Abstract
Choosing effective therapy for patients with malignant pheochromocytoma or paraganglioma (PPGL) is problematic and none of the options are curative. Although combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) is an established treatment option, only a limited number of case series have been reported in the literature. To determine the efficacy of CVD in patients treated at Tokyo Women's Medical University. Retrospective review of patients treated with CVD between 1989 and 2012 was conducted. Demographics, clinical presentation, imaging, and laboratory reports were reviewed and analyzed. Efficacy of CVD was ascertained from the biochemical and tumor responses. Twenty-three patients fulfilled study criteria and 6 of these were excluded due to inadequate follow-up or discontinuance by poor general condition or adverse effects. Thus, 17 cases were included in the study. The age and duration of the disease before initiation of CVD were 54.7 ± 12.0 years and 9.1 ± 8.1 years, respectively. The follow-up period after initiation of CVD ranged from 12 to 192 months (median, 60 months). Complete or partial biochemical and/or partial tumor response was achieved in 47.1 % (responders). No significant biochemical or tumor response was seen in 23.5 % and deterioration in biochemical and tumor outcomes was seen in 29.4 % (non-responders). No patient showed complete biochemical and tumor responses. In responders, these effects were documented within 4 months after initiation of CVD with a progression-free survival of 31 to 60 months (median, 40 months). Age at the first diagnosis with PPGL was younger (P < 0.05) and the lag time to eventual diagnosis of malignant disease was longer (P < 0.05) in responders than those in non-responders. The responders had improvements in hypertension and impaired glucose tolerance. Although CVD chemotherapy is not curative for patients with malignant PPGL, it does provide approximately half of the patients with biochemical, tumor, and hypertension benefits.
Keywords: Sunitinib, Tumor Response, Dacarbazine, Biochemical Response, Tumor Volume Reduction
Pheochromocytoma and paraganglioma (PPGL) arise from chromaffin tissue and most tumors are associated with hypersecretion of catecholamines and metanephrines. Approximately 10 % of adrenal medullary tumors (pheochromocytoma) and 15 to 30 % of paragangliomas arising from sympathetic ganglions are malignant [1, 2]. Malignant PPGL cannot be diagnosed by histologic criteria of the primary tumor, but rather is confirmed by presence of metastatic disease to non-chromaffin tissue (e.g., liver, lung, bone and lymph node). In some cases, the metastatic lesions develop slowly and the diagnosis of malignancy may occur several years or several decades after the initial surgery.
Symptoms that impair the performance status of patients with malignant PPGL include fluctuation of blood pressure, palpitation, and refractory constipation due to paralytic ileus. In addition, the major causes of death include cardiac failure and lethal arrhythmias. The symptoms in these patients are caused by catecholamine excess, which may be resistant to adrenergic blockade with α- and β-adrenergic receptor blockers. Morbidity and mortality are related to tumor burden and to catecholamine excess. To date, there is no cure for metastatic PPGL. Thus, controlling chronic overproduction of catecholamines or blocking their effects and slowing tumor growth are important treatment goals.
Surgical tumor debulking may be considered in those patients with good performance status, no evidence of massive multiple metastases, and a solitary target tumor [1, 3]. 131-I-MIBG radiotherapy is associated with tumor responses in 40 % of the patients [4] in low-dose cohorts and 80 % in high-dose cohorts [5]. However, the availability of 131-I-MIBG radiotherapy is limited in Japan. α-Methyl-paratyrosine, a catecholamine synthesis inhibitor [6, 7], is an effective medical treatment to limit excess catecholamine production. However, α-methyl-paratyrosine does not have cytotoxic effects and does not result in tumor volume reduction.
Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) is a widely available treatment option. However, it has been difficult to evaluate the efficacy of CVD chemotherapy because of the low prevalence of malignant PPGL. There are only a few reports [8–11], which address the effectiveness of CVD in this rare tumor. Herein, we evaluate the efficacy and clinical correlates of CVD chemotherapy in the treatment of patients with malignant PPGL.
Patients and Methods
Patients
Twenty-three patients with malignant PPGL were treated with CVD chemotherapy at Tokyo Women’s Medical University Hospital between 1989 and 2012. The diagnosis of malignant PPGL was based on clinical and/or pathological findings. All patients had metastatic lesions in non-chromaffin tissue including bone, liver, lung and lymph nodes. Germline mutation testing for mutations in genes that predispose to PPGL (e.g., succinate dehydrogenase) was not performed in any of the patients. Patients had not been treated with 131-I-MIBG radiotherapy, anti-neoplastic therapies other than CVD therapy, or α-methyl-paratyrosine for at least 2 years before or during CVD therapy.
Twenty-one patients had predominance of norepinephrine hypersecretion. Two patients had normal plasma and urine fractionated catecholamine levels; one of these two patients had increased plasma concentration of chromogranin A (CgA). The patients with catecholamine-producing tumors had associated hypertension and tachycardia and they were treated with α-adrenergic and β-adrenergic blockers and/or a calcium channel blocker. Five patients had diabetes mellitus, one patient was treated with insulin, and four patients were treated with strict diet management.
This study has been conducted in accordance with the Helsinki Declaration on human experimentation and guideline of epidemiologic study of Japanese Ministry of Health, Labor and Welfare.
Treatment with CVD Chemotherapy
CVD chemotherapy was administered to those patients with greater than third level in Eastern Cooperative Oncology Group Performance Status, adequate bone marrow function, satisfactory hepatic and renal function tests. CVD chemotherapy was administered as originally described by Averbuch et al. [8]: a combination of cyclophosphamide (750 mg/m2 body surface area) on day 1, vincristine (1.4 mg/m2 body surface area) on day 1 and dacarbazine (600 mg/m2 body surface area) on days 1 and 2 every 21–28 days. The dosage of vincristine was limited to 2.0 mg/m2 body surface area/day according to an official instruction of the medicine in Japan. Although there was no dose titration, the treatment intervals were modified to 60–90 days in some patients after the 5th cycle for personal reasons (e.g., work schedule, economic factors).
Evaluation of the Effects of the Treatment
Responses to CVD chemotherapy were evaluated on tumor response refer to RECIST 1.1 guidelines and the standard criteria described by Averbuch et al. [8], and on biochemical response according to modified the criteria by Averbuch et al. [8].
Tumor Response
Computed tomography (CT) scan, magnetic resonance imaging (MRI), and 131-I-MIBG or 123-I-MIBG scintigraphy were performed before and after CVD chemotherapy. Total volume of tumors more than 1.5 cm in diameter was estimated. Tumor response was classified as follows: complete response = disappearance of all measurable tumor; partial response = >25 % tumor reduction; minimal response = >25 % but <30 % tumor reduction; stable disease = no significant change in tumor; and, progressive disease = the appearance of new lesion(s) or increased size >20 % of total target lesions.
Biochemical Response
Plasma and urine norepinephrine were measured in the 21 patients with catecholamine-secreting PPGL. CgA was measured in one patient with non-catecholamine-secreting PPGL. One patient whose catecholamine and CgA levels were not elevated could not be evaluated for a biochemical response to CVD chemotherapy. Biochemical response was classified as follows: complete response = normalization of the biochemical tumor markers; partial response = >50 % reduction; minimal response = >25 and <50 % reduction; no change = <25 % reduction and <25 % increase; progressive disease = >25 % increase of the biochemical tumor markers.
Overall Effectiveness and Factors Contributed to Effectiveness
The patients who showed either minimal or partial biochemical response and/or minimal or partial tumor response were defined as responders and those who showed no response and progressive disease were defined as non-responders. Clinical factors that contributed to effectiveness were analyzed.
Adverse Effects
Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/Documentation/CTCAE_Governance_2010-03-11.pdf).
Statistical Analyses
All values are expressed as mean ± SD. The Mann–Whitney U test and Kruskal–Wallis test were used to compare mean values between two groups and three groups, respectively. Statistical analysis was performed with StatView software (Abacus Concepts, Berkeley, CA). A value of P < 0.05 was considered as statistically significant.
Results
Three cases were excluded from the analyses because of inadequate follow-up. CVD chemotherapy was discontinued in three cases because of poor general condition or adverse effects (e.g., severe bone marrow suppression and liver dysfunction). Thus, 17 cases (10 males and 7 females) formed our study group (Table 1). The mean age and duration of the disease at the initiation of CVD chemotherapy were 54.7 ± 12.0 years and 9.1 ± 8.1 years, respectively. The follow-up period after initiation of CVD chemotherapy ranged from 12 to 192 months with a median of 60 months. The mean ages at the first diagnosis of PPGL and of malignant disease were 41.3 ± 17.6 years and 47.3 ± 15.1 years, respectively. The mean interval between the diagnoses of PPGL to the detection of malignant disease was 6.5 ± 8.4 years. Primary tumors were located in adrenal glands in four patients, peri-adrenal in six patients, abdominal paraaortic in four patients, urinary bladder in two patients, and cardiac in one patient. Distribution of metastatic lesions is shown in Table 2.
Table 1.
Clinical characteristics of patients with malignant pheochromocytoma or paraganglioma treated with CVD chemotherapy
| All | Respondersa | Non-respondersb with stable disease | Non-respondersb with progressive disease | |
|---|---|---|---|---|
| Number of patients | 17 | 8 | 4 | 5 |
| Age (year) | 54.6 ± 12.6 | 50.1 ± 13.8 | 57.5 ± 11.7 | 59.4 ± 11.3 |
| Age at the diagnosis of PPGL (year) | 41.3 ± 17.6 | 31.9 ± 17.4 | 44.0 ± 17.3 | 50.8 ± 14.0c |
| Age at the diagnosis of malignant disease (year) | 47.3 ± 15.1 | 43.1 ± 14.8 | 48.5 ± 17.8 | 52.2 ± 15.0 |
| Sex; M/F | 11/6 | 5/3 | 2/2 | 2/3 |
| Localization of primary tumor | ||||
| Right or left adrenal gland | 4 | 1 | 2 | 1 |
| Peri-adrenal | 6 | 2 | 0 | 4 |
| Paraaortic | 4 | 3 | 1 | 0 |
| Urinary bladder | 2 | 1 | 1 | 0 |
| Heart | 1 | 1 | 0 | 0 |
| Duration of the disease prior to initiation of CVD chemotherapy (year) | 9.1 ± 8.1 | 12.8 ± 9.6 | 5.8 ± 5.0 | 5.8 ± 5.4c |
| Time interval between the diagnosis of PPGL and detection of malignant disease (year) | 6.5 ± 8.4 | 12.3 ± 10.1 | 4.5 ± 5.3 | 1.4 ± 1.3c |
Data shown as mean ± standard deviation
CVD cyclophosphamide, vincristine, dacarbazine; PPGL pheochromocytoma or paraganglioma
aResponders; patients with either any biochemical responses and/or any tumor responses
bNon-responders; patients with no clinically significant biochemical or tumor responses
c P < 0.05 vs. responders
Table 2.
The relationship between the overall efficacy of CVD chemotherapy and the localization of sites of metastatic disease
| Case no. | Bone | Retroperitoneum | Lung | Liver | Local recurrence | |
|---|---|---|---|---|---|---|
| Partial/minimal response | 1 | + | ||||
| 2 | + | |||||
| 3 | + | |||||
| 4 | + | |||||
| 5 | + | |||||
| 6 | + | + | ||||
| 7 | + | + | + | |||
| 8 | + | + | + | + | + | |
| No change | 1 | + | ||||
| 2 | + | + | + | |||
| 3 | + | + | + | + | ||
| 4 | + | + | + | + | ||
| Progressive disease | 1 | + | ||||
| 2 | + | |||||
| 3 | + | + | ||||
| 4 | + | + | + | |||
| 5 | + | + | + | + |
Tumor Response
Although no patient had a complete tumor response, eight patients (47.1 %) had a partial or minimal response (Fig. 1), four patients (23.5 %) had no change, and five patients (29.4 %) had progressive disease.
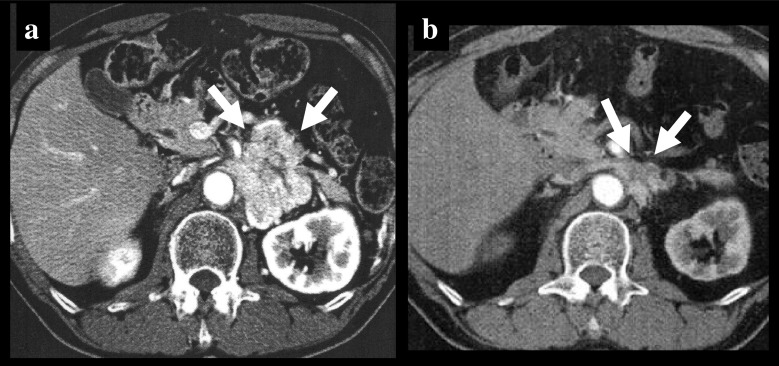
Fig. 1.
CT before (a) and 2 years after (b) CVD chemotherapy in a patient who showed partial response in a tumor. Arrows show anterior margins of left retroperitoneal tumor mass
Biochemical Response
Two patients (11.8 %) had a complete biochemical response, six patients (35.3 %) had a partial or minimal response, four patients (23.5 %) had no change, and five patients (29.4 %) had progressive disease. In the patients with partial biochemical response there was an improvement in diabetes mellitus and hypertension.
Overall Effectiveness
Complete or partial biochemical and/or partial or minimal tumor response was achieved in eight patients (47.1 %) (i.e., responders), including two patients with complete biochemical but partial tumor responses (Fig. 2a, b). Although the distinctive findings of tumor deposits on MRI T1-, T2-weighted images did not change, accumulation of 123-I-MIBG was reduced after CVD chemotherapy. No clinically significant biochemical or tumor responses were seen in four patients (23.5 %) and there was deterioration in biochemical and tumor responses in five patients (29.4 %) (i.e., non-responders). No patients showed complete biochemical and tumor responses.
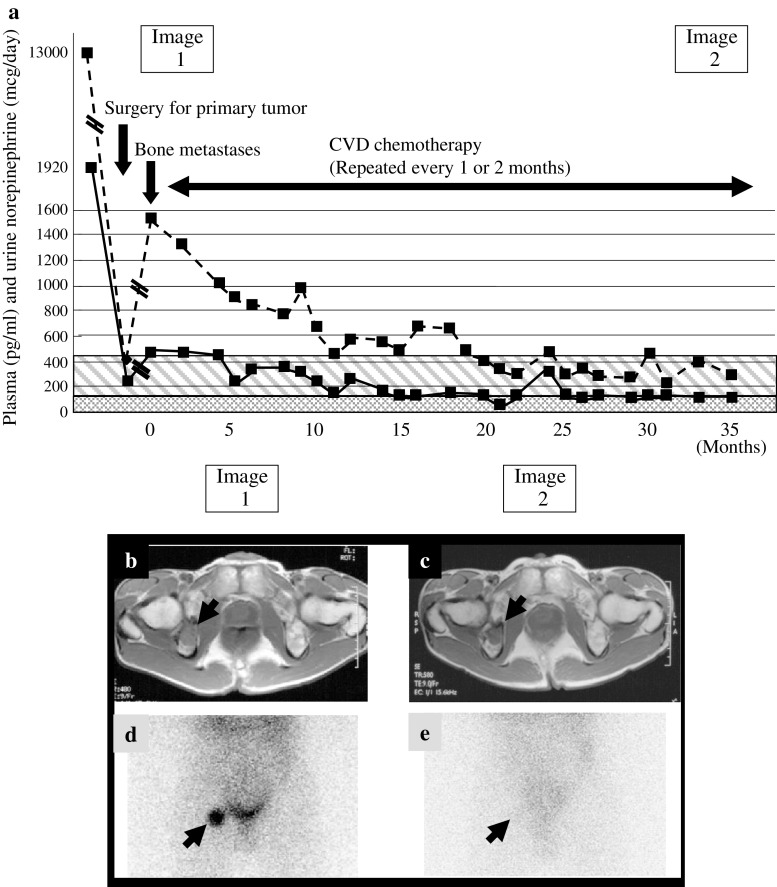
Fig. 2.
Clinical course (a) of a patient who showed dissociation between catecholamine levels and MRI findings (b, c). Despite biochemical responses there was no tumor response as measured by MRI. While the findings on 123-I-MIBG (d, e) did correlate with the decline in catecholamine levels during treatment with CVD. The solid line shows urine norepinephrine (in microgram per day) and the dotted line shows plasma norepinephrine (in picogram per milliliter)
In the eight responders, the positive effects of chemotherapy were detected from 1 to 4 months after initiation of CVD chemotherapy (median of 2.5 months); the period of stable disease (progression-free survival) was 31 to 60 months (median of 40 months). In the five non-responders, no effects of chemotherapy were detected though more than four cycles of CVD chemotherapy.
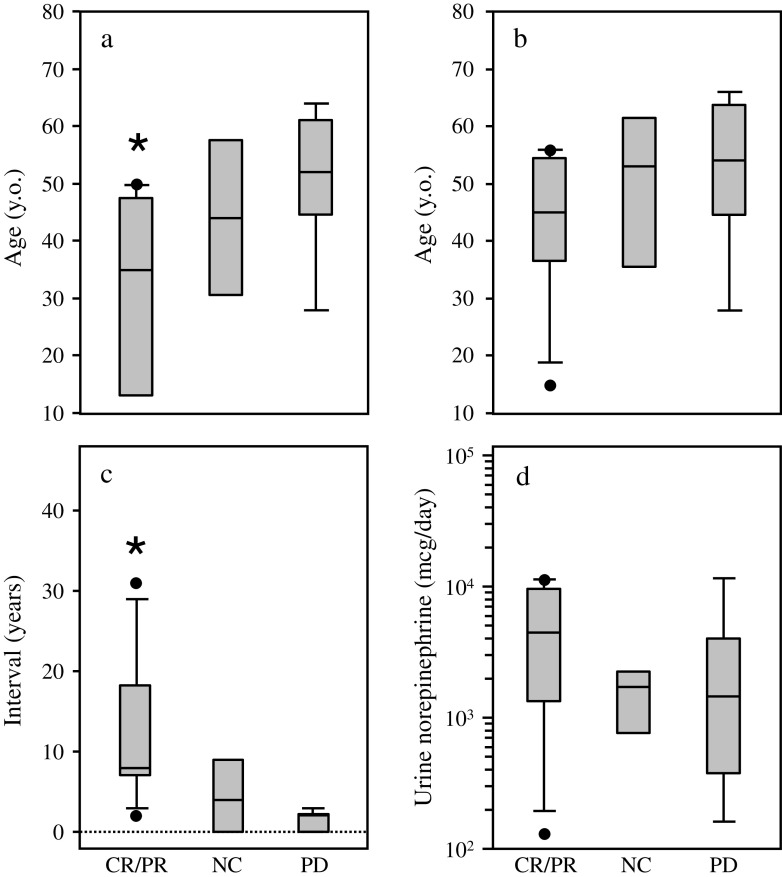
Compared to non-responders, the responders were younger at the time of diagnosis of PPGL (P < 0.05) (Fig. 3a) and they had a longer interval until malignant PPGL was detected (P < 0.05) (Fig. 3c). The age at diagnosis of malignant disease (Fig. 3b), catecholamine levels (Fig. 3d), and sites of metastatic disease were not correlated to the outcome of CVD chemotherapy (Table 2).
Fig. 3.
Box plots (showing median, 25th and 75th percentiles, and an outlier as a closed circle) of age at the first diagnosis of pheochromocytoma (a), age at diagnosis of malignant disease (b), interval between the diagnosis of pheochromocytoma or paraganglioma to the detection of malignant disease (c) and urine norepinephrine level in the patients who were treated with CVD chemotherapy. CR/PR complete or partial response, NC no change, PD progressive disease. *P < 0.05 vs. PD
Clinical Course After Progression-Free Survival
Three of eight responders were observed for less than 2 years after initiation of CVD chemotherapy and they were in progression-free survival. Three of eight responders died after relapse of disease, and the time interval between relapse of disease and death was from 4 to 7 years. Eight of nine non-responders died during follow-up; the time interval between discontinuing of CVD chemotherapy and death was from 4 to 7 years. Kaplan–Meier analysis showed that the 50 % survival was 6 years in the patients with complete or partial biochemical and/or partial tumor response (n = 8), 4 years in the patients with no significant biochemical or tumor response (n = 4), and 3 years in the patients with deterioration in biochemical and tumor responses (n = 5) (Fig. 4). There were no statistically significant differences in the mortality after initiation of CVD chemotherapy among three groups.
Fig. 4.
Kaplan–Meier curves which are shown for overall survival in patients with complete or partial biochemical and/or partial tumor response (dashed line), no significant biochemical or tumor response (dotted line) and deterioration in biochemical and tumor responses (solid line)
Benefits on Hypertension and Impaired Glucose Tolerance
The three patients with complete- and four patients with partial biochemical responses had improvements in hypertension, tachycardia and blood glucose. The improvements in blood pressure and blood glucose concentrations correlated the decline of urine and blood catecholamine levels. One patient was able to be withdrawn from insulin therapy. The patients who did not have biochemical response did not show any improvement in control of blood pressure, heart rate or glucose metabolism.
Adverse Effects
CVD chemotherapy was discontinued in three patients due to prolonged gastrointestinal symptoms (Grade 3), leukopenia (Grade 2), and liver dysfunction (Grade 2). In other patients, CVD chemotherapy was associated with leukopenia (Grade 2) and liver dysfunction (Grade 1) lasting for up to 1 week after each CVD cycle as major adverse effects. Gastrointestinal symptoms (Grade 2) and high fever (Grade 1) were classified as transient and mild adverse effects.
Discussion
In this report, we describe the response to CVD in 17 patients with malignant PPGL. Catecholamine or CgA levels normalized in two patients and either tumor volume or biochemical markers showed more than 30 % decrease in 47.1 % of the patients. Progression-free survival was 31 to 60 months. These outcomes are not inferior to other therapies that have been recently reported, such as tyrosine kinase inhibitor and other new anti-neoplastic therapies. For example, in a report of 17 patients with malignant PPGL [12], sunitinib treatment was associated with a partial response in three (17.6 %) patients and stable disease in five (29.4 %) patients, progression-free survival as determined by RECIST 1.1 criteria of 4.1 months. In addition, treatment with mTOR inhibitors have not been correlated with improved outcomes in patients with malignant PPGL [13].
In our study, the responders to CVD chemotherapy had a statistically significant longer interval from the time of first diagnosis of PPGL to the detection of metastasis compared to non-responders. This finding suggests that the impact of CVD chemotherapy might be greater in those patients with more slowly growing tumors. Thus, a relationship between rapid tumor proliferative potential and susceptibility to chemotherapeutic agents was not evident. We speculate that the effectiveness of CVD was attenuated in patients with more aggressive malignant PPGL because of limited cytoxicity of CVD. In addition, the efficacy of CVD chemotherapy was not correlated to the sites of metastatic disease or the baseline levels of catecholamines.
In every instance where there was a tumor volume reduction, there was an associated decrease in catecholamine levels. However, two patients showed normalization in catecholamine levels without the tumor volume reduction in their boney metastases—findings on T1–T2-weighted MRI did not change but the accumulation of 123-I-MIBG was reduced. Thus, we speculate that tumor cells may lose the ability to produce catecholamines by degeneration or fibrosis in the absence of tumor volume reduction. Therefore, the catecholamine response might be a better indicator of the effects of CVD chemotherapy than the tumor size on imaging. The effectiveness of CVD chemotherapy became obvious very early (1–4 months after initiating the CVD chemotherapy) in the responders—an observation that has been previously described [9]. Thus, it has been suggested that CVD chemotherapy should be discontinued if no biochemical or tumor responses are detected after 4–5 cycles.
The effectiveness of CVD chemotherapy in our study was not as high when compared to previous reports [8, 9]. Explanations for these different outcomes may relate to different dosages of CVD and differences in response to CVD due to an Asian genetic background. Averbuch et al. reported the dosage of cyclophosphamide and dacarbazine was increased by 10 % each cycle until myelosuppression was seen. However, we did not increase the doses of cyclophosphamide or dacarbazine because of the regulation of those medicines in our country. It has been reported that the treatment outcomes of anticancer drug could be differ due to racial genetic background [14]. Treatment outcomes may be impacted by variations of the expression of tumor growth factors and variations in genes that regulate drug metabolism and thus sensitivity to the drugs [14]. Moreover, it was reported that sunitinib was more beneficial in the patients with the germline mutations of the succinate dehydrogenase subunit (SDH) B gene [12]. Germline mutation testing was not performed in our patients. Although there are a few case reports of patients with SDHB mutations [15–17], the frequencies of mutations in SDHx has not been reported in Japanese PPGL patients. Therefore the relation between SDHx mutation and the effectiveness of CVD chemotherapy was not determined.
CVD chemotherapy is palliative [18], and after a period of progression-free survival, tumors grow and catecholamine increase despite continued CVD treatment. It has been reported that CVD chemotherapy did not affect the total survival in malignant PPGL [10]. In our study, although not statistically significant, the 50 % survival interval was better in the patients with complete or partial response than that in the patients with deterioration of disease. Another positive outcome of CVD chemotherapy in our study was improvement of control in blood pressure glucose metabolism in the responder group during progression-free survival.
There are some limitations of our study. Imaging studies do not always visualize all lesions in a patient. Accordingly, we may have some cases where the tumor response was not estimated precisely. In addition, CVD chemotherapy was not administered to all our patients with malignant PPGL. Because this study was a retrospective, nonrandomized, and the number of the patients was small, our results may be affected by selection bias. Due to the rarity of malignant PPGL, it would be difficult to perform a prospective study at one institution.
To date, there is no cure for malignant PPGL. Therefore, controlling chronic overproduction of catecholamines or blocking their effects is an important treatment goal. CVD chemotherapy is not associated with severe adverse effects and it does not need technical facilities that treatment with 131-I-MIBG requires. Our results suggest that CVD chemotherapy is a beneficial treatment with regard to the signs and symptoms related catecholamine excess, reduction in tumor volume, and increased progression-free survival.
In conclusion, we describe tumor response, biochemical response, and overall effectiveness of CVD chemotherapy in Asian patients with malignant PPGL. Although the efficacy was slightly inferior to that previously reported in Caucasian patients [8, 9, 19], the responders showed good control in blood pressure, heart rate and glucose metabolism during their progression-free survival.
Acknowledgments
Disclosure Summary
The authors have nothing to disclose.
References
- 1.O’Riordain DS, Young WF, Jr, Grant CS, Carney JA, van Heerden JA. Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg. 1996;20(7):916–921. doi: 10.1007/s002689900139. [DOI] [PubMed] [Google Scholar]
- 2.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr-Relat Cancer. 2007;14(3):569–585. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- 3.Mishra AK, Agarwal G, Kapoor A, Agarwal A, Bhatia E, Mishra SK. Catecholamine cardiomyopathy in bilateral malignant pheochromocytoma: successful reversal after surgery. Int J Cardiol. 2000;76(1):89–90. doi: 10.1016/S0167-5273(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 4.Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 5.Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S, Fitzgerald P. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27(25):4162–4168. doi: 10.1200/JCO.2008.21.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehnert H, Mundschenk J, Hahn K. Malignant pheochromocytoma. Front Horm Res. 2004;31:155–162. doi: 10.1159/000074663. [DOI] [PubMed] [Google Scholar]
- 7.Steinsapir J, Carr AA, Prisant LM, Bransome ED., Jr Metyrosine and pheochromocytoma. Arch Intern Med. 1997;157(8):901–906. doi: 10.1001/archinte.1997.00440290087009. [DOI] [PubMed] [Google Scholar]
- 8.Averbuch SD, Steakley CS, Young RC, et al. Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med. 1988;109(4):267–273. doi: 10.7326/0003-4819-109-4-267. [DOI] [PubMed] [Google Scholar]
- 9.Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, Pacak K, Fojo T. Treatment of malignant pheochromocytoma and paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113(8):2020–2028. doi: 10.1002/cncr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura K, Kimura H, Shimizu S, Kodama H, Okamoto T, Obara T, Takano K. Survival of patients with metastatic malignant pheochromocytoma and efficacy of combined cyclophosphamide, vincristine, and dacarbazine chemotherapy. J Clin Endocrinol Metab. 2009;94(8):2850–2856. doi: 10.1210/jc.2008-2697. [DOI] [PubMed] [Google Scholar]
- 11.Ayala-Ramirez M, Feng L, Habra MA, Rich T, Dickson PV, Perrier N, Phan A, Waguespack S, Patel S, Jimenez C. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: insights from the largest single-institutional experience. Cancer. 2012;118(11):2804–2812. doi: 10.1002/cncr.26577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayala-Ramirez M, Chougnet CN, Habra MA, Palmer JL, Leboulleux S, Cabanillas ME, Caramella C, Anderson P, Al Ghuzlan A, Waguespack SG, Deandreis D, Baudin E, Jimenez C. Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97(11):4040–4050. doi: 10.1210/jc.2012-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Druce MR, Kaltsas GA, Fraenkel M, Gross DJ, Grossman AB. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001) Horm Metab Res. 2009;41(9):697–702. doi: 10.1055/s-0029-1220687. [DOI] [PubMed] [Google Scholar]
- 14.Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol. 2010;11(1):75–84. doi: 10.1016/S1470-2045(09)70160-3. [DOI] [PubMed] [Google Scholar]
- 15.Sato H, Kanai G, Hirabayshi K, Kajiwara H, Itoh J, Osamura RY. L157X nonsense mutation of the succinate dehydrogenase subunit B gene in a Japanese patient with right paraaortic paraganglioma. Endocrine. 2010;38(1):18–23. doi: 10.1007/s12020-010-9365-x. [DOI] [PubMed] [Google Scholar]
- 16.Oishi Y, Nagai S, Yoshida M, Fujisawa S, Sazawa A, Shinohara N, Nonomura K, Matsuno K, Shimizu C. Mutation analysis of the SDHB and SDHD genes in pheochromocytomas and paragangliomas: identification of a novel nonsense mutation (Q168X) in the SDHB gene. Endocr J. 2010;57(8):745–750. doi: 10.1507/endocrj.K10E-023. [DOI] [PubMed] [Google Scholar]
- 17.Kodama H, Iihara M, Nissato S, Isobe K, Kawakami Y, Okamoto T, Takekoshi K. A large deletion in the succinate dehydrogenase B gene (SDHB) in a Japanese patient with abdominal paraganglioma and concomitant metastasis. Endocr J. 2010;57(4):351–356. doi: 10.1507/endocrj.K09E-324. [DOI] [PubMed] [Google Scholar]
- 18.Scholz T, Eisenhofer G, Pacak K, Dralle H, Lehnert H. Clinical review: current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 19.Patel SR, Winchester DJ, Benjamin RS. A 15-year experience with chemotherapy of patients with paraganglioma. Cancer. 1995;76(8):1476–1480. doi: 10.1002/1097-0142(19951015)76:8<1476::AID-CNCR2820760827>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]