Abstract
A phospho-tyrosine 537-ERα (p-Y537-ERα) antibody was validated for immunohistochemistry of formalin-fixed paraffin-embedded human breast cancer biopsies and used to interrogate multiple ER positive breast cancer cases present on tissue microarrays already constructed by the Manitoba Breast Tumor Bank. Nuclear p-Y537-ERα protein expression was positively associated with positive nodes (Spearman r = 0.20, P = 0.0002) and large tumor size (r = 0.13, P = 0.02). On univariate analysis, high levels of p-Y537 were associated with poor overall survival (HR = 1.65, 95% CI 1.08–2.52, P = 0.02) but not relapse free survival from breast cancer. The association with overall survival was not significant on multivariate analysis. These data support the relevance of phosphorylation at p-Y537-ERα in human breast cancers and add further support to the presence of a phosphorylation code for ERα in human breast cancer in vivo.
Keywords: Phosphorylation, Tyrosine 537, Estrogen receptor, Breast cancer, Tissue microarrays, Immunohistochemistry, Endocrine therapy
Introduction
Although phosphorylation of tyrosine 537 in the human estrogen receptor has been of interest and explored experimentally for several years, its detection in breast cancer cell lines has been inconsistent [1–3]. The detection of a mutation at this site in ERα in a biopsy sample from a human breast cancer metastasis [4], however, supports its potential relevance in human breast cancer in vivo. However, detection of phosphorylated Y537-ERα in human breast tumor biopsies has not been reported. We have optimized and validated an antibody specific for phosphorylation at Y537 on ERα for immunohistochemistry (IHC) of formalin-fixed paraffin-embedded (FFPE) breast tumor tissues and investigated p-Y537-ERα protein expression in multiple human breast cancer biopsies in order to establish relevance in human breast cancer in vivo.
Materials and Methods
Tissue Microarrays
The tissue microarrays (TMAs) used in this study were constructed prior to this study by the Manitoba Breast Tumor Bank (MBTB, CancerCare Manitoba and University of Manitoba) from over 5,000 cases that have been accrued over the period 1988 to present [5, 6]. Detailed descriptions of the MBTB, case selection, and TMA construction have been published previously [7]. There were over 450 cases represented on the original TMAs, but due to exhaustion of tumor cores from previous use of the TMAs or incomplete data for some cases, the tumor numbers (n) analyzed for some markers were less than 450. This is shown in the results section. MBTB is part of the Canadian Tumor Repository Network (http://www.CTRNet.ca) and embraces their policies and operating protocols. The MBTB operates with approval from the Research Ethics Board of the Faculty of Medicine, University of Manitoba.
Antibodies
The phospho-Y537-ERα antibody was a rabbit polyclonal affinity-purified antibody (1 mg/ml; Abcam Inc Cambridge MA) and was used at a 1/150 final dilution. Other phospho-ERα specific antibodies used for IHC were validated previously [8]. HER-2 (mouse monoclonal, clone CB11) and ERα (6F11, mouse monoclonal, Abcam, Cambridge, MA) were used as previously described [9].
Immunohistochemistry, Quantification, and Cut-Off Selection
Immunohistochemistry was performed as described previously [8], where 5 μm sections were submitted to heat-induced antigen retrieval in the presence of a tris/borate/EDTA buffer (CC1, Ventana Medical Systems, AZ, USA) using an automated tissue immunostainer (Discovery Staining Module, Ventana Medical Systems, AZ, USA). Specificity of the phospho-specific antibodies generally was determined in parallel using antibodies that had been immunoabsorbed (immunoneutralized) with ~30× excess phospho-specific peptide or non-phosphorylated peptide. Nuclear staining was assessed and H scores (0–300) were generated as previously reported [7, 8]. TMAs were evaluated by two investigators (GPS, PHW) and when discordance was found, cases were re-evaluated to reach consensus.
Statistical Methodology
Relapse-free survival was defined as the time from initial surgery to the date of clinically documented local or distant disease recurrence or death attributed to breast cancer (censors were other death and end of follow-up). Overall survival (OS) was defined as the time from initial surgery to the date of death attributable to breast cancer (censors were other deaths and end of follow-up). Survival was analyzed by Cox regression to examine hazard ratios (HR). Each model was tested and complied with the assumption of proportional hazard. Statistical analyses were performed using SAS™version 9.1. Analyses were performed with single predictors and in a multivariate model.
Results
Validation and Optimization of a Phospho-Tyrosine 537 ER Alpha Antibody for Immunohistochemistry
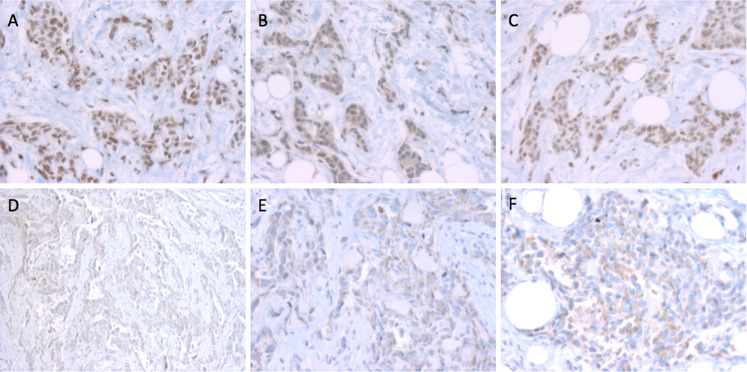
The antibody was screened for its ability to detect nuclear staining in ERα positive but not ERα negative breast tumors (Fig. 1a versus d–f). A breast cancer case showing strong nuclear staining was then chosen for further analysis. One section was stained with the p-Y537-ERα antibody (Fig. 1a) and loss of nuclear staining was observed in a serial section stained with antibody that had been immunoabsorbed with ~30× excess of the phospho-peptide used to generate the antibody (Fig. 1b), but not with excess non-phospho-peptide (Fig. 1c). It should be noted that while the excess phospho-peptide immunoneutralization lead to loss of nuclear staining (compare Fig. 1a to b), there appeared to be no loss of cytoplasmic staining (Fig. 1b), and indeed, an increase in cytoplasmic staining was sometimes seen (not shown).
Fig. 1.
Immunohistochemical validation of p-Y537-ERα phosphoantibodies in human breast cancer biopsy samples. a An ER+ (95 fmol/mg protein by ligand binding assay, LBA) breast tumor section stained with the p-Y537-ERα antibody showing strong, nuclear staining. b A serial section of the same tumor using p-Y537-ERα antibody pre-absorbed with excess of the phosphorylated peptide. c A serial section of the same tumor using p-Y537-ERα antibody pre-absorbed with excess of the non-phosphorylated ERα peptide. d–f Three different ERα negative (0 fmol/mg protein by LBA) breast tumors stained with the p-Y537-ERα antibody. All magnifications, ×500
Relationship of Phospho-Tyrosine 537 ERα to Known Prognostic Markers in Human Breast Tumor Samples
The validated p-Y537-ERα antibody was then used to stain TMA sections of FFPE ERα positive breast cancer cases. These TMAs have been previously used [7], and the clinical characteristics of these patients have been previously reported [7].
Statistically significant positive correlations were found with size (Spearman r = 0.13, P = 0.02, n = 348) and nodes involved (Spearman r = 0.20, P = 0.0002, n = 341). Furthermore, the median IHC score for p-Y537-ERα in tumors ≤2.5 cm was 85 and the median for tumors >2.5 cm was 180, and this difference was statistically significant (Mann–Whitney two-tailed, P = 0.02). The median IHC score for p-Y537-ERα in node negative patients was 70, and the median for node positive patients was 180. The difference in level of expression of p-Y537-ERα between the node negative and positive groups was statistically significant (Mann–Whitney two-tailed, P = 0.0002). Large size and positive nodal status are markers of poor prognosis; therefore, these data suggest that detection of high levels of p-Y537-ERα in primary breast tumors is associated with poor prognosis in this cohort of breast cancer patients.
Relationship of Phospho-Tyrosine 537 ERα to Other Phosphorylation Sites on ER Alpha in Human Breast Tumors
Previously, using the same TMAs, we had determined the expression of other known and novel phosphorylated sites in ERα [7, 8]. By comparing p-Y537-ERα expression with other phosphorylated sites in this cohort, p-Y537-ERα was observed to be positively correlated with p-T311-ERα (Spearman r = 0.41, P < 0.0001, n = 343) and p-S559-ERα (Spearman r = 0.24, P < 0.0001, n = 340). When the cases were dichotomized into high or low expression of p-T311-ERα using the median IHC score as a cut-off, the expression of p-Y537-ERα was significantly higher in the high p-T311-ERα versus the low p-T311-ERα (180 versus 40 median IHC score, Mann–Whitney two-tailed, P < 0.0001). Similarly, the expression of p-Y537 was significantly higher in the high p-S559-ERα versus the low p-S559-ERα expressing tumors (90 versus 25 median IHC score, Mann–Whitney two-tailed, P < 0.0001). These phosphorylation sites have been found to be previously associated with a poor prognosis in women treated with tamoxifen [7].
Relationship of Phospho-Tyrosine 537 ERα Detection in ER Positive Breast Cancers Patients Treated with Tamoxifen
The relationship of p-Y537-ERα to known and novel markers of poor prognosis in breast cancer suggested that p-Y537-ERα may also be associated with poor prognosis and potentially poor outcome to tamoxifen therapy. Since patients represented on these TMAs had surgery, plus or minus radiation, followed by adjuvant tamoxifen therapy with a median clinical follow-up was 99 months (range 9–217 months), the relationship of high versus low expression of p-Y537-ERα to clinical outcome was determined.
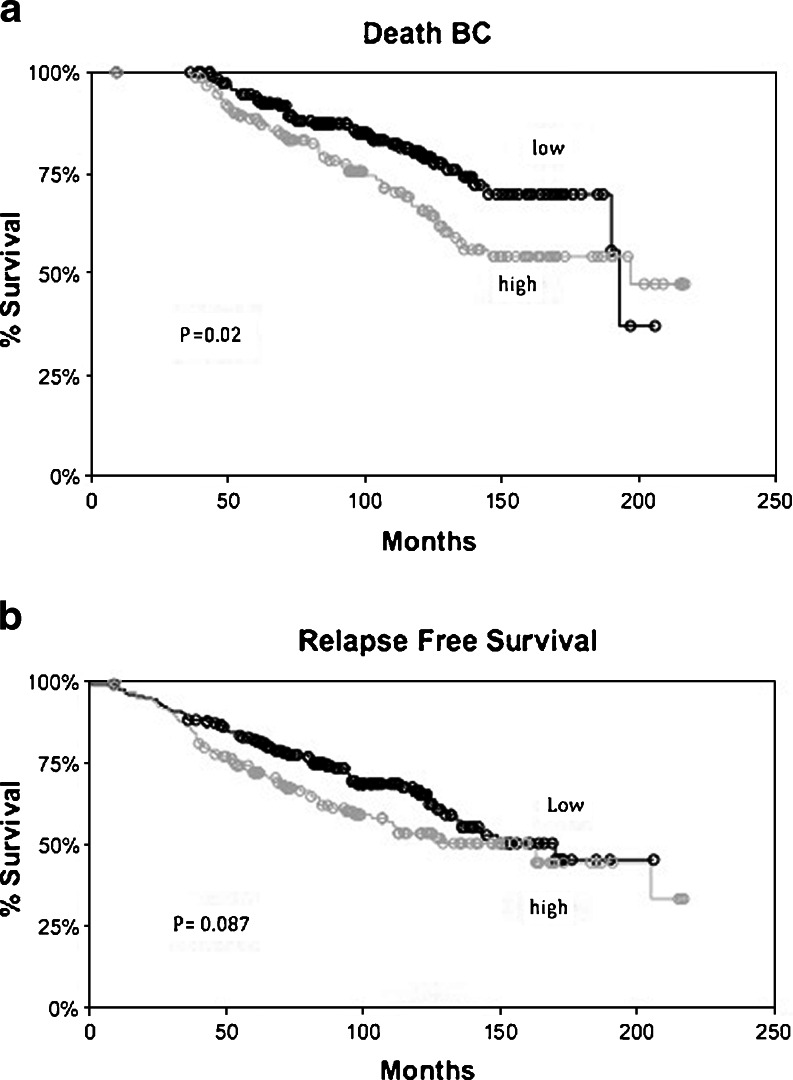
Univariate analysis for overall survival from breast cancer death (OS) is shown in Table 1. Detection of high levels of p-Y537-ERα was associated with a poorer OS from breast cancer in women treated with tamoxifen (Fig. 2a). The association of other parameters such as nodal status, size, PR levels, etc., with clinical outcome in this cohort has been previously reported [7]. Size was the only parameter that remained significantly associated with OS from breast cancer death after multivariate analysis. High expression of p-Y537-ERα was not found to be associated with relapse free survival (Table 2, Fig. 2b). These data suggest that high expression of p-Y537-ERα is not an independent factor associated with clinical outcome.
Table 1.
Overall survival from death due to breast cancer
| Predictor | N | HR | 95% C.I. | P |
|---|---|---|---|---|
| Age > 50 years | 420 | 1.67 | 0.74–3.81 | 0.22 |
| Size > 2.5 cm | 422 | 2.27 | 1.56–3.30 | <0.0001* |
| Node +ve | 415 | 2.06 | 1.42–3.00 | 0.0002* |
| Grade | 420 | 1.38 | 1.02–1.87 | 0.035* |
| PR LBA > 20 | 421 | 0.61 | 0.42–0.87 | 0.007* |
| p-Y537 > median | 348 | 1.65 | 1.08–2.52 | 0.02* |
| Multivariate analysis | ||||
| Age > 50 year | 337 | 1.37 | 0.42–4.43 | 0.60 |
| Size > 2.5 cm | 337 | 1.90 | 1.23–2.94 | 0.0038* |
| Node +ve | 337 | 1.58 | 1.02–2.45 | 0.04* |
| Grade | 337 | 1.40 | 0.99–1.97 | 0.056 |
| PR LBA > 20 | 337 | 0.67 | 0.44–1.03 | 0.065 |
| p-Y537 > median | 337 | 1.49 | 0.96–2.30 | 0.073 |
Fig. 2.
a Kaplan–Meier graphs of overall survival from breast cancer specific death with respect to expression p-Y537-ERα (high > median H score). b Kaplan–Meier graphs of relapse free survival from breast cancer recurrence or breast cancer specific death with respect to expression of p-Y537-ERα (high > median H score). P value represents the significance of a simple survival analysis without the proportional hazard assumption that was applied in the analyses presented in Tables 1 and 2
Table 2.
Relapse-free survival from recurrence or death due to breast cancer
| Predictor | N | HR | 95% C.I. | P |
|---|---|---|---|---|
| Age > 50 year | 420 | 1.57 | 0.80–3.07 | 0.19 |
| Size > 2.5 cm | 422 | 1.85 | 1.36–2.51 | <0.0001* |
| Node +ve | 415 | 2.02 | 1.48–2.76 | <0.0001* |
| Grade | 420 | 1.45 | 1.12–1.88 | 0.0055* |
| PR LBA > 20 | 421 | 0.60 | 0.44–0.81 | 0.001* |
| p-Y537 > median | 348 | 1.35 | 0.96–1.90 | 0.087 |
| Multivariate analysis | ||||
| Age > 50 year | 337 | 0.93 | 0.40–2.15 | 0.87 |
| Size > 2.5 cm | 337 | 1.64 | 1.16–2.33 | 0.0055* |
| Node +ve | 337 | 1.68 | 1.17–2.39 | 0.0044* |
| Grade | 337 | 1.43 | 1.07–1.91 | 0.015* |
| PR LBA > 20 | 337 | 0.60 | 0.42–0.86 | 0.0047* |
| p-Y537 > median | 337 | 1.22 | 0.86–1.73 | 0.28 |
Relationship of Phospho-Tyrosine 537 ERα Expression and HER2 Expression
The expression of HER2 was determined by scoring membrane staining intensity (0, 1, 2, 3) as previously described [9]. There appeared to be an inverse relationship between p-Y537-ERα and HER2 staining (Spearman r = −0.065, P = 0.024, n = 187). Furthermore, the median H score for p-Y537-ERα in tumors where HER2 was not detected was significantly higher than those tumors which were ≥2+ for HER2 staining (90 versus 45, P = 0.027). In addition, in a small group of tumors (n = 9) that were matched for ERα, PR, and grade, no significant difference was observed in p-Y537-ERα expression in the HER2 negative versus the HER2 over-expressing group (90 versus 120, P = 0.93 Mann–Whitney). These data suggest that phosphorylation of Y537 is unlikely to be due to HER2 over-expression in ER positive breast cancer.
Discussion
Post-translational modifications (PTMs) such as phosphorylation are known to be important in regulation of protein function [10]. ERα can be phosphorylated at multiple residues including serines, threonines, and tyrosines. We and others have previously been able to establish the potential relevance in vivo of phosphorylation at several serine and threonine residues by IHC mainly in breast cancer biopsy samples [7, 11–15]. While phosphorylation at tyrosines on ERα has been of interest due to the previously determined interactions of ERα, c-Src, and growth factor receptor kinases, tyrosine phosphorylation on ERα has only been performed to date using cultured cells [1, 3, 16]. However, the finding from Suzanne Fuquas laboratory that at least one breast cancer metastasis was found to express a missense mutant ERα such that an asparagine residue replaced tyrosine 537 [4] suggests that this residue and its phosphorylation may have a role in metastasis in breast cancer in vivo. Furthermore, this mutant displayed potent estrogen independent transcriptional activity that was minimally affected by either tamoxifen or ICI164384 [4], supporting a role of tyrosine phosphorylation in breast cancer progression and possibly antiestrogen resistance. Previous evidence suggests that phosphorylation at Y-537 increases the efficiency of estrogen binding [17], and site-directed mutagenesis experiments to eliminate phosphorylation at this site resulted in increased ligand-independent transcriptional activity that was still however inhibited by antiestrogen [16]. Other experiments using an excess of a 12 amino acid peptide containing p-Y537 of human ERα demonstrated that this disrupted the dimerization of ERα such that the receptor no longer bound to DNA containing an ERE using an electromobility shift assay [18]. Furthermore, Y537 is at the base of helix 12 in ERα. Upon ligand binding, the positioning of helix 12 is critical for coactivator recruitment [19]. Interestingly, mutation of Y537 to a serine was found to impart stability to recombinant ligand binding domain fragments of ERα to facilitate crystallization in the absence of ligand [20]. It appears that the structure of the amino acid at residue 537 as well as its phosphorylation can have significant functional consequences for ERα. The limited data currently available in the literature suggest that in particular, phosphorylation at Y537 can affect stability of the receptor dimer and coactivator recruitment. Therefore, it is tempting to speculate that persistent phosphorylation at Y537, which could be concluded from our ability to detect p-Y537 (often an unstable event) in breast tumor biopsy samples, could significantly alter the cyclical and dynamic interaction of the ligand-bound receptor with target gene promoters, which is known to be critical for regulation of gene transcription [21, 22]. This could lead in turn to deregulated estrogen signaling and increased risk of disease progression. These data together with the current detection of p-Y537 in human breast cancer biopsy samples, now strongly support the relevance of phosphorylation of Y-537 in breast cancer in vivo.
The association of pY537 with large tumor size and positive node status suggested that it might be a poor prognostic marker. This was supported by a significant association of detection of high levels of nuclear p-Y537 with a poorer overall survival. However, this relationship did not remain significant on multivariate analysis suggesting that p-Y537's association with tumor size and/or nodal status was driving the significance associated with univariate analysis. Recently, we reported that some phosphorylated sites on ERα were associated with good outcome, but others were associated with poor outcome in the same patient cohort. P-Y537 was found positively correlated with two other sites, p-T311 and p-S559 also associated with poor outcome [7]; however, if we added the presence of high p-Y537 to the P7-score that we had previously developed [7], to generate a P8-score, no further value for predicting prognosis was found. Interestingly, as was noted previously, the phospho-sites associated with poor outcome seemed to cluster in the C-terminal region while those associated with good outcome were located in the N-terminal region of ERα [7]. P-Y537 reinforces this pattern, although the functional significance remains to be determined. It is speculated that differential phosphorylation in the N- versus the C-terminal regions may influence how these two regions interact with each other to recruit co-factor complexes [23, 24].
The kinases involved in phosphorylation at Y537 in vivo are unknown. Experimental data from cells in culture have suggested that HER2 and/or Src-like kinases are possible candidates. However, the detection of activated c-Src in the nucleus of ER+ breast cancer patients treated with tamoxifen is generally associated with improved patient outcome [25]. In contrast, increased expression of HER2 and/or EGFR in breast tumors is associated with poor patient outcome and when co-expressed with ERα is also associated with tamoxifen resistance [26, 27]. In the current study, no association was found between p-Y537-ERα and increased HER2 expression, and EGFR was not measured in these tumors. Interestingly, p-Y537-ERα is required for the receptor to interact with the SH2 domain of c-Src and to regulate c-Src/EGFR-like signaling [28, 29], but such interactions appear to be associated most often with the extra-nuclear, so-called non-genomic actions of ERα, and in the present study, only nuclear localization of p-Y537-ERα was measured. Recently, the nuclear localization of receptor tyrosine kinases such as EGFR, HER2, and IGF1R has been reported [30, 31], and functional roles of nuclear localized receptor tyrosine kinases in transcription, DNA synthesis and cellular proliferation, DNA repair, chemo- and radio-resistance have been suggested [32]. Importantly, high levels of nuclear localized EGFR have been correlated with poor patient outcome in breast cancer [32] as well as in other cancer sites [31]. Since nuclear localized HER2 or EGFR was not assessed in this current study, it is possible that the nuclear localized HER2 and/or EGFR may have a role in phosphorylation of ERα at p-Y537. This exciting possibility requires further investigation.
Somatic ER mutations in primary breast cancer are relatively rare [33]. Due to the paucity of banked tissue from distant breast cancer metastases, studies in this regard are also rare. However, of interest is the observation that ER mutations were found in two out of eight breast cancer metastases analyzed by Fuqua's group [4]. Fuqua's group have also identified a more frequently occurring mutation in early breast cancers and interestingly this mutation alters a residue, K303, which can undergo post-translational modification [34]. In this case, the mutation (K303R) results in the inability of the site to be acetylated and also regulates the phosphorylation of a down-stream site S305 [35]. Such data underscore the importance of PTMs of ERα and suggest the possibility that altered regulation of PTMs may have important roles in breast cancer progression.
Acknowledgements
This work was supported by the Canadian Institute of Health Research (CIHR). We acknowledge the strong support of the Cancer Care Manitoba Foundation (CCMF) for our facilities at MICB. This study was also supported by the Manitoba Breast Tumor Bank, Winnipeg, Manitoba, funded in part by CCMF and CIHR. The authors have no known conflicts of interests either financial or personal between themselves and others that might bias the work.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Arnold S, Obourn J, Jaffe H, Notides A. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by Src family tyrosines in vitro. Mol Endocrinol. 1995;9:24–33. doi: 10.1210/me.9.1.24. [DOI] [PubMed] [Google Scholar]
- 2.Marquez DC, Lee J, Lin T, Pietras RJ. Epidermal growth factor receptor and tyrosine phosphorylation of estrogen receptor. Endocr. 2001;16(2):73–81. doi: 10.1385/ENDO:16:2:073. [DOI] [PubMed] [Google Scholar]
- 3.Pietras R, Arboleda J, Reese D, Wongvipat N, Pegram M, Ramos L, Gorman C, Parker M, Sliwkowski M, Slamon D. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone independent growth in human breast cancer cells. Oncogene. 1995;10(12):2435–2446. [PubMed] [Google Scholar]
- 4.Zhang QX, Borg A, Wolf DM, Oesterreich S, Fuqua SA. An estrogen receptor mutant with strong hormone-independent activity from a metastatic breast cancer. Cancer Res. 1997;57(7):1244–1249. [PubMed] [Google Scholar]
- 5.Watson P, Snell L, Parisien M. The NCIC-Manitoba Breast Tumor Bank: a resource for applied cancer research. CMAJ. 1996;155:281–283. [PMC free article] [PubMed] [Google Scholar]
- 6.Snell L, Watson P. Breast tissue banking: collection, handling, storage, and release of tissue for breast cancer research. Meth Mol Med. 2006;120:3–24. [PubMed] [Google Scholar]
- 7.Skliris GP, Nugent ZJ, Rowan BG, Penner CR, Watson PH, Murphy LC. A phosphorylation code for oestrogen receptor-alpha predicts clinical outcome to endocrine therapy in breast cancer. Endocr Relat Cancer. 2010;17(3):589–597. doi: 10.1677/ERC-10-0030. [DOI] [PubMed] [Google Scholar]
- 8.Skliris GP, Rowan BG, Al-Dhaheri M, Williams C, Troup S, Begic S, Parisien M, Watson PH, Murphy LC. Immunohistochemical validation of multiple phospho-specific epitopes for estrogen receptor alpha (ERalpha) in tissue microarrays of ERalpha positive human breast carcinomas. Breast Cancer Res Treat. 2009;118(3):443–453. doi: 10.1007/s10549-008-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weitsman G, Li L, Skliris G, Davie J, Ung K, Curtis-Snell L, Tomes L, Watson P, Murphy L. Estrogen receptor-alpha phosphorylated at Serine 118 is present at the promoters of estrogen-regulated genes and is not altered due to Her2 over-expression. Cancer Res. 2006;66:10162–10170. doi: 10.1158/0008-5472.CAN-05-4111. [DOI] [PubMed] [Google Scholar]
- 10.Weigel N, Moore N. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007;21:2311–2319. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- 11.Murphy LC, Cherlet T, Adeyinka A, Niu Y, Snell L, Watson P. Phospho-serine-118 estrogen receptor-alpha detection in human breast tumors in vivo. Clin Cancer Res. 2004;10:1354–1359. doi: 10.1158/1078-0432.CCR-03-0112. [DOI] [PubMed] [Google Scholar]
- 12.Gee J, Robertson J, Gutteridge E, Ellis I, Pinder S, Rubini M, Nicholson R. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Nishio M, Toyama T, Sugiura H, Kondo N, Kobayashi S, Fuji Y, Iwase H. Low phosphorylation of estrogen receptor alpha (ER{alpha}) serine 118 and high phosphorylation of ER{alpha} serine 167 improve survival in ER-positive breast cancer. Endocr Relat Cancer. 2008;15:755–763. doi: 10.1677/ERC-08-0078. [DOI] [PubMed] [Google Scholar]
- 14.Holm C, Kok M, Michalides R, Fles R, Koornstra RH, Wesseling J, Hauptmann M, Neefjes J, Peterse JL, Stal O, et al. Phosphorylation of the oestrogen receptor alpha at serine 305 and prediction of tamoxifen resistance in breast cancer. J Pathol. 2009;217(3):372–379. doi: 10.1002/path.2455. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Sarwar N, Peston D, Kulinskaya E, Shousha S, Coombes R, Ali S. Phosphorylation of estrogen receptor-alpha at Ser167 is indicative of longer disease-free and overall survival in breast cancer patients. Clin Cancer Res. 2007;13:5769–5776. doi: 10.1158/1078-0432.CCR-07-0822. [DOI] [PubMed] [Google Scholar]
- 16.Weis K, Ekena K, Thomas J, Lazennec G, Katzenellenbogen B. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol Endocrinol. 1996;10:1388–1398. doi: 10.1210/me.10.11.1388. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SF, Melamed M, Vorojeikina DP, Notides AC, Sasson S. Estradiol-binding mechanism and binding capacity of the human estrogen receptor is regulated by tyrosine phosphorylation. Mol Endocrinol. 1997;11(1):48–53. doi: 10.1210/me.11.1.48. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SF, Notides AC. An antiestrogen: a phosphotyrosyl peptide that blocks dimerization of the human estrogen receptor. Proc Natl Acad Sci USA. 1995;92(16):7475–7479. doi: 10.1073/pnas.92.16.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiau A, Barstad D, Loria P, Cheng L, Kushner P, Agard D, Greene G. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/S0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 20.Nettles KW, Bruning JB, Gil G, Nowak J, Sharma SK, Hahm JB, Kulp K, Hochberg RB, Zhou H, Katzenellenbogen JA, et al. NFkappaB selectivity of estrogen receptor ligands revealed by comparative crystallographic analyses. Nat Chem Biol. 2008;4(4):241–247. doi: 10.1038/nchembio.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid G, Hubner M, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11(1):695–707. doi: 10.1016/S1097-2765(03)00090-X. [DOI] [PubMed] [Google Scholar]
- 22.Metivier R, Penot G, Hubner M, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115(6):751–763. doi: 10.1016/S0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 23.Dutertre M, Smith C. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-alpha: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y, Kitamoto T, Masuhiro Y, Watanabe M, Kase T, Metzger D, Yanagisawa J, Kato S. p300 mediates functional synergism between AF-1 and AF-2 of estrogen receptor alpha and beta by interacting directly with the N-terminal A/B domains. J Biol Chem. 2000;275:15645–15651. doi: 10.1074/jbc.M000042200. [DOI] [PubMed] [Google Scholar]
- 25.Campbell EJ, McDuff E, Tatarov O, Tovey S, Brunton V, Cooke TG, Edwards J. Phosphorylated c-Src in the nucleus is associated with improved patient outcome in ER-positive breast cancer. Br J Cancer. 2008;99(11):1769–1774. doi: 10.1038/sj.bjc.6604768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newby J, Johnston S, Smith I, Dowsett M. Expression of epidermal growth factor receptor and c-erbB2 during the development of tamoxifen resistance in human breast cancer. Clin Cancer Res. 1997;3:1643–1651. [PubMed] [Google Scholar]
- 27.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23(11):2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 28.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varricchio L, Migliaccio A, Castoria G, Yamaguchi H, de Falco A, Di Domenico M, Giovannelli P, Farrar W, Appella E, Auricchio F. Inhibition of estradiol receptor/Src association and cell growth by an estradiol receptor alpha tyrosine-phosphorylated peptide. Mol Cancer Res. 2007;5(11):1213–1221. doi: 10.1158/1541-7786.MCR-07-0150. [DOI] [PubMed] [Google Scholar]
- 30.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3(9):802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 31.Aleksic T, Chitnis MM, Perestenko OV, Gao S, Thomas PH, Turner GD, Protheroe AS, Howarth M, Macaulay VM. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70(16):6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YN, Yamaguchi H, Hsu JM, Hung MC. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene. 2010;29(28):3997–4006. doi: 10.1038/onc.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barone I, Brusco L, Fuqua SA. Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res. 2010;16(10):2702–2708. doi: 10.1158/1078-0432.CCR-09-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuqua SA, Wiltschke C, Zhang QX, Borg A, Castles CG, Friedrichs WE, Hopp T, Hilsenbeck S, Mohsin S, O'Connell P, et al. A hypersensitive estrogen receptor-alpha mutation in premalignant breast lesions. Cancer Res. 2000;60(15):4026–4029. [PubMed] [Google Scholar]
- 35.Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64(24):9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]