce (Pinaceae) bark are an important site of defense reactions. Am. J. Bot. 85 (5), 601–615. doi: 10.2307/2446529 [DOI] [PubMed] [Google Scholar]
- Franceschi V. R., Krekling T., Christiansen E. (2002). Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am. J. Bot. 89 (4), 578–586. doi: 10.3732/ajb.89.4.578 [DOI] [PubMed] [Google Scholar]
- Franceschi V. R., Krokene P., Christiansen E., Krekling T. (2005). Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 167 (2), 353–376. doi: 10.1111/j.1469-8137.2005.01436.x [DOI] [PubMed] [Google Scholar]
- Galko J., Lalík M., Rell S., Nikolov C., Barta M., Pittner J., et al. (2022). Comprehensive comparison of treatments for controlling the large pine weevil (Hylobius abietis) in central Europe. Sci. Rep. 12 (1), 9673. doi: 10.1038/s41598-022-13729-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijari J., Nerg A.-M., Kainulainen P., Viiri H., Vuorinen M., Holopainen J. K. (2005). Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in scots pine seedlings. Entomol. Exp. Appl. 115 (1), 117–124. doi: 10.1111/j.1570-7458.2005.00263.x [DOI] [Google Scholar]
- Ikotun T. (1984). Production of oxalic acid by Penicillium oxalicum in culture and in infected yam tissue and interaction with macerating enzyme. Mycopathologia 88 (1), 9–14. doi: 10.1007/BF00439288 [DOI] [Google Scholar]
- Kassambara A. (2020) Ggpubr: “ggplot2” based publication ready plots (0.4.0). Available at: https://cran.r-project.org/package=ggpubr.
- Kassambara A. (2021) Rstatix: pipe-friendly framework for basic statistical tests. Available at: https://cran.r-project.org/package=rstatix.
- Kirisits T. (2004). “Fungal associates of European bark beetles with special emphasis on the ophiostomatoid fungi,” in Bark and wood boring insects in living trees in Europe, a synthesis. Eds. Lieutier F., Day K. R., Battisti A., Gregoire J.-C., Evans H. F. (Dordrecth: Kluwer Academic Publishers; ), 181–236. [Google Scholar]
- Kozlowski G., Buchala A., Métraux J.-P. (1999). Methyl jasmonate protects Norway spruce [Picea abies (L.) Karst.] seedlings against Pythium ultimum Trow. Physiol. Mol. Plant Pathol. 55 (1), 53–58. doi: 10.1006/pmpp.1999.0205 [DOI] [Google Scholar]
- Kozlowski G., Metraux J.-P. (1998). Infection of Norway spruce (Picea abies (L.) Karst.) seedlings with Pythium irregulare Buism.and Pythium ultimum Trow.: histological and biochemical responses. Eur. J. Plant Pathol. 104, 225–234. doi: 10.1023/A:1008620309431 [DOI] [Google Scholar]
- Krekling T., Franceschi V. R., Krokene P., Solheim H. (2004). Differential anatomical response of Norway spruce stem tissues to sterile and fungus infected inoculations. Trees 18 (1), 1–9. doi: 10.1007/s00468-003-0266-y [DOI] [Google Scholar]
- Krokene P. (2015). “Conifer defense and resistance to bark beetles,” in Bark beetles - biology and ecology of native and invasive species. Eds. Vega F. E., Hofstetter R. W. (Amsterdam; Boston; Heidelberg; London; New York; Oxford; Paris; San Diego; San Francisco; Singapore; Sydney; Tokyo: Academic Press; ), 177–207. doi: 10.1016/B978-0-12-417156-5.00005-8 [DOI] [Google Scholar]
- Krokene P., Christiansen E., Solheim H., Franceschi V. R., Berryman A. A. (1999). Induced resistance to pathogenic fungi in Norway spruce. Plant Physiol. 121 (2), 565–570. doi: 10.1104/pp.121.2.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokene P., Nagy N. E., Krekling T. (2008. a). “Traumatic resin ducts and polyphenolic parenchyma cells in conifers,” in Induced plant resistance to herbivory. Ed. Schaller A. (Berlin: Springer Netherlands; ), 147–169. doi: 10.1007/978-1-4020-8182-8_7 [DOI] [Google Scholar]
- Krokene P., Nagy N. E., Solheim H. (2008. b). Methyl jasmonate and oxalic acid treatment of Norway spruce: anatomically based defense responses and increased resistance against fungal infection. Tree Physiol. 28 (1), 29–35. doi: 10.1093/treephys/28.1.29 [DOI] [PubMed] [Google Scholar]
- Krokene P., Solheim H. (1998). Pathogenicity of four blue-stain fungi associated with aggressive and nonaggressive bark beetles. Phytopathology 88 (1), 39–44. doi: 10.1094/PHYTO.1998.88.1.39 [DOI] [PubMed] [Google Scholar]
- Krokene P., Solheim H., Christiansen E. (2001). Induction of disease resistance in Norway spruce (Picea abies) by necrotizing fungi. Plant Pathol. 50 (2), 230–233. doi: 10.1046/j.1365-3059.2001.00559.x [DOI] [Google Scholar]
- Kumari S., Tayal P., Sharma E., Kapoor R. (2014). Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiological Research 169 (11), 862–872. doi: 10.1016/j.micres.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Lenth R., Singmann H., Love J., Buerkner P., Herve M. (2022). “Emmeans: estiamted marginal means, aka least-squares means,” in R package version 1.7.0. [Google Scholar]
- Likar M., Regvar M. (2008). Early defence reactions in Norway spruce seedlings inoculated with the mycorrhizal fungus Pisolithus tinctorius (Persoon) Coker & Couch and the pathogen Heterobasidion annosum (Fr.) Bref. Trees 22, 861–868. doi: 10.1007/s00468-008-0247-2 [DOI] [Google Scholar]
- Liland K. H. (2022) Mixlm: mixed model ANOVA and statistics for education (1.2.6). Available at: https://cran.r-project.org/package=mixlm.
- Mageroy M. H., Christiansen E., Långström B., Borg-Karlson A.-K., Solheim H., Björklund N., et al. (2020. a). Priming of inducible defenses protects Norway spruce against tree-killing bark beetles. Plant Cell Environ. 43 (2), 420–430. doi: 10.1111/pce.13661 [DOI] [PubMed] [Google Scholar]
- Mageroy M. H., Wilkinson S. W., Tengs T., Cross H., Almvik M., Pétriacq P., et al. (2020. b). Molecular underpinnings of methyl jasmonate-induced resistance in Norway spruce. Plant Cell Environ. 43 (8), 1827–1843. doi: 10.1111/pce.13774 [DOI] [PubMed] [Google Scholar]
- Martin D., Tholl D., Gershenzon J., Bohlmann J. (2002). Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol. 129 (3), 1003–1018. doi: 10.1104/pp.011001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michavila Puente-Villegas S., Rodríguez-García A., Rubio F., Gil L., López R. (2021). Salicylic and citric acid as promising new stimulants for resin tapping in maritime pine (Pinus pinaster Ait.). For. Syst. 29, eSC07. doi: 10.5424/fs/2020293-16737 [DOI] [Google Scholar]
- Miller B., Madilao L. L., Ralph S., Bohlmann J. (2005). Insect-induced conifer defense. white pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol. 137 (1), 369–382. doi: 10.1104/pp.104.050187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira X., Zas R., Sampedro L. (2012). “Methyl jasmonate as chemical elicitor of induced responses and anti-herbivory resistance in young conifer trees,” in Plant defence: biological control. Eds. Mérillon J. M., Ramawat K. G. (Dordrecht: Springer Netherlands; ), 345–362. doi: 10.1007/978-94-007-1933-0_15 [DOI] [Google Scholar]
- Nagy N. E., Fossdal C. G., Krokene P., Krekling T., Lönneborg A., Solheim H. (2004). Induced responses to pathogen infection in Norway spruce phloem: changes in polyphenolic parenchyma cells, chalcone synthase transcript levels and peroxidase activity. Tree Physiol. 24 (5), 505–515. doi: 10.1093/TREEPHYS/24.5.505 [DOI] [PubMed] [Google Scholar]
- Nagy N. E., Franceschi V. R., Solheim H., Krekling T., Christiansen E. (2000). Wound-induced traumatic resin duct development in stems of Norway spruce (Pinaceae): anatomy and cytochemical traits. Am. J. Bot. 87 (3), 302–313. doi: 10.2307/2656626 [DOI] [PubMed] [Google Scholar]
- Neuwirth E. (2022). RColorBrewer: ColorBrewer palettes (1.1-3). [Google Scholar]
- Nilsson U., Luoranen J., Kolström T., Örlander G., Puttonen P. (2010). Reforestation with planting in northern Europe. Scand. J. For. Res. 25, 283–294. doi: 10.1080/02827581.2010.498384 [DOI] [Google Scholar]
- Örlander G., Nilsson U. (1999). Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scand. J. For. Res. 14, 341–354. doi: 10.1080/02827589950152665 [DOI] [Google Scholar]
- Puentes A., Zhao T., Lundborg L., Björklund N., Borg-Karlson A.-K. A.-K. (2021). Variation in methyl jasmonate-induced defense among Norway spruce clones and trade-offs in resistance against a fungal and an insect pest. Plant 12. doi: 10.3389/fpls.2021.678959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2022). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing; ). Available at: http://www.R-project.org. [Google Scholar]
- Rodrigues K., Fett-Neto A. (2009). Oleoresin yield of Pinus elliottii in a subtropical climate: seasonal variation and effect of auxin and salicylic acid-based stimulant paste. Ind. Crops Prod. 30, 316–320. doi: 10.1016/j.indcrop.2009.06.004 [DOI] [Google Scholar]
- Singh U., Sarma B., Singh D., Bahadur A. (2002). Studies on exudate-depleted sclerotial development in Sclerotium rolfsii and the effect of oxalic acid, sclerotial exudate, and culture filtrate on phenolic acid induction in chickpea (Cicer arietinum). Can. J. Microbiol. 48 (5), 443–448. doi: 10.1139/w02-040 [DOI] [PubMed] [Google Scholar]
- Tang Y., Horikoshi M., Li W. (2016). Ggfortify: unified interface to visualize statistical results of popular r packages. R J. 8 (2), 478–489. doi: 10.32614/RJ-2016-060 [DOI] [Google Scholar]
- Urbanek Krajnc A., Kristl J., Ivancic A. (2011). Application of salicylic acid induces antioxidant defense responses in the phloem of Picea abies and inhibits colonization by Ips typographus . For. Ecol. Managem. 261 (3), 416–426. doi: 10.1016/j.foreco.2010.10.027 [DOI] [Google Scholar]
- van Loon L. C. (1997). Induced resistance in plants and the role of pathogenesis-related proteins. Eur. J. Plant Pathol. 103 (9), 753–765. doi: 10.1023/A:1008638109140 [DOI] [Google Scholar]
- Wickham H. (2016). ggplot2: elegant graphics for data analysis (New York: Springer-Verlag; ). Available at: https://ggplot2.tidyverse.org. [Google Scholar]
- Wilkinson S. W., Dalen L. S., Skrautvol T. O., Ton J., Krokene P., Mageroy M. H. (2022). Transcriptomic changes during the establishment of long-term methyl jasmonate-induced resistance in Norway spruce. Plant Cell Environm. 45 (6), 1891–1913. doi: 10.1111/PCE.14320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zas R., Björklund N., Nordlander G., Cendán C., Hellqvist C., Sampedro L. (2014). Exploiting jasmonate-induced responses for field protection of conifer seedlings against a major forest pest, Hylobius abietis . For. Ecol. Managem. 313, 212–223. doi: 10.1016/j.foreco.2013.11.014 [DOI] [Google Scholar]
- Zeneli G., Krokene P., Christiansen E., Krekling T., Gershenzon J. (2006). Methyl jasmonate treatment of mature Norway spruce (Picea abies) trees increases the accumulation of terpenoid resin components and protects against infection by Ceratocystis polonica, a bark beetle-associated fungus. Tree Physiol. 26 (8), 977–988. doi: 10.1093/treephys/26.8.977 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang C., Su P., Liao X. (2015). Control effect and possible mechanism of the natural compound phenazine-1-carboxamide against Botrytis cinerea . PloS One 10 (10), e0140380. doi: 10.1371/journal.pone.0140380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Kandasamy D., Krokene P., Chen J., Gershenzon J., Hammerbacher A. (2019). Fungal associates of the tree-killing bark beetle, Ips typographus, vary in virulence, ability to degrade conifer phenolics and influence bark beetle tunneling behavior. Fungal Ecol. 38, 71–79. doi: 10.1016/j.funeco.2018.06.003 [DOI] [Google Scholar]
- Zulak K. G., Bohlmann J. (2010). Terpenoid biosynthesis and specialized vascular cells of conifer defense. J. Integr. Plant Biol. 52 (1), 86–97. doi: 10.1111/j.1744-7909.2010.00910.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of experimental treatments, tree measurements, and number of replicates. Different chemicals were applied on the lower stem bark of 2-year-old Norway spruce plants: MeJA = methyl jasmonate, OxA = oxalic acid, SA = salicylic acid, Tween20 = water with 0.1% Tween20 (control). Different chemical concentrations were used in different sub-experiments (e.g., MeJA100 = 100 mM MeJA). Four weeks after treatment, plants were inoculated with the fungus Endoconidiophora polonica or left intact. Symptoms of fungal infection and different anatomical parameters were measured in the stem five weeks after fungal inoculation. Tree height growth was measured at the time of fungal inoculation and at harvest.
Quantification of anatomical defense structures in the stem bark of 2-year-old Norway spruce plants 9 weeks after application of different chemical priming stimuli. Four weeks after application, three plants per treatment were inoculated with the fungus Endoconidiophora polonica and three plants were left intact. Upper panels: cross-sectional area of polyphenolic inclusions inside phloem parenchyma cells after treatment with high concentrations of oxalic acid (OxA; 200 mM), salicylic acid (SA; 500 mM) or methyl jasmonate (MeJA; 100 mM) (left panels) or different concentrations of MeJA (0, 5, 25, 50 or 100 mM with 0.1% Tween 20) (right panels). Control plants in the left panels were untreated or were treated with 0.1% Tween 20 (n = 3 + 3 = 6). Control plants in the right panels were treated with 0.1% Tween 20 (n = 3). Lower panels: cross-sectional area of cortical resin ducts (CD). Bars represent treatment group means ± 95% confidence interval of the mean. Points represent individual replicates. No significant differences were found between treatments (1-way ANOVA (~ treatment) followed by Tukey post-hoc, p < 0.05).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.